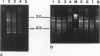
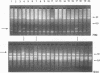
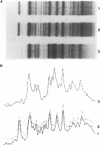
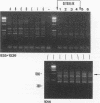
Abstract
Selected segments of any DNA molecule can be amplified exponentially by PCR. This technique provides a powerful tool to detect and identify minimal numbers of microorganisms. PCR is applicable both in diagnosis and in epidemiology. By amplification of hypervariable DNA domains, differences can be detected even among closely related strains. PCR fingerprinting is a valuable tool for medical microbiologists, epidemiologists, and microbial taxonomists. The current state of PCR-mediated genotyping is reviewed, and a comparison with conventional molecular typing methods is included. Because of its speed and versatility, PCR fingerprinting will play an important role in microbial genetics, epidemiology, and systematics.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akopyanz N., Bukanov N. O., Westblom T. U., Kresovich S., Berg D. E. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992 Oct 11;20(19):5137–5142. doi: 10.1093/nar/20.19.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annas G. J. Setting standards for the use of DNA-typing results in the courtroom--the state of the art. N Engl J Med. 1992 Jun 11;326(24):1641–1644. doi: 10.1056/NEJM199206113262418. [DOI] [PubMed] [Google Scholar]
- Aufauvre-Brown A., Cohen J., Holden D. W. Use of randomly amplified polymorphic DNA markers to distinguish isolates of Aspergillus fumigatus. J Clin Microbiol. 1992 Nov;30(11):2991–2993. doi: 10.1128/jcm.30.11.2991-2993.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barany F. The ligase chain reaction in a PCR world. PCR Methods Appl. 1991 Aug;1(1):5–16. doi: 10.1101/gr.1.1.5. [DOI] [PubMed] [Google Scholar]
- Bassam B. J., Caetano-Anollés G., Gresshoff P. M. DNA amplification fingerprinting of bacteria. Appl Microbiol Biotechnol. 1992 Oct;38(1):70–76. doi: 10.1007/BF00169422. [DOI] [PubMed] [Google Scholar]
- Bej A. K., Mahbubani M. H., Atlas R. M. Amplification of nucleic acids by polymerase chain reaction (PCR) and other methods and their applications. Crit Rev Biochem Mol Biol. 1991;26(3-4):301–334. doi: 10.3109/10409239109114071. [DOI] [PubMed] [Google Scholar]
- Ben-Ezra J., Johnson D. A., Rossi J., Cook N., Wu A. Effect of fixation on the amplification of nucleic acids from paraffin-embedded material by the polymerase chain reaction. J Histochem Cytochem. 1991 Mar;39(3):351–354. doi: 10.1177/39.3.1704393. [DOI] [PubMed] [Google Scholar]
- Bingen E., Boissinot C., Desjardins P., Cave H., Brahimi N., Lambert-Zechovsky N., Denamur E., Blot P., Elion J. Arbitrarily primed polymerase chain reaction provides rapid differentiation of Proteus mirabilis isolates from a pediatric hospital. J Clin Microbiol. 1993 May;31(5):1055–1059. doi: 10.1128/jcm.31.5.1055-1059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E., Denamur E., Lambert-Zechovsky N., Brahimi N., el Lakany M., Elion J. Rapid genotyping shows the absence of cross-contamination in Enterobacter cloacae nosocomial infections. J Hosp Infect. 1992 Jun;21(2):95–101. doi: 10.1016/0195-6701(92)90028-k. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., Schneider U., Thurston S. J. Fingerprinting genomes by use of PCR with primers that encode protein motifs or contain sequences that regulate gene expression. Mamm Genome. 1992;3(10):537–545. doi: 10.1007/BF00350618. [DOI] [PubMed] [Google Scholar]
- Boom R., Sol C. J., Salimans M. M., Jansen C. L., Wertheim-van Dillen P. M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990 Mar;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brousseau R., Saint-Onge A., Préfontaine G., Masson L., Cabana J. Arbitrary primer polymerase chain reaction, a powerful method to identify Bacillus thuringiensis serovars and strains. Appl Environ Microbiol. 1993 Jan;59(1):114–119. doi: 10.1128/aem.59.1.114-119.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck G. E., O'Hara L. C., Summersgill J. T. Rapid, simple method for treating clinical specimens containing Mycobacterium tuberculosis to remove DNA for polymerase chain reaction. J Clin Microbiol. 1992 May;30(5):1331–1334. doi: 10.1128/jcm.30.5.1331-1334.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstain J. M., Grimprel E., Lukehart S. A., Norgard M. V., Radolf J. D. Sensitive detection of Treponema pallidum by using the polymerase chain reaction. J Clin Microbiol. 1991 Jan;29(1):62–69. doi: 10.1128/jcm.29.1.62-69.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano-Anollés G., Bassam B. J., Gresshoff P. M. Primer-template interactions during DNA amplification fingerprinting with single arbitrary oligonucleotides. Mol Gen Genet. 1992 Nov;235(2-3):157–165. doi: 10.1007/BF00279356. [DOI] [PubMed] [Google Scholar]
- Cancilla M. R., Powell I. B., Hillier A. J., Davidson B. E. Rapid genomic fingerprinting of Lactococcus lactis strains by arbitrarily primed polymerase chain reaction with 32P and fluorescent labels. Appl Environ Microbiol. 1992 May;58(5):1772–1775. doi: 10.1128/aem.58.5.1772-1775.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassol S., Rudnik J., Salas T., Montpetit M., Pon R. T., Sy C. T., Read S., Major C., O'Shaughnessy M. V. Rapid DNA fingerprinting to control for specimen errors in HIV testing by the polymerase chain reaction. Mol Cell Probes. 1992 Aug;6(4):327–331. doi: 10.1016/0890-8508(92)90009-m. [DOI] [PubMed] [Google Scholar]
- Chakraborty R., Kidd K. K. The utility of DNA typing in forensic work. Science. 1991 Dec 20;254(5039):1735–1739. doi: 10.1126/science.1763323. [DOI] [PubMed] [Google Scholar]
- Colman S. D., Hu P. C., Bott K. F. Prevalence of novel repeat sequences in and around the P1 operon in the genome of Mycoplasma pneumoniae. Gene. 1990 Mar 1;87(1):91–96. doi: 10.1016/0378-1119(90)90498-g. [DOI] [PubMed] [Google Scholar]
- Compton J. Nucleic acid sequence-based amplification. Nature. 1991 Mar 7;350(6313):91–92. doi: 10.1038/350091a0. [DOI] [PubMed] [Google Scholar]
- Correia F. F., Inouye S., Inouye M. A 26-base-pair repetitive sequence specific for Neisseria gonorrhoeae and Neisseria meningitidis genomic DNA. J Bacteriol. 1986 Sep;167(3):1009–1015. doi: 10.1128/jb.167.3.1009-1015.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowhurst R. N., Hawthorne B. T., Rikkerink E. H., Templeton M. D. Differentiation of Fusarium solani f. sp. cucurbitae races 1 and 2 by random amplification of polymorphic DNA. Curr Genet. 1991 Nov;20(5):391–396. doi: 10.1007/BF00317067. [DOI] [PubMed] [Google Scholar]
- Czajka J., Bsat N., Piani M., Russ W., Sultana K., Wiedmann M., Whitaker R., Batt C. A. Differentiation of Listeria monocytogenes and Listeria innocua by 16S rRNA genes and intraspecies discrimination of Listeria monocytogenes strains by random amplified polymorphic DNA polymorphisms. Appl Environ Microbiol. 1993 Jan;59(1):304–308. doi: 10.1128/aem.59.1.304-308.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D., Schachter J., Dawson C. R., Stephens R. S. Comparison of the major outer membrane protein variant sequence regions of B/Ba isolates: a molecular epidemiologic approach to Chlamydia trachomatis infections. J Infect Dis. 1992 Aug;166(2):383–392. doi: 10.1093/infdis/166.2.383. [DOI] [PubMed] [Google Scholar]
- Devlin B., Risch N., Roeder K. Statistical evaluation of DNA fingerprinting: a critique of the NRC's report. Science. 1993 Feb 5;259(5096):748-9, 837. doi: 10.1126/science.8430323. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I. New molecular techniques for microbial epidemiology and the diagnosis of infectious diseases. J Infect Dis. 1990 Apr;161(4):595–602. doi: 10.1093/infdis/161.4.595. [DOI] [PubMed] [Google Scholar]
- Endtz H. P., Giesendorf B. A., van Belkum A., Lauwers S. J., Jansen W. H., Quint W. G. PCR-mediated DNA typing of Campylobacter jejuni isolated from patients with recurrent infections. Res Microbiol. 1993 Nov-Dec;144(9):703–708. doi: 10.1016/0923-2508(93)90034-y. [DOI] [PubMed] [Google Scholar]
- Espy M. J., Smith T. F., Persing D. H. Dependence of polymerase chain reaction product inactivation protocols on amplicon length and sequence composition. J Clin Microbiol. 1993 Sep;31(9):2361–2365. doi: 10.1128/jcm.31.9.2361-2365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete A., Bantle J. A., Halling S. M., Stich R. W. Amplification fragment length polymorphism in Brucella strains by use of polymerase chain reaction with arbitrary primers. J Bacteriol. 1992 Dec;174(23):7778–7783. doi: 10.1128/jb.174.23.7778-7783.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitani S., Komano T., Inouye S. A unique repetitive DNA sequence in the Myxococcus xanthus genome. J Bacteriol. 1991 Mar;173(6):2125–2127. doi: 10.1128/jb.173.6.2125-2127.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesendorf B. A., Goossens H., Niesters H. G., Van Belkum A., Koeken A., Endtz H. P., Stegeman H., Quint W. G. Polymerase chain reaction-mediated DNA fingerprinting for epidemiological studies on Campylobacter spp. J Med Microbiol. 1994 Feb;40(2):141–147. doi: 10.1099/00222615-40-2-141. [DOI] [PubMed] [Google Scholar]
- Giesendorf B. A., van Belkum A., Koeken A., Stegeman H., Henkens M. H., van der Plas J., Goossens H., Niesters H. G., Quint W. G. Development of species-specific DNA probes for Campylobacter jejuni, Campylobacter coli, and Campylobacter lari by polymerase chain reaction fingerprinting. J Clin Microbiol. 1993 Jun;31(6):1541–1546. doi: 10.1128/jcm.31.6.1541-1546.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson E., Clément J. M., Brutlag D., Hofnung M. A family of dispersed repetitive extragenic palindromic DNA sequences in E. coli. EMBO J. 1984 Jun;3(6):1417–1421. doi: 10.1002/j.1460-2075.1984.tb01986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagelberg E., Gray I. C., Jeffreys A. J. Identification of the skeletal remains of a murder victim by DNA analysis. Nature. 1991 Aug 1;352(6334):427–429. doi: 10.1038/352427a0. [DOI] [PubMed] [Google Scholar]
- Hayes L. J., Bailey R. L., Mabey D. C., Clarke I. N., Pickett M. A., Watt P. J., Ward M. E. Genotyping of Chlamydia trachomatis from a trachoma-endemic village in the Gambia by a nested polymerase chain reaction: identification of strain variants. J Infect Dis. 1992 Nov;166(5):1173–1177. doi: 10.1093/infdis/166.5.1173. [DOI] [PubMed] [Google Scholar]
- Hennessy K. J., Iandolo J. J., Fenwick B. W. Serotype identification of Actinobacillus pleuropneumoniae by arbitrarily primed polymerase chain reaction. J Clin Microbiol. 1993 May;31(5):1155–1159. doi: 10.1128/jcm.31.5.1155-1159.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Ames G. F., Barnes W. M., Clement J. M., Hofnung M. A novel intercistronic regulatory element of prokaryotic operons. Nature. 1982 Aug 19;298(5876):760–762. doi: 10.1038/298760a0. [DOI] [PubMed] [Google Scholar]
- Hulton C. S., Higgins C. F., Sharp P. M. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol Microbiol. 1991 Apr;5(4):825–834. doi: 10.1111/j.1365-2958.1991.tb00755.x. [DOI] [PubMed] [Google Scholar]
- Janse C. J., Ramesar J., Mons B. Chromosome translocation in Plasmodium berghei. Nucleic Acids Res. 1992 Feb 11;20(3):581–586. doi: 10.1093/nar/20.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayarao B. M., Bassam B. J., Caetano-Anollés G., Gresshoff P. M., Oliver S. P. Subtyping of Streptococcus uberis by DNA amplification fingerprinting. J Clin Microbiol. 1992 May;30(5):1347–1350. doi: 10.1128/jcm.30.5.1347-1350.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., MacLeod A., Tamaki K., Neil D. L., Monckton D. G. Minisatellite repeat coding as a digital approach to DNA typing. Nature. 1991 Nov 21;354(6350):204–209. doi: 10.1038/354204a0. [DOI] [PubMed] [Google Scholar]
- Kersulyte D., Woods J. P., Keath E. J., Goldman W. E., Berg D. E. Diversity among clinical isolates of Histoplasma capsulatum detected by polymerase chain reaction with arbitrary primers. J Bacteriol. 1992 Nov;174(22):7075–7079. doi: 10.1128/jb.174.22.7075-7079.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievits T., van Gemen B., van Strijp D., Schukkink R., Dircks M., Adriaanse H., Malek L., Sooknanan R., Lens P. NASBA isothermal enzymatic in vitro nucleic acid amplification optimized for the diagnosis of HIV-1 infection. J Virol Methods. 1991 Dec;35(3):273–286. doi: 10.1016/0166-0934(91)90069-c. [DOI] [PubMed] [Google Scholar]
- Kramer F. R., Lizardi P. M. Replicatable RNA reporters. Nature. 1989 Jun 1;339(6223):401–402. doi: 10.1038/339401a0. [DOI] [PubMed] [Google Scholar]
- Kuypers J. M., Critchlow C. W., Gravitt P. E., Vernon D. A., Sayer J. B., Manos M. M., Kiviat N. B. Comparison of dot filter hybridization, Southern transfer hybridization, and polymerase chain reaction amplification for diagnosis of anal human papillomavirus infection. J Clin Microbiol. 1993 Apr;31(4):1003–1006. doi: 10.1128/jcm.31.4.1003-1006.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Lawyer F. C., Stoffel S., Saiki R. K., Myambo K., Drummond R., Gelfand D. H. Isolation, characterization, and expression in Escherichia coli of the DNA polymerase gene from Thermus aquaticus. J Biol Chem. 1989 Apr 15;264(11):6427–6437. [PubMed] [Google Scholar]
- Lehmann P. F., Lin D., Lasker B. A. Genotypic identification and characterization of species and strains within the genus Candida by using random amplified polymorphic DNA. J Clin Microbiol. 1992 Dec;30(12):3249–3254. doi: 10.1128/jcm.30.12.3249-3254.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon E., Gutman P. D., Yao H. L., Minton K. W. A highly conserved repeated chromosomal sequence in the radioresistant bacterium Deinococcus radiodurans SARK. J Bacteriol. 1991 Mar;173(6):2137–2140. doi: 10.1128/jb.173.6.2137-2140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson G., Gutman G. A. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol. 1987 May;4(3):203–221. doi: 10.1093/oxfordjournals.molbev.a040442. [DOI] [PubMed] [Google Scholar]
- LiPuma J. J., Stull T. L., Dasen S. E., Pidcock K. A., Kaye D., Korzeniowski O. M. DNA polymorphisms among Escherichia coli isolated from bacteriuric women. J Infect Dis. 1989 Mar;159(3):526–532. doi: 10.1093/infdis/159.3.526. [DOI] [PubMed] [Google Scholar]
- Loudon K. W., Burnie J. P., Coke A. P., Matthews R. C. Application of polymerase chain reaction to fingerprinting Aspergillus fumigatus by random amplification of polymorphic DNA. J Clin Microbiol. 1993 May;31(5):1117–1121. doi: 10.1128/jcm.31.5.1117-1121.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski J. R., Weinstock G. M. Short, interspersed repetitive DNA sequences in prokaryotic genomes. J Bacteriol. 1992 Jul;174(14):4525–4529. doi: 10.1128/jb.174.14.4525-4529.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurier S. I., Audurier A., Marquet-Van der Mee N., Notermans S., Wernars K. A comparative study of randomly amplified polymorphic DNA analysis and conventional phage typing for epidemiological studies of Listeria monocytogenes isolates. Res Microbiol. 1992 Jun;143(5):507–512. doi: 10.1016/0923-2508(92)90097-8. [DOI] [PubMed] [Google Scholar]
- Mazurier S. I., Wernars K. Typing of Listeria strains by random amplification of polymorphic DNA. Res Microbiol. 1992 Jun;143(5):499–505. doi: 10.1016/0923-2508(92)90096-7. [DOI] [PubMed] [Google Scholar]
- Mazurier S., van de Giessen A., Heuvelman K., Wernars K. RAPD analysis of Campylobacter isolates: DNA fingerprinting without the need to purify DNA. Lett Appl Microbiol. 1992 Jun;14(6):260–262. doi: 10.1111/j.1472-765x.1992.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Myers L. E., Silva S. V., Procunier J. D., Little P. B. Genomic fingerprinting of "Haemophilus somnus" isolates by using a random-amplified polymorphic DNA assay. J Clin Microbiol. 1993 Mar;31(3):512–517. doi: 10.1128/jcm.31.3.512-517.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard C., Brousseau R., Mouton C. Application of polymerase chain reaction with arbitrary primer (AP-PCR) to strain identification of Porphyromonas (Bacteroides) gingivalis. FEMS Microbiol Lett. 1992 Aug 15;74(2-3):163–168. doi: 10.1111/j.1574-6968.1992.tb05360.x. [DOI] [PubMed] [Google Scholar]
- Nastasi A., Pignato S., Mammina C., Giammanco G. rRNA gene restriction patterns and biotypes of Shigella sonnei. Epidemiol Infect. 1993 Feb;110(1):23–30. doi: 10.1017/s0950268800050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton C. M., Wachsmuth I. K., Evins G. M., Kiehlbauch J. A., Plikaytis B. D., Troup N., Tompkins L., Lior H. Evaluation of 10 methods to distinguish epidemic-associated Campylobacter strains. J Clin Microbiol. 1991 Apr;29(4):680–688. doi: 10.1128/jcm.29.4.680-688.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persing D. H., Landry M. L. In vitro amplification techniques for the detection of nucleic acids: new tools for the diagnostic laboratory. Yale J Biol Med. 1989 Mar-Apr;62(2):159–171. [PMC free article] [PubMed] [Google Scholar]
- Peter J. B. The polymerase chain reaction: amplifying our options. Rev Infect Dis. 1991 Jan-Feb;13(1):166–171. doi: 10.1093/clinids/12.5.166. [DOI] [PubMed] [Google Scholar]
- Peterson W. L. Helicobacter pylori and peptic ulcer disease. N Engl J Med. 1991 Apr 11;324(15):1043–1048. doi: 10.1056/NEJM199104113241507. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A. Epidemiological typing methods for mycoses. Clin Infect Dis. 1992 Mar;14 (Suppl 1):S4–10. doi: 10.1093/clinids/14.supplement_1.s4. [DOI] [PubMed] [Google Scholar]
- Puchhammer-Stöckl E., Heinz F. X., Kundi M., Popow-Kraupp T., Grimm G., Millner M. M., Kunz C. Evaluation of the polymerase chain reaction for diagnosis of herpes simplex virus encephalitis. J Clin Microbiol. 1993 Jan;31(1):146–148. doi: 10.1128/jcm.31.1.146-148.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Higuchi R. G., Wilson A. C. Ancient DNA and the polymerase chain reaction. The emerging field of molecular archaeology. J Biol Chem. 1989 Jun 15;264(17):9709–9712. [PubMed] [Google Scholar]
- Ralph D., McClelland M., Welsh J., Baranton G., Perolat P. Leptospira species categorized by arbitrarily primed polymerase chain reaction (PCR) and by mapped restriction polymorphisms in PCR-amplified rRNA genes. J Bacteriol. 1993 Feb;175(4):973–981. doi: 10.1128/jb.175.4.973-981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley D. E., Samadpour M., Krieger J. N. Detection of variable DNA repeats in diverse eukaryotic microorganisms by a single set of polymerase chain reaction primers. J Clin Microbiol. 1991 Dec;29(12):2746–2751. doi: 10.1128/jcm.29.12.2746-2751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B. C., Dwyer B. Rapid, simple method for typing isolates of Mycobacterium tuberculosis by using the polymerase chain reaction. J Clin Microbiol. 1993 Feb;31(2):329–334. doi: 10.1128/jcm.31.2.329-334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rys P. N., Persing D. H. Preventing false positives: quantitative evaluation of three protocols for inactivation of polymerase chain reaction amplification products. J Clin Microbiol. 1993 Sep;31(9):2356–2360. doi: 10.1128/jcm.31.9.2356-2360.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sarafis K., Isaac-Renton J. Pulsed-field gel electrophoresis as a method of biotyping of Giardia duodenalis. Am J Trop Med Hyg. 1993 Jan;48(1):134–144. doi: 10.4269/ajtmh.1993.48.134. [DOI] [PubMed] [Google Scholar]
- Saulnier P., Bourneix C., Prévost G., Andremont A. Random amplified polymorphic DNA assay is less discriminant than pulsed-field gel electrophoresis for typing strains of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1993 Apr;31(4):982–985. doi: 10.1128/jcm.31.4.982-985.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal S. E., Jackson L. A., Daniels M. J. Use of tRNA consensus primers to indicate subgroups of Pseudomonas solanacearum by polymerase chain reaction amplification. Appl Environ Microbiol. 1992 Nov;58(11):3759–3761. doi: 10.1128/aem.58.11.3759-3761.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples G. J., Lloyd R. G. A novel repeated DNA sequence located in the intergenic regions of bacterial chromosomes. Nucleic Acids Res. 1990 Nov 25;18(22):6503–6508. doi: 10.1093/nar/18.22.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Boothroyd J. C. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature. 1992 Sep 3;359(6390):82–85. doi: 10.1038/359082a0. [DOI] [PubMed] [Google Scholar]
- Skakni L., Sardet A., Just J., Landman-Parker J., Costil J., Moniot-Ville N., Bricout F., Garbarg-Chenon A. Detection of Mycoplasma pneumoniae in clinical samples from pediatric patients by polymerase chain reaction. J Clin Microbiol. 1992 Oct;30(10):2638–2643. doi: 10.1128/jcm.30.10.2638-2643.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Cantor C. R. Purification, specific fragmentation, and separation of large DNA molecules. Methods Enzymol. 1987;155:449–467. doi: 10.1016/0076-6879(87)55030-3. [DOI] [PubMed] [Google Scholar]
- Snijders P. J., Meijer C. J., Walboomers J. M. Degenerate primers based on highly conserved regions of amino acid sequence in papillomaviruses can be used in a generalized polymerase chain reaction to detect productive human papillomavirus infection. J Gen Virol. 1991 Nov;72(Pt 11):2781–2786. doi: 10.1099/0022-1317-72-11-2781. [DOI] [PubMed] [Google Scholar]
- Struelens M. J., Bax R., Deplano A., Quint W. G., Van Belkum A. Concordant clonal delineation of methicillin-resistant Staphylococcus aureus by macrorestriction analysis and polymerase chain reaction genome fingerprinting. J Clin Microbiol. 1993 Aug;31(8):1964–1970. doi: 10.1128/jcm.31.8.1964-1970.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struelens M. J., Carlier E., Maes N., Serruys E., Quint W. G., van Belkum A. Nosocomial colonization and infection with multiresistant Acinetobacter baumannii: outbreak delineation using DNA macrorestriction analysis and PCR-fingerprinting. J Hosp Infect. 1993 Sep;25(1):15–32. doi: 10.1016/0195-6701(93)90005-k. [DOI] [PubMed] [Google Scholar]
- Struelens M. J., Maes N., Rost F., Deplano A., Jacobs F., Liesnard C., Bornstein N., Grimont F., Lauwers S., McIntyre M. P. Genotypic and phenotypic methods for the investigation of a nosocomial Legionella pneumophila outbreak and efficacy of control measures. J Infect Dis. 1992 Jul;166(1):22–30. doi: 10.1093/infdis/166.1.22. [DOI] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Subramanian P. S., Versalovic J., McCabe E. R., Lupski J. R. Rapid mapping of Escherichia coli::Tn5 insertion mutations by REP-Tn5 PCR. PCR Methods Appl. 1992 Feb;1(3):187–192. doi: 10.1101/gr.1.3.187. [DOI] [PubMed] [Google Scholar]
- Tibayrenc M., Neubauer K., Barnabé C., Guerrini F., Skarecky D., Ayala F. J. Genetic characterization of six parasitic protozoa: parity between random-primer DNA typing and multilocus enzyme electrophoresis. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1335–1339. doi: 10.1073/pnas.90.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upcroft P., Mitchell R., Boreham P. F. DNA fingerprinting of the intestinal parasite Giardia duodenalis with the M13 phage genome. Int J Parasitol. 1990 May;20(3):319–323. doi: 10.1016/0020-7519(90)90146-e. [DOI] [PubMed] [Google Scholar]
- Versalovic J., Koeuth T., Lupski J. R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991 Dec 25;19(24):6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. Potential genetic functions of tandem repeated DNA sequence blocks in the human genome are based on a highly conserved "chromatin folding code". Hum Genet. 1990 Mar;84(4):301–336. doi: 10.1007/BF00196228. [DOI] [PubMed] [Google Scholar]
- Wakefield A. E., Guiver L., Miller R. F., Hopkin J. M. DNA amplification on induced sputum samples for diagnosis of Pneumocystis carinii pneumonia. Lancet. 1991 Jun 8;337(8754):1378–1379. doi: 10.1016/0140-6736(91)93062-e. [DOI] [PubMed] [Google Scholar]
- Welsh J., Chada K., Dalal S. S., Cheng R., Ralph D., McClelland M. Arbitrarily primed PCR fingerprinting of RNA. Nucleic Acids Res. 1992 Oct 11;20(19):4965–4970. doi: 10.1093/nar/20.19.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Genomic fingerprinting using arbitrarily primed PCR and a matrix of pairwise combinations of primers. Nucleic Acids Res. 1991 Oct 11;19(19):5275–5279. doi: 10.1093/nar/19.19.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., Petersen C., McClelland M. Polymorphisms generated by arbitrarily primed PCR in the mouse: application to strain identification and genetic mapping. Nucleic Acids Res. 1991 Jan 25;19(2):303–306. doi: 10.1093/nar/19.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., Pretzman C., Postic D., Saint Girons I., Baranton G., McClelland M. Genomic fingerprinting by arbitrarily primed polymerase chain reaction resolves Borrelia burgdorferi into three distinct phyletic groups. Int J Syst Bacteriol. 1992 Jul;42(3):370–377. doi: 10.1099/00207713-42-3-370. [DOI] [PubMed] [Google Scholar]
- Wenzel R., Herrmann R. Repetitive DNA sequences in Mycoplasma pneumoniae. Nucleic Acids Res. 1988 Sep 12;16(17):8337–8350. doi: 10.1093/nar/16.17.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. J., Arnheim N., Erlich H. A. The polymerase chain reaction. Trends Genet. 1989 Jun;5(6):185–189. doi: 10.1016/0168-9525(89)90073-5. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990 Nov 25;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Barbour A. G., Bergström S., Burman N., Restrepo B. I., Rosa P. A., Schwan T., Soutschek E., Wallich R. Antigenic variation and strain heterogeneity in Borrelia spp. Res Microbiol. 1992 Jul-Aug;143(6):583–596. doi: 10.1016/0923-2508(92)90116-6. [DOI] [PubMed] [Google Scholar]
- Wilson M. A., Rimler R. B., Hoffman L. J. Comparison of DNA fingerprints and somatic serotypes of serogroup B and E Pasteurella multocida isolates. J Clin Microbiol. 1992 Jun;30(6):1518–1524. doi: 10.1128/jcm.30.6.1518-1524.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods C. R., Jr, Versalovic J., Koeuth T., Lupski J. R. Analysis of relationships among isolates of Citrobacter diversus by using DNA fingerprints generated by repetitive sequence-based primers in the polymerase chain reaction. J Clin Microbiol. 1992 Nov;30(11):2921–2929. doi: 10.1128/jcm.30.11.2921-2929.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods C. R., Versalovic J., Koeuth T., Lupski J. R. Whole-cell repetitive element sequence-based polymerase chain reaction allows rapid assessment of clonal relationships of bacterial isolates. J Clin Microbiol. 1993 Jul;31(7):1927–1931. doi: 10.1128/jcm.31.7.1927-1931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. P., Kersulyte D., Goldman W. E., Berg D. E. Fast DNA isolation from Histoplasma capsulatum: methodology for arbitrary primer polymerase chain reaction-based epidemiological and clinical studies. J Clin Microbiol. 1993 Feb;31(2):463–464. doi: 10.1128/jcm.31.2.463-464.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegle J. S., Su Y., Corcoran K. P., Nie L., Mayrand P. E., Hoff L. B., McBride L. J., Kronick M. N., Diehl S. R. Application of automated DNA sizing technology for genotyping microsatellite loci. Genomics. 1992 Dec;14(4):1026–1031. doi: 10.1016/s0888-7543(05)80126-0. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J. Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergeneric consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl Environ Microbiol. 1992 Jul;58(7):2180–2187. doi: 10.1128/aem.58.7.2180-2187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum A., Bax R., Peerbooms P., Goessens W. H., van Leeuwen N., Quint W. G. Comparison of phage typing and DNA fingerprinting by polymerase chain reaction for discrimination of methicillin-resistant Staphylococcus aureus strains. J Clin Microbiol. 1993 Apr;31(4):798–803. doi: 10.1128/jcm.31.4.798-803.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum A., De Jonckheere J., Quint W. G. Genotyping Naegleria spp. and Naegleria fowleri isolates by interrepeat polymerase chain reaction. J Clin Microbiol. 1992 Oct;30(10):2595–2598. doi: 10.1128/jcm.30.10.2595-2598.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum A., Homan W., Limper L., Quint W. G. Genotyping isolates and clones of Giardia duodenalis by polymerase chain reaction: implications for the detection of genetic variation among protozoan parasite species. Mol Biochem Parasitol. 1993 Sep;61(1):69–77. doi: 10.1016/0166-6851(93)90159-u. [DOI] [PubMed] [Google Scholar]
- van Belkum A., Quint W. G., de Pauw B. E., Melchers W. J., Meis J. F. Typing of Aspergillus species and Aspergillus fumigatus isolates by interrepeat polymerase chain reaction. J Clin Microbiol. 1993 Sep;31(9):2502–2505. doi: 10.1128/jcm.31.9.2502-2505.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum A., Ramesar J., Trommelen G., Uitterlinden A. G. Mini- and micro-satellites in the genome of rodent malaria parasites. Gene. 1992 Sep 1;118(1):81–86. doi: 10.1016/0378-1119(92)90251-j. [DOI] [PubMed] [Google Scholar]
- van Belkum A., Struelens M., Quint W. Typing of Legionella pneumophila strains by polymerase chain reaction-mediated DNA fingerprinting. J Clin Microbiol. 1993 Aug;31(8):2198–2200. doi: 10.1128/jcm.31.8.2198-2200.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum A., Trommelen G., Uitterlinden A. Minisatellite-like repeat sequences in the genome of rodent malaria parasites. Acta Leiden. 1991;60(1):83–91. [PubMed] [Google Scholar]