Abstract
The misconception that infertility is typically associated with the female is commonly faced in the management of infertile men. It is uncommon for a patient to present for an infertility evaluation with an abnormal semen analysis report before an extensive female partner workup has been performed. Additionally, a man is usually considered fertile based only on seminal parameters without a physical exam. This behavior may lead to a delay in both the exact diagnosis and in possible specific infertility treatment. Moreover, male factor infertility can result from an underlying medical condition that is often treatable but could possibly be life-threatening.
The responsibility of male factor in couple's infertility has been exponentially rising in recent years due to a comprehensive evaluation of reproductive male function and improved diagnostic tools. Despite this improvement in diagnosis, azoospermia is always the most challenging topic associated with infertility treatment. Several conditions that interfere with spermatogenesis and reduce sperm production and quality can lead to azoospermia. Azoospermia may also occur because of a reproductive tract obstruction. Optimal management of patients with azoospermia requires a full understanding of the disease etiology. This review will discuss in detail the epidemiology and etiology of azoospermia. A thorough literature survey was performed using the Medline, EMBASE, BIOSIS, and Cochrane databases. We restricted the survey to clinical publications that were relevant to male infertility and azoospermia. Many of the recommendations included are not based on controlled studies.
Keywords: Male Infertility, Azoospermia, Semen Analysis
INTRODUCTION
The development of intracytoplasmic sperm injection (ICSI) as an efficient therapy for severe male factor infertility has become an appropriate treatment for the majority of male reproductive tract deficiencies (1). Usually, even men with potentially treatable causes of infertility are treated with assisted reproductive techniques (ARTs) instead of specific therapy. However, once the diagnosis of azoospermia is established, no sperm can be found in the ejaculate; as a consequence, assisted reproduction cannot be applied due to the absence of sperm. Therefore, an understanding of azoospermia is very important for urologists.
Azoospermia, defined as the absence of sperm in the ejaculate, is identified in approximately 1% of all men and in 10 to 15% of infertile males (2). A precise diagnosis of azoospermia and systematic evaluation of the patient to establish the disease etiology are needed to guide appropriate management options and to determine the associated cost benefits, risks and prognosis for treatment success. Clinicians should also provide adequate counseling for the couple and generous support for patients with severe male factor infertility.
In the past, men with azoospermia were classified as infertile, and a sperm donor was initially considered one of the best options for conceiving. Currently, the knowledge that many causes of azoospermia can be reversed is widespread in the medical literature and practice. Thus, any trusted specialized assisted reproductive center will request a urologist/andrologist to provide sperm for an ART procedure. As a result, the urologist, even if he/she is not a specialist in the infertility field, is responsible for the adequate evaluation, diagnosis and treatment of the underlying condition whenever possible instead of only providing sperm extracted from the testicle or epididymis. This review discusses the most common causes of azoospermia that should be considered during the management of azoospermic men. We also included algorithms that will help to clarify and organize the etiologies and mechanisms of azoospermia.
DIAGNOSIS OF AZOOSPERMIA
Azoospermia is defined as the complete absence of sperm from the ejaculate. This diagnosis must be confirmed by centrifugation of a semen specimen for 15 min at room temperature with high-powered microscopic examination of the pellet and a centrifugation speed of at least 3,000 g (3).
The semen analysis should be performed according to the 2010 World Health Organization guidelines, and at least two semen samples obtained more than two weeks apart should be examined (3,4).
The finding of even small quantities of sperm in the centrifuged specimen excludes complete ductal obstruction and offers the potential for immediate sperm cryopreservation for ICSI cycles. Ron-El et al. showed that it is possible to detect sperm in 35% of the men who were thought to have nonobstructive azoospermia when a meticulous routine analysis of a centrifuged semen specimen was performed (5). These findings are interesting because patients routinely present with one or more semen analyses that were previously performed without using standardized centrifugation procedures. Urologists must always consider the need to repeat the seminal analysis to consistently confirm the diagnosis of azoospermia.
EVALUATION OF AZOOSPERMIC PATIENTS
Medical history
A complete evaluation should include a complete medical and surgical history, history of childhood illnesses (such as viral orchitis or cryptorchidism) and genital trauma, medications and allergies, and an inspection of past infections, such as sexually transmitted diseases (6). It is important to assess gonadotoxin exposures and prior radiation therapy or chemotherapy. In addition, approximately 1% of male infertility cases may be a consequence of a serious or potentially fatal disorder (7). Thus, it is always important to recognize that infertility may be the initial manifestation of a severe medical condition (8).
Physical examination
A general physical examination is an essential part of the evaluation of an azoospermic man, and the patient should be examined in the supine and standing position in a warm room. A cold room causes contraction of the dartos and makes the examination difficult.
The presence of clinical varicocele should be investigated and correctly classification, as some recent studies have shown that varicocele grade is related to the prognosis of treatment (9-12). These findings suggest that high-grade varicocele could be more frequently related to azoospermia, whereas varicocele grade I is not sufficient to explain the entire disease etiology.
Appropriate sexual development must be assessed. Androgen deficiency should be suspected in the presence of diminished body hair distribution, gynecomastia or eunuchoid proportions (13). During the physical examination, men who are incompletely masculinized can be identified by excessively long extremities that occurred due to the absence of adequate epiphyseal closure at the time of puberty; this characteristic is observed in men with Kallmann's or Klinefelter's syndrome (14). The thyroid must be palpated, and the heart and lungs should be auscultated. The breasts should be observed and palpated for gynecomastia, which can be related to estrogen-secreting testicular tumors or adrenal tumors. The abdomen must be cautiously palpated. In addition to the general physical examination, a particular focus must be given to the genitalia, as described below.
Palpation of the testes and measurement of their size is mandatory. Normal adult testicular measurements have been established to be at least 4.6 cm in length and 2.6 cm in width, resulting in a volume ranging from 18 to 20 cm3. Because 85% of the testicular volume is associated with sperm production, a decreased testicular size indicates impaired spermatogenic potential (15). The testes of patients with nonobstructive azoospermia will typically measure less than 15 cm3 in volume, and the epididymis will be flat. In the vast majority of patients, obstructive azoospermia may be easily distinguished from nonobstructive azoospermia through a thorough analysis of clinical diagnostic parameters. Ninety-six percent of men with obstructive azoospermia had follicle-stimulating hormone (FSH) levels of 7.6 mIU/ml or less or a testicular long axis greater than 4.6 cm. Conversely, 89% of men with nonobstructive azoospermia had FSH levels greater than 7.6 mIU/ml or a testicular long axis of 4.6 cm or less. Based on these results, we believe that an isolated diagnostic testicular biopsy is rarely indicated (16).
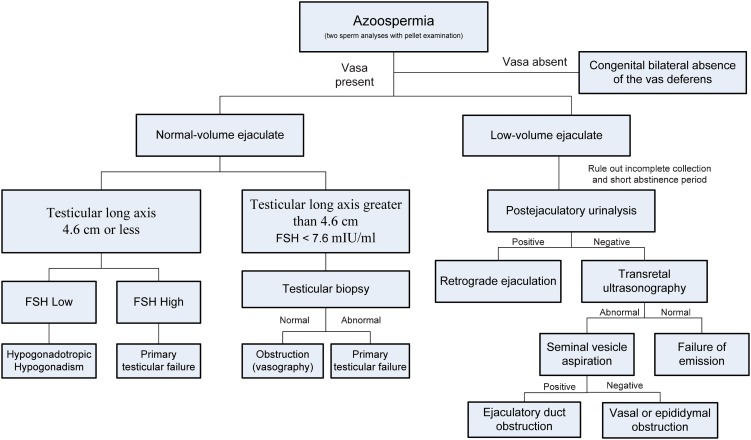
The presence and consistency of both the vasa and epididymides should be evaluated. Palpation can complete the diagnosis of a bilateral congenital absence of the vas deferens, and scrotal exploration is not needed to make the diagnosis (Figure 1) (17).
Figure 1.
Algorithm for the evaluation of patients with azoospermia.
A digital rectal exam is necessary to look for masses and to examine the size and consistency of the prostate. Under normal conditions, the seminal vesicles may not be palpable but may be prominent in the setting of ejaculatory duct obstruction.
Endocrine evaluation
An endocrinologic evaluation of patients who have severe male factor infertility leads to specific diagnoses and treatment strategies in a large population of infertile men (18). Although some authors recommend routine screening of the male hypothalamic-pituitary-gonadal axis in all patients, endocrine screening of men with sperm counts of less than 10 million/mL based on serum testosterone and FSH levels alone will detect the vast majority of clinically significant endocrinopathies (19). In addition to cases of seminal parameter abnormalities, an investigation of those patients presenting with impaired sexual function or other clinical findings suggestive of endocrinopathy, such as a marked reduction in testicular size or gynecomastia, is recommended (20).
If the testosterone level is low, a more complete evaluation will be necessary to analyze total and free testosterone, luteinizing hormone (LH), prolactin and estradiol levels. The information obtained from a complete endocrine profile may help to elucidate the etiology (21).
Semen analysis
Azoospermic patients with a normal ejaculate volume may have either obstruction of the reproductive system or abnormalities in spermatogenesis. Azoospermic men with a low semen volume and normal-sized testes may have ejaculatory dysfunction or ejaculatory duct obstruction. All patients presenting with absent ejaculation or low-volume ejaculation (<1.5 ml) should be asked to repeat the semen analysis and provide a postejaculation urine specimen. It is important to keep in mind that the majority of seminal fluid is contributed by the seminal vesicle. The ejaculated volume is an essential tool in the evaluation of an azoospermic patient, and the use of a diagnostic algorithm may prevent mistakes (Figure 1).
Diagnostic testis biopsy
Testicular histology is the only definitive way to diagnose azoospermia. However, the pattern of the testis tissue is heterogeneous, and spermatogenesis most often occurs only in focal areas; therefore, a biopsy is rarely used as a diagnostic tool (22). Usually, testicular characteristics and laboratory findings are suggestive of nonobstructive azoospermia. Thus, testicular sperm extraction (TESE) can be performed at the same time as ART in a specialized assisted reproduction center, which allows for sperm cryopreservation during the procedure and avoids a testicular biopsy.
In select patients with azoospermia, a normal testicular size, a palpable vas deferens and normal serum FSH levels, testis biopsies may be required to differentiate obstruction from disorders of spermatogenesis (Figure 1) (4). A normal testicular biopsy is pathognomonic for obstruction, and a vasography should be indicated to identify the site of the obstruction. Furthermore, scrotal exploration or endoscopic intervention may be required (Figure 1).
ETIOLOGIES OF AZOOSPERMIA
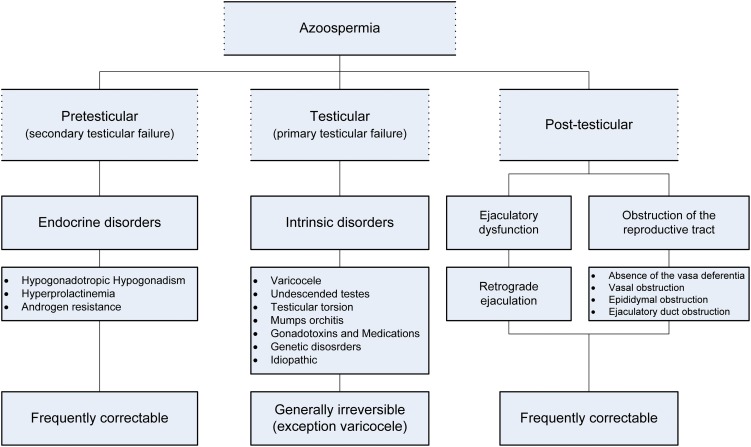
Although there are many causes of azoospermia, the etiologies of this disorder fall into three general categories: pretesticular, testicular and post-testicular. Pretesticular causes of azoospermia are endocrine abnormalities that adversely affect spermatogenesis. Testicular etiologies involve intrinsic disorders of spermatogenesis inside the testes. The post-testicular causes of azoospermia include obstruction of the ductal system at any location of the male reproductive tract. Every etiology of azoospermia is associated with a different prognosis, ranging from returns of production to simply finding sperm in the reproductive tract. The pretesticular and post-testicular abnormalities that cause azoospermia are commonly treatable, which may facilitate the restoration of fertility potential. Conversely, testicular disorders are generally irreversible, and the success rates for interventions associated with intrinsic testicular abnormalities are significantly lower. The etiologies, mechanisms and prognoses of azoospermia are summarized in Figure 2.
Figure 2.
Etiologies, mechanisms and prognoses of azoospermia.
Pretesticular causes
Pretesticular causes, also called secondary testicular failure, usually result from pathological endocrine conditions. Although an uncommon cause of male subfertility, up to 3% of infertile men will have an underlying endocrinopathy (19).
Hypogonadotropic hypogonadism
Typical causes of hypogonadotropic hypogonadism (HGH) include Kallmann's syndrome, pituitary trauma, pituitary tumors and anabolic steroid use. HGH is a rare cause of male infertility, and the disorder can be classified as congenital or acquired (23).
Kallmann's syndrome is a congenital cause of HGH and is associated with a malformation of the midline cranial structures (24). In this syndrome, the pathophysiology is a defect at the level of the hypothalamic secretion of gonadotropin-releasing hormone (GnRH) due to the failure of the GnRH-releasing neurons to migrate to the olfactory lobe during development. Kallman syndrome is the most frequently reported form of congenital HGH, occurring in between 1:10,000 and 1:60,000 births (21). Segregation analysis in familial cases has demonstrated diverse inheritance patterns, suggesting the existence of several genes that regulate GnRH secretion. Genetic defects have been demonstrated in the KAL gene, located in the Xp22.3 region, that explain the X-linked form of the disease (25). The clinical presentation of Kallmann's syndrome depends on the degree of hypogonadism. Most patients undergo delayed puberty, although those with less severe defects may present with a normal phenotype and mild subfertility. Other findings include anosmia, cleft palate and small testes. In more severe cases, congenital deafness, asymmetry of the cranium and the face, cerebellar dysfunction, cryptorchidism, and renal abnormalities may be present.
Acquired causes of HGH include pituitary tumors, pituitary trauma, panhypopituitarism and anabolic steroid use. Exogenous testosterone inhibits the hypothalamic-pituitary gonadal (HPG) axis and results in azoospermia by inhibiting gonadotropins via the feedback loop of the HPG axis, resulting in hypogonadism and infertility (18). Excess exogenous androgens from anabolic steroid use impair spermatogenesis by suppressing FSH levels and by depressing intratesticular testosterone levels (26).
The initial evaluation of patients with suspected HGH may include a pituitary MRI to rule out a pituitary tumor (27). Pituitary tumors can cause local destruction of the anterior pituitary. The serum prolactin level should be measured, and hyperprolactinemia must be ruled out or treated before initiating gonadotropin replacement therapy. In patients with acquired HGH, normal spermatogenesis can usually be restored by treatment with exogenous gonadotropins or GnRH (28).
Hyperprolactinemia
Hyperprolactinemia is a form of HGH caused by excessive prolactin secretion (6). An excess of prolactin inhibits the hypothalamic secretion of GnRH and has been implicated as a cause of reproductive and sexual dysfunction. Routine screening of infertile men for hyperprolactinemia has not been shown to be useful (29). Prolactin has negligible physiological effects in men and is produced in the anterior pituitary. Hyperprolactinemia suppresses both FSH and LH and may be caused by medications, concurrent medical illnesses, tricyclic antidepressants, some antihypertensives, stress, or pituitary tumors (macroadenoma or microadenoma); the cause may also be idiopathic (30). The most common medications that induce hyperprolactinemia are phenothiazines, imipramine, methyldopa, and reserpine (21). The most common causes of hyperprolactinemia are prolactin-secreting microadenomas (<10 mm) and prolactin-secreting macroadenomas (>10 mm) (31). Symptoms of prolactinomas include infertility, depressed libido, galactorrhea, headache, fatigue, and erectile dysfunction. In patients with prolactin-secreting pituitary adenomas, gonadotropin and testosterone levels are commonly suppressed, whereas prolactin levels are elevated. The level of prolactin elevation provides insight into the type of pathology. Prolactin levels greater than 250 ng/ml, between 100 and 250 ng/ml, between 25 and 100 ng/ml, and between 0 and 25 ng/ml most commonly correspond to macroadenoma, microadenoma, pituitary stalk compression and normal levels, respectively (6).
Androgen resistance
Androgen resistance occurs in approximately 1:60,000 births. More than 300 mutations have been found in the androgen receptor gene located on the X chromosome (Xq11-q12) (32). In addition to well-recognized mutations localized in its 8 exons, mutations in the gene promoter region have also been reported (33). Because many mutations exist, the syndrome is clinically variable and ranges from phenotypic females (complete androgen insensitivity) to normally virilized but infertile males (partial and minimal androgen insensitivity). Depending on the intensity of the defect, serum testosterone levels can be low, normal or high. It has been reported that as many as 40% of men with low or no sperm counts may have subtle androgen receptor abnormalities as the primary cause (34).
Modern genetic research on the androgen receptor gene has also led to interesting new clinical correlations with male infertility. The androgen receptor gene has 8 exons, and it is known that a critical region of CAG nucleotide repeats, usually 15-30 in number, can be found in exon 1 (35). Extension of this repeat region results in spinal and bulbar muscular atrophy (Kennedy disease), a neurodegenerative disease that begins around age 30; this disorder involves muscle cramping and atrophy, including infertility from testicular atrophy. There is now enough evidence to propose that subtle alterations in this CAG repeat region may also be the cause of some cases of idiopathic infertility. Yoshida et al. recently detected longer than normal CAG nucleotide repeats in normally virilized men with idiopathic azoospermia (36). Furthermore, Casella et al. found that the testicular histology in azoospermic patients is associated with the polyglutamine length of the androgen receptor gene (37).
Testicular etiologies
Testicular etiologies, broadly termed as primary testicular failure, are intrinsic disorders of spermatogenesis. Direct testicular pathology may derive from varicocele-induced testicular damage, undescended testes, testicular torsion, mumps orchitis, gonadotoxic effects from medications, genetic abnormalities and idiopathic causes. Primary testicular failure in conjunction with azoospermia, commonly termed nonobstructive azoospermia, is best managed by harvesting testicular sperm for eventual ICSI. However, the exact etiology should be determined whenever possible, and treatment may improve the success rates of sperm retrieval.
Varicocele
Currently, there is convincing evidence in the literature that varicoceles produce a progressive harmful effect on the testis, and varicocelectomy has been shown to prevent the progressive decline in testicular function and reverse the damage (38-40). Additionally, varicocele repairs have been documented to improve pregnancy rates and ART outcomes (41-43). However, identifying the individuals with varicocele who will benefit from varicocele treatment remains a challenge for andrologists.
Azoospermia in association with a varicocele occurs in between 5 and 10% of men (44-46). Tulloch, in 1955, was the first to report the recovery of sperm in the ejaculate and also subsequent pregnancy after varicocelectomy in a primarily azoospermic patient (47). At that time, these findings elicited renewed attention to varicocele treatment. However, it is still unknown why varicocele can have a devastating effect, leading to azoospermia, in some patients while 75% of men presenting with varicocele have normal semen findings (48,49). The large range of influence that varicocele exerts on testicular function suggests that there is currently no adequate diagnostic method with which to evaluate men presenting with clinical varicocele.
Although sperm can be found in the ejaculate of azoospermic men following varicocele repair in 21 to 55% of cases, spontaneous pregnancies are extremely rare (45,46,49,50). However, varicocelectomy in this population may avoid the need for more invasive procedures, such as TESE, by providing sperm via ejaculation for ICSI (51). Moreover, the fertilizing ability and ICSI success rates have been described as superior when fresh motile ejaculated sperm are used compared with sperm provided by testicular biopsy or microsurgical TESE (4,52).
Azoospermic patients may experience only intermittent sperm production that results in a temporary induction of spermatogenesis. Thus, semen cryopreservation is strongly recommended after the initial improvement following surgery (53).
Azoospermic men with varicocele must decide whether to undergo varicocelectomy or microsurgical testicular sperm extraction with ICSI. Many of these patients will ultimately require ICSI, especially those with Sertoli cell-only patterns or maturation arrest at the spermatocyte stage (45,49,50,54). However, more than half of these men with maturation arrest at the spermatid stage or hypospermatogenesis can provide postoperative motile sperm via ejaculation (55,56). Therefore, a testicular biopsy during varicocele repair in azoospermic men provides histological data that can be used as a predictor of sperm appearance in the ejaculate after surgery and of success in sperm retrieval (45,50,57). Additionally, this information may assist couples in deciding how long to wait following varicocelectomy prior to proceeding with ART.
The finding of a genetic etiology in infertile men with varicocele suggests that in such patients, Yq microdeletion screening should be performed to provide a proper diagnosis and to avoid unnecessary procedures that will most likely fail to improve testicular function due to a molecular/genetic basis (58).
Undescended testes
Undescended testes are the most common genital malformation in boys and are noted in 2.7% of newborns and up to 0.8% of 1-year-olds (59). It is important to differentiate cryptorchid testes from retractile testes, a circumstance involving hyperactive cremasteric muscles that cause the testes to periodically reside in the inguinal canal or high scrotum. Suggested mechanisms for cryptorchidism-induced subfertility include testicular dysgenesis, an impaired endocrine axis, immunologic damage, and obstruction (60). Early treatment can potentially minimize the risk of infertility, and the success depends on the initial position of the testicle (61). This condition should be treated hormonally and/or surgically before the child's first birthday; furthermore, the parents must be well informed about the risk, mainly because treatment before the age of 13 years does not seem to reduce the risk of malignancy (62).
Although the majority of men with a history of unilateral undescended testes are capable of paternity, testicular volume and age at orchiopexy are independent predictors of fertility potential and sperm retrieval in men with a history of cryptorchidism (60,63). The incidence of azoospermia after treatment for undescended testes is approximately 13 and 34% in unilateral and bilateral cryptorchidism, respectively (60). However, a 30 and 80% incidence of azoospermia results from untreated unilateral and bilateral undescended testes, respectively (60).
Testicular torsion
Testicular torsion occurs in approximately 1:4,000 males before the age of 25 years (64). This disorder demands immediate surgical exploration, and the risks of nonoperative management are well documented (65). Testicular preservation is usually achieved if surgical exploration is performed within 6 hours after the onset of symptoms. In addition to duration, other factors, such as the degree of rotation, have been related to testicular salvage (66,67). The most significant complication of testicular torsion is a loss of the testis, which may lead to impaired fertility (68). Severe oligospermia or azoospermia is rare after unilateral testicular torsion; however, these conditions are possible when the contralateral testis has experienced any previous abnormality, such as orchiopexy for an undescended testis (69).
Endocrine testicular function is expected to be normal in the event of a lost gonad. Conversely, the exocrine testicular function (spermatogenesis) is commonly affected (70,71). Patients with testicular torsion seem to have bilateral abnormalities that result in decreased spermatogenesis (72). It is unclear whether these abnormalities are due to an autoimmune process occurring after the rupture of the hemato-testicular barrier, leading to the formation of antisperm antibodies, or as a result of reperfusion-induced injury to the testis (71,73,74). Contralateral testicular biopsies are abnormal in up to 88% of cases at the time of torsion; therefore, some abnormalities are believed to be present even before the onset of torsion (68).
Mumps orchitis
Since the introduction of a vaccine against the mumps virus, there has been a reduced risk of mumps and its complications. On the other hand, mumps orchitis should still be suspected in cases of scrotal swelling, as there has been a recent increase in mumps orchitis among pubertal and postpubertal males (75).
Pubertal mumps orchitis occurs unilaterally in 67% of patients and bilaterally in 33% (76). Testicular atrophy occurs in 36% of those affected bilaterally, whereas infertility occurs in just 13% (77). However, prepubertal mumps orchitis has little effect on future fertility (76). Thus, it is important that urologists/andrologists are familiar with the diagnosis, treatment and complications of this condition.
Gonadotoxins and medications
Drugs and medications may harm male fertility through four distinct mechanisms: 1) direct gonadotoxic effects, 2) alteration of the hypothalamic-pituitary-gonadal (HPG) axis, 3) ejaculation dysfunction, and 4) reduction in libido. Meanwhile, gonadotoxins affect spermatogenesis by direct injury to germ cells in the testis or by interfering with the function of the Sertoli cells (78). For this reason, a detailed history of medications, including prescribed, over-the-counter, illicit, and nutraceutical drugs, should be obtained. The vast majority of these medications are not able to cause azoospermia, but special attention should be given to patients using or being administered exogenous androgens, antiandrogens, chemotherapy agents or radiation therapy, as well as to patients exposed to environmental toxins, such as pesticides, fumigants, insecticides and solvents.
Genetic
Chromosomal disorders are encountered at a higher frequency in the infertile compared with the fertile population (79). These chromosome alterations can currently be diagnosed in 15% of azoospermic and 5% of oligospermic men and represent one of the most common genetic defects in infertile men (80,81). Therefore, it is important that these men undergo genetic testing prior to the use of their sperm for ART.
a- Klinefelter Syndrome
Klinefelter Syndrome (KS) is 45 times more common in men seeking infertility treatment than in the general male population and is the most common numerical chromosome anomaly observed in male infertility, occurring in between 1:500 and 1:1000 males (82). KS is characterized by X chromosome polysomy, with X disomy being the most common variant (47,XXY). Ninety percent of men with KS have non-mosaic X chromosome polysomes (83). Advanced maternal and paternal age have been associated with an increased risk of KS, and the number of sex-chromosome disomic sperm (24,XY) in the fathers of boys with KS increases markedly as paternal age increases (84,85).
It is well known that men with KS present with a wide range of phenotypes and socioeconomic backgrounds, and these differences are the main reason for delayed diagnoses. It is estimated that only 10% of adolescents with KS are diagnosed before puberty (86). The classical phenotype of men with KS is characterized by tall eunuchoid body proportions; low testosterone levels; thin facial and pubic hair; small, hard testicles; a micropenis; sterility; and mild to moderate cognitive deficits (87). Affected men are also predisposed to diabetes mellitus, varicose veins, and chronic bronchitis, and they have a higher mortality rate due to breast cancer and non-Hodgkin lymphoma (88). Patients are commonly azoospermic, but testicular sperm extraction (TESE) may reveal spermatozoa in approximately 69% of KS men (89). Live births of children with normal karyotypes have been reported (90,91). The mosaic variant is less severe, and patients with this variant can present with normal testicular size, complete spermatogenesis and the presence of ejaculated sperm.
b- 47,XYY Syndrome
This syndrome is caused by paternal nondisjunction during meiosis that results in YY sperm, occurring in approximately 1:1000 (92). These patients typically have a tall stature, demonstrate decreased intelligence and exhibit antisocial behavioral characteristics. Most men with this syndrome are azoospermic or severely oligospermic, and testis biopsy findings range from Sertoli cell-only to maturation arrest patterns (93). Unlike patients with KS, serum testosterone levels are normal in these patients.
c- XX Male Syndrome
Etiologically, 90% of these patients present with a translocation of the SRY (testis-determining region) from the Y to the X chromosome, occurring in approximately 1:20,000 (94). In the other 10%, either the translocation occurs to an autosomal chromosome, or the individual is SRY-negative (94). Typically, these patients have normal male external and internal genitalia and a normal hormonal profile. Because there is no translocation of the entire AZF region, these patients are azoospermic and therefore not candidates for testicular sperm extraction; these individuals should be provided with the option of donor sperm-assisted reproduction.
d- Mixed Gonadal Dysgenesis
Patients with mixed gonadal dysgenesis normally have a mosaic 45,X0/46,XY genotype and anatomically have a testis on one side and a streak gonad on the other (92). Although this is a rare syndrome, the normally formed testis is often cryptorchidic and devoid of viable germ cells. The streak gonad is at risk of developing gonadoblastoma or seminoma and should be surgically removed (95). There are varying degrees of ambiguity of the external genitalia.
e- Y-chromosome microdeletions
Tiepolo and Zuffardi were the first to publish a report on six azoospermic patients carrying deletions of the Y chromosome and postulated that factors controlling human spermatogenesis (azoospermia factor; AZF) may be located on the distal portion of the euchromatin segment of the long arm of the Y chromosome (Yq11) (96). Twenty years later, with the development of molecular biology technologies, the first spermatogenesis gene was identified, and the following three distinct intervals within the AZF region were mapped (from proximal to distal): Yq (AZFa, AZFb and AZFc) (97,98).
Closest to the centromere, the AZFa region contains two genes that are important in the spermatogenesis process: DDX3Y (also known as DBY) and USP9Y (99). Microdeletion in the AZFa region occurs in almost 1% of men with nonobstructive azoospermia. Spermatogenic failure is the critical clinical consequence, and to date, there are no data in the literature to support the finding of sperm TESE (100).
At first, the AZFb and AZFc regions seemed to be distinct and nonoverlapping; however, the precise characterization of the P5 to P1 interval has revealed that these regions are actually overlapping and correspond to different sites of ectopic homologous recombination within this region (101).
Detected in approximately 1:4000 men and found in 13% of azoospermic and 6% of severely oligospermic men, AZFc is the most common microdeletion region (102). An AZFc microdeletion removes several genes that occupy this region, with the four copies of DAZ (Deleted in AZoospermia) being more clinically significant than with only one copy deleted (103,104). Oates et al. have offered the most pertinent clinical correlations to date regarding AZFc microdeletions. In their sample of 42 men, 38% were severely oligospermic and 62% were azoospermic. Of the azoospermic men, 67% had some level of spermatogenesis as detected from the testis biopsy; however, 19% of the overall group did not have sperm available from either the ejaculate or testis tissue (105).
As previously described, the AZFb region is not distinct from the AZFc region but corresponds to another possible position of ectopic homologous recombination within that region that extends from the P5 palindrome to the P1 palindrome. The AZFb microdeletion is 6.2 Mb long, starting in the P5 palindrome and ending in the proximal portion of the P1 palindrome. The AZFb/AZFc microdeletion is longer (7.7 Mb), with its origin in P5 (as for AZFb) and its termination at the distal end of P1 (101). The greatest clinical meaning of these microdeletions associated is that there is a small likelihood of sperm retrieval by TESE (100). Combined, these two microdeletions may be found in approximately 1 to 3% of the NOA population (106).
In a very elegant review of the literature, Foresta et al. found that almost 5,000 infertile men had been analyzed for the presence of Y microdeletions from 1992 to 2001. They found that the prevalence of deletions increases with more strict patient selection criteria. For instance, in unselected oligozoospermic men, the prevalence is 2.9%. The prevalence increases to 11.6% if idiopathic oligozoospermic patients are selected and to 14.3% if idiopathic severe oligozoospermia patients are included. In the same manner, unselected azoospermic patients show a deletion rate of 7.3%, but the exclusion of patients with obstructive azoospermia causes the prevalence to rise to 10.5% and to 18% if only idiopathic forms are considered. Furthermore, if patients are selected on the basis of their testicular structure, the prevalence is 24.7% in cases of idiopathic severe oligozoospermia with a testicular picture of severe hypospermatogenesis and 34.5% in cases of idiopathic azoospermia with a testicular histology of Sertoli cell-only syndrome (107).
Post-testicular causes of azoospermia
Post-testicular causes of azoospermia are due to either the obstruction of sperm delivery or ejaculatory dysfunction. The clinical management of obstructive azoospermia depends on its cause and also must take into account any coexisting infertility factors in the female partner (108). Therefore, both partners should be carefully evaluated before making any treatment recommendations. Men presenting with obstructive azoospermia may father children in one of two ways: surgical correction of the obstruction, which may allow the couple to conceive naturally; or retrieval of sperm directly from the epididymis or testis, followed by the use of ART (108). The surgical management of obstructive azoospermia varies with the site of obstruction and depends on the presence of pathological conditions, such as the absence of the vasa deferentia, vasal obstruction and ejaculatory duct obstruction. These conditions are described separately below.
Absence of the vasa deferentia
Congenital bilateral absence of the vas deferens (CBAVD) is found in 1% of infertile men and in up to 6% of those with obstructive azoospermia (109). There are two possible mechanisms responsible for this condition: 1) mutations of the cystic fibrosis transmembrane regulator gene (CFTR) and 2) abnormalities in the differentiation of the mesonephric duct (110).
Cystic fibrosis (CF) is the most common autosomal recessive disease in Caucasians, with an incidence of 1:2500 births and a carrier frequency of 1:20 (33). The CFTR gene (7q31.2) contains 27 exons and is 250 base pairs in length. A three-base-pair deletion in exon 10 (delta F508) is the most common mutation found in the Caucasian population (111), but there are more than 800 different mutations that have been described. Another common mutation consists of an intron 8 anomaly, called 5T (112). Normally, seven to nine thymidines are present in this region; a reduction to the five-thymidine variant decreases the efficiency of the splicing of exon 9 and eventually leads to a 10-50% reduction in CFTR mRNA (113). Eighty percent of men with CBAVD and 43% of men with a congenital unilateral absence of the vas deferens have detectable CFTR gene mutations (17,113).
The clinical features of CBAVD include normal testis size and preservation of spermatogenesis. The caput epididymis is always present, but the corpus and the cauda are found only occasionally. Seminal vesicles are often absent or atrophic but may also be enlarged or cystic. The ejaculate is acidic and low in volume [<1 ml].
Any insult to the Wolffian duct before week seven of gestation may impair urinary and reproductive tract formation including partial epididymal aplasia, seminal vesicle aplasia or hypoplasia, which may lead to a low ejaculate volume. Secondary findings include ipsilateral renal agenesis in 11% of patients with CBAVD and in 26% of patients with unilateral vasal absence (110). Imaging confirmation of renal agenesis is imperative in patients with unilateral absence of the vas deferens or in those with CBAVD lacking detectable CFTR gene mutations.
Spermatozoa can be easily retrieved from the caput epididymis for use in conjunction with ICSI by using either microsurgical or percutaneous procedures (114). If the CFTR mutation is also present in the female, a preimplantation diagnosis can be performed to avoid the birth of a CF child or a CBAVD male.
Vasal obstruction
The most frequent cause of nonpurposeful vasal obstruction is inadvertent injury during the performance of a hernia repair. This complication more frequently occurs when performed in infancy but can occur after any inguinal procedure where the vas and cord are manipulated (115). The diagnosis is suspected when examination reveals normal testicular size and the epididymis is full and firm. Instead of a direct vasal injury, the postoperative inflammatory response caused by the mesh may entrap and obstruct the inguinal vas deferens. The exact frequency of this problem is presently unknown. An estimated 80% of inguinal hernia operations involve the placement of a knitted polypropylene mesh to form a ‘tension-free' herniorrhaphy. The prosthetic mesh induces a chronic foreign-body fibroblastic response, creating scar tissue that imparts strength to the floor and leads to fewer recurrences. However, little is known regarding the long-term effects of the polypropylene mesh on the vas deferens, especially with regard to fertility (116).
The most common cause of obstruction of the vas deferens is vasectomy performed for elective sterilization (117). Approximately 500,000 vasectomies are performed in the United States each year (118). It is not surprising, therefore, that the most common indication to perform a vasovasostomy is to reverse a prior vasectomy to restore a man's fertility due to a new marriage.
Epididymal obstruction
Young's syndrome is a triad of disorders that encompasses chronic sinusitis, bronchiectasis and obstructive azoospermia (119). Patients with this syndrome have only mildly impaired respiratory function and normal spermatogenesis. The pathophysiology of the condition is unclear but may involve abnormal ciliary function or abnormal mucus quality. The exact cause of azoospermia is not completely elucidated but is most likely due to obstruction of the epididymis by inspissated secretions. The diagnosis is based on the occurrence of chronic sinopulmonary infections and persistent azoospermia with normal spermatogenesis, after excluding cystic fibrosis and immotile-cilia syndrome. The sperm appear to be normal in patients with Young's syndrome, and paternity has been documented in these patients (119).
Ejaculatory duct obstruction
Ejaculatory duct obstruction is a pathological condition characterized by the obstruction of one or both ejaculatory ducts and may be either congenital or acquired (120,121). In 1973, Farley and Barnes initially described ejaculatory duct obstruction, which is responsible for 1 to 5% of male infertility cases (122,123). Although first described in azoospermic men showing complete blockage, it is now clear that obstruction may manifest in several ways, including azoospermia and oligoasthenospermia (124).
Congenital causes include extrinsic compression of the ejaculatory ducts by Müllerian (utricular) or Wolffian (diverticular) cysts. Acquired causes may be secondary to iatrogenic trauma (postsurgical), prostatic calcification, seminal vesicle calculi or infected-related scar tissue. Although there are no pathognomonic findings associated with ejaculatory duct obstruction, quite a few clinical findings are highly suggestive of this condition. Men with this condition present with low-volume azoospermia; dilated seminal vesicles; and normal secondary sex characteristics, testes size, and hormonal profiles. Based on a suspicious semen analysis results, a transrectal ultrasound may be performed to confirm the diagnosis. Ejaculatory duct obstruction must not be confused with an obstruction of the vas deferens; approximately 80% of the volume of the semen is the gel-like fluid originating from the seminal vesicles, whereas the fraction from the testicles and epididymis, which contain the spermatozoa, accounts for only 5 to 10% of the volume of the semen. Thus, vasal obstruction usually does not influence the ejaculate volume (125).
Disorders of Ejaculation
Although this is a relatively unusual cause of male infertility, disorders of ejaculation present an interesting challenge to the treating physician (126). Ejaculatory dysfunction includes a variety of disorders with individualized treatments. Ejaculatory dysfunction should be suspected in any patient with a low volume (<1.0 ml) of or absent ejaculate and should be distinguished from anorgasmia. Retrograde ejaculation can be defined as the abnormal backward flow of semen into the bladder with ejaculation; the etiology may be anatomic, neurogenic, pharmacologic or idiopathic. Pharmacological agents implicated in retrograde ejaculation include neuroleptics, tricyclic antidepressants, alpha-blockers used in the treatment of prostatism and certain antihypertensives (126-128). The diagnosis of retrograde ejaculation is made by examining the post-ejaculate urine for sperm. Although specific criteria have not been established for a positive post-ejaculate urinalysis, the finding of greater than 10 to 15 sperm per high-power field confirms the presence of retrograde ejaculation. In contrast, sperm will not be present in the urine of a patient with failure of emission, which must be diagnosed clinically.
EXPERT COMMENTARY
Azoospermia may be due to inadequate hormonal stimulation, impaired spermatogenesis or an obstruction. In the majority of cases, the assessment of both physical and laboratory information, including semen volume, testicular volume, the presence of bilateral vas deferens and serum FSH level, will aid in the differentiation between the three categories.
Seminal plasma is a potential source of biomarkers for many disorders of the male reproductive system, including male infertility. In the future, the identification and characterization of different proteins expressed in the seminal plasma of men with normal and impaired spermatogenesis may aid in the elucidation of the molecular basis of male infertility and possibly azoospermia (129).
Over the past three decades, revolutionary treatments for infertility using assisted reproductive technologies have been developed. The first major development occurred in 1978 with the birth of baby Louise Brown, who was conceived by in vitro fertilization. The next major development involved gamete micromanipulation reproduction techniques, such as intracytoplasmic sperm injection (1,130).
These highly complex technologies have been applied with increasing frequency in the treatment of couples around the world. Over a million children have been conceived from assisted conception worldwide as of 2005 (131). All of these newer techniques appear to be increasingly less 'natural', and there is a dearth of information on these children beyond the neonatal period, especially with regard to fertile potential. Additionally, unlike most therapeutic procedures used in medicine, ARTs did not undergo meticulous safety testing prior to clinical use.
There is no doubt that ICSI overcomes natural barriers and results in the transmission of possible genetic abnormalities to offspring. Infertility could be, in some way, a natural mechanism to block the transmission of these undesirable genetic traits to offspring. Although researchers believe that approximately 75% or more cases of infertility have a genetic basis, our current ability to diagnose these defects remains limited (132). However, before the development of ART, these men could not have reproduced by any means.
Today, approximately 28% of azoospermic men presenting with genetic alterations can be diagnosed, allowing for adequate counseling before proceeding to ART (133). However, many patients with ‘idiopathic' azoospermia are thought to have a contributing but as yet unidentifiable genetic cause. As such, there exists a risk of transmission of these abnormalities to offspring. As urologists, we are increasingly expected to be experts on the potential genetic basis of azoospermia. Genetic testing and counseling should always be considered in the management of these couples prior to treatment to allow the couples to make an informed decision as to whether to use the husband's sperm.
Karyotyping and Y-chromosome microdeletion analysis should be offered to all men with azoospermia due to primary testicular failure prior to the use of ART with their sperm. Because Y microdeletions are the most common molecularly defined causes of spermatogenic failure, significant numbers of Y-deleted boys could be expected to be fathered through ICSI. It has been demonstrated that spermatozoa from an oligozoospermic subject carrying a Yq deletion are able to fertilize oocytes in vitro, suggesting that sperm carrying a deletion possess all the characteristics required to regulate capacitation, the acrosome reaction, and the ability to penetrate and fertilize the oocyte (134). Although no other health problems are associated with microdeletion of the Y chromosome, few data exist on the phenotypes of the sons of fathers with these genetic abnormalities. Therefore, Y chromosome analysis should be offered to men who have nonobstructive azoospermia or severe oligospermia prior to performing an ICSI with their sperm.
The simple palpation of the vas deferens could bring attention to a wide range of lethal syndromes that include the congenital absence of the vas deferens. If the doctor suspects any such syndrome, genetic testing for CFTR mutations in the female partner should be offered before proceeding with treatments that could utilize the sperm of a man with CBAVD. Currently, the recommendation is that if the female partner tests positive for a CFTR mutation, the male should be tested as well. However, if the female partner has a negative test for CFTR mutations, testing of the male partner is optional.
Interest has been renewed in genetic studies to determine the underlying causes of idiopathic male infertility, but the overwhelming trend has been to sidestep improvements in diagnostic evaluation in favor of a more expensive, although more efficacious, option, namely ART (18). Wherever possible, an accurate diagnosis of the etiology of azoospermia is important prior to the initiation of the appropriate treatment. It is also imperative that urologists work intimately with reproductive specialists because the timing and coordination of care may help achieve the ultimate goals, as well as the better management of azoospermia to maximize sperm quality, in these patients. In an ideal world, cause-specific therapy of male-factor infertility would decrease the use of ART, thereby avoiding the costs, risks, complications, and treatment of the unaffected female partner.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–8. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 2.Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol. 1989;142(1):62–5. doi: 10.1016/s0022-5347(17)38662-7. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Geneva: WHO Press; 2010. World Health Organization: WHO Laboratory manual for the examination and processing of human semen - 5th ed. [Google Scholar]
- 4.Schlegel PN. Causes of azoospermia and their management. Reprod Fertil Dev. 2004;16(5):561–72. doi: 10.10371/RD03087. [DOI] [PubMed] [Google Scholar]
- 5.Ron-El R, Strassburger D, Friedler S, Komarovski D, Bern O, Soffer Y, et al. Extended sperm preparation: an alternative to testicular sperm extraction in non-obstructive azoospermia. Hum Reprod. 1997;12(6):1222–6. doi: 10.1093/humrep/12.6.1222. [DOI] [PubMed] [Google Scholar]
- 6.Burrows PJ, Schrepferman CG, Lipshultz LI. Comprehensive office evaluation in the new millennium. Urol Clin North Am. 2002;29(4):873–94. doi: 10.1016/s0094-0143(02)00091-5. [DOI] [PubMed] [Google Scholar]
- 7.Honig SC, Lipshultz LI, Jarow J. Significant medical pathology uncovered by a comprehensive male infertility evaluation. Fertil Steril. 1994;62(5):1028–34. [PubMed] [Google Scholar]
- 8.Shefi S, Turek PJ. Definition and current evaluation of subfertile men. Int Braz J Urol. 2006;32(4):385–97. doi: 10.1590/s1677-55382006000400002. [DOI] [PubMed] [Google Scholar]
- 9.Steckel J, Dicker AP, Goldstein M. Relationship between varicocele size and response to varicocelectomy. J Urol. 1993;149(4):769–71. doi: 10.1016/s0022-5347(17)36203-1. [DOI] [PubMed] [Google Scholar]
- 10.Jarow JP, Ogle SR, Eskew LA. Seminal improvement following repair of ultrasound detected subclinical varicoceles. J Urol. 1996;155(4):1287–90. [PubMed] [Google Scholar]
- 11.Unal D, Yeni E, Verit A, Karatas OF. Clomiphene citrate versus varicocelectomy in treatment of subclinical varicocele: a prospective randomized study. Int J Urol. 2001;8(5):227–30. doi: 10.1046/j.1442-2042.2001.00289.x. [DOI] [PubMed] [Google Scholar]
- 12.Cocuzza M, Athayde KS, Alvarenga C, Srougi M, Hallak J. Grade 3 Varicocele in Fertile Men: A Different Entity. J Urol. in press. doi: 10.1016/j.juro.2011.11.114. [DOI] [PubMed] [Google Scholar]
- 13.Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male. Clinics. 2011;66(4):691–700. doi: 10.1590/S1807-59322011000400026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bettinger HF, Robinson B. The Klinefelter-Reifenstein-Albright syndrome. Med J Aust. 1946;2(13):446–9. [PubMed] [Google Scholar]
- 15.Lipshultz LI, Corriere JN., Jr Progressive testicular atrophy in the varicocele patient. J Urol. 1977;117(2):175–6. doi: 10.1016/s0022-5347(17)58387-1. [DOI] [PubMed] [Google Scholar]
- 16.Schoor RA, Elhanbly S, Niederberger CS, Ross LS. The role of testicular biopsy in the modern management of male infertility. J Urol. 2002;167(1):197–200. [PubMed] [Google Scholar]
- 17.Mickle J, Milunsky A, Amos JA, Oates RD. Congenital unilateral absence of the vas deferens: a heterogeneous disorder with two distinct subpopulations based upon aetiology and mutational status of the cystic fibrosis gene. Hum Reprod. 1995;10(7):1728–35. doi: 10.1093/oxfordjournals.humrep.a136164. [DOI] [PubMed] [Google Scholar]
- 18.Kim HH, Schlegel PN. Endocrine manipulation in male infertility. Urol Clin North Am. 2008;35(2):303–18, x. doi: 10.1016/j.ucl.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Sigman M, Jarow JP. Endocrine evaluation of infertile men. Urology. 1997;50(5):659–64. doi: 10.1016/S0090-4295(97)00340-3. [DOI] [PubMed] [Google Scholar]
- 20.Report on evaluation of the azoospermic male. Fertil Steril. 2006;86(5 Suppl 1):S210–5. doi: 10.1016/j.fertnstert.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Sussman EM, Chudnovsky A, Niederberger CS. Hormonal evaluation of the infertile male: has it evolved. Urol Clin North Am. 2008;35(2):147–55, vii. doi: 10.1016/j.ucl.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14(1):131–5. doi: 10.1093/humrep/14.1.131. [DOI] [PubMed] [Google Scholar]
- 23.Liu PY, Handelsman DJ. The present and future state of hormonal treatment for male infertility. Hum Reprod Update. 2003;9(1):9–23. doi: 10.1093/humupd/dmg002. [DOI] [PubMed] [Google Scholar]
- 24. Cummingham GRLL. P K, edDiseases of the testies and male sex organs Basic Clinical Endocrinology 1986New York: John Wiley and Sons; 263–78.In: [Google Scholar]
- 25.Maya-Nunez G, Zenteno JC, Ulloa-Aguirre A, Kofman-Alfaro S, Mendez JP. A recurrent missense mutation in the KAL gene in patients with X-linked Kallmann's syndrome. J Clin Endocrinol Metab. 1998;83(5):1650–3. doi: 10.1210/jcem.83.5.4817. [DOI] [PubMed] [Google Scholar]
- 26.McLachlan RI, O'Donnell L, Meachem SJ, Stanton PG, de K, Pratis K, et al. Hormonal regulation of spermatogenesis in primates and man: insights for development of the male hormonal contraceptive. J Androl. 2002;23(2):149–62. [PubMed] [Google Scholar]
- 27.Gilbaugh JH, 3rd, Lipshultz LI. Nonsurgical treatment of male infertility. An update. Urol Clin North Am. 1994;21(3):531–48. [PubMed] [Google Scholar]
- 28.Cocuzza M, Agarwal A. Nonsurgical treatment of male infertility: specific and empiric therapy. Biologics. 2007;1(3):259–69. [PMC free article] [PubMed] [Google Scholar]
- 29.Eggert-Kruse W, Schwalbach B, Gerhard I, Tilgen W, Runnebaum B. Influence of serum prolactin on semen characteristics and sperm function. Int J Fertil. 1991;36(4):243–51. [PubMed] [Google Scholar]
- 30.Siddiq FM, Sigman M. A new look at the medical management of infertility. Urol Clin North Am. 2002;29(4):949–63. doi: 10.1016/s0094-0143(02)00085-x. [DOI] [PubMed] [Google Scholar]
- 31.Jane JA, Jr, Laws ER., Jr The surgical management of pituitary adenomas in a series of 3,093 patients. J Am Coll Surg. 2001;193(6):651–9. doi: 10.1016/s1072-7515(01)01101-2. [DOI] [PubMed] [Google Scholar]
- 32.Bhasin S, Ma K, Sinha I, Limbo M, Taylor WE, Salehian B. The genetic basis of male infertility. Endocrinol Metab Clin North Am. 1998;27(4):783–805, viii. doi: 10.1016/s0889-8529(05)70041-4. [DOI] [PubMed] [Google Scholar]
- 33.Mak V, Jarvi KA. The genetics of male infertility. J Urol. 1996;156(4):1245–56. discussion 56-7. [PubMed] [Google Scholar]
- 34.Aiman J, Griffin JE, Gazak JM, Wilson JD, MacDonald PC. Androgen insensitivity as a cause of infertility in otherwise normal men. N Engl J Med. 1979;300(5):223–7. doi: 10.1056/NEJM197902013000503. [DOI] [PubMed] [Google Scholar]
- 35.Kupker W, Schwinger E, Hiort O, Ludwig M, Nikolettos N, Schlegel PN, et al. Genetics of male subfertility: consequences for the clinical work-up. Hum Reprod. 1999;14 Suppl 1:24–37. doi: 10.1093/humrep/14.suppl_1.24. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida KI, Yano M, Chiba K, Honda M, Kitahara S. CAG repeat length in the androgen receptor gene is enhanced in patients with idiopathic azoospermia. Urology. 1999;54(6):1078–81. doi: 10.1016/s0090-4295(99)00312-x. [DOI] [PubMed] [Google Scholar]
- 37.Casella R, Maduro MR, Misfud A, Lipshultz LI, Yong EL, Lamb DJ. Androgen receptor gene polyglutamine length is associated with testicular histology in infertile patients. J Urol. 2003;169(1):224–7. doi: 10.1016/S0022-5347(05)64073-6. [DOI] [PubMed] [Google Scholar]
- 38.Marmar JL, Benoff S. Varicoceles. J Urol. 2006;175(3 Pt 1):818–9. doi: 10.1016/S0022-5347(05)00831-1. [DOI] [PubMed] [Google Scholar]
- 39.Cocuzza M, Cocuzza MA, Bragais FM, Agarwal A. The role of varicocele repair in the new era of assisted reproductive technology. Clinics. 2008;63(3):395–404. doi: 10.1590/S1807-59322008000300018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cocuzza M, Athayde KS, Agarwal A, Pagani R, Sikka SC, Lucon AM, et al. Impact of clinical varicocele and testis size on seminal reactive oxygen species levels in a fertile population: a prospective controlled study. Fertil Steril. 2008;90(4):1103–8. doi: 10.1016/j.fertnstert.2007.07.1377. [DOI] [PubMed] [Google Scholar]
- 41.Esteves SC, Oliveira FV, Bertolla RP. Clinical outcome of intracytoplasmic sperm injection in infertile men with treated and untreated clinical varicocele. J Urol. 2010;184(4):1442–6. doi: 10.1016/j.juro.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal A, Deepinder F, Cocuzza M, Agarwal R, Short RA, Sabanegh E, et al. Efficacy of varicocelectomy in improving semen parameters: new meta-analytical approach. Urology. 2007;70(3):532–8. doi: 10.1016/j.urology.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Marmar JL, Agarwal A, Prabakaran S, Agarwal R, Short RA, Benoff S, et al. Reassessing the value of varicocelectomy as a treatment for male subfertility with a new meta-analysis. Fertil Steril. 2007 doi: 10.1016/j.fertnstert.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Czaplicki M, Bablok L, Janczewski Z. Varicocelectomy in patients with azoospermia. Arch Androl. 1979;3(1):51–5. doi: 10.3109/01485017908985048. [DOI] [PubMed] [Google Scholar]
- 45.Matthews GJ, Matthews ED, Goldstein M. Induction of spermatogenesis and achievement of pregnancy after microsurgical varicocelectomy in men with azoospermia and severe oligoasthenospermia. Fertil Steril. 1998;70(1):71–5. doi: 10.1016/s0015-0282(98)00108-3. [DOI] [PubMed] [Google Scholar]
- 46.Kim ED, Leibman BB, Grinblat DM, Lipshultz LI. Varicocele repair improves semen parameters in azoospermic men with spermatogenic failure. J Urol. 1999;162(3 Pt 1):737–40. doi: 10.1097/00005392-199909010-00031. [DOI] [PubMed] [Google Scholar]
- 47.Tulloch WS. Varicocele in subfertility; results of treatment. Br Med J. 1955;2(4935):356–8. doi: 10.1136/bmj.2.4935.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. World Health Organization. Fertil Steril. 1992;57(6):1289–93. [PubMed] [Google Scholar]
- 49.Kadioglu A, Tefekli A, Cayan S, Kandirali E, Erdemir F, Tellaloglu S. Microsurgical inguinal varicocele repair in azoospermic men. Urology. 2001;57(2):328–33. doi: 10.1016/s0090-4295(00)00908-0. [DOI] [PubMed] [Google Scholar]
- 50.Cocuzza M, Pagani R, Lopes RI, Athayde KS, Lucon AM, Srougi M, et al. Use of subinguinal incision for microsurgical testicular biopsy during varicocelectomy in men with nonobstructive azoospermia. Fertil Steril. 2009;91(3):925–8. doi: 10.1016/j.fertnstert.2007.12.065. [DOI] [PubMed] [Google Scholar]
- 51.Schlegel PN, Goldstein M. Alternate indications for varicocele repair: non-obstructive azoospermia, pain, androgen deficiency and progressive testicular dysfunction. Fertil Steril. 2011;96(6):1288–93. doi: 10.1016/j.fertnstert.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 52.Aboulghar MA, Mansour RT, Serour GI, Fahmy I, Kamal A, Tawab NA, et al. Fertilization and pregnancy rates after intracytoplasmic sperm injection using ejaculate semen and surgically retrieved sperm. Fertil Steril. 1997;68(1):108–11. doi: 10.1016/s0015-0282(97)81484-7. [DOI] [PubMed] [Google Scholar]
- 53.Pasqualotto FF, Lucon AM, Hallak J, Goes PM, Saldanha LB, Arap S. Induction of spermatogenesis in azoospermic men after varicocele repair. Hum Reprod. 2003;18(1):108–12. doi: 10.1093/humrep/deg032. [DOI] [PubMed] [Google Scholar]
- 54.Lee JS, Park HJ, Seo JT. What is the indication of varicocelectomy in men with nonobstructive azoospermia. Urology. 2007;69(2):352–5. doi: 10.1016/j.urology.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Esteves SC, Glina S. Recovery of spermatogenesis after microsurgical subinguinal varicocele repair in azoospermic men based on testicular histology. Int Braz J Urol. 2005;31(6):541–8. doi: 10.1590/s1677-55382005000600005. [DOI] [PubMed] [Google Scholar]
- 56.Schlegel PN, Kaufmann J. Role of varicocelectomy in men with nonobstructive azoospermia. Fertil Steril. 2004;81(6):1585–8. doi: 10.1016/j.fertnstert.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 57.Su LM, Palermo GD, Goldstein M, Veeck LL, Rosenwaks Z, Schlegel PN. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia: testicular histology can predict success of sperm retrieval. J Urol. 1999;161(1):112–6. [PubMed] [Google Scholar]
- 58.Moro E, Marin P, Rossi A, Garolla A, Ferlin A. Y chromosome microdeletions in infertile men with varicocele. Mol Cell Endocrinol. 2000;161(1-2):67–71. doi: 10.1016/s0303-7207(99)00226-9. [DOI] [PubMed] [Google Scholar]
- 59.Mathers MJ, Sperling H, Rubben H, Roth S. The undescended testis: diagnosis, treatment and long-term consequences. Dtsch Arztebl Int. 2009;106(33):527–32. doi: 10.3238/arztebl.2009.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grasso M, Buonaguidi A, Lania C, Bergamaschi F, Castelli M, Rigatti P. Postpubertal cryptorchidism: review and evaluation of the fertility. Eur Urol. 1991;20(2):126–8. doi: 10.1159/000471680. [DOI] [PubMed] [Google Scholar]
- 61.Pettersson A, Richiardi L, Nordenskjold A, Kaijser M, Akre O. Age at surgery for undescended testis and risk of testicular cancer. N Engl J Med. 2007;356(18):1835–41. doi: 10.1056/NEJMoa067588. [DOI] [PubMed] [Google Scholar]
- 62.Docimo SG, Silver RI, Cromie W. The undescended testicle: diagnosis and management. Am Fam Physician. 2000;62(9):2037–44. 47–8. [PubMed] [Google Scholar]
- 63.Raman JD, Schlegel PN. Testicular sperm extraction with intracytoplasmic sperm injection is successful for the treatment of nonobstructive azoospermia associated with cryptorchidism. J Urol. 2003;170(4 Pt 1):1287–90. doi: 10.1097/01.ju.0000080707.75753.ec. [DOI] [PubMed] [Google Scholar]
- 64.Williamson RC. Torsion of the testis and allied conditions. Br J Surg. 1976;63(6):465–76. doi: 10.1002/bjs.1800630618. [DOI] [PubMed] [Google Scholar]
- 65.Watkin NA, Reiger NA, Moisey CU. Is the conservative management of the acute scrotum justified on clinical grounds. Br J Urol. 1996;78(4):623–7. doi: 10.1046/j.1464-410x.1996.16321.x. [DOI] [PubMed] [Google Scholar]
- 66.Heindel RM, Pakyz RE, Reinking LN, Cosentino MJ. The effect of various degrees of unilateral spermatic cord torsion on fertility in the rat. J Urol. 1990;144(2 Pt 1):366–9. doi: 10.1016/s0022-5347(17)39462-4. [DOI] [PubMed] [Google Scholar]
- 67.Arap M, Cocuzza M, Mesquita J, Arap S. Testicular torsion - Analysis of 30 cases comparing doppler ultrasound to pre operatory clinical diagnosis. Acta Urol Port. 2000;17:55–8. [Google Scholar]
- 68.Visser AJ, Heyns CF. Testicular function after torsion of the spermatic cord. BJU Int. 2003;92(3):200–3. doi: 10.1046/j.1464-410x.2003.04307.x. [DOI] [PubMed] [Google Scholar]
- 69.Daehlin L, Ulstein M, Thorsen T, Hoisaeter PA. Follow-up after torsion of the spermatic cord. Scand J Urol Nephrol. 1996;179:139–42. [PubMed] [Google Scholar]
- 70.Cosentino MJ, Nishida M, Rabinowitz R, Cockett AT. Histological changes occurring in the contralateral testes of prepubertal rats subjected to various durations of unilateral spermatic cord torsion. J Urol. 1985;133(5):906–11. doi: 10.1016/s0022-5347(17)49278-0. [DOI] [PubMed] [Google Scholar]
- 71.Lievano G, Nguyen L, Radhakrishnan J, Fornell L, John E. New animal model to evaluate testicular blood flow during testicular torsion. J Pediatr Surg. 1999;34(6):1004–6. doi: 10.1016/s0022-3468(99)90778-9. [DOI] [PubMed] [Google Scholar]
- 72.Krarup T. The testes after torsion. Br J Urol. 1978;50(1):43–6. doi: 10.1111/j.1464-410x.1978.tb02764.x. [DOI] [PubMed] [Google Scholar]
- 73.Becker EJ, Prillaman HM, Turner TT. Microvascular blood flow is altered after repair of testicular torsion in the rat. J Urol. 1997;157(4):1493–8. [PubMed] [Google Scholar]
- 74.Arap MA, Vicentini FC, Cocuzza M, Hallak J, Athayde K, Lucon AM, et al. Late Hormonal Levels, Semen Parameters and Presence of Antisperm Antibodies in Patients Treated for Testicular Torsion. J Androl. 2007 doi: 10.2164/jandrol.106.002097. [DOI] [PubMed] [Google Scholar]
- 75.Davis NF, McGuire BB, Mahon JA, Smyth AE, O'Malley KJ, Fitzpatrick JM. The increasing incidence of mumps orchitis: a comprehensive review. BJU Int. 2010;105(8):1060–5. doi: 10.1111/j.1464-410X.2009.09148.x. [DOI] [PubMed] [Google Scholar]
- 76.Werner CA. Mumps orchitis and testicular atrophy; a factor in male sterility. Ann Intern Med. 1950;32(6):1075–86. doi: 10.7326/0003-4819-32-6-1075. [DOI] [PubMed] [Google Scholar]
- 77.Werner CA. Mumps orchitis and testicular atrophy; occurrence. Ann Intern Med. 1950;32(6):1066–74. doi: 10.7326/0003-4819-32-6-1066. [DOI] [PubMed] [Google Scholar]
- 78.Nudell DM, Monoski MM, Lipshultz LI. Common medications and drugs: how they affect male fertility. Urol Clin North Am. 2002;29(4):965–73. doi: 10.1016/s0094-0143(02)00079-4. [DOI] [PubMed] [Google Scholar]
- 79.Tournaye H, Staessen C, Liebaers I, Van Assche E, Devroey P, Bonduelle M, et al. Testicular sperm recovery in nine 47,XXY Klinefelter patients. Hum Reprod. 1996;11(8):1644–9. doi: 10.1093/oxfordjournals.humrep.a019462. [DOI] [PubMed] [Google Scholar]
- 80.Pandiyan N, Jequier AM. Mitotic chromosomal anomalies among 1210 infertile men. Hum Reprod. 1996;11(12):2604–8. doi: 10.1093/oxfordjournals.humrep.a019178. [DOI] [PubMed] [Google Scholar]
- 81.Peschka B, Leygraaf J, Van der Ven K, Montag M, Schartmann B, Schubert R, et al. Type and frequency of chromosome aberrations in 781 couples undergoing intracytoplasmic sperm injection. Hum Reprod. 1999;14(9):2257–63. doi: 10.1093/humrep/14.9.2257. [DOI] [PubMed] [Google Scholar]
- 82.Van Assche E, Bonduelle M, Tournaye H, Joris H, Verheyen G, Devroey P, et al. Cytogenetics of infertile men. Hum Reprod. 1996;11 Suppl 4:1–24. doi: 10.1093/humrep/11.suppl_4.1. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 83.Graham JM, Jr, Bashir AS, Stark RE, Silbert A, Walzer S. Oral and written language abilities of XXY boys: implications for anticipatory guidance. Pediatrics. 1988;81(6):795–806. [PubMed] [Google Scholar]
- 84.Lowe X, Eskenazi B, Nelson DO, Kidd S, Alme A, Wyrobek AJ. Frequency of XY sperm increases with age in fathers of boys with Klinefelter syndrome. Am J Hum Genet. 2001;69(5):1046–54. doi: 10.1086/323763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eskenazi B, Wyrobek AJ, Kidd SA, Lowe X, Moore D, 2nd, Weisiger K. Sperm aneuploidy in fathers of children with paternally and maternally inherited Klinefelter syndrome. Hum Reprod. 2002;17(3):576–83. doi: 10.1093/humrep/17.3.576. [DOI] [PubMed] [Google Scholar]
- 86.Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter's syndrome. Lancet. 2004;364(9430):273–83. doi: 10.1016/S0140-6736(04)16678-6. [DOI] [PubMed] [Google Scholar]
- 87.Paduch DA, Bolyakov A, Cohen P, Travis A. Reproduction in men with Klinefelter syndrome: the past, the present, and the future. Semin Reprod Med. 2009;27(2):137–48. doi: 10.1055/s-0029-1202302. [DOI] [PubMed] [Google Scholar]
- 88.Swerdlow AJ, Schoemaker MJ, Higgins CD, Wright AF, Jacobs PA. Cancer incidence and mortality in men with Klinefelter syndrome: a cohort study. J Natl Cancer Inst. 2005;97(16):1204–10. doi: 10.1093/jnci/dji240. [DOI] [PubMed] [Google Scholar]
- 89.Schiff JD, Palermo GD, Veeck LL, Goldstein M, Rosenwaks Z, Schlegel PN. Success of testicular sperm extraction [corrected] and intracytoplasmic sperm injection in men with Klinefelter syndrome. J Clin Endocrinol Metab. 2005;90(11):6263–7. doi: 10.1210/jc.2004-2322. [DOI] [PubMed] [Google Scholar]
- 90.Bourne H, Stern K, Clarke G, Pertile M, Speirs A, Baker HW. Delivery of normal twins following the intracytoplasmic injection of spermatozoa from a patient with 47,XXY Klinefelter's syndrome. Hum Reprod. 1997;12(11):2447–50. doi: 10.1093/humrep/12.11.2447. [DOI] [PubMed] [Google Scholar]
- 91.Hinney B, Guttenbach M, Schmid M, Engel W, Michelmann HW. Pregnancy after intracytoplasmic sperm injection with sperm from a man with a 47,XXY Klinefelter's karyotype. Fertil Steril. 1997;68(4):718–20. doi: 10.1016/s0015-0282(97)00280-x. [DOI] [PubMed] [Google Scholar]
- 92.Turek PJ, Pera RA. Current and future genetic screening for male infertility. Urol Clin North Am. 2002;29(4):767–92. doi: 10.1016/s0094-0143(02)00090-3. [DOI] [PubMed] [Google Scholar]
- 93.Wong EC, Ferguson KA, Chow V, Ma S. Sperm aneuploidy and meiotic sex chromosome configurations in an infertile XYY male. Hum Reprod. 2008;23(2):374–8. doi: 10.1093/humrep/dem377. [DOI] [PubMed] [Google Scholar]
- 94.Van der Auwera B, Van Roy N, De Paepe A, Hawkins JR, Liebaers I, Castedo S, et al. Molecular cytogenetic analysis of XX males using Y-specific DNA sequences, including SRY. Hum Genet. 1992;89(1):23–8. doi: 10.1007/BF00207036. [DOI] [PubMed] [Google Scholar]
- 95.Denes FT, Cocuzza MA, Schneider-Monteiro ED, Silva FA, Costa EM, Mendonca BB, et al. The laparoscopic management of intersex patients: the preferred approach. BJU Int. 2005;95(6):863–7. doi: 10.1111/j.1464-410X.2005.05417.x. [DOI] [PubMed] [Google Scholar]
- 96.Tiepolo L, Zuffardi O. Localization of factors controlling spermatogenesis in the nonfluorescent portion of the human Y chromosome long arm. Hum Genet. 1976;34(2):119–24. doi: 10.1007/BF00278879. [DOI] [PubMed] [Google Scholar]
- 97.Vogt P, Chandley AC, Hargreave TB, Keil R, Ma K, Sharkey A. Microdeletions in interval 6 of the Y chromosome of males with idiopathic sterility point to disruption of AZF, a human spermatogenesis gene. Hum Genet. 1992;89(5):491–6. doi: 10.1007/BF00219172. [DOI] [PubMed] [Google Scholar]
- 98.Vogt PH, Edelmann A, Kirsch S, Henegariu O, Hirschmann P, Kiesewetter F, et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet. 1996;5(7):933–43. doi: 10.1093/hmg/5.7.933. [DOI] [PubMed] [Google Scholar]
- 99.Wimmer R, Kirsch S, Weber A, Rappold GA, Schempp W. The Azoospermia region AZFa: an evolutionar y view. Cytogenet Genome Res. 2002;99(1-4):146–50. doi: 10.1159/000071586. [DOI] [PubMed] [Google Scholar]
- 100.Hopps CV, Mielnik A, Goldstein M, Palermo GD, Rosenwaks Z, Schlegel PN. Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod. 2003;18(8):1660–5. doi: 10.1093/humrep/deg348. [DOI] [PubMed] [Google Scholar]
- 101.Repping S, Skaletsky H, Lange J, Silber S, Van Der Veen F, Oates RD, et al. Recombination between palindromes P5 and P1 on the human Y chromosome causes massive deletions and spermatogenic failure. Am J Hum Genet. 2002;71(4):906–22. doi: 10.1086/342928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reijo R, Alagappan RK, Patrizio P, Page DC. Severe oligozoospermia resulting from deletions of azoospermia factor gene on Y chromosome. Lancet. 1996;347(9011):1290–3. doi: 10.1016/s0140-6736(96)90938-1. [DOI] [PubMed] [Google Scholar]
- 103.Lepretre AC, Patrat C, Mitchell M, Jouannet P, Bienvenu T. No partial DAZ deletions but frequent gene conversion events on the Y chromosome of fertile men. J Assist Reprod Genet. 2005;22(4):141–8. doi: 10.1007/s10815-005-4910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saxena R, de Vries JW, Repping S, Alagappan RK, Skaletsky H, Brown LG, et al. Four DAZ genes in two clusters found in the AZFc region of the human Y chromosome. Genomics. 2000;67(3):256–67. doi: 10.1006/geno.2000.6260. [DOI] [PubMed] [Google Scholar]
- 105.Oates RD, Silber S, Brown LG, Page DC. Clinical characterization of 42 oligospermic or azoospermic men with microdeletion of the AZFc region of the Y chromosome, and of 18 children conceived via ICSI. Hum Reprod. 2002;17(11):2813–24. doi: 10.1093/humrep/17.11.2813. [DOI] [PubMed] [Google Scholar]
- 106.Oates RD. The genetic basis of male reproductive failure. Urol Clin North Am. 2008;35(2):257–70, ix. doi: 10.1016/j.ucl.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 107.Foresta C, Moro E, Ferlin A. Y chromosome microdeletions and alterations of spermatogenesis. Endocr Rev. 2001;22(2):226–39. doi: 10.1210/edrv.22.2.0425. [DOI] [PubMed] [Google Scholar]
- 108.The management of infertility due to obstructive azoospermia. Fertil Steril. 2008;90(5 Suppl):S121–4. doi: 10.1016/j.fertnstert.2008.08.096. [DOI] [PubMed] [Google Scholar]
- 109.Ferlin A, Raicu F, Gatta V, Zuccarello D, Palka G, Foresta C. Male infertility: role of genetic background. Reprod Biomed Online. 2007;14(6):734–45. doi: 10.1016/s1472-6483(10)60677-3. [DOI] [PubMed] [Google Scholar]
- 110.McCallum T, Milunsky J, Munarriz R, Carson R, Sadeghi-Nejad H, Oates R. Unilateral renal agenesis associated with congenital bilateral absence of the vas deferens: phenotypic findings and genetic considerations. Hum Reprod. 2001;16(2):282–8. doi: 10.1093/humrep/16.2.282. [DOI] [PubMed] [Google Scholar]
- 111.Uzun S, Gokce S, Wagner K. Cystic fibrosis transmembrane conductance regulator gene mutations in infertile males with congenital bilateral absence of the vas deferens. Tohoku J Exp Med. 2005;207(4):279–85. doi: 10.1620/tjem.207.279. [DOI] [PubMed] [Google Scholar]
- 112.Lebo RV, Grody WW. Variable penetrance and expressivity of the splice altering 5T sequence in the cystic fibrosis gene. Genetic testing. 2007;11(1):32–44. doi: 10.1089/gte.2006.9997. [DOI] [PubMed] [Google Scholar]
- 113.Claustres M. Molecular pathology of the CFTR locus in male infertility. Reprod Biomed Online. 2005;10(1):14–41. doi: 10.1016/s1472-6483(10)60801-2. [DOI] [PubMed] [Google Scholar]
- 114.Tournaye H. Surgical sperm recovery for intracytoplasmic sperm injection: which method is to be preferred. Hum Reprod. 1999;14 Suppl 1:71–81. doi: 10.1093/humrep/14.suppl_1.71. [DOI] [PubMed] [Google Scholar]
- 115.Matsuda T, Horii Y, Yoshida O. Unilateral obstruction of the vas deferens caused by childhood inguinal herniorrhaphy in male infertility patients. Fertil Steril. 1992;58(3):609–13. doi: 10.1016/s0015-0282(16)55272-8. [DOI] [PubMed] [Google Scholar]
- 116.Shin D, Lipshultz LI, Goldstein M, Barme GA, Fuchs EF, Nagler HM, et al. Herniorrhaphy with polypropylene mesh causing inguinal vasal obstruction: a preventable cause of obstructive azoospermia. Ann Surg. 2005;241(4):553–8. doi: 10.1097/01.sla.0000157318.13975.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Costabile RA, Spevak M. Characterization of patients presenting with male factor infertility in an equal access, no cost medical system. Urology. 2001;58(6):1021–4. doi: 10.1016/s0090-4295(01)01400-5. [DOI] [PubMed] [Google Scholar]
- 118.Marquette CM, Koonin LM, Antarsh L, Gargiullo PM, Smith JC. Vasectomy in the United States, 1991. Am J Public Health. 1995;85(5):644–9. doi: 10.2105/ajph.85.5.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Handelsman DJ, Conway AJ, Boylan LM, Turtle JR. Young's syndrome. Obstructive azoospermia and chronic sinopulmonary infections. N Engl J Med. 1984;310(1):3–9. doi: 10.1056/NEJM198401053100102. [DOI] [PubMed] [Google Scholar]
- 120.Pryor JP, Hendry WF. Ejaculatory duct obstruction in subfertile males: analysis of 87 patients. Fertil Steril. 1991;56(4):725–30. doi: 10.1016/s0015-0282(16)54606-8. [DOI] [PubMed] [Google Scholar]
- 121.Goldwasser BZ, Weinerth JL, Carson CC., 3rd Ejaculatory duct obstruction: the case for aggressive diagnosis and treatment. J Urol. 1985;134(5):964–6. doi: 10.1016/s0022-5347(17)47550-1. [DOI] [PubMed] [Google Scholar]
- 122.Porch PP., Jr Aspermia owing to obstruction of distal ejaculatory duct and treatment by transurethral resection. J Urol. 1978;119(1):141–2. doi: 10.1016/s0022-5347(17)57412-1. [DOI] [PubMed] [Google Scholar]
- 123.Farley S, Barnes R. Stenosis of ejaculatory ducts treated by endoscopic resection. J Urol. 1973;109(4):664–6. doi: 10.1016/s0022-5347(17)60510-x. [DOI] [PubMed] [Google Scholar]
- 124.Smith JF, Walsh TJ, Turek PJ. Ejaculatory duct obstruction. Urol Clin North Am. 2008;35(2):221–7, viii. doi: 10.1016/j.ucl.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 125.Hopps CV, Goldstein M, Schlegel PN. The diagnosis and treatment of the azoospermic patient in the age of intracytoplasmic sperm injection. Urol Clin North Am. 2002;29(4):895–911. doi: 10.1016/s0094-0143(02)00083-6. [DOI] [PubMed] [Google Scholar]
- 126.Schuster TG, Ohl DA. Diagnosis and treatment of ejaculatory dysfunction. Urol Clin North Am. 2002;29(4):939–48. doi: 10.1016/s0094-0143(02)00080-0. [DOI] [PubMed] [Google Scholar]
- 127.Hendry WF. Disorders of ejaculation: congenital, acquired and functional. Br J Urol. 1998;82(3):331–41. doi: 10.1046/j.1464-410x.1998.00758.x. [DOI] [PubMed] [Google Scholar]
- 128.Debruyne FM. Alpha blockers: are all created equal. Urology. 2000;56(5 Suppl 1):20–2. doi: 10.1016/s0090-4295(00)00744-5. [DOI] [PubMed] [Google Scholar]
- 129.Davalieva K, Kiprijanovska S, Noveski P, Plaseski T, Kocevska B, Broussard C, et al. Proteomic analysis of seminal plasma in men with different spermatogenic impairment. Andrologia. doi: 10.1111/j.1439-0272.2012.01275.x. "in press". [DOI] [PubMed] [Google Scholar]
- 130.Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- 131.Bonduelle M, Wennerholm UB, Loft A, Tarlatzis BC, Peters C, Henriet S, et al. A multi-centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conception. Hum Reprod. 2005;20(2):413–9. doi: 10.1093/humrep/deh592. [DOI] [PubMed] [Google Scholar]
- 132.Alukal JP, Lamb DJ. Intracytoplasmic sperm injection (ICSI)--what are the risks. Urol Clin North Am. 2008;(35(2)):277–88. ix–x. doi: 10.1016/j.ucl.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.In't Veld PA, Halley DJ, van Hemel JO, Niermeijer MF, Dohle G, Weber RF. Genetic counselling before intracytoplasmic sperm injection. Lancet. 1997;350(9076):490. doi: 10.1016/s0140-6736(05)63078-4. [DOI] [PubMed] [Google Scholar]
- 134.Rossato M, Ferlin A, Garolla A, Pistorello M, Foresta C. Case report: high fertilization rate in conventional in-vitro fertilization utilizing spermatozoa from an oligozoospermic subject presenting microdeletions of the Y chromosome long arm. Mol Hum Reprod. 1998;4(5):473–6. doi: 10.1093/molehr/4.5.473. [DOI] [PubMed] [Google Scholar]