Abstract
This systematic review examines critically the scientific basis for Canada's Physical Activity Guide for Healthy Active Living for adults. Particular reference is given to the dose-response relationship between physical activity and premature all-cause mortality and seven chronic diseases (cardiovascular disease, stroke, hypertension, colon cancer, breast cancer, type 2 diabetes (diabetes mellitus) and osteoporosis). The strength of the relationship between physical activity and specific health outcomes is evaluated critically. Literature was obtained through searching electronic databases (e.g., MEDLINE, EMBASE), cross-referencing, and through the authors' knowledge of the area. For inclusion in our systematic review articles must have at least 3 levels of physical activity and the concomitant risk for each chronic disease. The quality of included studies was appraised using a modified Downs and Black tool. Through this search we identified a total of 254 articles that met the eligibility criteria related to premature all-cause mortality (N = 70), cardiovascular disease (N = 49), stroke (N = 25), hypertension (N = 12), colon cancer (N = 33), breast cancer (N = 43), type 2 diabetes (N = 20), and osteoporosis (N = 2). Overall, the current literature supports clearly the dose-response relationship between physical activity and the seven chronic conditions identified. Moreover, higher levels of physical activity reduce the risk for premature all-cause mortality. The current Canadian guidelines appear to be appropriate to reduce the risk for the seven chronic conditions identified above and all-cause mortality.
Introduction
There is considerable literature supporting the importance of habitual physical activity in the primary and secondary prevention of varied chronic conditions [1-16]. Routine physical activity is thought to be of benefit for over 25 chronic conditions [17]. Seven chronic diseases in particular have been associated with a physically inactive lifestyle including coronary artery disease, stroke, hypertension, colon cancer, breast cancer, type 2 diabetes (diabetes mellitus) and osteoporosis [18-20].
Canada has played a leading role in the development of physical activity guidelines for individuals across the lifespan. This includes the development (in 1998) of "Canada's Physical Activity Guide to Healthy Active Living" for adults between the ages of 20 and 55 yr [21], which was followed by "Canada's Physical Activity Guide to Healthy Active Living for Older Adults" [22], and "Canada's Physical Activity Guide to Healthy Active Living for Children and Youth" [23]. The adult guidelines (which are now approximately 10 years old) state generally that 20-55 yr adults should accumulate 60 min of daily physical activity or 30 min of moderate to vigorous exercise on at least 4 days a week [18,19].
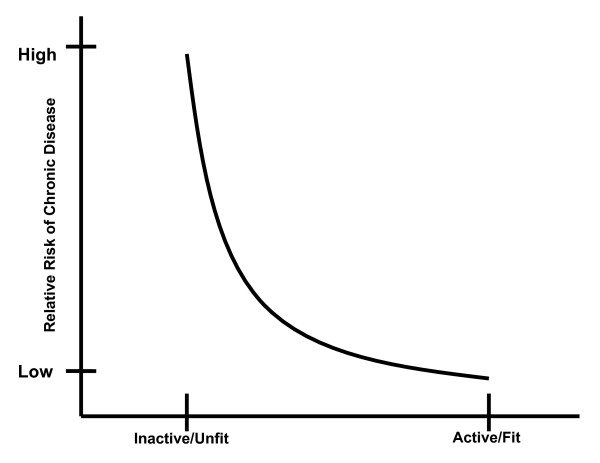
We reported recently that Canada's adult guidelines were consistent with other international guidelines and were supported by a compelling body of literature [18,19]. We revealed strong evidence that routine physical activity was effective in the primary prevention of cardiovascular disease, stroke, hypertension, breast cancer, colon cancer, type 2 diabetes and osteoporosis. Moreover, physical activity appears to play an important role in the prevention of obesity and obesity-related co-morbidities. However, implicit in the adult guidelines is the belief that there is a dose-response relationship between physical activity and the associated health benefits. Moreover, a central belief in these guidelines and most international physical activity guidelines is that the dose-response relationship is curvilinear with the greatest health benefits seen in physically inactive individuals who become "more physically active." In fact, a consistent pattern (shown in Figure 1) has been hypothesized, wherein there are marked changes in health status with relatively minor increments in physical activity/fitness in individuals that are the least active/fit. Generally, the health benefits have been thought to level off at the upper end of the physical activity/fitness continuum (Figure 1). However, recent work (such as that provided by Gledhill and Jamnik in the Canadian Physical Activity and Lifestyle Approach) has speculated that there are likely multiple dose-response curves for various endpoints [24].
Figure 1.
Theoretical relationship between the risk for chronic disease and physical activity/fitness.
The primary purpose of this systematic review was to examine critically the current literature to determine whether or not a dose-response relationship exists between habitual physical activity and chronic disease. In particular, we sought to determine whether the key messaging "Every little bit counts, but more is even better - everyone can do it!" of the adult physical activity guidelines is supported by a strong body of evidence.
Due to the breadth of literature, we have chosen to focus on the relationship between physical activity and all-cause mortality, and the seven chronic conditions that are thought to be reduced greatly with habitual physical activity (i.e., cardiovascular disease (excluding stroke), stroke, hypertension, colon cancer, breast cancer, type 2 diabetes and osteoporosis) (see Table 1). Owing to the nature of the physical activity guidelines, the emphasis of this paper was on primary prevention, despite the clear evidence that routine physical activity is also an effective secondary preventative strategy against many chronic conditions [16,18,19]. Accordingly, our primary objectives were to examine the evidence for a dose-response relationship between: 1) physical activity and all-cause mortality, and 2) physical activity and incidence of the following chronic conditions (cardiovascular disease (except stroke), stroke, hypertension, type 2 diabetes, colon cancer, breast cancer, and osteoporosis.
Table 1.
Relative risks (RR) and population attributable risks (PAR%) for physical inactivity in Canada, Australia, and the USA.
| Canada | Australia | USA | ||||
|---|---|---|---|---|---|---|
| Disease | RR | PAR% | RR | PAR% | RR | PAR% |
| CHD | 1.45 | 19.4 | 1.5 | 18 | 2.0 | 22 |
| Stroke | 1.60 | 24.3 | 2.0 | 16 | na | Na |
| Hypertension | 1.30 | 13.8 | na | na | 1.5 | 12 |
| Colon Cancer | 1.41 | 18.0 | 1.5 | 19 | 2.0 | 22 |
| Breast Cancer | 1.31 | 14.2 | 1.1 | 9 | 1.2 | 5 |
| Type 2 Diabetes | 1.50 | 21.1 | 1.3 | 13 | 1.5 | 12 |
| Osteoporosis | 1.59 | 24.0 | 1.4* | 18* | 2.0 | 18* |
Methods
Criteria for considering studies for this review
Our research team utilized a rigorous, systematic, and evidence-based approach to examine critically the levels of evidence on physical activity and the risk for premature mortality and chronic disease. Any studies that evaluated the relationship between at least three different levels of physical activity and mortality or incidence of chronic disease were eligible for inclusion. Therefore, excluded studies included those that examined only the most active versus least active populations (e.g., sedentary/inactive vs. physically active). Any form of physical activity/exercise measurement (e.g., self-report, pedometer, accelerometer, maximal aerobic power (VO2 max)) was eligible for inclusion. The key outcomes were mortality and incidence of chronic disease. Only published, English language studies examining adults (e.g., 19-65 yr) were included. Participants must have previously been healthy (asymptomatic) adults without established chronic disease. There was no restriction according to study design.
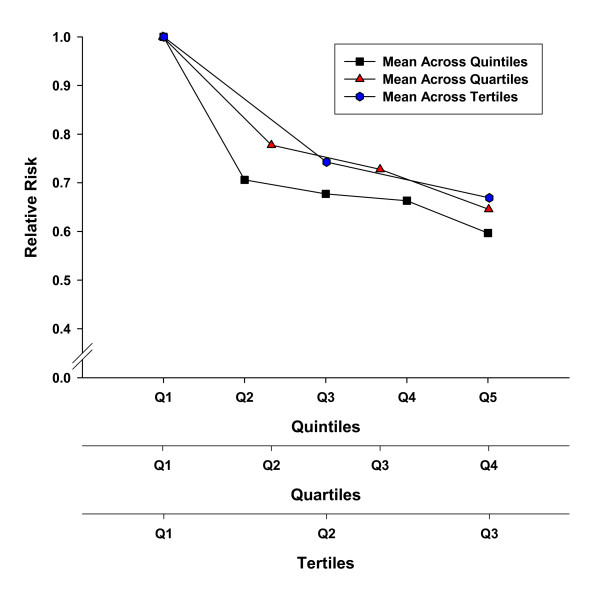
To examine the relative risk reductions associated with physical activity, we calculated the mean and median risk reductions across studies focusing on the highest level versus the lowest level of physical activity/fitness. For each study we also determined whether or not a dose-response relationship was present (i.e., reflecting a progressive decrease in the risk with increasing physical activity/fitness levels).
Search strategy
Literature searches were conducted in the following electronic bibliographical databases:
• MEDLINE (1950-March 2008, OVID Interface);
• EMBASE (1980- March 2008, OVID Interface),
• CINAHL (1982- March 2008, OVID Interface);
• PsycINFO (1840- March 2008, Scholars Portal Interface);
• Cochrane Library (-March 2008),
• SPORTDiscus (-March 2008).
The Medical Subject Headings (MeSH) were kept broad. See tables 2, 3, 4, 5, 6, 7, 8 and 9 for the complete search strategy and keywords used. The electronic search strategies were created and carried out by researchers experienced with systematic reviews of the literature (DW and LN). The citations and applicable electronic versions of the article (where available) were downloaded to an online research management system (RefWorks, Bethesda, Maryland, USA).
Table 2.
Results of the MEDLINE literature search regarding all-cause mortality.
| # | Searches (28 Feb 2008) | Results |
|---|---|---|
| 1 | exp Physical Fitness/ | 15236 |
| 2 | Motor Activity/ | 49721 |
| 3 | exp Physical Endurance/ | 15383 |
| 4 | exp Exercise/ | 57742 |
| 5 | exp Exertion/ | 88903 |
| 6 | exp Sports/ | 71887 |
| 7 | exp exercise therapy/ | 17231 |
| 8 | exp exercise tolerance/ | 4192 |
| 9 | exp health behaviour/ | 59409 |
| 10 | leisure time physical activity.mp | 996 |
| 11 | occupational physical activity.mp | 190 |
| 12 | exp Pliability/ | 2279 |
| 13 | exp Muscle Strength/ | 5717 |
| 14 | musc$ power.mp | 965 |
| 15 | exp Back/ | 12821 |
| 16 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 291635 |
| 17 | dose-response.mp | 321066 |
| 18 | intensity.mp | 142881 |
| 19 | volume.mp | 298471 |
| 20 | exp Energy Metabolism/ | 206808 |
| 21 | exp oxygen consumption/ | 83352 |
| 22 | exp time factors/ | 763712 |
| 23 | 17 or 18 or 19 or 20 or 21 or 22 | 1651633 |
| 24 | 16 and 23 | 67698 |
| 25 | exp Mortality/ | 190058 |
| 26 | all cause mortality.mp | 4618 |
| 27 | 25 or 26 | 192720 |
| 28 | 24 and 27 | 421 |
| 29 | limit 28 to (english and humans and "all adult (19 plus years) | 279 |
Table 3.
Results of the MEDLINE literature search regarding cardiovascular disease.
| Search # | Searches (3 Mar 2008) | Results |
|---|---|---|
| 1 | exp Physical Fitness/ | 15244 |
| 2 | Motor Activity/ | 49751 |
| 3 | exp Physical Endurance/ | 15408 |
| 4 | exp Exercise/ | 57806 |
| 5 | exp Exertion/ | 88967 |
| 6 | exp Sports/ | 71931 |
| 7 | exp exercise therapy/ | 17243 |
| 8 | exp exercise tolerance/ | 4205 |
| 9 | exp health behaviour/ | 59467 |
| 10 | leisure time physical activity.mp | 998 |
| 11 | occupational physical activity.mp | 191 |
| 12 | exp Pliability/ | 2289 |
| 13 | exp Muscle Strength/ | 5731 |
| 14 | musc$ power.mp | 965 |
| 15 | exp Back/ | 12822 |
| 16 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 291817 |
| 17 | dose-response.mp | 321198 |
| 18 | intensity.mp | 142955 |
| 19 | volume.mp | 298620 |
| 20 | exp Energy Metabolism/ | 206886 |
| 21 | exp oxygen consumption/ | 83387 |
| 22 | exp time factors/ | 764091 |
| 23 | 17 or 18 or 19 or 20 or 21 or 22 | 1652372 |
| 24 | 16 and 23 | 67760 |
| 25 | exp Cardiovascular Diseases/ | 1411730 |
| 26 | exp Heart diseases/ | 675083 |
| 27 | exp Myocardial infarction/ | 116070 |
| 28 | exp Death, Sudden Cardiac/ | 6772 |
| 29 | exp Coronary Artery Disease/ | 18137 |
| 30 | exp Coronary Disease/ | 144236 |
| 31 | exp Vascular Diseases | 1018275 |
| 32 | 25 or 26 or 27 or 28 or 29 or 30 or 31 | 1411730 |
| 33 | 24 and 32 | 9603 |
| 34 | limit 33 to (english language and humans and "all adult (19 plus years)") | 5544 |
Table 4.
Results of the MEDLINE literature search regarding stroke.
| Search # | Searches (29 Feb 2008) | Results |
|---|---|---|
| 1 | exp Physical Fitness/ | 15241 |
| 2 | Motor Activity/ | 49744 |
| 3 | exp Physical Endurance/ | 15387 |
| 4 | exp Exercise/ | 57764 |
| 5 | exp Exertion/ | 88921 |
| 6 | exp Sports/ | 71907 |
| 7 | exp exercise therapy/ | 17237 |
| 8 | exp exercise tolerance/ | 4196 |
| 9 | exp health behaviour/ | 59430 |
| 10 | leisure time physical activity.mp | 996 |
| 11 | occupational physical activity.mp | 190 |
| 12 | exp Pliability/ | 2288 |
| 13 | exp Muscle Strength/ | 5720 |
| 14 | musc$ power.mp | 965 |
| 15 | exp Back/ | 12821 |
| 16 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 291718 |
| 17 | dose-response.mp | 321133 |
| 18 | intensity.mp | 142919 |
| 19 | volume.mp | 298526 |
| 20 | exp Energy Metabolism/ | 206837 |
| 21 | exp oxygen consumption/ | 83359 |
| 22 | exp time factors/ | 763871 |
| 23 | 17 or 18 or 19 or 20 or 21 or 22 | 1651958 |
| 24 | 16 and 23 | 67720 |
| 25 | exp Stroke/ | 45243 |
| 26 | exp Cerebrovascular Disorders/ | 196243 |
| 27 | exp Brain Ischemia/ | 58943 |
| 28 | exp Brain Infarction/ or exp Cerebral Infarction | 21357 |
| 29 | exp Infarction, Middle Cerebral Artery/ or exp Intracranial Aneurysm/ or exp Subarachnoid | 46725 |
| 30 | Hemorrhage/ or exp Cerebral Hemorrhage/exp Ischemic Attack, Transient/ | 14753 |
| 31 | 25 or 26 or 27 or 28 or 29 or 30 | 196243 |
| 32 | 24 and 31 | 692 |
| 33 | limit 32 to (english language and humans and "all adult (19 plus years)") | 291 |
Table 5.
Results of the MEDLINE literature search regarding hypertension.
| Search # | Searches (3 Mar 2008) | Results |
|---|---|---|
| 1 | exp Physical Fitness/ | 15244 |
| 2 | Motor Activity/ | 49751 |
| 3 | exp Physical Endurance/ | 15408 |
| 4 | exp Exercise/ | 57806 |
| 5 | exp Exertion/ | 88967 |
| 6 | exp Sports/ | 71931 |
| 7 | exp exercise therapy/ | 17243 |
| 8 | exp exercise tolerance/ | 4205 |
| 9 | exp health behaviour/ | 59467 |
| 10 | leisure time physical activity.mp | 998 |
| 11 | occupational physical activity.mp | 191 |
| 12 | exp Pliability/ | 2289 |
| 13 | exp Muscle Strength/ | 5731 |
| 14 | musc$ power.mp | 965 |
| 15 | exp Back/ | 12822 |
| 16 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 291817 |
| 17 | dose-response.mp | 3211987 |
| 18 | intensity.mp | 142955 |
| 19 | volume.mp | 298620 |
| 20 | exp Energy Metabolism/ | 206886 |
| 21 | exp oxygen consumption/ | 83387 |
| 22 | exp time factors/ | 764091 |
| 23 | 17 or 18 or 19 or 20 or 21 or 22 | 1652372 |
| 24 | exp Hypertension/ | 168466 |
| 25 | exp Blood Pressure/ | 205571 |
| 26 | exp Blood Pressure Determination/ or exp Blood Pressure Monitoring, Ambulatory/ or exp Blood | 18244 |
| 27 | Pressure Monitors/24 or 25 or 26 | 336025 |
| 28 | 16 and 23 and 27 | 5647 |
| 29 | limit 28 to (english language and humans and "all adult (19 plus years)") | 3642 |
Table 6.
Results of the MEDLINE literature search regarding colon cancer.
| Search # | Searches (3 Mar 2008) | Results |
|---|---|---|
| 1 | exp Physical Fitness/ | 15244 |
| 2 | Motor Activity/ | 49751 |
| 3 | exp Physical Endurance/ | 15408 |
| 4 | exp Exercise/ | 57806 |
| 5 | exp Exertion/ | 88967 |
| 6 | exp Sports/ | 71931 |
| 7 | exp exercise therapy/ | 17243 |
| 8 | exp exercise tolerance/ | 4205 |
| 9 | exp health behaviour/ | 59467 |
| 10 | leisure time physical activity.mp | 998 |
| 11 | occupational physical activity.mp | 191 |
| 12 | exp Pliability/ | 2289 |
| 13 | exp Muscle Strength/ | 5731 |
| 14 | musc$ power.mp | 965 |
| 15 | exp Back/ | 12822 |
| 16 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 291817 |
| 17 | dose-response.mp | 321198 |
| 18 | intensity.mp | 142955 |
| 19 | volume.mp | 298620 |
| 20 | exp Energy Metabolism/ | 206886 |
| 21 | exp oxygen consumption/ | 83387 |
| 22 | exp time factors/ | 764091 |
| 23 | 17 or 18 or 19 or 20 or 21 or 22 | 1652372 |
| 24 | exp Colonic Neoplams/ | 51780 |
| 25 | exp Rectal Neoplasms/ | 28011 |
| 26 | exp Colorectal Neoplasms/ | 99982 |
| 27 | exp Colorectal Neoplasms/, Hereditary Nonpolyposis/ or exp Intestinal Neoplasms. | 117563 |
| 28 | 24 or 25 or 26 or 27 | 117563 |
| 29 | 16 and 23 and 28 | 108 |
| 30 | limit 29 to (53nglish language and humans and "all adult (19 plus years)") | 77 |
Table 7.
Results of the MEDLINE literature search regarding breast cancer.
| Search # | Searches (28 Feb 2008) | Results |
|---|---|---|
| 1 | exp Physical Fitness/ | 15236 |
| 2 | Motor Activity/ | 49721 |
| 3 | exp Physical Endurance/ | 15383 |
| 4 | exp Exercise/ | 57742 |
| 5 | exp Exertion/ | 88903 |
| 6 | exp Sports/ | 71887 |
| 7 | exp exercise therapy/ | 17231 |
| 8 | exp exercise tolerance/ | 4192 |
| 9 | exp health behaviour/ | 59409 |
| 10 | leisure time physical activity.mp | 996 |
| 11 | occupational physical activity.mp | 190 |
| 12 | exp Pliability/ | 2279 |
| 13 | exp Muscle Strength/ | 5717 |
| 14 | musc$ power.mp | 965 |
| 15 | exp Back/ | 12821 |
| 16 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 291635 |
| 17 | dose-response.mp | 321066 |
| 18 | intensity.mp | 142881 |
| 19 | volume.mp | 298471 |
| 20 | exp Energy Metabolism/ | 206808 |
| 21 | exp oxygen consumption/ | 83352 |
| 22 | exp time factors/ | 763712 |
| 23 | 17 or 18 or 19 or 20 or 21 or 22 | 1651633 |
| 24 | exp Breast Neoplasms/ | 149817 |
| 25 | 16 and 23 and 24 | 296 |
| 26 | limit 25 to (54 nglish language and humans and "all adult (19 plus years)" | 216 |
Table 8.
Results of the MEDLINE literature search regarding type 2 diabetes.
| Search # | Searches (29 Feb 2008) | Results |
|---|---|---|
| 1 | exp Physical Fitness/ | 15241 |
| 2 | Motor Activity/ | 49744 |
| 3 | exp Physical Endurance/ | 15387 |
| 4 | exp Exercise/ | 57764 |
| 5 | exp Exertion/ | 88921 |
| 6 | exp Sports/ | 71907 |
| 7 | exp exercise therapy/ | 17237 |
| 8 | exp exercise tolerance/ | 4196 |
| 9 | exp health behaviour/ | 59430 |
| 10 | leisure time physical activity.mp | 996 |
| 11 | occupational physical activity.mp | 190 |
| 12 | exp Pliability/ | 2288 |
| 13 | exp Muscle Strength/ | 5720 |
| 14 | musc$ power.mp | 965 |
| 15 | exp Back/ | 12821 |
| 16 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 291718 |
| 17 | dose-response.mp | 321133 |
| 18 | intensity.mp | 142919 |
| 19 | volume.mp | 298526 |
| 20 | exp Energy Metabolism/ | 206837 |
| 21 | exp oxygen consumption/ | 83359 |
| 22 | exp time factors/ | 763871 |
| 23 | 17 or 18 or 19 or 20 or 21 or 22 | 1651958 |
| 24 | 16 and 23 | 67720 |
| 25 | exp Blood Glucose/or exp Diabetes Mellitus, Type 2/ | 132583 |
| 26 | exp Hyperglycemia/ | 16214 |
| 27 | exp Glucose Intolerance/ or exp Glucose Tolerance Test/ | 24986 |
| 28 | exp Hyperinsulinism/ | 30490 |
| 29 | 25 or 26 or 27 or 28 | 165157 |
| 30 | 29 and 24 | 3006 |
| 31 | Limit 30 to (english language and humans and "all adult (19 plus years)") | 1985 |
Table 9.
Results of the MEDLINE literature search regarding osteoporosis.
| Search # | Searches (29 feb 2008) | Results |
|---|---|---|
| 1 | exp Physical Fitness/ | 15241 |
| 2 | Motor Activity/ | 49744 |
| 3 | exp Physical Endurance/ | 15387 |
| 4 | exp Exercise/ | 57764 |
| 5 | exp Exertion/ | 88921 |
| 6 | exp Sports/ | 71907 |
| 7 | exp exercise therapy/ | 17237 |
| 8 | exp exercise tolerance/ | 4196 |
| 9 | exp health behaviour/ | 59430 |
| 10 | leisure time physical activity.mp | 996 |
| 11 | occupational physical activity.mp | 190 |
| 12 | exp Pliability/ | 2288 |
| 13 | exp Muscle Strength/ | 5720 |
| 14 | musc$ power.mp | 965 |
| 15 | exp Back/ | 12821 |
| 16 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 291718 |
| 17 | dose-response.mp | 321133 |
| 18 | intensity.mp | 142919 |
| 19 | volume.mp | 298526 |
| 20 | exp Energy Metabolism/ | 206837 |
| 21 | exp oxygen consumption/ | 83359 |
| 22 | exp time factors/ | 763871 |
| 23 | 17 or 18 or 19 or 20 or 21 or 22 | 1651958 |
| 24 | exp Osteoporosis, Postmenopausal/ or exp Osteoporosis/ | 31532 |
| 25 | exp Fractures, Bone/ or exp Bone Density/ | 125269 |
| 26 | exp Bone Diseases/ or exp Bone Diseases, Metabolic/ | 308084 |
| 27 | exp "Bone and bones"/ | 369634 |
| 28 | exp Tensile Strength/ | 12050 |
| 29 | exp Compressive Strength | 2838 |
| 30 | 24 or 25 or 26 or 27 or 28 or 29 | 642158 |
| 31 | 16 and 23 and 30 | 2138 |
| 32 | limit 31 to (english language and humans and "all adult (19 plus years)") | 1193 |
Screening
Two reviewers (LN and SC) screened independently the title and abstract of the citations to identify potential articles for inclusion. Duplicate citations were removed. The reviewers were not blinded to the authors or journals. Biographies of key studies and reviews in the field were also cross-referenced for further articles. For those articles that appeared relevant, the full text was obtained and data was extracted using a common template. In cases of disagreement, discussion with a third reviewer (DW) was used to achieve consensus. Full (100%) consensus was achieved. All studies that were excluded during the citation and full-article screening processes were recorded along with the reasons for exclusion.
Data Extraction
Two reviewers (LN and SC) completed standardized data extraction forms, which were verified by two other reviewers (DW and SB). We extracted information regarding the study design, the country where the study was conducted, the participant characteristics, the sample size, the objectives of the study, the methodologies employed, the major outcomes (i.e., mortality, incidence of chronic disease, physical activity levels/classifications), and the comments and conclusions made based on the findings of the study. The reviewers were not blinded to the journal or the author names when extracting information from the articles.
Level of Evidence
The approach used to establish the level and grade of evidence was consistent with that used during creation of the "Canadian clinical practice guidelines on the management and prevention of obesity in adults and children" [25]. The level of evidence provides information regarding the strength of the evidence in favour of physical activity/exercise in the primary prevention of premature mortality and the seven chronic diseases of primary interest. This evaluation process is based on a pre-defined and objective criteria (see Table 10).
Table 10.
The levels and grade of evidence scaling criteria applied to the articles.
| Level of Evidence | Criteria |
|---|---|
| Level 1 | Randomized control trials without important limitations |
| Level 2 | • Randomized control trials with important limitations |
| • Observational studies (non-randomized clinical trials or cohort studies) with overwhelming evidence | |
| Level 3 | Other observational studies (prospective cohort studies, case-control studies, case series) |
| Level 4 | Inadequate or no data in population of interest |
| Anecdotal evidence or clinical experience | |
| Grade of Evidence | Criteria |
| Grade A | Strong recommendation (action can apply to most individuals in most circumstances) |
| • Benefits clearly outweigh risks (or vice-versa) | |
| • Evidence is at Level 1, 2, or 3 | |
| Grade B | Weak recommendation (action may differ depending on individual's characteristics or other circumstances) |
| • Unclear if benefits outweigh risks | |
| • Evidence is at Level 1, 2, or 3 | |
| Grade C | Consensus recommendation (alternative actions may be equally reasonable) |
| • Unclear if benefits outweigh risks | |
| • Evidence is at Level 3 or 4 | |
The grade for each article provides information regarding whether physical activity is effective in the primary prevention of the varied conditions evaluated (Table 10). Where applicable this grade informs the reader about the potential risk of the physical activity. A study that receives the highest grading would indicate that the benefits clearly outweigh the risks and receive a strong recommendation.
Quality Assessment
The quality of each study was also established using the procedures of Gorber et al. [26]. Owing to the fact that only observational study designs were included in our systematic review, we used the Downs and Black [27] scale to assess the quality of non-randomized investigations. Similar to the work of Prince et al. [28] we chose to include the most relevant components of the scoring tool. Therefore, a modified version of the Downs and Black checklist was used with the final checklist consisting of 15 items with a maximum score of 15 points. Higher points reflected a superior quality of investigation.
Results
Physical Inactivity and All-Cause Mortality
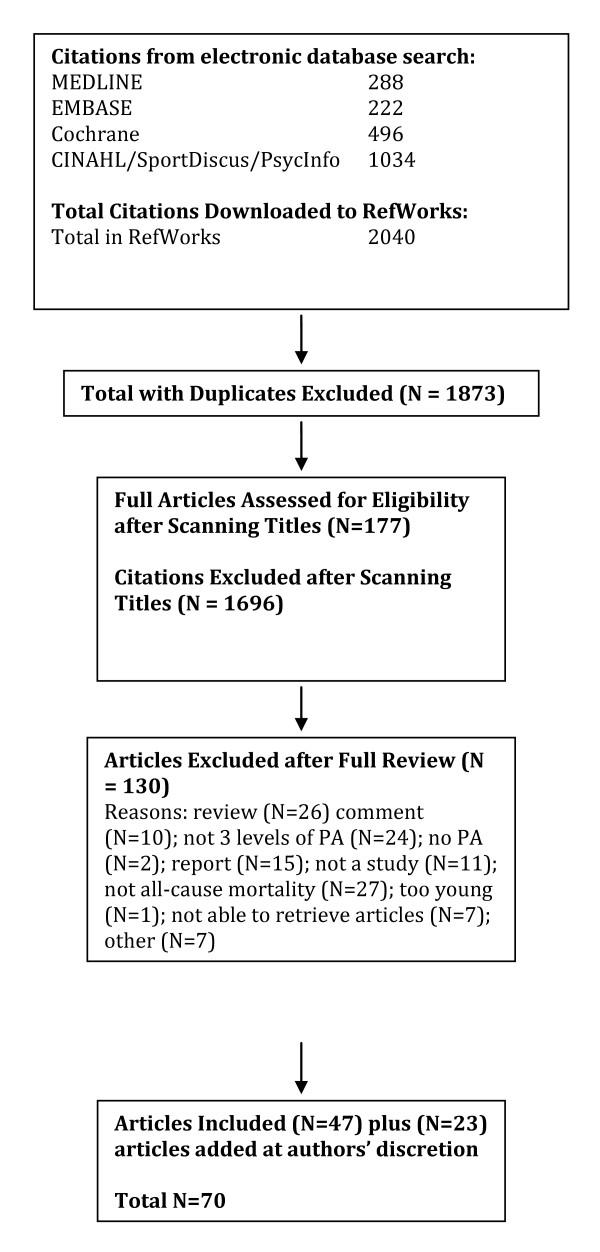
A total of 2040 citations were identified during the electronic database search (Figure 2). Of these citations, 288 were identified in MEDLINE, 222 in EMBASE, 496 in Cochrane, and 1034 in the CINAHL/SportDiscus/PsychInfo search. A total of 167 duplicates were found, leaving a total of 1873 unique citations. A total of 1696 articles were excluded after scanning, leaving a total of 177 articles for full review. From these articles 130 were excluded after full review leaving 47 articles for inclusion in the systematic review. An additional 23 articles were added to the review based on the authors' knowledge of the area. The reasons for exclusion included review articles (n = 26), commentary (n = 10), did not report 3 levels of physical activity (n = 24), no objective measure of physical activity (n = 2), report (n = 15), not a formal study (n = 11), not related to all-cause mortality (n = 27), the participants were too young (n = 1), not able to retrieve articles (n = 7), and other (n = 7). Therefore, a total of 70 articles were included in the systematic review of the literature regarding the relationship between physical activity and premature mortality.
Figure 2.
Results of the Literature Search for All-Cause Mortality.
The majority of the studies included in our systematic review were prospective cohort investigations (Table 11). These studies involved a total of 1,525,377 participants; averaging 21,791 participants per study (range 302-252,925). There were a total of 111,125 reported cases of premature all-cause mortality (ranging per study from 43-10,952). The total length of study follow-up for the prospective cohort studies averaged 11.1 yr (ranging from 0.5-28 yr). The articles were published over a 22 yr period ranging from 1985 to 2007. These studies involved large samples of men and women from regions throughout the world.
Table 11.
Studies examining the relationship between physical activity and all-cause mortality.
| Publication Country Study Design Quality Score | Objective | Population | Methods | Outcome | Comments and Conclusions |
|---|---|---|---|---|---|
| Blair et al 1989 [7] | To study physical fitness (PF) and risk of all-cause mortality in men and women. | • n = 13,344 (10,224 men; 3,120 women) | Baseline and 8 year follow-up | • 283 deaths | Low levels of PF increase the risk for premature mortality. |
| • Sex: Men and women | Adjusted risk ratio (RR), 95% confidence interval (CI) | ||||
| USA | • Age: 20->60 years (yr) | PF assessment: Maximal treadmill exercise test. | |||
| Prospective cohort | • Characteristics: Participants were given a preventative Medicine examination including maximal treadmill exercise test | Fitness categorized into quintiles: | Men | ||
| D & B score = 12 | Q1 = least fit | • Q1 = 3.44 (2.05-5.77) | |||
| Q2 | • Q2 = 1.37 (0.76-2.50) | ||||
| Q3 | • Q3 = 1.46 (0.81-2.63) | ||||
| Q4 | • Q4 = 1.17 (0.63-2.17) | ||||
| Q5 = most fit | • Q5 = 1.00 (referent) | ||||
| Women | |||||
| • Q1 = 4.65 (2.22-9.75) | |||||
| • Q2 = 2.42 (1.09-5.37) | |||||
| • Q3 = 1.43 (0.60-3.44) | |||||
| • Q4 = 0.76 (0.27-2.11) | |||||
| • Q5 = 1.00 (referent) | |||||
| Myers et al 2004 [32] | To determine the effects of PF and physical activity (PA) on all-cause mortality. | • n = 6,213 | Baseline and mean 5.5 ± 2.0 year follow-Up | • 1,256 deaths | Being fit or active is associated with >50% reductions in mortality risk. |
| • Sex: Men | |||||
| USA | • Age: Mean 59.0 ± 11.2 yr | PF Level hazard ratio (HR) (95% CI) | |||
| • Characteristics: Men referred for exercise testing | PF assessment: Treadmill test to measure VO2 peak | • G1 = 1.00 (referent) | PF predicted mortality more strongly than PA. | ||
| Prospective cohort | • G2 = 0.59 (0.52-0.68) | ||||
| • G3 = 0.46 (0.39-0.55) | |||||
| • G4 = 0.28 (0.23-0.34) | Increasing PA (by 1000 kcal/wk or 1 MET) confers a mortality benefit of 20%. | ||||
| D & B score = 12 | PA assessment: Self reported PA divided into 4 groups | ||||
| PA Level HR (95% CI) | |||||
| G1 = Lowest level | • G1 = 1.00 (referent) | ||||
| G2 | • G2 = 0.63 (0.36-1.10) | ||||
| G3 | • G3 = 0.42 (0.23-0.78) | ||||
| G4 = Highest level | • G4 = 0.38 (0.19-0.73) | ||||
| Blair et al 1995 [36] | To evaluate the relationship between changes in PF and risk of mortality in men. | • n = 9,777 | 4.9 year mean follow-up | • 223 deaths | Men who maintained or increased adequate PF had a reduced risk for all-cause mortality than individuals who were consistently unfit. |
| • Sex: Men | |||||
| • Age: 20-82 yr | RR (95% CI) | ||||
| USA | • Characteristics: Participants were given a preventative medicine examination including maximal treadmill exercise test | PF assessment: Maximal exercise test at baseline and follow-up | • G1 = 1.00 (referent) | ||
| Prospective cohort | • G2 = 0.56 (0.41-0.75) | ||||
| • G3 = 0.52 (0.38-0.70) | |||||
| • G4 = 0.33 (0.23-0.47) | |||||
| D & B score = 13 | Groups based on changes in PF | ||||
| G1 = unfit to unfit | |||||
| G2 = unfit to fit | |||||
| G3 = fit to unfit | |||||
| G4 = fit to fit | |||||
| Bijnen et al 1999 [37] | To examine the association of PA at baseline and 5 years | • n = 472 | 1985 and 1990 | • 118 deaths | Recent levels of PA were more important for mortality risk than PA 5 years previously. |
| • Sex: Men | |||||
| • Age: >65 yr | PA assessment: Questionnaire, divided into tertiles: Lowest Middle Highest | Multivariate adjusted RR (95% CI) | |||
| Netherlands | previously with all- cause mortality risk in a cohort of elderly Dutch men. | • Characteristics: Mostly independently living elders (~95%) | PA in 1985: Lowest tertile = 1.00 (referent) Middle tertile | ||
| Retrospective cohort | • Zutphen Elderly Study | • Total activity = 1.25 (0.79- 1.99) | Becoming or remaining sedentary increased the mortality risk. | ||
| D & B score = 12 | • Walking = 0.97 (0.60-1.57) | ||||
| • Bike = 0.97 (0.59-1.57) | |||||
| • Gardening = 0.66 (0.39-1.10) | |||||
| • Other = 1.08 (0.66-1.78) | |||||
| • Heavy activity = 0.73 (0.45-1.17) | |||||
| • Non heavy activity = 0.89 (0.57-1.40) | |||||
| Highest tertile | |||||
| • Total activity = 1.25 (0.73-2.12) | |||||
| • Walking = 0.94 (0.58-1.55) | |||||
| • Bike = 1.07 (0.61-1.88) | |||||
| • Gardening = 0.77 (0.42-1.39) | |||||
| • Other = 1.24 (0.74-2.07) | |||||
| • Heavy activity = 0.76 (0.44-1.32) | |||||
| • Non heavy activity = 0.94 (0.58-1.53) | |||||
| PA in 1990: | |||||
| Lowest tertile = 1.00 (referent) | |||||
| Middle tertile | |||||
| • Total activity = 0.56 (0.35-0.89) | |||||
| • Walking = 0.82 (0.51-1.32) | |||||
| • Bike = 0.49 (0.29-0.82) | |||||
| • Gardening = 1.67 (1.00-2.79) | |||||
| • Other = 0.93 (0.53-1.65) | |||||
| • Heavy activity = 1.19 (0.73-1.92) | |||||
| • Non heavy activity = 0.61 (0.38-0.99) | |||||
| Highest tertile | |||||
| • Total activity = 0.44 (0.25-0.80) | |||||
| • Walking = 1.17 (0.70-1.96) | |||||
| • Bike = 0.43 (0.23-0.80) | |||||
| • Gardening = 1.03 (0.55-1.94) | |||||
| • Other = 0.74 (0.44-1.23) | |||||
| • Heavy activity = 0.72 (0.40-1.31) | |||||
| • Non heavy activity = 0.65 (0.40-1.05) | |||||
| Gregg et al 2003 [39] | To examine the relationship of changes in PA and mortality among older women. | • n = 9,518 | Baseline (1986-1988) and median 10.6 year follow-up (1992-1994) | • 2,218 deaths | Increasing and maintaining PA levels could lengthen life for older women but appears to provide less benefit for women aged at least 75 years and those with poor health status. |
| • Sex: Women | PA Assessment: Questionnaire, divided into quintiles of PA (kcal/wk) | ||||
| • Age: ≥ 65 yr | Multivariate adjusted HRR | ||||
| USA | • Characteristics: White community dwelling participants from 4 US research centres | (95% CI): Quintiles of total | |||
| Q1= <163 | PA | ||||
| Prospective cohort | Q2 = 163-503 | • Q1 = 1.00 (referent) | |||
| Q3 = 504-1045 | • Q2 = 0.73 (0.64-0.82) | ||||
| Q4 = 1046-1906 | • Q3 = 0.77 (0.68-0.87) | ||||
| D & B score = 13 | Q5 = ≥ 1907 | • Q4 = 0.62 (0.54-0.71) | |||
| • Q5 = 0.68 (0.59-0.78) | |||||
| Walking HRR (95% CI) | |||||
| • Q1 = 1.00 (referent) | |||||
| Quintiles of walking(kcal/wk) | • Q2 = 0.91 (0.81-1.02) | ||||
| Q1 = <70 | • Q3 = 0.78 (0.68-0.88) | ||||
| Q2 = 70-186 | • Q4 = 0.71 (0.63-0.82) | ||||
| Q3 = 187-419 | • Q5 = 0.71 (0.62-0.82) | ||||
| Q4 = 420-897 | |||||
| Q5 = 898 | |||||
| Multivariate adjusted HRR (95% CI) | |||||
| Change in activity level: Sedentary at baseline | |||||
| • Staying sedentary = 1.00 (referent) | |||||
| • Became active = 0.52 (0.40-0.69) | |||||
| Mod / high active at baseline | |||||
| • Became sedentary = 0.92 (0.77-1.09) | |||||
| • Stayed active = 0.68 (0.56-0.82) | |||||
| Wannamethee et al 1998 [40] | To study the relationship between heart rate, PA and all- cause mortality. | • n = 5,934 | Baseline (1978-1980) and 12-14 year follow-up | • 219 deaths | Maintaining or taking up light or moderate PA reduces mortality in older men. |
| • Sex: Men | |||||
| • Age: Mean 63 yr | Multivariate adjusted RR (95% CI), | ||||
| UK | • Characteristics: Healthy, sedentary(4,311 were considered "healthy" in 1992) | PA assessment: Questionnaire, split into groups | PA | ||
| Prospective cohort | • The British Regional Heart Study | • G1 = 1.00 (referent) | |||
| • G2 = 0.61 (0.43-0.86) | |||||
| • G3 = 0.50 (0.31-0.79) | |||||
| D & B score = 12 | PA score | • G4 = 0.65 (0.45-0.94) | |||
| G1 = | |||||
| Inactive/occasional | Regular walking | ||||
| G2 = Light | • G1 = 1.00 (referent) | ||||
| G3 = Moderate | • G2 = 1.15 (0.73-1.79) | ||||
| G4 = Moderately | • G3 = 1.06 (0.75-1.50) | ||||
| vigorous/Vigorous | • G4 = 0.97 (0.65-1.46) | ||||
| Regular walking (min/d) | • G5 = 0.62 (0.37-1.05) | ||||
| G1 = 0 | Recreational activity | ||||
| G2 = <20 | • G1 = 1.00 (referent) | ||||
| G3 = 21-40 | • G2 = 0.95 (0.43-1.07) | ||||
| G4 = 41-60 | • G3 = 0.68 (0.43-1.07) | ||||
| G5 = ≥ 60 | • G4 = 0.34 (0.35-1.00) | ||||
| Recreational activity, 4 groups | Sporting activity | ||||
| G1 = Inactive/fairly Inactive | • G1 = 1.00 (referent) | ||||
| G2 = Average 4 hr/weekend | • G2 = 0.50 (0.25-1.03) | ||||
| G3 = Fairly active >4 h/weekend | • G3 = 0.88 (0.64-1.23) | ||||
| G4 = Very active | |||||
| Sporting activity, 3 Groups | |||||
| G1 = None | |||||
| G2 = Occasional | |||||
| G3 = >1 time/month | |||||
| Paffenbarger et al 1986 [63] | To examine the PA and life-style characteristics of Harvard alumni for the relationship with all-cause mortality. | • n = 16,936 | 12-16 year follow-up (1962 to 1978) | • 1,413 deaths | The findings suggest a protective effect of exercise against all-cause mortality. |
| • Sex: Men | Age adjusted RR (95% CI): | ||||
| • Age: 35-74 | |||||
| USA | • Characteristics: Harvard alumni | Records of freshman year physical examinations and records of intercollegiate sport | Those who walked | ||
| Prospective cohort | • G1 = 1.00 (referent) | ||||
| • G2 = 0.85 | |||||
| • G3 = 0.79 | |||||
| D & B score = 14 | Trend p = 0.0009 | ||||
| PA assessment: Mailed questionnaires surveying post college | Physical Activity Index (95% CI): | ||||
| PA | • G1 = 1.00 (referent) | ||||
| • G2 = 0.78 | |||||
| • G3 = 0.73 | |||||
| • G4 = 0.63 | |||||
| Exercise reported: Walking (miles/wk) 3 | • G5 = 0.62 | ||||
| groups | • G6 = 0.52 | ||||
| G1 = <3 | • G7 = 0.46 | ||||
| G2 = 3-8 | • G8 = 0.62 | ||||
| G3 = ≥ 9 | |||||
| Trend p = <0.0001 | |||||
| PA index (kcal/wk) 3 groups: | |||||
| G1 = <500 | |||||
| G2 = 500-999 | |||||
| G3 = 1000-1499 | |||||
| G4 = 1500-1999 | |||||
| G5 = 2000-2499 | |||||
| G6 = 2500-2999 | |||||
| G7 = 3000-3499 | |||||
| G8 = >3500 | |||||
| Cox proportional hazard models | |||||
| Schnohr et al 2007 [64] | To determine the impact of walking duration and intensity on all-cause mortality. | • n = 7,308 (3,204 male; 4,104 female) | Baseline and an average of 12 year | • 1,391 deaths | The findings indicate that the relative intensity and not duration of walking is the most important in relation to all-cause mortality. |
| Denmark | • Sex: Male and female | follow-up | Multivariate adjusted HR (95% CI): | ||
| • Age: 20-93 yr | PA assessment: Questionnaire, 4 durations and 3 intensities | ||||
| Prospective cohort | • Characteristics: Participants with no history of CHD, stroke or cancer and who had no difficulty in walking | Men | |||
| D & B score = 12 | • The Copenhagen City Heart Study | • G1 = 1.00 (referent) | |||
| • G2 = 0.38 (0.25-0.58) | |||||
| • G3 = 0.38 (0.18-0.79) | |||||
| Duration (hours/day) | • G4 = 0.69 (0.44-1.07) | ||||
| 1 = <0.5 | • G5 = 0.37 (0.26-0.54) | ||||
| 2 = 0.5-1 | • G6 = 0.33 (0.18-0.61) | ||||
| 3 = 1-2 | • G7 = 0.78 (0.50-1.23) | ||||
| 4 = >2 | • G8 = 0.41 (0.29-0.59) | ||||
| • G9 = 0.33 (0.20-0.54) | |||||
| Intensity | • G10 = 0.43 (0.22-0.82) | ||||
| Slow intensity (SI) | • G11 = 0.42 (0.29-0.60) | ||||
| Average intensity (AI) | • G12 = 0.28 (0.16-0.48) | ||||
| Fast intensity (FI) | |||||
| Women | |||||
| 12 groups | • G1 = 1.00 (referent) | ||||
| G1 = 1 and SI | • G2 = 0.82 (0.52-1.29) | ||||
| G2 = 1 and AI | • G3 = 0.78 (0.27-2.21) | ||||
| G3 = 1 and FI | • G4 = 1.22 (0.82-1.81) | ||||
| G4 = 2 and SI | • G5 = 0.74 (0.52-1.05) | ||||
| G5 = 2 and AI | • G6 = 0.56 (0.33-0.96) | ||||
| G6 = 2 and FI | • G7 = 0.94 (0.60-1.47) | ||||
| G7 = 3 and SI | • G8 = 0.87 (0.61-1.23) | ||||
| G8 = 3 and AI | • G9 = 0.48 (0.28-0.83) | ||||
| G9 = 3 and FI | • G10 = 0.88 (0.40-1.88) | ||||
| G10 = 4 and SI | • G11 = 0.64 (0.44-0.95) | ||||
| G11 = 4 and AI | • G12 = 0.38 (0.21-0.69) | ||||
| G12 = 4 and FI | |||||
| Kushi et al 1997 [65] | To evaluate the association between PA and all-cause mortality in postmenopausal women. | • n = 40,417 | 7 year follow-up | • 2,260 deaths | The results demonstrate a graded inverse association between PA and all-cause mortality in postmenopausal women. |
| • Sex: Women | |||||
| • Age: 55-69 yr | PA assessment: Questionnaire for frequency of moderate and vigorous LTPA | Multivariate adjusted Frequency of moderate PA per week RR (95% CI): | |||
| USA | • Characteristics: Postmenopausal Iowa women | ||||
| Prospective cohort | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.71 (0.63-0.79) | |||||
| D & B score = 13 | Divided by frequency/week | • G3 = 0.63 (0.56-0.71) | |||
| • G4 = 0.59 (0.51-0.67) | |||||
| G1 = Rarely/never | Trend p = <0.001 | ||||
| G2 = 1 time/week to a few times/month | |||||
| Frequency of vigorous PA per week | |||||
| G3 = 2-4 times/week | |||||
| G4 = >4 times/week | • G1 = 1.00 (referent) | ||||
| • G2 = 0.83 (0.69-0.99) | |||||
| • G3 = 0.74 (0.59-0.93) | |||||
| Activity index | • G4 = 0.62 (0.42-0.90) | ||||
| G1 = Low | Trend p = 0.009 | ||||
| G2 = Medium | |||||
| G3 = High | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.77 (0.69-0.86) | |||||
| • G3 = 0.68 (0.60-0.77) | |||||
| Trend p = <0.001 | |||||
| Paffenbarger et al 1993 [67] | To analyze changes in the lifestyles of Harvard College alumni and the association of these changes with mortality. | • n = 10,269 | Baseline (1977) and 8 year follow-up (1985) | • 476 deaths | Beginning moderately vigorous sports activity was associated with lower rates of death from all causes among middle aged and older men. |
| • Sex: Men | |||||
| • Age: 45-84 yr (in 1977) | Beginning moderate sports activity was associated with 23% lower risk of death (95% CI 4%-42%, p = 0.015) than those not taking up moderate activity | ||||
| USA | • Characteristics: Participants with no reported life- threatening disease | PA Assessment: Questionnaire -- blocks walked daily, stairs climbed daily and type, frequency and duration of weekly sports and recreational activities | |||
| Prospective cohort | |||||
| D & B score = 13 | |||||
| Physical activity index (kcal/wk) | |||||
| Sports and recreational activities | |||||
| Light <4.5 METs | |||||
| Moderate >4.5 METs | |||||
| Weekly lists of deaths were obtained from the Harvard college alumni office | |||||
| Proportional hazard models with Poisson regression methods | |||||
| Katzmarzyk and Craig 2002 [154] | To quantify the relationship between musculoskeletal fitness and all-cause mortality. | • n = 8,116 (3,933 male; 4,183 female) | Baseline (1981) and | • 238 deaths | Some components of musculoskeletal fitness are predictive of mortality. |
| 13 year follow-up | |||||
| • Sex: Men and women | RR (95% CI) adjusted for age, smoking status, body mass and VO2max | ||||
| Canada | Musculoskeletal fitness (sit ups, push ups, grip strength, sit and reach) measures divided into quartiles | ||||
| • Age: 20-69 yr | Q1 = lowest | Sit ups | |||
| Prospective cohort | • Characteristics: Participants who had musculoskeletal fitness measurements taken | Q2 | Men | ||
| Q3 | • Q1 = 2.72 (1.56-4.64) | ||||
| Q4 = highest | • Q2 = 1.32 (0.73-2.41) | ||||
| D & B score = 11 | • Q3 = 1.61 (0.90-2.87) | ||||
| • Q4 = 1.00 (referent) | |||||
| • Canadian Fitness Survey | |||||
| Cox proportional hazard ratio model | Women | ||||
| • Q1 = 2.26 (1.15-4.43) | |||||
| • Q2 = 2.24 (1.07-4.67) | |||||
| • Q3 = 1.27 (0.59-2.72) | |||||
| • Q4 = 1.00 (referent) | |||||
| Push-ups | |||||
| Men | |||||
| • Q1 = 1.25 (0.77-2.05) | |||||
| • Q2 = 1.17 (0.71-1.90) | |||||
| • Q3 = 0.94 (0.55-1.62) | |||||
| • Q4 = 1.00 (referent) | |||||
| Women | |||||
| • Q1 = 0.61 (0.32-1.17) | |||||
| • Q2 = 0.81 (0.45-1.47) | |||||
| • Q3 = 0.87 (0.48-1.58) | |||||
| • Q4 = 1.00 (referent) | |||||
| Grip strength (kg) | |||||
| Men | |||||
| • Q1 = 1.49 (0.86-2.59) | |||||
| • Q2 = 1.42 (0.82-2.45) | |||||
| • Q3 = 1.59 (0.95-2.68) | |||||
| • Q4 = 1.00 (referent) | |||||
| Women | |||||
| • Q1 = 1.08 (0.58-1.99) | |||||
| • Q2 = 0.62 (0.44-1.56) | |||||
| • Q3 = 1.25 (0.70-2.23) | |||||
| • Q4 = 1.00 (referent) | |||||
| Sit and reach (cm) | |||||
| Men | |||||
| • Q1 = 1.06 (0.64-1.74) | |||||
| • Q2 = 1.01 (0.61-1.66) | |||||
| • Q3 = 1.20 (0.74-1.95) | |||||
| • Q4 = 1.00 (referent) | |||||
| Women | |||||
| • Q1 = 1.18 (0.66-2.10) | |||||
| • Q2 = 1.07 (0.60-1.91) | |||||
| • Q3 = 0.77 (0.44-1.46) | |||||
| • Q4 = 1.00 (referent) | |||||
| Andersen et al 2000 [163] | To evaluate the relationship between levels of OPA, LTPA, cycling to work and sports participation and all-cause mortality. | • n = 30,640 (17,265 men; 13,375 women) | 14.5 year follow-up | • 8,549 deaths | LTPA was inversely associated with all-cause mortality in both men and women in all age groups. |
| PA assessment: Questionnaire for LTPA, divided into: | Incidence of all-cause mortality and PA | ||||
| Denmark | • Sex: Men and women | ||||
| Prospective cohort | • Age: 20-93 years (yr) | Multivariate adjusted RR (95% CI) | |||
| G1 = Low | |||||
| • Characteristics: Participants of the Copenhagen City Heart Study, Glostrup Population Study and Copenhagen Male Study | G2 = Moderate | ||||
| D & B score = 13 | G3 = High | Age 20-44 yr | |||
| Men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.73 (0.56-0.96) | |||||
| • G3 = 0.74 (0.55-1.01) | |||||
| Women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.75 (0.54-1.04) | |||||
| • G3 = 0.66 (0.42-1.05) | |||||
| Age 45-64 yr | |||||
| Men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.75 (0.67-0.84) | |||||
| • G3 = 0.75 (0.67-0.85) | |||||
| Women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.73 (0.65-0.83) | |||||
| • G3 = 0.66 (0.56-0.77) | |||||
| Age >65 yr | |||||
| Men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.62 (0.53-0.73) | |||||
| • G3 = 0.60 (0.50-0.72) | |||||
| Women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.52 (0.45-0.61) | |||||
| • G3 = 0.49 (0.39-0.61) | |||||
| All age groups | |||||
| Men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.72 (0.66-0.78) | |||||
| • G3 = 0.71 (0.65-0.78) | |||||
| Women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.65 (0.60-0.71) | |||||
| • G3 = 0.59 (0.52-0.67) | |||||
| Barengo et al 2004 [164] | To investigate whether moderate or high LTPA are associated with reduced CVD and all-cause mortality, independent of CVD risk factors and other forms of PA in men and women. | • n = 31,677 (15,853 men; 16,824 women) | 20 year follow-up | HRR (95% CI) | Moderate and high levels of LTPA and OPA are associated with reduced premature all-cause mortality. |
| • Sex: Men and women | PA assessment: Questionnaire self administered to measure OPA, LTPA and commuting activity | LTPA | |||
| Finland | • Age: 30-59 yr | • 1.00 (referent) = low | |||
| • Characteristics: Participants from eastern and south-western Finland | • 0.91 (0.84-0.98) = mod, Men | ||||
| Prospective cohort | |||||
| • 0.79 (0.70-0.90) = high, Men | |||||
| D & B score = 14 | • 0.89 (0.81-0.98) = mod, women | ||||
| • 0.98 (0.83-1.16) = high, women | |||||
| OPA | |||||
| • 1.00 (referent) = low | |||||
| • 0.75 (0.68-0.83) = mod, men | |||||
| • 0.77 (0.71-0.84) = active, men | |||||
| • 0.79 (0.70-0.89) = mod, women | |||||
| • 0.78 (0.70-0.87) = active, women | |||||
| Bath 2003 [165] | To examine differences between older men and women on the self-rated health mortality relationship. | • n = 1,042 (406 men; 636 women at baseline) | Baseline, 4 and 12 years post | Number of deaths: At 4 years 242 (106 men; 136 women) | The self-rated health-mortality relationship can be explained by health and related factors among older men and women. |
| UK | • Sex: Men and women | • At 12 years 665 (287 men; 378 women) | |||
| Prospective cohort | • Age: >65 yr | ||||
| • Characteristics: Community-dwelling Elderly | General physical health 14-item health index (Ebrahin et al 1987) scoring from 0-14 (no health problems -- multiple health problems) |
Multivariate adjusted HR (95% CI) | |||
| D & B score = 11 | |||||
| • The Nottingham Longitudinal Study of Activity and Ageing | |||||
| Men after 4 years | |||||
| • High = 1.00 (referent) | |||||
| • Med = 1.19 (0.61-2.33) | |||||
| PA assessment: Self-rated health surveys, divided into 3 levels of PA: | • Low = 1.51 (0.75-3.03) | ||||
| High | Women after 4 years | ||||
| Medium | • High = 1.00 (referent) | ||||
| Low | • Med = 1.03 (0.58-1.82) | ||||
| • Low = 1.51 (0.86-2.67) | |||||
| Men after 12 years | |||||
| Cox proportional hazards regression Models | • High = 1.00 (referent) | ||||
| • Med = 1.28 (0.94-1.74) | |||||
| • Low = 1.13 (0.82-1.55) | |||||
| Women after 12 years | |||||
| • High = 1.00 (referent) | |||||
| • Med = 1.20 (0.90-1.61) | |||||
| • Low = 1.23 (0.93-1.62) | |||||
| Bijnen et al 1998 [166] | To describe the association between PA and mortality (CVD, stroke, all-cause) in elderly men. | • n = 802 | 10 year follow-up | • 373 deaths | PA may protect against all- cause mortality in elderly men |
| • Sex: Men | |||||
| • Age: 64-84 yr | PA assessment: Questionnaire, divided into groups: | Multivariate adjusted RR (95% CI) | |||
| Netherlands | • Characteristics: Retired Dutch men | ||||
| • G1 = 1.00 (referent) | |||||
| Prospective cohort | G1 = Lowest | • G2 = 0.80 (0.63-1.02) | |||
| G2 = Middle | • G3 = 0.77 (0.59-1.00) | ||||
| G3 = Highest | p = 0.04 | ||||
| D & B score = 12 | |||||
| Blair et al 1993 [167] | To evaluate the relationship of sedentary living habits to all-cause mortality in women. | • n = 3,120 | Baseline and 8 year follow-up | • 43 deaths | There is a graded inverse relationship between PF and all-cause mortality in women. |
| • Sex: Women | |||||
| • Age: Not available | Age adjusted death rates (per 10,000 person years) by fitness | ||||
| USA | • Characteristics: Participants were given a preventative medicine examination | PF assessment: PF measured via maximal treadmill exercise test; | |||
| Prospective | • Low Fitness = 40 | The lack of relationship between PA and death rate was believed to be due to an inadequate assessment of PA. | |||
| • Mod Fitness = 16 | |||||
| D & B score = 14 | • High Fitness = 7 | ||||
| PA assessment: Questionnaire | |||||
| No difference between levels of PA | |||||
| Blair et al 1996 [168] | To review the association of PF to all-cause and CVD mortality. | • n = 32,421 (25,341 men; 7,080 women) | Baseline and average 8 year follow-up (range 0.1-19.1 years) | • 601 deaths in men | The study observed a steep inverse gradient of death rates across low, moderate and high PF levels. The association was strong and remained after adjustment for potential confounding factors. |
| • 89 deaths in women | |||||
| • Sex: Men and women | |||||
| USA | • Age: 20-80 yr (mean 43 yr) | RR (95% CI) in low PF vs. | |||
| PF assessment: Treadmill test; duration was used to assign participants to sex specific groups: | high PF | ||||
| Prospective cohort | • Characteristics: Participants were excluded if they did not reach 85% of their age predicted maximal heart rate on the maximal exercise treadmill test | Men | |||
| • 1.52 (1.28-1.82) | |||||
| Women | |||||
| D & B score = 14 | • 2.10 (1.36-3.26) | ||||
| Low (least fit 20%) | Adjusted deaths per 10,000 person years according to PF | ||||
| Moderate (next 40%) | |||||
| High (most fit 40%) | Men | ||||
| • Aerobics Center Longitudinal Study | Proportional hazard modeling | • Low = 49 | |||
| • Med = 27 | |||||
| • High = 23 | |||||
| Women | |||||
| • Low = 29 | |||||
| • Med = 13 | |||||
| • High = 14 | |||||
| Boyle et al 2007 [169] | To examine the association between PA and the risk of incident disability, including impairment in activities of daily living and instrumental activities of daily living in community based older persons free from dementia. | • n = 1,020 | 2.6 year follow-up | • 156 deaths | The risk of death decreased 11% with each hour of PA/wk. |
| • Sex: Men and women | |||||
| • Age: 54-100 yr | PA assessment: Questionnaire, hr/wk of PA Incidence of all-cause mortality | HR for all-cause mortality | |||
| USA | • Characteristics: Participants from 40 retirement communities across Chicago | The risk of death was 11% lower for each hr/wk of PA | |||
| Prospective cohort | |||||
| D & B score = 13 | • Rush Memory and Aging Project | ||||
| Bucksch et al 2005 [170] | To examine the effect of moderately intense PA on all-cause mortality. | • n = 7,187 (3,742 men; 3,445 women) | Baseline (1984-1986) and 12-14 yr follow-up (1998) | • 943 deaths | Participants who achieved recommended amounts of MPA or VPA were at a significantly lower risk of death than their sedentary counterparts. |
| • Sex: Men and women | RR (95% CI) for achieving recommended PA vs. not achieving recommendation | ||||
| Germany | • Age: 30-69 yr | ||||
| Prospective cohort | • Characteristics: Participants were healthy and physically active during leisure time | PA assessment: Questionnaire (Minnesota Leisure Time Physical Activity questionnaire) divided into groups based on: Achieving recommended amount of MPA (30 min, 5 d/wk (≥2.5 h/wk)) | |||
| Women | |||||
| • MPA = 0.65 (0.51-0.82) | |||||
| D & B score = 13 | • VPA = 0.78 (0.57-1.08) | ||||
| • MPA or VPA = 0.60 (0.47-0.75) | |||||
| Men | |||||
| • MPA = 0.90 (0.77-1.01) | |||||
| • VPA = 0.74 (0.61-0.90) | |||||
| • MPA or VPA = 0.80 (0.68-0.94) | |||||
| Achieving recommended amount of VPA (20 min, 3 d/wk (≥ 1 h/wk)) | |||||
| RR (95% CI) for volume of lifestyle activities (kcal/kg/wk) | |||||
| Volume of lifestyle activities (kcal/kg/wk) | Women | ||||
| G1 = 0 | • G1 = 1.00 (referent) | ||||
| G2 = <14 | • G2 = 0.79 (0.57-1.08) | ||||
| G3 = 14-33.5 | • G3 = 0.68 (0.50-0.94) | ||||
| G4 = ≥ 33.5 | • G4 = 0.57 (0.41-0.79) | ||||
| p < 0.001 | |||||
| Men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.98 (0.76-1.17) | |||||
| • G3 = 0.80 (0.63-1.00) | |||||
| • G4 = 0.91 (0.74-1.13) | |||||
| p = 0.20 | |||||
| Adjusted for age, other recommendation, social class, smoking, BMI, cardio risk factor index, alcohol intake, chronic disease index and dietary factors | |||||
| Bucksch and Helmert 2004 [171] | To examine LTPA and premature death in the general population of former West Germany. | • n = 7,187 (3,742 men; 3,445 women) | Baseline (1984-1986) and 12-14 year follow-up (1998) | • 943 deaths | LTPA is inversely associated with all-cause mortality in men and women. |
| • Sex: Men and women | RR (95% CI) | ||||
| • Age: 30-69 yr | Men, LTPA | ||||
| Germany | • Characteristics: Participants were selected on the basis of the German Cardiovascular Prevention Study | PA assessment: Questionnaire (Minnesota Leisure Time Physical Activity questionnaire) divided into groups based on: LTSA (h/wk) | • G1 = 1.00 (referent) | ||
| • G2 = 0.85 (0.78-0.93) | |||||
| Prospective cohort | • G3 = 0.64 (0.50-0.82) | ||||
| • G4 = 0.70 (0.54-0.91) | |||||
| p < 0.001 | |||||
| D & B score = 14 | • The National Health Survey of the German Federal Institute of Population Research (1984-1998) | Men, LTPA index | |||
| G1 = 0 | • G1 = 1.00 (referent) | ||||
| G2 = <1 | • G2 = 0.92 (0.70-1.23) | ||||
| G3 = 1-2 | • G3 = 0.89 (0.69-1.17) | ||||
| G4 = >2 | • G4 = 0.61 (0.44-0.84) | ||||
| p <0.01 | |||||
| The LTSA-index (kcal/kg/wk) | |||||
| G1 = 0 | Women, LTPA | ||||
| G2 = 1-10 | • G1 = 1.00 (referent) | ||||
| G3 = 10-25 | • G2 = 0.93 (0.82-1.04) | ||||
| G4 = >25 | • G3 = 0.69 (0.48-0.98) | ||||
| • G4 = 0.57 (0.35-0.94) | |||||
| Mortality -- Records from the mandatory population registries | p < 0.01 | ||||
| Women, LTPA index | |||||
| • G1 = 1.00 (referent) | |||||
| Cox proportional hazard regression model | • G2 = 0.68 (0.45-1.01) | ||||
| • G3 = 0.79 (0.51-1.21) | |||||
| • G4 = 0.46 (0.25-0.85) | |||||
| p < 0.01 | |||||
| Adjusted for age, social class, smoking, BMI, cardio risk factor index, alcohol intake, chronic disease index and dietary factors | |||||
| Carlsson et al 2006 [172] | To investigate the association between PA and mortality in post-menopausal women. | • n = 27,734 | Baseline (1997) and 2-7 year follow-up (1999-2004) | • 1,232 deaths | The study indicates that even fairly small amounts of activity will reduce mortality in older women. |
| • Sex: Women | |||||
| • Age: 51-83 yr | RR (95% CI) adjusted for lifestyle and medical problems | ||||
| Sweden | • Characteristics: Women who participated in a population based Screening programme in 1987 | ||||
| Prospective cohort | PA assessment: Questionnaires for: METs/day, different PA (walking/biking), LTPA, OPA, household PA, TV watching and reading | ||||
| PA (METs/day) | |||||
| • >50 = 1.00 (referent) | |||||
| D & B score = 12 | • 45-50 = 1.05 (0.77-1.42) | ||||
| • The Swedish Mammography Cohort | • 40-45 s = 1.09 (0.81-1.46) | ||||
| • 45-40 = 1.26 (0.94-1.70) | |||||
| • <35 = 2.56 (1.85-3.53) | |||||
| Mortality -- Records from the National Population Register | |||||
| Different PA | |||||
| Walking/biking (min/d) | |||||
| • > 90 = 1.00 (referent) | |||||
| • 60-90 = 1.01 (0.76-1.34) | |||||
| • 40-60 = 0.92 (0.70-1.20) | |||||
| • 20-40 = 0.96 (0.75-1.23) | |||||
| • <20 = 1.16 (0.90-1.50) | |||||
| • Almost never = 1.94 (1.51-2.50) | |||||
| LTPA (hr/wk) | |||||
| • >5 = 1.00 (referent) | |||||
| • 4-5 = 0.95 (0.74-1.22) | |||||
| • 2-3 = 1.02 (0.83-1.26) | |||||
| • 1 = 1.09 (0.88-1.36) | |||||
| • <1 = 1.91 (1.56-2.35) | |||||
| OPA | |||||
| • Heavy manual labour = 1.00 (referent) | |||||
| • Walking/lifting/ a lot carrying = 0.96 (0.55-1.70) | |||||
| • Walking/lifting/ not a lot carrying = 1.00 (0.60-1.68) | |||||
| • Mostly standing = 0.91 (0.52-1.61) | |||||
| • Seated 50% of time = 0.97 (0.58-1.62) | |||||
| • Mostly sedentary = 1.93 (1.15-3.25) | |||||
| Household work (hr/d) | |||||
| • >8 h/d = 1.00 (referent) | |||||
| • 7-8 = 0.68 (0.49-0.93) | |||||
| • 5-6 = 0.66 (0.51-0.87) | |||||
| • 3-4 = 0.83 (0.64-1.06) | |||||
| • 1-2 = 0.89 (0.69-1.15) | |||||
| • <1 = 1.73 (1.30-2.32) | |||||
| Adjusted for age | |||||
| Crespo et al 2002 [173] | To study the relationship between PA and obesity with all- cause mortality in Puerto Rican men. | • n = 9,136 (1962-1965) | Baseline and 12 year follow-up | • 1,445 deaths | Some PA is better than none in protecting against all-cause mortality. The benefits are independent of body weight. |
| Puerto Rico | • Sex: Men | PA assessment: Questionnaire, divided into 4 groups based on METs | Multivariate OR (95% CI) adjusted for age | ||
| G1 = low | |||||
| G2 | |||||
| G3 | |||||
| G4 = high | |||||
| Prospective cohort | • Age: 35-79 yr | Multivariate logistic function model | • C1 = 1.00 (referent) | ||
| D & B score = 12 | • Characteristics: Participants with no known coronary heart disease | • C2 = 0.67 (0.57-0.78) | |||
| • The Puerto Rico Heart Health Program | • C3 = 0.63 (0.54-0.74) | ||||
| • C4 = 0.54 (0.46-0.64) | |||||
| p < 0.0001 | |||||
| Multivariate adjusted OR (95% CI) | |||||
| • C1 = 1.00 (referent) | |||||
| • C2 = 0.68 (0.58-0.79) | |||||
| • C3 = 0.63 (0.54-0.75) | |||||
| • C4 = 0.55 (0.46-0.65) | |||||
| p < 0.0001 | |||||
| Davey Smith et al 2000 [174] |
To examine the relationship of PA and various causes of death. | • n = 6,702 (at baseline) | Baseline (1969-1970) and 25 year follow-up | • 926 deaths | In the study, an inverse association of both LTPA and walking pace with mortality from all-causes was seen. |
| UK | • Sex: Men | PA assessment: Questionnaire with 3 groups for walking pace (Slower, same, faster) and 3 groups for LTPA (inactive, moderately active, active) | Age adjusted RR (95% CI) for walking pace | ||
| Prospective cohort | • Age: 40-64 yr | • Slower = 2.47 (2.2-2.8) | |||
| D & B score = 13 | • Characteristics: Participants from rural northern Japan | • Same = 1.35 (1.2-1.5) | |||
| • Whitehall study | • Faster = 1.00 (referent) p < 0.001 |
||||
| Fully adjusted RR (95% CI) for walking pace | |||||
| • Slower = 1.87 (1.6-2.1) | |||||
| • Same = 1.21 (1.1-1.3) | |||||
| • Faster = 1.00 (referent) p < 0.001 |
|||||
| Age adjusted RR (95% CI) for LTPA | |||||
| • Inactive = 1.44 (1.3-1.6) | |||||
| • Mod = 1.13 (1.0-1.2) | |||||
| • Active = 1.00 (referent) p < 0.001 |
|||||
| Fully adjusted RR (95% CI) for LTPA | |||||
| • Inactive = 1.20 (1.1-1.3) | |||||
| • Mod = 1.07 (1.0-1.2) | |||||
| • Active = 1.00 (referent) p < 0.001 |
|||||
| Eaton et al 1995 [175] | To determine whether self-reported PA predicts a decreased rate of CHD and all- cause mortality in middle aged men. | • n = 8,463 | 21 year follow-up | • 2,593 deaths | Baseline levels of self- reported LTPA predicted a decreased rate of CHD and all-cause mortality. |
| Europe, Israel, mid eastern Asia, Northern Africa | • Sex: Men | PA assessment: Questionnaire for LTPA | Age adjusted RR (95% CI) LTPA | ||
| Prospective cohort | • Age: ≥40 yr | G1 = Sedentary | • G1 = 1.00 (referent) | ||
| D & B score = 12 | • Characteristics: Government employees without known CVD | G2 = Light | • G2 = 0.84 (0.74-0.94) | ||
| G3 = Light daily | • G3 = 0.81 (0.73-0.90) | ||||
| G4 = Heavy | • G4 = 0.84 (0.72-0.98) | ||||
| OPA | |||||
| Questionnaire for OPA | • G1 = 1.00 (referent) | ||||
| G1 = Sitting | • G2 = 0.99 (0.88-1.12) | ||||
| G2 = Standing | • G3 = 1.09 (0.99-1.20) | ||||
| G3 = Walking | • G4 = 1.16 (1.03-1.30) | ||||
| G4 = Physical labour | |||||
| Fang et al 2005 [176] | To assess the association of exercise and CVD outcome among persons with different blood pressure status. | • n = 9,791 (3,819 men; 5,972 women) | 17 year follow-up | Incidence of all-cause mortality and PA | A significant effect of exercise on mortality in normotensive subjects was not found. |
| USA | • Sex: Men and women | PA assessment: Questionnaire with 3 groups | Multivariate adjusted HR (95% CI) | ||
| Prospective cohort | • Age:25-74 yr | G1 = Least exercise | • G1 = 1.00 (referent) | ||
| D & B score = 12 | • Characteristics: Non- institutionalized participants | G2 = Moderate exercise | • G2 = 0.75 (0.53-1.05) | ||
| G3 = Most exercise | • G3 = 0.71 (0.45-1.12) | ||||
| Fried et al 1998 [177] | To determine the disease, functional and personal characteristics that jointly predict mortality. | • n = 5,886 | 5 year follow-up | • 646 deaths | PA was a predictor of 5-year mortality. |
| USA | • Sex: Men and women | PA assessment: Self reported exercise (5 groups) | Incidence of all-cause mortality and PA | ||
| Prospective cohort | • Age: ≥65 yr | MPA or VPA (kJ/wk) | Multivariate adjusted RR (95% CI) | ||
| D & B score = 11 | • Characteristics: Community dwelling elders | G1 = ≤282 | • G1 = 1.00 (referent) | ||
| G2 = 283-1789 | • G2 = 0.78 (0.60-1.00) | ||||
| G3 = 1790-4100 | • G3 = 0.81 (0.63-1.05) | ||||
| G4 = 4101-7908 | • G4 = 0.72 (0.55-0.93) | ||||
| G5 = >7908 | • G5 = 0.56 (0.43-0.74) p < 0.005 |
||||
| Fujita et al 2004 [178] | To examine the relationship between walking duration and all-cause mortality in a Japanese cohort. | • n = 41,163 (20,004 men; 21,159 women) | Baseline (1990) and 11 year follow-up (2001) | • 1,879 deaths | Time spent walking was associated with a reduced risk for all-cause mortality. |
| Japan | • Sex: Men and women | PA assessment: Questionnaire Walking, 3 levels: | Age and sex adjusted RR (95% CI) for time spent walking (hr/d) | ||
| G1 = ≤30 min | |||||
| G2 = 30 min to 1 hr | |||||
| G3 = ≥1 hr | |||||
| Prospective cohort | • Age: 40-64 yr | Cox proportional hazard model | Whole group | ||
| D & B score = 13 | • Characteristics: Healthy, sedentary | • G1 = 1.22 (1.09-1.35) | |||
| • G2 = 1.09 (0.95-1.22) | |||||
| • G3 = 1.00 (referent) p < 0.001 |
|||||
| Men only | |||||
| • G1 = 1.14 (1.00-1.30) | |||||
| • G2 = 1.03 (0.90-1.19) | |||||
| • G3 = 1.00 (referent p = 0.061 | |||||
| Women only | |||||
| • G1 = 1.40 (1.16-1.68) | |||||
| • G2 = 1.23 (1.01-1.49) | |||||
| • G3 = 1.00 (referent) p < 0.001 |
|||||
| RR (95% CI) for time spent walking (hr/d) (adjusted for age, education, marital status, past history of diseases, smoking, drinking, BMI and dietary variables) | |||||
| Whole group | |||||
| • G1 = 1.17 (1.04-1.31) | |||||
| • G2 = 1.06 (0.93-1.20) | |||||
| • G3 = 1.00 (referent) p = 0.011 |
|||||
| Men | |||||
| • G1 = 1.08 (0.94-1.25) | |||||
| • G2 = 0.98 (0.84-1.14) | |||||
| • G3 = 1.00 (referent) p = 0.318 |
|||||
| Women | |||||
| • G1 = 1.38 (1.12-1.70) | |||||
| • G2 = 1.24 (1.00-1.54) | |||||
| • G3 = 1.00 (referent) p < 0.001 |
|||||
| Glass et al 1999 [179] | To examine any association between social activity, productive activity and PA and mortality in older people. | • n = 2,761 (1,169 men; 1,143 women) | 13 year follow-up | Incidence of all-cause mortality by fitness activity quartile | More active elderly people were less likely to die than those who were less active. |
| USA | • Sex: Men and women | PA assessment: Interview, Amount of activity | 13 yr mortality by amount of activity | ||
| Prospective cohort | • Age: ≥ 65 yr | G1 = Low | • G1 = 74.0 | ||
| D & B score = 12 | • Characteristics: Healthy elders | G2 = Low-medium | • G2 = 69.8 | ||
| G3 = Medium-high | • G3 = 62.4 | ||||
| G4 = High | • G4 = 55.2 | ||||
| Gulati et al 2003 [180] | To determine whether exercise capacity is a predictor for all-cause mortality in asymptomatic women. | • n = 5,721 | Baseline (1992) and 8 year follow-up (2000) | • 180 deaths | This study confirmed that exercise capacity is an independent predictor of death in asymptomatic women, greater than what has been previously established among men. |
| USA | • Sex: Women | PF Assessment: Treadmill stress test Exercise capacity (METs) G1 = <5 G2 = 5-8 G3 = >8 |
For every 1 MET increase there was a reduced death risk of 17% (p < 0.001) | ||
| Prospective cohort | • Age: Mean 52 ± 11 yr | Age-adjusted RR | |||
| D & B score = 11 | • Characteristics: Asymptomatic women | • G1 = 2.0 (1.3-3.2) | |||
| • St James Women Take Heart Project | • G2 = 1.6 (1.1-2.4) | ||||
| • G3 = 1.00 (referent) | |||||
| Adjusted for Framingham Risk Score |
|||||
| • G1 = 3.1 (2.1-4.8) | |||||
| • G2 = 1.9 (1.3-2.9) | |||||
| • G3 = 1.00 (referent) | |||||
| Haapanen et al 1996 [181] | To examine the association between LTPA and all-cause mortality. | • n = 1,072 | Baseline and a 10 yr 10 month follow-up |
• 168 deaths | Low PA is a risk factor for all-cause mortality. |
| Finland | • Sex: Men | PA assessment: Self-reported LTPA, divided into 4 groups by EE (kJ/wk) G1 = 0-3349 G2 = 3350-6279 G3 = 6280-8791 G4 = >8791 |
RR (95% CI) according to EE group | ||
| Prospective cohort | • Age: 35-63 yr | Mortality--National Death Index search |
• G1 = 2.74 (1.46-5.14) | ||
| D & B score = 14 | • Characteristics: Healthy, sedentary | Cox proportional HR | • G2 = 1.10 (0.55-2.21) | ||
| • G3 = 1.74 (0.87-3.50) | |||||
| • G4 = 1.00 (referent) | |||||
| Hakim et al 1998 [182] | To examine the association between walking and mortality in retired men. | • n = 707 | Baseline and 12 yr follow-up | • 208 deaths | The findings in older physically capable men indicate that regular walking is associated with a lower overall mortality rate. |
| USA | • Sex: Men | RR (95% CI) according to distance walked | |||
| Prospective cohort | • Age: 61-81 yr | Adjusted for age | |||
| D & B score = 12 | • Characteristics: Retired non-smoking men who were physically capable of participating in low intensity activities on a daily basis | PA assessment: Questionnaire Distance walked (miles/day) | • G1 vs. G3 = 1.9 (1.3-2.9) | ||
| G1 = 0.0-0.9 | • G1 vs. G3 = 1.6 (1.2-2.2) | ||||
| G2 = 1.0-2.0 | • G2 vs. G3 = 1.2 (0.8-1.7) | ||||
| G3 = 2.1-8.0 | Trend p = 0.002 | ||||
| • Honolulu Heart Program | |||||
| Adjusted for risk factors | |||||
| • G1 vs. G3 = 1.8 (1.2-2.7) | |||||
| • G1 vs. G2 = 1.5 (1.1-2.1) | |||||
| • G2 vs. G3 = 1.1 (0.8-1.7) | |||||
| Trend p = 0.01 | |||||
| Hillsdon et al 2004 [183] | To examine whether VPA is associated with all-cause mortality. | • n = 10,522 (4,929 men; 5,593 women) | >10 year follow-up | • 825 deaths | Questionnaire respondents who reported engaging in VPA less than twice a week experienced a 37% reduced risk of all-cause mortality compared with respondents who reported a lower frequency of VPA. |
| • Sex: Men and women | PA assessment: Questionnaire for frequency of VPA | Age and sex adjusted RR (95% CI) | |||
| UK | • Age: 35-64 yr | G1 = Never, <1 time/month | |||
| • Characteristics: Healthy, sedentary | G2 = <2 times/wk | • G1 = 1.00 (referent) | |||
| Prospective Cohort | • OXCHECK study | G3 = >2 times/wk | • G2 = 0.57 (0.42-0.79) | ||
| • G3 = 0.72 (0.54-0.95) | |||||
| D & B score = 11 | Fully adjusted RR (95% CI) | ||||
| • G1 = 1.00 (referent) | |||||
| Mortality -- Recorded from the Office of National Statistics | • G2 = 0.63 (0.45-0.89) | ||||
| • G3 = 0.81 (0.60-1.09) | |||||
| Cox proportional HR | |||||
| Hu et al 2005 [184] | To examine the association of PA and BMI and their combined effect with the risk of total, CVD and cancer mortality. | • n = 47,212 (22,528 men; 24,684 women) | 17.7 year follow-up | • 7,394 deaths | Regular PA is an important indicator for decreased risk of all-cause mortality. PA has a strong independent effect on mortality. |
| • Sex: Men and women | |||||
| Finland | • Age:25-64 yr | PA assessment: Questionnaire for PA level, divided into 3 groups | Adjusted HR (95% CI) | ||
| • Characteristics: Participants from eastern Finland | Men | ||||
| Prospective cohort | • G1 = 1.00 (referent) | ||||
| • G2 = 0.74 (0.68-0.81) | |||||
| G1 = Low | • G3 = 0.63 (0.58-0.70) | ||||
| D & B score = 12 | G2 = Moderate | Trend p = <0.001 | |||
| G3 = High | |||||
| Women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.64 (0.58-0.70) | |||||
| • G3 = 0.58 (0.52-0.64) | |||||
| Trend p = <0.001 | |||||
| Hu et al 2004 [185] | To examine the association of BMI and PA with death. | • n = 116,564 | Baseline (1976) and | • 10,282 deaths | Reduced PA is a strong and independent predictor of death. |
| • Sex: Women | 24 year follow-up | ||||
| • Age: 30-55 yr | Multivariate RR (95% CI) by PA (hr/wk) | ||||
| USA | • Characteristics: Females free of known CVD and cancer | PA assessment: Questionnaire for PA level, divided into 3 groups (hr/week) | • G1 = 1.00 (referent) | ||
| G1 = ≥ 3.5 | • G2 = 1.18 (1.10-1.26) | ||||
| Prospective cohort | G2 = 1.0-3.4 | • G3 = 1.52 (1.41-1.63) | |||
| D & B score = 11 | G3 = <1.0 | Multivariate RR (95% CI) by PA adjusted for BMI | |||
| • G1 = 1.00 (referent) | |||||
| BMI (kg/m2) | • G2 = 1.14 (1.06-1.22) | ||||
| G1 = <25 | • G3 = 1.44 (1.34-1.55) | ||||
| G2 = 25-29 | |||||
| G3 = 30 | |||||
| Cox proportional HR | |||||
| Kampert et al 1996 [186] | To examine PF and PA in relation to all-cause and cancer mortality. | • n = 32,421 (25,341 men; 7,080 women) | Baseline (1970) and ~8 year follow-up (1989) | • 690 deaths | The data support the hypothesis that an active and fit way of life delays death. |
| • Sex: Men and women | Adjusted RR (95% CI) by quintiles of activity | ||||
| USA | • Age: 20-88 yr (mean ~43) | ||||
| Prospective cohort | • Characteristics: Predominantly white and from the middle and upper socioeconomic strata | PA assessment: Questionnaire, divided into quintiles of activity (min/wk) | Men | ||
| • Sedentary = 1.00 (referent) | |||||
| • C1-2 = 0.71 (0.58-0.97) | |||||
| D & B score = 13 | • C3 = 0.83 (0.59-1.16) | ||||
| Male activity categories | • C4 = 0.57 (0.30-1.08) | ||||
| • C5 = 0.92 (0.29-2.88) | |||||
| Sedentary = 855 | Trend p = 0.011 | ||||
| C1-2 = 1,072 | |||||
| C3 = 1,292 | Women | ||||
| C4 = 1,453 | • Sedentary = 1.00 (referent) | ||||
| C5 = 1,601 | • C1-2 = 0.68 (0.39-1.17) | ||||
| • C3 = 0.39 (0.09-1.65) | |||||
| Females activity categories | • C4-5 = 1.14 (0.27-4.80) | ||||
| Sedentary = 605 | Trend p = 0.217 | ||||
| C1-2 = 792 | |||||
| C3 = 979 | |||||
| C4-5 = 1,158 | |||||
| Cox proportional HR | |||||
| Kaplan et al 1996 [187] | To assess LTPA and its association with all cause mortality. | • n = 6,131 (3298 men; 2833 women) | 28 year follow-up | • 1,226 deaths | The data provide further support for the importance of PA and indicate that the protective effect of PA is a robust one. |
| • Sex: Men and women | PA assessment: Three questions about PA, with scores 0 (never), 2 (sometimes) or 4 (often). | Incidence of all-cause mortality and PA | |||
| USA | • Age: 16-94 yr | ||||
| • Characteristics: Northern Californian adults | |||||
| Prospective cohort | Death rates/1000 person years | ||||
| Men | |||||
| D & B score = 13 | • T1 = 24.68 | ||||
| Tertiles of PA score | • T2 = 11.37 | ||||
| T1 = 0-2 | • T3 = 7.59 | ||||
| T2 = 4-6 | Women | ||||
| T3 = 8-12 | • T1 = 18.03 | ||||
| • T2 = 7.66 | |||||
| • T3 = 3.88 | |||||
| Khaw et al 2006 [188] | To examine the relationship between PA patterns over 1 year and total mortality. | • n = 22,191 (9,984 men; 12,207 women) | 8 year follow-up | • 1,553 deaths | Even very moderate levels of usual PA are associated with reductions in mortality. |
| • Sex: Men and women | PA assessment: Questionnaire, divided into 4 groups of PA | Incidence of all-cause mortality and PA | |||
| UK | • Age: 45-79 yr | Adjusted RR (95% CI) | |||
| • Characteristics: Community living participants | All | ||||
| Prospective cohort | G1 = Inactive | • G1 = 1.00 (referent) | |||
| G2 = Moderately inactive | • G2 = 0.83 (0.73-0.95) | ||||
| D & B score = 13 | • G3 = 0.68 (0.58-0.80) | ||||
| G3 = Moderately active | • G4 = 0.68 (0.57-0.81) | ||||
| G4 = Active | Age <65 | ||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.01 (0.78-1.31) | |||||
| • G3 = 0.81 (0.62-1.07) | |||||
| • G4 = 0.82 (0.62-1.09) | |||||
| Age >65 | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.77 (0.66-0.91) | |||||
| • G3 = 0.65 (0.53-0.79) | |||||
| • G4 = 0.64 (0.50-0.80) | |||||
| Kohl et al 1996 [189] | To determine the association of maximal exercise hemodynamic responses with risk of all-cause mortality. | • n = 26,621 (20,387 men; 6,234 women) | Average 8.1 year follow-up | • 348 deaths in men and 66 in women | The results suggest an exaggerated SBP or an attenuated heart rate response to maximal exercise may indicate an elevated risk for mortality. |
| • Sex: Men and women | |||||
| USA | • Age: Male mean 42.2 yr; female mean 41.9 Yr | Adjusted RH (95% CI) by maximal exercise test HR | |||
| Prospective cohort | Men | ||||
| • Characteristics: Apparently healthy patients of a preventive medicine centre | PF assessment: Maximal exercise test HR (bpm), divided into 4 Groups: | • Q1 = 1.00 (referent) | |||
| G1 = <171 | • Q2 = 0.61 (0.44-0.85) | ||||
| D & B score = 12 | G2 = 171-178 | • Q3 = 0.69 (0.51-0.93) | |||
| G3 = 179-188 | • Q4 = 0.60 (0.41-0.87) | ||||
| G4 = >188 | Trend p<0.05 | ||||
| Women | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 1.23 (0.65-2.32) | |||||
| • Q3 = 0.69 (0.30-1.63) | |||||
| • Q4 = 0.71 (0.22-2.24) | |||||
| Trend p>0.05 | |||||
| Kujala et al 1998 [190] | To investigate LTPA and mortality in a cohort of twins. | • n = 15,902 (7,925 men; 7,977 women) | Baseline 1975 and death outcome from 1977-1994 | • 1,253 deaths | LTPA is associated with reduced mortality, even after genetic and other familial factors are taken into account. |
| • Sex: Men and women | HR (95% CI) | ||||
| Finland | • Age: 25-64 yr | ||||
| • Characteristics: Healthy, Finnish same sex twins | PA assessment: Questionnaire, quintiles of fitness in MET hours/day | Adjusted for age and sex | |||
| Prospective cohort | • Sedentary = 1.00 (referent) | ||||
| • OE = 0.71 (0.62-0.81) | |||||
| • The Finnish Twin Cohort | • CE = 0.57 (0.45-0.74) | ||||
| D & B score = 13 | Q1 = <58 | Trend p = 0.001 | |||
| Q2 = 59-1.29 | |||||
| Q3 = 1.30-2.49 | Adjusted for age, sex, smoking | ||||
| Q4 = 2.50-4.49 | |||||
| Q5 = >4.50 | • Sedentary = 1.00 (referent) | ||||
| • OE = 0.76 (0.67-0.87) | |||||
| Categorized into: | • CE = 0.68 (0.53-0.88) | ||||
| -Sedentary | |||||
| -Occasional exerciser (OE) | Trend p = 0.001 | ||||
| -Conditioning exerciser (CE) | Adjusted for age, sex, smoking, occupational group, alcohol | ||||
| • Sedentary = 1.00 (referent) | |||||
| • OE = 0.80 (0.69-0.91) | |||||
| • CE = 0.76 (0.59-0.98) | |||||
| Trend p = 0.002 | |||||
| HR (95% CI) among 434 same sex twin pairs compared with sedentary category in 1975 | |||||
| • Sedentary = 1.00 (referent) | |||||
| • OE = 0.66 (0.46-0.94) | |||||
| • CE = 0.44 (0.23-0.83) | |||||
| Trend p = 0.005 | |||||
| Adjusted for smoking | |||||
| • Sedentary = 1.00 (referent) | |||||
| • OE = 0.70 (0.48-1.01) | |||||
| • CE = 0.56 (0.29-1.09) | |||||
| Trend p = 0.04 | |||||
| Adjusted for smoking, occupational group, alcohol | |||||
| • Sedentary = 1.00 (referent) | |||||
| • OE = 0.73 (0.50-1.07) | |||||
| • CE = 0.56 (0.29-1.11) | |||||
| Trend p = 0.06 | |||||
| OR (95% CI) in quintiles among 434 same sex twin pairs compared with sedentary category in 1975 | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.85 | |||||
| • Q3 = 0.72 | |||||
| • Q4 = 0.68 | |||||
| • Q5 = 0.60 | |||||
| LaCroix et al 1996 [191] | To determine whether walking is associated with a reduced risk of CVD hospitalization and death in older adults. | • n = 1,645 (615 men; 1030 women) | 4.2 year follow-up | RR (95% CI) by category of walking | Walking more than 4 hr/wk was associated with a reduced risk of mortality from all-causes. |
| • Sex: Men and women | PA assessment: Questionnaire for walking h/wk, divided into 3 groups | ||||
| USA | • Age: ≥65 yr | G1 = <1 hr/week | Men | ||
| Characteristics: Participants from a group health co-operative | G2 = 1-4 hr/week | • G1 = 1.00 (referent) | |||
| Prospective cohort | G3 = >4 hr/week | • G2 = 0.78 (0.43-1.45) | |||
| • G3 = 0.89 (0.49-1.62) | |||||
| D & B score = 12 | Women | ||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.50 (0.28-0.90) | |||||
| • G3 = 0.48 (0.25-0.83) | |||||
| Age 65-74 yr | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.81 (0.40-1.61) | |||||
| • G3 = 1.13 (0.60-2.15) | |||||
| Age ≥75 yr | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.63 (0.37-1.08) | |||||
| • G3 = 0.46 (0.25-0.84) | |||||
| High functioning | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.73 (0.38-1.41) | |||||
| • G3 = 0.89 (0.48-1.65) | |||||
| Limited functioning | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.60 (0.34-1.05) | |||||
| • G3 = 0.51 (0.28-0.92) | |||||
| Lam et al 2004 [192] | To investigate the relationship LTPA and mortality in Hong Kong. | • n = 24,079 cases (13,778 men; 10,301 women); | 10 years prior | Multivariate adjusted OR (95% CI) by LTPA | The data confirm and extend previous findings in Caucasian populations on the association between LTPA and longevity. |
| PA assessment: | Men | ||||
| Hong Kong | • n = 13,054 controls (3,918 men; 9,136 women) | Questionnaire for LTPA, divided into 3 groups | • G1 = 1.00 (referent) | ||
| • G2 = 0.60 (0.54-0.67) | |||||
| Case-Control | • G3 = 0.66 (0.60-0.73) | ||||
| • Sex: Men and women | G1 = <1 times per month | ||||
| D & B score = 12 | • Age: ≥35 yr | Women | |||
| • Characteristics: All ethnic Chinese | G2 = 1-3 times per month | • G1 = 1.00 (referent) | |||
| • G2 = 0.81 (0.74-0.88) | |||||
| G3 = ≥4 times per month | • G3 = 0.71 (0.66-.077) | ||||
| Lan et al 2006 [193] | To investigate the relationship between exercise and all-cause mortality. | • n = 2,113 (1,081 men; 1,032 women) | Baseline and 2 year follow-up | • 197 deaths | Older persons are recommended to expend at least 1000 kcal/wk through regular exercise for mortality reduction. |
| • Sex: Men and women | HR (95% CI) by LTPA frequency | ||||
| Taiwan | • Age: ≥65 yr | PA assessment: Questionnaire for LTPA (frequency/wk) | |||
| Prospective cohort | • Characteristics: Non-institutionalized elders | Adjusted for age and sex | Protection of exercise against death also increases with the number of activities. | ||
| G1 = Sedentary | • G1 = 1.00 (referent) | ||||
| • Taiwan National Health Interview Survey | G2 = 1 time/wk | • G2 = 0.49 (0.36-0.67) | |||
| D & B score = 13 | G3 = ≥2 times/wk | • G3 = 0.20 (0.09-0.46) | |||
| Trend p = <0.001 | |||||
| Questionnaire for EE (kcal/wk), divided into 5 groups: | Multivariate adjusted | ||||
| • G1 = 1.00 (referent) | |||||
| G1 = Sedentary | • G2 = 0.70 (0.50-0.98) | ||||
| G2 = <500 | • G3 = 0.35 (0.15-0.82) | ||||
| G3 = 500-999 | Trend p = 0.014 | ||||
| G4 = 1000-1999 | |||||
| G5 = ≥2000 | |||||
| HR (95% CI) by EE | |||||
| Adjusted for age and sex | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.64 (0.41-1.01) | |||||
| • G3 = 0.55 (0.35-0.85) | |||||
| • G4 = 0.30 (0.17-0.53) | |||||
| • G5 = 0.24 (0.12-0.48) | |||||
| Trend p <0.001 | |||||
| Multivariate adjusted | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.80 (0.49-1.30) | |||||
| • G3 = 0.74 (0.46-1.17) | |||||
| • G4 = 0.50 (0.27-0.90) | |||||
| • G5 = 0.43 (0.21-0.87) | |||||
| Trend p = 0.043 | |||||
| Laukkanen et al 2001 [194] | To examine the relationship between maximal oxygen uptake and overall mortality. | • n = 1,294 | Baseline and 10.7 year follow-up | • 124 deaths | PF has a strong, graded, inverse association with overall mortality. |
| • Sex: Men | Adjusted RR (95% CI) by quartile | ||||
| Finland | • Age: 42.0-61.3 yr (mean 52.1) | ||||
| • Characteristics: Men free from CVD, COPD, and cancer at baseline | PF assessment: Exercise tolerance test, 4 groups by maximal oxygen uptake (ml/kg/min) | ||||
| Prospective cohort | Maximal oxygen uptake | ||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.47 (0.71-3.01) | |||||
| D & B score = 14 | • G3 = 2.79 (1.44-5.39) | ||||
| G1 = >37.1 | • G4 = 3.85 (2.02-7.32) | ||||
| G2 = 32.3-37.1 | Linear trend p = <0.001 | ||||
| G3 = 27.6-32.2 | |||||
| G4 = <27.6 | Test duration | ||||
| • G1 = 1.00 (referent) | |||||
| Test duration (min) | • G2 = 2.22 (1.08-4.55) | ||||
| G1 = >11.2 | • G3 = 2.23 (1.11-4.49) | ||||
| G2 = 9.6-11.2 | • G4 = 3.94 (2.01-7.74) | ||||
| G3 = 8.2-9.5 | Linear trend p<0.001 | ||||
| G4 = <8.2 | |||||
| Lee and Paffenbarger 2000 [195] | To compare various levels of PA with mortality. | • n = 13,485 | Baseline and 15 year follow-up | • 2,539 deaths | The study provides some support for recommendations that emphasize MPA. A benefit of VPA is also evident. |
| • Sex: Men | |||||
| • Age: Mean 57.5 yr | RR (95% CI) | ||||
| • Characteristics: Men who matriculated as undergraduates in 1916-1950 | PA assessment: | • G1 = 1.00 (referent) | |||
| USA | Questionnaires for LTPA index (including walking, stair climbing, sports and recreational activity), | • G2 = 0.80 (0.72-0.88) | |||
| • G3 = 0.74 (0.65-0.83) | |||||
| Prospective cohort | • G4 = 0.80 (0.69-0.93) | ||||
| • The Harvard Alumni Health Study | • G5 = 0.73 (0.64-0.84) | ||||
| Trend p = <0.001 | |||||
| D & B score = 12 | 5 groups (kJ/wk) | ||||
| G1 = <4200 | |||||
| G2 = 4200-8399 | |||||
| G3 = 8400-12599 | |||||
| G4 = 12600-16799 | |||||
| G5 = ≥ 16800 | |||||
| Lee et al 1995 [196] | To examine the independent association of vigorous and non-vigorous PA with longevity. | • n = 17,321 | Follow-up 22-26 years | • 3,728 deaths | There is a graded inverse relationship between PA and mortality. Vigorous, but not non-vigorous activities are associated with longevity. |
| • Sex: Men | |||||
| • Age: Mean 46 yr | PA assessment: Questionnaires for EE (kJ/wk), quintiles | RR (95% CI) by EE (kJ/wk) | |||
| USA | • Characteristics: Harvard University alumni, without self-reported physician diagnosed cardiovascular disease, cancer or chronic obstructive pulmonary disease | Q1= 1.00 (referent) | |||
| • Q2 = 0.94 (0.86--1.04) | |||||
| Prospective cohort | Q1 = ≤ 630 | • Q3 = 0.95 (0.86--1.05) | |||
| Q2 = 630-1680 | • Q4 = 0.91 (0.83 - 1.01) | ||||
| Q3 = 1680-3150 | • Q5 = 0.91 (0.82-1.00) | ||||
| D & B score = 12 | Q4 = 3150-6300 | ||||
| Q5 = >6300 | RR (95% CI) by EE (Vigorous activity, kJ/wk) | ||||
| • Q1 = 1.00 (referent) | |||||
| • The Harvard Alumni Health Study | • Q2 = 0.88 (0.82-0.96) | ||||
| • Q3 = 0.92 (0.82-1.02) | |||||
| • Q4 = 0.87 (0.77-0.99) | |||||
| • Q5 = 0.87 (0.78-0.97) | |||||
| Lee et al 2004 [197] | To investigate the effect of various PA patterns on all-cause mortality. | • n = 8,421 | Baseline 1988 and follow-up 1993 | • 1,234 deaths | The results suggest that regular PA generating 1000 kcal/wk or more should be recommended for lowering mortality rates. Among those with no major risk factors, even 1-2 episodes per week generating 1000 kcal or more can postpone mortality. |
| • Sex: Men | |||||
| • Age: Mean 66 yr | Age adjusted RR (95% CI) by PA pattern | ||||
| USA | • Characteristics: Participants free of major chronic disease | PA assessment: Questionnaire for PA (kcal/wk), 4 groups | |||
| • G1 = 1.00 (referent) | |||||
| Prospective cohort | • G2 = 0.75 (0.63-0.90) | ||||
| G1 = <500 | • G3 = 0.82 (0.63-1.07) | ||||
| • The Harvard Alumni Health Study | (Sedentary) | • G4 = 0.61 (0.53-0.69) | |||
| D & B score = 11 | G2 = 500-999 | ||||
| (Insufficiently active) | Multivariate adjusted | ||||
| G3 = ≥ 1000 | |||||
| (Weekend warrior) | • G1 = 1.00 (referent) | ||||
| G4 = Regularly active | • G2 = 0.75 (0.62-0.91) | ||||
| • G3 = 0.85 (0.65-1.11) | |||||
| • G4 = 0.64 (0.55-0.73) | |||||
| Leitzmann et al 2007 [198] | To examine PA guidelines in relation to mortality. | • n = 252,925 (142,828 male; 110,097 women) | Baseline and 6 month follow-up | • 7,900 deaths | Following PA guidelines is associated with lower risk of death. Mortality benefit may also be achieved by engaging in less than recommended activity levels. |
| USA | • Sex: Men and women | PA assessment: Questionnaire for MPA and VPA, 5 groups each MPA (h/wk) | Multivariate adjusted RR (95% CI) according to activity | ||
| • Age: 50-71 yr | MPA | ||||
| Prospective cohort | • Characteristics: Participants free of CVD, cancer or emphysema | • G1 = 1.00 (referent) | |||
| • The National Institute of Health-American Association of Retired Persons | • G2 = 0.85 (0.79-0.93) | ||||
| • G3 = 0.79 (0.74-0.85) | |||||
| D & B score = 13 | G1 = sedentary | • G4 = 0.76 (0.71-0.82) | |||
| G2 = <1 | • G5 = 0.68 (0.63-0.74) | ||||
| G3 = 1-3 | Trend p = <0.001 | ||||
| G4 = 4-7 | VPA | ||||
| G5 = >7 | |||||
| VPA (frequency/wk) | • G1 = 1.00 (referent) | ||||
| G1 = inactive | • G2 = 0.77(0.71-0.83) | ||||
| G2 = <1 | • G3 = 0.77 (0.72-0.82) | ||||
| G3 = 1-2 | • G4 = 0.68 (0.63-0.73) | ||||
| G4 = 3-4 | • G5 = 0.71 (0.66-0.77) | ||||
| G5 = ≥ 5 | Trend p = <0.001 | ||||
| Cox proportional HR | |||||
| Leon et al 1997 [199] | To examine the long-term association of LTPA and risk of death from coronary heart disease and all-causes. | • n = 12,138 | 16 year follow-up | • 1,904 deaths | The data suggest that a relatively small amount of daily moderate intensity LTPA can reduce premature mortality in middle-aged and older men at high risk for CHD. |
| • Sex: Men | |||||
| • Age: 35-57 yr | PA assessment: Minnesota LTPA questionnaire, categorized by frequency/month and average duration, deciles (min/d) | Multivariate adjusted RR (95% CI) by deciles of LTPA | |||
| USA | • Characteristics: Men who at entry to the study were free of clinical evidence of CHD or other serious medical problems but were at the upper 10%-15% of a CHD probability score distribution derived from the FHS data | ||||
| Prospective cohort | • D1 = 1.00 (referent) | ||||
| • D2-4 = 0.85 (0.73-0.99) | |||||
| • D5-7 = 0.87 (0.75-1.02) | |||||
| D & B score = 12 | • D8-10 = 0.83 (0.71-0.97) | ||||
| D1 = 4.9 | |||||
| D2-4 = 22.7 | |||||
| D5-7 = 53.9 | |||||
| D8-10 = 140.4 | |||||
| • Multiple Risk Factor Intervention Trial | Cox proportional HR | ||||
| Lissner et al 1996 [200] | To examine the relationship of OPA and LTPA on all-cause mortality in women. | • n = 1,405 | Baseline and 20 year follow-up | • 277 deaths | Decreases in PA as well as low initial levels are strong risk factors for mortality. |
| • Sex: Women | |||||
| • Age: 38-60 yr | RR (95% CI) by LTPA | ||||
| Sweden | • Characteristics: Free from major disease at baseline | PA assessment: Questionnaire for OPA and LTPA, 3 groups | |||
| 20 year follow-up | |||||
| Prospective cohort | LTPA during age 20-38 years | ||||
| • The Gothenburg Prospective Study of Women | • Low = 1.00 (referent) | ||||
| G1 = Low | • Med = 0.66 (0.34-1.26) | ||||
| D & B score = 10 | G2 = Medium | • High = 0.46 (0.21-1.01) | |||
| G3 = High | |||||
| LTPA during age 39-60 years | |||||
| Proportional hazard regression | • Low = 1.00 (referent) | ||||
| • Med = 0.56 (0.35-0.90) | |||||
| • High = 0.44 (0.22-0.91) | |||||
| LTPA during the past 12 months | |||||
| • Low = 1.00 (referent) | |||||
| • Med = 0.56 (0.39-0.82) | |||||
| • High = 0.45 (0.24-0.86) | |||||
| 20 year follow-up | |||||
| OPA during age 20-38 years | |||||
| • Low = 1.00 (referent) | |||||
| • Med = 0.59 (0.18-1.87) | |||||
| • High = 0.50 (0.16-1.58) | |||||
| OPA during age 39-60 years | |||||
| • Low = 1.00 (referent) | |||||
| • Med = 0.66 (0.21-2.08) | |||||
| • High = 0.47 (0.14-1.52) | |||||
| OPA during the past 12 months | |||||
| • Low = 1.00 (referent) | |||||
| • Med = 0.28 (0.17-0.46) | |||||
| • High = 0.24 (0.14-0.43) | |||||
| Manini et al 2006 [201] | To determine whether energy expenditure is associated with all-cause mortality in older adults. | • n = 302 (150 men; 152 women) | Mean follow-up of 6.15 years | • 55 deaths | Free-living activity EE was strongly associated with lower risk of mortality. |
| • Sex: Men and women | HR (95% CI) by tertiles of PA EE | ||||
| USA | • Age: 70-82 yr | PA assessment: Questionnaire, divided into tertiles of PA EE (kcal/d) | |||
| Prospective cohort | • Characteristics: High-functioning community dwelling elders | Adjusted for age, sex, race and study site | |||
| T1 = <521 | • T1 = 1.00 (referent) | ||||
| D & B score = 13 | T2 = 521-770 | • T2 = 0.63 (0.29-1.18) | |||
| T3 = >770 | • T3 = 0.37 (0.15-0.76) | ||||
| Trend p = 0.009 | |||||
| Adjusted for age, sex, race, study site, weight, height, percent body fat and sleep duration | |||||
| • T1 = 1.00 (referent) | |||||
| • T2 = 0.57 (0.30-1.09) | |||||
| • T3 = 0.31 (0.14-0.69) | |||||
| Trend p = 0.004 | |||||
| Adjusted for age, sex, race, study site, self rated health, education, smoking, CVD, lung disease, diabetes, hip or knee osteoarthritis, osteoporosis, cancer and depression | |||||
| • T1 = 1.00 (referent) | |||||
| • T2 = 0.65 (0.33-1.28) | |||||
| • T3 = 0.33 (0.15-0.74) | |||||
| Trend p = 0.007 | |||||
| Matthews et al 2007 [202] | To determine the effects of exercise and non-exercise PA on mortality. | • n = 67,143 | Baseline and an average of 5.7 year follow-up | • 1,091 deaths | Overall PA levels are an important determinant of longevity. |
| • Sex: Women | |||||
| • Age: 40-70 yr | RR (95% CI) | ||||
| China | • Characteristics: Women without heart disease, stroke or cancer | ||||
| PA assessment: Interview to report (MET h/d), 4 groups Overall activity | Multivariate adjustment | ||||
| Prospective cohort | Overall activity (MET hr/d) | ||||
| • G1 = 1.00 (referent) | |||||
| • The Shanghai Women's Health Study | • G2 = 0.81 (0.69-0.96) | ||||
| D & B score = 12 | G1 = ≤ 9.9 | • G3 = 0.67 (0.57-0.80) | |||
| G2 = 10.0-13.6 | • G4 = 0.61 (0.51-0.73) | ||||
| G3 = 13.7-18.0 | Trend p = 0.000 | ||||
| G4 = ≥ 18.1 | |||||
| Adult exercise (MET hr/d) | |||||
| Adult exercise | • G1 = 1.00 (referent) | ||||
| G1 = 0 | • G2 = 0.84 (0.74-0.96) | ||||
| G2 = 0.1-3.4 | • G3 = 0.77 (0.59-0.99) | ||||
| G3 = 3.5-7.0 | • G4 = 0.64 (0.36-1.14) | ||||
| G4 = ≥ 7.1 | Trend p = 0.008 | ||||
| Cox proportional hazard models | |||||
| Menotti and Seccareccia 1985 [203] | To investigate the relationship between OPA and all-cause mortality. | • n = 99,029 | Baseline and 5 year follow-up | • 2,661 deaths | The results suggest that PA may play a role in the prediction of fatal events. |
| • Sex: Men | |||||
| • Age: 40-59 yr | |||||
| • Characteristics: Men employed on the Italian railway system | PA assessment: Questionnaire Men at risk classified by 3 levels of PA and 3 levels of job responsibility, combined to create 8 groups of PA-job responsibility | Age adjusted death rates per 1000 over 5 years classified by PA only | |||
| Italy | • Sedentary = 26.20 | ||||
| Prospective cohort | • Moderate = 27.05 | ||||
| • Heavy = 27.35 | |||||
| D & B score = 12 | Age adjusted death rates per 1,000 over 5 years classified by PA and job responsibility | ||||
| G1 = sedentary -- low | • G1 = 30.00 | ||||
| G2 = sedentary -- med | • G2 = 25.20 | ||||
| G3 = sedentary -- high | • G3 = 25.80 | ||||
| G4 = moderate -- low | • G4 = 26.30 | ||||
| G5 = moderate -- med | • G5 = 28.50 | ||||
| G6 = moderate -- high | • G6 = 25.80 | ||||
| G7 = heavy -- low | • G7 = 26.90 | ||||
| G8 = heavy -- med | • G8 = 30.80 | ||||
| Mensink et al 1996 [204] | To compare various indices for PA and their association with cardiovascular risk factors as well as total and CVD mortality. | • n = 15,436 (7,689 men; 7797 women) | 5-8 year follow-up | Incidence of all-cause mortality and PA | An inverse relation of PA and total mortality. |
| Germany | • Sex: Men and women | PA assessment: Questionnaire Total activity, 3 groups | Adjusted RR (95% CI) | ||
| • Age: 25-69 yr | |||||
| Prospective cohort | • Characteristics: Participants from communities in Western Germany | Total activity, men | |||
| G1 = Low | • G1 = 1.00 (referent) | ||||
| G2 = Moderate | • G2 = 0.56 (0.30-1.04) | ||||
| D & B score = 12 | G3 = High | • G3 = 0.78 (0.42-1.44) | |||
| Total activity, women | |||||
| LTPA, 3 groups | • G1 = 1.00 (referent) | ||||
| G1 = Low | • G2 = 1.24 (0.60-2.58) | ||||
| G2 = Moderate | • G3 = 1.29 (0.58-2.85) | ||||
| G3 = High | |||||
| Conditioning activity, 3 groups | LTPA, men | ||||
| • G1 = 1.00 (referent) | |||||
| G1 = No activity | • G2 = 0.61 (0.35-1.05) | ||||
| G2 = Moderate | • G3 = 0.79 (0.48-1.31) | ||||
| G3 = High | LTPA, women | ||||
| • G1 = 1.00 (referent) | |||||
| Sports activity, 4 groups | • G2 = 0.94 (0.51-1.75) | ||||
| • G3 = 0.81 (0.44-1.49) | |||||
| G1 = no sports | |||||
| G2 = <1 hour | Conditioning activity, men | ||||
| G3 = 1-2 hours | • G1 = 1.00 (referent) | ||||
| G4 = >2 hours | • G2 = 0.76 (0.44-1.34) | ||||
| • G3 = 0.67 (0.36-1.25) | |||||
| Conditioning activity, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.38 (0.13-1.06) | |||||
| • G3 = 0.80 (0.42-1.54) | |||||
| Sports Activity, men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.49 (0.26-0.95) | |||||
| • G3 = 0.57 (0.30-1.09) | |||||
| • G4 = 0.36 (0.16-0.79) | |||||
| Sports activity, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.38 (0.12-1.23) | |||||
| • G3 = 0.52 (0.23-1.17) | |||||
| • G4 = 0.28 (0.07-1.17) | |||||
| Morgan and Clarke 1997 [205] | To assess the value of broadly based customary PA scores in predicting 10-year mortality in elderly people. | • n = 1,042 (407 men; 635 women) | 10 year follow-up | Incidence of all-cause mortality and PA | A wide range of customary or habitual PA, can provide indices showing both cross sectional and predictive validity for 10 year mortality. |
| • Sex: Men and women | PA assessment: Questionnaire for PA, 3 groups | ||||
| UK | • Age: ≥65 yr | HR (95% CI) | |||
| • Characteristics: British elders | Men | ||||
| Prospective cohort | G1 = Low | • G1 = 1.59 (1.12-2.25) | |||
| • Nottingham Longitudinal Study of Activity and Aging | G2 = Intermediate | • G2 = 1.35 (0.96-1.89) | |||
| G3 = High | • G3 = 1.00 (referent) | ||||
| D & B score = 12 | Women | ||||
| • G1 = 2.07 (1.53-2.79) | |||||
| • G2 = 1.53 (1.12-2.09) | |||||
| • G3 = 1.00 (referent) | |||||
| Myers et al 2002 [206] | To compare PF and PA levels with all-cause mortality. | • n = 6,213 | Baseline and mean 6.2 ± 3.7 year follow-up | • 1,256 deaths | Exercise capacity is a more powerful predictor of mortality among men than other established risk factors for CVD. |
| • Sex: Men | |||||
| • Age: Mean 59 ± 11 yr | Age adjusted RR (95% CI) by quintile | ||||
| USA | • Characteristics: Participants with a normal exercise test result (n = 2,534) and participants with an abnormal exercise test or CVD or both (n = 3,679) | ||||
| PF assessment: Treadmill test for VO2 peak, divided into quintiles (METs) | • Q1 = 4.5 (3.0-6.8) | ||||
| Prospective cohort | • Q2 = 2.4 (1.5-3.8) | ||||
| • Q3 = 1.7 (1.1-2.8) | |||||
| • Q4 = 1.3 (0.7-2.2) | |||||
| D & B score = 12 | Q1 = Lowest level | • Q5 = 1.00 (referent) | |||
| 1.0-5.9 | |||||
| Q2 | |||||
| Q3 | |||||
| Q4 | |||||
| Q5 = Highest level | |||||
| ≥13.0 | |||||
| Ostbye et al 2002 [207] | To analyze the effect of smoking and other modifiable risk factors on ill health, defined in a multidimensional fashion. | • n = 12,956 | 6 year follow-up | • 782 deaths | Quitting smoking and increasing exercise levels are the lifestyle interventions most likely to improve overall health. |
| • Sex: Men and women | |||||
| • Age: 50-60 yr | PA assessment: Questionnaire for PA, 4 groups | Incidence of all-cause mortality and PA | |||
| USA | • Characteristics: Participants from the Health and Retirement Study (HRS) only | ||||
| Prospective cohort | G1 = Sedentary | Death rates (95% CI) per 1000 population/yr | |||
| G2 = Light | |||||
| G3 = Moderate | • G1 = 20.6 (17.8-24.0) | ||||
| D & B score = 13 | G4 = Heavy | • G2 = 9.1 (8.1-9.5) | |||
| • G3 = 8.3 (7.5-9.2) | |||||
| • G4 = 4.4 (3.5-5.6) | |||||
| Paffenbarger et al 1994 [208] | To study the adoption or maintenance of PA and other optional lifestyle patterns for their influence on mortality rates of Harvard College alumni. | • n = 14,786 | Follow-up between | • 2,343 deaths | Adopting a physically active lifeway delays mortality and extends longevity. |
| • Sex: Men | 1977 and 1988 | ||||
| • Age: 45-84 yr (in 1977) | RR (95% CI) of mortality according to PA | ||||
| USA | PA assessment: Questionnaire for blocks walked daily, stairs climbed daily and type, frequency and duration of weekly sports and recreational activities | ||||
| Characteristics: Harvard College alumni | |||||
| Prospective cohort | Physical activity index (kcal/wk) | ||||
| • G1 = 1.00 (referent) | |||||
| D & B score = 14 | • G2 = 1.13 (1.01-1.26) | ||||
| • G3 = 0.72 (0.64-0.82) | |||||
| • G4 = 0.77 (0.69-0.85) | |||||
| Physical activity index (kcal/wk) Sports and recreational activities were scored according to intensity and duration | Walking (km/wk) | ||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.21 (1.08-1.35) | |||||
| • G3 = 0.94 (0.83-1.07) | |||||
| • G4 = 0.89 (0.78-1.01) | |||||
| Moderately vigorous sports play (METs) | |||||
| Light < 4.5 METs | |||||
| Moderate ≥ 4.5 METs | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.11 (0.93-1.33) | |||||
| • G3 = 0.73 (0.65-0.81) | |||||
| • G4 = 0.72 (0.64-0.80) | |||||
| Adjusted for potential confounding influences | |||||
| Richardson et al 2004 [209] | To investigate the impact of a sedentary lifestyle on all-cause mortality. | • n = 9,611 (4,642 men; 4,969 women) | Baseline (1992) and 8 year follow-up | • 810 deaths | A sedentary lifestyle is associated with a higher risk of death in pre- retirement aged adults. |
| • Sex: Men and women | OR (95% CI) | ||||
| USA | • Age: 51-61 yr | PA assessment: Questionnaire for PA, 3 groups: | • G1 = 1.00 (referent) | ||
| Prospective cohort | • Characteristics: Participants born between 1931-1941 and who not institutionalized in 1992 | • G2 = 0.64 (0.52-0.81) | |||
| G1 = Sedentary | • G3 = 0.62 (0.44-0.85) | ||||
| G2 = occasional or light | p = 0.01 | ||||
| D & B score = 13 | G3 = Regular MVPA | ||||
| • Health and Retirement Study | |||||
| Rockhill et al 2001 [210] | To determine the association between recreational PA and mortality in women. | • n = 80,348 | Baseline (1980) and follow-up between 1982-1996 | • 4,871 deaths | People who are more physically active are at reduced mortality risk relative to those who are less active. |
| • Sex: Women | |||||
| • Age: 30-55 yr | Multivariate adjusted RR (95% CI) by (hr/wk) | ||||
| USA | • Characteristics: Free from CVD or cancer at baseline | ||||
| • Nurses Health Study | PA assessment: Questionnaire in 1980 and up-dated every 2- 4 years, 5 groups of PA (hr/wk) | • G1 = 1.00 (referent) | |||
| Prospective cohort | • G2 = 0.82 (0.76-0.89) | ||||
| • G3 = 0.75 (0.69-0.81) | |||||
| • G4 = 0.74 (0.68-0.81) | |||||
| D & B score = 11 | • G5 = 0.71 (0.61-0.82) | ||||
| p<0.001 | |||||
| G1 = <1 | |||||
| G2 = 1-1.9 | |||||
| G3 = 2-3.9 | |||||
| G4 = 4-6.9 | |||||
| G5 = ≥7 | |||||
| Rosengren and Wilhelmsen 1997 [211] | To investigate the effect of OPA and LTPA on risk of death. | • n = 7,142 | Baseline (1970-1973) and 20 year follow-up | • 2,182 deaths | The study demonstrates the protective effect of LTPA on mortality. |
| • Sex: Men | |||||
| • Age: 47-55 yr | Unadjusted RR (95% CI) | ||||
| • Characteristics: Without symptomatic CHD | PA assessment: Postal questionnaires, 3 groups: | • G1 = 1.00 (referent) | |||
| Sweden | • G2 = 0.74 (0.68-0.82) | ||||
| • G3 = 0.73 (0.68-0.79) | |||||
| Prospective cohort | G1 = Sedentary | ||||
| G2 = Moderately active | Multivariate adjustment | ||||
| G3 = Regular exercise | • G1 = 1.00 (referent) | ||||
| D & B score = 13 | • G2 = 0.84 (0.77-0.93) | ||||
| • G3 = 0.83 (0.77-0.90) | |||||
| Schnohr et al 2003 [212] | To assess the associations of regular LTPA and changes in LTPA with risk of death. | • n = 7,023 (4,471 men; 5,676 women) | 18 year follow-up | • 2,725 deaths | Maintaining or adopting a moderate or high degree of PA was associated with lower risk of death. |
| • Sex: Men and women | PA assessment: Questionnaire, 9 groups | Incidence of all-cause mortality and PA and changes in PA | |||
| Denmark | • Age: 20-79 yr | ||||
| • Characteristics: Participants from the Copenhagen City Heart Registered Population | |||||
| Prospective cohort | G1 = Low--low | ||||
| G2 = Low--moderate | Adjusted RR (95% CI) | ||||
| G3 = Low--high | Men | ||||
| D & B score = 12 | G4 = Moderate- low | • G1 = 1.00 (referent) | |||
| G5 = Moderate-Moderate | • G2 = 0.64 (0.49-0.83) | ||||
| • G3 = 0.64 (0.47-0.87) | |||||
| G6 = Moderate-high | • G4 = 0.73 (0.56-0.96) | ||||
| G7 = High-low | • G5 = 0.71 (0.57-0.88) | ||||
| G8 = High-moderate | • G6 = 0.64 (0.51-0.81) | ||||
| G9 = High-high | • G7 = 1.11 (0.76-1.62) | ||||
| • G8 = 0.66 (0.51-0.85) | |||||
| • G9 = 0.61 (0.48-0.76) | |||||
| Women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.75 (0.57-0.97) | |||||
| • G3 = 0.72 (0.50-1.05) | |||||
| • G4 = 0.70 (0.54-0.91) | |||||
| • G5 = 0.64 (0.52-0.79) | |||||
| • G6 = 0.58 (0.45-0.73) | |||||
| • G7 = 0.72 (0.48-1.07) | |||||
| • G8 = 0.61 (0.47-0.80) | |||||
| • G9 = 0.66 (0.51-0.85) | |||||
| Schnohr et al 2004 [213] | To examine whether the relationship between established risk factors and mortality differs with socioeconomic status as measured by level of education. | • n = 30,635 (16,236 men; 14,399 women) | 16 year follow-up | • 10,952 deaths | The study shows the strong predictive effect of PA on mortality is independent of education level. |
| • Sex: Men and women | Socioeconomic status assessment: level of education | Incidence of all-cause mortality and PA stratified by years of education | |||
| Denmark | • Age: 20-93 yr | ||||
| • Characteristics: Participants from the Copenhagen City Heart Registered Population | |||||
| Prospective cohort | PA assessment: Questionnaire | Deaths <8 years of education | |||
| D & B score = 12 | Men | ||||
| 4 groups of PA | G1 = 916 | ||||
| G1 = none or very little | G2 = 1693 | ||||
| G2 = 2-4 h/wk of LPA | G3 = 1012 | ||||
| G3 = >4 h/wk of LPA or 2-4 h/wk of high level activity | G4 = 67 | ||||
| G4 = Competition level or >4 h/wk of hard level activity | Women | ||||
| • G1 = 872 | |||||
| • G2 = 1298 | |||||
| • G3 = 346 | |||||
| • G4 = 10 | |||||
| 8-11 years of education | |||||
| Men | |||||
| • G1 = 432 | |||||
| • G2 = 1040 | |||||
| • G3 = 616 | |||||
| • G4 = 33 | |||||
| Women | |||||
| • G1 = 363 | |||||
| • G2 = 852 | |||||
| • G3 = 268 | |||||
| • G4 = 10 | |||||
| >11 years of education | |||||
| Men | |||||
| • G1 = 104 | |||||
| • G2 = 302 | |||||
| • G3 = 182 | |||||
| • G4 = 11 | |||||
| Women | |||||
| • G1 = 48 | |||||
| • G2 = 129 | |||||
| • G3 = 61 | |||||
| • G4 = 3 | |||||
| Schnohr et al 2006 [214] | To investigate the association between LTPA and mortality. | • n = 4,894 (2,136 men; 2,758 women) | Baseline (1976) and start of follow-up in 1981-1983 (to 2000) | • 1,787 deaths | Long-term moderate or high PA was associated with significantly lower mortality in men and women. |
| • Sex: Men and women | RR (95% CI) | ||||
| Denmark | • Age: 20-79 yr | ||||
| • Characteristics: Healthy males and women | Unadjusted | ||||
| Prospective cohort | PA assessment: Survey for LTPA, 3 groups: | • G1 = 1.00 (referent) | |||
| • G2 = 0.64 (0.56-0.73) | |||||
| • The Copenhagen City Heart Study | • G3 = 0.56 (0.48-0.65) | ||||
| D & B score = 13 | G1 = Low | Trend p < 0.001 | |||
| G2 = Mod | |||||
| G3 = High | Multivariate adjustment | ||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.78 (0.68-0.89) | |||||
| • G3 = 0.75 (0.64-0.87) | |||||
| Trend p = 0.001 | |||||
| Schooling et al 2006 [215] | To examine how a Comprehensive assessment of baseline health status affects the relationship between obesity or PA and mortality. | • n = 54,088 (17,849 men; 36,239 women) | 4.1 year follow-up | • 3,819 deaths | PA, which normally has a negative relationship with adiposity, had the largest impact on survival for the health states, with the strongest inverse relationship between BMI and mortality. |
| • Sex: Men and women | PA assessment: Interview for PA min/d, 3 groups | Incidence of all-cause mortality and PA | |||
| Hong Kong | • Age: ≥ 65 yr | ||||
| Prospective cohort | • Characteristics: Chinese elders | G1 = None | Adjusted HR (95% CI) | ||
| G2 = ≤ 30 min/d | • G1 = 1.00 (referent) | ||||
| G3 = ≥ 30 min/d | • G2 = 0.83 (0.76-0.91) | ||||
| D & B score = 13 | • G3 = 0.73 (0.67-0.80) | ||||
| Trend p<0.001 | |||||
| Sundquist et al 2004 [216] | To study the association between varying levels of PA and all-cause mortality in the elderly. | • n = 3,206 (1,414 men; 1,792 women) | Baseline (1988-1989) and follow-up in 2000 | • 1,806 deaths | Even occasional PA decreases the risk of mortality among elderly people. |
| • Sex: Men and women | PA assessment: Questionnaire for PA, 5 groups | Age-adjusted HR (95% CI) | |||
| Sweden | • Age: ≥65 yr | Men | |||
| • Characteristics: Non-institutionalized elders | • G1 = 1.00 (referent) | ||||
| Prospective cohort | • G2 = 0.74 (0.62-0.87) | ||||
| G1 = none | • G3 = 0.57 (0.44-0.73) | ||||
| The Swedish Annual Level-of-Living Survey (Statistics Sweden) | G2 = occasionally | • G4 = 0.51 (0.41-0.64) | |||
| D & B score = 12 | G3 = once per week | • G5 = 0.60 (0.44-0.82) | |||
| G4 = twice per week | Women | ||||
| G5 = vigorously at least twice per week | • G1 = 1.00 (referent) | ||||
| • G2 = 0.70 (0.59-0.82) | |||||
| • G3 = 0.59 (0.46-0.77) | |||||
| Cox proportional HR | • G4 = 0.47 (0.35-0.62) | ||||
| • G5 = 0.54 (0.31-0.94) | |||||
| Men and women | |||||
| Multivariate adjustment | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.72 (0.64-0.81) | |||||
| • G3 = 0.60 (0.50-0.71) | |||||
| • G4 = 0.50 (0.42-0.59) | |||||
| • G5 = 0.60 (0.46-0.79) | |||||
| Talbot et al 2007 [217] | To investigate how changes in LTPA affect all-cause mortality. | • n = 2,092 (1,316 men; 776 women) | Baseline in 1958 for males and in 1978 for females and an average follow-up of 21.2 ± 9.4 years for men and 10.2 ± 5.6 years for women | • 628 deaths (538 male; 90 female) | Greater declines in total and high-intensity LTPA are independent predictors of all-cause mortality. |
| • Sex: Men and women | |||||
| USA | • Age: 19-<90 yr | RR (95% CI) for standard deviation of rate of change in LTPA | |||
| Prospective cohort | • Characteristics: Community residents, generally with above average income, high education and with good or excellent self related health | (If RR is <1 then a SD increase is associated with decrease mortality. If RR is >1, then a SD increase is associated with increase in mortality) | |||
| D & B score = 13 | PA assessment: Questionnaire for LTPA (METs min/24 h), 3 groups | ||||
| The Baltimore Longitudinal Study of Aging | G1 = low | ||||
| G2 = medium | Multivariate adjustment | ||||
| G3 = high | Men <70 years | ||||
| Rate of change (ROC) | • G1 = 0.96 (0.84-1.08) | ||||
| • G2 = 0.91 (0.79-1.04) | |||||
| • G3 = 0.42 (0.33-0.53) | |||||
| • ROC low = 0.90 (0.80-1.01) | |||||
| • ROC med = 1.01 (0.90-1.14) | |||||
| • ROC high = 0.78 (0.65-0.94) | |||||
| Men >70 years | |||||
| • G1 = 0.95 (0.82-1.10) | |||||
| • G2 = 0.89 (0.76-1.05) | |||||
| • G3 = 0.78 (0.62-0.97) | |||||
| • ROC low = 1.07 (0.93-1.24) | |||||
| • ROC med = 1.13 (1.00-1.27) | |||||
| • ROC high = 0.91 (0.75-1.12) | |||||
| Women <70 years | |||||
| • G1 = 0.75 (0.53-1.07) | |||||
| • G2 = 0.61 (0.36-1.03) | |||||
| • G3 = 0.80 (0.50-1.30) | |||||
| • ROC low = 1.02 (0.74-1.40) | |||||
| • ROC med = 1.38 (0.86-2.28) | |||||
| • ROC high = 0.90 (0.63-1.27) | |||||
| Women >70 years | |||||
| • G1 = 0.85 (0.63-1.15) | |||||
| • G2 = 0.78 (0.39-1.59) | |||||
| • G3 = 0.62 (0.32-1.22) | |||||
| • ROC low = 1.10 (0.85-1.42) | |||||
| • ROC med = 0.96 (0.46-2.03) | |||||
| • ROC high = 0.70 (0.40-1.22) | |||||
| Trolle-Lagerros et al 2005 [218] | To quantify the effect of PA on overall mortality in younger women and to assess the effect of past versus current activity. | • n = 99,099 | 11.4 year follow-up | • 1,313 deaths | Current PA substantially reduces mortality among women. The association is observed even with low levels of PA and is accentuated with increased PA. |
| • Sex: Women | |||||
| • Age: 30-49 yr | PA assessment: Questionnaire using a 5 point scale, 5 groups | Incidence of all-cause mortality and PA past and current | |||
| Sweden and Norway | • Characteristics: Participants from Norway and one region of Sweden | ||||
| Retrospective cohort | G1 = Sedentary | Adjusted HR (95% CI) | |||
| G2 = Low | PA at enrolment | ||||
| G3 = Moderate | • G1 = 1.00 (referent) | ||||
| D & B score = 13 | G4 = High | • G2 = 0.78 (0.61-1.00) | |||
| G5 = Vigorous | • G3 = 0.62 (0.49-0.78) | ||||
| • G4 = 0.58 (0.44-0.75) | |||||
| • G5 = 0.46 (0.33-0.65) | |||||
| Trend p<0.0001 | |||||
| PA at age 30 yr | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.79 (0.55-1.15) | |||||
| • G3 = 0.90 (0.64-1.28) | |||||
| • G4 = 0.98 (0.68-1.42) | |||||
| • G5 = 0.96 (0.65-1.44) | |||||
| Trend p = 0.22 | |||||
| PA at age 14 yr | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.95 (0.66-1.38) | |||||
| • G3 = 0.96 (0.69-1.34) | |||||
| • G4 = 0.88 (0.62-1.25) | |||||
| • G5 = 1.06 (0.75-1.51) | |||||
| Trend p = 0.62 | |||||
| Villeneuve et al 1998 [219] | To examine the relationship between PF, PA and all-cause mortality. | • n = 14,442 (6,246 men; 8,196 women) | Baseline (1981) and 7 year follow-up | RR (95% CI) by EE, multivariate adjustment | There was a reduction in mortality risk associated with even modest participation in activities of low intensity. |
| • Sex: Men and women | |||||
| Canada | • Age: 20-69 yr | PA assessment: Questionnaire for EE (kcal/kg/day), 5 groups | LTPA, men | ||
| • Characteristics: Asymptomatic for CVD | • G1 = 1.00 (referent) | ||||
| Prospective cohort | • G2 = 0.81 (0.59-1.11) | ||||
| • G3 = 0.79 (0.54-1.13) | |||||
| Canadian Fitness Survey | G1 = 0-<0.5 | • G4 = 0.86 (0.61-1.22) | |||
| D & B score = 11 | G2 = 0.5-<1.5 | • G5 = 0.82 (0.65-1.04)* | |||
| G3 = 1.5-<3.0 | |||||
| G4 = ≥ 3.0 | Non vigorous LTPA, men | ||||
| G5 = ≥ 0.5 | • G1 = 1.00 (referent) | ||||
| PF levels: | • G2 = 0.81 (0.56-1.17) | ||||
| Recommended | • G3 = 0.70 (0.44-1.13) | ||||
| Minimum | • G4 = 0.82 (0.53-1.27) | ||||
| • G5 = 0.78 (0.59-1.04)* | |||||
| Undesirable Refusal | |||||
| LTPA, women | |||||
| Multivariate Poisson regression analysis | • G1 = 1.00 (referent) | ||||
| • G2 = 0.94 (0.69-1.30) | |||||
| • G3 = 0.92 (0.64-1.34) | |||||
| • G4 = 0.71 (0.45-1.11) | |||||
| • G5 = 0.88 (0.68-1.04)* | |||||
| Non vigorous LTPA, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.97 (0.69-1.36) | |||||
| • G3 = 0.87 (0.57-1.33) | |||||
| • G4 = 0.72 (0.43-1.21) | |||||
| • G5 = 0.89 (0.67-1.17)* | |||||
| RR (95% CI) by fitness levels, adjusted for age, sex and smoking Recommended = 1.00 (referent) | |||||
| • Minimum = 1.02 (0.69-1.51) | |||||
| • Undesirable = 1.52 (0.72-3.18) | |||||
| • Refusal = 1.04 (0.45-2.39) | |||||
| Weller and Corey 1998 [220] | To study the relationship between PA and mortality in women. | • n = 6,620 | Baseline and 7 year follow-up | • 449 deaths | PA is inversely associated with risk of death in women. |
| • Sex: Women | |||||
| • Age: ≥;30 yr | OR (95% CI) | ||||
| Canada | • Characteristics: Without known heart disease | PA assessment: Questionnaires for: EE (kcal/kg/d), quartiles | |||
| • Canadian Fitness Survey | EE (kcal/kg/d) | ||||
| Prospective cohort | • Q1 = 1.00 (referent) | ||||
| • Q2 = 0.91 (0.66-1.25) | |||||
| Q1 = lowest | • Q3 = 0.94 (0.72-1.23) | ||||
| D & B score = 11 | Q2 = | • Q4 = 0.89 (0.67-1.17) | |||
| Q3 = | |||||
| Q4 = highest | LTPA levels | ||||
| LTPA, 3 groups | • G1 = 1.00 (referent) | ||||
| G1 = Sedentary | • G2 = 0.63 (0.46-0.86) | ||||
| G2 = Mod | • G3 = 0.76 (0.59-0.98) | ||||
| G3 = High | |||||
| Walking | |||||
| Walking, 3 groups | • G1 = 1.00 (referent) | ||||
| G1 = < half the time | • G2 = 0.64 (0.49-0.82) | ||||
| G2 = half the time | • G3 = 0.64 (0.47-0.86) | ||||
| G3 = > half the time | |||||
| Yu et al 2003 [221] | To examine the relationship between LTPA and all-cause mortality. | • n = 1,975 | Baseline and 10 year follow-up | • 252 deaths | The study found a strong inverse association between heavy LTPA and all-cause mortality. |
| UK | • Sex: Men | ||||
| • Age: 49-64 yr | Age adjusted HR (95% CI) | ||||
| • Characteristics: Without a history of CHD at baseline | PA assessment: Questionnaire (Minnesota LTPA index, kcal/d), 3 group | • G1 = 1.00 (referent) | |||
| • G2 = 0.73 (0.54-0.99) | |||||
| Prospective cohort | • G3 = 0.74 (0.55-1.04) | ||||
| Trend p = 0.046 | |||||
| D & B score = 11 | G1 = Light to no activity | Multivariate adjusted | |||
| G2 = Moderate activity | • G1 = 1.00 (referent) | ||||
| G3 = Heavy activity | • G2 = 0.79 (0.58-1.08) | ||||
| • G3 = 0.76 (0.56-1.04) | |||||
| Trend p = 0.083 | |||||
D & B score, Downs and Black quality score; PF, physical fitness; YR, years; RR, risk ratio; 95% CI, 95% confidence interval; PA, physical activity; VO2 peak, peak oxygen consumption; HR, hazard ratio; min/d, minutes per day; kcal/wk, kilocalories per week; LTPA, leisure-time physical activity; MET, metabolic equivalent; VO2 max, maximal oxygen consumption; OPA, occupational physical activity; CVD, cardiovascular disease; hr/wk, hours per week; MPA, moderate physical activity; kcal/kg/wk, kilocalories per kilogram per week; kJ/wk, kilojoules per week; EE, energy expenditure; G, groups; EE, energy expenditure; BMI, body mass index; C, class; kg/m2, kilogram by meters squared; HR, heart rate; BPM, beats per minute; MVPA, moderate to vigorous physical activity; OR, odds ratio; Q, quartile or quintile; RCT, randomized clinical trial; T, tertiles; TPA, total physical activity; VPA, vigorous physical activity; mL/kg/min, milliliters per kilogram per minute.
We observed a mean 31% lower risk for all-cause mortality in the most active individuals. The median risk reduction was 32%. It is important to highlight that many of these studies included women, with sub-analyses that revealed similar risk reductions between sexes. Our findings are consistent with previous reports [15,16,29-31]. The majority (90%) of the studies supported the health benefits of physical activity demonstrating a significant risk reduction in physically active individuals. The level of evidence would be considered to be a Level 2A based on the presence of overwhelming evidence from observational trials. The studies examined were generally of a good quality with a mean (and median) score of 12 out of 15 (range 10-14).
A clear dose-response relationship was also observed with marked reductions in the risk for all-cause mortality occurring with relatively small increments in physical activity (Figure 3). To examine more closely the temporal relationship between physical activity and all-cause mortality we calculated the (unadjusted) relative risks associated with incremental levels of physical activity/fitness using the reported cases of all-cause mortality and the number of participants (per group) in each investigation. In some instances, we were required to calculate the number of participants based on the reported incidence rates and person years, or based on data obtained directly from the authors (2 investigations). We were not able to obtain this information in 18 investigations, and as such this analysis was restricted to the remaining 52 investigations. There was considerable variability in the methods of classifying the physical activity/fitness levels of the participants. Accordingly, Figure 3 illustrates the mean relative risk reduction according to three separate study types including those that subdivided participants into tertiles, quartiles and quintiles, respectively. This figure demonstrates clearly the dose-response relationship between physical activity and all-cause mortality. Collectively, the literature is consistent indicating that the current Canadian guidelines (approximately 4.2 MJ/wk, 1000 kcal/wk) are associated with a 20-30% lower risk for premature all-cause mortality, with greater health benefits with high volumes and/or intensities of activity. In our analyses it was apparent that the greatest differences in risk occurred between the lowest adjacent activity/fitness categories, suggesting that sedentary individuals can markedly reduce their risk for all-cause mortality with relatively minor increments in physical activity. This is consistent with the current messaging of Canada's physical activity guidelines.
Figure 3.
Mean relative risk reduction in all-cause mortality across physical activity/fitness categories.
The strength of the relationship between physical fitness and premature mortality has been well-established [6,32,33]. In our analyses there were greater risk reductions in studies that took objective measures of physical fitness. We observed an average risk reduction of approximately 45%, which was consistent between men and women. A risk reduction of greater than 50% was not uncommon in these studies. For instance, Myers et al. (2004) reported that being fit or physically active was associated with greater than 50% lower mortality risk in men. They also noted that a 4.2 MJ/wk (1000 kcal/wk) increase in physical activity, or a 1 metabolic equivalent (MET) higher physical fitness level was associated with a mortality benefit of around 20%. It is also important to highlight that longitudinal studies evaluating changes in physical activity or fitness have revealed a lower premature mortality risk [16,34-41]. As we previously reported, routine physical activity or elevated physical fitness also appears to reduce the risk for premature mortality in individuals with risk factors for chronic disease [42,43].
Implications
Since the seminal work of Morris and colleagues (in the 1950s [44,45]) and the early work of Paffenbarger (in the 1970s [46,47]) there has been considerable research (especially epidemiological evidence) documenting the health benefits of engaging in routine physical activity and/or being physically fit [17,48]. Both physical activity (a behaviour) and physical fitness (an attained state) appear to be related to health status in a dose-dependent fashion, with physical fitness demonstrating the strongest relationship [18,19]. Numerous reports indicate that physical inactivity and/or low physical fitness are associated with an increased risk for chronic disease and premature all-cause and disease-specific mortality [2,43,49-51]. Some of the most compelling research includes the relationship between physical activity/fitness and all-cause mortality. As demonstrated below and in Table 11 and Figure 1, this literature is extensive.
The assessment of the relationship between all-cause mortality is complicated by the inclusion of deaths related to suicides, homicide, and accidents [18,19,52]. Nonetheless, the available evidence is incontrovertible; individuals who are habitually physically active and/or physically fit are at a markedly reduced risk for premature all-cause mortality [15,16,18,19]. In Canada, physical inactivity is a major cause of premature mortality from diseases of the cardiovascular system (33.3%), cancers (29.1%), and type 2 diabetes (3.5%) [53]. Globally, physical inactivity has been linked with 2 million premature deaths per year, including 22% of cases of coronary heart disease, and 10-16% of cases of breast cancer, colon cancer, rectal cancer and type 2 diabetes [54]. As such, the promotion of the health benefits of physical activity is of paramount importance for the effective prevention of chronic disease and premature mortality on a national and international scale.
In summary, there is a clear dose-response relationship between physical activity and premature all-cause mortality. Physically active individuals have an approximate risk reduction of 31% in comparison to physically inactive individuals. When objective measures of aerobic fitness are taken the risk reductions are even greater approximating 45%.
Recommendation #1
For a reduced risk for premature mortality, it is recommended that individuals should participate in 30 min or more of moderate to vigorous exercise on most days of the week. Greater health benefits appear to occur with higher volumes and/or intensities of activity. [Level 2, Grade A]
Primary Prevention of Cardiovascular Disease
In our systematic search of the literature, a total of 9408 citations were identified during the electronic database search (Figure 4). Of these citations, 5973 were identified in MEDLINE, 2561 in EMBASE, 193 in Cochrane, and 681 in the CINAHL/SportDiscus/PsychInfo search. A total of 923 duplicates were found, leaving a total of 8485 unique citations. A total of 8138 articles were excluded after scanning, leaving a total of 347 articles for full review. An additional 20 articles were added through cross-referencing. From these articles 319 were excluded after full review leaving 33 articles for inclusion in the systematic review. The reasons for exclusion included non-experimental studies (n = 45), only effect on cardiovascular disease risk factors (n = 115), did not report 3 levels of physical activity (n = 12), subjects less than 18 yr of age (n = 4), reviews, summaries, dissertations, thesis, and abstracts (n = 30), clinical population (n = 14), not on cardiovascular disease or did not fit definition of cardiovascular disease (n = 78), and other (n = 19). Therefore, a total of 49 articles were included in the systematic review of the literature regarding the relationship between physical activity and the incidence of cardiovascular disease.
Figure 4.
Results of the Literature Search for Cardiovascular Disease.
The majority of the studies included in our systematic review were prospective cohort investigations (Table 12). These studies involved a total of 726,474 participants; averaging 12,313 participants per study (range 680-88,393). There were a total of 34,815 reported cases of cardiovascular disease (ranging per study from 42-2,596). The total length of study follow-up for the prospective cohort studies averaged 14.1 yr (ranging from 2-29 yr). The articles were published over a 32 yr period ranging from 1975 to 2007. These studies involved large samples of men and women from regions throughout the world.
Table 12.
Studies examining the relationship between physical activity and cardiovascular disease.
| Publication Country Study Design Quality Score | Objective | Population | Methods | Outcome | Comments and Conclusions |
|---|---|---|---|---|---|
| Paffenbarger and Hale 1975 [47] | To evaluate the role of PA in reducing coronary mortality among longshoreman | • n = 6,351 | 22 years of follow up, or until reached the age of 75 yr | RR (95% CI) Sudden death | VPA is associated with reduced risk of coronary mortality, particularly sudden cardiac death. |
| USA | • Sex: Men | • G1 = 1.00 (referent) | |||
| • Age: 35-74 yr | • G2 = 3.5 | ||||
| • Characteristics: Longshoreman | PA assessment: Energy and oxygen cost requirements of longshoring jobs | • G3 = 2.8 | |||
| Prospective cohort | Delayed death | ||||
| • G1 = 1.00 (referent) | |||||
| D & B score = 12 | • G2 = 1.4 | ||||
| Activity level | • G3 = 1.5 | ||||
| G1 = Heavy (5.2-7.5 kcal/min) | Unspecified death | ||||
| G2 = Moderate (2.4-5.0 kcal/min) | • G1 = 1.00 (referent) | ||||
| G3 = Light (1.5-2.0 kcal/min) | • G2 = 1.1 | ||||
| • G3 = 1.6 | |||||
| Outcome measure: Death from CHD | |||||
| Manson et al 2002 [56] | To compare the roles of walking and vigorous exercise in the prevention of CV events in a large, ethnically diverse cohort of postmenopausal women. | • n = 73,743 | Enrolment from 1994-98 Clinic visit for baseline screening, | • Number of New Cases: 345 | Both walking and VPA are associated with substantial reductions in the incidence of CHD events. |
| USA | • Sex: Women | • Total Number of CVD events: 1551 | |||
| • Age: 50-79 yr | |||||
| • Characteristics: Healthy, Post Menopausal | Age adjusted RR (95% CI) Total exercise (MET-hr/wk) | ||||
| PA assessment: Questionnaire for: Total exercise (MET- hr/wk) | |||||
| Prospective cohort | G1 = 0-2.4 | • G1 = 1.00 (referent) | |||
| • Women's Health Initiative Observational Study | G2 = 2.5-7.2 | • G2 = 0.73 (0.53-0.99) | |||
| G3 = 7.3-13.4 | • G3 = 0.69 (0.51-0.95) | ||||
| D & B score = 12 | G4 = 13.5-23.3 | • G4 = 0.68 (0.50-0.93) | |||
| G5 = ≥ 23.4 | • G5 = 0.47 (0.33-0.67) | ||||
| p = <0.001 | |||||
| Walking (MET-hr/wk) | |||||
| Walking (MET-hr/wk) | • G1 = 1.00 (referent) | ||||
| G1 = None | • G2 = 0.71 (0.53-0.96) | ||||
| G2 = 0.1-2.5 | • G3 = 0.60 (0.44-0.83) | ||||
| G3 = 2.6-5.0 | • G4 = 0.54 (0.39-0.76) | ||||
| G4 = 5.1-10.0 | • G5 = 0.61 (0.44-0.84) | ||||
| G5 > 10 | p = 0.004 | ||||
| Time for VPA (min) | Vigorous exercise | ||||
| G1 = None | • G1 = 1.00 (referent) | ||||
| G2 = 1-60 | • G2 = 1.12 (0.79-1.60) | ||||
| G3 = 61-100 | • G3 = 0.56 (0.32-0.98) | ||||
| G4 = 101-150 | • G4 = 0.73 (0.43-1.25) | ||||
| G5 = >150 | • G5 = 0.58 (0.34-0.99) | ||||
| p = 0.008 | |||||
| Outcome Measure: Incidence of CVD and CHD | |||||
| Wisloff et al 2006 [58] | To study the association between the amount and intensity of exercise and CVD mortality. | • n = 56,072 (27,143 men; 28,929 women) | Length of follow-up: 16 ± 4 yr | • Number of Cases: 1,603 male, 993 female | Men and women who exercise to a moderate degree and spend less than the recommended energy (< 1000 kcal/wk) are at lower risk of dying from heart disease than those who never exercise. |
| Norway | • Sex: Men and women | PA assessment: Questionnaire for LTPA, 4 groups | Multivariate RR (95% CI) | ||
| Prospective cohort | • Age: ≥ 20 yr | Men | |||
| • Characteristics: Free form CVD | Men | • Q1 = 1.00 (referent) | |||
| Q1 = None | • Q2 = 0.66 (0.50-0.87) | ||||
| D & B score = 12 | • HUNT study | Q2 = 1/wk >30 min high | • Q3 = 0.83 (0.65-1.06) | ||
| Q3 = 2-3/wk > 30 min high | • Q4 = 0.77 (0.59-1.01) | ||||
| Q4 = ≥ 4/wk > 30 min high | Women | ||||
| • Q1 = 1.00 (referent) | |||||
| Women | • Q2 = 0.63 (0.31-1.29) | ||||
| Q1 = None | • Q3 = 0.66 (0.32-1.34) | ||||
| Q2 = 1/wk ≤ 30 min low | • Q4 = 0.86 (0.45-1.62) | ||||
| Q3 = 1/wk ≤ 30 min high | |||||
| Q4 = 2-3/wk ≤ 30 min low | |||||
| Outcome Measure: Ischaemic heart disease mortality | |||||
| Cox proportional HR | |||||
| Lee et al 2001 [59] | To examine the relationship between PA (specifically walking) and CHD among women, including those at high risk for CHD. | • n = 39,372 | Recruitment of Participants: Sept 1992-May 1995 | • Number of Cases: 244 | Even light to moderate activity is associated with lower CHD rates in women. |
| USA and Puerto Rico | • Sex: Women | ||||
| • Age: ≥ 45 yr | Multivariate RR (95% CI) Time spent walking | ||||
| • Characteristics: Healthy | PA assessment: Questionnaires Divided into 4 or 5 groups: | • G1 = 1.00 (referent) | |||
| • Women's Health Study | • G2 = 0.86 (0.57-1.29) | As little as 1 hour of walking per week predicted lower risk. | |||
| Prospective cohort | • G3 = 0.49 (0.28-0.86) | ||||
| • G4 = 0.48 (0.29-0.78) | |||||
| p = <0.001 | |||||
| D & B score = 12 | Time spent walking | ||||
| G1 = No regular walking | Walking pace | ||||
| G2 = 1-59 min/wk | • G1 = 1.00 (referent) | ||||
| G3 = 1.0-1.5 h/wk | • G2 = 0.56 (0.32-0.97) | ||||
| G4 = ≥ 2.0 h/wk | • G3 = 0.71 (0.47-1.05) | ||||
| Walking pace (km/h) | • G4 = 0.52 (0.30-0.90) | ||||
| G1 = No regular walking | p = 0.02 | ||||
| G2 = 3.2 | |||||
| G3 = 3.2-4.7 | |||||
| G4 = ≥ 4.8 | EE (kcal/wk) | ||||
| • Q1 = 1.00 (referent) | |||||
| EE (kcal/wk) | • Q2 = 0.79 (0.56-1.12) | ||||
| G1 = 200 | • Q3 = 0.55 (0.37-0.82) | ||||
| G2 = 200-599 | • Q4 = 0.75 (0.50-1.12) | ||||
| G3 = 600-1499 and | p = 0.03 | ||||
| G4 = 1500 or more | |||||
| Energy expended VPA (kcal/wk) | |||||
| Energy expenditure for VPA (kcal/wk) | • G1 = 1.00 (referent) | ||||
| G1 = No vigorous, <200 kcal/wk | • G2 = 0.65 (0.46-0.91) | ||||
| G2 = No vigorous, ≥ 200 kcal/wk | • G3 = 1.18 (0.79-1.78) | ||||
| • G4 = 0.96 (0.60-1.55) | |||||
| • G5 = 0.63 (0.38-1.04) | |||||
| G3 = Vigorous, 1-199 kcal/wk | |||||
| G4 = Vigorous, 200-499 kcal/wk | |||||
| G5 = Vigorous, ≥ 500 kcal/wk | |||||
| Paffenbarger et al 1993 [67] | To analyze changes in the lifestyle of Harvard Alumni and the associations of these changes to mortality. | • n = 10,269 | Baseline measure in 1962 or 1967 with a follow up in 1977 | Alumni who increased their PA index to 2000 kcal or more per week had a 17% lower risk of death from CHD then those who were sedentary (p = 0.507) | Moderately vigorous sports activity was associated with lower rates of death from CHD among middle aged and older men |
| • Sex: Men | |||||
| • Age: 45-84 yr | |||||
| USA | • Characteristics: Health, Harvard College Alumni | ||||
| Prospective cohort | PA assessment: Mailed questionnaires included questions on type, duration, intensity, frequency of PA. | Men who took up moderate took up moderately vigorous activity had a 41% lower risk than those who continued not to engage in such activity (p = 0.044) | |||
| D & B score = 13 | Outcome Measure: CHD deaths between 1977 and 1985 | ||||
| Cox proportional hazards model | |||||
| Poisson regression methods | |||||
| The Mantel extension of the Mantel-Haenszel test | |||||
| Haapanen et al 1997 [77] | To examine the association between duration and intensity of LTPA and the risk of CHD. | • n = 2,840 (1,500 men; 1,340 women) | Length of Follow-up: 10 yrs | • Incident Rates (per 1000 person-years) for CHD = 108 for men and 75 for women. | Total EE had an inverse and independent association with risk of CHD in middle aged Finnish men but not among women. |
| Finland | • Sex: Men and women | PA assessment: Questionnaire for LTPA EE (kcal/wk) | Multivariate RR (95% CI) LTPA and CHD mortality | ||
| Prospective cohort | • Age: 35-63 yr | Men | |||
| • Characteristics: Healthy | Men | • G1 = 1.98 | |||
| G1 = 0-1100 | • G2 = 1.33 | ||||
| D & B score = 13 | G2 = 1101-1900 | • G3 = 1.00 (referent) | |||
| G3 = >1900 | |||||
| Women | |||||
| Women | • G1 = 1.25 | ||||
| G1 = 0-900 | • G2 = 0.73 | ||||
| G2 = 901-1500 | • G3 = 1.00 (referent) | ||||
| G3 = >1500 | |||||
| Outcome Measure: CHD mortality | |||||
| Cox proportional HR | |||||
| Barengo et al 2004 [164] | To investigate whether moderate or high LTPA are associated with a reduced CVD and all-cause mortality, independent of CVD risk factors and other forms of PA in men and women. | • n = 31,677 (15,853 men; 16,824 women) | 20 year follow-up | • Number of Cases (Men): 1,661 | Moderate and high levels of LTPA and OPA are associated with reduced CVD mortality. |
| PA assessment: Questionnaire for LTPA and OPA, 3 groups | • Number of Cases (Women): 778 | ||||
| Finland | • Sex: Men and women | HR (95% CI) LTPA, men | |||
| Prospective cohort | • Age: 30-59 | G1 = Low activity | • G1 = 1.00 (referent) | ||
| • Characteristics: Participant from eastern and south-western Finland | G2 = Moderate activity | • G2 = 0.91 (0.82-1.00) | |||
| D & B score = 14 | G3 = High activity | • G3 = 0.83 (0.69-0.99) | |||
| LTPA, women (referent) | |||||
| • G1 = 1.00 | |||||
| • G2 = 0.83 (0.71-0.96) | |||||
| • G3 = 0.89 (0.68-1.18) | |||||
| OPA, men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.75 (0.64-0.87) | |||||
| • G3 = 0.77 (0.69-0.87) | |||||
| OPA, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.73 (0.60-0.88) | |||||
| • G3 = 0.77 (0.65-0.91) | |||||
| Bijnen et al 1998 [166] | To describe the association between the PA pattern of elderly men and CHD mortality. | • n = 802 | Length of Follow-up: 10 | • Number of Cases: 90 | PA did not show a protective effect on death from CHD. |
| • Sex: Men | |||||
| • Age: 64-84 yr | PA assessment: Questionnaire, divided into 3 groups | RR (95% CI) | |||
| Netherlands | • Characteristics: Free from Serious Illness | • G1 = 1.00 (referent) | |||
| • G2 = 0.63 (0.38-1.05) | |||||
| Prospective cohort | G1 = Lowest | • G3 = 0.85 (0.51-1.44) | |||
| • Ethnicity: Dutch | G2 = Middle | ||||
| • Zutphen Elderly Study | G3 = Highest | ||||
| D & B score = 13 | Outcome Measure: CHD Mortality | ||||
| Cox Proportional HR | |||||
| Davey-Smith et al 2000 [174] | To examine the association between two measures of physical activity (LTPA and usual walking pace) with cause specific mortality (CHD). | • n = 6,702 | Length of Follow-up: 25 yrs | • Number of Cases: 955 | Inverse associations of both LTPA and walking pace with mortality from CHD were seen. |
| • Sex: Men | |||||
| • Age: 40-64 yr | RR (95% CI) by walking pace | ||||
| England | • Whitehall Study | PA assessment: Questionnaire during examination for walking pace and LTPA | • G1 = 1.45 (0.9-2.2) | ||
| • G2 = 1.30 (1.1-1.6) | |||||
| Prospective cohort | • G3 = 1.00 (referent) | ||||
| p < 0.01 | |||||
| D & B score = 11 | Walking pace | Multivariate RR (95% CI) by LTPA level | |||
| G1 = Slower | • G1 = 1.24 (1.0-1.5) | ||||
| G2 = Same | • G2 = 0.94 (0.8-1.2) | ||||
| G3 = Faster | • G3 = 1.00 | ||||
| p < 0.05 | |||||
| LTPA | |||||
| G1 = Inactive | |||||
| G2 = Moderate | |||||
| G3 = Active | |||||
| Outcome Measure: CHD Mortality | |||||
| Cox Proportional HR | |||||
| Eaton et al 1995 [175] | To determine whether self reported PA predicts a decreased risk of CHD. | • n = 8,463 (LTPA), 8,418 (OPA) | Length of Follow-up: 21 yrs | • Number of Cases: 709 | Baseline levels of self reported LTPA predicted a decreased rate of CHD. |
| Age adjusted RR (95% CI) by LTPA level | |||||
| USA | • Sex: Men | PA assessment: Interview | • G1 = 1.00 (referent) | ||
| • Age: 40 yr | • G2 = 0.79 (0.63-0.99) | ||||
| Prospective cohort | • Characteristics: Healthy, free of CHD | LTPA | • G3 = 0.73 (0.59-0.89) | ||
| G1 = Sedentary | • G4 = 0.71 (0.52-0.98) | ||||
| G2 = Light | |||||
| D & B score = 11 | Ethnicity: Israeli | G3 = Light Daily | Age adjusted RR (95% CI) by OPA level | ||
| G4 = Heavy | • G1 = 1.00 (referent) | ||||
| • G2 = 0.99 (0.75-1.18) | |||||
| OPA | • G3 = 0.94 (0.78-1.12) | ||||
| G1 = Sitting | • G4 = 0.87 (0.67-1.10) | ||||
| G3 = Walking | |||||
| G4 = Physical Labour | |||||
| Outcome Measure: CHD Death | |||||
| Cox Proportional HR | |||||
| Hillsdon et al 2004 [183] | To examine whether a short, easily administered measure of PA is associated with the risk of death from all causes and specific causes. | • n = 10,522 (4,929 men; 5,593 women) | Length of Follow-up: > 10 yrs | • Number of Cases: 155 | Self reported VPA is associated with the risk of future mortality. |
| Multivariate RR (95% CI) by PA level | |||||
| UK | • Sex: Men and women | PA assessment: Questionnaire, 3 groups: | • G1 = 1.00 (referent) | ||
| • G2 = 0.46 (0.19-1.12) | |||||
| Prospective cohort | • Age: 35-64 yr | G1 = Never / <1 time/month | • G3 = 0.96 (0.53-1.75) | ||
| • Characteristics: no history of chest pain | |||||
| G2 = <2 times/wk | |||||
| D & B score = 11 | G3 = ≥ 2 times/wk | ||||
| Outcome Measure: IHD mortality | |||||
| Cox proportional HR | |||||
| Leon et al 1997 [199] | To study the relationship of PA to CHD in a well defined population at above average risk for CHD over a 16 yr observation period. | • n = 12,138 | Follow up for 16 years | Age Adjusted RR (95% CI) | A relatively small amount (10-36 min/d) of daily moderate intensity LTPA can significantly reduce premature mortality from CHD in middle aged men at high risk for CHD. |
| USA | • Sex: Men | • G1 = 1.00 (referent) | |||
| • Age: 35-57 yr | PA assessment: Questionnaire at baseline (Minnesota LTPA questionnaire), divided/grouped into deciles of LTPA (min/d) | • G2 = 0.71 (0.56-0.91) | |||
| • Characteristics: Free of CHD but in the upper 10-15% of a CHD probability risk score | • G3 = 0.75 (0.59-0.96) | ||||
| • G4 = 0.69 (0.54-0.96) | |||||
| Prospective cohort | Multivariate adjusted RR (95% CI) | ||||
| • G1 = 1.00 (referent) | |||||
| D & B score = 11 | G1 = D1: (0-9 min/d) | • G2 = 0.75 (0.54-0.96) | |||
| Multiple risk factor intervention trial | G2 = D2-4: (10-36 min/d) | • G3 = 0.81 (0.64-1.04) | |||
| G3 = D5-7: (37-75 min/d) | • G4 = 0.75 (0.59-0.96) | ||||
| G4 = D8-10: (76-359 min/d) | |||||
| Outcome Measure: CHD Mortality | |||||
| Rosengren et al 1997 [211] | To examine the long term effect of OPA and LTPA on the risk of death from CHD. | • n = 7,142 | Length of Follow-up: 20 yrs | Number of Cases: 684 | There appears to be a protective effect of LTPA on CHD-related death. |
| • Sex: Men | |||||
| • Age: 47-55 yr | Multivariate RR (95% CI) for LTPA | ||||
| Sweden | • Characteristics: Swedish men | PA assessment: Questionnaire for LTPA, 3 groups | • G1 = 1.00 (referent) | ||
| • G2 = 0.84 (0.71-1.00) | |||||
| Prospective cohort | • G3 = 0.84 (0.73-0.96) | ||||
| G1 = Sedentary | |||||
| G2 = Moderately active | |||||
| D & B score = 13 | G3 = Regular exercise | ||||
| Outcome Measure: CHD death | |||||
| Proportional HR | |||||
| Schnohr et al 2006 [214] | To describe the associations between different levels of LTPA and subsequent causes of death. | • n = 4,894 (2,136 men; 2,758 women) | Participants included in the study were only those whose PA levels did not change over 5 years | • Number of Cases: 292 | There was an inverse and significant dose- response association between LTPA and CHD-related mortality. |
| Adjusted RR (95% CI) Whole group | |||||
| Denmark | • Sex: Men and women | • G1 = 1.00 (referent) | |||
| • G2 = 0.71 (0.51-0.99) | |||||
| Prospective cohort | Age: 20--79 yr | PA assessment: | • G3 = 0.56 (0.38-0.82) | ||
| • Characteristics: Healthy | Questionnaire LTPA | ||||
| D & B score = 12 | • Copenhagen City Heart Study | G1 = <4 METS | Men | ||
| G2 = 4-6 METS | • G1 = referent | ||||
| G3 = >6 METS | • G2 = survived 4.9 yrs longer | ||||
| • G3 = survived 6.8 yrs longer | |||||
| Cox proportional HR | |||||
| Women | |||||
| • G1 = referent | |||||
| • G2 = survived 5.5 yrs longer | |||||
| • G3 = survived 6.4 yrs longer | |||||
| Weller et al 1998 [220] | To examine the relationship between PA and mortality. | • n = 6,620 | Length of Follow-up: 7 yrs | • Number of Cases: 109 | LTPA is inversely associated with risk of fatal MI. |
| • Sex: Women | |||||
| • Age: ≥ 30 yr | PA assessment: | OR (95% CI) by LTPA | |||
| Canada | • Characteristics: Canadian Women | Questionnaire, 4 groups for LTPA (kcal/kg/day) and non-LTPA (kcal/kg/day) | • Q1 = 1.00 (referent) | ||
| • Q2 = 0.61 (0.07-1.19) | |||||
| Prospective cohort | • Q3 = 0.84 (0.52-1.37) | ||||
| • Q4 = 0.63 (0.36-1.09) | |||||
| D & B score = 9 | LTPA (kcal/kg/day) | OR (95% CI) by non-LTPA | |||
| Q1 = ≥ 0 | • Q1 = 1.00 (referent) | ||||
| Q2 = ≥ 0.1 | • Q2 = 0.71 (0.44-1.16) | ||||
| Q3 = ≥ 0.5 | • Q3 = 0.57 (0.33-0.97) | ||||
| Q4 = ≥ 1.6 | • Q4 = 0.49 (0.26-0.92) | ||||
| Non-LTPA (kcal/kg/day) | |||||
| Q1 = ≥ 0 | |||||
| Q2 = ≥ 2.8 | |||||
| Q3 = ≥ 5.9 | |||||
| Q4 = ≥ 9.9 | |||||
| Outcome Measure: Fatal MI | |||||
| Logistic regression analysis | |||||
| Yu et al 2003 [221] | To examine the optimal intensity of LTPA to decrease the risk of CHD mortality in middle aged British men. | • n = 1,975 | 10 year follow-up | • Number of Cases: 82 | Strong significant inverse relationship between heavy LTPA and CHD mortality. |
| • Sex: Men | PA assessment: Questionnaire (Minnesota LTPA questionnaire), 3 groups | Multivariate adjusted HR (95% CI) | |||
| • Age: 49-64 yr | • G1 = 1.00 (referent) | ||||
| UK | • Characteristics: Healthy, no previous history of CHD | • G2 = 0.74 (0.44-1.25) | |||
| • G3 = 0.55 (0.31-0.98) | |||||
| Prospective cohort | p = 0.039 | Relationship was not significant for low- moderate intensity LTPA and OPA. | |||
| • Caerphilly collaborative heart study | Total activity level (kcal/day) | ||||
| D & B score = 11 | G1 = 0.0 - 161.6 | ||||
| G2 = 161.8 - 395.3 | |||||
| G3 = 395.5 - 2747.2 | |||||
| Cox proportional HR | |||||
| Altieri et al 2004 [222] | To assess the possible protective role of PA on CHD. | • n = 985 (507 men; 478 women) | PA assessment: Questionnaire for OPA, divided into quartiles | Number of Cases: 507 | LTPA from 15-19 yrs as well as OPA from 30 - 39 yrs both have a significant inverse relationship with risk of non fatal acute MI. |
| OR (95% CI) for CHD and OPA | |||||
| Italy | • Sex: Men and women | Q1 = lowest | • Q1 = 1.00 (referent) | ||
| Q2 | • Q2 = 0.63 (0.39-1.03) | ||||
| Case Control | • Age: < 79 yr | Q3 | • Q3 = 0.56 (0.35-0.90) | ||
| • Characteristics: Case: Patients admitted to Hospital with non-fatal Acute MI. Controls: Patients admitted to hospital for acute condition unrelated to known or potential risk factors for acute MI | Q4 = highest | • Q4 = 0.57 (0.34-0.95) | |||
| D & B score = 11 | p = 0.045 | ||||
| Outcome Measure: Non Fatal acute MI | |||||
| Unconditional logistic regression | |||||
| Batty et al 2003 [223] | To examine the relationship between physical activity and three mortality endpoints in healthy persons. | • n = 6,474 | Length of Follow-up: 25 yr | • Number of Cases: 837 | A suggestion that the symptomatic nature of ischemia appeared to modify the affects of |
| • Sex: Men | • Number of Dropouts: 158 | ||||
| • Age: 40-64 yr | PA assessment: Questionnaire for LTPA, divided into 3 groups: | ||||
| UK | • Characteristics: British civil servants who underwent a resting ECG | HR (95% CI) for CHD and LTPA | |||
| • G1 = 1.14 (0.9-1.4) | PA on total and CHD mortality. | ||||
| Prospective cohort | G1 = Inactive | • G2 = 0.94 (0.8-1.1) | |||
| G2 = Moderate | • G3 = 1.00 (referent) | ||||
| G3 = Active | |||||
| D & B score = 13 | |||||
| Outcome Measure: CHD mortality | |||||
| Cox Proportional HR | |||||
| Chen and Millar [224] | To examine the potential protective effect of LTPA on the incidence of heart disease and depression. | • n = 15,670 | Length of Follow-up: 2 yrs | • 100 cases | Regular and at least MPA can be beneficial to heart health. |
| • Sex: Men and women | |||||
| • Age: ≥ 20 yr | PA assessment: EE from self administered questionnaire, 4 groups (kcal/kg/day) | Adjusted OR (95% CI) | |||
| Canada | • Characteristics: Healthy and free from heart disease | • G1 = 5.0 (1.84-13.59) | |||
| • G2 = 3.7 (1.26-10.67) | |||||
| Prospective cohort | • G3 = 1.00 (referent) | ||||
| G1 = Sedentary | • G4 = 1.3 (0.41-3.89) | ||||
| G2 = Light (<1.5) | |||||
| D & B score = 11 | National Population Health Survey | G3 = Moderate (1.5-2.9) | |||
| G4 = Active (≥ 3) | |||||
| Outcome Measure: CHD incidence | |||||
| Multiple logistic regression | |||||
| Conroy et al 2005 [225] | To examine the relationship between 1) PA during young adulthood and middle age, and 2) PA during each time period and CHD during middle age and older women. | • n = 37,169 | Length of Follow-up: 9 yrs | • Number of Cases: 477 | PA during middle age predicts lower risk of CHD |
| • Sex: Women | |||||
| • Age: ≥ 45 yr | Multivariate RR (95% CI) Baseline PA and incidence of CHD | ||||
| US | • Characteristics: Healthy women health professionals | PA assessment: Questionnaire for EE (kcal/wk) and months/yr | |||
| • Women's Health Study | • G1 = 1.00 (referent) | ||||
| Cohort study | • G2 = 0.62 (0.48-0.80) | ||||
| • G3 = 0.61 (0.48-0.79) | |||||
| D & B score = 11 | Baseline PA (kcal/wk) | • G4 = 0.61 (0.46-0.81) | |||
| G1 = <200 | p = <0.001 | ||||
| G2 = 200-599 | |||||
| G3 = 600-1499 | Past PA and incidence of CHD | ||||
| G4 = ≥ 1500 | • G1 = 1.00 (referent) | ||||
| • G2 = 0.76 (0.57-1.02) | |||||
| Past PA | • G3 = 0.95 (0.72-1.24) | ||||
| Months per year | • G4 = 1.04 (0.78-1.39) | ||||
| G1 = 0 | • G5 = 0.81 (0.58-1.14) | ||||
| G2 = 1-3 | |||||
| G3 = 4-6 | |||||
| G4 = 7-9 | |||||
| G5 = 10-12 | |||||
| Outcome Measure: Incidence of CHD | |||||
| Cox proportional hazard regression | |||||
| Dorn et al 1999 [226] | To examine the long-term relationships between total PA and mortality from all causes and CHD in the general population. | • n = 1,461 (698 men; 763 women) | Length of Follow-up: 29 years | • Number of Cases: 109 men, 81 women | PA favorably influences mortality risks in non- obese men and younger women. |
| USA | • Sex: Men and women | PA assessment: Questionnaire | Multivariate RR (95% CI) for PAI in non- obese men | ||
| Prospective cohort | • Age: 15-96 yr | • 0.40 (0.19-0.88) for 1 kcal/kg/h | |||
| • Characteristics: | Outcome Measure: CHD | Multivariate RR (95% CI) for PAI in obese men | |||
| Healthy, free from CHD, diabetes, and Stroke. | Mortality | • 1.86 (0.86-4.03) for 1 kcal/kg/h | |||
| D & B score = 11 | |||||
| Cox Proportional Hazard | |||||
| Ratio | Multivariate RR (95% CI) for PAI in women < 60 yrs | ||||
| • Ethnicity: White. | • 0.42 (0.11-1.52) for 1 kcal/kg/h | ||||
| Multivariate RR (95% CI) for PAI in women > 60 yrs | |||||
| • 1.78 (0.77-4.09) for 1 kcal/kg/h | |||||
| Folsom et al 1997 [227] | To examine the association of PA at baseline with CHD incidence. | • n = 13,999 (6,166 men; 7833 women) | Length of Follow-up: 4-7 yrs | • Number of Cases: 223 men, 97 women, | No significant relationships. |
| Multivariate RR (95% CI) LTPA, men | |||||
| USA | • Sex: Men and women | PA assessment: Questionnaire during home interview, divided into quartiles of LTPA and sports activity | |||
| • Q1 = 1.00 (referent) | |||||
| Prospective cohort | • Age: 45-64 yr | • Q2 = 1.08 (0.75-1.55) | |||
| • Characteristics: no CHD at baseline | • Q3 = 0.83 (0.51-1.36) | ||||
| • Q4 = 0.89 (0.59-1.35) | |||||
| D & B score = 9 | Q1 = Low | ||||
| • Ethnicity: Black and non Black | Q2 | LTPA, women | |||
| Q3 | • Q1 = 1.00 (referent) | ||||
| • Atherosclerosis Risk in Communities Study | Q4 = High | • Q2 = 0.74 (0.42-1.31) | |||
| • Q3 = 1.07 (0.55-2.09) | |||||
| Outcome Measure: CHD incidence Poisson Regression | • Q4 = 0.64 (0.34-1.24) | ||||
| Multivariate RR (95% CI) Sports, men | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 1.15 (0.79-1.68) | |||||
| • Q3 = 1.03 (0.68-1.54) | |||||
| • Q4 = 0.83 (0.56-1.23) | |||||
| Sports, women | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.99 (0.58-1.67) | |||||
| • Q3 = 0.64 (0.32-1.27) | |||||
| • Q4 = 0.72 (0.37-1.38) | |||||
| Fransson et al 2004 [228] | To estimate the influence of LTPA and OPA on acute MI. | • n = 4069 (2,742 men; 1,327 women) | PA assessment: Questionnaire for LTPA, 5 groups | • Number of Cases: 1,204 men, 550 women | Exercise seems to reduce the risk of MI. |
| Sweden | • Sex: Men and Women | G1 = Seldom | OR (95% CI) | ||
| G2 = Sometimes | |||||
| Case Control | • Age: 45-70 yr | G3 = 1×/wk | LTPA, men | ||
| • Characteristics: Cases: Diagnosed with acute MI | G4 = 2-3×/wk | • G1 = 1.00 (referent) | |||
| D & B score = 12 | G5 = >3×/wk | • G2 = 0.76 (0.61-0.95) | |||
| • G3 = 0.67 (0.51-0.88) | |||||
| • G4 = 0.63 (0.49-0.83) | |||||
| • Stockholm Heart Epidemiology | Questionnaire for total physical activity, 3 groups | • G5 = 0.53 (0.38-0.73) | |||
| G1 = Passive | |||||
| G2 = Somewhat active | LTPA, women | ||||
| G3 = Active | • G1 = 1.00 (referent) | ||||
| Questionnaire for sitting at work, 3 groups | • G2 = 0.69 (0.49-0.98) | ||||
| • G3 = 0.38 (0.25-0.58) | |||||
| G1 = Less than half the time | • G4 = 0.62 (0.38-1.01) | ||||
| G2 = About half the time | • G5 = 0.31 (0.15-0.66) | ||||
| G3 = More than half the time | Total physical activity, men | ||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.66 (0.47-0.94) | |||||
| Outcome Measure: Acute MI | • G3 = 0.46 (0.31-0.69) | ||||
| Total physical activity, women | |||||
| Conditional and unconditional logistics regression | • G1 = 1.00 (referent) | ||||
| • G2 = 0.34 (0.22-0.53) | |||||
| • G3 = 0.16 (0.07-0.37) | |||||
| Sitting at work, men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.91 (0.73-1.15) | |||||
| • G3 = 0.90 (0.72-1.12) | |||||
| Sitting at work, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.77 (0.51-1.17) | |||||
| • G3 = 0.47 (0.31-0.69) | |||||
| Fransson et al 2006 [229] | To evaluate whether LTPA compensates for the increased risk of acute MI associated with overweight and obesity. | • n = 4069 (2,742 men; 1,327 women) | PA Assessment: Questionnaire for LTPA, 3 groups | Number of Cases: 1204 men, 550 women | Regular LTPA seems to provide protection against MI and non- fatal MI. |
| Multivariate OR (95% CI) for acute MI | |||||
| Sweden | • Sex: Men and women | G1 = Very little /occasional walks | LTPA, men | ||
| • G1 = 1.00 (referent) | |||||
| Case Control | • Age: 45-70 yr | G2 = Occasional / once per week | • G2 = 0.70 (0.58-0.84) | ||
| • Characteristics: Cases: had acute MI | • G3 = 0.57 (0.46-0.71) | ||||
| D & B score = 12 | G3 = Twice per week or more | LTPA, women | |||
| • G1 = 1.00 (referent) | |||||
| Outcome measure: Acute MI | • G2 = 0.52 (0.40-0.68) | ||||
| • G3 = 0.44 (0.30-0.65) | |||||
| Multivariate OR (95% CI) for non-fatal MI | |||||
| Conditional and unconditional logistics regression | LTPA, men | ||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.79 (0.65-0.96) | |||||
| • G3 = 0.63 (0.50-0.79) | |||||
| LTPA, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.64 (0.48-0.86) | |||||
| • G3 = 0.58 (0.39-0.87) | |||||
| Haapanen-Niemi 2000 [230] | To investigate the independent associations and the possible interaction of BMI LTPA and perceived physical performance and functional capacity with the risk of mortality. | • n = 2,212 (1,090 men; 1,122 women) | Length of Follow-up: 16 yrs | • Number of Cases: 208 all cause deaths, 54% of those CVD. 73% of CVD deaths due to CHD | Increase perceived PF is associated with a reduced risk of CHD mortality in men. |
| Finland | • Sex: Men and women | PA assessment: Postal Survey | |||
| Multivariate RR (95% CI) | |||||
| Prospective cohort | • Age: 35-63 yr | Total LTPA energy expenditure (kcal/wk) | Total LTPA EE index and CHD mortality, men | ||
| • Characteristics: Healthy |
• G1 = 1.00 (referent) | ||||
| G1 = High | • G2 = 0.88 (0.44-1.76) | ||||
| D & B score = 13 | • Ethnicity: Finnish |
G2 = Moderate | • G3 = 1.70 (0.90-3.21) | ||
| G3 = Low | p = 0.056 | ||||
| Perceived physical fitness compared to age-mates | Multivariate RR (95% CI) Perceived physical fitness, men | ||||
| G1 = Better | • G1 = 1.00 (referent) | ||||
| G2 = Similar | • G2 = 2.82 (1.06-7.46) | ||||
| G3 = Worse | • G3 = 4.64 (1.56-13.84) | ||||
| Outcome Measure: CHD mortality | p = 0.011 | ||||
| Total LTPA EE index and CHD mortality, women | |||||
| Cox proportional HR | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.43 (0.16-1.16) | |||||
| • G3 = 1.17 (0.51-2.68) | |||||
| p = 0.046 | |||||
| Multivariate RR (95% CI) Perceived physical fitness, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.82 (0.32-2.16) | |||||
| • G3 = 1.89 (0.57-6.27) | |||||
| p = 0.154 | |||||
| Kannel et al 1986 [231] | To examine the role of low levels of OPA and LTPA in the development of CV morbidity and mortality over the short and long term. | • n = 1,166 | Length of Follow-up: 24 yrs | • Number of Cases: 220 mortality, 371 morbidity | Rate of CHD Mortality and Morbidity decreases with increased level of PA but no association was found with physical demand of work |
| • Sex: Men | |||||
| • Age: 45-65 yr | |||||
| USA | • Characteristics: | PA assessment: Questionnaire during examination | Cumulative 24 year age adjusted rate per 1000 people | ||
| Prospective cohort | 24 hr PA index for LTPA CHD mortality | ||||
| PA index: | • G1 = 255 | ||||
| D & B score = 11 | G1 = <29 | • G2 = 184 | |||
| G2 = 30-34 | • G3 = 152 | ||||
| G3 = >34 | p < 0.01 | ||||
| Physical demand of work | 24 hr PA index for LTPA CHD incidence | ||||
| G1 = Sedentary | • G1 = 414 | ||||
| G2 = Light | • G2 = 353 | ||||
| G3 = Medium | • G3 = 311 | ||||
| G4 = Heavy | |||||
| Outcome Measure: CHD mortality and Morbidity | Physical demand of work and CHD mortality | ||||
| • G1 = 216 | |||||
| Cox proportional HR | • G2 = 209 | ||||
| • G3 = 169 | |||||
| • G4 = 170 | |||||
| Physical demand of work and CHD incidence: | |||||
| • G1 = 355 | |||||
| • G2 = 405 | |||||
| • G3 = 307 | |||||
| • G4 = 325 | |||||
| Kaprio et al 2000 [232] | To examine the contribution of genetic and other familial factors to the relationship between LTPA and CHD. | • n = 8,205 | Length of Follow-up: 18 yrs | • Number of Cases: 723 | LTPA compared to being sedentary helps prevent CHD in men. |
| • Sex: Men | |||||
| • Age: 25-69 yr | Multivariate RR (95% CI) | ||||
| Finland | • Characteristics: Same sex twin pairs, free of CVD | PA assessment: Questionnaire for LTPA, 3 groups: | • G1 = 1.00 (referent) | ||
| • G2 = 0.84 (0.70-1.01) | |||||
| Prospective cohort | • G3 = 0.68 (0.50-0.92) | ||||
| G1 = Sedentary | p = 0.010 | ||||
| G2 = Occasional | |||||
| D & B score = 12 | Exercisers | ||||
| G3 = Conditioning | |||||
| Exercisers | |||||
| Outcome Measure: Hospitalization or death from CHD | |||||
| Poisson regression | |||||
| Lakka et al 1994 [233] | To investigate the independent associations of LTPA and maximal oxygen uptake with the risk of acute MI. | • n = 1,166 | Baseline examination: 1984-1989 | Conditioning LTPA and VO2 max had an inverse, graded and independent association with the risk | |
| • Sex: Men | |||||
| • Age: 42-61 yr | Adjusted RH (95% CI) by conditioning PA level | ||||
| Finland | • Characteristics: Healthy with normal ECG | PA assessment: Questionnaire for conditioning PA (h/wk), 3 groups (h/wk) | |||
| G1 = <0.7 | • G1 = 1.00 (referent) | ||||
| Prospective cohort | G2 = 0.7 | • G2 = 1.11 (0.58-2.12) | |||
| • Kuopio Ischaemic Heart Disease Risk Factor Study | G3 = >2.2 | • G3 = 0.31(0.12-0.85) | |||
| D & B score = 13 | Adjusted RG (95% CI) by VO2 max | ||||
| • G1 = 1.00 | |||||
| PF assessment: VO2 max (ml/kg/min) | • G2 = 0.76 (0.38-1.50) | ||||
| • G3 = 0.26 (0.10-0.68) | |||||
| G1 = <28.0 | |||||
| G2 = 28.0-33.6 | |||||
| G3 = >33.6 | |||||
| Outcome event: acute MI | |||||
| Cox proportional HR | |||||
| Laukkanen at al 2004 [234] | To determine whether VO2peak predicts CVD morbidity and mortality in a sample of men as related to conventional risk factors, medications or underlying chronic disease. | • 1,294 healthy; 1,057 unhealthy | PF Assessment: VO2 peak (ml/kg/min) measured by exercise test with an electrically braked cycle ergometer, divided into quartiles | • Number of Cases: 204 CV deaths, 323 non-fatal coronary events | Dose-response relationship between directly measured PF and CVD death among healthy men at baseline. |
| Finland | • Sex: Men | Healthy men with low VO2 peak (lowest quartile) had an increased risk | |||
| • Age: 42-60 yr | |||||
| Prospective cohort | • Characteristics: Healthy and not healthy participants | ||||
| Q1 = <27.6 | Adjusted RR (95% CI) by PF quartile Fatal MI | ||||
| Q2 = 27.6-32.2 | Unfit men with unfavorable risk profiles are the risk group that would benefit the most from preventative measures. | ||||
| D & B score = 11 | Q3 = 32.3-37.1 | • 3.29 (0.86-12.90) | |||
| • Kuopio Ischaemic Heart Disease Risk Factor Study | Q4 = >37.2 | ||||
| Non-Fatal MI | |||||
| Outcome Measure: Incidence of fatal and non fatal CVD during 13 year follow-up | • 2.16 (1.12-4.18) | ||||
| Cox proportional HR | |||||
| Lee at al 2000 [235] | To investigate whether different durations of exercise episode are associated with different risk of CHD. | • n = 7,307 | Baseline survey in 1988 | • Number of Cases: 482 | Longer durations of PA bouts are not associated with decreased CHD risk compared with shorter bouts, once total EE is taken into account. |
| • Sex: Men | |||||
| USA | • Age: Mean 66.1 ± 7.5 | PA assessment: Survey for EE (kJ/wk), divided into 5 groups and episodes of PA (min), divided into 6 groups | Multivariate adjusted RR (95% CI) by EE | ||
| • G1 = 1.00 (referent) | |||||
| • Characteristics: Healthy | • G2 = 0.80 (0.57-1.12) | ||||
| • G3 = 0.80 (0.55-1.16) | |||||
| Prospective cohort | • Harvard Alumni Study | • G4 = 0.74 (0.47-1.17) | |||
| • G5 = 0.62 (0.41-0.94) | |||||
| D & B score = 12 | Energy expenditure (kJ/wk) | As long as the total EE is similar, more frequent shorter bouts or longer less frequent bouts have an equivalent reduction in CHD risk. | |||
| G1 = <4,200 | Multivariate adjusted RR (95% CI) by duration of PA episode | ||||
| G2 = 4,200-8,399 | |||||
| G3 = 8,400-12,599 | |||||
| G4 = 12,600-16,799 | • G1 = 1.00 (referent) | ||||
| G5 = ≥ 16,800 | • G2 = 1.15 (0.70-1.87) | ||||
| • G3 = 1.01 (0.68-1.51) | |||||
| • G4 = 1.11 (0.67-1.84) | |||||
| Duration of PA episode (min) | • G5 = 1.18 (0.77-1.80) | ||||
| G1 = None | • G6 = 1.25 (0.83-1.87) | ||||
| G2 = 1-15 | |||||
| G3 = 16-30 | |||||
| G4 = 31-45 | |||||
| G5 = 46-60 | |||||
| G6 = >60 | |||||
| Outcome Measure: Fatal and Non Fatal CHD | |||||
| Proportional hazards regression | |||||
| Lee et al 2003 [236] | To investigate whether moderate- intensity exercise is associated with reduced CHD. | • n = 7,337 | PA assessment: Survey rating usual level of exertion when exercising, divided into tertiles | • Number of Cases: 551 | Inverse association between relative intensity of PA and the risk of CHD. |
| USA | • Sex: Male | Multivariate adjustment RR (95% CI) | |||
| • Age: Mean 66.1 yr | • T1 = 1.00 (referent) | ||||
| • T2 = 0.87 (0.70-1.09) | |||||
| • Characteristics: Healthy | • T3 = 0.92 (0.75-1.14) | ||||
| Prospective cohort | Energy expenditure (kcal/wk) | ||||
| Harvard Alumni Study | |||||
| T1 = <1000 | |||||
| D & B score = 13 | T2 = 1000-2499 | ||||
| T3 = ≥ 2500 | |||||
| Cox proportional HR | |||||
| Lemaitre et al 1999 [237] | To investigate whether regular participation in moderate intensity activity confers overall protection from sudden primary cardiac arrest. | • n = 355 cases, 503 controls | PA assessment: Interview (with spouses) for LTPA, 7 groups | • 355 cases | Participation in moderate intensity LTPA was associated with a decreased risk of primary cardiac arrest. |
| • Sex: Men and women | RR (95% CI) | ||||
| USA | G1 = No activity | • G1 = 1.00 (referent) | |||
| • Age: 25-74 yr | G2 = Gardening only≤ 60 min/wk | • G2 = 0.52 (0.21-1.28) | |||
| Case control | • Characteristics: Previously healthy prior to primary cardiac arrest. Control Subjects: Individually matched to case patients on age (within 7 years) and sex at a ratio of about 2:1 were randomly selected from community by random-digit dialing | G3 = Gardening only > 60 min/wk | • G3 = 0.34 (0.13 0.89) | ||
| G4 = Walking ≤ 60 min/wk | • G4 = 0.45 (0.17-1.19) | ||||
| D & B score = 11 | G5 = Walking > 60 min/wk | • G5 = 0.27 (0.11-0.67) | |||
| G6 = Moderate intensity | • G6 = 0.31 (0.13-0.74) | ||||
| LTPA (not walking or gardening) | G7 = 0.34 (0.16-0.75) | ||||
| G7 = High intensity LTPA | |||||
| Logistic regression analysis | |||||
| Lemaitre et al 1995 [238] | To examine whether LTPA decreases the risk of MI in postmenopausal women. | • n = 1,193 | PA assessment: Phone interview for LTPA, divided into quartiles of EE (mean kcal/wk) | • Number of Cases: 268 | Risk of MI among postmenopausal women is decreased by 50% with modest LT energy expenditures, equivalent to 30-45 min of walking for exercise three times per week |
| • Sex: Women | |||||
| • Age: Mean 67 yr | Multivariate RR (95% CI) | ||||
| USA | • Q1 = 1.00 (referent) | ||||
| • Characteristics: Postmenopaus al Cases: Diagnosed with non-fatal MI Controls: free from MI | Q1 = 71 | • Q2 = 0.52 (0.34-0.80) | |||
| Case control | Q2 = 472 | • Q3 = 0.40 (0.26-0.63) | |||
| Q3 = 1183 | • Q4 = 0.40 (0.25-0.63) | ||||
| D & B score = 11 | Q4 = 3576 | p = <0.001 | |||
| Outcome Measure: Diagnosed with non-fatal MI | |||||
| Logistic regression analysis | |||||
| Li et al 2006 [239] | To examine independent and joint associations of PA and adiposity with CHD incidence. | • n = 88,393 | Length of Follow-up: 20 yrs | • Number of Cases: 2,358 | Physical inactivity independently contributes to the development of CHD in women. |
| • Sex: Women | • Number of Dropouts: <2% lost to follow-contributes to the development of CHD in women. | ||||
| USA | • Age: 34-59 yr | up | |||
| • Characteristics: Nurses | PA assessment: Questionnaire for LTPA (hr/wk), 3 groups | ||||
| Prospective cohort | Multivariate HR (95% CI) | ||||
| • Nurses' Health Study | • G1 = 1.00 (referent) | ||||
| G1 = ≥3.5 | • G2 = 1.34 (1.18-1.51) | ||||
| D & B score = 12 | G2 = 1-3.49 | • G3 = 1.43 (1.26-1.63) | |||
| G3 = <1 | |||||
| Outcome Measure: CHD incidence | |||||
| Cox proportional HR | |||||
| Lemaitre et al 1995 [240] | To evaluate the effect of PA on MI occurrence. | • n = 1,107 (726 controls, 381 cases) | PA assessment: Questionnaire, 3-5 groups depending on variable | OR (95% CI), | PA level was inversely associated with occurrence of MI in both sexes, although the association presented a significant linear trend only for women; in men it suggested a u-shaped relation. |
| Total PA, men | |||||
| Portugal | • Sex: Men and women | • G1 = 1.00 (referent) | |||
| Total PA (MET hr/day), men | • G2 = 0.54 (0.33-0.88) | ||||
| Case control | • Age: ≥ 40 yr | • G3 = 0.34 (0.20-0.59) | |||
| • Characteristics: Case: Admitted to Hospital and diagnosed with first episode of MI Control: Healthy, no history of CHD | G1 = 28.3-32.1 | • G4 = 0.59 (0.36-0.98) | |||
| D & B score = 12 | G2 = 32.2-33.3 | • G5 = 0.90 (0.56-1.45) | |||
| G3 = 33.4-36.5 | Trend p = 0.827 | ||||
| G4 = 36.6-40.3 | Total PA, women | ||||
| G5 = 40.4-83.1 | • Q1 = 1.00 (referent) | ||||
| Total PA (MET hr/day), women | • Q2 = 0.39 (0.21-0.73) | ||||
| Q1 = 28.9-32.7 | • Q3 = 0.33 (0.17-0.64) | ||||
| Q2 = 32.8-34.1 | • Q4 = 0.22 (0.11-0.47) | ||||
| Q3 = 34.2-37.8 | p = <0.001 | ||||
| Q4 = 37.8-70.6 | |||||
| Sport participation, men | |||||
| Sport participation (MET hr/day), men | • G1 = 1.00 (referent) | ||||
| G1 = 0.0 | • G2 = 0.36 (0.19-0.69), | ||||
| G2 = 0.1-1.0 | • G3 = 0.72 (0.41-1.26), | ||||
| G3 = 1.1-2.0 | • G4 = 0.42 (0.23-0.76), | ||||
| G4 = 2.1-3.6 | • G5 = 0.31 (0.16-0.62) | ||||
| G5 = 3.7-15.4 | p = <0.001 | ||||
| Lovasi et al 2007 [241] | To investigate the shape of the relationship between LTPA and MI risk. | • n = 4,094 | PA assessment: Telephone interview (Minnesota LTPA Questionnaire) | • Number of Cases: 697 | Time engaged in LTPA, even non strenuous LTPA was associated with a lower risk of MI, and the shape of this relationship was non- linear |
| • Sex: Men and women | Adjusted OR (95% CI) | ||||
| USA | • Age: 64 ± 9 yr | LTPA and non fatal CHD | |||
| • Characteristics: Group Health Cooperative Members | • G1 = 1.00 (referent) | ||||
| Case control | LTPA | • G2 = 0.88 (0.66-1.17) | |||
| G1 = None | • G3 = 0.62 (0.46-0.83) | ||||
| D & B score = 11 | G2 = <2 | • G4 = 0.61 (0.45-0.82) | |||
| G3 = 2-5 | • G5 = 0.59 (0.44-0.80) | ||||
| G4 = 5-9 | |||||
| G5 = >9 h/wk | Adjusted RR (95% CI) Strenuous LTPA and non Fatal CHD | ||||
| Strenuous LTPA | • G1 = 1.00 (referent) | ||||
| G1 = None | • G2 = 0.76 (0.59-0.99) | ||||
| G2 = non strenuous LTPA | • G3 = 0.53 (0.40-0.70) | ||||
| G3 = Any Strenuous | |||||
| LTPA | |||||
| Outcome measure: non fatal CHD | |||||
| Logistic regression | |||||
| Manson et al 1999 [242] | To assess the comparative roles of walking and vigorous exercise in the prevention of coronary events in women. | • n = 72,488 | PA assessment: | • Number of Cases: 645 coronary events | Both walking and VPA are associated with a substantial reductions in incidence of CHD. Risk reductions for each were similar hen total PAy was similar. Walking 3 or more hours per week could reduce the risk of CHD by 30-40%. |
| • Sex: Women | Questionnaire with detailed information on PA. | ||||
| • Age: 40-65 yr | Multivariate RR (95% CI) by total PA score | ||||
| USA | • Characteristics: Healthy, no Previous history of CHD | • G1 = 1.00 (referent) | |||
| • G2 = 0.88 (0.71-1.10) | |||||
| Prospective cohort | Total PA score | • G3 = 0.81(0.64-1.02) | |||
| G1 = 1-2.0 | • G4 = 0.74 (0.58-0.95) | ||||
| Nurses' Health Study | G2 = 2.1-4.6 | • G5 = 0.66 (0.51-0.86) | |||
| D & B score = 12 | G3 = 4.7-10.4 | p = 0.002 | |||
| G4 = 10.5-21.7 | |||||
| G5 = >21.7 | |||||
| Multivariate RR (95% CI) by walking activity | |||||
| • G1 = 1.00 (referent) | |||||
| Walking, in those who did not participate in VPA: (MET hr/wk) | • G2 = 0.78 (0.57-1.06) | ||||
| G1 = 0.5 | • G3 = 0.88 (0.65-1.21) | ||||
| G2 = 0.6-2.0 | • G4 = 0.70 (0.51-0.95) | ||||
| G3 = 2.1-3.8 | • G5 = 0.65 (0.47-0.91) | ||||
| G4 = 3.9-9.9 | p = 0.02 | ||||
| G5 = ≥ 10 | |||||
| Multivariate RR (95% CI) by walking pace | |||||
| • 1.00 (referent) | |||||
| Walking pace (mph) | • 0.75 (0.59-0.96) | ||||
| G1 = <2.0 | • 0.64 (0.47-0.88) | ||||
| G2 = 2.0-2.9 | |||||
| G3 = ≥ 3.0 | |||||
| Mora et al 2007 [243] | To investigate whether differences in several CV risk factors mediate the effect of PA on reduced risk of CVD. | • n = 27,055 | 10.9 ± 1.6 yr of follow up | • Number of Cases: 640 | There remained a borderline significant inverse association between PA and risk of CHD after adjustment for all sets of risk factors. |
| • Sex: Women | |||||
| • Age: ≥ 45 yr | PA assessment: Questionnaires at study entry for categories of EE from PA (kcal/wk), 4 groups | HR (95% CI), basic model | |||
| USA | • Characteristics: Healthy | • G1 = 1.00 (referent) | |||
| • G2 = 0.84 (0.67-1.06) | |||||
| Prospective cohort | • Women's health study | • G3 = 0.76 (0.61-0.96) | |||
| • G4 = 0.62 (0.48-0.82) | |||||
| G1 = <200 | p = 0.001 | ||||
| D & B score = 13 | G2 = 200-599 | While all sets of risk factors should some mediation on the effect of PA on CHD none made the relationship insignificant | |||
| G3 = 600-1499 | Multivariate adjusted HR (95% CI) | ||||
| G4 = ≥ 1500 | • G1 = 1.00 (referent) | ||||
| • G2= 0.71 (0.58-0.87) | |||||
| Outcome measure: | • G3 = 0.64 (0.52-0.78) | ||||
| Incidence of CVD and | • G4 = 0.48 (0.38-0.62) | ||||
| p = <0.001 | |||||
| Cox proportional HR | |||||
| O'Connor et al 1995 [244] | To examine the association between intensity of exercise and CHD risk. | • n = 680 (532 men and 148 women) | PA assessment: Home interview for PA, divided into quartiles | • Number of Cases: 340 | Significant inverse association between PA level and the risk of non fatal MI in men, which persisted after adjustment for other risk factors. |
| Adjusted OR (95% CI) by PA level, men | |||||
| • Q1 = 1.00 (referent) | |||||
| USA | • Sex: Men and women | Q1 = Lowest | • Q2 = 0.60 (0.32-1.13) | ||
| Q2 | • Q3 = 0.41 (0.21-0.78) | ||||
| Case control | • Age: < 76 yr | Q3 | • Q4 = 0.41 (0.22-0.77) | ||
| • Characteristics: Cases: Diagnosed MI (non-fatal), no previous history of CHD. Controls: no history of CHD. | Q4 = Highest | p = 0.003 | |||
| D & B score = 12 | Outcome Measure: non-fatal MI | Adjusted OR (95% CI) by PA level, women | |||
| • Q1 = 1.00 (referent) | |||||
| Moderate- vigorous sports men Cut-points kcal/wk | • Q2 = 1.07 (0.27-4.17) | ||||
| Q1 = Lowest | • Q3 = 2.02 (0.56-7.38) | ||||
| Q2 | • Q4 = 1.29 (0.31-5.35) | ||||
| Q3 | p = 0.51 | ||||
| Q4 = Highest | |||||
| Adjusted OR (95% CI) by moderate-vigorous sports, men | |||||
| • Q1 = 1.00 (referent) | |||||
| Moderate- vigorous sports Women | • Q2 = 1.12 (0.60-2.10) | ||||
| Cut-points kcal/wk | • Q3 = 0.61 (0.30-1.24) | ||||
| Q1 = Lowest | • Q4 = 0.43 (0.20-0.92) | ||||
| Q2 | p = 0.02 | ||||
| Q3 | |||||
| Q4 = Highest | Adjusted OR (95% CI) by moderate-vigorous sports, women | ||||
| Logistic regression analysis | • Q1 = 1.00 (referent) | ||||
| • Q2 = 1.31 (0.37-4.66) | |||||
| • Q3 = 1.90 (0.44-8.28) | |||||
| • Q4 = 0.35 (0.07-1.84) | |||||
| p = 0.62 | |||||
| Rastogi et al 2004 [245] | To examine the relation between PA and CHD risk in India. | • n = 1,050 | PA assessment: Questionnaire | Number of Cases: 350 | Observed a strong and dose dependent inverse association between LTPA and non fatal CHD. |
| • Sex: Men and women | Multivariate OR (95% CI) by LTPA | ||||
| USA | • Age: 21-74 yr | LTPA (MET min/d) | • G1 = 1.00 (referent) | ||
| • Characteristics: Cases: Diagnosed with MI (non fatal) Controls: non- cardiac patients | G1 = 0 | • G2 = 0.96 (0.59-1.55) | |||
| Case control | G2 = 0-145 | • G3 = 0.44 (0.27-0.71) | |||
| D & B score = 12 | G3 = ≥145 | p = 0.001 | |||
| Sedentary time (min/d) | Multivariate OR (95% CI) by sedentary time | ||||
| G1 = <70 | • G1 = 1.00 (referent) | ||||
| G2 = 70-130 | • G2 = 1.15 (0.68-1.95) | ||||
| G3 = 130-215 | • G3 = 1.04 (0.61-1.76) | ||||
| G4 = ≥215 | • G4 = 1.88 (1.09-3.21) | ||||
| p = 0.02 | |||||
| Outcome Measure: Non-fatal MI | |||||
| Conditional logistic regression | |||||
| Rodriguez et al 1994 [246] | To examine the relationship between PA and 23 yr incidence of CHD morbidity and mortality. | • n = 7,074 | 23 year follow-up | • Number of Cases: 789 | PA was associated with a significant reduction in the risk of CHD morbidity and mortality. |
| • Sex: Men | |||||
| • Age: 45-64 yr | PA assessment: Questionnaire for PA index, divided into tertiles | Age adjusted RR (95% CI), CHD incidence | |||
| USA | • Characteristics: Japanese- American living in Oahu, Hawaii in 1965, < 65 years to reduce effect of retirement on PA levels | • T1 = 1.00 (referent) | |||
| • T2 = 1.01 (.86-1.19) | |||||
| Prospective cohort | T1 = Low | • T3 = 0.83 (0.86-1.19) | These data support the hypothesis that PA is associated with a favorable profile of CVD risk factors. | ||
| T2 = Moderate | |||||
| T3 = High | Multivariate adjusted RR (95% CI), CHD incidence | ||||
| D & B score = 11 | Cox proportional regression model | • T1 = 1.00 (referent) | |||
| • T2 = 1.07 (0.90-1.26) | This study did not show a dose- response relationship since the medium tertile of PA showed increased rates of CHD compared to the inactive group. | ||||
| • The Honolulu Heart Program | • T3 = 0.95 (0.80-1.14) | ||||
| Age adjusted RR (95% CI), CHD mortality | |||||
| • T1 = 1.00 (referent) | |||||
| • T2 = 1.12 (0.88-1.44) | |||||
| • T3 = 0.74 (0.56-0.97) | |||||
| Multivariate adjusted RR (95% CI) | |||||
| • T1 = 1.00 (referent) | |||||
| • T2 = 1.19 (0.93-1.53) | |||||
| • T3 = 0.85 (0.65-1.13) | |||||
| Rothenbacher et al 2003 [247] | To estimate the risk for CHD associated with LTPA. | • n = 791 (312 cases; 479 controls) | PA assessment: Interview | Number of Cases: 312 | LTPA showed a clear inverse association with risk of CHD. |
| LTPA (h/wk) | Multivariate OR (95% CI), LTPA | ||||
| Germany | • Sex: Men and Women | G1 = 0 | Winter | ||
| G2 = <1 | • G1 = 1.00 (referent) | ||||
| Case control | Age: 40-68 yr | G3 = 1-2 | • G2 = 0.48 (0.27-0.84) | ||
| Characteristics: Cases: stable CHD diagnosed within 2 years, no recent MI, Controls: no history of CHD. | G4 = >2 | • G3 = 0.54 (0.369-0.82) | |||
| D & B score = 12 | • G4 = 0.27 (0.19-0.47) | ||||
| Workday activity by | |||||
| bike/foot, (min/workday) | Summer | ||||
| G1 = <15 | • G1 = 1.00 (referent) | ||||
| G2 = 15-30 | • G2 = 0.85 (0.47-1.53) | ||||
| G3 = 30-60 | • G3 = 0.60 (0.38-0.95) | ||||
| G4 = >60 | • G4 = 0.39 (0.26-0.59) | ||||
| Outcome Measure: non fatal CHD | Multivariate OR (95% CI), workday activity by bike/foot | ||||
| Unconditional logistic regression, linear regression model | • G1 = 1.00 (referent) | ||||
| • G2 = 0.53 (0.30-0.93) | |||||
| • G3 = 0.36 (0.21-0.62) | |||||
| • G4 = 0.58 (0.36-0.94) | |||||
| Seccareccia and Menotti 1992 [248] | To examine the relationship between OPA and the risk of CHD death. | • n = 1,621 | 25 year of follow-up | • 189 cases | Increase in OPA is inversely related to risk of CHD death. |
| • Sex: Men | |||||
| • Age: 40-59 yr | PA assessment: Questionnaire for OPA (kcal/d), 3 groups | Age Standardized CHD and deaths rates: | |||
| • Characteristics: Healthy | • G1 = 18.9 ± 3.1 | ||||
| Italy | • G2 = 13.1 ± 1.7 | ||||
| G1 = Sedentary, < 2400 | • G3 = 11.0 ± 0.9 | ||||
| Prospective cohort | G2 = Moderate, 2400-3199 | ||||
| D & B score = 11 | G3 = Heavy ≥ 3200 | ||||
| Indicators of PF including HR, vital capacity, FEV in 3/4 of sec, and corrected arm circumference (minus contribution of fat). | |||||
| End Point: Fatal CHD | |||||
| Sesso et al 2000 [249] | To examine the association of the quantity and intensity of PA with CHD risk and the impact of other coronary risk factors. | • n = 12,516 | PA assessment: Questionnaire | Number of Cases: 2,135 | L-Shaped association between PA and the risk of CHD, with a reduction in CHD risk of approximately 20% for total PA levels >4200 kJ/wk |
| • Sex: Men | |||||
| • Age: 39-88 yr | Multivariate HR (95% CI) | ||||
| USA | • Characteristics: Healthy | PA Index (kJ/wk) | • G1 = 1.00 (referent) | ||
| G1 = <2100 | • G2 = 0.90 (0.79-1.03) | ||||
| Prospective cohort | • Harvard Alumni | G2 = 2100-4199 | • G3 = 0.81 (0.71-0.92) | ||
| Study | G3 = 4200-8399 | • G4 = 0.80 (0.69-0.93) | |||
| G4 = 8400-12599 | • G5 = 0.81 (0.71-0.94) | ||||
| D & B score = 12 | G5 = >12600 | p = 0.003 | Suggests that vigorous activities are associated with a reduced risk of CHD, whereas moderate or light PA has no clear association with risk of CHD. | ||
| Cox proportional HR | |||||
| Sundquist et al 2005 [250] | To examine the long term effect of LTPA on incident cases of CHD. | • n = 5,196 (2,645 men, 2,551 women) | PA assessment: Questionnaire Levels of PA | Age and sex adjusted RR (95% CI) | Positive long term effect of LTPA on CHD risk among men and women. |
| • Q1 = 1.00 (referent) | |||||
| Sweden | • Sex: Men and women | Q1 = None | • Q2 = 0.72 (0.51-1.00) | ||
| Q2 = Occasionally | • Q3 = 0.64 (0.46-0.89) | ||||
| Prospective cohort | Age: 35-74 yr | Q3 = 1-2 times per week | • Q4 = 0.46 (0.29-0.74) | ||
| • Characteristics: Those not hospitalized for CHD in the last 2 years and those who rate their general health as poor were excluded | Q4 = Vigorous ≥2 times per week | Multivariate adjusted RR (95% CI) | |||
| D & B score = 11 | Outcome Measure: Fatal or non fatal CHD | • Q1 = 1.00 (referent) | |||
| • Q2 = 0.76 (0.55-1.07) | |||||
| • Q3 = 0.74 (0.53-1.04) | |||||
| • Q4 = 0.59 (0.37-0.95) | |||||
| Cox regression model | |||||
| Talbot et al 2002 [251] | To examine the contributions of LTPA and aerobic fitness to the risk of coronary events in healthy younger and older adults. | • n = 689 | Surveys began in 1960 and were completed on every visit | • Number of Cases: 63 | In younger men PF predicts a reduced risk of CHD but not LTPA. |
| • Sex: Men | |||||
| • Age: | After adjusting for coronary risk factors there was: | ||||
| USA | 51.6 ± 16.8 yr | ||||
| • Characteristics: Community dwelling | PA assessment: Survey for LTPA (97 activities) at every visit. | RR: 0.53 (p < 0.001) and | In older men, high intensity LTPA and PF appear to be of similar importance in reducing CHD risk. | ||
| Prospective cohort | RR: 0.61 (p = 0.024) in older men. | ||||
| D & B score = 12 | • Baltimore Longitudinal Study of Aging | PF assessment: Treadmill VO2 max test on alternate visits | Total LTPA was unrelated to coronary risk in either age group. | ||
| With 3 levels of LTPA intensity substituted for total LTPA: | |||||
| Unpaired t-tests and chi square tests. Cox Proportional hazards Analysis | RR = 0.39 for tertile 3 vs. tertile 1 | ||||
| Tanasescu et al 2002 [252] | To assess the amount, type and intensity of PA in relation to risk of CHD in men. | • n = 44,452 | PA assessment: Questionnaire | • Number of Cases: 1,700 | Total PA, running, weight training, and walking were associated with a reduced risk for CVD. |
| • Sex: Men | |||||
| • Age: 40-75 yr | Age adjusted HR (95% CI) by total PA | ||||
| USA | • Characteristics: Health professionals, no history of CHD and in good health | Total PA (MET hr/wk) | • Q1 = 1.00 (referent) | ||
| Q1 = 0-6.32 | • Q2 = 0.85 (0.74 0.98) | ||||
| Prospective cohort | Q2 = 6.33-14.49 | • Q3 = 0.78 (0.67-0.92) | |||
| Q3 = 14.50-25.08 | • Q4 = 0.72 (0.62-0.83) | The average exercise intensity was associated with a reduced risk (independent of total PA). | |||
| Q4 = 25.09-41.98 | • Q5 = 0.58 (0.49-0.68) | ||||
| D & B score = 11 | Q5 = > 41.99 | p = .001 | |||
| • Health Professionals follow-up study | Exercise intensity (METs) | Age adjusted HR (95% CI) by exercise intensity | |||
| G1 = Low-1-4 | • G1 = .00 (referent) | ||||
| G2 = Mod.-4-6 | • G2 = 0.94 (0.83-1.04) | ||||
| G3 = High 6-12 | |||||
| Walking pace independent of total volume of PA (mph) | • G3 = 0.83 (0.72-0.97) | ||||
| p = 0.02 | |||||
| Q1 = <2 | Age adjusted HR (95% CI) by walking pace | ||||
| Q2 = 2-3 | • Q1 = 1.00 (referent) | ||||
| Q3 = 3-4 | • Q2 = 0.72 (0.54-0.94) | ||||
| Q4 = > 4 | • Q3 = 0.61 (0.45-0.81) | ||||
| • Q4 = 0.51 (0.31-0.84) | |||||
| Outcome Measure: Nonfatal MI or Fatal CHD occurring during follow-up | p <0.001 | ||||
| Cox proportional HR | |||||
| Vatten et al 2006 [253] | To investigate whether obesity- related CV mortality could be modified by PA. | • n = 54,284 (27,769 men; 26,515 women) | Length of Follow-up: 16 years | • Number of Cases: 2,462 | Increased PA reduces the risk of death in women, but not in men. |
| Multivariate HR (95% CI), men | |||||
| Norway | • Sex: Men and women | PA assessment: | • Q1 = 1.00 (referent) | ||
| Questionnaire | • Q2 = 1.01 (0.89-1.16) | ||||
| Prospective cohort | Age: ≥ 20 yr | Divided into 4 groups | • Q3 = 0.98 (0.84-1.14) | ||
| • Characteristics: Free from CVD at baseline | Q1 = High | • Q4 = 1.18 (1.00-1.38) | |||
| Q2 = Medium | p = 0.11 | ||||
| D & B score = 12 | Q3 = Low | ||||
| • HUNT study | Q4 = Never | Multivariate HR (95% CI), women | |||
| Outcome Measure: Ischemic heart disease mortality | • Q1 = 1.00 (referent) | ||||
| • Q2 = 1.23 (1.01-1.51) | |||||
| • Q3 = 1.54 (1.24-1.91) | |||||
| • Q4 = 1.52 (1.23-1.88) | |||||
| Cox proportional HR | p <0.001 | ||||
| Wagner et al 2002 [254] | To investigate if the association between PA patterns and incidence of coronary events could explain the gradient in CHD observed between 2 countries. | • n = 9,758 | Length of Follow-up: 5 yrs | Number of Cases: 167 hard CHD, 154 angina events | Beneficial effect of LTPA EE on hard CHD incidence in middle aged men. |
| • Sex: Men and women | PA assessment: Questionnaire for LTPA, 3 groups: | Number of Dropouts: < 2% | |||
| Ireland/France | • Age: 50-59 yr | ||||
| • Characteristics: Healthy at Baseline | HR (95% CI), hard events | ||||
| Prospective cohort | G1 = Lowest | • G1 = 1.00 (referent) | |||
| G2 = Middle | • G2 = 0.73 (0.51-1.05) | ||||
| G3 = Highest | • G3 = 0.66 (0.46-0.96) | ||||
| D & B score = 12 | Outcome Measure: CHD hard events and Angina | p = 0.04 | |||
| HR (95% CI), angina | |||||
| • G1 = 1.00 (referent) | |||||
| Cox proportional HR | • G2 = 0.83 (0.55-1.25) | ||||
| • G3 = 1.28 (0.88-1.86) | |||||
| p = 0.10 | |||||
D & B score, Downs and Black quality score; YR, years; G, groups; CHD, coronary heart disease; RR, risk ratio; 95% CI, 95% confidence interval; PA, physical activity; VPA, vigorous physical activity; CV, cardio vascular; MET, metabolic equivalent; kcal/wk, kilocalories per week; Q, quartile or quintile; km/h, kilometers per hour; LTPA, leisure-time physical activity; HR, hazard ratio; OPA, occupational physical activity; kcal/kg/day kilocalories per kilogram per day; MI, myocardial infarction; ECG, electrocardiogram; kcal/kg/h kilocalories per kilogram per hour; mph, miles per hour; CVD, cardiovascular disease.
Similar to the all-cause mortality data, the risk for cardiovascular disease demonstrates a graded inverse dose-response relationship to physical activity and fitness. The relative reduction in the incidence of cardiovascular disease averages 33% (median risk reduction of 36%), with greater risk reductions in studies that employed objective measures of aerobic fitness. It is not uncommon for studies to demonstrate a 50% or higher risk reduction when an objective measure of physical fitness was taken (Table 12). The importance of physical activity may actually be underestimated owing to multivariate control for many confounding factors (as discussed previously) and the fact that effects of within-person variation in physical activity are often not considered [55]. The relative risk reduction appears to be similar for men and women, and also appear to extend to non-Caucasian populations [56]. Some evidence also exists indicating that small amounts of physical activity are associated with lower cardiovascular-disease related mortality [57,58]. Similar to all-cause mortality, physical activity confers health benefits independent of other known risk factors [42,59]. Collectively, the level of evidence would be considered to be Level 2A based on the presence of overwhelming evidence from observational trials. The quality of the investigations was generally high with a mean (and median) Downs and Black score of 12 (range 9-14).
Implications
Research in the field began with the landmark work of Morris and colleagues, which demonstrated that men in physically demanding occupations (bus conductors and postmen) had a significantly lower risk of heart disease than individuals who worked in less demanding jobs (bus drivers and office workers) [45]. Since then considerable research has examined the relationship between physical activity and the risk for cardiovascular disease. In fact, several systematic reviews of the literature have been developed regarding the role of habitual physical activity in the primary and secondary prevention of cardiovascular disease [33,60-62]. The research to date has been consistent and compelling, habitual physical activity reduces markedly the risk for cardiovascular disease.
Based on the available literature, there is compelling evidence that the recommendation of 30 min of moderate intensity exercise on most days of the week (equivalent to 4.2 MJ/wk or 1000 kcal/wk) reaches a threshold associated with significant reductions in cardiovascular-related mortality [32,63]. Brisk walking has also been shown to be preferable to a slower pace [64]. However, weekly exercise volumes of less than 4.2 MJ (1000 kcal) may be cardio-protective [14,59,65-67]. For instance, Lee et al. (2001) found that as little as 1 hr/wk of walking was associated with a 50% lower cardiovascular disease mortality in one sample of women. Wisloff et al. [58] reported that a single weekly bout of self-reported high intensity exercise was associated with a lower risk of cardiovascular death relative to those reporting no activity in both men (RR = 0.61, 95% CI = 0.49-0.75), and women (RR = 0.49, 95% CI = 0.27-0.89). Moreover, no additional benefit was seen with higher durations or frequency of exercise sessions [58]. The authors stated that this evidence challenges "current recommendations that require at least 1000 kcal of caloric expenditure per week to achieve exercise-induced protection against premature cardiovascular death." However, this research is in fact supportive of the Canadian guidelines which recognize the potential health benefits of low volumes of physical activity as reflected by the statement "Every little bit counts, but more is even better - everyone can do it!" It however should be noted that the statement "more is even better" is supported by a strong evidence base.
Recommendation #2
For a reduced risk for cardiovascular disease-related events and mortality, it is recommended that individuals participate in 30 min or more of moderate to vigorous exercise on most days of the week. Greater health benefits appear to occur with high volume and/or intensities of activity. Health benefits may also occur with as little as one hr of brisk walking per week. [Level 2, Grade A]
The Primary Prevention of Stroke
Stroke affects a significant proportion of Canadian society with approximately 50,000 new cases each year [68]. The relationship between physical activity and the risk for stroke is compelling, supporting at least a 25-30% risk reduction in the most active individuals [31]. In fact, in a review of the literature Katzmarzyk and Janssen [20] reported that lack of physical activity carried a relative risk of 1.60 (95% CI = 1.42-1.80) for stroke, similar to or higher than that for coronary heart disease (1.45), hypertension (1.30), colon cancer (1.41), breast cancer (1.31), type 2 diabetes (1.50), and osteoporosis (1.59).
In our systematic review of the literature, a total of 1104 citations were identified during the electronic database search (Figure 5). Of these citations, 405 were identified in MEDLINE, 183 in EMBASE, 227 in Cochrane, and 289 in the CINAHL/SportDiscus/PsychInfo search. A total of 13 duplicates were found, leaving a total of 1091 unique citations. A total of 1011 articles were excluded after scanning, leaving a total of 80 articles for full review. An additional 9 articles were retrieved through cross-referencing and the authors' knowledge of the field. From these articles 64 were excluded after full review leaving 25 articles for inclusion in the systematic review. The reasons for exclusion included non-experimental/weak design (poor execution introducing bias) (n = 16), did not contain three levels of physical activity or not possible to determine dose-response relationship (n = 14), reviews, summaries, meta-analyses (n = 17), dissertations, thesis, abstracts (n = 8), and other (n = 9). Therefore, a total of 25 articles were included in the systematic review of the literature regarding the relationship between physical activity and the primary prevention of stroke (Table 13).
Figure 5.
Results of the Literature Search for Stroke.
Table 13.
Studies examining the relationship between physical activity and stroke.
| Publication Country Study Design Quality Score | Objective | Population | Methods | Outcome | Comments and Conclusions |
|---|---|---|---|---|---|
| Wisloff et al 2006 [58] | To assess exercise amount and intensity in relation to subsequent CVD mortality (including stroke). | • n = 27,143 men, 28,929 women | 16 year follow up | Multivariate adjusted RR (95% CI) Men | Both high and low- intensity exercise may be associated with a reduced risk of stroke in both men and women. |
| Norway | • Sex: Men and women | PA Assessment: Questionnaire | G1 = 1.00 (referent) | ||
| • Age: ≥ 20 yr | G2 = 0.90 (0.70-1.17) | ||||
| • Characteristics: free from CVD | PA | G3a = 0.90 (0.64-1.26) | |||
| • HUNT Study | G1 = None | G3b = 0.59 (0.27-1.27) | |||
| G2 = <1/wk | G3c = 0.62 (0.40-0.95) | ||||
| G3a = 1/wk ≤ 30 min low | G3d = 0.51 (0.31-0.86) | ||||
| G3b = 1/wk ≤ 30 min high | G4a = 0.72 (0.49-1.05) | ||||
| G3c = 1/wk > 30 min low | G4b = 0.63 (0.31-1.30) | ||||
| Prospective cohort | G3d = 1/wk > 30 min high | G4c = 1.02 (0.72-1.44) | |||
| G4a = 2-3/wk ≤ 30 min low | G4d = 0.59 (0.37-0.92) | ||||
| G4b = 2-3/wk ≤ 30 min high | G5a = 0.97 (0.70-1.36) | ||||
| D & B score = 12 | G4c = 2-3/wk > 30 min low | G5b = 0.68 (0.27-1.66) | |||
| G4d = 2-3/wk > 30 min high | G5c = 0.81 (0.65-1.20) | ||||
| G5a = ≥ 4/wk ≤ 30 min low | G5d = 0.67 (0.49-1.11) | ||||
| G5b = ≥ 4/wk ≤ 30 min high | |||||
| G5c = ≥ 4wk > 30 min low | RR (95% CI) Women | ||||
| G5d = ≥ 4/wk > 30 min high | G1 = 1.00 (referent) | ||||
| Outcome Measure: IHD mortality | G2 = 1.01 (0.81-1.25) | ||||
| Cox proportional HR | G3a = 0.88 (0.68-1.15) | ||||
| G3b = 0.98 (0.46-2.10) | |||||
| G3c = 0.63 (0.42-0.94) | |||||
| G3d = 1.00 (0.50-1.98) | |||||
| G4a = 0.91 (0.70-1.17) | |||||
| G4b = 1.44 (0.78-2.65) | |||||
| G4c = 0.62 (0.44-0.88) | |||||
| G4d = 0.77 (0.36-1.66) | |||||
| G5a = 0.74 (0.56-0.99) | |||||
| G5b = 0.40 (0.10-1.62) | |||||
| G5c = 0.63 (0.45-0.89) | |||||
| G5d = 0.51 (0.21-1.26) | |||||
| Abbott et al 2003 [69] | To examine the way in which risk factor effects on the incidence of thromboembolic and hemorrhagic stroke can change over a broad range of ages. | • n = 7,589 | 6, 15 and 26 year follow up | Incidence rates per 1000 of stroke: | The protective effect of PA on reducing risk of stroke increased with age. |
| USA | • Sex: Men | • G1 = 9.0 (49) | |||
| • Age: 45-93 yr | PA assessment: Using PA index over a 24 hour period PA information collected at study enrolment 1965-1968 and updated at physical examinations that occurred at 6, 15 and 26 years into follow-up. | • G2 = 17.8 (124) | |||
| Prospective cohort | • Characteristics: Free from CHD and stroke at enrolment; Japanese ancestry living on the island of Oahu, Hawaii. | Grouped into 4 age groups, yr: | • G3 = 33.4 (112) | ||
| D & B score = 14 | • Honolulu Heart Program | G1 = 45-54 | • G4 = 48.1 (111) | ||
| G2 = 55-64 | Incidence of stroke event increased with advancing age p <0.001 | ||||
| G3 = 65-74 | There appeared to be a small protective effect within each age group. Inverse relations increased with age (p = 0.046). The protective effect of PA became significant in men >77 years (p = 0.032) | ||||
| G4 = 75-93 | |||||
| Outcome Measure: diagnosis of fatal and non fatal stroke during 26 years of follow-up | |||||
| Cox proportional HR | |||||
| Gillium et al 1996 [70] | To examine the relationship between recreational and non-recreational PA and risk of stroke. | • n = 2,368 men, 2,713 women | 11.6 year follow up | Number of Cases: 249 white women, 270 white men, 104 black | Sedentary behaviour was found to be associated with increased risk of stroke. |
| USA | • Sex: Men and women | PA assessment: Questionnaire divided into tertiles: | |||
| • Age: 45-74 yr | T1 = Low | RR (95% CI) Black men and women Recreational PA | |||
| Prospective cohort | • Ethnicity: Black and white | T2 = Medium | • T1 = 1.33 (0.67-2.63) | ||
| D & B score = 12 | • NHANES I | T3 = High | • T2 = 1.33 (0.63-2.79) | ||
| • T3 = 1.00 (referent) | |||||
| Outcome Measure: Total Stroke | Non-recreational PA | ||||
| Cox proportional HR | • T1 = 1.40 (0.90-2.16) | ||||
| • T2 = 1.41 (0.74-2.70) | |||||
| • T3 = 1.00 (referent) | |||||
| RR (95% CI) White men age 45-64 Recreational PA | |||||
| • T1 = 1.24 (0.63-2.41) | |||||
| • T2 = 1.17 (0.61-2.27 | |||||
| • T3 = 1.00 (referent) | |||||
| Non-recreational PA | |||||
| • T1 = 1.07 (0.40-2.86) | |||||
| • T2 = 1.75 (1.04-2.96) | |||||
| • T3 = 1.00 (referent) | |||||
| RR (95% CI) White women age 45-64 Recreational PA | |||||
| • T1 = 3.13 (0.95-10.32) | |||||
| • T2 = 1.80 (0.52-6.22) | |||||
| • T3 = 1.00 (referent) | |||||
| Non-recreational PA | |||||
| • T1 = 3.51 (1.66-7.46) | |||||
| • T2 = 1.07 (0.57-1.99) | |||||
| • T3 = 1.00 (referent) | |||||
| RR (95% CI) White men age 65-74 Recreational PA | |||||
| • T1 = 1.29 (0.58-1.88) | |||||
| • T2 = 0.86 (0.58-1.28) | |||||
| • T3 = 1.00 (referent) | |||||
| Non-recreational | |||||
| • T1 = 1.82 (1.15-2.88) | |||||
| • T2 = 1.20 (0.88-1.64) | |||||
| • T3 = 1.00 (referent) | |||||
| RR (95% CI) White women age 65-75 Recreational PA | |||||
| • T1 = 1.55 (0.95-2.53) | |||||
| • T2 = 1.27 (0.76-2.12) | |||||
| • T3 = 1.00 (referent) | |||||
| Non-recreational PA | |||||
| • T1 = 1.82 (1.10-3.02) | |||||
| • T2 = 1.42 (1.01-2.00) | |||||
| • T3 = 1.00 (referent) | |||||
| Lee and Blair 2002 [71] | To examine the association between PF and stroke mortality in men. | • n = 16,878 | Baseline medical evaluation between 1971 and 1994 with average follow up period of 10 years | Average estimated maximal METs | Moderate and high levels of PF were associated with lower risk of stroke mortality in men. |
| • Sex: Men | • T1 = 8.5 MET | ||||
| • Age: 40-87 yrs | • T2 = 10.5 MET | ||||
| USA | • Aerobics Center Longitudinal Study | • T3 = 13.1 MET | |||
| Prospective cohort | PF assessment: Maximal exercise tolerance test, divided into tertiles | RR (95% CI) adjusted for age and exam year | |||
| • T1 = 1.00 (referent) | |||||
| D & B score = 13 | T1 = Low | • T2 = 0.35 (0.16-0.77) | |||
| T2 = Moderate | • T3 = 0.28 (0.11-0.71) | ||||
| T3 = High | Trend p = 0.005 | ||||
| Cox proportional HR | |||||
| Hu et al 2000 [72] | To examine the association between PA and risk of total stroke and stroke sub- types in women. | • n = 72,488 | Baseline measurement in 1986 with follow-up questionnaire in 1988 and 1992 | • 407 cases of stroke (258 ischemic strokes, 67 subarachnoid hemorrhages, 42 intracerebral hemorrhages, and 40 strokes of unknown type) | PA, including moderate-intensity exercise such as walking, is associated with a substantial reduction in risk of total and ischemic stroke in a dose- response manner. |
| • Sex: Women | |||||
| • Age:40-65 yr | |||||
| USA | • Characteristics: Nurses | ||||
| Prospective cohort | • Nurses' Health Study | PA assessment: Questionnaire for total PA (MET h/wk), divided into quintiles, walking activity (MET h/wk), divided into quintiles and walking pace | Multivariate RR (95% CI) for total stroke by total PA level | ||
| • Q1 = 1.00 (referent) | |||||
| D & B score = 13 | • Q2 = 0.98 | ||||
| • Q3 = 0.82 | |||||
| • Q4 = 0.74 | |||||
| • Q5 = 0.66 | |||||
| Total PA (MET h/wk) | |||||
| p = 0.005 | |||||
| Q1 = 0 - 2.0 | |||||
| Q2 = 2.1 - 4.6 | |||||
| Multivariate RR (95% CI) for ischemic Stroke by total PA level | |||||
| Q3 = 4.7 - 10.4 | |||||
| Q4 = 10.5-21.7 | |||||
| • Q1 = 1.00 (referent) | |||||
| Q5 = > 21.7 | |||||
| • Q2 = 0.87 | |||||
| Walking activity (MET h/wk) | • Q3 = 0.83 | ||||
| Q1 = 0.5 | • Q4 = 0.76 | ||||
| Q2 = 0.6 - 2.0 | • Q5 = 0.52 | ||||
| Q3 = 2.1 - 3.8 | p = 0.003 | ||||
| Q4 = 3.9 - 10 | |||||
| Q5 = 10 | Multivariate RR (95% CI) for total stroke by walking activity | ||||
| Walking pace (mph) | • Q1 = 1.00 (referent) | ||||
| G1 < 2.0 | • Q2 = 0.76 | ||||
| G2 = 2-2.9 | • Q3 = 0.78 | ||||
| G3 3.0 | • Q4 = 0.70 | ||||
| • Q5 = 0.66 | |||||
| Outcome measure: Stroke incidence | p = 0.01 | ||||
| Multivariate RR (95% CI) for ischemic stroke by walking activity | |||||
| Pooled logistic regression | |||||
| Cox proportional HR | • Q1 = 1.00 (referent) | ||||
| • Q2 = 0.77 | |||||
| • Q3 = 0.75 | |||||
| • Q4 = 0.69 | |||||
| • Q5 = 0.60 | |||||
| p = 0.02 | |||||
| Multivariate RR (95% CI) for total stroke by usual Walking Pace | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.81 | |||||
| • G3 = 0.49 | |||||
| p < 0.001 | |||||
| Multivariate RR (95% CI) for ischemic stroke by usual walking pace | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.71 | |||||
| • G3 = 0.47 | |||||
| p < 0.001 | |||||
| Lee et al 1999 [74] | To examine the association between exercise and stroke risk. | • n = 21,823 | 11.1 year follow up | Number of Cases: 533 | VPA is associated with a decreased risk of stroke in men. |
| • Sex: Men | |||||
| • Age: 40-84 yr | PA assessment: Questionnaire for frequency of VPA, divided into 4 groups | Multivariate RR1 (95% CI) for total stroke by VPA | |||
| USA | |||||
| • G1 = 1.00 (referent) | |||||
| Prospective cohort | • G2 = 0.79 (0.61-1.03) | Inverse association with PA seemed to be mediated through beneficial effects on body weight, BP, cholesterol and glucose tolerance. | |||
| G1 < 1 time/week | • G3 = 0.80 (0.65-0.99) | ||||
| G2 = 1 time/week | • G4 = 0.79 (0.61-1.03) | ||||
| D & B score = 13 | G3 = 2-4 times/week | p = 0.04 | |||
| G4 ≥ 5 times/week | RR2 (95% CI) for total stroke by VPA | ||||
| • G1 = 1.00 (referent) | |||||
| RR1 = adjusted for smoking, alcohol consumption, history of angina and parental history of MI at <60 years | • G2 = 0.81 (0.61-1.07) | ||||
| • G3 = 0.88 (0.70-1.10) | |||||
| • G4 = 0.86 (0.65-1.13) | |||||
| p = 0.25 | |||||
| RR2 (95% CI) for ischemic stroke by | |||||
| RR2 = adjusted for all of the above plus, BMI, history of, hypertension, high cholesterol and diabetes | |||||
| VPA | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.90 (0.66-1.22) | |||||
| • G3 = 0.95 (0.74-1.22) | |||||
| • G4 = 0.97 (0.71-1.32) | |||||
| Outcome Measure: Total Stroke (Ischemic and Hemorrhagic) | p = 0.81 | ||||
| RR2 (95% CI) for hemorrhagic stroke by VPA | |||||
| Cox proportional HR | • G1 = 1.00 (referent) | ||||
| • G2 = 0.54 (0.25-1.13) | |||||
| • G3 = 0.71 (0.41-1.23) | |||||
| • G4 = 0.54 (0.26-1.15) | |||||
| p = 0.10 | |||||
| Bijnen et al 1998 [166] | To describe the association between the PA patterns of elderly men and stroke mortality. | • n = 802 | 10 year follow up | Number of Cases: 47 | No significant finding |
| • Sex: Men | |||||
| • Age:64-84 yr | PA assessment: | Multivariate adjusted RR (95% CI) | |||
| Denmark | • Characteristics: Not all free from previous stroke | Questionnaire for LTPA, divided into tertiles | • T1= 1. 00 (referent) | ||
| • T2 = 0.65 (0.33-1.25) | |||||
| Prospective cohort | T1 = Lowest | • T3 = 0.55 (0.24-1.26) | |||
| T2 | p = 0.12 | ||||
| T3 = Highest | |||||
| D & B score = 15 | |||||
| Outcome Measure: Stroke Mortality | |||||
| Cox proportional HR | |||||
| Schnohr et al 2006 [214] | To describe the association between different levels of LTPA and subsequent causes of death (stroke). | • n = 2136 men, 2,758 women | 5 year follow up | RR (95% CI), univariate | Although RR for of death from stroke was below 1 for both moderate and high compared with low PA, this association did not reach the level of statistical significance. |
| • G1 = 1.00 (referent) | |||||
| • Sex: Men and women | PA assessment: | • G2 = 0.64 (0.39-1.05) | |||
| Copenhagen | • Age: 20 -- 79 yr | Questionnaire for LTPA, | • G3 = 0.70 (0.41-1.21) | ||
| • Characteristics: Healthy, PA level did not change between 2 examinations, 5 years apart | divided into 3 groups | Trend p = 0.4 | |||
| Prospective cohort | G1 = Low PA (<4 METS) | ||||
| G2 = Moderate PA (4-6 | RR (95% CI), multivariate: | ||||
| METS) | • G1 = 1.00 (referent) | ||||
| D & B score = 13 | G3 = High PA (>6 METS) | • G2 = 0.67 (0.40-1.12) | |||
| • Copenhagen City Heart Study | • G3 = 0.76 (0.43-1.34) | ||||
| Multivariate Analysis Kaplan-Meier Plots | Trend p = 0.6 | ||||
| Linear, Logistical and Cox Regression. | |||||
| Vatten et al 2006 [253] | To investigate whether obesity- related CV mortality could be modified by PA. | • n = 26,515 men, 27,769 women | 16 year follow up | Number of Cases: 994 women, 771 men | Lower levels of TPA are associated with an increased risk of stroke. |
| • Sex: Men and women | PA assessment: Questionnaire for total amount of PA, divided into 4 groups | ||||
| Norway | • Age: 20 yr | Multivariate HR (95% CI), men | |||
| • Characteristics: Free from CVD at baseline | • Q1 = 1.00 (referent) | ||||
| Prospective cohort | • Q2 = 1.05 (0.85-1.30) | ||||
| • HUNT study | G1 = High | • Q3 = 1.21 (0.95-1.54) | |||
| G2 = medium | • Q4 = 1.35 (1.05-1.74) | ||||
| D & B score = 14 | G3 = low | p = 0.009 | |||
| G4 = never | |||||
| Multivariate HR (95% CI), women | |||||
| Outcome Measure: Stroke mortality | • Q1 = 1.00 (referent) | ||||
| • Q2 = 1.16 (0.93-1.45) | |||||
| • Q3 = 1.45 (1.14-1.86) | |||||
| Cox proportional HR | |||||
| • Q4 = 1.45 (1.14-1.83) | |||||
| p < 0.001 | |||||
| Agnarsson et al 1999 [255] | To examine the association of LTPA and pulmonary function with the risk of stroke. | • n = 4,484 | Length of Follow-up: 10.6 ± 3.6 years | Number of Cases: 249 | Apparent protective effect of regular continued LTPA in middle age men on the risk of ischemic stroke. |
| • Sex: Men | |||||
| • Age: 45-80 | Adjusted for age and smoking RR (95% CI) for total stroke by LTPA level | ||||
| Iceland | • Characteristics: no history of Stroke | PA assessment: Questionnaire for LTPA (h/wk) and type of activity (intensity), each divided into 3 groups | |||
| Prospective cohort | • Reykjavik Study | • G1 = 1.00 (referent) | |||
| • G2 = 0.84 (0.63-1.13) | |||||
| • G3 = 0.73 (0.40-1.35) | |||||
| D & B score = 13 | LTPA summer/winter | ||||
| G1 = none | Adjusted for age and smoking RR (95% CI) for ischemic stroke by LTPA level | ||||
| G2 = ≤ 5 h/wk | |||||
| G3 = ≥ 6 h/wk | |||||
| • G1 = 1.00 (referent) | |||||
| Type of Activity | • G2 = 0.72 (0.51-1.01) | ||||
| G1 = none | |||||
| • G3 = 0.78 (0.41-1.48) | |||||
| G2 = low intensity | |||||
| G3 = high Intensity | |||||
| RR (95% CI) for total stroke by type of activity | |||||
| Outcome Measure: Total and ischemic Stroke | • G1 = 1.0,0 (referent) | ||||
| • G2 = 0.75 (0.53-1.08) | |||||
| • G3 = 1.10 (0.78-1.57) | |||||
| Cox proportional HR | |||||
| RR (95% CI) for ischemic stroke by type of activity | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.72 (0.44-1.07) | |||||
| • G3 = 0.96 (0.64-1.44) | |||||
| Ellekjaer et al 2000 [256] | To examine the association between different levels of LTPA and stroke mortality in middle-aged and elderly women. | • n = 14,101 | Baseline 1984-1986: 2 self administered questionnaires and clinical measurements included in the screening program. | Number of cases: 457 | This study demonstrates a consistent, negative association between PA and stroke mortality in women. |
| • Sex: Women | |||||
| • Age: 50 yr | Multivariate RR (95% CI), all age groups | ||||
| Norway | • Characteristics: free from stroke at baseline | ||||
| • G1 = 1.00 (referent) | |||||
| Prospective cohort | • G2 = 0.77 | ||||
| PA assessment: Questionnaire for LTPA, divided into 3 groups | • G3 = 0.52 | ||||
| D & B score = 14 | Multivariate RR (95% CI), age 50--69 years | ||||
| G1 = low | The most active women had approx. 50% lower risk of death from stroke compare to inactive women. | ||||
| G2 = medium | • G1 = 1.00 (referent) | ||||
| G3 = high | • G2 = 0.57 | ||||
| • G3 = 0.42 | |||||
| Outcome Measure: Death from stroke | p = 0.0021 | ||||
| Multivariate RR (95% CI), age 70-79 years | |||||
| Cox proportional HR | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.79 | |||||
| • G3 = 0.56 | |||||
| p = 0.0093 | |||||
| Multivariate RR (95% CI), age 80-101 years | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.91 | |||||
| • G3 = 0.57 | |||||
| p = 0.1089 | |||||
| Evenson et al 1999 [257] | To examine the relationship between PA and ischemic stroke risk. | • n = 14,575 | 7.2 year follow up | Number of Cases: 189 | PA was weakly associated with a reduced risk of ischemic stroke among middle aged adults. |
| • Sex: Men and women | Number of Dropouts: 0% | ||||
| • Age: 45-64 yr | PA assessment: Questionnaire (Baecke questionnaire) | ||||
| USA | • Atherosclerosis Risk in Communities Study | Sport, Incidence of Ischemic Stroke | |||
| Prospective cohort | Multivariate adjusted RR (95% CI) by sport | ||||
| Outcome Measure: | |||||
| Ischemic Stroke | • Q1 = 1.00 (referent) | ||||
| D & B score = 14 | • Q3= 0.83 (0.52-1.32) | ||||
| Multivariate Poisson and Cox proportional HR | |||||
| Multivariate adjusted RR (95% CI) by LTPA | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = | |||||
| • Q3 = 0.89 (0.57-1.37) | |||||
| Multivariate adjusted RR (95% CI) by OPA | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = | |||||
| • Q3 = 0.69 (0.47-1.00) | |||||
| Haheim et al 1993 [258] | To determine the risk factors of stroke incidence and mortality. | • n = 14,403 | Baseline Screening from May 1972- December 1973. | HR (95% CI) for stroke incidence | Increased LTPA is associated with a reduced risk of stroke incidence but not mortality. |
| • Sex: Men | • G1 = 1.00 (referent) | ||||
| • Age: 40-49 yr | • G2 = 0.64 (0.38-1.08) | ||||
| Norway | PA assessment: Questionnaire for LTPA, divided into groups | • G3 = 0.36 (0.15-0.80) | |||
| Prospective cohort | HR (95% CI) for stroke mortality | ||||
| G1 = Sedentary | • G1 = 1.00, (referent) | ||||
| G2 = Moderate | • G2 = 0.82 (0.33-2.35) | ||||
| D & B score = 14 | G3 = Intermediate or Great | • G3 = 0.29 (0.03-1.51) | |||
| Outcome Measure: Incidence of stroke morbidity and mortality until study end date, December 31, 1984. | |||||
| Cox proportional HR | |||||
| Hu et al 2005 [259] | To assess the relationship of different types of PA with total and type-specific stroke risk. | • n = 47,721 | PA assessement: Mailed questionnaire for LTPA, OPA and commuting PA, divided into groups as follows: | RR (95% CI) by LTPA, men | A high level of LTPA reduces the risk of all subtypes of stroke. Daily active commuting also reduces the risk of ischemic stroke. |
| • Sex: Men and women | • G1 = 1.00 (referent) | ||||
| • G2 = 0.83 | |||||
| Finland | • Age: 25-64 | • G3 = 0.72 | |||
| • Characteristics: Healthy at baseline | p < 0.001 | ||||
| Prospective cohort | |||||
| LTPA levels: | RR (95% CI) by LTPA, women | ||||
| G1 = Low | • G1 = 1.00 (referent) | ||||
| D & B score = 13 | G2 = Moderate | • G2 = 0.86 | |||
| G3 = High | • G3 = 0.75 | ||||
| p = 0.007 | |||||
| OPA: | |||||
| G1 = Light | RR (95% CI) by LTPA, men and women | ||||
| G2 = Moderate | |||||
| G3 = Hard | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.85 | |||||
| Commuting PA: | |||||
| G1 = Motorized or no work, | • G3 = 0.73 | ||||
| G2 = walking or cycling 1-29 min G3 = walking or cycling ≥ 30 min. | p <0.001 | ||||
| RR (95% CI) by OPA, men | |||||
| • Not significant | |||||
| Outcome Measure: Incidence of fatal or non-fatal stroke occurring during follow-up until end of 2003. Mean follow-up of 19 years. | |||||
| RR (95% CI) by OPA, women | |||||
| • Not significant | |||||
| RR (95% CI) by OPA, men and women | |||||
| • G1 = 1.00 (referent) | |||||
| Cox proportional hazard | • G2 = 0.90 | ||||
| • G3 = 0.87 | |||||
| p = 0.007 | |||||
| RR (95% CI) by commuting PA, men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.91 | |||||
| • G3 = 0.85 | |||||
| p = 0.047 | |||||
| RR (95% CI) by commuting PA, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.86 | |||||
| • G3 = 0.85 | |||||
| p = 0.018 | |||||
| RR (95% CI) by commuting PA, men and women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.89 | |||||
| • G3 = 0.85 | |||||
| p = 0.002 | |||||
| Kiely et al 1994 [260] | To examine the influence of increased PA on stroke risk in members of the Framingham study cohort. | • n = 1,897 men 2,299 women | Baseline measurement in 1954-1955 and follow up in either 1968-1969 or 1971- 1972 | Multivariate adjusted RR (95% CI) at first examination, men (mean age 50 years) | Medium and high levels of PA among men are protective against stroke relative to low levels. |
| • Sex: Men and women | |||||
| USA | • G1 = 1.00 (referent) | ||||
| • Age: 28-62 yr | • G2 = 0.90 (0.62-1.31) p = 0.59 | ||||
| Prospective cohort | • Characteristics: Free from stroke | PA assessment: Questionnaire for metabolic work done during a typical 24 hr period, divided into 3 groups | • G3 = 0.84 (0.59-1.18) p = 0.31 | ||
| Multivariate adjusted RR (95% CI) at first examination, women (mean age 50 years) | Protective effect of PA was slightly less for high levels of PA compared to medium levels for older men. | ||||
| D & B score = 12 | |||||
| G1 = Low | • G1 = 1.00 (referent) | ||||
| G2 = Medium | • G2 = 1.21 (0.89-1.63) p = 0.23 | ||||
| G3 = High | • G3 = 0.89 (0.60-1.31) p = 0.54 | ||||
| Outcome Measure: Incidence of stroke, as defined by the first occurrence of atherothrombotic brain infarctions, cerebral embolism or other type of stroke, during 32 years of follow-up. | |||||
| Multivariate adjusted RR (95% CI) at second examination, men (mean age 63 years) | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.41 (0.24-0.89) p = 0.0007 | |||||
| • G3 = 0.53 (0.34-0.84) p = 0.007 | |||||
| Multivariate adjusted RR (95% CI) at second examination, women (mean age 64 years) | |||||
| Cox proportional HR | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.97 (0.64-1.47) p = 0.67 | |||||
| • G3 = 1.21 (0.75-1.96) p = 0.43 | |||||
| Krarup et al 2007 [261] | To compare the reported level of PA performed during the week preceding an ischemic stroke with that of community controls. | • n = 127 cases 301 controls | PA assessment: | Univariate OR (95% CI) | Stroke patients are less physically active in the week preceding an ischemic stroke when compared to age and sex-matched controls. Increasing PASE score was inversly, log-linearly and significantly associated with OR for ischemic stroke. |
| Questionnaire about PA 1 week prior to stroke (cases) and 1 week prior to questionnaire (controls), divided into PASE scores and quartiles | PASE Score | ||||
| • Sex: Men and women | • Q1 = 1.00 (referent) | ||||
| Denmark | • Q2 = 0.51 (0.28-0.95) | ||||
| • Age: ≥ 40 yr | • Q3 = 0.27 (0.14-0.54) | ||||
| Case control | • Characteristics: Case: Stroke Patients (20% had history of Stroke), Controls: 4% had history of stroke | • Q4 = 0.08 (0.03-0.20) | |||
| D & B score = 14 | Q1 = 0-49 | Multivariate OR (95% CI) PASE Score | |||
| Q2 = 50-99 | |||||
| Q3 = 100-149 | • Q1 = 1.00 (referent) | ||||
| Q4 = 150+ | • Q2 = 0.53 (0.26-1.08) | ||||
| • Q3 = 0.27 (0.12-0.59) | |||||
| Outcome measure: | |||||
| Ischemic stroke | • Q4 = 0.09 (0.03-0.25) | ||||
| Chi squared Kruskal-Wallis Statistics Multivariate conditional logistic regression | |||||
| Kurl et al 2003 [262] | To examine the relationship of PF with subsequent incidence of stroke. Also to compare PF with conventional risk factors as a predictor for future stroke. | • n = 2,011 | Baseline examinations conducted between March 1984 and December 1989 with average follow up period of 11 years | Multivariate HR (95% CI), any stroke | Low PF was associated with an increased risk of any stroke and ischemic stroke. |
| • Sex: Men | • Q1 = 1.00 (referent) | ||||
| • Age: 42, 48, 54 or 60 yrs | • Q2 = 1.39 (0.70-2.77) | ||||
| Finland | • Q3 = 1.32 (0.66-2.65) | ||||
| • Characteristics: Free from stroke or pulmonary disease • Kuopio Ischaemic Heart Disease Risk Factor Study |
• Q4 = 2.30 (1.18-4.06) | ||||
| Prospective cohort | Trend p = 0.01 | ||||
| PF assessment: Maximal exercise test on cycle ergometer. VO2 max (ml/kg/min) divided into quartiles | |||||
| Multivariate HR (95% CI), ischemic stroke | |||||
| D & B score = 14 | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 1.28 (0.56-2.94) | |||||
| • Q3 = 1.64 (0.74-3.65) | |||||
| Q1 = >35.3 | |||||
| • Q4 = 2.40 (1.09-5.25) | |||||
| Q2 = 30.3-35.3 | |||||
| Trend p = 0.01 | |||||
| Q3 = 25.2-30.2 | |||||
| Q4 = <25.2 | |||||
| Outcome Measure: Stroke incidence | |||||
| Cox proportional HR | |||||
| Myint et al 2006 [263] | To examine the association between a combination of OPA and LTPA with risk of subsequent stroke. | • n = 22,602 | Baseline measurement in | Model A: Used all 4 categories of PA | Higher levels of PA assessed using a single simple pragmatic tool based on both OPA and LTPA is associated with reduced stroke risk. |
| • Sex: Men | 1993-1997 | HR (95% CI), men and women | |||
| • Age: 40-79 yr | • G1 = 1.00 (referent) | ||||
| UK | • Characteristics: Healthy at baseline | PA assessment: Questionnaire for PA (includes LTPA and OPA) divided into 4 groups | • G2 = 0.78 (0.61-1.00) | ||
| • G3 = 0.66 (0.49-0.91) | |||||
| Prospective cohort | • European Prospective Investigation in Cancer-Norfolk | • G4 = 0.70 (0.49-0.99) | |||
| p = 0.024 | |||||
| D & B score = 11 | G1 = Inactive | HR (95% CI), men | |||
| G2 = moderately inactive | • G1 = 1.00 (referent) | ||||
| G3 = moderately active | • G2 = 0.75 (0.52-1.09) | ||||
| G4 = active | |||||
| • G3 = 0.55 (0.35-0.86) | |||||
| • G4 = 0.67 (0.43-1.05) | |||||
| Outcome Measure: Incidence of fatal and non fatal stroke. | |||||
| p = 0.41 | |||||
| Women not significant p = 0.50 | |||||
| Cox proportional HR | |||||
| Model B: Used 3 categories of PA (G3 and G4 combined combined) | |||||
| HR (95% CI), men and women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.78 (0.61-1.00) | |||||
| • G3 = 0.68 (0.52-0.88) | |||||
| p = 0.009 | |||||
| HR (95% CI), men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.75 (0.52-1.09), | |||||
| • G3 = 0.61 (0.43-0.86) | |||||
| p = 0.019 | |||||
| Women not significant p = 0.34 | |||||
| Noda et al 2005 [264] | To examine the impact of exercise on CVD (stroke) mortality in Asian populations. | • n = 31,023 men, 42,242 women | 9.7 year follow up | Number of Cases: 186 men, 141 women | PA through walking and sports participation may reduce the risk of mortality from ischemic stroke |
| • Sex: Men and women | PA assessment: Questionnaire for PA (walking and sports participation (h/day), divided into quartiles: | Number of Dropouts: 3.4% | |||
| Japan | • Age: 40 -79 yr | ||||
| • Ethnicity: Asian | Multivariate adjusted HR (95% CI) by duration of walking PA, men | ||||
| Prospective cohort | |||||
| • Q1 = 1.03 (0.63-1.69) | |||||
| Q1 = <0.5 | • Q2 = 1.00 (referent) | ||||
| D & B score = 13 | Q2 = 0.5 | • Q3 = 0.56 (0.35-0.91) | |||
| Q3 = 0.6-0.9 | • Q4 = 0.71 (0.49-1.02) | ||||
| Q4 = >1.0 | |||||
| Multivariate adjusted HR (95% CI) by duration of walking PA, women | |||||
| Outcome Measure: Death from ischemic stroke | |||||
| • Q1 = 1.38 (0.82-2.33) | |||||
| • Q2 = 1.00 (referent) | |||||
| Cox proportional HR | |||||
| • Q3 = 0.56 (0.32-0.97) | |||||
| • Q4 = 0.73 (0.48-1.13) | |||||
| Multivariate adjusted HR (95% CI) by sport PA, men | |||||
| • Q1 = 1.34 (0.86-2.08) | |||||
| • Q2 = 1.00 (referent) | |||||
| • Q3 = 1.22 (0.66-2.25) | |||||
| • Q4 = 0.84 (0.45-1.57) | |||||
| Multivariate adjusted HR (95% CI) by sport PA, women | |||||
| • Q1 = 1.07 (0.64-1.77) | |||||
| • Q2 = 1.00 (referent) | |||||
| • Q3 = 0.62 (0.25-1.58) | |||||
| • Q4 = 0.73 (0.31-1.70) | |||||
| Paganini-Hill and Barreto 2001 [265] | To identify risk factors and preventative measures for stroke in elderly men and women. | • n = 4,722 men, 8,532 women | Baseline survey in 1981- 1982. | Multivariate adjusted RR (95% CI) for total hemorrhagic occlusion by exercise, men | Emphasized role of lifestyle modification in the primary prevention of stroke. |
| • Sex: Men and women | |||||
| PA assessment: Questionnaire on amount of hours per day of exercise | • Q1 = 1.00 (referent) | ||||
| USA | Age: 44-101 yr | • Q2 = 0.88 | |||
| • Characteristics: no previous history of stroke. Residence of a retirement community in Southern California | Q3 = 0.83 | ||||
| Prospective cohort | G1 = <0.5 | ||||
| G2 = <0.1 | Multivariate adjusted RR (95% CI) for total hemorrhagic occlusion by exercise, women | ||||
| G3 = 1+ | |||||
| D & B score = 13 | |||||
| Outcome Measure: Incidence of hemorrhagic occlusion strokes up until December 31, 1998. | • Q1 = 1.00 (referent) | ||||
| • Q2 = 0.91 | |||||
| • Q3 = 0.85 | |||||
| Poisson Regression 40 year follow up | |||||
| Pitsavos et al 2004 [266] | To investigate the interaction between PA in men with LVH on stroke mortality. | • n = 489 | Number of cases: 67 | PA reduced the risk of stroke in men without LVH. | |
| • Sex: Men | |||||
| PA assessment: Questionnaire | RR (95% CI) | ||||
| USA | • Age: 40-59 yr | • G1 = 1.00 (referent) | |||
| • Characteristics: Those without LVH | G1 = Sedentary | • G2 = 0.64 (0.45-0.91) | |||
| Prospective cohort | G2 = Moderate | • G3 = 0.72 (0.51-1.02) | |||
| • Corfu Cohort (Greece) from Seven Countries Study | G3 = Hard | ||||
| D & B score = 12 | Outcome Measure: Stroke mortality | ||||
| Cox proportional HR | |||||
| Sacco et al 1998 [267] | To investigate the association between LTPA and ischemic stroke. | • n = 369 case, 678 control | Case Subjects were recruited during hospitalization, self referral or from monitoring non hospitalized stroke. Controls were eligible if they had never been diagnosed with stroke and were >39 years. | LTPA was related to a decreased occurrence of ischemic stroke in elderly, multiethnic, urban subjects. | |
| • Sex: Men and women | O R (95% CI) for duration of LTPA and stroke | ||||
| USA | |||||
| • Age: > 39 yr | • G1 = 1.00 (referent) | ||||
| Case control | • Characteristics: Case Subjects: Diagnosed with first cerebral infarction after July 1, 1993. Control Subjects: Never diagnosed with stroke | • G2 = 0.42 | |||
| • G3 = 0.35 | |||||
| D & B score = 14 | • G4 = 0.31 | ||||
| PA assessment: | |||||
| Questionnaire | |||||
| Divided into duration of LTPA (h/wk) | |||||
| • Northern Manhattan Stroke Study | |||||
| G1 = 0 | |||||
| G2 = <2 | |||||
| G3 = 2-<5 | |||||
| G4 = ≥ 5 | |||||
| Multivariate conditional logistic regression Baseline data collection from 1982-1983 in East Boston (MA), New Haven (CT) and Iowa and Washington counties (IA). | |||||
| Simonsick et al 1993 [268] | To examine the association between recreational PA among physically capable older adults and incidence of selected chronic diseases and mortality over 3 and 6 years. | • n = 1,815 | After 3 years Iowa | No consistent relationship between PA and stroke was found after 3 or 6 years across all 3 population cohorts. | |
| • Sex: Men and women | |||||
| • Age: ≥ 65 yrs | OR (95% CI) Stroke and activity level | ||||
| USA | • Characteristics: Physically capable to do heavy work around the house, walk up and down a flight of stairs and walk a half mile without help. | • T1 = 0.22 (0.08-0.61) | |||
| • T2 = 1.05 (0.60-1.84) | |||||
| Prospective cohort | • T3 = 1.00 (Referent) | ||||
| PA assessment: Questionnaire | |||||
| New Haven | |||||
| D & B score = 12 | T1 = High | OR (95% CI) Stroke and activity level | |||
| T2 = Moderate and | • T1 = 1.06 (0.38-2.95) | ||||
| T3 = Inactive | • T2 = 1.26 (0.54-2.92) | ||||
| • Established Populations for Epidemiologic Studies of the Elderly | • T3 = 1.00 (Referent) | ||||
| Outcome Measure: Stroke incidence during 3 and 6 year follow-ups. | |||||
| East Boston | |||||
| OR (95% CI) Stroke and activity level | |||||
| • T1 = 0.59 (0.17-1.95) | |||||
| Logistic Regression | |||||
| • T2 = 1.08 (0.52-2.27) | |||||
| • T3 = 1.00 (Referent) | |||||
| After 6 years | |||||
| Iowa | |||||
| OR (95% CI) Stroke and activity level | |||||
| • T1 = 0.56 (0.31-1.00) | |||||
| • T2 = 0.97 (0.64-1.48) | |||||
| • T3 = 1.00 (Referent) | |||||
| New Haven | |||||
| OR (95% CI) Stroke and activity level | |||||
| • T1 = 1.05 (0.52-2.12) | |||||
| • T2 = 1.29 (0.72-2.32) | |||||
| • T3 = 1.00 (Referent) | |||||
| East Boston | |||||
| OR (95% CI) Stroke and activity level | |||||
| • T1 = 1.21 (0.56-2.61) | |||||
| • T2 = 1.73 (0.98-3.06) | |||||
| • T3 = 1.00 (Referent) | |||||
| Thrift et al 2002 [269] | To examine whether intracerebral hemorrhage is associated with dynamic or static exercise. | • n = 662 | PA assessment: Interview, divided into 3 groups: frequency of vigorous activity | Number of Cases: 331 | Findings not significant after multivariate analysis. |
| • Sex: Men and women | |||||
| • Age: 18-80 yr | Multivariate OR (95% CI) by frequency of VPA | ||||
| Australia | • Characteristics: Cases: first episode ofintracerebral hemorrhage Controls: Neighbours of cases | ||||
| G1 = Never | • G1 = 1.00 (referent) | ||||
| Case control | G2 = Rarely | • G2 = 0.68 (0.36-1.27) | |||
| G3 = Once or more per month | • G3 = 0.66 (0.39-1.11) | ||||
| D & B score = 14 | p = 0.094 | ||||
| OPA level | Multivariate OR (95% CI) by OPA level | ||||
| G1 = Sedentary | • G1 = 1.00 (referent) | ||||
| G2 = Light to moderate | • G2 = 0.94 (0.59-1.48), p = 0.773 | ||||
| G3 = Heavy | • G3 = 1.18 (0.57-2.46), p = 0.650 | ||||
| Outcome Measure: Intracerebral hemorrhage | |||||
| Multiple logistic regression | |||||
D & B score, Downs and Black quality score; YR, years; wk, week; CVD, cardiovascular disease; G, groups; PA, physical activity; CHD, coronary heart disease; RR, risk ratio; 95% CI, 95% confidence interval; T, tertile; PF, physical fitness; MET, metabolic equivalent; Q, quartile or quintile; OPA, occupational physical activity; LTPA, leisure-time physical activity; HR, hazard ratio; VPA, vigorous physical activity; LVH, left ventricular hypertrophy.
The data providing dose-response information is all observational in nature, involving both case control and cohort investigations. These studies (predominantly prospective cohort designs) included a total of 479,336 participants; averaging 17,753 subjects per study (range 428-73,265). There were a total of 12,361 reported cases of stroke (ranging per study from 32-2,863). The total length of study follow-up for the prospective cohort studies averaged 13.2 yr (ranging from 6-26 yr). The articles were published over a 14 yr period ranging from 1993 to 2007. These studies involved large samples of men and women from regions throughout the world including studies from the USA (11), UK (2), Iceland (1), Denmark (2), Norway (4), Netherlands (1), Finland (2), Japan (1), Australia (1) and Greece (1). Very few studies [69,70] examined non-Caucasian participants.
We found strong evidence that physical activity was associated with a reduced risk for stroke. The level of evidence was consistent with a Level 3A classification. We observed an average risk reduction of 31% across all studies (median = 29%). In comparison to cardiovascular disease, there was more variability in the risk reductions in stroke in the highest activity/fitness group. The quality of the investigations was also generally quite good with a mean (and median) Downs and Black score of 13 (range 11-15).
The risk reductions appear to be even greater in studies that assessed physical fitness directly. For instance, in data from the Aerobics Center Longitudinal Study [71] the high fitness group (estimated peak METs = 13.1) and the moderate fitness group (estimated peak METs 10.5) had significantly lower risks of stroke mortality (68 and 63%, respectively) than the least fit men (estimated peak METs 8.5).
A dose-response relationship did emerge when examining the literature. However, as illustrated by others this was extremely variable amongst studies and varied according to the type of stroke (ischemic or haemorrhagic) [52]. For instance, 12 studies (46%) revealed a dose-response relationship in one or more measures of occupational and/or leisure-time physical activity and the risk for stroke. It is difficult to determine the minimal and optimal physical activity dosage for the prevention of stroke. Brisk walking has been associated with a lower risk of total and ischemic stroke [72]. In the Harvard Alumni study, the risk of stroke was lower at a weekly energy expenditure of 4.2-8.4 MJ/wk (1000-1999 kcal/wk) (RR = 0.76 (95% CI, 0.59 to 0.98)). With expenditures of 8.4-12.6 MJ/wk (2000-2999 kcal/wk) the RR dropped to 0.54 (0.38 to 0. 76) [73]. Thus, the recommended daily expenditure of Canada's physical activity guidelines is sufficient to reduce the risk for stroke. Further research is required to clearly determine the risk reductions at exercise volumes less than 4.2 MJ/wk (1000 kcal/wk).
In summary, the results of these studies (taken as a whole) indicate that occupation- and leisure time-related physical activity are inversely related to the risk for stroke. Both physically active men and women have a lower risk of stroke, and it appears that this benefit may be present for both ischemic and haemorrhagic stroke [74]. The relationship between physical activity and stroke appears to be consistent between men and women. Unfortunately, relatively limited data exists in non-Caucasian populations.
Recommendation #3
For a reduced risk of stroke, it is recommended that individuals should participate in 30 min or more of moderate to vigorous exercise on most days of the week. Brisk walking appears to be protective against the development of stroke. It remains to be determined whether lower volumes of physical activity lead to a reduced risk for stroke. [Level 3, Grade A]
Primary Prevention of Hypertension
A total of 6287 citations were identified during the electronic database search (Figure 6). Of these citations, 4054 were identified in MEDLINE, 1360 in EMBASE, 253 in Cochrane, and 620 in the CINAHL/SportDiscus/PsychInfo search. A total of 40 duplicates were found, leaving a total of 6247 unique citations. A total of 6167 articles were excluded after scanning, leaving a total of 80 articles for full review. An additional five articles were found through cross-referencing and the reviewers' personal files. From these articles 72 were excluded after full review for the following reasons: weak design (n = 4), did not contain three levels of physical activity or not possible to determine dose-response relationship (n = 19), reviews, summaries, meta-analyses (n = 8), not dealing with hypertension (n = 2), only reported on changes in blood pressure (n = 27), clinical population (n = 7), and other (n = 6). Therefore, a total of 12 articles were included in the systematic review of the literature regarding the relationship between physical activity and the primary prevention of hypertension. The majority of the literature examining the dose-response (for at least three levels of physical activity/fitness) involved prospective cohort analyses (83%).
Figure 6.
Results of the Literature Search for Hypertension.
As shown in Table 14, 12 investigations examined the dose-response (i.e., three or more levels) relationship between physical activity and the incidence of hypertension. This involved a total of 112,636 participants, averaging 10,240 subjects per study (range 1,243-41,837). There were a total of 11,441 reported cases of hypertension (ranging per study from 118-2,936). The total length of study follow-up averaged 8.6 yr (ranging from 0-16 yr). The articles were published over a 24 yr period ranging from 1983 to 2007.
Table 14.
Studies examining the relationship between physical activity and hypertension.
| Publication Country Study Design Quality Score | Objective | Population | Methods | Outcome | Comments and Conclusions |
|---|---|---|---|---|---|
| Rankinen et al 2007 [75] | To investigate the contributions of DNA sequence variation in candidate genes, PF and BMI, as well as their interactions to the incidence of hypertension. | • n = 629 cases; 605 controls | 10 year follow up | PF showed the strongest association with HTN risk among all subjects as well as sex-specific models. Each 1- MET increment in PF was associated with 19% (12- 14%), 16% (9-22%), 32% (17- 45%) risk reduction in all subjects, men and women respectively. | PF is a significant predictor of the risk of hypertension. |
| USA | • Sex: Men and women | All subjects required to have 2 clinic visits at least 2 years apart. | |||
| Case control | • Age: Case: 43.3 (9.2) yr Control: 42.7 (8.9) yr | PF assessment: treadmill test (Blake protocol) | |||
| D & B score = 13 | • Characteristics: Healthy with BP 134/86 mmHg or less at their first clinic visit. Cases: those who developed hypertension during the follow-up period. Controls were those who did not develop hypertension | Outcome measure: Incidence of hypertension during follow-up. Incident cases of hypertension were defined as physician diagnosed hypertension with medication or SBP ≥ 140 mmHg and/of DBP ≥ 90 mmHg t-tests and chi-square tests Logistic regression modelling | When divided into quartiles on the basis of sex specific MET cut-offs, the third and fourth quartiles had a 58% (41-71%) and 63% (47-75%) lower risk of hypertension compared to the 1st quartile. | ||
| Pereira et al 1999 [76] | To examine PA and incident hypertension in men and women. | • n = 7,459 | PA Assessment: Questionnaire for leisure, sport and work index, divided into quartiles | White Men | There is an inverse association between PA and incident hypertension in White middle aged men. White men in the highest quartiles of sport and leisure activity had statistically significant reductions in the odds of developing hypertension of 23 and 34% respectively, compared to men in the lower quartiles. |
| USA | • Sex: Men and women | Q1 = Lowest | Leisure Index Model 1 | ||
| Prospective cohort | • Age: 45-65 yr | Q2 | • Q1 = 1.00 (referent) | ||
| D & B score = 12 | • Characteristics: No history of angina, MI, evidence of MI, angioplasty or other CV surgery or hypertension | Q3 | • Q2 = 0.95 (0.70-1.28) | ||
| • Atherosclerosis Risk in Communities Study | Q4 = Highest | • Q3 = 0.83 (0.63-1.09) | |||
| Model 1 adjusted for: Age, education, baseline BP and study centre | • Q4 = 0.64 (0.46-0.89) | ||||
| Model 2 adjusted for: Covariates in model 1 and smoking, alcohol consumption, parental history of hypertension, energy, sodium, potassium and caffeine intake, BMI, waist to hip ratio, menopausal status and hormone use | Trend p = 0.01 | ||||
| Outcome Measure: Incidence of hypertension as defined as a SBP 140 mmHg and/or a DBP 90 mmHg or use of antihypertensive medications. | Leisure Index Model 2 | ||||
| Unconditional logistic regression Orthogonal polynomial coefficients | • Q1 = 1.00 (referent) | ||||
| • Q2 = 0.99 (0.72-1.35) | |||||
| • Q3 = 0.86 (0.65-1.13) | |||||
| • Q4 = 0.66 (0.47-0.94) | |||||
| Trend p = 0.01 | |||||
| Women | |||||
| Sport Index Model 1 | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 1.26 (0.78-2.05) | |||||
| • Q3 = 1.06 (0.61-1.84) | |||||
| • Q4 = 1.92 (1.12-3.29) | |||||
| Trend p = 0.04 | |||||
| Men | |||||
| Sport Index Model 1 | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 1.23 (0.91-1.66) | |||||
| • Q3 = 0.92 (0.70-1.22) | |||||
| • Q4 = 0.74 (0.54-1.02) | |||||
| Trend p = 0.02 | |||||
| Sport Index Model 2 | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 1.26 (0.93-1.71) | |||||
| • Q3 = 0.95 (0.71-1.26) | |||||
| • Q4 = 0.77 (0.55-1.08) | |||||
| Trend p = 0.05 | |||||
| Haapanen et al 1997 [77] | To assess the association between PA and hypertension. | • n = 732 men; 796 women | 10 year follow up (1980 baseline) | Age adjusted incidence rates ofhypertension Total energy expenditure High as referent: | Increased EE during LTPA and increased intensity of these activities were associated with reduced risk for incident hypertension (age adjusted) in men but not women. |
| Finland | • Sex: Men and women | PA assessement: Questionnaire for EE (kcal/wk), divided into tertiles | |||
| Prospective cohort | • Age: 35-65 years | Male | Male: | ||
| D & B score = 11 | • Characteristics: Free of hypertension at baseline. Excluded those unable to participate in regular PA due to poor health | T1 = Low = 0-1100 | • T1 = 1.00 (referent) | ||
| T2 = Medium = 1101-1900 | • T2 = 1.66 | ||||
| T3 = High >1900 | • T3 = 1.73 | ||||
| Trend p = 0.021 | |||||
| Female | Female: | ||||
| T1 = Low = 0-900 | • T1 = 1.00 (referent) | ||||
| T2 = Medium = 901-1500 | • T2 = 0.94 | ||||
| T3 = High = >1500 | • T3 = 1.16 | ||||
| Outcome measure: Incidence of hypertension through self reported diagnosis and death certificates | Trend p = 0.648 | ||||
| Cox proportional HR | |||||
| Paffenbarger et al 1983 [78] | To examine the relationship of student and alumnus PA patterns and other characteristics with incident hypertension. | • n = 14,998 | PA Assessment: Questionnaire for PA based on number of stairs ascended, blocks walked and hours per week of light and vigorous sports play, yard work etc. | There was no significantly reduced risk for hypertension in men who climbed 50 plus stairs per day (compared to < 50 stairs); who walked 5 plus blocks per day (compared to < 5 blocks); or who played light sports (compared to those who did not). | Contemporary vigorous exercise was inversely related to hypertension risk. |
| USA | • Sex: Men | ||||
| Prospective cohort | • Age: 35-74 yr | Outcome measure: Diagnosis of hypertension by physicians using criteria of SBP > 160 mmHg and/or DBP > 95 mmHg | The 59% of men who did engage in vigorous sports were at 35% greater risk of hypertension than the 41% who did not. | ||
| RR = 1.35 | |||||
| Trend p = <0.001 | |||||
| D & B score = 12 | • Characteristics: free of hypertension Harvard Alumni Study | Multivariate estimates | Alumni on the low side of the physical activity index (< 2000 kcal/wk) had a 30% increased risk of hypertension then those ≥ 2000 kcal/wk. | ||
| RR = 1.30 | |||||
| Trend p = 0.004 | |||||
| Paffenbarger et al 1997 [79] | To investigate the quantity and intensity of energy expenditure required to delay hypertension and prevent premature death. | • n = 6,390 | PA Assessment: Questionnaire for weekly sports play, divided into tertiles | RR (95% CI) | Lack of vigorous sports play independently increased the risk of developing hypertension. |
| USA | • Sex: Men | T1 = None | • T1 = 1.00 (referent) | ||
| Prospective cohort | • Age: 45-84 yr | T2 = Light Only (< 4.5 METs) | • T2 = 1.04 (0.77-1.40) | ||
| D & B score = 12 | • Characteristics: Free of hypertension, CHD, diabetes, COPD and potentially malignant cancer in 1977 | T3 = Moderately vigorous (≥ 4.5 METs) | • T3 = 0.77 (0.62-0.96) | ||
| • Harvard Alumni Study | Outcome measure: Incident hypertension | Trend p = 0.004 | |||
| Hu et al 2004 [81] | To discover whether regular PA can reduce the risk of hypertension in normal weight and overweight men and women. | • n = 8,302 men; 9,139 women | 11 year follow up | Multivariate adjusted HR (95% CI), men | Regular PA can reduce the risk of hypertension. The protective effect of PA was observed in both sexes regardless of level of obesity. |
| Finland | • Sex: Men and women | PA assessement: Questionnaire for OPA, LTPA and commuting PA, divided into tertiles | • T1 = 1.00 (referent) | ||
| Prospective cohort | Age: 25-64 yr | T1 = Low | • T2 = 0.63 | ||
| D & B score = 13 | Characteristics: Healthy and free of hypertension at baseline | T2 = Medium | • T3 = 0.59 | ||
| T3 = High | Trend p = < 0.001 | ||||
| Outcome Measure: Incidence of drug treated hypertension | Multivariate adjusted HR (95% CI), women | ||||
| Cox proportional HR | • T1 = 1.00 | ||||
| • T2 = 0.82 | |||||
| • T3 = 0.71 | |||||
| Trend p = 0.005 | |||||
| Gu et al 2007 [82] | To determine the 8-year incidence of HTN and its risk factors among Chinese adults. | • n = 10,525 | Baseline Examination in 1991 with 8 year follow up | RR (95% CI), men | Increasing PA has the potential to reduce incidence of hypertension. |
| China | • Sex: Men and women | PA assessment: Questionnaire administered by trained staff, divided into groups | • G1 = 1.00 (referent) | ||
| Prospective cohort | Age: ≥ 40 yr | G1 = Low | • G2 = 1.12 (0.86-1.46) | ||
| D & B score = 13 | Characteristics: Healthy and free from hypertension at baseline. | G2 = Medium | • G3 = 1.27 (1.10-1.47) | ||
| G3 = High | RR (95% CI), women | ||||
| Outcome measure: HTN as defined at SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg or current use of antihypertensive medication | • G1 = 1.00 (referent) | ||||
| t-tests, chi squared tests, Cochran-Armitage modeling, Modified Poisson approach | • G2 = 1.14 (0.98-1.34) | ||||
| • G3 = 1.22 (1.02-1.45) | |||||
| Hayashi et al 1999 [83] | To investigate the association of the duration of the walk to work and LTPA with the risk for hypertension. | • n = 6,017 | PA assessment: Questionnaire on health related behaviours and exercise Walk time to work | RR (95% CI) Frequency walk time to work (minutes | The duration of walk to work was associated with a decreased risk of hypertension even after adjustment. |
| Japan | • Sex: Men | T1 = 0-10 min | • T1 = 1.00 (referent) | Regular PA (at least once weekly) was inversely related to the risk of incident hypertension | |
| Prospective cohort | • Age: 35-60 yr | T2 = 11-20 min | • T2 = 0.65 (0.47-0.90) | ||
| D & B score = 12 | • Characteristics: Free from HTN at baseline. All employees at gas company in Osaka Japan. All had sedentary jobs. | T3 = ≥ 21 min | • T3 = 0.72 (0.59-0.88) | ||
| Outcome measure: Diagnosed with hypertension (as defined by a SBP ≥ 160 mmHg, a DBP ≥ 95 mmHg, or use of antihypertensive medication) | Trend p = < 0.001 | ||||
| Cox proportional HR | |||||
| Nakanishi et al 2005 [84] | To examine the relationship of overall PA to the risk of developing hypertension in normotensive Japanese male office workers over a 7 year observation period. | • n = 2,548 | 7 year follow up | Multivariate adjusted RR (95% CI) by PA level only | The rate of rise in both SBP and DBP in each follow-up year decreased with higher EE and that the risk of developing hypertension decreased in a dose dependent manner with higher daily life activity level. |
| Japan | • Sex: Men | Q1 = 1.00 (referent) | Analysis stratified by the presence of or absence of a risk factor showed the negative association of daily life activity with the risk of developing hypertension for men at both low and high risk. This tendency was also observed among men in all 3 categories of normotension. | ||
| Prospective cohort | • Age: 35-59 yr | PA assessment: 1-day activity record and reported the type and frequency on a weekly basis of LTPA, divided into quartiles (kcal/kg/d) | Q2 = 0.84 (0.72-0.98) | ||
| D & B score = 12 | • Characteristics Healthy at baseline. No hypertension or CHD. All office workers for a Japanese company | • Q1 = <33.3 | • Q3 = 0.75 (0.63-0.88) | ||
| • Q2 = 33.3-36.9 | • Q4 = 0.54 (0.45-0.64) | ||||
| • Q3 = 37.0-40.3 | Trend p = < 0.001 | ||||
| • Q4 = 40.4 | Multivariate adjusted RR (95% CI) by PA level, low normal BP | ||||
| 3 categories of normotensive BP Low Normal: SBP < 120, DBP < 80 Normal: SBP 120-130, DBP 80- 85 High Normal: SBP 130-139 DBP 85-89 | • Q1 = 1.00 (referent) | ||||
| 3 categories of normotensive BP Low Normal: SBP < 120, DBP < 80 Normal: SBP 120-130, DBP 80- 85 High Normal: SBP 130-139 DBP 85-89 | • Q2 = 0.70 (0.47-1.05) | ||||
| Cox proportional hazard model | • Q3 = 0.55 (0.37-0.83) | ||||
| • Q4 = 0.43 (0.28-0.65) | |||||
| Trend p = <0.001 | |||||
| Multivariate adjusted RR (95% CI) by PA level, normal | |||||
| BP | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.89 (0.68-1.16) | |||||
| • Q3 = 0.69 (0.52-0.91) | |||||
| • Q4 = 0.50 (0.37-0.68) | |||||
| Trend p = <0.001 | |||||
| Multivariate adjusted RR (95% CI) by PA level, high normal BP | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.86 (0.69-1.07) | |||||
| • Q3 = 0.88 (0.69-1.11) | |||||
| • Q4 = 0.60 (0.46-0.78) | |||||
| Trend p = 0.001 | |||||
| Foy et al 2006 [85] | To examine whether insulin resistance is associated with the effect of vigorous or moderate PA on baseline BP. | • n = 1,599 | Baseline examination in 1992-1993 | Unadjusted OR (95% CI) | Participants who meet or exceed current caloric expenditure recommendations for VPA demonstrate significantly less hypertension than do sedentary or underactive individuals. |
| USA | • Sex: Men and women | PA assessment: VPA over the past year was determined via a 1-year recall of physical activity (kcal/d), divided into 3 groups | • T1 = 1.00 (referent) | ||
| Cross sectional | • Age: 40-69 yr | • T1 = O | • T2 = 0.69 (0.53-0.88) | ||
| D & B score = 12 | • Characteristics: Community dwelling adults | • T2 = 1-149 kcal/day | • T3 = 0.57 (0.45-0.74) | ||
| • Insulin Resistance Atherosclerosis Study | • T3 = >150 kcal/day | • Trend p = < 0.001 | |||
| Adjusted OR (95% CI) | |||||
| • T1 = 1.00 (referent) | |||||
| • T2 = 0.82 (0.62-1.09) | |||||
| • T3 = 0.73 (0.55-0.98) | |||||
| Trend p = 0.004 | |||||
| Folsom et al 1990 [270] | To examine the relationship between fat distribution and the 2-yr incidence of hypertension and stroke. | • n = 41,837 | Baseline mailed survey in 1986: Pa assessment: Questionnaire for LTPA | • 978 cases | High PA reduced the risk of hypertension only before adjusting for other factors. |
| USA | • Sex: Women | T1 = Low | Age Adjusted RR (95% CI) | ||
| Prospective cohort | • Age: 55-69 years (yr) | T2 = Medium | • T1 = 1.00 (referent) | ||
| D & B score = 12 | • Characteristics: All free of HTN at baseline | T3 = High | • T2 = 0.9 (0.7-1.1) | ||
| Mantel-Haenszel method | • T3 = 0.7 (0.6-0.9) | ||||
| Multiple logistic regression | |||||
| Levenstein et al 2001 [271] | To examine the effects of a variety of psychosocial factors on the development of HTN in men and women in the general population. | • n = 1,031 men, 1,326 women | Questionnaires in 1965 and 1974, cohort followed until 1994 | LTPA predictor of hypertension OR (95% CI) | Risk of HTN was reduced with increases in LTPA in women. |
| USA | • Sex: Men and women | PA assessment: LTPA rated on a scale of 0-16 points and analysed as a continuous variable | • All Subjects: 0.94 (0.91-0.97) | ||
| Prospective cohort | • Characteristics: Free of hypertension at baseline | Outcome measure: Incidence of hypertension (defined as those who are taking antihypertensive medications) | • Women: 0.90 (0.87-0.94) | ||
| D & B score = 13 | • Alameda cohort study | Logistic regression analysis | • Men: 0.98 (0.94-1.02) | ||
D & B score, Downs and Black quality score; YR, years; PF, physical fitness; BMI, body mass index; MET, metabolic equivalent; PA, physical activity; MI, myocardial infarction; G, groups; Q, quartile or quintile; 95% CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; EE, energy expenditure; kcal/wk, kilocalories per week; T, tertile; RR, risk ratio; HR, hazard ratio; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; OPA, occupational physical activity; LTPA, leisure-time physical activity; BP, blood pressure; kcal/day, kilocalories per day.
All studies reviewed demonstrated positive effects of physical activity on the risk for hypertension. Of these studies all (7; 58%) revealed an inverse and graded relationship between hypertension and at least one measure of physical activity or fitness. Across all studies, when comparing the most active/fit group versus the least active/fit group we found an average RR of 0.68 (median = 0.70, range 0.37 to 0.90). Therefore, we observed that physical activity/fitness was associated with an average risk reduction of 32% for hypertension. It should be noted that the study [75] demonstrating the largest risk reduction (63%) evaluated cardiorespiratory fitness directly during a maximal treadmill test. This supports research (as discussed previously) which indicates that physical fitness is a better predictor of chronic disease than physical activity [6,18,19,32,33]. Taken as a whole, the level of evidence can be classified as Level 3A. The quality of studies was generally good with a mean Downs and Black score of 11 (median = 11, range = 10-12).
Five studies showed variable results (i.e., no clearly defined dose-response) while generally supporting the inverse relationship between physical activity/fitness and hypertension [76-80]. The variability in the response appears to be the result of different activity/fitness classifications and/or differing subject populations. For instance, some studies revealed that the dose-response relationships differed between genders and/or ethnicities [76,77]. Pereira et al. [76] revealed a 30% reduction in the risk for hypertension in the most active white men. There were graded dose-response relationships between indices of both leisure and sport activities in the white men.
However, there was a lack of association between physical activity and hypertension in white women and African American men and women. Similarly, Haapenen et al. [77] revealed a stronger association in men than in women. However, it should be noted clearly that other studies included in this systematic review evaluated women demonstrating a graded response [81]. Moreover, several studies were conducted with non-Caucasian populations and demonstrated a dose-dependent benefit [82-85]. In fact, data was obtained from varied regions of the world including USA (7), Japan (2), China (1), and Finland (1). Therefore, there is evidence to suggest that the protective effects of physical activity with respect to hypertension are transferable to women and non-Caucasian populations. However, further research is clearly warranted that examines the relationship between physical activity and hypertension in persons of different ethnicities. Moreover, further research is needed to determine the effects of impact of socio-economic status on the observed relationships.
Some studies have indicated that vigorous activity is required to reduce the risk for hypertension. For instance, Paffenbarger [78] revealed that Harvard Alumni who did not engage in vigorous sports play were at a 35% higher risk for developing hypertension. However, there was no difference in the risk for hypertension in men who climbed >50 stairs per day, walked more than 5 city blocks daily, or engaged in light sports only. Similarly, the Paffenbarger and Lee [79] study revealed that moderately vigorous sports play was associated with a lower risk for hypertension, but physical activity (kcal/wk), walking distance (km/wk) and the amount of stairs climbed (floors/wk) were not significant predictors of the risk for hypertension. Collectively, this research group concluded that these findings highlighted the importance of the intensity of effort.
However, it should be noted that many of the studies in our systematic review observed the protective effect with moderate intensity physical activities. Findings from randomized controlled trials have also provided strong evidence that moderate intensity aerobic exercise is sufficient to reduce blood pressure and the risk for hypertension, particularly in at risk individuals [86,87]. The American College of Sports Medicine [88] recently advocated that to prevent hypertension, individuals should exercise on most, and preferably all, days of the week at a moderate intensity, for 30 min or more per day (continuous or accumulated). They also recommended supplementing endurance type activities with resistance exercise. This is supported by research indicating that moderate intensity resistance training can reduce blood pressure [89]. Collectively, this research and our current summary of the dose-response literature indicates that physical activity levels that are of a moderate to vigorous intensity are sufficient to lead to marked reductions in the risk for hypertension.
Implications
The impact of hypertension on North American society is enormous. In the US, 31% of non-institutionalized adults over the ages of 20 are currently thought to have hypertension [90]. In Canada, approximately 20% of adults report a diagnosis of hypertension including over 4 million Canadians [91-93]. It has been estimated that a 55 yr old Canadian with normal blood pressure has a greater than 90% chance of developing hypertension before the age of 80 yr [92]. The primary prevention of hypertension is of paramount importance to the attenuation of the risks and costs associated with hypertension and related comorbidities.
There is clear evidence that routine physical activity and/or increased physical fitness reduce greatly the risk for hypertension in both normotensive and hypertensive individuals [18,19]. Extensive research has been conducted in the area including numerous prospective trials and various randomized controlled trials. Numerous reviews of the literature (of epidemiological and randomized controlled trials) have supported an inverse relationship between physical activity/fitness and in the incidence of hypertension [20,87,89,94-102]. In a recent systematic review of the prospective literature, Katzmarzyk and Janssen (2004) calculated that physically inactive individuals were at a 30% higher risk for hypertension (RR = 1.30 (95% CI = 1.16-1.46)) with a population attributable risk of 13.8% in Canada [20]. Acute bouts of exercise have also been shown to lead to transient changes in blood pressure that are potentially of health benefit [98]. For instance, blood pressure is often reduced after a single exercise session for 12-22 hr [88,103].
It is clear that routine physical activity is effective in both the primary and secondary prevention of hypertension. However, the optimal dosage of physical activity/exercise remains somewhat unclear. Our review of the literature examined critically the relationship between multiple levels of physical activity/fitness and the incidence of hypertension (in individuals without diagnosed hypertension). As identified above this evidence was compelling supporting the protective effects of habitual physical activity in the primary prevention of hypertension.
Recommendation #4
For a reduced risk for hypertension, it is recommended that individuals should participate in 30 min or more of moderate to vigorous exercise on most days of the week. [Level 3, Grade A]
Primary Prevention of Colon and Breast Cancer
Colon Cancer
In our systematic search of the colon cancer literature, a total of 252 citations were identified during the electronic database search (Figure 7). Of these citations, 83 were identified in MEDLINE, 44 in EMBASE, 25 in Cochrane, and 100 in the CINAHL/SportDiscus/PsychInfo search. A total of 15 duplicates were found, leaving a total of 237 unique citations. A total of 164 articles were excluded after screening, leaving a total of 73 articles for full review. From these articles 47 were excluded after full-text review leaving 26 articles for inclusion, and an additional 7 articles were added from the authors' personal files. The reasons for exclusion included non-experimental/weak design (n = 8), reviews, summaries, meta-analyses (n = 13), editorial/comment (n = 3), not dealing specifically with colon cancer (n = 4), did not contain three levels of physical activity or not possible to determine dose-response relationship (n = 9), and other (n = 10). Therefore, a total of 33 articles were included in the systematic review of the literature regarding the relationship between physical activity and the primary prevention of colon cancer.
Figure 7.
Results of the Literature Search for Colon Cancer.
These studies involved a total of 1,433,103 participants; averaging 43,427 participants per study (range 142-413,044). There were a total of 17,959 reported cases of colon cancer (ranging per study from 93-1,993). The total length of study follow-up for the prospective cohort studies averaged 10.7 yr (ranging from 4-26 yr). The articles were published over a 23 yr period ranging from 1985 to 2008. These studies involved large samples of men and women from regions throughout the world.
A dose-dependency of this relationship was present in the majority of the studies. When comparing the most active/fit group versus the least active/fit group we found a mean risk reduction of 30% (median = 32%) across all studies. The most compelling literature was that which evaluated the relationship between moderate-to-vigorous leisure time physical activity. Based on the literature reviewed and the volume of activity assessed it would appear that Canada's guidelines for physical activity are sufficient to lower the risk for the development of colon cancer in asymptomatic adults. The level of evidence would be considered to be Level 2A. The studies were generally of a higher quality with a mean Downs and Black score of 13 (median = 14, range = 11-15).
It should be noted that there was considerable variability in the findings and conclusions of the studies (Table 15). As discussed later, the literature was further confounded by the fact that the relative risks associated with physical activity were often controlled (through multivariate analyses) for various potential confounding factors, which may actually inappropriately decrease the level of risk reduction associated with physical activity [31]. Moreover, similar to other chronic conditions this literature was limited greatly by the lack of consistent physical activity assessment and description. In many instances, it was difficult to determine the actual absolute volume and/or intensity of activity for each category of comparison. However, despite these limitations the results of these studies (taken as a whole) indicate that both occupation- and leisure time-related physical activity are inversely related to the risk of colon cancer.
Table 15.
Studies examining the relationship between physical activity and colon cancer.
| Publication Country Study Design Quality Score | Objective | Population | Methods | Outcome | Comments and Conclusions |
|---|---|---|---|---|---|
| Hou et al 2004 [272] | To examine the effect of various forms of PA on colon cancer risk, with particular attention to commuting PA. | • n = 931 case, 1,552 control | PA assessment: Interview for the following variables | • Number of cases: 931 | Regular frequent PA over a long period of time reduces risk of CC. |
| China | • Sex: Men and women | Multivariate OR (95% CI) by OPA, men | |||
| • G1 = 1.00 (referent) | |||||
| Case control | • Age: 30-74 yr | OPA (kJ/min) | • G2 = 1.23 (0.93-1.64) | ||
| D & B score = 14 | • Characteristics: Case: diagnosed with CC. controls: selected randomly from residents of urban Shanghai. | G1 = <8 | • G3 = 0.81 (0.59-1.19) | ||
| G2 = 8-12 | p = 0.10 | ||||
| G3 = >12 | |||||
| Commuting PA (MET hr/wk) | Multivariate OR (95% CI) by OPA, women | ||||
| G1 = <48.3 | • G1 = 1.00 (referent) | ||||
| G2 = 48.3-94.3 | • G2 = 0.96 (0.69-1.16) | ||||
| G3 = >94.3 | • G3 = 0.64 (0.39-1.02) | ||||
| p = 0.009 | |||||
| LTPA (MET hr/wk) | Multivariate OR (95% CI) Commuting PA, men | ||||
| G1 = < 9.2 | • G1 = 1.00 (referent) | ||||
| G2 = 9.2-13.6 | • G2 = 1.11 (0.31-1.23) | ||||
| G3 = >13.6 | • G3 = 0.52 (0.27-0.87) | ||||
| Outcome Measure: incident CC | p<0.001 | ||||
| Multiple logistic regression | Multivariate OR (95% CI) Commuting PA, women | ||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.87 (0.42-1.52) | |||||
| • G3 = 0.56 (0.21-0.91) | |||||
| p = 0.007 | |||||
| Multivariate OR (95% CI) LTPA, men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.17 (0.13-1.95) | |||||
| • G3 = 0.72 (0.41-1.07) | |||||
| p = 0.06 | |||||
| Multivariate OR (95% CI) LTPA, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.03 (0.41-1.59) | |||||
| • G3 = 0.84 (0.13-2.25) | |||||
| p = 0.15 | |||||
| Boutron-Ruault et al 2001 [273] | To determine which step of the adenoma-carcinoma pathway was influenced by OPA and recreational PA. | • n = 480 | PA assessment: Questionnaire and classified into 3 groups | Number of cases: 171 | A sedentary lifestyle was associated with a high risk of CC. |
| France | • Sex: Men and women | G1 = Low | Age and gender adjusted OR (95% CI), OPA | ||
| Case control | • Age: 30-79 years | G2 = Medium | • G1 = 1.00 (referent) | ||
| D & B score = 13 | • Characteristics: Cases had 1stdiagnosis of colorectal adenoma, controls were polyp free. | G3 = High | • G2 = 1.3 (0.8-2.0) | ||
| • G3 = 0.5 (0.3-0.9) | |||||
| p = 0.005 | |||||
| Outcome Measure: Incident CC | |||||
| Multiple logistic regression | Age and gender adjusted OR (95% CI), LTPA | ||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.7 (0.4-1.1) | |||||
| • G3 = 0.3 (0.2-0.5) | |||||
| p = <0.0001 | |||||
| Age and gender adjusted OR (95% CI), Global PA | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.8 (0.5-1.2) | |||||
| • G3 = 0.3 (0.2-0.6) | |||||
| p = 0.0003 | |||||
| Brownson et al 1991 [274] | To investigate the risks of 16 cancer types in relation to OPA. | • n = 17,147 | PA assessment: Medical records and classified into 3 groups: | Number of cases: 1,838 | OPA is inversely related to risk of CC. |
| • Sex: Men | Multivariate OR (95% CI) | ||||
| USA | • Age: ≥ 20 yr | OPA | G1 = 1.00 (referent) | ||
| • Characteristics: White, working | G1 = Low - Activity required <20% of time | G2 = 1.2 (1.0-1.5) | |||
| Case controll | G2 = Moderate - Activity required 20-80% of time | G3 = 1.1 (1.0-1.3) | |||
| D & B score = 15 | G3 = High - Activity required >80% of time | p = 0.05 | |||
| Outcome Measure: CC | |||||
| Maximum likelihood estimates | |||||
| Calton et al 2006 [275] | To examine the relationship between PA and colon cancer risk in women. | • n = 31,783 | 11 year follow up | Number of cases: 243 | Results do not support the hypothesis that PA is related to a lower incidence of CC in women. |
| USA | • Sex: Women | PA Assessment: Questionnaire / Phone interviews for the following variables, divided into 4 or 5 groups | |||
| Prospective cohort | • Age: 61.1 yr | Multivariate RR (95% CI), TPA | |||
| D & B score = 12 | • Characteristics: Free from cancer at baseline | • G1 = 1.00 (referent) | |||
| • G2 = 1.45 (0.98-2.15) | |||||
| • G3 = 1.16 (0.77-1.75) | |||||
| • G4 = 1.27 (0.84-1.91) | |||||
| • G5 = 1.15 (0.76-1.75) | |||||
| p = 0.77 | |||||
| TPA (MET h/d) | |||||
| G1 = 34.0-48.5 | Multivariate RR (95% CI), MPA | ||||
| G2 = 48.51-54.3 | • G1 = 1.00 (referent) | ||||
| G3 = 54.31-59.0 | • G2 = 1.23 (0.82-1.83) | ||||
| G4 = 59.1-64.9 | • G3 = 1.47 (0.99-2.21) | ||||
| G5 = 65.0-98.1 | • G4 = 0.94 (0.61-1.46) | ||||
| • G5 = 1.07 (0.70-1.62) | |||||
| MPA (h/d) | p = 0.80 | ||||
| G1 = 0-3.0 | |||||
| G2 = 3.01-5.0 | |||||
| G3 = 5.01-6.70 | Multivariate RR (95% CI), VPA | ||||
| G4 = 6.71-8.14 | • Q1 = 1.00 (referent) | ||||
| G5 = 8.15-18.0 | • Q2 = 1.19 (0.85-1.66) | ||||
| • Q3 = 0.87 (0.59-1.29) | |||||
| VPA (h/d) | • Q4 = 1.10 (0.78-1.55) | ||||
| Q1 = 0 | p = 0.80 | ||||
| Q2 = 0.1-1.0 | |||||
| Q3 = 1.1-2.0 | |||||
| Q4 = 2.1-14.0 | |||||
| Outcome Measure: Incidence of CC | |||||
| Cox proportional HR | |||||
| Chao et al 2004 [276] | To examine how the characteristics of recreational PA affect its association with colon cancer incidence among older. | • n = 151,174 (70,403 men; 80,771 women) | 7 year follow up | Number of cases: 940 | Increased amounts of time spent in recreational PA is associated with substantially lower risk of CC. |
| USA | • Sex: Men and women | PA assessment: Questionnaire for the following variables | Multivariate RR (95% CI) by recreational PA, men | ||
| Prospective cohort | • Age: mean 63 yr | • G1 = 1.00 (referent) | |||
| D & B score = 12 | • Cancer prevention study II Nutrition Cohort | • G2 = 0.91 (0.69-1.19) | |||
| Recreational PA (h/wk) | • G3 = 0.72 (0.52-1.01) | ||||
| G1 = None | • G4 = 0.86 (0.64-1.15) | ||||
| G2 = <2 | • G5 = 0.77 (0.54-1.08) | ||||
| G3 = 2-3 | • G6 = 0.58 (0.39- 0.87) | ||||
| G4 = 4-6 | p = 0.007 | ||||
| G5 = 7 | |||||
| G6 = ≥ 8 | Multivariate RR (95% CI) by recreational PA, women | ||||
| Recreational (MET h/wk) | • G1 = 1.00 (referent) | ||||
| G1 = None | • G2 = 1.01 (0.70-1.44) | ||||
| G2 = <7, 7-13 | • G3 = 1.01 (0.68-1.49) | ||||
| G3 = 14-23 | • G4 = 0.97 (0.66-1.43) | ||||
| G4 = 24-29 | • G5 = 1.03 (0.65-1.65) | ||||
| G5 = ≥ 30 | • G6 = 0.65 (0.39-1.11) | ||||
| p = 0.14 | |||||
| Walking (h/wk) | |||||
| Q1 = None | |||||
| Q2 = <4 | Multivariate RR (95% CI) by recreational PA, men and women | ||||
| Q3 = 4-6 | • G1 = 1.00 (referent) | ||||
| Q4 = ≥ 7 | • G2 = 0.94 (0.75-1.16) | ||||
| • G3 = 0.83 (0.65-1.07) | |||||
| Walking plus other | • G4 = 0.89 (0.71-1.12) | ||||
| activities (h/wk) | • G5 = 0.85 (0.64-1.12) | ||||
| Q1 = None | • G6 = 0.60 (0.44-0.83) | ||||
| Q2 = <4 | p = 0.002 | ||||
| Q3 = 4-6 | |||||
| Q4 = ≥ 7 | Multivariate RR (95% CI) by MET h/wk men | ||||
| Outcome Measure: Incidence of CC | • G1 = 1.00 (referent) | ||||
| • G2 = 0.90 (0.68-1.18) | |||||
| Cox proportional HR | • G3 = 0.83 (0.59-1.16) | ||||
| • G4 = 0.75 (0.55-1.01) | |||||
| • G5 = 0.86 (0.63-1.19) | |||||
| • G6 = 0.60 (0.41-0.87) | |||||
| p = 0.005 | |||||
| Multivariate RR (95% CI) by MET h/wk women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.02 (0.71-1.46) | |||||
| • G3 = 0.98 (0.65-1.47) | |||||
| • G4 = 1.0 (0.68-1.47) | |||||
| • G5 = 0.94 (0.60-1.48) | |||||
| • G6 = 0.77 (0.48-1.24) | |||||
| p = 0.15 | |||||
| Multivariate RR (95% CI) by MET h/wk men and women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.93 (0.75-1.16) | |||||
| • G3 = 0.88 (0.68-1.13) | |||||
| • G4 = 0.84 (0.66-1.06) | |||||
| • G5 = 0.89 (0.68-1.15) | |||||
| • G6 = 0.65 (0.49-0.87) | |||||
| p = 0.002 | |||||
| Multivariate RR (95% CI) by walking, Men | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.87 (0.66-1.15) | |||||
| • Q3 = 0.83 (0.60-1.16) | |||||
| • Q4 = 0.88 (0.61-1.25) | |||||
| p = 0.34 | |||||
| Multivariate RR (95% CI) by walking, women | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 1.00 (0.70-1.44) | |||||
| • Q3 = 1.08 (0.71-1.63) | |||||
| • Q4 = 1.18 (0.71-1.95) | |||||
| p = 0.41 | |||||
| Multivariate RR (95% CI) by walking plus other activities, men | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.73 (0.53-1.02) | |||||
| • Q3 = 0.85 (0.58-1.24) | |||||
| • Q4 = 0.53 (0.36-0.78) | |||||
| p = 0.02 | |||||
| Multivariate RR (95% CI) by walking plus other activities, women | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.99 (0.67-1.47) | |||||
| • Q3 = 0.72 (0.43-1.19) | |||||
| • Q4 = 0.59 (0.36-0.98) | |||||
| p = 0.07 | |||||
| Colbert et al 2001 [277] | To examine the association between OPA and LTPA and colon cancer in male smokers. | • n = 29,133 | 12 year follow-up | Number of cases: 152 | OPA is protective against CC in a dose-response manner. |
| USA | • Sex: Men | ||||
| Prospective cohort | • Age: 50-69 yr | PA assessment: Interview for OPA and LTPA | Multivariate RR (95% CI) by OPA | ||
| D & B score = 13 | • Characteristics: Smokers | • G1 = 0.61 (0.39-0.98) | |||
| • Alpha- Tocopherol, Beta-Carotene Cancer Prevention Study | • G2 = 1.00 (referent) | ||||
| • G3 = 0.60 (0.34-1.04) | |||||
| OPA | • G4 = 0.45 (0.26-0.78) | ||||
| G1 = Non-worker | p = 0.003 | ||||
| G2 = Sedentary | |||||
| G3 = Light | Multivariate RR (95% CI), by LTPA | ||||
| G4 = Moderate | • G1 = 1.00 (referent) | ||||
| • G2 = 0.82 (0.59-1.13) | |||||
| LTPA | |||||
| G1 = Sedentary | |||||
| G2 = Active | |||||
| Outcome Measure: incident CC | |||||
| Cox proportional HR | |||||
| Dosemeci et al 1993 [278] | To examine associations between PA and cancer sites among workers in Turkey. | • n = 6,236 (3,486 cases in men and 379 cases in women; 2,127 control men and 244 control women) | PA assessment: Stanford Occupational Classification code system. | Number of cases: 93 | Occupational EE is inversely related to risk of CC. |
| Turkey | • 93 cases for CC | Multivariate OR (95% CI) by total occupational EE | |||
| Case control | • Sex: Men and women | • G1 = 1.6 (0.9-2.8) | |||
| • Age: not indicated | Total Occupational EE (kj/min) | • G2 = 1.1 (0.6-2.0) | |||
| D & B score = 13 | • Characteristics: All hospitalized Cases: Diagnosed with CC. Controls: included subjects diagnosed as non-cancers and cancers which there is no suggestion of an association with PA. | G1 = <8 | • G3 = 1.0 (referent) | ||
| G2 = 8-12 | p = 0.04 | ||||
| G3 = >12 | When adjusted for socioeconomic status p = 0.03 | ||||
| Sitting time at work (h/d) Levels: | Multivariate OR (95% CI) by sitting time at work | ||||
| G1 = <2 | • G1 = 1.00 (referent) | ||||
| G2 = 2-6 | • G2 = 1.5 (0.9-2.5) | ||||
| G3 = >6 | • G3 = 1.5 (0.8-3.0) | ||||
| p = 0.03 | |||||
| Outcome Measure: Incident CC | When adjusted for socioeconomic status p = 0.03 | ||||
| Maximum likelihood estimates | |||||
| Friedenreich et al 2006 [279] | To investigate the role of PA in the development of colon cancer. | • n = 413,044 | 4 year follow-up | Multivariate RR (95% CI), TPA | Inverse association between PA and risk of CC, particularly for right sided tumours. |
| • Sex: Men and women | PA assessment: modified Baecke Questionnaire | • Q1 = 1.00 (referent) | |||
| UK | • Age: 35-70 yr | • Q2 = 0.92 (0.76-1.12) | |||
| • Characteristics: Free of cancer at baseline | • Q3 = 0.86 (0.70-1.04) | ||||
| Prospective cohort | • European Prospective Investigation into Nutrition and Cancer. (EPIC) | TPA | • Q4 = 0.78 (0.59-1.03) | ||
| Q1 = Inactive | p = 0.04 | ||||
| D & B score = 14 | Q2 = Moderately inactive | Multivariate RR (95% CI), TPA and right sided CC | |||
| Q3 = Moderately active | • Q1 = 1.00 (referent) | ||||
| Q4 = Active | • Q2 = 1.79 (0.59-1.06) | ||||
| Household PA (MET-h/wk) | • Q3 = 0.64 (0.47-0.86) | ||||
| Q1 = <19.5 | • Q4 = 0.65 (0.43-1.00) | ||||
| Q2 = 19.5-39.6 | p = 0.004 | ||||
| Q3 = 39.6-73.9 | |||||
| Q4 = ≥ 73.9 | Multivariate RR (95% CI), household PA and right sided CC | ||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.97 (0.75-1.27) | |||||
| Outcome Measure: Incident CC | • Q3 = 0.84 (0.64-1.12) | ||||
| • Q4 = 0.74 (0.54-1.02) | |||||
| p = 0.05 | |||||
| Cox proportional HR | |||||
| Giovannucci et al 1995 [280] | To examine the association between PA and colon cancer. | • n = 47,723 | 6 year follow-up | Multivariate RR (95% CI) | A moderate level of PA was related to a substantially lower risk of CC in this cohort of middle age to elderly men. |
| • Sex: Men | • G1 = 1.00 (referent) | ||||
| • Age: 40-75 yr | PA assessment: Questionnaire | • G2 = 0.73 (0.48-1.10) | |||
| USA | • Characteristics: Health professionals | • G3 = 0.94 (0.63-1.39) | |||
| • G4 = 0.78 (0.51-1.20) | |||||
| Prospective cohort | • Health Professionals Follow-up Study | Outcome Measure: Incidence of colon cancer | • G5 = 0.53 (0.32-0.88) | ||
| p = 0.03 | |||||
| D & B score = 12 | |||||
| Mantel-Haeszel estimator and logistic regression | |||||
| Isomura et al 2006 [281] | To examine the relationship of OPA, LTPA, commuting, housework and shopping with colorectal cancer risk. | • n = 1545 (778 cases, 767 controls) | PA assessment: Questionnaire and interview for the following variables | • Number of cases: 778 | Adds to the evidence that PA confers decreased risk of CC, especially of distal CC in both men and women. |
| Japan | • Sex: Men and women | Multivariate OR (95% CI) for all CC by OPA, men | |||
| • G1 = 1.00 (referent) | |||||
| Case control | • Age: 20-74 yr | OPA, men | • G2 = 0.9 (0.6-1.4) | ||
| • Characteristics: Free from cancer at baseline | G1 = Sedentary | • G3 = 0.7 (0.4-1.0) | |||
| D & B score = 12 | G2 = Moderate | p = 0.06 | |||
| . | G3 = Hard | ||||
| • Fukuoka colorectal cancer study | Multivariate OR (95% CI) for proximal | ||||
| OPA, women | CC by OPA, men | ||||
| G1 = Sedentary | • G1 = 1.00 (referent) | ||||
| G2 = Active | • G2 = 1.2 (0.6-2.2) | ||||
| • G3 = 0.7 (0.4-1.4) | |||||
| Total non-OPA, men (MET-h/wk) | p = 0.45 | ||||
| G1 = 0.0 | Multivariate OR (95% CI) for distal CC by OPA, men | ||||
| G2 = 0.1-15.9 | • G1 = 1.00 (referent) | ||||
| G3 = 16.0 | • G2 = 0.8 (0.4-1.4) | ||||
| • G3 = 0.6 (0.4-1.0) | |||||
| p = 0.047 | |||||
| Total non-OPA women (MET hr/wk) | |||||
| G1 = 0.0 | |||||
| G2 = 0.1-15.9 | |||||
| G3 = 16.0 | Multivariate OR (95% CI) for all CC by non-OPA, men | ||||
| Moderate or hard non-OPA, men (MET hr/wk) | • G1 = 1.00 (referent) | ||||
| G1 = 0.0 | • G2 = 0.9 (0.6-1.4) | ||||
| G2 = 0.1-14.9 | • G3 = 0.8 (0.5-1.2) | ||||
| G3 = ≥15.0 | p = 0.22 | ||||
| Multivariate OR (95% CI) for proximal CC by non-OPA, men | |||||
| Moderate or hard non-OPA, women (MET hr/wk) | • G1 = 1.00 (referent) | ||||
| G1 = 0.0 | • G2 = 1.2 (0.6-2.1) | ||||
| G2 = 0.1-14.9 | • G3 = 0.9 (0.5-1.7) | ||||
| G3 = 15.0 | p = 0.69 | ||||
| Outcome Measure: Incident CC | Multivariate OR (95% CI) for distal CC by non-OPA, men | ||||
| • G1 = 1.00 (referent) | |||||
| Multiple logistic regression analysis | • G2 = 0.8 (0.5-1.3) | ||||
| • G3 = 0.7 (0.4-1.1) | |||||
| p = 0.19 | |||||
| Multivariate OR (95% CI) for all CC by non-OPA, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.9 (0.5-1.5) | |||||
| • G3 = 0.8 (0.5-1.4) | |||||
| p = 0.45 | |||||
| Multivariate OR (95% CI) for proximal CC by non-OPA, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.5 (0.7-3.3) | |||||
| • G3 = 1.6 (0.7-3.6) | |||||
| p = 0.41 | |||||
| Multivariate OR (95% CI) for distal CC by non-OPA, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.7 (0.4-1.3) | |||||
| • G3 = 0.6 (0.3-1.1) | |||||
| p = 0.12 | |||||
| Multivariate OR (95% CI) for all CC by moderate or hard non-OPA, men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.8 (0.6-1.2) | |||||
| • G3 = 0.8 (0.5-1.1) | |||||
| p = 0.24 | |||||
| Multivariate OR (95% CI) for proximal CC by moderate or hard non-OPA, men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.1 (0.6-2.1) | |||||
| • G3 = 1.0 (0.6-1.8) | |||||
| p = 0.99 | |||||
| Multivariate OR (95% CI) for distal CC by moderate or hard non-OPA, men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.7 (0.4-1.1) | |||||
| • G3 = 0.7 (0.4-1.0) | |||||
| p = 0.12 | |||||
| Multivariate OR (95% CI) for all CC by moderate or hard non-OPA, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.0 (0.6-1.6), | |||||
| • G3 = 0.8 (0.5-1.4) | |||||
| p = 0.35 | |||||
| Multivariate OR (95% CI) for proximal CC by moderate or hard non-OPA, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.3 (0.6-2.5) | |||||
| • G3 = 1.3 (0.6-2.7) | |||||
| p = 0.59 | |||||
| Multivariate OR (95% CI) for distal CC by moderate or hard non-OPA, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.8 (0.5-1.5) | |||||
| • G3 = 0.5 (0.3-1.1) | |||||
| p = 0.41 | |||||
| Johnsen at el 2006 [282] | To investigate the effects of OPA on colon cancer incidence. | • n = 54,478 (28,356 men, 26,122 women) | 7.6 year follow-up | • Number of cases: 140 women, 157 men | No support for the hypothesis that OPA measured by MET-score may be associated with a lower risk of CC. |
| • Sex: Men and women | PA assessment: Questionnaire for OPA by MET score, 4 groups | • Number of dropouts: <0.8% | |||
| Denmark | • Age: 50-64 yr | Multivariate RR (95% CI), men | |||
| Prospective cohort | • Characteristics: Free of Cancer at baseline | Q1 = Sitting | • Q1 = 1.00 (referent) | ||
| • Diet, Cancer and Health Study | Q2 = Standing | • Q2 = 1.11 (0.69-1.77) | |||
| Q3 = Manual | • Q3 = 1.17 (0.77-1.79) | ||||
| D & B score = 13 | Q4 = Not working | • Q4 = 0.95 (0.58-1.55) | |||
| Outcome Measure: Incidence of colon cancer | Multivariate RR (95% CI), women | ||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 1.15 (0.68-1.93) | |||||
| • Q3 = 1.34 (0.83-2.16) | |||||
| Cox proportional HR | • Q4 = 0.96 (0.60-1.53) | ||||
| Larsen et al 2006 [283] | To examine the relationship between PA and colorectal cancer. | • n = 6,961 | PA assessment: Questionnaire (scored from 2-12), divided into quartiles: | Number of cases: 108 | Inactivity was not a significant risk factor for advanced colonic neoplasia. |
| • Sex: Men and women | RR (95% CI) | ||||
| Norway | • Age: 50-64 | • Q1 = 1.00 (referent) | |||
| • Characteristics: No history of colorectal surgery, radiotherapy, cardiopulmonary disease, anticoagulant therapy, coronary episode. | Q1 = 2-4 | • Q2 = 0.61 (0.32-1.16) | |||
| Cross-sectional evaluation within a randomized controlled trial | Q2 = 5 | • Q3 = 0.75 (0.45-1.26) | |||
| Q3 = 6 | • Q4 = 0.56 (0.34-0.92) | ||||
| Q4 = 7-12 | p = 0.04 | ||||
| Outcome Measure: Positive test for colonic neoplasia | Multivariate RR (95% CI) | ||||
| D & B score = 13 | • Q1 = 1.00 (referent) | ||||
| • Q2 = 0.64 (0.33-1.25) | |||||
| • Q3 = 0.82 (0.47-1.43) | |||||
| Multivariate logistic regression analysis | • Q4 = 0.67 (0.39-1.16) | ||||
| p = 0.23 | |||||
| Larsson et al 2006 [284] | To investigate the association between PA and colorectal cancer. | • n = 45,906 | 7.1 year follow-up | Number of cases: 309 (133 proximal, 138 distal) | Results support a role of PA in reducing the risk of CC. |
| • Sex: Men | |||||
| • Age: 45-79 yr | PA assessment: Questionnaire for the following variables | ||||
| Sweden | • Characteristics: Free of cancer at baseline | Multivariate HR (95% CI) by LTPA | |||
| • Q1 = 1.00 (referent) | |||||
| Prospective cohort | • Q2 = 0.66 (0.43-1.02) | ||||
| LTPA (min/day) | • Q3 = 0.68 (0.46-1.01) | ||||
| Q1 = <10 | • Q4 = 0.56 (0.37-0.83) | ||||
| D & B score = 14 | Q2 = 10-29 | p = 0.01 | |||
| Q3 = 30-59 | |||||
| Q4 = ≥ 60 | Multivariate HR (95% CI) by home/housework PA | ||||
| Home/housework PA (h/day) | • Q1 = 1.00 (referent) | ||||
| • Q2 = 0.75 (0.58-0.97) | |||||
| Q1 = none | • Q3 = 0.75 (0.58-0.97) | ||||
| Q2 = <1 | • Q4 = 0.68 (0.48-0.96) | ||||
| Q3 = 1-2 | p = 0.01 | ||||
| Q4 = ≥ 3 | |||||
| Incidence of Proximal CC(h/day) | Multivariate HR (95% CI) for distal CC by LTPA | ||||
| G1 = <1 | • Q1 = 1.00 (referent) | ||||
| G2 = 1-2 | |||||
| G3 = ≥ 3 | • Q2 = 0.51 (0.28-0.93) | ||||
| • Q3 = 0.50 (0.29-0.87) | |||||
| • Q4 = 0.40 (0.22-0.70) | |||||
| p = 0.01 | |||||
| Outcome Measure: Incident CC | |||||
| Multivariate HR (95% CI) for proximal CC by home/housework PA | |||||
| Cox proportional HR | • G1 = 1.00 (referent) | ||||
| • G2 = 0.78 (0.53-1.14) | |||||
| • G3 = 0.50 (0.29-0.89) | |||||
| p = 0.02 | |||||
| Lee and Paffenbarger 1994 [285] | To predict cancer risk using prospective assessments of PA. | • n = 17,607 | 26 year follow-up | • Number of cases: 280 | Found a trend, of borderline statistical significance toward decreasing CC risk with increasing PA. |
| • Sex: Men | • Number of dropouts: 14% | ||||
| • Age: 30-79 yr | PA assessment: Questionnaire for PA level (kcal/wk) | ||||
| USA | • Characteristics: Healthy at baseline | Multivariate RR (95% CI), Model A: PA in 1962/1966 and updated in 1977 | |||
| • Harvard College Alumni | • G1 = 1.00 (referent) | ||||
| Prospective cohort | G1 = <1000 | • G2 = 1.07 (0.81-1.42) | |||
| G2 = 1000-2499 | • G3 = 1.08 (0.81-1.46) | ||||
| G3 = ≥ 2500 | p = 0.58 | ||||
| D & B score = 13 | |||||
| Outcome Measure: Incidence of fatal and non fatal CC | Multivariate RR (95% CI), Model B: PA in both 1962/1966 and 1977 | ||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.75 (0.42-1.35) | |||||
| Cox proportional HR | • G3 = 0.94 (0.54-1.64) | ||||
| p = 0.76 | |||||
| Lee et al 1997 [286] | To investigate whether PA alters the risk of developing CC in men. | • n = 20,614 | 10.9 year follow-up | Number of cases: 217 | Data does not support the hypothesis that PA is related inversely to risk of developing CC. |
| • Sex: Men | |||||
| • Age: 40-84 yrs | PA assessment: Questionnaire for the following variables | Multivariate RR (95% CI), frequency of | |||
| USA | • Characteristics: Physicians, free of cancer at baseline | PA at baseline | |||
| • G1 = 1.00 (referent) | |||||
| Prospective cohort | • G2 = 1.1 (0.7-1.7) | ||||
| Frequency of PA at baseline (times/week) | • G3 = 1.2 (0.8-1.6) | ||||
| Physicians Health Study | • G4 = 1.1 (0.7-1.6) | ||||
| D & B score = 15 | G1 = <1 | p = 0.6 | |||
| G2 = 1 | |||||
| G3 = 2-4 | RR (95% CI), frequency of PA at baseline and 36 months | ||||
| G4 = 5+ | |||||
| • G1 = 1.00 (referent) | |||||
| Frequency of PA at baseline and 36 months | • G2 = 1.2 (0.5-2.7) | ||||
| G1 = 1/<1 | • G3 = 1.4 (0.9-2.3) | ||||
| G2 = <1/1+ | • G4 = 1.3 (0.9-2.0) | ||||
| G3 = 1+/< 1 | |||||
| G4 = 1+/1+ | |||||
| Outcome Measure: Incidence of fatal and non-fatal CC | |||||
| Cox proportional HR | |||||
| Lee et al 2007 [287] | To examine the association between PA and the risk of developing CRC in Japanese men and women. | • n = 65,022 | 6 year follow-up | Number of cases: 154 proximal CC, 166 distal CC | PA may prevent CC among Japanese men. |
| • Sex: Men and women | |||||
| Japan | • Age: 40-69 yr | Multivariate RR (95% CI) for CC men | |||
| • Characteristics | PA assessment: Questionnaire for PA level (median MET hr/d) | • Q1 = 1.00 (referent) | |||
| Prospective cohort | • Ethnicity: Japanese | Q1 = 28.25 | • Q2 = 0.87 (0.61-1.26) | ||
| Q2 = 33.25 | • Q3 = 0.62 (0.41-0.95) | ||||
| Q3 = 35.25 | • Q4 = 0.58 (0.39-0.87) | ||||
| D & B score = 13 | Q4 = 43.75 | p = 0.006 | |||
| Outcome Measure: Incidence of CC | Multivariate RR (95% CI) for proximal CC men | ||||
| Cox proportional HR | • Q1 = 1.00 (referent) | ||||
| • Q2 = 0.89 (0.52-1.51) | |||||
| • Q3 = 0.44 (0.22-0.86) | |||||
| • Q4 = 0.29 (0.14-0.60) | |||||
| p < 0.001 | |||||
| Multivariate RR (95% CI) for distal CC Men | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.92 (0.54-1.54) | |||||
| • Q3 = 0.75 (0.42-1.33) | |||||
| • Q4 = 0.89 (0.53-1.51) | |||||
| p = 0.685 | |||||
| PA level and incidence of CC women | |||||
| Total CC | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 1.03 (0.65-1.64) | |||||
| • Q3 = 0.91 (0.57-1.47) | |||||
| • Q4 = 0.89 (0.54-1.49) | |||||
| p = 0.610 | |||||
| Proximal CC women | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 1.14 (0.61-2.12) | |||||
| • Q3 = 1.01 (0.53-1.89) | |||||
| • Q4 = 0.55 (0.24-1.26) | |||||
| p = 0.151 | |||||
| Distal CC women | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 1.09 (0.52-2.29) | |||||
| • Q3 = 0.77 (0.34-1.74) | |||||
| • Q4 = 1.37 (0.66-2.85) | |||||
| p = 0.401 | |||||
| Longnecker et al 1995 [288] | To examine the relationship between OPA and vigorous LTPA and the risk of cancer of the right colon and rectum. | • n = 242 rectal cancer and 703 controls | PA assessment: Interview for vigorous LTPA and OPA (coded and self-reported), divided into groups: | Number of cases: 163 | The amount of time spent at vigorous LTPA was associated with a decreased risk of cancer of the right colon. |
| • Sex: Men | RR (95% CI) by vigorous LTPA | ||||
| USA | • Age: ≥ 31 yr | • G1 = 1.00 (referent) | |||
| • Characteristics: Case: Diagnosed with adenocarcinoma of the right colon or rectum. Controls: Both community and hospital. No history of large bowel cancer. | • G2 = 0.73 (0.23-2.29) | ||||
| Case control | • G3 = 0.47 (0.16-1.36) | ||||
| Vigorous LTPA (h/wk) | • G4 = 0.60 (0.35-1.00) | ||||
| D & B score = 14 | G1 = 0 | p = 0.03 | |||
| G2 = ≤ 0.5 | |||||
| G3 = 1 | Multivariate OR (95% CI) by vigorous | ||||
| G4 = >1 | LTPA | ||||
| • G1 = 1.00 (referent) | |||||
| Coded lifetime OPA | • G2 = 0.81 (0.26-2.54) | ||||
| G1 = Sedentary | • G3 = 0.36 (0.11-1.14) | ||||
| G2 = light work | • G4 = 0.57 (0.33-0.97) | ||||
| G3 = moderate | p = 0.06 | ||||
| G4 = heavy | |||||
| Self reported lifetime | Multivariate OR (95% CI) by coded lifetime OPA | ||||
| OPA | |||||
| G1 = Sedentary | • G1 = 1.00 (referent) | ||||
| G2 = light work | • G2 = 0.79 (0.39-1.61) | ||||
| G3 = more than light work | • G3 = 0.79 (0.36-1.74) | ||||
| • G4 = 0.99 (0.30-3.22) | |||||
| p = 0.42 | |||||
| Outcome Measure: Diagnosed with CC | |||||
| Multivariate OR (95% CI) by self reported lifetime OPA | |||||
| Conditional Logistic Regression | • G1 = 1.00 (referent) | ||||
| • G2 = 0.85 (0.41-1.76) | |||||
| • G3 = 0.68 (0.31-1.52) | |||||
| p = 0.15 | |||||
| Mai et al 2007 [289] | To examine in detail the relationship between recreational PA and invasive CC among women. | • n = 120,147 | 7 year follow-up | Number of cases: 395 | Modest inverse association between recreational PA and CC. |
| • Sex: Women | |||||
| • Age: 22-84 yr | PA assessment: Questionnaire | RR (95% CI) by MPA over past 3 years | |||
| USA | • Characteristics: no prior history of CC | • G1 = 1.00 (referent) | |||
| • G2 = 0.95 (0.72-1.24) | |||||
| Prospective cohort | MPA over past 3 yrs (h/wk/yr) | • G3 = 0.78 (0.62-0.97) | |||
| • California Teachers Study | p = 0.02 | ||||
| G1 = 0-0.50 | |||||
| D & B score = 15 | G2 = 0.51-1.99 | RR (95% CI) by strenuous + moderate (lifetime) PA: | |||
| G3 = ≥ 2.00 | • G1 = 1.00 (referent) | ||||
| • G2 = 0.79 (0.56-1.11) | |||||
| Strenuous + Moderate (lifetime) PA (h/wk/yr) | • G3 = 0.64 (0.44-0.93) | ||||
| p = 0.04 | |||||
| G1 = 0.0-0.50 | |||||
| G2 = 0.51-3.99 | |||||
| G3 = ≥ 4.00 | |||||
| Outcome Measure: Incidence of invasive adenocarcinoma of the colon | |||||
| Cox proportional HR | |||||
| Martinez et al 1997 [290] | To examine whether LTPA could significantly influence the risk of CC in women. | • n = 89,448 | 6 year follow-up | Number of cases: 212 | Significant inverse association between LTPA and incidence of CC in women. |
| • Sex: Women | |||||
| • Age: 30-55 yr | PA assessment: Questionnaire for LTPA | Multivariate RR (95% CI) for all CC | |||
| USA | • Characteristics: Nurses, free from cancer at baseline | • G1 = 1.00 (referent) | |||
| G1 = <2 | • G2 = 0.71 (0.44-1.15) | ||||
| Prospective | G2 = 2-4 | • G3 = 0.78 (0.50-1.20) | |||
| cohort | G3 = 5-10 | • G4 = 0.67 (0.42-1.07) | |||
| G4 = 11-21 | • G5 = 0.54 (0.33-0.90) | ||||
| D & B score = 14 | G5 = >21 | p = 0.03 | |||
| Outcome Measure: Incidence of CC | Multivariate RR (95% CI) for distal CC | ||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.92 (0.48-1.79) | |||||
| Mantel-Haenszel Estimator and logistic regression models | • G3 = 0.81 (0.43-1.55) | ||||
| • G4 = 0.71 (0.36-1.41) | |||||
| • G5 = 0.31 (0.12-0.77) | |||||
| p = 0.01 | |||||
| Multivariate RR (95% CI) for proximal | |||||
| CC | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.54 (0.23-1.22) | |||||
| • G3 = 0.79 (0.40-1.56) | |||||
| • G4 = 0.62 (0.30-1.32) | |||||
| • G5 = 0.77 (0.38-1.58) | |||||
| p = 0.67 | |||||
| Nilsen et al 2008 [291] | To study the separate associations of recreational PA with the incidence of, and mortality from cancer in the ascending, transverse, descending and sigmoid segments of the colon. | • n = 59,369 | 17 year follow-up | Number of cases: 736 | Strong inverse associations between recreational PA and risk of cancer morbidity and mortality of the transverse and sigmoid colon but no association for cancer in the ascending and descending colon. |
| • Sex: Men and women | PA assessment: Questionnaire for frequency and duration of recreational PA | HR (95% CI) by frequency of recreational PA, men | |||
| Norway | • Age: not indicated | • G1 = 1.00 (referent) | |||
| Prospective cohort | • Characteristics: Free from cancer at baseline | • G2 = 0.84 (0.60-1.19) | |||
| • Nord-Trondelag Health Study | • G3 = 0.82 (0.58-1.17) | ||||
| • G4 = 0.81 (0.57-1.15) | |||||
| D & B score = 14 | Frequency of Recreational PA (times per week) | • G5 = 0.77 (0.54-1.09) | |||
| G1 = none | p = 0.18 | ||||
| G2 = <1 | HR (95% CI) by frequency of | ||||
| G3 = 1 | recreational PA, women | ||||
| G4 = 2-3 | • G1 = 1.00 (referent) | ||||
| G5 = ≥ 4 | • G2 = 0.91 (0.66-1.25) | ||||
| • G3 = 0.79 (0.57-1.09) | |||||
| Duration of recreational PA (min per exercise) | • G4 = 0.66 (0.47-0.92) | ||||
| • G5 = 0.99 (0.72-1.36) | |||||
| G1 = none | p = 0.35 | ||||
| G2 = <15 | |||||
| G3 = 15-30 | HR (95% CI) by duration of recreational | ||||
| G4 = 31-60 | PA, men | ||||
| G5 = >60 | • G1 = 1.00 (referent) | ||||
| • G2 = 1.07 (0.71-1.60) | |||||
| Intensity of recreational PA | • G3 = 0.80 (0.57-1.12) | ||||
| • G4 = 0.68 (0.48-0.97) | |||||
| G1 = none | • G5 = 0.74 (0.50-1.08) | ||||
| G2 = Low | p = 0.02 | ||||
| G3 = Moderate/High | HR (95% CI) by duration of recreational PA, women | ||||
| Summary score for recreational PA | • G1 = 1.00 (referent) | ||||
| G1 = None | • G2 = 0.85 (0.59-1.23) | ||||
| G2 = Low | • G3 = 0.81 (0.60-1.09) | ||||
| G3 = High | • G4 = 0.73 (0.53-1.01) | ||||
| By subsite-specific (transverse colon, decending colon, sigmoid colon) CC | • G5 = 0.84 (0.53-1.34) | ||||
| p = 0.10 | |||||
| HR (95% CI) by intensity of recreational PA, men | |||||
| Levels of REC PA: | |||||
| G1 = None | • G1 = 1.00 (referent) | ||||
| G2 = < 1 x/wk | • G2 = 0.83 (0.62-1.12) | ||||
| G3 = low score | • G3 = 0.74 (0.52-1.06) | ||||
| G4 = high score | p = 0.11 | ||||
| Outcome Measure: incidence of fatal and non fatal CC | HR (95% CI) by intensity of recreational PA, women | ||||
| Cox proportional HR | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.77 (0.59-1.01) | |||||
| • G3 = 0.89 (0.60-1.32) | |||||
| p = 0.33 | |||||
| HR (95% CI) by summary score for recreational PA, men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.85 (0.62-1.16) | |||||
| • G3 = 0.69 (0.48-0.98) | |||||
| p = 0.06 | |||||
| HR (95% CI) by summary score for recreational PA, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.86 (0.64-1.01) | |||||
| • G3 = 0.72 (0.53-0.98) | |||||
| p = 0.03 | |||||
| HR (95% CI) by total CC and recreational PA, incidence | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.88 (0.70-1.12) | |||||
| • G3 = 0.87 (0.70-*1.08) | |||||
| • G4 = 0.73 (0.58-0.92) | |||||
| p = 0.009 | |||||
| HR (95% CI) by subsite specific CC and recreational PA, death | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.87 (0.64-1.18) | |||||
| • G3 = 0.79 (0.59-1.04) | |||||
| • G4 = 0.56 (0.41-0.78) | |||||
| p <0.001 | |||||
| HR (95% CI) for transverse CC incidence and recreational PA | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.75 (0.44-1.28) | |||||
| • G3 = 0.66 (0.41-1.08) | |||||
| • G4 = 0.44 (0.25-0.78) | |||||
| p = 0.004 | |||||
| HR (95% CI) for transverse CC death and recreational PA | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.73 (0.36-1.49) | |||||
| • G3 = 0.40 (0.19-0.82) | |||||
| • G4 = 0.33 (0.14-0.76) | |||||
| p = 0.002 | |||||
| HR (95% CI) for sigmoid CC incidence and recreational PA | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.88 (0.59-1.32) | |||||
| • G3 = 0.68 (0.46-1.01) | |||||
| • G4 = 0.48 (0.31-0.75) | |||||
| p <0.001 | |||||
| HR (95% CI) for sigmoid CC death and recreational PA | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.78 (0.45-1.35) | |||||
| • G3 = 0.51 (0.30-0.87) | |||||
| • G4 = 0.29 (0.15-0.56) | |||||
| p <0.001 | |||||
| Schnohr et al 2005 [292] | To assess the association between LTPA and incidence of cancer in the general population. | • n = 28,259 (15,043 men,13,216 women) | 14 year follow-up | • Number of cases: 215 men, 108 women | For the most active men, VPA was associated with a non-significant lower risk of CC. |
| Denmark | PA assessment: Questionnaire for LTPA | Multivariate RR (95% CI), men | |||
| • Sex: Men and women | G1 = Low | • G1 = 1.00 (referent) | |||
| Prospective cohort | G2 = Moderate | • G2 = 1.08 (0.74-1.57) | |||
| • Age: 20-93 yr | G3 = Vigorous | • G3 = 0.72 (0.47-1.11) | |||
| D & B score = 13 | • Characteristics: Free from cancer at baseline | Outcome Measure: Incidence of CC | p =0.06 | ||
| Multivariate RR (95% CI), women | |||||
| • Copenhagen Heart Study, The Copenhagen County Centre of Preventive Medicine and the Copenhagen Male Study | • G1 = 1.00 (referent) | ||||
| Cox proportional HR | • G2 = 1.02 (0.70-1.50) | ||||
| • G3 = 0.90 (0.56-1.46) | |||||
| p = 0.68 | |||||
| Slattery et al 1988 [293] | To assess the relationship of PA and diet with the development of CC in Utah. | • n = 229 cases, 384 controls | PA assessment: Interview for the following variables | • Number of cases: 229 | PA shows an inverse relationship with incidence of CC. |
| USA | • Sex: Men and women | Multivariate OR (95% CI) by TPA, men | |||
| Case control | • Age: 40-79 yr | • Q1 = 1.00 (referent) | |||
| • Characteristics: Case: Diagnosed with CC Controls: no history of cancer | TPA | • Q2 = 1.19 (0.67-2.13) | |||
| Q1 = Low | • Q3 = 0.88 (0.48-1.69) | ||||
| Q2 | • Q4 = .70 (0.38-1.29) | ||||
| D & B score = 13 | Q3 | ||||
| Q4 = high | Multivariate OR (95% CI) by TPA, women | ||||
| Intense PA | • Q1 = 1.00 (referent) | ||||
| G1 = none | • Q2 = 0.97 (0.56-1.69) | ||||
| G2 = low | • Q3 = 0.91 (0.52-1.60) | ||||
| G3 = high | • Q4 = 0.48 (0.27-0.87) | ||||
| Non-intense PA | Multivariate OR (95% CI) by intense PA, men | ||||
| Q1 = Low | |||||
| Q2 | • G1 = 1.00 (referent) | ||||
| Q3 | • G2 = 0.83 (0.40-1.75) | ||||
| Q4 = high | • G3 = 0.27 (0.11-0.65) | ||||
| Outcome Measure: Diagnosed with CC | Multivariate OR (95% CI) by intense PA, women | ||||
| Multiple logistic regression analysis | • G1 = 1.00 (referent) | ||||
| • G2 = 0.55 (0.23-1.34) | |||||
| Multivariate OR (95% CI) by non-intense PA, men | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 1.40 (0.76-2.57) | |||||
| • Q3 = 0.93 (0.51-1.72) | |||||
| • Q4 = 1.25 (0.68-2.29) | |||||
| Multivariate OR (95% CI) by non-intense PA, women | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 1.09 (0.62-1.90) | |||||
| • Q3 = 0.94 (0.53-1.66) | |||||
| • Q4 = 0.53 (0.29-0.95) | |||||
| Slattery et al 1997 [294] | To examine the relationship between weekly PA patterns (source, duration and frequency) and CC. | • n = 1,993 cases, 2,410 controls | PA Assessment: Interview, adapted CARDIA PA history | Number of cases: 1,993 | High level of leisure time VPA during the past 20 yrs was associated with a reduced risk of CC in both men and women. The same associations were not observed with leisure time MPA. |
| USA | • Sex: Men and women | Multivariate OR (95% CI) by recent leisure time VPA, men | |||
| Case control | • Age: 30-79 yr | Recent leisure time | • Q1 = 1.00 (referent) | ||
| • Characteristics: Cases: diagnosed with first primary CC. Controls: no history of CC | VPA | • Q2 = 0.80 (0.64-1.01) | |||
| Q1 = None | • Q3 = 0.84 (0.66-1.05) | ||||
| D & B score = 14 | Q2 | • Q4 = 0.69 (0.55-0.87) | |||
| Q3 | |||||
| Q4 = High | Multivariate OR (95% CI) by recent leisure time VPA, women | The greatest inverse association was observed when activities were performed for longer periods of time per session. | |||
| The Three Centered Diet, Activity and Lifestyle Colon Cancer Study | Leisure time VPA | • Q1 = 1.00 (referent) | |||
| Q1 = Low | • Q2 = 0.79 (0.61-1.02) | ||||
| Q2 | • Q3 = 0.83 (0.64-1.07) | ||||
| Q3 | • Q4 = 0.86 (0.67-1.10) | ||||
| Q4 = High | |||||
| Current PA (min) | Multivariate OR (95% CI) by leisure time VPA, men | ||||
| G1 = <30 | |||||
| G2 = 30-60 | • Q1 = 1.00 (referent) | ||||
| G3 = ≥ 60 | • Q2 = 0.97 (0.76-1.25) | ||||
| • Q3 = 0.86 (0.67-1.09) | |||||
| LTPA (ranked by time per session) | • Q4 = 0.61 (0.47-0.79) | ||||
| Q1 = None | Multivariate OR (95% CI) by leisure time VPA, women | ||||
| Q2 = Low - <30 min | |||||
| Q3 = moderate - 30-60 min | • Q1 = 1.00 (referent) | ||||
| Q4 = high ->60 min | • Q2 = 0.75 (0.59-0.95) | ||||
| • Q3 = 0.68 (0.53-0.87) | |||||
| Number of activity session per week | • Q4 = 0.63 (0.48-0.83) | ||||
| G1 = None | Multivariate OR (95% CI) by current MPA time per week | ||||
| G2 = 1 | |||||
| G3 = 2-4 | • Q1 = 1.00 (referent) | ||||
| G4 = 5-7 | • Q2 = 1.00 (0.83-1.21) | ||||
| G5 = >7 | • Q3 = 0.90 (0.76-1.07) | ||||
| • Q4 = 0.92 (0.77-1.10) | |||||
| Outcome Measure: Diagnosed with CC | Multivariate OR (95% CI) by current VPA time per week | ||||
| Unconditional regression models | • Q1 = 1.00 (referent) | ||||
| • Q2 = 0.90 (0.73-1.12) | |||||
| • Q3 = 0.89 (0.71-1.10) | |||||
| • Q4 = 0.83 (0.69-0.98) | |||||
| Multivariate OR (95% CI) by leisure time MPA time per session | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 1.20 (0.91-1.59) | |||||
| • Q3 = 1.09 (0.83-1.42) | |||||
| • Q4 = 1.08 (0.82-1.42) | |||||
| Multivariate OR (95% CI) by leisure time VPA time per session | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.86 (0.74-0.99) | |||||
| • Q3 = 0.76 (0.64-0.90) | |||||
| • Q4 = 0.68 (0.52-0.87) | |||||
| Multivariate OR (95% CI) by number of MPA sessions/wk | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.02 (0.79-1.30) | |||||
| • G3 = 0.86 (0.72-1.02) | |||||
| • G4 = 0.91 (0.81-1.14) | |||||
| • G5 = 1.02 (0.82-1.27) | |||||
| Multivariate OR (95% CI) by number of VPA sessions/wk | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.72 (0.56-0.92) | |||||
| • G3 = 0.87 (0.73-1.03) | |||||
| • G4 = 1.00 (0.81-1.25) | |||||
| • G5 = 0.84 (0.61-1.15) | |||||
| Slattery et al 1997 [295] | To determine how physical inactivity interacts with other components of energy balance in determining risk of CC. | • n = 1,993 cases, 2,410 controls | PA Assessment: Interview for lifetime VPA (PA index) | Number of cases: 1,993 | These results support previous findings that physical inactiity is associated with an increased risk of developing CC. |
| USA | • Sex: Men and women | Q1 = 10-12 | Multivariate OR (95% CI), men | ||
| • Age: 30-79 yr | Q2 = 7-9 | • Q1 = 1.00 (referent) | |||
| • Characteristics: Cases: diagnosed with first primary CC. Controls: no history of CC | Q3 = 4-6 | • Q2 = 1.60 (1.11-1.75) | |||
| Case control | • The Three Centered Diet, Activity and Lifestyle Colon Cancer Study | Q4 = <4 | • Q3 = 1.59 (1.26-2.01) | ||
| D & B score = 14 | • Q4 = 1.63 (1.26-2.12) | ||||
| Outcome Measure: Diagnosed with CC | Multivariate OR (95% CI), women | ||||
| • Q1 = 1.00 | |||||
| • Q2 = 1.14 (0.86-1.52) | |||||
| Unconditional regression models | • Q3 = 1.13 (0.85-1.49 | ||||
| • Q4 = 1.59 (1.21-2.10) | |||||
| Takahashi et al 2007 [296] | To investigate the association between time spent walking each day and the risk of CRC. | • n = 20,519 men, 21,469 women | 7 year follow-up | • Number of cases: 101 | Time spent walking per day was associated with a lower risk of colon cancer in men but not in women. |
| • Sex: Men and women | PA assessment: Questionnaire for time spent walking (h/day) | • Number of dropouts: 3.5% | |||
| Japan | • Age: 40-64 yr | Multivariate RR (95% CI), men | |||
| • Characteristics: Free from cancer at baseline | • G1 = 1.00 (referent) | ||||
| Prospective cohort | G1 = <0.5 | • G2 = 0.72 (0.43-1.21) | |||
| G2 = 0.5-1 | • G3 = 0.38 (0.22-0.64) | ||||
| G3 = >1 | p < 0.001 | ||||
| D & B score = 12 | Outcome Measure: Incidence of CC | Time spent walking and incidence of CC | |||
| Multivariate RR (95% CI), women | |||||
| Cox proportional HR | • G1 = 1.00 | ||||
| • G2 = 2.68 (0.94-7.68) | |||||
| • G3 = 1.79 (0.64-4.96) | |||||
| p = 0.42 | |||||
| Tang et al 1999 [297] | To investigate the association between PA, water intake and risk of CRC in a hospital based case controlled study. | • n = 163 cases, 163 controls | PA assessment: Interview | • Number of cases: 163 | Found a negative association between LTPA and the risk of CC among men. |
| • Sex: Men and women | Multivariate RR (95% CI), men | ||||
| Taiwan | LTPA METs | • G1 = 1.00 (referent) | |||
| • Age: 33-80 yr | G1 = Sedentary | • G2 = 2.22 (0.68-7.21) | |||
| Case control | • Characteristics: Cases: Hospital patients diagnosed with colorectal cancer Controls: Hospital patients in hospital for other reasons, free of CRC. | G2 = Moderate (< 20 MET) | • G3 = 0.19 (0.05-0.77) | ||
| D & B score = 14 | G3 = Active (≥20 MET) | p = 0.03 | |||
| Multivariate RR (95% CI), women | |||||
| Outcome Measure: Diagnosis of CC | • G1 = 1.00 (referent) | ||||
| • G2 = 0.52 (0.13-2.03) | |||||
| • G3 = 0.63 (0.18-2.18) | |||||
| Conditional logistic regression analysis | p = 0.48 | ||||
| Tavani et al 1999 [298] | To investigate the relationship between PA and risk of CC in both sexes at different ages. | • n = 5,379 (1,225 cases and 4,154 controls) | PA assessment: Questionnaire on activity at work and during leisure time | • Number of cases: 537 women, 688 men | The study confirms that OPA is protective against CC. |
| Italy | • Sex: Men and women | Multivariate OR (95% CI) for CC by OPA at age 15-19 yr, men | |||
| Case control | • Age: 19-74 yr | G1 = Highest | • G1 = 1.00 (referent) | ||
| G2 | • G2 = 0.89 (0.64-1.23) | ||||
| D & B score = 13 | G3 | • G3 = 0.72 (0.54-0.97) | |||
| G4 | • G4 = 0.54 (0.40-0.74) | ||||
| G5 = Lowest | • G5 = 0.47 (0.31-0.71) | ||||
| p < 0.01 | |||||
| OPA at 30-39 yrs old | |||||
| Q1 = Highest | Multivariate OR (95% CI) for CC by OPA at age 15-19 yr, women | ||||
| Q2 | • G1 = 1.00 (referent) | ||||
| Q3 | • G2 = 0.73 (0.55-0.96) | ||||
| Q4 = Lowest | • G3 = 0.91 (0.69-1.21) | ||||
| Outcome Measure: Diagnosis of CC | • G4 = 0.62 (0.44-0.89) | ||||
| Unconditional multiple Logistic Regression | p < 0.05 | ||||
| Multivariate OR (95% CI) for CC by OPA at age 30-39 yr, men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.01 (0.75-1.37) | |||||
| • G3 = 0.79 (0.59-1.06) | |||||
| • G4 = 0.71 (0.52-0.97) | |||||
| • G5 = 0.64 (0.44-0.93) | |||||
| p < 0.01 | |||||
| Multivariate OR (95% CI) for CC by OPA at age 30-39 yr, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.65 (0.46-0.93) | |||||
| • G3 = 0.57 (0.41-0.79) | |||||
| • G4 = 0.49 (0.33-0.72) | |||||
| p < 0.01 | |||||
| Multivariate OR (95% CI) for CC by OPA at age 50-59 yr, men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.06 (0.78-1.43) | |||||
| • G3 = 0.85 (0.63-1.14) | |||||
| • G4 = 0.68 (0.49-0.95) | |||||
| • G5 = 0.69 (0.45-1.05) | |||||
| p < 0.01 | |||||
| Multivariate OR (95% CI) for CC by OPA at age 50-59 yr, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.69 (0.47-1.00) | |||||
| • G3 = 0.68 (0.46-1.00) | |||||
| • G4 = 0.75 (0.47-1.20) | |||||
| p = > 0.05 | |||||
| Multivariate OR (95% CI) for ascending CC by OPA at age 30-39 yr No significant associations for men or women | |||||
| Multivariate OR (95% CI) for transverse and descending CC by OPA at age 30-39 yr, men | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.92 (0.51-1.67) | |||||
| • Q3 = 0.76 (0.43-1.37) | |||||
| • Q4 = 0.46 (0.24-0.87) | |||||
| p < 0.05 | |||||
| Multivariate OR (95% CI) for transverse and descending CC by OPA at age 30-39 yr, women | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.51 (0.23-1.10) | |||||
| • Q3 = 0.39 (0.19-0.80) | |||||
| • Q4 = 0.29 (0.12-0.71) | |||||
| p < 0.01 | |||||
| Multivariate OR (95% CI) for sigmoid CC by OPA at age 30-39 yr, men | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 1.02 (0.65-1.57) | |||||
| • Q3 = 0.78 (0.51-1.20) | |||||
| • Q4 = 0.54 (0.34-0.85) | |||||
| p < 0.01 | |||||
| Multivariate OR (95% CI) for sigmoid CC by OPA at age 30-39 yr, women | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.62 (0.36-1.05) | |||||
| • Q3 = 0.71 (0.44-1.15) | |||||
| • Q4 = 0.58 (0.32-1.03) | |||||
| p > 0.05 | |||||
| Thune et al 1996 [299] | To examine the association between self-reported OPA and LTPA and the subsequent risk of CC. | • n = 81,516 (53,242 men, 28,274 women) | 16.3 year follow up | Number of cases: 236 men, 99 women | An inverse dose-response relationship between TPA and risk of CC was observed in women. In men this inverse dose-response was found only for those 45 yrs or older at study entry. |
| PA assessment: Questionnaire for TPA (OPA plus recreational PA (combined) | Multivariate RR (95% CI) for total CC, men | ||||
| Norway | • Sex: Men and women | G1 = Sedentary | • G1 = 1.00 (referent) | ||
| Prospective cohort | • Age: 20-49 yr | G2 = Moderate | • G2 = 1.18 (0.76-1.82) | ||
| • Characteristics: Free from cancer at baseline | G3 = Active | • G3 = 0.97 (0.63-1.50) | |||
| D & B score = 14 | p = 0.49 | ||||
| Multivariate RR (95% CI) for total CC, women | |||||
| Outcome Measure: Incidence of CC | • G1 = 1.00 (referent) | ||||
| Cox proportional HR | • G2 = 0.97 (0.33-2.77) | ||||
| • G3 = 0.63 (0.39-1.04) | |||||
| p = 0.04 | |||||
| Multivariate RR (95% CI) for proximal CC, men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.16 (0.57-2.34) | |||||
| • G3 = 0.96 (0.47-1.93) | |||||
| p = 0.64 | |||||
| Multivariate RR (95% CI) for proximal CC, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.22 (0.51-2.94) | |||||
| • G3 = 0.62 (0.30-1.28) | |||||
| p = 0.10 | |||||
| Multivariate RR (95% CI) for distal CC, men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.29 (0.72-2.33) | |||||
| • G3 = 0.99 (0.55-1.80) | |||||
| p = 0.53 | |||||
| Multivariate RR (95% CI) for distal CC, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.84 (0.32-2.17) | |||||
| • G3 = 0.61 (0.30-1.23) | |||||
| p = 0.15 | |||||
| Multivariate RR (95% CI) for total CC, men < 45 yrs at entry | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 2.02 (0.78-5.21) | |||||
| • G3 = 2.23 (0.88-5.66) | |||||
| p = 0.13 | |||||
| Multivariate RR (95% CI) for total CC, women < 45 yrs at entry | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.96 (0.39-2.40) | |||||
| • G3 = 0.62 (0.31-1.23) | |||||
| p = 0.13 | |||||
| Multivariate RR (95% CI) for total CC, men ≥ 45 yrs at entry | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.96 (0.59-1.58) | |||||
| • G3 = 0.66 (0.40-1.10) | |||||
| p = 0.04 | |||||
| Multivariate RR (95% CI) for total CC, women ≥ 45 yrs at entry | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.99 (0.41-2.39) | |||||
| • G3 = 0.66 (0.33-1.33) | |||||
| p = 0.19 | |||||
| Vena et al 1985 [300] | To assess the relationship between lifetime OPA and the risk of CC. | • n = 1,641 (210 cases, 1,431 control) | PA assessment: Questionnaire | • Number of cases: 210 | CC risk increased with increasing amount and proportion of time in jobs involving only sedentary or light work. |
| USA | • Sex: Men | Number of work years in jobs with sedentary or light work (yr) | OR (95% CI) by number of work years in jobs with sedentary or light work | ||
| Case control | • Age: 30-79 yr | G1 = None | • G1 = 1.00 (referent) | ||
| • Characteristics: Cases: admitted to hospital. Diagnosis of CC Controls: Admitted to hospital. Diagnosed with non-neoplastic non-digestive diseases | G2 = 1-20 | • G2 = 1.49 | |||
| D & B score = 15 | G3 = >20 | • G3 = 1.97 | |||
| OR (95% CI) by proportion of years in jobs with sedentary or light work | |||||
| Proportion of years in jobs with sedentary or light work | • G1 = 1.00 (referent) | ||||
| • G2 = 1.53 | |||||
| • G3 = 1.58 | |||||
| G1 = None | • G4 = 2.10 | ||||
| G2 = 0.01-0.50 | |||||
| G3 = 0.41-0.99 | OR (95% CI) by proportion of life in jobs with sedentary or light work | ||||
| G4 = 1.00 (referent) | |||||
| • G1 = 1.00 (referent) | |||||
| Proportion of life in jobs with sedentary or light work | • G2 = 1.66 | ||||
| • G3 = 1.83 | |||||
| G1 = None | |||||
| G2 = 0.01-0.40 | |||||
| G3 = 0.41-1.00 | |||||
| Outcome Measure: diagnosed with CC | |||||
| Multiple logistic regression | |||||
| Vetter et al 1992 [301] | To investigate the influence of OPA on the risk of CC in a developing country. | • n = 87 men cases, 13 women cases, 371 controls | PA assessment: Questionnaire Job title and industry names | Number of cases: 87 men, 13 women | This study presents a weak inverse association between CC and PA. |
| USA | • Sex: Men and women | OR (95% CI) Sitting time and CC | Only 2 of the 4 measures of activity showed evidence of an increased CC risk for sedentary jobs (time spent sitting and occupational EE) though neither was statistically significant. | ||
| Case control | • Age: 14-97 yr | Levels (Sitting time, energy expenditure | • G1 = 1.00 (referent) | ||
| D & B score = 11 | • Characteristics: Cases: Diagnosed with CC Controls: cancer cases other then colon, rectum and lung cancer. | G1 = High | • G2 = 1.0 (0.5-2.0) | ||
| G2 = Moderate | • G3 = 1.5 (0.7-2.9) | ||||
| G3 = Sedentary | p = 0.145 | ||||
| Outcome Measure: Diagnosed with CC | OR (95% CI) Energy Expenditure and CC | ||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.5 (0.7-3.3) | |||||
| • G3 = 1.6 (0.8-3.6) | |||||
| p = 0.143 | |||||
| White et al 1996 [302] | To assess the relationship between PA and CC among men and women. | • n = 871 (251 men, 193 women cases. 233 men & 194 women controls) | PA assessment: Phone interview | • Number of cases: 251 men & 193 women | The results of this study show modest support that recreational PA is associated with a reduced risk of CC. |
| USA | Total PA (episodes/wk) | RR (95% CI) by total PA, men | |||
| G1 = 0 | • G1 = 1.00 (referent) | ||||
| Case control | • Sex: Men and women | G2 = <1 | • G2 = 0.81 (0.45-1.44) | ||
| G3 = 1-<2 | • G3 = 0.53 (0.30-0.94) | ||||
| D & B score = 14 | • Age: 30-62 yr | G4 = 2-< 4 | • G4 = 0.57 (0.33-1.00) | ||
| • Characteristics: Cases: Diagnosed with CC, no previous history or CC or inflammatory bowel | G5 = ≥ 4 | • G5 = 0.57 (0.40-1.11) | |||
| Moderate-high intensity PA (epsiodes/wk) | p = 0.03 | ||||
| RR (95% CI) by total PA, women | |||||
| G1 = 0 | • G1 = 1.00 (referent) | ||||
| G2 = <1 | • G2 = 1.17 (0.57-2.40) | ||||
| G3 = 1-<2 | • G3 = 1.27 (0.65-2.45) | ||||
| Controls: No history of CC or inflammatory bowel | G4 = ≥ 2 | • G4 = 0.59 (0.34-1.04) | |||
| • G5 = 1.09 (0.61-1.97) | |||||
| High intensity PA (episodes/wk) | p = 0.52 | ||||
| G1 = 0 | RR (95% CI) by total PA, men and women | ||||
| G2 = <1 | • G1 = 1.00 (referent) | ||||
| G3 = ≥ 1 | • G2 = 0.94 (0.60-1.47) | ||||
| METS/wk | • G3 = 0.77 (0.50-1.19) | ||||
| Q1 = 0 | • G4 = 0.57 (0.39-0.85) | ||||
| Q2 = <7.30 | |||||
| Q3 = 7.30-17.88 | • G5 = 0.83 (0.57-1.22) | ||||
| Q4 = ≥ 17.88 | p = 0.04 | ||||
| Outcome Measure: Diagnosed with CC | RR (95% CI) by moderate-high intensity PA, men | ||||
| • G1 = 1.00 (referent) | |||||
| Unconditional logistic regression | • G2 = 0.84 (0.49-1.43) | ||||
| • Q3 = 0.75 (0.42-1.36) | |||||
| • Q4 = 0.66 (0.41-1.05) | |||||
| p = 0.07 | |||||
| RR (95% CI) by moderate-high intensity PA, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.07 (0.58-1.97) | |||||
| • G3 = 1.00 (0.51-1.98) | |||||
| • G4 = 0.74 (0.42-1.29) | |||||
| p = 0.37 | |||||
| RR (95% CI) by moderate-high intensity PA, men and women | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.93 (0.62-1.39) | |||||
| • Q3 = 0.86 (0.55-1.34) | |||||
| • Q4 = 0.70 (0.49-1.00) | |||||
| p = 0.05 | |||||
| RR (95% CI) by high intensity PA, men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.85 (0.48-1.52) | |||||
| • G3 = 0.57 (0.35-0.92) | |||||
| p = 0.02 | |||||
| RR (95% CI) by high intensity PA, Women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.02 (0.51-2.04) | |||||
| • G3 = 0.74 (0.43-1.28) | |||||
| p = 0.31 | |||||
| RR (95% CI) by high intensity PA, men and women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.93 (0.59-1.44) | |||||
| • G3 = 0.64 (0.45-0.92) | |||||
| p = 0.02 | |||||
| RR (95% CI) by METs/wk, men | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.64 (0.38-1.07) | |||||
| • Q3 = 0.59 (0.37-0.96) | |||||
| • Q4 = 0.69 (0.42-1.13) | |||||
| p = 0.05 | |||||
| RR (95% CI) by METs/wk, women | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.87 (0.51-1.49) | |||||
| • Q3 = 1.20 (0.69-2.08) | |||||
| • Q4 = 0.74 (0.41-1.34) | |||||
| p = 0.62 | |||||
| RR (95% CI) by METs/wk, women | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.73 (0.50-1.06) | |||||
| • Q3 = 0.80 (0.56-1.16) | |||||
| • Q4 = 0.73 (0.50-1.06) | |||||
| p = 0.08 | |||||
| Wolin et al 2007 [303] | To assess the relationship between PA and risk of CC in women. | • n = 79,295 | 16 year follow-up | Number of cases: 547 (245 distal, 302 proximal) Number of dropouts: 10% | A significant inverse association exists between PA, including moderate intensity, such as walking, and risk of CC in women that is more pronounced for distal tumours. |
| • Sex: Women | |||||
| • Age: 40-65 yr | PA assessment: Questionnaire | ||||
| USA | • Characteristics: Nurses, no history of CC, ulcerative colitis and Crohn's disease | ||||
| Prospective cohort | Level of PA | Multivariate RR (95% CI) for distal CC by level of PA | |||
| G1 = <2 | • G1 = 1.00 (referent) | ||||
| G2 = 2.1-4.5 | • G2 = 0.93 (0.64-1.36) | ||||
| D & B score = 14 | G3 = 4.6-10.3 | • G3 = 0.99 (0.68-1.44) | |||
| Nurses' Health Study | G4 = 10.4 - 21.4 | • G4 = 0.87 (0.59-1.29 | |||
| G5 = ≥ 21.5 | • G5 = 0.54 (0.34-0.84) | ||||
| MPA or VPA (hr/wk) | p = 0.004 | ||||
| G1 = 0 | Multivariate RR (95% CI) for proximal CC by level of PA not significant p = 0.77 | ||||
| G2 = <1 | |||||
| G3 = 1-1.9 | |||||
| G4 = 2-3.9 | |||||
| G5 = ≥ 4 | Multivariate RR (95% CI) for all CC by MPA or VPA | ||||
| Outcome Measure: Fatal and non fatal CC | • G1 = 1.00 (referent) | ||||
| • G2 = 0.85, (0.64-1.14) | |||||
| Cox proportional HR | • G3 = 0.74 (0.53-1.04) | ||||
| • G4 = 0.56 (0.33-0.94) | |||||
| p = 0.01 | |||||
| Multivariate RR (95% CI) for distal CC by MPA or VPA | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.10 (0.73-1.66) | |||||
| • G3 = 0.63 (0.36-1.10) | |||||
| • G4 = 0.51 (0.22-1.17) | |||||
| p = 0.04 | |||||
| Multivariate RR (95% CI) for proximal CC by MPA or VPA not significant p = 0.12 | |||||
| Zhang et al 2006 [304] | To examine the relationship between LTPA and OPA and the risk of CC by anatomic site and to evaluate their joint effect on the risk of CC. | • n = 585 cases 2,172 controls | PA assessment: Questionnaire for the following variables | Number of cases: 585 | Found a significant inverse association between reported LTPA and risk of CC with a slightly stronger association for the right colon than the left in both men and women. |
| USA | • Sex: Men and women | Multivariate OR (95% CI) by moderate- strenuous LTPA, men and women | |||
| • Age: 40-85 yr | Moderate-Strenuous LTPA | • G1 = 1.00 (referent) | |||
| Case control | • Characteristics: Case: diagnosed with CC Control: no history of CC. | G1 = <1 month | • G2 = 0.7 (0.5-1.1) | ||
| G2 = 1-4 months | • G3 = 0.6 (0.4-0.8) | ||||
| D & B score = 15 | G3 = ≥ 2 weeks | p = 0.003 | |||
| Multivariate OR (95% CI) by moderate- strenuous LTPA, men | The joint effect of OPA and LTPA suggested that the risk was lowest for those with high OPA and non-OPA. | ||||
| • G1 = 1.00 (referent) | |||||
| Outcome Measure: CC | • G2 = 0.9 (0.5-1.7) | ||||
| • G3 = 0.5 (0.3-0.9) | |||||
| Unconditional logistic regression models | p = 0.02 | ||||
| Multivariate OR (95% CI) by moderate-strenuous LTPA, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G3 = 0.5 (0.3-1.0) | |||||
| • G3 = 0.6 (0.4-0.9) | |||||
| p = 0.02 | |||||
| Multivariate OR (95% CI) by moderate-strenuous LTPA, men and women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.7 (0.5-1.1) | |||||
| • G3 = 0.8 (0.6-1.1) | |||||
| p = 0.53 | |||||
| Multivariate OR (95% CI) by moderate-strenuous LTPA, men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.9 (0.5-1.5) | |||||
| • G3 = 0.8 (0.6-1.2) | |||||
| p = 0.55 | |||||
| Multivariate OR (95% CI) by moderate- strenuous LTPA, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.6 (0.3-1.1) | |||||
| • G3 = 0.8 (0.5-1.2) | |||||
| p = 0.62 | |||||
| Multivariate OR (95% CI) by moderate- strenuous LTPA and OPA, OPA-Low | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.5 (0.3-0.9) | |||||
| • G3 = 0.8 (0.5-1.2) | |||||
| p = 0.41 | |||||
| Multivariate OR (95% CI) by moderate-strenuous LTPA and OPA, OPA-Medium | |||||
| • G1 = 0.7 (0.5-1.1) | |||||
| • G2 = 0.7 (0.4-1.3) | |||||
| • G3 = 0.5 (0.3-0.8) | |||||
| p = 0.04 | |||||
| Multivariate OR (95% CI) by moderate-strenuous LTPA and OPA, OPA-High | |||||
| • G1 = 0.9 (0.5-1.6) | |||||
| • G2 = 0.6 (0.3-1.3) | |||||
| • G3 = 0.5 (0.3-0.8) | |||||
D & B score, Downs and Black quality score; YR, years; PA, physical activity; OPA, occupational physical activity; kJ/min, kilojoules per minute; G, groups; MET, metabolic equivalent; HR, hazard ratio; RR, risk ratio; OR, odds ratio; 95% CI, confidence interval; LTPA, leisure-time physical activity; CC, colon cancer; TPA, total physical activity; MDA, moderate physical activity; h/d, hours per day; VPA, vigorous physical activity; h/wk, hours per week.
Breast Cancer
As reviewed eloquently by others, the epidemiological evidence relating physical activity to a decreased incidence of breast cancer is persuasive. A recent systematic review of the literature found that more than 60 observational trials have examined the relationship between physical activity and breast cancer [31]. Previous reviews of the literature have revealed compelling and consistent findings indicating that habitual physical activity is associated with a reduced risk for breast cancer ranging from 20-80% [31,104].
Various investigations have attempted to evaluate the dose-response relationship between physical activity and the incidence of breast cancer (Table 16). Despite the volume of evidence available questions still remain regarding the minimal and optimal volume of exercise required to reduce the risk for breast cancer. As discussed by others [31,104] the findings are as varied as the investigations.
Table 16.
Studies examining the relationship between physical activity and breast cancer.
| Publication Country Study Design Quality Score | Objective | Population | Methods | Outcome | Comments and Conclusions |
|---|---|---|---|---|---|
| Rockhill et al 1999 [106] | To examine the effect of PA on the risk for BC. | • n = 121,701 | PA assessment: Self-reported LTPA, grouped into hr/wk | 3,137 cases of BC | Women who engaged in 7 or more hours per week of MVPA had a 20% lower risk of BC. An inverse dose-response relationship existed between PA and BC incidence. |
| • Sex: Women | |||||
| • Age: 30-55 yr | RR (95% CI) for BC and LTPA | ||||
| USA | • 16-yr follow-up | G1 = <1 | |||
| • Characteristics: Free of BC | G2 = 1.0-1.9 | • G1 = 1.00 (referent) | |||
| Prospective cohort | G3 = 2.0-3.9 | • G2 = 0.88 (0.79-0.98) | |||
| • The nurses Health Study | G4 = 4.0-6.9 | • G3 = 0.89 (0.81-0.99) | |||
| D & B score = 13 | G5 = ≥7 | • G4 = 0.85 (0.77-0.94) | |||
| Multivariate pooled logistic regression | • G5 = 0.82 (0.70-0.97) | ||||
| Trend p = 0.004 | |||||
| Sesso et al 1998 [107] | To examine the association between PA and BC among postmenopausal women. | • n = 1,566 | 31-yr follow-up | 109 cases of BC | There is an inverse relationship between PA and BC in post-menopausal women. |
| • Sex: Women | |||||
| USA | • Age: 45.5 | PA assessment: Questionnaire at baseline, divided into tertiles (kcal/wk) | RR (95% CI) for BC and PA | ||
| • Characteristics: Free of BC | |||||
| Prospective cohort | • T1 = 1.00 (referent) | ||||
| T1 = <500 | • T2 = 0.92 (0.58-1.45) | ||||
| D & B score = 14 | T2 = 500-999 | ||||
| T3 = ≥ 1,000 | • T3 = 0.73 (0.46-1.14) | ||||
| RR (95% CI), post-menopausal women only | |||||
| • T1 = 1.00 (referent) | |||||
| • T2 = 0.95 (0.58-1.57) | |||||
| • T3 = 0.49 (0.28-0.86) | |||||
| Dosemeci et al 1993 [278] | To conduct a multiple-site case-control study of 15 cancers to examine associations between PA, SES, and these cancer sites among workers. | • n = 2,643 control group | Cases: obtained from an oncological treatment center from 1979-1984 | 31 men had BC and 241 women had BC | This study shows the sitting-time index showed an elevated risk of female BC for sedentary jobs without SES adjustment. |
| • n = 2,127 men and n = 244 women) | |||||
| • Sex: Men and women | |||||
| Turkey | • Characteristics: Cases - diagnosed with one of the 15 cancers being examined. Control Group - subjects diagnosed with non-cancers, cancers of the buccal cavity, esophagus, liver, bone, soft tissue, brain, lymphoma and other cancer sites for which there is no suggestion on an association with PA | Adjusted SES OR (95%CI), men | |||
| Controls: pulled from the same hospital as the cases | |||||
| Case control | • G1 = 1.40 (0.60-3.90) | ||||
| D & B score = 12 | PA assessment: OPA (kJ/min) | • G2 = 1.10 (0.40-3.10) | |||
| G1 = <8 | • G3 = 1.00 (referent) | The slightly elevated risk of male BC was based on a small number and disappeared when the risk was adjusted for SES. | |||
| G2 = 8-12 | Trend p = 0.34 | ||||
| G3 = >12 | |||||
| Adjusted SES OR (95%CI), women | |||||
| Gart's method and Mantel's chi-square test | |||||
| • G1 = 1.10 (0.60-2.10) | |||||
| • G2 = 0.90 (0.50-1.80) | |||||
| • G3 = 1.00 (referent) | |||||
| Trend p = 0.23 | |||||
| Bernstein et al 1994 [305] | To determine whether young women who regularly participate in PA during their reproductive years had a reduced risk of BC. | • n = 1,090 (545 cases; 545 controls) | PA assessment: Questionnaire for overall participation in PA after menarche (h/wk), PA within 10 years after menarche (h/wk), each divided into 5 groups: | Adjusted OR (95% CI) by PA after menarche | PA may substantially reduce a women's lifetime risk of BC. |
| • Sex: Women | • G1 = 1.00 (referent) | ||||
| USA | • Age: ≤ 40 yr | • G2 = 0.95 (0.64-1.41) | |||
| • Characteristics: White women matched for age and parity | |||||
| Case control | • G3 = 0.65 (0.45-0.96) | ||||
| D & B score = 15 | G1 = none | • G4 = 0.80 (0.54-1.17) | |||
| G2 = 0.1-0.7 | |||||
| G3 = 0.8-1.6 | • G5 = 0.42 (0.27-0.64) | ||||
| G4 = 1.7-3.7 | |||||
| G5 = ≥ 3.8 | Trend p = 0.0001 | ||||
| Logistic regression | Adjusted OR (95% CI) by PA within 10 years after menarche | ||||
| • None = 1.00 (referent) | |||||
| • 0.1-1.2 = 0.93 (0.63-1.38) | |||||
| • 1.3-2.9 = 0.78 (0.52-1.19) | |||||
| • 3.0-5.5 = 0.69 (0.45-1.05) | |||||
| • ≥5.6 = 0.70 (0.47-1.06) | |||||
| Trend p = 0.027 | |||||
| Adjusted OR (95% CI) by PA after menarche, nulliparous women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.81 (0.42-1.57) | |||||
| • G3 = 0.65 (0.35-1.21) | |||||
| • G4 = 0.94 (0.53-1.67) | |||||
| • G5 = 0.73 (0.38-1.41) | |||||
| Trend p = 0.43 | |||||
| Adjusted OR (95% CI) by PA after menarche, parous women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.06 (0.65-1.74) | |||||
| • G3 = 0.65 (0.40-1.06) | |||||
| • G4 = 0.70 (0.42-1.18) | |||||
| • G5 = 0.38 (0.16-0.50) | |||||
| Trend p < 0.0001 | |||||
| Bernstein et al 2005 [306] | To examine the relationship between BC risk and lifetime and time- or age-specific measures of LTPA among white and black women. | • n = 9,187 (4,538 cases; 4,649 control) | Cases: histologically confirmed cases of BC | 4,538 cases of BC | This study supports an inverse association between PA and BC among black women and among white women. |
| • Sex: Women | |||||
| • Age: 35-64 | Multivariate adjusted OR (95%CI) annual MET h/wk, White participants | ||||
| USA | • Ethnicity: White (including Hispanics) or Black | Controls: random-digit dialing methods | |||
| • Characteristics: Case Group: histologically confirmed cases of invasive BC | |||||
| Case control | • Control Group: healthy | • G1 = 1.00 (referent) | |||
| PA assessment: | • G2 = 0.84 (0.71-0.99) | ||||
| D & B score = 13 | Questionnaire for lifetime PA (MET h/wk), divided into 5 groups | • G3 = 0.89 (0.75-1.04) | |||
| G1 = Inactive | • G4 = 0.82 (0.69-0.97) | The relationship appears to be similar between black and white women. | |||
| G2 = ≤ 2.2 | • G5 = 0.81 (0.69-0.96) | ||||
| G3 = 2.3-6.6 | Trend p = 0.09 | ||||
| G4 = 6.7-15.1 | |||||
| G5 = ≥ 15.2 | |||||
| Unconditional logistic regression modeling | Multivariate adjusted OR (95%CI) annual MET h/wk, Black participants | ||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.11 (0.91-1.35) | |||||
| • G3 = 0.83 (0.67-1.03) | |||||
| • G4 = 0.79 (0.63-0.99) | |||||
| • G5 = 0.77 (0.62-0.95) | |||||
| Trend p = 0.003 | |||||
| Carpenter et al. 1999 [307] | To examine whether lifetime exercise activity is related to BC risk in post-menopausal women. | • n = 2,027 (1,123 case; 904 control) | Cases: diagnosed with primary invasive or in situ BC | 1,123 cases of BC | Strenuous exercise appears to reduce BC risk among post-menopausal women who do not gain sizable amounts of weight during adulthood. |
| • Sex: Women | Multivariate adjusted OR (95%CI) | ||||
| USA | • Age: 55-64 yr | ||||
| • Ethnicity: White (including Hispanic) | Controls: individually matched to each case patient based on birth date and race | • G1 = 1.00 (referent) | |||
| Case control | • Characteristics: post-menopausal, English-speaking, born in USA, Canada or Western Europe | • G2 = 0.88 (0.72-1.07) | |||
| D & B score = 15 | • G3 = 0.55 (0.37-0.83) | ||||
| PA assessment: Questionnaire for lifetime PA (MET hr/wk), divided into 3 groups | Trend p = 0.01 | ||||
| G1 = no activity | |||||
| G2 = 0.1-17.59 | |||||
| G3 = ≥17.6 | |||||
| Conditional logistic regression | |||||
| Carpenter et al 2003 [308] | To examine the effects of obesity and lifetime exercise patterns on post-menopausal BC risk according to family history. | • n = 3,511 (cases n = 1,883, controls) n = 1,628 | PA assessment: Interview for the following PA variables | 1,883 cases of BC | Exercise independent of body size seemed to exert a protective effect primarily among women with a negative family history. |
| • Sex: Women | Adjusted OR (95% CI) by lifetime exercise between menarche and reference date (MET hr/wk) | ||||
| USA | • Age: 55-72 | ||||
| • Characteristics: Postmenopausal Women | Lifetime exercise between menarche and reference date (MET hr/wk) | ||||
| Case control | |||||
| D & B score = 15 | G1 = 0 | • G1 = 1.00 (referent) | |||
| G2 = 0.1-3.74 | • G2 = 0.85 (0.71-1.03) | ||||
| G3 = 3.75-8.74 | |||||
| G4 = 8.75-17.59 | • G3 = 0.87 (0.69-1.10) | ||||
| G5 = ≥17.60 | |||||
| • G4 = 1.02 (0.79-1.30) | |||||
| Average exercise activity in 10 years prior to reference date (MET hr/wk) | |||||
| • G5 = 0.66 (0.48-0.90) | |||||
| G1 = 0 | Trend p = 0.07 | ||||
| G2 = 0.1-6.9 | |||||
| G3 = 7.0-13.9 | Adjusted OR (95% CI) by average exercise activity in 10 years prior to reference date (MET hr/wk) | ||||
| G4 = 14.0-24.4 | |||||
| G5 = ≥24.5 | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.93 (0.71-1.22) | |||||
| • G3 = 0.92 (0.70-1.19) | |||||
| • G4 = 0.86 (0.65-1.11) | |||||
| • G5 = 0.75 (0.55-1.02) | |||||
| Trend p = 0.05 | |||||
| Chang et al 2006 [309] | To address the independent and combined effects of energy intake, BMI, and PA on BC incidence in women. | • n = 27,541 | 9.3 year follow-up (median 4.9 yr) | 764 women developed BC | The study suggests that energy intake, BMI and physical inactivity are each independently and positively associated with BC risk. |
| • Sex: Women | |||||
| USA | • Age: 55-74 | ||||
| • Characteristics: no history of any cancer (nonmelanoma skin cancer patients were included in the trial) | PA assessment: Questionnaire for vigorous PA (h/wk), divided into 6 groups | Multivariate adjusted RR (95%CI) | |||
| Prospective cohort | • Prostate, Lung, colorectal, and Ovarian Cancer Screening Trial | G1 = 0 | |||
| G2 = <1 | • G1 = 1.00 (referent) | ||||
| D & B score = 13 | G3 = 1 | • G2 = 0.89 (0.69-1.15) | |||
| G4 = 2 | • G3 = 0.96 (0.73-1.26) | ||||
| G5 = 3 | • G4 = 0.90 (0.70-1.16) | ||||
| G6 = ≥4 | • G5 = 1.02 (0.79-1.30) | ||||
| • G6 = 0.78 (0.61-0.99) | |||||
| Cox proportional HR | Trend p = 0.153 | ||||
| Colditz et al 2003 [310] | To evaluate the relationship between PA and risk of pre-menopausal BC by type of activity and within subgroups of adiposity and oral contraceptive use. | • n = 110,468 | PA assessment: Self report on 8 activities (walking or hiking, jogging (>10 min mile), running, Biking, racquet sports, lap swimming, calisthenics/aerobics other aerobic activities) to calculate MET scores (MET hrwk), divided into 5 groups: | Total cases diagnosed n = 849 | These data among pre-menopausal women suggest that there is no overall association between PA and risk of BC. The findings also suggest that the effect of PA could be substantially modified by the underlying degree of adiposity. |
| • Sex: Women | |||||
| USA | • Age: 25-42 | ||||
| • Characteristics: pre-menopausal, no history of cancer other than nonmelanoma skin cancer | Multivariate adjusted RR (95% CI) | ||||
| Prospective cohort | • G1 = 1.00 (referent) | ||||
| D & B score = 12 | • G2 = 1.05 (0.82-1.34) | ||||
| • G3 = 0.96 (0.75-1.23) | |||||
| G1 = <3 | • G4 = 1.05 (0.80-1.37) | ||||
| G2 = 3-8.9 | |||||
| G3 = 9-17.9 | • G5 = 1.07 (0.84-1.36) | ||||
| G4 = 18-26.9 | |||||
| G5 = ≥27 | Trend p = 0.69 | ||||
| Cox proportional HR | |||||
| Coogan et al 1997 [311] | To evaluate the effect of OPA on BC risk. | • n = 11,646 (4,863 cases and 6,783 controls). | PA assessment: Telephone interview to estimate OPA, divided into tertiles: | 4,863 cases of BC | There was evidence of a graded inverse relationship between the intensity of work related activity and the incidence of BC. |
| OR (95% CI) | |||||
| USA | • Sex: Women | T1 = Sedentary | • T1 = 1.00 (referent) | ||
| • Age: <74 yr | T2 = Medium activity jobs | • T2 = 0.86 (0.77-0.97) | |||
| Case control | T3 = Heavy jobs | ||||
| • T3 = 0.82 (0.63-1.08) | |||||
| D & B score = 14 | Logistic regression models | ||||
| Coogan and Aschengrau 1999 [312] | To evaluate the effect of OPA on BC risk. | • n = 903 (233 case; 670 control) | PA assessment: Telephone interview to estimate OPA, divided into tertiles: | 233 cases of BC | There was no evidence that holding a job of medium/heavy activity reduced BC. |
| • Sex: Women | OR (95%CI) | ||||
| • Age: <50 - 80+ | T1 = Exclusively sedentary | • T1 = 1.00 (referent) | |||
| USA | • Ethnicity: White, Black or Other | T2 = Exclusively light | • T2 = 1.20 (0.70-1.90) | ||
| • Characteristics: must have worked outside the home. Cases: All incident cases of BC reported to the Massachusetts Cancer Registry from 1983 to 1986 were eligible | T3 = Exclusively medium or heavy | • T3 = 0.90 (0.40-1.90) | The study was limited by OPA misclassification and by the lack of information on LTPA. | ||
| Case control | Trend p = 0.63 | ||||
| D & B score = 14 | Miettinen's test-based method and Fisher's exact method | ||||
| Dallal et al 2007 [313] | To examine the relationship between LTPA and invasive and in situ BC among women. | • n = 110,599 | 6.6 yr follow-up | 2,649 cases of invasive | The results support a protective role of strenuous long-term exercise activity against invasive and in situ BC and suggest differing effects by hormone receptor status. |
| • Sex: Women | BC | ||||
| USA | • Age: 20-79 | PA assessment: Self-reported participation in moderate and strenuous activities to estimate annual strenuous physical activity (hr/wk), divided into quintiles | 593 cases of in situ BC | ||
| • Ethnicity: White, Black, Hispanic, Asian, American Indian or other | |||||
| Prospective cohort | • Characteristics: California resident at baseline and no history of BC | Multivariate adjusted RR (95% CI) for invasive BC | |||
| D & B score = 12 | • California Teachers Study cohort | • Q1 = 1.00 (referent) | |||
| • Q2 = 0.93 (0.85-1.02) | |||||
| Q1 = 0.00-0.50 | |||||
| Q2 = 0.51-2.00 | • Q3 = 0.88 (0.78-0.99) | ||||
| Q3 = 2.01-3.50 | |||||
| Q4 = 3.51-5.00 | • Q4 = 1.02 (0.88-1.18) | ||||
| Q5 = >5 | |||||
| • Q5 = 0.80 (0.69-0.94) | |||||
| Cox proportional HR | |||||
| Trend p = 0.02 | |||||
| Multivariate adjusted RR (95% CI) for in situ BC | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.96 (0.79-1.17) | |||||
| • Q3 = 0.86 (0.67-1.11) | |||||
| • Q4 = 0.95 (0.70-1.30) | |||||
| • Q5 = 0.69 (0.48-0.98) | |||||
| Trend p = 0.40 | |||||
| Dirx et al 2001 [314] | To evaluate the relationship between PA and BC risk with specific emphasis on interaction with other aspects of energy balance. | • n = 62,537 | 7.3 yr follow-up | 1,208 cases of incident BC | The current study supports the hypotheses that PA is related inversely to BC risk in postmenopausal women. |
| • Sex: Women | |||||
| Netherlands | • Age: 55-69 | PA assessment: Questionnaire for total recreational PA (min/day), divided into quartiles | |||
| • Characteristics: healthy, postmenopausal | Multivariate adjusted RR (95% CI) | ||||
| Case study | |||||
| • Q1 = 1.00 (referent) | |||||
| D & B score = 11 | Q1 = <30 | • Q2 = 0.84 (0.67-1.07) | |||
| Q2 = 30-60 | |||||
| Q3 = 61-90 | • Q3 = 0.78 (0.60-1.00) | ||||
| Q4 = >90 | |||||
| • Q4 = 0.76 (0.58-0.99) | |||||
| Exponentially distributed failure time regression models | |||||
| Trend p = 0.003 | |||||
| Dorn et al 2003 [315] | To examine the associations between LTPA and OPA across the lifespan and pre- and post-menopausal BC. | • n = 1,550 (740 case; 810 control) | Cases: women diagnosed and histologically confirmed with BC | 740 cases of BC | The study supports the hypothesis that strenuous LTPA is associated with a reduced risk of BC risk in both pre- and post menopausal women |
| USA | • Sex: Women | Multivariate adjusted OR (95%CI), pre- menopausal | |||
| • Age: 40-85 | |||||
| Case control | • Characteristics: Case Group -- histologically confirmed incidence of BC. Control Group -- healthy | Controls: randomly selected and frequency matched on age and county with the cases. | |||
| • G1 = 1.00 (referent) | |||||
| D & B score = 13 | • G2 = 0.94 (0.64-1.38) | ||||
| • G3 = 0.73 (0.44-1.22) | |||||
| PA assessment: Questionnaire for lifetime strenuous PA (hr/yr) | • G4 = 1.07 (0.57-2.02) | ||||
| Trend p = 0.82 | |||||
| G1 = 0 | |||||
| G2 = 1-273 | |||||
| G3 = 274-545 | Multivariate adjusted OR (95%CI), post-menopausal | ||||
| G4 = >546 | |||||
| Logistic regression | • G1 = 1.00 (referent) | ||||
| • G2 = 0.85 (0.61-1.19) | |||||
| • G3 = 0.73 (0.45-1.17) | |||||
| • G4 = 0.78 (0.47-1.29) | |||||
| Trend p = 0.19 | |||||
| Drake 2001 [316] | To evaluate PA as a predictor of BC and describe BC risk factors in this sample. | • n = 4,520 | PA assessment: Self-report of type, intensity, duration and frequency of walking, jogging, biking, stationary biking, swimming, dancing, racket sports, stretching, participating in other exercise, calisthenics, weight-lifting and treadmill exercises, divided into groups | 150 incident cases of breast cancer | Increased frequency of a specific PA (jogging) was found to have an important protective role in BC incidence. |
| • Sex: Women | |||||
| USA | • Age: 21-86 | ||||
| • Characteristics: no diagnosis of BC at entry | OR (95% CI) for BC and PA | ||||
| Prospective cohort | |||||
| D & B score = 11 | • Aerobic Center Longitudinal Study | Activity type | |||
| • G1 = 1.32 | |||||
| • G2 = 1.08 | |||||
| • G3 = 1.35 | |||||
| Trend p = 0.05 | |||||
| G1 = Aerobic (job, bike, aerobic dance) | |||||
| G2 = Moderate (golf, walk) | |||||
| G3 = Weight training | |||||
| Chi-square | |||||
| Friedenreich et al 2001 [317] | To examine the type and dose of PA and the time periods in life when PA may be specifically associated with BC risk. | • n = 2,470 (1,233 case; 1,237 control) | Cases: in situ and invasive cases of BC from 1995-1997 | 1,233 cases of BC | This study provides evidence that lifetime PA reduces risk of post-menopausal BC. |
| Canada | • Sex: Women | OR (95%CI), pre-menopausal | |||
| Case Control | • Age: ≤ 80 | Controls: matched to cases on age and place of residence | • Q1 = 1.00 (referent) | ||
| • Characteristics: Case Group - Alberta residents, English speaking, capable of completing an in-person interview. Control Group -- no history of cancer diagnoses excluding nonmelanoma skin cancer | • Q2 = 1.15 (0.78- 1.70) | ||||
| D & B score = 13 | PA assessment: Questionnaire for lifetime PA (MET hr/wk/yr), divided into quartiles by menopausal status | • Q3 = 1.15 (0.78- 1.69) | |||
| Pre-menopausal | • Q4 = 1.07 (0.72- 1.61) | ||||
| Q1 = <86.6 | Trend p = 0.50 | ||||
| Q2 = 86.6-108.3 | OR (95%CI), post- menopausal | ||||
| Q3 = 108.3-134.9 | • Q1 = 1.00 (referent) | ||||
| Q4 = ≥ 134.9 | • Q2 = 0.73 (0.55- 0.98) | ||||
| Post-menopausal | • Q3 = 0.75 (0.56- 1.00) | ||||
| Q1 = <104.8 | • Q4 = 0.70 (0.52- 0.94) | ||||
| Q2 = 104.8-128.1 | Trend p = 0.003 | ||||
| Q3 = 128.1-160.9 | |||||
| Q4 = ≥ 160.9 | |||||
| Logistic regression | |||||
| Friedenreich et al 2001 [318] | To examine the influence of frequency, duration, and intensity of PA on risk of BC and to compare BC risks associated with self-reported versus assigned intensity of PA. | • n = 2,470 (1,233 case; 1,237 control) | Cases: in situ and invasive cases of BC | 1,233 cases of BC | This study found that moderate- intensity activities were the major contributors to the decrease in BC risk found in this study. |
| Canada | • Sex: Women | Multivariate adjusted OR (95% CI), pre- menopausal | |||
| Case control | • Age: ≤ 80 | Controls: matched to cases on age and place of residence | • Q1 = 1.00 (referent) | ||
| D & B score = 13 | • Characteristics: Case Group -- resident of Alberta, English speaking and able to complete an in-person interview. Control Group -- free of any cancer diagnosis excluding nonmelanoma skin cancer | PA assessment: Questionnaire for lifetime PA questionnaire (MET hr/wk/yr), divided into quartiles | • Q2 = 1.19 (0.80- 1.76) | ||
| Q1 = <28.8 | • Q3 = 1.33 (0.90- 1.96) | ||||
| Q2 = 28.8-35.4 | • Q4 = 1.07 (0.71- 1.62) | ||||
| Q3 = 35.4-42.7 | Trend p = 0.52 | ||||
| Q4 = ≥ 42.7 | Multivariate adjusted OR (95% CI), post- Menopausal | ||||
| Logistic regression | • Q1 = 1.00 (referent) | ||||
| • Q2 = 0.85 (0.64- 1.14) | |||||
| • Q3 = 0.83 (0.62- 1.10) | |||||
| • Q4 = 0.69 (0.51- 0.93) | |||||
| Trend p = 0.006 | |||||
| Friedenreich and Rohan 1995 [319] | To describe the association between LTPA and BC. | • n = 902 (451 case; 451 control) | Cases: first diagnosis of BC in 1982 and 1984 | Adjusted OR (95%CI), pre-menopausal | This study found some evidence (of borderline statistical significance) that recreational PA is associated with decreased risk of BC. |
| Australia | • Sex: Women | • Q1 = 1.00 (referent) | |||
| Case control | • Age: 20-74 yr | Controls: Randomly selected from the electoral roll, matched on date of birth to each case | • Q2 = 0.77 (0.36- 1.65) | ||
| D & B score = 13 | • Characteristics: Australian women | PA assessment: Self reported PA (kcal/wk), divided into quartiles | • Q3 = 0.48 (0.22- 1.03) | ||
| Q1 = 0 | • Q4 = 0.60 (0.30- 1.17) | ||||
| Q2 = 1-2,000 | Trend p = 0.09 | ||||
| Q3 = 2000-4000 | Adjusted OR (95%CI), post-menopausal | ||||
| Q4 = >4000 | • Q1 = 1.00 (referent) | ||||
| Logistic regression models | • Q2 = 0.74 (0.46- 1.18) | ||||
| • Q3 = 0.88 (0.53- 1.48) | |||||
| • Q4 = 0.73 (0.44- 1.20) | |||||
| Trend p = 0.32 | |||||
| Gammon et al 1998 [320] | To examine the association between LTPA and BC among young women. | • n = 3,173 (1,668 case; 1,505 control) | Cases: women diagnosed with BC between 1990-1992 | 1,668 cases of BC | The study's data does not support the hypothesis of a reduced risk of BC among young women with increased recreational PA in adolescence, young adulthood or during the year prior to the interview, or with the average PA over the three time periods |
| USA | • Sex: Women | Multivariate adjusted OR (95%CI) | |||
| Case control | • Age: <45 | Controls: were matched to cases by age group and geographic center | • Q1 = 1.00 (referent) | ||
| D & B score = 13 | • Characteristics: Case Group -- diagnosed with invasive or in situ BC. Control Group - healthy | PA assessment: Questionnnaire for recreational PA, for ages 12- 13 yr, age 20 yr and 1 year prior to the interview. Divided into quartiles MET score | • Q2 = 0.79 (0.63- 0.98) | ||
| Q1 = 1.62-18.07 | • Q3 = 0.98 (0.79- 1.22) | ||||
| Q2 = 18.08-30.00 | • Q4 = 1.01 (0.81- 1.25) | ||||
| Q3 = 30.01-42.95 | Trend p = 0.42 | ||||
| Q4 = 42.96-98.00 | |||||
| Logistic regression | |||||
| Gilliland et al 2001 [321] | To investigate the relationship of PA with BC risk in Hispanic and non- Hispanic White women | • n = 1,556 (712 case; 844 control) | Cases: diagnosed with BC between 1992-1994 | 712 cases of BC | Hispanic and non- Hispanic women with high PA during non-OPA were at substantially reduced risk of BC. |
| USA | • Sex: Women | ||||
| Case control | • Age: between 35-74 at diagnosis | Controls: matched on ethnicity, age and seven health planning districts | Adjusted OR (95%CI), pre-menopausal Hispanic | ||
| D & B score = 13 | • Ethnicity: Hispanic and non-Hispanic White | PA assessment: Self-reported non-OPA (MET hr/wk score) | • G1 = 1.00 (referent) | ||
| • Characteristics: Case Group -- diagnosed with in situ or invasive BC and residents of New Mexico at time of diagnosis. Control Group -- healthy | G1 = <25 | • G2 = 1.17 (0.53- 2.55) | |||
| G2 = 25-50 | • G3 = 0.49 (0.22- 1.07) | ||||
| G3 = 50-80 | • G4 = 0.29 (0.12- 0.72) | ||||
| G4 = ≥ 80 | Trend p < 0.001 | ||||
| Logistic regression | Adjusted OR (95%CI), pre-menopausal non- Hispanic | ||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.35 (0.64- 2.85) | |||||
| • G3 = 1.44 (0.67- 3.10) | |||||
| • G4 = 1.13 (0.49- 2.61) | |||||
| Trend p = 0.741 | |||||
| Adjusted OR (95%CI), post-menopausal Hispanic | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.74 (0.40- 1.36) | |||||
| • G3 = 0.37 (0.18- 0.75) | |||||
| • G4 = 0.38 (0.18- 0.77) | |||||
| Trend p = 0.002 | |||||
| Adjusted OR (95%CI), post-menopausal non- Hispanic | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.45 (0.26- 0.78) | |||||
| • G3 = 0.49 (0.28- 0.86) | |||||
| • G4 = 0.45 (0.24- 0.85) | |||||
| Trend p = 0.019 | |||||
| Hsing et al 1998 [322] | To evaluate the role of selected demographic, lifestyle, and anthropometric factors in the risk for male BC. | • n = 690 (178 case; 512 control) | Cases: selected from 18,733 decedents included in the 1986 NMFS conducted by the US | 178 cases of BC | This study suggests that obesity increases the risk of male BC, possibly through hormonal mechanisms, while dietary factors, PA and SES indicators also deserve further investigation. |
| USA | • Sex: Men | National Center for Health Statistics (NCHS) | Adjusted OR (95%CI) | ||
| • Age: 25-74 | • G1 = 1.00 (referent) | ||||
| Case control | • Ethnicity: Black and White | • G2 = 0.60 (0.30- 1.10) | |||
| D & B score = 12 | • Characteristics: Case Group -- deceased. Control Group -- dying (or deceased) of causes other than BC | Controls: selected from male decedents dying of causes other than BC | • G3 = 1.30 (0.80- 2.00) | ||
| PA assessment: Questionnaire (frequency and intensity), divided into groups | |||||
| G1 = Regular | |||||
| G2 = Irregular | |||||
| G3 = Hardly any | |||||
| Logistic regression analysis | |||||
| Hu et al 1997 [323] | To study breast cancer focusing on breast-feeing, body weight, and PA as well as reproductive histories on pre- and post- menopausal Japanese women. | • n = 526 (157 case; 369 control) | Cases: Histologically confirmed cases of BC from 1989-1993. | 157 cases of BC | Reduced risk of pre- menopausal BC was associated with high EE in PA during teenage years, although the trend was not statistically significant. |
| Japan | • Sex: Women | Unadjusted RR (95%CI), pre-menopausal | |||
| Case control | • Age: 26-75 | • G1 = 1.00 (referent) | |||
| D & B score = 13 | • Characteristics: Case Group -- histologically confirmed cases of BC and resident of Gifu prefecture at time of diagnosis. Control Group -- no breast disease or hormone- related (ovarian, endometrial and thyroid) cancers | Controls: individuals who had the screening test for BC during the same period | • G2 = 0.74 (0.38- 1.44) | ||
| PA assessment: Questionnaire for TPA (kcal/wk), divided into groups | • G3 = 1.01 (0.54- 1.87) | ||||
| G1 = 0 | Trend p = 0.876 | ||||
| G2 = 1-649 | Unadjusted RR (95%CI), post-menopausal: | ||||
| G3 = ≥ 650 | • G1 = 1.00 (referent) | ||||
| • G2 = 1.53 (0.69- 3.54) | |||||
| Logistic regression models | • G3 = 1.39 (0.61- 3.13) | ||||
| John et al 2003 [324] | To examine BC risk in relation to lifetime histories of MPA and VPA including LTPA, transportation household and outdoor chores, and OPA in a multiethnic population. | • n = 2,870 (1,277 case; 1,593 control) | Cases: diagnosed between 1995-1998 | 1,277 cases of BC | This study supports previous reports of a reduced risk of BC in physically active women. |
| USA | • Sex: Women | Multivariate adjusted OR (95%CI), pre- menopausal Latinas | |||
| Case control | • Age: 35-79 | • G1 = 1.00 (referent) | |||
| D & B score = 12 | • Ethnicity: Latina, African-American and White | Controls: randomly selected according race/ethnicity and age distribution of cases | • G2 = 0.84 (0.49- 1.45) | ||
| PA assessment: In-person interview for lifetime PA (hr/wk), divided into groups | • G3 = 0.73 (0.42- 1.28) | ||||
| Pre-menopausal | Multivariate adjusted OR (95%CI), pre- menopausal African Americans | ||||
| G1 = <9.1 | • G1 = 1.00 (referent) | ||||
| G2 = 9.1-20.7 | • G2 = 1.00 (0.55- 1.84) | ||||
| G3 = ≥ 20.7 | • G3 = 0.68 (0.35- 1.34) | ||||
| Post-menopausal | |||||
| G1 = <9.6 | |||||
| G2 = 9.6-21.6 | |||||
| G3 = ≥ 21.7 | |||||
| Logistic regression modeling | Multivariate adjusted OR (95%CI), pre- menopausal Whites | ||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.82 (0.42- 1.58) | |||||
| • G3 = 0.76 (0.36- 1.61) | |||||
| Multivariate adjusted OR (95%CI), post- menopausal Latinas | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.82 (0.55- 1.24) | |||||
| • G3 = 0.81 (0.54- 1.22) | |||||
| Multivariate adjusted OR (95%CI), post- menopausal African Americans | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.78 (0.52- 1.17) | |||||
| • G3 = 0.71 (0.47- 1.07) | |||||
| Multivariate adjusted OR (95%CI), post- menopausal Whites | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.94 (0.64- 1.37) | |||||
| • G3 = 0.91 (0.60- 1.41) | |||||
| Kruk 2007 [325] | To examine the association between all types of PA and BC risk among Polish women. | • n = 590 (268 case; 322 control) |
PA assessment: Questionnaire for lifetime PA (MET hr/wk/yr), divided into groups | 268 cases of BC | The results of this study provide evidence of an inverse association between PA and the risk of BC. |
| Poland | • Sex: Women | G1 = <110 | Multivariate adjusted OR (95%CI), pre- menopausal | ||
| Case control | • Age: 35-75 yr | G2 = 110-150 | • G1 = 1.00 (referent) | ||
| D & B score = 13 | • Characteristics: Polish women. Cases: identified from the Szczecin Regional Cancer Registry. Controls: matched on age and place of residence | G3 = >150 | • G2 = 0.45 (0.14- 1.44) | ||
| Logistic regression analysis | • G3 = 0.44 (0.14- 1.37) | ||||
| Trend p = 0.42 | |||||
| Multivariate adjusted OR (95%CI), post- menopausal | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.60 (0.33- 1.09) | |||||
| • G3 = 0.31 (0.21- 0.70) | |||||
| Trend p = 0.002 | |||||
| Kruk 2007 [326] | To examine the relationship between LTPA and BC risk. | • n = 822 (cases n = 257, control n = 565 | PA assessment: Questionnaire for LTPA (METs), divided into groups | Adjusted OR (95% CI) | The findings provide further support to the hypothesis that increased LTPA throughout life is associated with a decreased risk of BC. |
| Poland | • Sex: Women | G1 = Low | • G1 = 1.00 (referent) | ||
| Case control | • Age: 35-93 yr | G2 = Medium | • G2 = 0.57 (0.36- 0.89) | ||
| D & B score = 13 | G3 = High | • G3 = 0.22 (0.14- 0.35) | |||
| Trend p < 0.0001 | |||||
| Lahmann et al 2007 [327] | To examine the association of PA with pre- and post-menopausal BC risk. | • n = 218,169 | Baseline and 6.4 year follow-up | 3,423 cases of BC | Increasing PA reduces BC risk. |
| Europe (9 countries) | • Sex: Women | Multivariate adjusted HR (95% CI) by TPA, pre- menopausal | |||
| Prospective cohort | • Age: 20-80 | • Q1 = 1.00 (referent) | |||
| D & B score = 12 | • The European Prospective Investigation into Cancer and nutrition study | PA assessment: Interviews and questionnaire for TPA and recreational PA, each divided into quartiles | • Q2 = 1.02 (0.84- 1.24) | ||
| TPA Index | • Q3 = 0.84 (0.68- 1.04) | ||||
| Q1 = Inactive | • Q4 = 1.02 (0.77- 1.36) | ||||
| Q2 = Moderately inactive | Trend p = 0.267 | ||||
| Q3 = Moderately active | Multivariate adjusted HR (95% CI) by TPA, Post- menopausal | ||||
| Q4 = Active | • Q1 = 1.00 (referent) | ||||
| Recreational PA (MET hr/wk) | • Q2 = 0.89 (0.79- 1.00) | ||||
| Q1 = <14 | • Q3 = 0.84 (0.74- 0.96) | ||||
| Q2 = 14-24 | • Q4 = 0.92 (0.76- 1.12) | ||||
| Q3 = 25-42 | Trend p = 0.06 | ||||
| Q4 = >42 | Multivariate adjusted HR (95% CI) by recreational PA, pre-menopausal | ||||
| Cox proportional index | • Q1 = 1.00 (referent) | ||||
| • Q2 = 0.91 (0.75- 1.10) | |||||
| • Q3 = 0.95 (0.78- 1.14) | |||||
| • Q4 = 0.94 (0.76- 1.15) | |||||
| Trend p = 0.580 | |||||
| Multivariate adjusted HR (95% CI) by recreational PA, post-menopausal | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 1.05 (0.94- 1.17) | |||||
| • Q3 = 0.92 (0.83- 1.03) | |||||
| • Q4 = 0.96 (0.85- 1.08) | |||||
| Trend p = 0.176 | |||||
| Lee et al 2001 [328] | To examine the association between PA and BC risk. | • n = 39,322 | Baseline and 4 year follow- up | 411 cases of BC | The data suggest that PA during middle age and older is not uniformly associated with decreased BC risk. Among post- menopausal women only, higher levels of PA may decrease the risk of BC. |
| USA | • Sex: Women | ||||
| • Age: ≥ 45 yr | Multivariate adjusted RR (95% CI) by PA, all women | ||||
| Prospective cohort | • Characteristics: Healthy women | PA assessment: Questionnaire | • Q1 = 1.00 (referent) | ||
| D & B score = 12 | Women's Health Study | PA (kJ/wk), divided into quartiles | • Q2 = 1.04 (0.77- 1.40) | ||
| Q1 = <840 | • Q3 = 0.86 (0.64- 1.17) | ||||
| Q2 = 840-2519 | • Q4 = 0.80 (0.58- 1.12) | ||||
| Q3 = 2520-6299 | Trend p = 0.11 | ||||
| Q4 = ≥ 6300 | Multivariate adjusted RR (95% CI) by PA, post- menopausal only | ||||
| VPA (kJ/wk), divided into quintiles | • Q1 = 1.00 (referent) | ||||
| Q1 = none | • Q2 = 0.97 (0.68- 1.39) | ||||
| Q2 = 1-839 | • Q3 = 0.78 (0.54- 1.12) | ||||
| Q3 = 840-2099 | • Q4 = 0.67 (0.44- 1.02) | ||||
| Q4 = 2100-4199 | Trend p = 0.03 | ||||
| Q5 = ≥ 4200 | Multivariate adjusted RR (95% CI) by VPA, all women | ||||
| Proportional hazard regression | • Q1 = 1.00 (referent) | ||||
| • Q2 = 1.02 (0.70- 1.48) | |||||
| • Q3 = 1.11 (0.78- 1.58) | |||||
| • Q4 = 0.97 (0.66- 1.44) | |||||
| • Q5 = 0.98 (0.69- 1.40) | |||||
| Trend p = 0.98 | |||||
| Multivariate adjusted RR (95% CI) by VPA, post- menopausal only | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.93 (0.57- 1.50) | |||||
| • Q3 = 0.91 (0.57- 1.47) | |||||
| • Q4 = 0.93 (0.57- 1.50) | |||||
| • Q5 = 0.76 (0.47- 1.24) | |||||
| Trend p = 0.29 | |||||
| Magnusson et al 2005 [329] | To report the relationship between pre-menopausal BC, body fatness at age 10 years and in adulthood, and sports participation during puberty, late adolescence and early adulthood from three related case-control studies. | • n = 3,108 (1,560 cases; 1,548 controls) | PA assessment: Interview for sports participation (h/wk in the following age categories (12-14 yr, 16-18 yr, 20-30 yr, 12-30 yr, around age of diagnosis) | Adjusted RR (95% CI), 12-14 yr | An inverse association between body fatness but not PA at a young age and the risk of BC in pre-menopausal women. |
| UK | • Sex: Women | • G1 = 1.00 (referent) | |||
| Case control | • Age: Study 1 = 36 yr, study 2 = 36-45 yr, study 3 = 46-54 yr | • G2 = 1.04 (0.93- 1.17) | |||
| D & B score = 13 | • Characteristics: White women with no previous malignancy, mental handicap or illness | Sports participation (h/wk) | • G3 = 1.03 (0.93- 1.14) | ||
| G1 = 0-1 | Trend p = 0.95 | ||||
| G2 = 2-3 | Adjusted RR (95% CI), 16-18 yr | ||||
| G3 = ≥ 4 | • G1 = 1.00 (referent) | ||||
| • G2 = 0.95 (0.83- 1.09) | |||||
| • G3 = 0.89 (0.79- 1.02) | |||||
| Trend p = 0.20 | |||||
| Adjusted RR (95% CI), 20-30 yr | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.90 (0.76- 1.08) | |||||
| • G3 = 1.01 (0.81- 1.26) | |||||
| Trend p = 0.73 | |||||
| Adjusted RR (95% CI), 12-30 yr | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.99 (0.89- 1.11) | |||||
| • G3 = 1.01 (0.88- 1.16) | |||||
| Trend p = 0.94 | |||||
| Adjusted RR (95% CI), around age of diagnosis | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.84 (0.71- 1.00) | |||||
| • G3 = 1.06 (0.86- 1.32) | |||||
| Trend p = 0.82 | |||||
| Malin et al 2005 [330] | To evaluate a pattern of behavioral exposures indicating positive energy balance would be associated with increased BC risk. | • n = 3,015 (1,459 cases; 1,556 control) | PA assessment: Questionnaire for PA (MET hr/d/yr), divided into groups | OR (95% CI) | The study suggests that promotion of behavioral patterns that optimize energy balance maybe a viable option for BC prevention. |
| China | • Sex: Women | G1 = 0 | • G1 = 1.86 (1.44- 2.41) | ||
| Case control | • Age: Mean ~47 yr | G2 = 0.1-2.92 | • G2 = 1.33 (0.96- 1.83) | ||
| D & B score = 12 | • Characteristics: Residents of urban Shanghai | G3 = >2.92 | • G3 = 1.00 (referent) | ||
| Shanghai Breast Cancer Study | |||||
| Margolis et al 2005 [331] | To study the association between PA and incident invasive BC. | • n = 99,504 | Baseline and 9.1 year follow-up | 1,166 cases of BC | No evidence of a protective effect of PA on BC risk was found. |
| Norway/Sweden | • Sex: Women | ||||
| Prospective cohort | • Age: 30-49 (mean 41 yr) | PA assessment: Questionnaire for PA using a 5 point scale and for competitive PA (years of participation), each divided into groups | Multivariate adjusted RR (95% CI) by PA level, at enrollment | ||
| D & B score = 13 | • The Norwegian- Swedish Women's Lifestyle and Health Study | PA level (5 point scale) | • G1 = 1.00 (referent) | ||
| G1 = None | • G2 = 1.35 (0.96- 1.90) | ||||
| G2 = Low | • G3 = 1.26 (0.91- 1.74) | ||||
| G3 = Moderate | • G4 = 1.19 (0.85- 1.67) | ||||
| G4 = High | • G5 = 1.24 (0.85- 1.82) | ||||
| G5 = Vigorous | Trend p = 0.85 | ||||
| Competitive PA (years) | Multivariate adjusted RR (95% CI) by PA level, at age 30 | ||||
| G1 = None | • G1 = 1.00 (referent) | ||||
| G2 = 1-4 | • G2 = 1.03 (0.64- 1.66) | ||||
| G3 ≥ 5 | • G3 = 1.16 (0.74- 1.81) | ||||
| • G4 = 1.06 (0.67- 1.68) | |||||
| •G5 = 1.20 (0.77- 1.95) | |||||
| Trend p = 0.60 | |||||
| Multivariate adjusted RR (95% CI) by PA level, at age 14 | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.93 (0.62- 1.39) | |||||
| • G3 = 0.94 (0.65- 1.35) | |||||
| • G4 = 1.07 (0.73- 1.55) | |||||
| • G5 = 1.05 (0.72- 1.54) | |||||
| Trend p = 0.14 | |||||
| Multivariate adjusted RR (95% CI) by years of competitive PA | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.21 (0.95- 1.54) | |||||
| • G3 = 0.95 (0.75- 1.19) | |||||
| Trend p = 0.96 | |||||
| McTiernan et al 1996 [332] | To investigate the relationship between LTPA and BC. | • n = 1,029 (cases n = 537, controls n = 492) | PA assessment: Questionnaire (Minnesota LTPA Questionnaire) for LTPA (hr/wk), divided into groups | Adjusted OR (95% CI) by LTPA during adulthood, all ages and menopausal status | The results indicate a weak negative association between PA and risk of BC in middle-aged women. |
| USA | • Sex: Women | G1 = None | • G1 = 1.00 (referent) | ||
| Case control | • Age: 50-64 | G2 = 0.1-1.5 | • G2 = 1.1 (0.7-1.6) | ||
| D & B score = 13 | G3 = 1.6-2.5 | • G3 = 0.7 (0.4-1.1) | |||
| G4 = 2.6-3.5 | • G4 = 0.7 (0.4-1.1) | ||||
| G5 = 3.6-5.0 | • G5 = 0.6 (0.4-0.9) | ||||
| G6 = >5 | • G6 = 1.1 (0.7-1.6) | ||||
| Calculated categories of EE (total time x intensity code) | Trend p = 0.29 | ||||
| G1 = Lowest | Adjusted OR (95% CI) by LTPA during adulthood, aged ≥ 55 yr, post-menopausal only | ||||
| G6 = Highest | • G1 = 1.00 (referent) | ||||
| • G2 = 0.8 (0.5-1.3) | |||||
| • G3 = 0.5 (0.3-0.9) | |||||
| • G4 = 0.6 (0.4-1.1) | |||||
| • G5 = 0.4 (0.2-0.8) | |||||
| • G6 = 0.8 (0.5-1.3) | |||||
| Trend p = 0.03 | |||||
| Adjusted OR (95% CI) by category of total EE in adulthood, all ages and menopausal status | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.2 (0.8-2.0) | |||||
| • G3 = 0.9 (0.6-1.3) | |||||
| • G4 = 0.6 (0.4-0.9) | |||||
| • G5 = 0.9 (0.6-1.5) | |||||
| • G6 = 0.9 (0.6-1.4) | |||||
| Trend p = 0.25 | |||||
| Adjusted OR (95% CI) by category of total EE in adulthood, aged ≥ 55 yr, post-menopausal only | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.8 (0.4-1.4) | |||||
| • G3 = 0.7 (0.4-1.2) | |||||
| • G4 = 0.5 (0.3-0.8) | |||||
| • G5 = 0.8 (0.5-1.3) | |||||
| • G6 = 0.6 (0.4-1.0) | |||||
| Trend p = 0.009 | |||||
| McTiernan et al 2003 [333] | To examine the association between current and past LTPA and incidence of BC in post-menopausal women. | • n = 74,171 | Baseline and mean follow-up of 4.7 years | 1,780 cases of BC | Increased PA is associated with reduced risk for BC in post-menopausal women. |
| USA | • Sex: Women | PA assessment: Questionnaire for TPA (MET hr/wk), moderate or strenuous PA (hr/wk) and strenuous PA (hr/wk), each divided into groups | Adjusted RR (95% CI) by TPA | Longer duration provides the most benefit however need not be strenuous. | |
| Prospective cohort | • Age: 50-79 | TPA (MET hr/wk) | • G1 = 1.00 (referent) | ||
| D & B score = 13 | • Characteristics: Women from the Women's Health Initiative Observational Study | G1 = none | • G2 = 0.90 (0.77-1.07) | ||
| G2 = 0-5.0 | • G3 = 0.82 (0.68-0.97) | ||||
| G3 = 5.1-10.0 | • G4 = 0.89 (0.76-1.00) | ||||
| G4 = 10.1-20.0 | • G5 = 0.83 (0.70-0.98) | ||||
| G5 = 20.1-40 | • G6 = 0.78 (0.62-1.00) | ||||
| G6 = ≥ 40.0 | Trend p = 0.03 | ||||
| Moderate or strenuous PA (hr/wk) | Adjusted RR (95% CI) by TPA, | ||||
| G1 = none | BMI ≤ 24.13 | ||||
| G2 = ≤ 1 | • G1 = 1.00 (referent) | ||||
| G3 = 1.1-2.0 | • G2 = 0.78 (0.57-1.10) | ||||
| G4 = 2.1-3.0 | • G3 = 0.70 (0.51-0.97) | ||||
| G5 = 3.1-4.0 | • G4 = 0.80 (0.60-1.10) | ||||
| G6 = 4.1-7.0 | • G5 = 0.68 (0.51-0.92) | ||||
| G7 = >7.0 | • G6 = 0.63 (0.43-0.93) | ||||
| Strenuous PA (hr/wk) | Trend p = 0.03 | ||||
| G1 = none | Adjusted RR (95% CI) by TPA, BMI 24.14-28.44 | ||||
| G2 = ≤ 1.0 | • G1 = referent | ||||
| G3 = 1.1-2.0 | • G2 = 0.72 (0.53-0.98) | ||||
| G4 = 2.1-4.0 | • G3 = 0.78 (0.57-1.10) | ||||
| G5 = >4.0 | • G4 = 0.77 (0.58-1.00) | ||||
| Cox proportional hazard ratio | • G5 = 0.85 (0.64-1.10) | ||||
| • G6 = 0.78 (0.52-1.20) | |||||
| Trend p = 0.74 | |||||
| Adjusted RR (95% CI) by TPA, BMI >28.44 | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.10 (0.88-1.50) | |||||
| • G3 = 0.90 (0.67-1.20) | |||||
| • G4 = 1.00 (0.79-1.30) | |||||
| • G5 = 0.89 (0.65-1.20) | |||||
| • G6 = 0.94 (0.57-1.60) | |||||
| Trend p = 0.30 | |||||
| Adjusted RR (95% CI) by current moderate or strenuous PA | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.92 (0.78-1.10) | |||||
| • G3 = 0.91 (0.79-1.10) | |||||
| • G4 = 0.94 (0.81-1.10) | |||||
| • G5 = 0.99 (0.83-1.20) | |||||
| • G6 = 0.91 (0.78-1.10) | |||||
| • G7 = 0.79 (0.63-0.99) | |||||
| Trend p = 0.12 | |||||
| Adjusted RR (95% CI) by current strenuous PA | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.94 (0.80-1.10) | |||||
| • G3 = 0.95 (0.80-1.10) | |||||
| • G4 = 0.93 (0.78-1.10) | |||||
| • G5 = 0.91 (0.67-1.20) | |||||
| Trend p = 0.25 | |||||
| Navarro Silvera et al 2006 [334] | To study the independent and combined associations of VPA, energy consumption and BMI with risk of subsequent BC. | • n = 40,318 in analysis (49,613 prior to exclusion) | Baseline and 16.4 year follow-up | 1,673 cases of BC from the 40,318 included in the analysis (2,545 cases from total prior to exclusion) | The results of the study suggest that BC risk may vary according to various combinations of the components of energy balance. |
| Canada | • Sex: Women | PA assessment: Questionnaire for VPA (min/d), divided into groups | Adjusted HR (95% CI) by VPA | ||
| Prospective cohort | • Age: 40-59 | G1 = none | • G1 = 1.00 (referent) | ||
| D & B score = 13 | • Characteristics: Canadian women with no history of BC | G2 = Any | • G2 = 0.98 (0.85-1.13) | ||
| • National Breast Screening Study (NBSS) | G3 = 0-30 | • G3 = 1.06 (0.88-1.27) | |||
| G4 = 30-60 | • G4 = 0.98 (0.83-1.16) | ||||
| G5 > 60 | • G5 = 0.93 (0.78-1.10) | ||||
| Cox proportional hazard ratio | Trend p = 0.38 | ||||
| Adjusted HR (95% CI) by VPA, pre-menopausal | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.91 (0.75-1.10) | |||||
| • G3 = 1.02 (0.80-1.31) | |||||
| • G4 = 0.88 (0.70-1.11) | |||||
| • G5 = 0.87 (0.68-1.09) | |||||
| Trend p = 0.23 | |||||
| Adjusted HR (95% CI) by VPA, post- menopausal | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.06 (0.87-1.30) | |||||
| • G3 = 1.08 (0.81-1.42) | |||||
| • G4 = 1.11 (0.87-1.41) | |||||
| • G5 = 1.00 (0.78-1.29) | |||||
| Trend p = 0.96 | |||||
| Patel et al 2003 [335] | To examine the association between various measures of PA and post-menopausal BC risk. | • n = 72,608 | Baseline and 5 year follow-up | 1,520 cases of breast cancer | The study shows a lower risk of post- menopausal BC is associated with regular PA. |
| USA | • Sex: Women | PA assessment: Questionnaire for LTPA (METs hr/wk) at various times during life, divided into groups | Adjusted RR (95% CI), LTPA at study entry | ||
| Prospective cohort | • Age: 50-74 | G1 = none | • G1 = 0.86 (0.70- 1.04) | ||
| D & B score = 14 | • Characteristics: Postmenopausal women | G2 = 0.1-6.9 | • G2 = 1.00 (referent) | ||
| • The American Cancer Society Cancer Prevention Study II (CPS-II) Nutritional Cohort | G3 = 7.0-17.5 | • G3 = 0.92 (0.81- 1.04) | |||
| G4 = 17.6-31.5 | • G4 = 0.94 (0.81- 1.09) | ||||
| G5 = 31.6-42.0 | • G5 = 0.77 (0.56- 1.06) | ||||
| G6 = >42.0 | • G6 = 0.71 (0.49- 1.02) | ||||
| LTPA at 10 years prior to study, calculated MET score and categorized into groups: | Trend p = 0.08 (among active women p = 0.03) | ||||
| None | Adjusted RR (95% CI), LTPA at age 40 yr | ||||
| Slight | • G1 = 1.03 (0.88- 1.21) | ||||
| Moderate | • G2 = 1.00 (referent) | ||||
| Heavy | • G3 = 1.05 (0.92- 1.20) | ||||
| Cox proportional hazard ratio | • G4 = 1.01 (0.87-1.18) | ||||
| • G5 = 1.16 (0.92- 1.46) | |||||
| • G6 = 0.79 (0.61- 1.03) | |||||
| Trend p = 0.31 (among active women p = 0.36) | |||||
| Adjusted RR (95% CI), LTPA at 10 years prior to study entry | |||||
| • None = 0.80 (0.51- 1.25) | |||||
| • Slight = 1.00 (referent) | |||||
| • Moderate = 0.93 (0.83-1.04) | |||||
| • Heavy = 0.87 (0.68- 1.13) | |||||
| Trend p = 0.32 (among active women, trend p = 0.16) | |||||
| Patel et al 2003 [336] | To evaluate the association between lifetime LTPA and BC risk. | • n = 1,183 (cases n = 616) n = 567, controls n = 616) | PA assessment: Calendar reporting for lifetime exercice activity (MET h/wk), divided into groups | Adjusted OR (95% CI) | The findings suggest that PA may modify the risk of in situ BC particularly in women without a family history of BC. |
| USA | • Sex: Women | G1 = None | • G1 = 1.00 (referent) | ||
| Case control | • Age: 35-64 | G2 = 0.0-3.0 | • G2 = 0.70 (0.48- 1.03) | ||
| D & B score = 14 | • Characteristics: White and Black women | G3 = 3.0-8.0 | • G3 = 0.65 (0.44- 0.96) | ||
| G4 = 8.0-16.0 | • G4 = 0.61 (0.41- 0.92) | ||||
| G5 = 16.0-32.0 | • G5 = 0.63 (0.40- 0.98) | ||||
| G6 = >32.0 | • G6 = 0.65 (0.39- 1.08) | ||||
| Unconditional logistical regressioin | Trend p = 0.27 (among exercisers only p = 0.81) | ||||
| Rintala et al 2002 [337] | To obtain an estimate of BC incidence in association with self-rated OPA. | • n = 680,000 | PA assessment: Self-reported OPA in 5 classes (1=low, 5=high) | 17,986 cases of BC | The results support the hypothesis that OPA, if high enough, markedly reduced BC risk. |
| Finland | • Sex: Women | Class 1 = Jobs sitting and light hand tasks | Adjusted RR (95% CI), age 25-39 years | ||
| Prospective cohort | • Age: Women born in 1930-1969 | Class 2 = Handling of heavier items (conveyor belt) | • C1+2 = 1.00 (referent) | ||
| D & B score = 11 | • Characteristics: Finish women | Class 3 = Jobs involving body motion | • C3 = 0.99 (0.85- 1.17) | ||
| Class 4 = Jobs involving walking up stairs or long distances, bending and carrying | • C4 = 0.90 (0.76- 1.07) | ||||
| Class 5 = Same as class 4 except heavy tasks were performed for most of the day | • C5 = 0.68 (0.51- 1.93) | ||||
| Poisson regression models | Trend | ||||
| Adjusted RR (95% CI), age 40-54 years | |||||
| • C1+2 = 1.00 (referent) | |||||
| • C3 = 1.02 (0.94- 1.11) | |||||
| • C4 = 0.99 (0.91- 1.09) | |||||
| • C5 = 0.84 (0.70- 1.00) | |||||
| Trend | |||||
| Adjusted RR (95% CI), age ≥ 55 years | |||||
| • C1+2 = 1.00 (referent) | |||||
| • C3 = 1.01 (0.96- 1.07) | |||||
| • C4 = 1.04 (0.98- 1.11) | |||||
| • C5 = 0.82 (0.71- 0.94) | |||||
| Trend | |||||
| Rockhill et al 1998 [338] | To examine the association between PA at two different times in life and BC risk. | • n = 372 | Baseline and 6 year follow-up | 372 cases of BC | The findings do not support a link between PA in late adolescence or in the recent past and BC risk among young adult women. |
| USA | • Sex: Women | PA assessment: Questionnaire for MVPA (h/wk) | Multivariate adjusted RR (95% CI) | ||
| Prospective cohort | • Age: 25-42 | G1 = <1 | • G1 = 1.00 (referent) | ||
| D & B score = 12 | • Characteristics: Nurses | G2 = 1.0-1.9 | • G2 = 1.1 (0.8-1.4) | ||
| • The Nurses Health Study | G3 = 2.0-3.9 | • G3 = 1.1 (0.8-1.4) | |||
| G4 = 4.0-6.9 | • G4 = 1.0 (0.7-1.4) | ||||
| G5 = ≥ 7 | • G5 = 1.1 (0.8-1.5) | ||||
| Logistic regression | |||||
| Slattery et al 2007 [339] | To evaluate the BC risk associated with TPA and VPA at ages 15, 30 and 50 years and the referent year prior to diagnosis/selection. | • n = 4,850 Non-Hispanic white: n = 3,128 (cases n = 1,527 controls n = 1601); Hispanic American Indian: n = 1,722 (cases n = 798, controls n = 924) | PA assessment: Questionnaire for TPA (activity score) and lifetime VPA (h/wk) | 1527 cases of BC (non- Hispanic white; 798 cases of BC (Hispanic American Indian) | The data suggest that PA is important in reducing risk of BC in non-Hispanic white and Hispanic American Indian women. |
| USA | • Sex: Women | TPA score | |||
| Case control | • Age: <50 yr | G1 = 0-3 | |||
| D & B score = 12 | • Characteristics: Non- Hispanic white and Hispanic American Indian | G2 = 4-6 | OR (95% CI) by TPA score, non-Hispanic white | ||
| G3 = 7-9 | • G1 = 1.00 (referent) | ||||
| G4 = 10-12 | • G2 = 0.78 (0.52- 1.17) | ||||
| Lifetime VPA | • G3 = 0.84 (0.57- 1.22) | ||||
| G1 = None | • G4 = 0.70 (0.44- 1.12) | ||||
| G2 = <1.0 | Trend p = 0.26 | ||||
| G3 = 1.0-2.9 | OR (95% CI) by TPA score, Hispanic American Indian | ||||
| G4 = ≥ 3.0 | • G1 = 1.00 (referent) | ||||
| Multivariable logistic regression | • G2 = 1.49 (0.98- 2.26) | ||||
| • G3 = 1.21 (0.80- 1.84) | |||||
| • G4 = 0.97 (0.53- 1.76) | |||||
| Trend p = 0.90 | |||||
| OR (95% CI) by lifetime VPA, non-Hispanic white | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.66 (0.36- 1.23) | |||||
| • G3 = 0.73 (0.40- 1.34) | |||||
| • G4 = 0.69 (0.37- 1.27) | |||||
| Trend p = 0.68 | |||||
| OR (95% CI) by lifetime VPA, Hispanic American Indian | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.15 (0.67- 1.96) | |||||
| • G3 = 1.19 (0.70- 2.03) | |||||
| • G4 = 1.09 (0.62- 1.90) | |||||
| Trend p = 0.84 | |||||
| Sprague et al 2007 [340] | To investigate the relationship between LTPA and strenuous OPA and BC risk. | • n = 15,710 (1,689 cases in situ; 6,391 invasive and 7,630 controls) | PA assessment: Questionnaire for lifetime TPA (hr/wk and MET hr/wk), divided into groups | Adjusted OR (95% CI) for in situ BC by lifetime TPA (hr/wk) | The results provide further evidence that for most women, PA may reduce the risk of invasive BC. |
| USA | • Sex: Women | Lifetime total PA (hr/wk) | • G1 = 1.00 (referent) | ||
| Case control | • Age: 20-69 | G1 = 0 | • G2 = 0.92 (0.72-1.19) | ||
| D & B score = 13 | • The Collaborative Breast Cancer Study | G2 = 0.1-15.0 | • G3 = 0.83 (0.62-1.13) | ||
| G3 = 15.1-30.0 | • G4 = 0.86 (0.59- 1.24) | ||||
| G4 = > 30.0 | Trend p = 0.22 | ||||
| MET hr/wk | Adjusted OR (95% CI) for in situ BC by lifetime TPA (MET hr/wk) | ||||
| G1 = 0.0 | • G1 = 1.00 (referent) | ||||
| G2 = 0.1-62.5 | • G2 = 0.93 (0.72- 1.20) | ||||
| G3 = 62.6-125.0 | • G3 = 0.82 (0.61- 1.10) | ||||
| G4 = >125.0 | • G4 = 0.82 (0.57- 1.17) | ||||
| Trend p = 0.10 | |||||
| Adjusted OR (95% CI) for invasive BC by lifetime TPA (hr/wk) | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.88 (0.76- 1.03) | |||||
| • G3 = 0.87 (0.73- 1.05) | |||||
| • G4 = 0.85 (0.67- 1.07) | |||||
| Trend p = 0.22 | |||||
| Adjusted OR (95% CI) for invasive BC by lifetime TPA (MET hr/wk) | |||||
| • G1 = 1.00 (referent) | |||||
| •G2 = 0.89 (0.76- 1.04) | |||||
| • G3 = 0.82 (0.68- 0.99) | |||||
| • G4 = 0.88 (0.71- 1.09) | |||||
| Trend p = 0.12 | |||||
| Steindorf et al 2003 [341] | To clarify the relationship between PA and BC risk. | • n = 1,246 (360 cases; 886 controls) | PA assessment: Computer assisted telephone interview for TPA (MET hr/wk) at various ages | 360 cases of BC | The data do not suggest an inverse association between PA and BC risk in pre-menopausal women. |
| Germany | • Sex: Women | TPA at age 12-19 yr | Multivariate adjusted OR (95% CI) by TPA at age 12-19 yr | ||
| Case control | • Age: Mean. cases 41.9 yr; controls 42.5 yr | G1 = 13.0-55.7 | • G1 = 1.00 (referent) | ||
| D & B score = 13 | G2 = 55.8-88.7 | • G2 = 1.07 (0.75- 1.52) | |||
| G3 = 88.8-134.0 | • G3 = 1.00 (0.70- 1.42) | ||||
| G4 = 134.1-695.9 | • G4 = 0.73 (0.50- 1.07) | ||||
| TPA at age 20-30 yr | Trend p = 0.44 | ||||
| G1 = 6.4-69.0 | Multivariate adjusted OR (95% CI) by TPA at age 20-30 yr | ||||
| G2 = 69.1-109.0 | • G1 = 1.00 (referent) | ||||
| G3 = 109.1-160.4 | • G2 = 0.95 (0.67- 1.37) | ||||
| G4 = 160.5-728.8 | • G3 = 0.85 (0.59- 1.23) | ||||
| TPA at age 12-30 yr (both) | • G4 = 0.96 (0.67- 1.39) | ||||
| G1 = 17.2-70.4 | Trend p = 0.32 | ||||
| G2 = 70.5-104.0 | Multivariate adjusted OR (95% CI) by TPA at age 12-30 yr | ||||
| G3 = 104.1-145.5 | • G1 = 1.00 (referent) | ||||
| G4 = 145.6-564.4 | • G2 = 0.97 (0.68- 1.38) | ||||
| Logistic regression | • G3 = 0.68 (0.46- 0.99) | ||||
| G4 = 0.94 (0.65- 1.35) | |||||
| Trend p = 0.29 | |||||
| Tehard et al 2006 [342] | To investigate the type, duration, frequency and intensity of PA required to reduce the risk of BC. | • n = 90,509 | Baseline and follow-up every 2 years for 12 years | 3,424 cases of BC | BC risk was reduced, especially with VPA. |
| France | • Sex: Women | PA assessment: Questionnaire for various PA variables, all divided into groups | Multivariate adjusted RR (95% CI) by TPA | ||
| Prospective cohort | • Age: 40-65 | TPA (MET hr/wk) | • G1 = 1.00 (referent) | ||
| D & B score = 13 | • Characteristics: French women insured with Mutuelle Generale de l'Education Nationale | G1 = <28.3 | • G2 = 1.05 (0.93- 1.17) | ||
| • E3N Cohort Study | G2 = 28.3-41.8 | • G3 = 0.94 (0.83- 1.05) | |||
| G3 = 41.8-57.8 | • G4 = 0.90 (0.80- 1.02) | ||||
| Trend p < 0.05 | |||||
| G4 = ≥ 57.8 | Multivariate adjustedRR (95% CI) by total recreational PA | ||||
| Total recreational PA (MET hr/wk) | • G1 = 1.00 (referent) | ||||
| G1 = Inactive | • G2 = 0.82 (0.71- 0.93) | ||||
| G2 = <16.0 | • G3 = 0.94 (0.84- 1.06) | ||||
| G3 = 16.0-22.3 | • G4 = 0.88 (0.79- 0.98) | ||||
| G4 = 22.3-33.8 | • G5 = 0.81 (0.72- 0.92) | ||||
| G5 = ≥33.8 = 0.81 | Trend p < 0.01 | ||||
| Walking (min/d) | Multivariate adjusted RR (95% CI) by walking duration | ||||
| G1 = <500 | • G1 = 1.00 (referent) | ||||
| G2 = 500-2000 | • G2 = 1.03 (0.95- 1.11) | ||||
| G3 = >2000 | • G3 = 0.91 (0.81- 1.02) | ||||
| MPA (hr/wk) | Trend p = 0.45 | ||||
| G1 = Inactive | Multivariate adjusted RR | ||||
| G2 = 0 | (95% CI) by MPA | ||||
| G3 = 1-4 | • G1 = 1.00 (referent) | ||||
| G4 = 5-13 | • G2 = 0.80 (0.60- 1.05) | ||||
| G5 = 14 | • G3 = 0.87 (0.79- 0.94) | ||||
| VPA (hr/wk) | • G4 = 0.86 (0.74- 0.99) | ||||
| G1 = Inactive | • G5 = 0.89 (0.65- 1.24) | ||||
| G2 = 0 | Trend p<0.01 | ||||
| G3 = 1-2 | Multivariate adjusted RR (95% CI) by VPA | ||||
| G4 = 3-4 | • G1 = 1.00 (referent) | ||||
| G5 = 5 | • G2 = 0.90 (0.81- 0.99) | ||||
| Cox proportional hazard ratio | • G3 = 0.88 (0.79- 0.97) | ||||
| • G4 = 0.82 (0.71- 0.95) | |||||
| • G5 = 0.62 (0.49- 0.78) | |||||
| Trend p < 0.0001 | |||||
| Thune et al 1997 [343] | To investigate whether everyday exercise is related to the risk of BC. | • n = 25,624 | Baseline and mean follow- up of 14 years | 351 cases of BC (110 pre-menopausal and 251 post-menopausal women) | LTPA and OPA are associated with a reduced risk of BC. |
| Norway | • Sex: Women | PA assessment: Self-reported LTPA and OPA, divided into groups | • G1 = 1.00 (referent) | Adjusted RR (95% CI) by LTPA | |
| Prospective cohort | • Age: 20-54 | LTPA | • G2 = 0.93 (0.71- 1.22) | ||
| D & B score = 14 | G1 = Sedentary | • G3 = 0.63 (0.42- 0.95) | |||
| G2 = Moderate | Trend p = 0.04 | ||||
| G3 = Regular exercise OPA | Adjusted RR (95% CI) by LTPA, pre- menopausal | ||||
| G1 = Sedentary | • G1 = 1.00 (referent) | ||||
| G2 = Walking | • G2 = 0.77 (0.46- 1.27) | ||||
| G3 = Lifting | • G3 = 0.53 (0.25- 1.14) | ||||
| G4 = Heavy manual labor | Trend p = 0.10 | ||||
| During work | Adjusted RR (95% CI) by LTPA, post- menopausal | ||||
| Pre-menopausal | • G1 = 1.00 (referent) | ||||
| G1 = Sedentary | • G2 = 1.00 (0.72- 1.39) | ||||
| G2 = Walking | • G3 = 0.67 (0.41- 1.10) | ||||
| G3 = Lifting or heavy manual labor | Trend p = 0.15 | ||||
| Adjusted RR (95% CI) by OPA | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.84 (0.63- 1.12) | |||||
| • G3 = 0.74 (0.52- 1.06) | |||||
| • G4 = 0.48 (0.25- 0.92) | |||||
| Trend p = 0.02 | |||||
| Adjusted RR (95% CI) by OPA, pre- menopausal | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.82 (0.50- 1.34) | |||||
| • G3 = 0.48 (0.24- 0.95) | |||||
| Trend p = 0.03 | |||||
| Adjusted RR (95% CI) by OPA, post- menopausal | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.87 (0.61- 1.24) | |||||
| • G3 = 0.78 (0.52- 1.18) | |||||
| Trend p = 0.24 | |||||
| Zheng et al 1993 [344] | To assess the role of OPA in the risk of BC. | • n= 3,783 (BC = 2,736) | PA assessment: Interview for OPA, divided into groups | 2,736 cases of BC | Women with low OPA had an increased risk of BC; the incidence of BC was reduced in women with high-activity jobs. |
| China | • Sex: Women | G1 = Low | Standardized incidence ratios | ||
| D & B score = 9 | • Age: 30 | G2 = Moderate | • G1= 131 | ||
| G3 = High | • G2 = 95 | ||||
| • G3 = 79 | |||||
D & B score, Downs and Black quality score; YR, years; PA, physical activity; BC, breast cancer; LTPA, leisure-time physical activity; g, group; HR, hazard ratio; RR, risk ratio; OR, odds ratio; 95% CI, confidence interval; T, tertile; MET, metabolic equivalent; MET/wk, metabolic equivalent per week; OPA, occupational physical activity; MET h/wk/yr, metabolic equivalent per hour per week per year; kcal/wk, kilocalories per week; TPA, total physical activity; VPA, vigorous physical activity.
In our systematic search of the literature, a total of 571 citations were identified during the electronic database search (Figure 8). Of these citations, 228 were identified in MEDLINE, 89 in EMBASE, 56 in Cochrane, and 198 in the CINAHL/SportDiscus/PsychInfo search. A total of 46 duplicates were found, leaving a total of 571 unique citations. A total of 411 articles were excluded after scanning, leaving a total of 114 articles for full review. From these articles 77 were excluded after full review leaving 37 articles for inclusion in the systematic review. An additional 6 articles were found through the reviewers' personal files. The reasons for exclusion included not containing three levels of physical activity or not possible to determine dose-response relationship (n = 1), reviews, summaries, meta-analyses (n = 20), report (n = 5), editorial/comment (n = 21), not a research article (N = 11), not dealing specifically with breast cancer (n = 4), not relevant (n = 5), not primary prevention (n = 3), and other (n = 10). Therefore, a total of 43 articles were included in the systematic review of the literature regarding the relationship between physical activity and the primary prevention of breast cancer.
Figure 8.
Results of the Literature Search for Breast Cancer.
The data providing dose-response information is all observational in nature, involving both case control and cohort investigations. These studies involved a total of 1,861,707 participants averaging 44,326 subjects per study (range 526-680,000). There were a total of 80,247 reported cases of breast cancer (ranging per study from 109-17,986). The total length of study follow-up for the prospective cohort studies averaged 10.5 yr (ranging from 4-31 yr). The articles were published over a 14 yr period ranging from 1993-2007. These studies involved large samples of men and women from regions throughout the world.
The literature with respect to the primary prevention of breast cancer is as compelling as that found with respect to colon cancer. There is strong evidence that routine physical activity is associated with a reduced risk for the development of breast cancer. However, this literature is also confounded by many shortcomings (similar to other cancer literature) including considerable variability in the statistical analyses employed, the physical activity measurement tools used, and the experimental designs.
The overall risk reduction for breast cancer for individuals that are habitually physically active (at or above Canada's guidelines for physical activity) is thought to approximate 20-40% [31,105]. In our analyses, we found very similar findings. When comparing the most active group versus the least active group we found a mean (and median) risk reduction of 20% across all studies. The level of evidence would be considered to be Level 2A. Generally, the articles were of high quality with a mean Downs and Black score of 13 (median = 13, range = 9-14).
A dose-dependency of this relationship is also generally present in the majority of the studies. For instance, greater than 50% studies revealed a dose-response relationship in one or more measures of occupational and/or leisure-time physical activity and the risk for breast cancer. Moreover, the majority of studies demonstrated the greatest risk reduction at the highest activity level. With respect to the minimal and optimal volume of exercise required, Lee [105] stated that 30-60 min/day of moderate-to-vigorous physical activity is required to decrease the risk for breast cancer. This belief is strongly supported by the literature. However, others have shown significant risk reductions at lower exercise volumes. For instance, Rockhill et al. [106] showed significant reductions (12% or greater) in the risk for breast cancer in women who accumulated at least 1 hr of moderate or vigorous physical activity per week. Similarly, Sesso et al. (1998) revealed that there was an 8% reduction in the risk for breast cancer with a relatively small energy expenditure of 500-999 kcal/wk. Further risk reductions were observed with higher energy expenditures (= 1000 kcal/wk = 51% reduction in the risk). As discussed above, Monninkhof et al. revealed a 6% decrease in breast cancer risk for each additional hour of physical activity per week [104]. Taken as a whole, it would therefore appear that Canada's guidelines for physical activity are more than appropriate for reducing the risk for breast cancer. Further research however is required to determine the minimal volume of exercise that is effective in the primary prevention of breast cancer.
Implications
There is a preponderance of data linking physical inactivity to site-specific cancers, particularly of the breast and colon [31,104-109]. The protective effects of physical activity also appear with other forms of cancer (such as endometrial cancer) [110]. In an important review of the literature Lee revealed that physically active women have a 20-30% lower risk of breast cancer, and physically active men and women have a 30-40% lower risk of colon cancer [105]. A more recent systematic review of the literature revealed a 20-80% lower risk of breast cancer in post-menopausal women [104], with a weaker association in pre-menopausal women. Considering data from both pre- and post-menopausal women the authors demonstrated that physically active individuals had a 15-20% lower risk of breast cancer. Monninkhof et al. also reported a 6% lower risk of breast cancer for each additional hour of physical activity per week [104]. This level of risk reduction was also supported by the U.S. Department of Health and Human Services during its recent evaluation of the literature [31].
Our current reviews of the literature support previous work in the field including the finding of a dose-response relationship between physical activity and cancers of the breast and colon [104,105,109]. It would appear that 30-60 min/day of moderate-to-vigorous physical activity is associated with a lower risk of breast and colon cancer.
Recommendation #5
For a reduced risk for site specific cancers (such as colon cancer and breast cancer), it is recommended that individuals should participate in 30 min or more of moderate to vigorous exercise on most days of the week. [Level 2, Grade A]
Primary Prevention of Type 2 Diabetes
In comparison to other chronic conditions, there is relatively limited literature examining the relationship between multiple levels of physical activity/fitness and the incidence of type 2 diabetes. All of the literature examining the dose-response (for at least three levels of physical activity/fitness) involved prospective cohort analyses. A total of 3655 citations were identified during the electronic database search (Figure 9). Of these citations, 2038 were identified in MEDLINE, 1116 in EMBASE, 118 in Cochrane, and 372 in the CINAHL/SportDiscus/PsychInfo search. A total of 614 duplicates were found, leaving a total of 3041 unique citations. A total of 2865 articles were excluded after scanning, leaving a total of 176 articles for full review. From these articles 156 were excluded after full review leaving 20 articles for inclusion in the systematic review of the literature regarding the relationship between physical activity and type 2 diabetes. The reasons for exclusion included non-experimental/weak design (N = 18), three levels of physical activity not reported (N = 16), reviews, summaries, or meta-analyses (N = 41), not related to type 2 diabetes (N = 71), and other (N = 10).
Figure 9.
Results of the Literature Search for Diabetes.
As shown in Table 17, 20 investigations examined the dose-response (i.e., three or more levels) relationship between physical activity and the incidence of type 2 diabetes. This involved a total of 624,952 subjects, averaging 32,892 subjects per study (range 1,543-87,907). There were a total of 19,325 cases of type 2 diabetes (ranging per study from 78-4,030). The total length of follow-up averaged 9.3 yr (ranging from 3 -16.8 yr). The articles were published over a 16 yr period ranging from 1991 to 2007.
Table 17.
The relationship between physical activity and the development of type 2 diabetes.
| Publication Country Study Design Quality Score | Objective | Population | Methods | Outcome | Comments and Conclusions |
|---|---|---|---|---|---|
| Haapanen et al 1997 [77] | To examine the association of PA and the risk of CHD, hypertension and T2D. | • n = 1,340 men, 1,500 women | 10 yr follow-up | Number of cases: 118 | LTPA has a preventive effect on T2D. |
| • Age: 35-63 yr | PA assessment: Self-reported | Age-adjusted RR (95% CI), men | |||
| Finland | LTPA (kcal/wk), divided into groups | • G1 = 1.54 (0.83-2.84) | |||
| • G2 = 1.21 (0.63-2.31) | |||||
| Prospective cohort | • G3 = 1.00 (referent) | ||||
| p = 0.374 | |||||
| Men | |||||
| D & B score = 14 | G1 = 0-1100 | Age-adjusted RR (95% CI), women | |||
| G2 = 1101-1900 | • G1 = 2.64 (1.28-5.44) | ||||
| G3 = >1900 | • G2 = 1.17 (0.50-2.70) | ||||
| • G3 = 1.00 (referent) | |||||
| Women (kcal/wk) | p < 0.006 | ||||
| G1 = 0-900 | |||||
| G2 = 901-1500 | |||||
| G3 = >1500 | |||||
| Cox proportional HR | |||||
| Hu et al 2003 [111] | To examine the relationship between sedentary behaviours (particularly prolonged television watching) and risk of obesity and T2D in women. | • n = 68,497 (diabetes specific analyses) | 6 yr follow-up | Number of cases: 1515 | Sedentary behaviours (especially television watching) are associated with an increased risk for obesity and T2D. |
| USA | • n = 50,277 (obesity specific analyses) | PA assessment: Self-reported PA and sedentary behaviour | Each 2-h/d increment in TV watching was associated with a 23% (95% CI, 17%-30%) increase in obesity and a 14% (95% CI, 5%- 23%) increase in risk of T2D | ||
| Prospective cohort | |||||
| • Age: 30-55 yr | Outcome measure: onset of obesity and T2D | Each 2-h/d increment in sitting at work was associated with a 5% (95% CI, 0%-10%) increase in obesity and a 7% (95% CI, 0%- 16%) increase in T2D | Light to moderate PA was associated with a significantly lower risk for obesity and T2D. | ||
| D & B score = 13 | • Sex: Women | Multivariate analyses adjusting for age, smoking, dietary factors, and other covariates | Standing or walking around at home (2 h/d) was associated with a 9% (95% CI, 6%-12%) reduction in obesity and a 12% (95% CI, 7%- 16%) reduction in T2D | ||
| • Characteristics: | Each 1 hour per day of brisk walking was associated with a 24% (95% CI, 19%-29%) reduction in obesity and a 34% (95% CI, 27%- 41%) reduction in T2D | ||||
| Free of T2D, CVD, or cancer at baseline | |||||
| • Nurses' Health Study | |||||
| Manson et al 1992 [112] | To examine the association between regular exercise and the subsequent development of T2D. | • n = 21,271 | 5 yr follow-up | Number of cases: 285 | Exercise appears to reduce the development of T2D even after adjusting for BMI. |
| • Sex: Men | PA assessment: Questionnaire Fpr VPA (enough to develop sweat) | ||||
| • Age: 40-84 yr | The age-adjusted incidence of T2D: | ||||
| USA | • Characteristics: | • 369 cases per 100,000 person- years in men who engaged in VPA less than once weekly • 214 cases per 100,000 person- years in those exercising at least five times per week (p trend < 0.001) |
|||
| Free of diagnosed diabetes, CVD and cancer at baseline | |||||
| Prospective cohort | |||||
| D & B score = 14 | Exercise frequency (times/wk) | ||||
| G1 = < Weekly | |||||
| G2 = At least weekly | |||||
| Age-adjusted RR (95% CI) by exercise frequency | |||||
| Times per week | |||||
| G1 = 0 | • G1 = 1.00 (referent) | ||||
| G2 = 1 | • G2 = 0.64 (0.51- 0.82) | ||||
| G3 = 2-4 | |||||
| G4 = >5 | Age-adjusted RR (95% CI) by exercise frequency | ||||
| • G1 = 1.00 (referent) | |||||
| Outcome measure: Incidence T2D | • G2 = 0.77 (0.55-1.07) | ||||
| • G3 = 0.62 (0.46-0.82) | |||||
| • G4 = 0.58 (0.40-0.84) | |||||
| Age- and BMI-adjusted RR (95% | |||||
| CI) by exercise frequency | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.71 (0.56- 0.91) | |||||
| Age- and BMI-adjusted RR (95% CI) by exercise frequency | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.78 (0.56-1.09) | |||||
| • G3 = 0.68 (0.51-0.90) | |||||
| • G4 = 0.71 (0.49-1.03) | |||||
| Hu et al. 2001[114] | To examine the relationship between dietary and lifestyle factors in relation to the risk for T2D. | • n = 84,941 | 16 yr follow-up | Number of cases: 3300 | The majority of T2D could be prevented through healthy living. |
| • Sex: Women | |||||
| • Age: 40-75 yr | PA assessment: Questionnaire For PA (h/wk), divided into groups | Multivariate-adjusted RR (95%) | |||
| USA | • Characteristics: participants had no history of diabetes, CVD, or cancer. | • Q1 = 1.00 (referent) | |||
| • Q2 = 0.89 (0.77-1.02) | |||||
| Retrospective cohort | • Q3 = 0.87 (0.75-1.00) | ||||
| Q1 = <0.5 | • Q4 = 0.83 (0.71-0.96) | ||||
| Q2 = 0.5--1.9 | • Q5 = 0.71 (0.56--0.90) | ||||
| D & B score = 13 | Nurses' Health Study | Q3 = 2.0--3.9 | |||
| Q4 = 4.0--6.9 | |||||
| Q5 = ≥7.0 | |||||
| Outcome measure: Incidence of T2D | |||||
| Cox regression | |||||
| Sato et al 2007 [116] | To examine the relationship between walking to work and the development of T2D. | • n = 8,576 | 4 yr follow-up | Number of cases: 878 | The duration of a walk to work is an independent predictor of the risk for T2D. |
| • Sex: Men | |||||
| • Age: 40--55 yr | PA assessment: For time spent walking to work, divided into tertiles | OR (95% CI) | |||
| Japan | • Kansai Healthcare Study | • T1 = 1.00 (referent) | |||
| • T2 = 0.86 (0.70-1.06) | |||||
| Prospective cohort | T1 = 0-10 min | • T3 = 0.73 (0.58-0.92) | |||
| T2 = 11-20 min | Significant difference was seen between ≤ 10 min and ≤ 20 min only (p = 0.007) | ||||
| T3 = ≥20 min | |||||
| D & B score = 14 | |||||
| Outcome measure: Incidence of T2D | |||||
| Hu G et al 2003 [117] | To examine the relationship of OPA, commuting and LTPA with the incidence of T2D. | • n = 14,290 | PA assessment: Questionnaire For OPA, LTPA and commuting PA | Multivariate adjusted HR (95% Cl) for OPA, men | Moderate and high OPA, commuting PA or LTPA significantly reduces risk of T2D in middle aged adults. |
| • Sex: Men and women | |||||
| • G1 = 1.00 (referent) | |||||
| Finland | • Age: 35-64 yr | • G2 = 0.67 (0.44-1.01) | |||
| • Characteristic: | OPA | • G3 = 0.73 (0.52-1.02) | |||
| Prospective cohort | Asymptomatic for stroke, CHD, or diabetes at baseline. | G1 = Light (sitting) | |||
| G2 = Moderate (standing, walking) | Multivariate adjusted HR (95% Cl) for OPA, women | ||||
| D & B score = 12 | G3 = Active (walking, lifting) | • G1 = 1.00 (referent) | |||
| • G2 = 0.72 (0.46-1.12) | |||||
| • G3 = 0.78 (0.52-1.18) | |||||
| Commuting PA (min/d) | |||||
| G1 = None | Multivariate adjusted HR (95% Cl) for OPA, men and women | ||||
| G2 = 1-29 | |||||
| G3 = ≥ 30 | |||||
| G1 = 1.00 (referent) | |||||
| G2 = 0.70 (0.52-0.96) | |||||
| LTPA | |||||
| G3 = 0.74 (0.57-0.95) | |||||
| • G1 = Low (inactive) | |||||
| • G2 = Moderate (walking, cycling >4 hr/wk) | |||||
| Multivariate adjusted HR (95% Cl) for commuting PA, men | |||||
| • G3 = High (running, jogging >3 hr/wk) | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 1.00 (0.71-1.42) | |||||
| Outcome measure: incidence of T2D | • G3 = 0.75 (0.46-1.23) | ||||
| Multivariate adjusted HR (95% Cl) for commuting PA, women | |||||
| Cox proportional HR | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.94 (0.63-1.42) | |||||
| • G3 = 0.57 (0.34-0.96) | |||||
| Multivariate adjusted HR (95% Cl) for commuting PA, men and women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.96 (0.74-1.25) | |||||
| • G3 = 0.64 (0.45-0.92) | |||||
| Multivariate adjusted HR (95% Cl) for LTPA, men | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.78 (0.57-1.06) | |||||
| • G3 = 0.84 (0.52-1.37) | |||||
| Multivariate adjusted HR (95% Cl) for LTPA, women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.81 (0.58-1.15) | |||||
| • G3 = 0.85 (0.43 -1.66) | |||||
| Multivariate adjusted HR (95% Cl) for LTPA, men and women | |||||
| • G1 = 1.00 (referent) | |||||
| • G2 = 0.81 (0.64-1.20) | |||||
| • G3 = 0.84 (0.57-1.25) | |||||
| Hsia et al 2005 [118] USA | To evaluate the relationship between PA and the incidence of T2D in a large, diverse group of older women. | • n = 87,907 | PA assessment: Questionnaire for frequency and duration of 4 walking speeds and 3 other activities classified by intensity (light, moderate, strenuous) | Number of cases: 2,271 | There is a strong inverse relationship between PA and T2D. There is a stronger relationship between PA and T2D in Caucasian women than in minority women. This may be explained by less precise risk estimates in minority women. |
| • Sex: Women | |||||
| • Age: White 63.8 ± 7.3, African American 61.9 ± 7.3, Hispanic 60.5 ± 7.1, Asian/Pacific Islander 63.7 ± 7.6, American Indian 61.5 ± 8.0 | Multivariate adjusted HR (95% CI) by walking, Caucasian | ||||
| • Q1 = 1.00 (referent) | |||||
| Prospective cohort | • Q2 = 0.85 (0.74-0.87) | ||||
| • Q3 = 0.87 (0.75-1.01) | |||||
| • Q4 = 0.75 (0.64-0.89) | |||||
| D & B score = 11 | Q1 = Low | • Q5 = 0.74, (0.62-0.89) | |||
| Q2 = | Trend p < 0.001 | ||||
| Q3 = | |||||
| Q4 = | Multivariate adjusted HR (95% CI) by TPA, Caucasian | ||||
| Q5 = High | |||||
| • Ethnicity: White n = 74,240; African American n = 6,465; Hispanic n = 3,231; Asian/Pacific Islander 2,445; American Indian n = 327 | • Q1 = 1.00 (referent) | ||||
| Cox proportional HR | |||||
| • Q2 = 0.88 (0.76- 1.01) | |||||
| • Q3 = 0.74 (0.64- 0.87) | |||||
| • Q4 = 0.80 (0.68- 0.94) | |||||
| • Q5 = 0.67 (0.56- 0.81) Trend p = 0.002 | |||||
| • Characteristics: participants had no history of diabetes, were not on any antidiabetic medications | |||||
| • Women's Health Initiative | |||||
| Wannamethee et al 2000 [120] | To examine the role of components of the insulin resistance syndrome in the relationship between PA and the incidence of T2D and CHD. | • n = 5,159 | 16.8 yr follow-up | Number of cases: 196 | The relationship between PA and T2D appears to be mediated by serum insulin and components of the insulin resistance syndrome. However, these factors do not appear to explain the inverse relationship between PA and T2D. |
| • Sex: Men | |||||
| • Age: 40-59 yr | PA assessment: Questionnaire for TPA Physical activity groups were identified and scored: | Multivariate adjusted RR (95% CI) | |||
| England, Wales and Scotland | • Characteristics: No history of heart disease, diabetes or stroke | Q1 = 1.00 (referent) | |||
| Q2 = 0.66 (0.42-1.02) | |||||
| Q3 = 0.65 (0.41-1.03) | |||||
| Prospective cohort | Q4 = 0.48 (0.28-0.83) | ||||
| Q1 = None | Q5 = 0.46 (0.27-0.79) | ||||
| Q2 = Occasional | p < 0.005 | ||||
| D & B score = 14 | Q3 = Light | ||||
| Q4 = Moderate | |||||
| Q5 = Moderately vigorous/vigorous | MPA (sporting activity once a week or frequent lighter- intensity activities such as walking, gardening, do-it yourself projects) are sufficient to produce a significant reduction in risk of both CHD and T2D. | ||||
| The men were classified according to current smoking status, alcohol consumption, and social class | |||||
| Cox proportional HR | |||||
| Manson et al 1991 [121] | To examine the association between regular VPA and the incidence of T2D. | • n = 87,253 | 8 yr follow-up | Number of cases: 1303 Women who engage in VPA at least once per week had reduced adjusted RR of T2D RR = 0.66 (0.6- 0.75) | PA is promising in the primary prevention of T2D. |
| • Sex: Women | |||||
| • Age: 34-59 yr | PA assessment: | ||||
| USA | • Characteristics: Free of diagnosed diabetes, cardiovascular disease and cancer | Questionnaire | |||
| Frequency of weekly exercise (0-+4) | |||||
| Prospective cohort | |||||
| The reduction in risk remained significant after adjustment for BMI RR = 0.84 (0.75-0.95) | |||||
| D & B score = 13 | Analysis also restricted to the first 2 yr after the assessment of PA level and to symptomatic diabetes | ||||
| When analysis was restricted to the first 2 years after ascertainment of PA level and to symptomatic disease as the outcome, the age- adjusted RR of those who exercised was 0.50, and age and body-mass index adjusted RR was 0.69 (0.48-1.0) | |||||
| Multivariate adjustments for age, body-mass index, family history of diabetes, and other variables did not alter the reduced risk found with exercise | |||||
| Multivariate analysis | Family history of diabetes did not modify the effect of exercise, and risk reduction with exercise was evident among both obese and non-obese women | ||||
| Helmrich et al 1994 [122] | To examine the relationship between PA and the development of T2D. | • n = 5,990 | 98,524 man-years of follow-up (1962-1976) | Number of cases: 202 | Increased PA is effective in preventing T2D. |
| • Sex: Men | |||||
| • Age: 39-68 yr | RR (95% CI) by blocks walked per day | ||||
| USA | • Characteristics: healthy, asymptomatic | PA assessment: Questionnaire for LTPA (walking, stair climbing, sports etc; kcal/wk) Blocks walked/day | The protective benefit is especially pronounced in those individuals who have the highest risk of disease. | ||
| • T1 = 1.00 (referent) | |||||
| Further review of the data reported by Helmich et al. 1991 | • T2 = 1.30 | ||||
| University of Pennsylvania Alumni Health Study | • T3 = 0.92 | ||||
| p = 0.80 | |||||
| LTPA (kcal/wk) kcal were assigned to each activity and added together | LTPA was inversely related to the development of T2D | ||||
| Prospective cohort | |||||
| Same findings to that reported in 1991 | |||||
| D & B score = 14 | Lowest < 500 | ||||
| Highest ≥ 3500 | |||||
| Blocks walked/day | |||||
| T1 = <5 | |||||
| T2 = 5-14 | |||||
| T3 = ≥15 | |||||
| Cox proportional HR | |||||
| Helmrich et al 1991 [123] | To examine the Relationship between PA and the Subsequent development of T2D. | • n = 5,990 | 98,524 man-years of follow-up (1962-1976) | Number of cases: 202 | Increased PA is effective in preventing T2D. |
| • Sex: Men | |||||
| • Age: 39-68 yr | LTPA was inversely related to the development of type 2 diabetes | ||||
| USA | • Characteristics: healthy, asymptomatic | PA assessment: Questionnaire for LTPA kcal/wk: stairs climbed/day and blocks walked/day, divided into groups | The protective benefit is especially pronounced in those individuals who have the highest risk of disease. | ||
| Prospective cohort | RR (95% CI) by sports played | ||||
| • University of Pennsylvania Alumni Health Study | • G1 = 1.00 (referent) | ||||
| • G2 = 0.90 | |||||
| D & B score = 13 | • G3 = 0.69 | ||||
| • G4 = 0.65 | |||||
| All activities LTPA | Trend p = 0.02 | ||||
| Q1 = <500 | |||||
| Q2 = 500-999 | RR (95% CI) by Flights of stairs climbed/day | ||||
| Q3 = 1000-1499 | |||||
| Q4 = 1500-1999 | • T1 = <5 = 1.00 (referent) | ||||
| Q5 = 2000-2499 | • T2 = 0.78 | ||||
| Q6 = 2500-2999 | |||||
| • T3 = 0.75 | |||||
| Q7 = 3000-3499 | |||||
| Trend p = 0.07 | |||||
| Q8 = ≥ 3500 | |||||
| RR (95% CI) by Blocks walked/day | |||||
| Sports played | |||||
| • T1 = 1.00 (referent0 | |||||
| G1 = None | |||||
| • T2 = 1.31 | |||||
| G2 = Moderate | |||||
| G3 = Vigorous | • T3 = 0.93 Trend p = 0.80 |
||||
| G4 = Moderate and Vigorous | |||||
| Age adjusted RR (95% CI) by all activities | |||||
| Stairs climbed per day | |||||
| T1 = <5 | • Q1 = 1.00 (referent) | ||||
| T2 = 5-14 | • Q2 = 0.94 | ||||
| T3 = ≥ 15 | • Q3 = 0.79 | ||||
| • Q4 = 0.78 | |||||
| Blocks walked per day | • Q5 = 0.68 | ||||
| T1 = <5 | • Q6 = 0.90 | ||||
| T2 = 5-14 | • Q7 = 0.86 | ||||
| T3 = ≥ 15 | • Q8 = 0.52 | ||||
| p = 0.01 for trend | |||||
| Cox proportional HR | |||||
| Age adjusted RR (95% CI) by all activities except vigorous sports | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.97 | |||||
| • Q3 = 0.87 | |||||
| • Q4 = 0.92 | |||||
| • Q5 = 0.75 | |||||
| • Q6 = 1.29 | |||||
| • Q7 = 1.03 | |||||
| • Q8 = 0.48 | |||||
| Trend p = 0.07 | |||||
| Age adjusted RR (95% CI) by vigorous sports only | |||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.69 | |||||
| • Q3 = N/A | |||||
| • Q4 = 0.53 | |||||
| • Q5 = 0.86 | |||||
| • Q6 = 0.56 | |||||
| • Q7 = 0.40 | |||||
| • Q8 = 0.46 | |||||
| Trend p = 0.05 | |||||
| Wei et al 1999 [124] | To determine whether PF is associated with risk for impaired fasting glucose and T2D. | • n = 8,633 | 6 yr follow-up | Number of cases: 149 | High PF is associated with a reduced risk for impaired fasting glucose and T2D. |
| USA | • Sex: Men | ||||
| • Age: 43.5 yr | PF assessment: Maximal treadmill exercise test (METs), divided into 3 groups | 593 patients developed impaired fasting glucose | |||
| • Characteristics: Non-diabetic men | OR (95% CI) for developing glucose intolerance | ||||
| Prospective cohort | T1 = Low | • T1 = 1.9 (1.5--2.4) | |||
| T2 = Moderate | • T2 = 1.5 (1.2--1.8) | ||||
| T3 = High | • T3 = 1.00 (referent) | ||||
| D & B score = 12 | Outcome measure: Incidence of impaired fasting glucose and T2D | OR (95% CI) for developing T2D | |||
| • T1 = 3.7 (2.4 --5.8) | |||||
| • T2 = 1.7 (1.1--2.7) | |||||
| • T3 = 1.00 (referent) | |||||
| Statistics: GLM | |||||
| Katzmarzyk et al 2007 [126] | To examine the relationships among adiposity, PA, PF and the development of T2D in a diverse sample of Canadians. | • n = 1,543 (709 men and 834 women) | 6 yr follow-up | Number of cases: 78 (37 in men, 41 in women) | Adiposity and PF are important predictors of the development of T2D. |
| Canada | • Sex: Men and women | PF assessment: Questionnaire | PA was associated with 23% lower odds of developing diabetes and maximal METs was also associated with significantly lower odds of developing diabetes (OR = 0.28) | ||
| Prospective cohort | • Age: 36.8 - 37.5 | PA assessment: LTPA Questionnaire | |||
| D & B score = 13 | • Characteristics: Free of diabetes at baseline | ||||
| • Canadian Physical Activity Longitudinal Study | |||||
| Burchfiel et al 1995 [345] | To examine the relationship between PA and T2D. | • n = 6,815 | 6 yr follow-up | Number of cases: 391 | PA is associated inversely and independently with incident T2D. |
| USA | • Sex: Men (Japanese- American) | PA assessment: Questionnaire PA index (based on intensity and duration of activity) | The age-adjusted 6-year cumulative incidence of diabetes decreased progressively with increasing quintile of physical activity from 73.8 to 34.3 per 1,000 (p < 0.0001, trend) | ||
| • Age: 45-68 yr | Levels of activity: | ||||
| Prospective cohort | • Characteristics: Free of diabetes at entry | Q1 = Basal - Sleeping reclining | |||
| D & B score = 13 | • The Honolulu Heart Program | Q2 = Sedentary | |||
| Q3 = Slight - Casual walking | |||||
| Q4 = Moderate -- Gardening | |||||
| Q5 = Heavy - Lifting, shoveling | |||||
| Outcome measure: Self-reported T2D (clinically recognized) | |||||
| Dziura et al 2004 [346] | To determine the prospective relation between reports of habitual PA, 3-year change in body weight, and the subsequent risk of T2D in an older cohort. | • n = 2,135 | PA assessment: Questionnaire for 4 types of activities (walking, gardening/housework, physical exercises, active sports or swimming) and frequency of participation measured with a PA score: | 118 cases of T2D | Observation of an inverse relationship between reported PA and rate of T2DM. |
| USA | • Sex: Men and women | Incident density of T2D = 6.6/1000 person years | |||
| • Age: ≥ 65 yr | |||||
| Prospective cohort | • Ethnicity: 83% White, 15% African American, 2% Non-white | Diabetes (n = 118) PA score: 2.17 ± 1.7 'Some' PA: 78% | Subjects reporting some PA at baseline experienced a rate of T2D over 50% lower relative to those reporting no PA. | ||
| D & B score = 12 | • Characteristics: Healthy asymptomatic | Never (score 0) Sometimes (score 1) Often (score 2) | Non-Diabetes (n = 2017) PA score: 2.34 ± 1.7 'Some' PA: 84% | ||
| Pearson product moment correlation coefficient and Cox proportional HR | |||||
| Hu et al. 1999 [347] | To quantify the dose-response relationship between total PA and incidence of T2D in women. | • n = 70,102 | 8 yr of follow-up | Number of cases: 1419 | Increased PA is associated with substantial reduction in risk of T2D including PA of moderate intensity and duration. |
| USA | • Sex: Women | PA assessment: Questionnaire for TPA (MET hr/wk) and VPA (6 METs) | Multivariate-adjusted RR (95% CI) of by TPA | ||
| • Age: 40-65 yr | • Q1 = 1.0 (referent) | ||||
| Prospective cohort | To examine the health benefits of walking in comparison to more vigorous activity. | • Characteristics: participants had no history of diabetes, CVD, or cancer | TPA (MET hr/wk) | • Q2 = 0.77 (0.66-0.90) | |
| D & B score = 12 | Nurses' Health Study | Q1 = 0-2.0 | • Q3 = 0.75 (0.65-0.88) | ||
| Q2 = 2.1-4.6 | • Q4 = 0.62 (0.52-0.73) | ||||
| Q3 = 4.7-10.4 | • Q5 = 0.54 (0.45-0.64) | ||||
| Q4 = 10.5-21.7 | Trend p < 0.001 | ||||
| • Q5 = ≥ 21.8 | |||||
| MET score | Multivariate-adjusted RR (95% CI) among women who did not perform vigorous exercise (MET's): | ||||
| Q1 = ≤ 0.5 | • Q1 = 1.0 (referent) | ||||
| Q2 = 0.6-2.0 | • Q2 = 0.91 (0.75-1.09) | ||||
| Q3 = 2.1-3.8 | • Q3 = 0.73 (0.59-0.90) | ||||
| Q4 = 3.9-9.9 | • Q4 = 0.69 (0.56-0.86) | ||||
| Q5 = ≥ 10.0 | • Q5 = 0.58 (0.46-0.73) | ||||
| Outcome measures: | Trend p < 0.001 | ||||
| Incidence of T2D | |||||
| Hu et al 2001 [348] | To examine the role of prolonged television watching on the risk for T2D. | • n = 37,918 | 10 year follow-up | Number of cases: 1058 | Increasing PA is associated with a significant reduction in risk for T2D, whereas a sedentary lifestyle indicated by prolonged TV watching is related directly to increased risk. |
| USA | • Sex: Men | ||||
| • Age: 40-75 yr | PA assessment: Questionnaire for PA (MET hr/wk) and TV watching (h/wk), each divided into quintiles | Multivariate-adjusted RR (95% CI) by PA | |||
| Prospective cohort | • Characteristics: participants had no history of diabetes, CVD, or cancer | Q1 = 0-5.9 | • Q1 = 1.00 (referent) | ||
| D & B score = 11 | • Health Professionals' Follow-up Study | Q2 = 6.0-13.7 | • Q2 = 0.78 (0.66 -- 0.93) | ||
| Q3 = 13.8-24.2 | • Q3 = 0.65 (0.54 -- 0.78) | ||||
| Q4 = 24.3-40.8 | • Q4 = 0.58 (0.48 -- 0.70) | ||||
| Q5 = ≥ 40.9 | • Q5 = 0.51 (0.41 -- 0.63) | ||||
| Trend p < 0.001 | |||||
| Time spent watching television per week (h/wk) | Multivariate-adjusted RR (95% CI) by TV time | ||||
| Q1 = 0-1 | • Q1 = 1.00 (referent) | ||||
| Q2 = 2-10 | • Q2 = 1.66 (1.15 - 2.39) | ||||
| Q3 = 11-20 | • Q3 = 1.64 (1.12 - 2.41) | ||||
| Q4 = 21-40 | • Q4 = 2.16 (1.45 - 3.22) | ||||
| Q5 = >40 | • Q5 = 2.87 (1.46 - 5.65) | ||||
| Trend p < 0.001 | |||||
| Rana et al 2007 [349] | To examine the individual and combined association of obesity and physical inactivity with the incidence of T2D. | • n = 68,907 | 16 yr follow-up | Number of cases: 4,030 | This study found that obesity and physical inactivity independently contributed to the development of T2D. |
| USA | • Sex: Women | ||||
| Prospective cohort | • Age: 30-55 years age range in 1976 (note: 1986 was the baseline year for the study) | PA assessment: Questionnaire for average amount of time/week MET hours per week spent in MVPA (≥ 3 METs), divided into quintiles | Multivariate-adjusted RR (95% CI) by MVPA: | The benefits of PA were not limited to lean women; among those who were overweight and obese, physically active women tended tobe at lower risk for T2D than sedentary women. | |
| D & B score = 12 | • Characteristics: No history of diabetes, CVD or cancer | Q1 = <2.1 | • Q1 = 2.37 (2.15--2.16) | ||
| • Nurses' Health Study | Q2 = 2.1-4.6 | • Q2 = 1.92 (1.73--2.13) | |||
| Q3 = 4.7-10.4 | • Q3 = 1.48 (1.34--1.64) | ||||
| Q4 = 10.5-21.7 | • Q4 = 1.40 (1.26--1.55) | ||||
| Q5 = ≥ 21.8 | • Q5 = 1.00 (referent) | ||||
| Trend p < 0.001 | |||||
| Cox proportional HR | |||||
| Sawada et al 2003 [350] | To examine the association between PF and the incidence of T2D. | • n = 4,747 | 14 yr follow-up | Number of cases: 280 | Low PF is associated with a higher risk for the development of T2D. |
| Japan | • Sex: Men | ||||
| • Age: 20-40 yr | PF assessment: Maximal aerobic power estimate ml/kg/min using a submaximal cycle ergometer test, divided into quartiles | Age-adjusted RR (95% CI) | |||
| Prospective cohort | • Characteristics: Free of diabetes, CVD, hypertensin, tuberculosis, and gastrointestinal disease at baseline | Q1 = 32.4 ± 3.1 | • Q1 = 1.00 (referent) | ||
| D & B score = 13 | Q2 = 38.0 ± 2.5 | • Q2 = 0.56 (0.42-- 0.75) | |||
| Q3 = 42.4 ± 3.0 | • Q3 = 0.35 (0.25-- 0.50) | ||||
| Q4 = 51.1 ± 6.2 | • Q4 = 0.25 (0.17-- 0.37) | ||||
| Trend p < 0.001 | |||||
| Outcome measure: Incidence of T2D | Multivariate adjusted RR (95% CI) | ||||
| • Q1 = 1.00 (referent) | |||||
| • Q2 = 0.78 (0.58--1.05) | |||||
| • Q3 = 0.63 (0.45--0.89) | |||||
| • Q4 = 0.56 (0.37--0.84) | |||||
| Trend p = 0.001 | |||||
| Cox proportional HR | |||||
| Weinstein et al 2004 [351] | To examine the relative contributions and joint association of PA and BMI with T2D. | • n = 37,878 | 6.9 year follow up | Number of cases: 1,361 | Although BMI and physical inactivity are independent predictors of incident diabetes, the magnitude of the association with BMI was greater than with PA in combined analyses. These findings underscore the critical importance of adiposity as a determinant of T2D. |
| USA | • Sex: Women | PA assessment: Questionnaire for walking per week (h/wk) and TPA (kcal/wk), divided into groups and quartiles respectively | Multivariate-adjusted HR (95% CI) by time spent walking | ||
| • Age: 45+ years | • G1 = 1.00 (referent) | ||||
| Prospective cohort | • Health care professionals | • G2 = 0.95 (0.82-1.10) | |||
| D & B score = 12 | • Characteristics: No history of CVD, cancer or diabetes | • G3 = 0.87 (0.73 -1.02) | |||
| • G4 = 0.66 (0.54-0.81) | |||||
| • G5 = 0.89 (0.73-1.09) | |||||
| Walking per week (h/wk) | Trend p = 0.004 | ||||
| G1 = no walking | Multivariate-adjusted HR (95% CI) by TPA | ||||
| G2 = <1 | • Q1 = 1.00 (referent) | ||||
| G3 = 1-1.5 | • Q2 = 0.91 (0.79-1.06) | ||||
| G4 = 2-3 | • Q3 = 0.86 (0.74-1.01) | ||||
| G5 = ≥ 4 | • Q4 = 0.82 (0.70-0.97) | ||||
| TPA (kcal/wk) | Trend p = 0.01 | ||||
| Q1 < 200 | |||||
| Q2 = 200-599 | |||||
| Q3 = 600-1,499 | |||||
| Q4 ≥ 1500 | |||||
| Cox proportional HR | |||||
D & B score, Downs and Black quality score; YR, years; PA, physical activity; CHD, coronary heart disease; T2D, type 2 diabetes; LTPA, leisure-time physical activity; g, group; kcal/wk, kilocalories per week; HR, hazard ratio; RR, risk ratio; OR, odds ratio; 95% CI, confidence interval; CVD, cardiovascular disease; OPA, occupational physical activity; PF, physical fitness; MET, metabolic equivalent; MET/wk, metabolic equivalent per week.
Of these studies 100% revealed an inverse relationship between type 2 diabetes and levels of physical activity or fitness. When comparing the most active/fit group versus the least active/fit group we found an average risk reduction of 42% (median = 44%). Therefore in our analyses the most physically active/fit had a 42% lower risk of developing type 2 diabetes. The majority (84%) of these studies revealed incremental reductions in the risk for type 2 diabetes with increasing activity/fitness levels. Therefore, the health benefits with respect to type 2 diabetes prevention appear to continue across the physical activity/fitness continuum. Similar to other clinical conditions, the dose-response relationship is such that small changes in activity levels yield marked reductions in the risk for type 2 diabetes. The health benefits of exercise appear to be particularly prevalent in individuals at high risk for developing type 2 diabetes (e.g., those with a high body mass index, the metabolic syndrome, a history of hypertension and/or a family history of type 2 diabetes). The level of evidence relating physical activity to the primary prevention of type 2 diabetes would be considered to be Level 2A. The quality of the investigations was generally high with a mean (and median) Downs and Black score of 13 (range 11-14).
As with other conditions is it difficult to separate the effects of volume and intensity of exercise. However, small changes in activity levels clearly can have a large effect on the risk for and incidence of type 2 diabetes. For instance, Hu and coworkers [111] revealed that nurses (n = 68,497) who engaged in 1 hr/day of brisk walking had 24% less obesity and 34% less type 2 diabetes (over a 6-year follow-up). These authors estimated that approximately 30% of new cases of obesity and 43% of new cases of type 2 diabetes could be prevented by adopting an active lifestyle including less than 10 hr/wk of television watching and ≥ 30 min/d of brisk walking. Similarly, over a 5-year period, male physicians who exercised vigorously at least once weekly had a 29% lower incidence of type 2 diabetes than individuals who did not exercise regularly [112]. These authors also revealed that physical activity that was sufficient to cause sweating was associated with a lower incidence of type 2 diabetes. Other research has also demonstrated that moderate-to-vigorous physical activity (≥ 5.5 METs for at least 40 min per week) and/or aerobic fitness levels above 31 mL·kg-1·min-1 are associated with a lower risk of type 2 diabetes in middle-aged men [113] with the effect being the greatest in high-risk individuals. Therefore, it would appear that Canada's recommendations for physical activity are sufficient to reduce the risk for type 2 diabetes.
In 2001, Hu et al. [114] reported very interesting and compelling research regarding the role of lifestyle factors in the development of type 2 diabetes. Using data from the Nurses' Health Study, they defined a low-risk group according to five lifestyle factors including BMI, a healthy diet, the participation in moderate-to-vigorous physical activity for at least 30 min per day, no current smoking, and the consumption of an average of at least one-half serving of an alcoholic beverage per day. They revealed that the women in the low risk group had a RR for type 2 diabetes of only 0.09 (CI 0.05-0.17) in comparison to the rest of the cohort. They also found that 91% of the cases of type 2 diabetes in this cohort (CI 83-95%) could be attributed to the five lifestyle factors. This research provided compelling evidence that the majority of type 2 diabetes could be prevented through healthy living [115].
As reviewed in Table 17 there is evidence that leisure-time, occupational and commuting-related leisure time activities significantly reduce the risk for the development of type 2 diabetes. For instance, a recent study by Sato and colleagues [116] revealed that the walking distance to work was directly related to the incidence of type 2 diabetes in 8,576 Japanese men followed for 4 years. The risk reduction was approximately 27% in the participants who walked to work for ≥21 min compared to those who did so for ≥10 min. These findings are similar to that found by Hu et al. who reported that moderate occupational, commuting and leisure-time physical activities all had a significant inverse relationship to risk in middle-aged men and women [117].
Although ethnicity is often not reported in the current research, the studies examined in our systematic review came from a variety of countries and regions. Data was obtained from studies from the USA, Canada, UK, Japan, and Finland. For instance, Hsia et al. (2005) conducted a prospective 5-year study of 87,907 post-menopausal women, finding a strong graded inverse relationship between physical activity and type 2 diabetes. The relationship was stronger in "Caucasian" than in minority (African-American, Hispanic or Asian) women. The authors postulated this finding might reflect less precise risk assessments in minority women [118]. As we have outlined previously, further research is clearly warranted that examines the relationship between physical activity and type 2 diabetes in persons of different ethnicities. Moreover, further research is needed to determine the effects of socio-economic status on the observed relationships. Nonetheless, the research is compelling, habitual physical activity appears to be highly effective in the primary prevention of type 2 diabetes.
Implications
In 1992, the consensus panel from the International Conference on Physical Activity, Fitness and Health (held in Toronto, Canada) [17] indicated that physical activity can effectively reduce the risk for, and incidence of, type 2 diabetes. Over 15 years later, the research is compelling; habitual physical activity is an effective primary preventative strategy against the development of type 2 diabetes [111-113,118-123]. As shown in our analyses, numerous observational studies have revealed that regular physical activity is associated with a lower risk of developing type 2 diabetes [111-113,118-123]. Moreover, increased aerobic fitness is inversely associated with the risk of type 2 diabetes [113,124]. It is also apparent that both aerobic and resistance type activities reduce the risk for type 2 diabetes [125,126].
Although it is difficult to determine the dose-response between physical activity and type 2 diabetes in the majority of the current randomized controlled trials, these trials have revealed important findings. Influential exercise and/or lifestyle intervention trials have demonstrated clearly the health benefits of physical activity/exercise in the prevention of type 2 diabetes. For instance, in the Diabetes Prevention Program (US), 3,234 high-risk participants were randomly assigned to one of three groups: a) a placebo control, b) metformin drug therapy (850 mg twice daily), and c) a lifestyle intervention. The authors revealed that the lifestyle intervention (including physical activity for at least 150 minutes per week) was more effective than metformin (alone) (respective reductions in incidence 58% and 31%) [127]. Similarly, Tuomilehto et al. (2001) conducted a randomized controlled trial with middle-aged, overweight subjects with impaired glucose tolerance (172 males and 350 females). The authors reported a significant reduction in the incidence of type 2 diabetes in the intervention group (which received advice regarding moderate intensity exercise (30 min/day) and dietary control). The lifestyle intervention reduced the risk of type 2 diabetes by approximately 54% in women and 63% in men [128]. In a review of the literature, Williamson et al. revealed modest weight loss via diet and physical activity reduced the incidence of type 2 diabetes in high risk individuals by 40-60% over a 3-4 year period [129]. Collectively, the epidemiological and randomized controlled trials provide compelling evidence supporting the role of habitual physical activity in the primary prevention of type 2 diabetes.
Recommendation #6
For a reduced risk for type 2 diabetes, it is recommended that individuals should participate in 30 min or more of moderate to vigorous exercise on most days of the week. [Level 2, Grade A]
Primary Prevention of Osteoporosis
The protective effects of physical activity and exercise training on bone health are well documented. In fact, the relationship between indicators of bone health (such as bone mineral density or bone mineral content) and physical activity have been evaluated extensively (see Table 18). Numerous exercise intervention trials have revealed that aerobic and resistance activities have a beneficial effect on bone mineral density across the lifespan [16]. In fact, several systematic reviews of the literature [130-135] and major consensus statements [136] have shown clearly the potential benefits of both aerobic and resistance training on bone health (particularly in post-menopausal women). It has been estimated that exercise interventions prevent or reverse at least 1% of bone loss per year in the lumbar spine and the femoral neck of pre- and post-menopausal women [130,137].
Table 18.
Studies examining the relationship between physical activity and osteoporosis.
| Publication Country Study Design Quality Score | Objective | Population | Methods | Outcome | Comments and Conclusions |
|---|---|---|---|---|---|
| Robitaille et al 2008 [150] | To assess the relationship between the physician- diagnosised osteoporosis and family history and examine whether osteoporosis risk factors account for this relationship. | • n = 8,073 | PA assessment: Questionnaire. Level of PA was expressed in MET (hr/wk) | Prevalence of reported osteoporosis in US women by PA level | Prevalence of osteoporosis declines with increasing PA in a dose-response manner. |
| • Sex: Women | |||||
| • Age: ≥ 20 yrs | |||||
| USA | • Characteristics: American women | ||||
| • Study: NHANES (1999-2004) | G1 = 0 | PA level (% prevalence) | |||
| Cross-sectional | G2 = <30 | • G1 = 11.0 (9.8-12.4) | |||
| G3 = ≥ 30 | • G2 = 7.1 (6.0-8.4) | ||||
| D & B score = 10 | • G3 = 3.9 (2.8-5.4) | ||||
| Muscle strengthening activities were expressed in frequency/wk Times/week | p < 0.001 | ||||
| PA level (age adjusted) | |||||
| • G1 = 8.9 (7.7-10.1) | |||||
| G1 = 0 | • G2 = 8.4 (7.3-9.7) | ||||
| G2 = <2 | • G3 = 6.2 (4.4-8.5) | ||||
| G3 = ≥ 2 | p < 0.01 | ||||
| Criteria for diagnosis of osteoporosis: Self-reported physician diagnosed | Muscle strengthening (%prevalence) | ||||
| • G1 = 8.1 (7.2-9.1) | |||||
| Chi-square | • G2 = 3.1 (1.7-5.5) | ||||
| • G3 = 7.4 (5.8-9.4) | |||||
| p < 0.001 | |||||
| Muscle strengthening (age adjusted) | |||||
| • G1 = 7.8 (6.9-8.7) | |||||
| • G2 = 6.7 (3.8-11.8) | |||||
| • G3 = 9.5 (7.6-11.9) | |||||
| p < 0.05 | |||||
| Keramat et al 2008 [151] | To assess risk factors for osteoporosis in postmenopausal women from selected BMD centers in Iran and India. | • Iran n = 363; 178 case, 185 control | Study period 2002 -- 2005 | OR (95% CI) of osteoporosis in exercisers vs. non-exercisers. Iran (age adjusted) | Exercise was shown as protective factor in both countries and it remained significant after adjustment for age weight and height in Iran. |
| • India n = 354; 203 case, 151 control | PA assessment: Questionnaire. PA was categorized as exercises, other exercises (e.g., swimming, aerobics, weight training) and walking | ||||
| Iran and India | |||||
| • Sex: Women | • Exercises = 0.4 (0.2-0.7) | ||||
| Case control | • Age: Iran Case = 58.2 (7.1) yr; Iran Control = 55.7 (6.0) yr; India Case = 58.9 (8.1) yr; India Control = 56.4 (7.5) yr | BMD assessment: DEXA | • Other exercises = 0.4 (0.2-0.6) | ||
| • Characteristics: Cases had BMD > 2.5 SD below average of young normal bone density in L1-L4 spine region and/or total femoral region. Controls had BMD < 1 SD below normal | Multinominal logistic regression | • Regular Walking = 0.5 (0.3- 0.8) | |||
| D & B score = 11 | Walking and other exercises were shown as protective factors in Iranian subjects. | ||||
| Iran (age, weight, height adjusted) | |||||
| • Exercises = 0.4 (0.2-0.7) | |||||
| • Other exercises = 0.3 (0.2-0.6) | |||||
| • Regular Walking = 0.4 (0.2- 0.8) I | |||||
| ndia (age adjusted) | |||||
| • Exercises = 0.4 (0.3-0.9) | |||||
| • Other exercises = NS | |||||
| • Regular Walking = NS | |||||
| India (age, weight, height adjusted) | |||||
| • Exercises = NS | |||||
| • Ethnicity: Indian and Iranian | • Other exercises = NS | ||||
| • Regular Walking = 0.4 (0.2- 0.8) | |||||
D & B score, Downs and Black quality score; YR, years; MET/wk, metabolic equivalent per week; G, groups; PA, physical activity; BMD, bone mineral density; SD, standard deviation; DEXA, dual energy x-ray absorptiometry; NS, not significant.
Exercise has also been shown to significantly reduce the risk and/or number of falls in comparison to inactive controls [138-142]. Moreover, fracture risk and/or incidence has been shown to be reduced in active individuals [143-145]. Case-control studies of older persons who suffered a hip fracture have revealed that these individuals had significantly lower physical activity levels throughout adulthood [136,146]. Observational studies have also revealed an inverse relationship between the incidence of fractures and physical activity [147-149]. For instance, Joakimsen et al. revealed lower fracture rates in individuals who performed more weight-bearing activities [148]. Similarly, Kujala et al. [147] in a 21-year prospective study revealed that intense activity was associated with a lower incidence of hip fracture (Hazard Ratio = 0.38, 95% CI = 0.16-0.91). Feskanich et al. (2002) also revealed that moderate physical activity was inversely related to the risk of hip fracture in postmenopausal women [149]. In a review of observational trials, Katzmarzyk and Janssen [20] revealed that the fracture risk was markedly higher in habitually inactive individuals (RR = 1.59 (95% CI = 1.40-1.80)) with a population attributable risk of 24% in Canada.
There is clear evidence that exercise training is of benefit for bone health and accordingly reduces the risk for osteoporosis. However, remarkably limited research has actually examined the relationship between routine physical activity and the prevalence and/or incidence of osteoporosis (Figure 10). In our systematic search of the osteoporosis literature, a total of 3655 citations were identified during the electronic database search (Figure 7). Of these citations, 1888 were identified in MEDLINE, 236 in EMBASE, 82 in Cochrane, and 481 in the CINAHL/SportDiscus/PsychInfo search. A total of 276 duplicates were found, leaving a total of 2411 unique citations. A total of 2059 articles were excluded after screening, leaving a total of 352 articles for full review. From these articles all 352 were excluded after full-text review. The reasons for exclusion included non-experimental/weak design (n = 87), did not contain three levels of physical activity or not possible to determine dose-response relationship (n = 38), reviews, summaries, meta-analyses (n = 39), not dealing specifically with osteoporosis (n = 21), only on change in bone mineral density (N = 123), clinical population (N = 10), bone metabolism (N = 13), fractures (N = 3), population < 18 yrs (N = 11), and other (N = 7). An additional 2 articles were found through the authors' knowledge of the field.
Figure 10.
Results of the Literature Search for Osteoporosis.
As identified in our systematic search, the majority of the literature has dealt with the relationship between physical activity and indicators of bone health and/or the incidence of fractures. However, a recent observational trial [150] has provided evidence supporting the ability of physical activity to reduce the incidence of osteoporosis. For instance, Robitaille et al. revealed a dose-response relationship between physical activity level and the prevalence of reported osteoporosis in 8073 women aged ≥ 20 yr in the National Health and Nutrition Examination Survey, 1999-2004 [150]. Those performing no physical activity were at a higher risk than those who engaged in moderate (<30 MET hr/wk) and high (>30 MET hr/wk) levels of physical activity. There was a dose-response relationship with the highest physical activity group having the lowest prevalence of osteoporosis. Similarly, Keramat et al. [151] in a case-control investigation revealed that physical activity was protective against the development of osteoporosis.
At this time it is difficult to define clearly the actual dose-response required to cause a reduction in the incidence of osteoporosis. It is clear that bone adaptations to exercise are load dependent and site specific [9,10,16,152]. As such, physical activities that involve significantly loading/impact are often advocated for the prevention of osteoporosis. It is has been shown that running 15-20 miles per week is associated with bone mineral accrual or maintenance. Longer distances however may be associated with reduced bone mineral density [136].
Feskanich et al. reported that the risk of hip fracture was lowered by 6% for each increase of 3 MET-hours per week of activity (or 1 hr/wk of walking at an average pace) [149]. There was a linear reduction with increasing physical activity level. Walking for at least 4 hr/wk was also associated with a 41% lower risk of hip fracture compared to less than 1 hr/wk [149]. The work of Robitaille et al. also indicated that moderate levels of physical activity are sufficient to reduce the prevalence of osteoporosis [150].
In summary, there is preliminary evidence to indicate that the current Canadian physical activity guidelines are sufficient to maintain and/improve bone health. However, further research is clearly required, in particular research that examines the relationship between physical activity and the incidence of osteoporosis in both men and women from varied ethnic backgrounds. Currently, the level of evidence would be considered to be at a Level 3A. The quality of the investigations was generally low with a mean (and median) Downs and Black score of 11.
Recommendation #7
For a reduced risk for osteoporosis, it is recommended that individuals should participate in load bearing activities for 30 min or more on most days of the week. [Level 3, Grade A]
Other Considerations
Musculoskeletal Fitness and Health
In the present analyses, all indices of physical activity/fitness were incorporated into our systematic reviews. Although the majority of the data is related to aerobic activities, it should be noted that many of these activities also had a significant musculoskeletal component. Moreover, direct measurements of musculoskeletal fitness were included in various studies included in our review. Although there is limited information available (in comparison to aerobic activities) there is compelling evidence that musculoskeletal fitness is also positively associated with health status [9,10,16].
Warburton and colleagues [9,10] in two reviews of the literature reported that enhanced musculoskeletal fitness is associated positively with glucose homeostasis, bone health, functional independence, mobility, psychological well-being, and overall quality of life and negatively associated with fall risk, morbidity and premature mortality. They also reported that interventions that increase musculoskeletal fitness also have a significant positive effect on the health status of the individuals with a low musculoskeletal reserve (e.g., the frail elderly).
In an evaluation of the current literature some key findings emerge. Grip strength has particularly been shown to be inversely related to premature mortality and/or morbidity (e.g., functional limitations) [153-156]. Rantanen et al. (1998) reported that those individuals with the lowest grip strength had a higher rate of mortality at a younger age (over a 27- year period) than their counterparts with higher muscular strength. Furthermore, they revealed that those with a faster rate of decline in muscular strength (>1.5% per year) or a very low grip strength (<21 kg) had a greater incidence of chronic diseases, such as type 2 diabetes, stroke, arthritis, coronary heart disease, and pulmonary disorders. It was shown that those in the lowest grip strength tertile had an 8-fold increased risk for disability. Individuals with high muscular strength have also been shown to develop less functional limitations in comparison to their counterparts with lower strength over a 5-year period [157].
Katzmarzyk and colleagues [126,154,158] in Canada have also demonstrated a positive relationship between musculoskeletal fitness and health status. For instance, Katzmarzyk and Craig (2002) revealed that there was a significantly higher risk of premature mortality in the lower quartile of sit-ups in both men (RR = 2.72) and women (RR = 2.26). Grip strength was also predictive of mortality in men (RR = 1.49), but not women. In a recent study, Mason et al. revealed that musculoskeletal fitness was a significant predictor of weight gain over a 20-year period [158]. Importantly, they also reported that individuals with low musculoskeletal fitness had 78% greater odds of significant weight gain (≥ 10 kg) compared to those with high musculoskeletal fitness. These studies provide direct support for the inclusion of resistance and flexibility training in Canada's physical activity guidelines for adults [3,159].
Recommendation #8
For improved health status and reduced risk for chronic disease and disability, it is recommended that individuals should include daily activities that tax the musculoskeletal system [Level 2, Grade A]
Limitations
It is important to note that for each chronic condition, the methods used to determine the relationship between physical activity and the specific clinical outcome were often quite varied. For instance, early work in the field generally controlled for few confounding variables (such as age). In comparison, current literature often controls for a myriad of potential confounding variables. These discrepancies make the comparison of the relative risk reductions between studies and across clinical conditions more difficult. Moreover, the multivariate analyses (controlling for various potential confounding factors) may inappropriately decrease the level of risk reduction associated with physical activity and the clinical endpoint [31]. This is owing to the fact that some of the health benefits associated with physical activity may be mediated through these variables [31].
There was often considerable variability in the findings and major conclusions of the studies examined. Often the available literature was limited by the lack of a clear standard for assessing physical activity. In many instances, it was extremely difficult to determine the actual dosage of physical activity used to group the participants. This lack of clarity makes it very difficult to clearly define the dose-response relationship between physical activity and various chronic conditions.
Conclusions
There is incontrovertible evidence that regular exercise is an effective preventative strategy against premature mortality, cardiovascular disease, stroke, hypertension, colon cancer, breast cancer, and type 2 diabetes. There is also compelling indirect evidence to support the protective effects of physical activity with respect to osteoporosis. In many instances the dose-response relationship is linear with further health benefits with increasing levels of activity. The current Canadian physical activity guidelines for adults are sufficient to reduce the risk for multiple chronic diseases simultaneously. The acknowledgement that every bit of exercise counts towards health benefits (with greater benefits at higher energy expenditures) is consistent with the literature and a reasonable message to promote to the general population. However, further investigation is likely required to evaluate the relationship between physical activity and health status in non-Caucasian populations.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DW was responsible for the conceptualization and design of the systematic review, the generation of the systematic review terms, oversaw the data collection, evaluated each article included in the review, and was responsible for creating and revising the manuscript. SC was involved in the data collection, the critical review of the articles, the creation of the tables contained in the article and the revision of the manuscript. AI assisted with the data collection, the critical review of the articles, and the creation and the revision of tables in the manuscript. LN assisted with the generation of the systematic review terms, the retrieval of articles, and the generation and revision of the tables. SB was involved in the conceptualization and design of the systematic review, the generation of the systematic review terms, oversaw the data collection, the review of the articles, and was responsible for creating and revising the manuscript. All authors have read and approved the final manuscript.
Contributor Information
Darren ER Warburton, Email: darrenwb@interchange.ubc.ca.
Sarah Charlesworth, Email: sacjazz@hotmail.com.
Adam Ivey, Email: adamivey@interchange.ubc.ca.
Lindsay Nettlefold, Email: lindsay.nettlefold@gmail.com.
Shannon SD Bredin, Email: bredin@interchange.ubc.ca.
Acknowledgements
Production of this paper has been made possible through a financial contribution from the Public Health Agency of Canada. The views expressed herein do not necessarily represent the views of the Public Health Agency of Canada. The leadership and administrative assistance was provided by the Canadian Society for Exercise Physiology (CSEP). Dr. Warburton is supported by a Canadian Institutes of Health Research New Investigator award and a Michael Smith Foundation for Health Research Clinical Scholar award. We are indebted to the work conducted by the staff from the CSEP Health & Fitness Program of BC and Physical Activity Support Line (PAL; http://www.physicalactivityline.com) in the systematic review of the literature and the development of tables for this manuscript and the companion paper by Paterson and Warburton [160].
References
- Bouchard C, Shephard RJ. In: Physical activity fitness and health: International proceedings and consensus statement. Bouchard C, Shephard RJ, Stephens T, editor. Champaign, IL: Human Kinetics; 1994. Physical activity fitness and health: the model and key concepts; pp. 77–88. [Google Scholar]
- Blair SN, Brodney S. Effects of physical inactivity and obesity on morbidity and mortality: current evidence and research issues. Med Sci Sports Exerc. 1999;7:S646–662. doi: 10.1097/00005768-199911001-00025. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. Position stand: Exercise and physical activity for older adults. Med Sci Sports Exerc. 1998;7:992–1008. doi: 10.1097/00005768-199806000-00033. [DOI] [PubMed] [Google Scholar]
- McAuley E. In: Physical activity, fitness and health: the consensus knowledge. Bouchard C, Shephard RJ, Stephens T, editor. Champaign, IL: Human Kinetics; 1994. Physical activity and psychosocial outcomes; pp. 551–568. [Google Scholar]
- Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, Skidmore B, Stone JA, Thompson DR, Oldridge N. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;7:682–692. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc. 2001;7:S379–399. doi: 10.1097/00005768-200105001-01549. discussion S419-320. [DOI] [PubMed] [Google Scholar]
- Blair SN, Kohl HW, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;7:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS Jr, Hyde RT, Hsieh CC, Wing AL. Physical activity, other life-style patterns, cardiovascular disease and longevity. Acta Med Scand Suppl. 1986;7:85–91. doi: 10.1111/j.0954-6820.1986.tb08936.x. [DOI] [PubMed] [Google Scholar]
- Warburton DE, Gledhill N, Quinney A. Musculoskeletal fitness and health. Can J Appl Physiol. 2001;7:217–237. doi: 10.1139/h01-013. [DOI] [PubMed] [Google Scholar]
- Warburton DE, Gledhill N, Quinney A. The effects of changes in musculoskeletal fitness on health. Can J Appl Physiol. 2001;7:161–216. doi: 10.1139/h01-012. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Healthy People 2000: National Health Promotion and Disease Prevention Objectives. Washington, D.C.: U.S. Department of Health and Human Services; 1991. [Google Scholar]
- Puett DW, Griffin MR. Published trials of nonmedicinal and noninvasive therapies for hip and knee osteoarthritis. Ann Intern Med. 1994;7:133–140. doi: 10.7326/0003-4819-121-2-199407150-00010. [DOI] [PubMed] [Google Scholar]
- Shephard RJ. Absolute versus relative intensity of physical activity in a dose-response context. Med Sci Sports Exerc. 2001;7:S400–418. doi: 10.1097/00005768-200106001-00008. discussion S419-420. [DOI] [PubMed] [Google Scholar]
- Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc. 2001;7:S459–471. doi: 10.1097/00005768-200106001-00016. discussion S493-454. [DOI] [PubMed] [Google Scholar]
- Warburton DE, Nicol C, Bredin SS. Prescribing exercise as preventive therapy. CMAJ. 2006;7:961–974. doi: 10.1503/cmaj.1040750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton DE, Nicol C, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;7:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C, Shephard RJ, Stephens T. In: Physical activity fitness and health: International proceedings and consensus statement. Bouchard C, Shephard RJ, Stephens T, editor. Champaign, IL: Human Kinetics; 1994. The consensus statement; pp. 9–76. [Google Scholar]
- Warburton DER, Katzmarzyk PT, Rhodes RE, Shephard RJ. Evidence-informed physical activity guidelines for Canadian adults. Appl Physiol Nutr Metab. 2007;7:S17–74. doi: 10.1139/H07-168. [DOI] [PubMed] [Google Scholar]
- Warburton DER, Katzmarzyk PT, Rhodes RE, Shephard RJ. Evidence-informed physical activity guidelines for Canadian adults. Can J Pub Health. 2007;7:S16–S68. [PubMed] [Google Scholar]
- Katzmarzyk PT, Janssen I. The economic costs associated with physical inactivity and obesity in Canada: an update. Can J Appl Physiol. 2004;7:90–115. doi: 10.1139/h04-008. [DOI] [PubMed] [Google Scholar]
- Health Canada and Canadian Society for Exercise Physiology. Canada's Physical Activity Guide to Healthy Active Living. Ottawa, ON: Health Canada (Cat. No. H39-429/1998-1E); 1998. http://www.paguide.com [Google Scholar]
- Health Canada and Canadian Society for Exercise Physiology. Canada's Physical Activity Guide to Healthy Active Living for Older Adults. Ottawa, ON: Health Canada; 1999. http://www.paguide.com [Google Scholar]
- Health Canada and Canadian Society for Exercise Physiology. Canada's Physical Activity Guide to Healthy Active Living for Children and Youths. Ottawa, ON: Health Canada; 2002. http://www.paguide.com [Google Scholar]
- Canadian Society for Exercise Physiology. Canadian Physical Activity, Fitness and Lifestyle Approach. 3. Ottawa: Canadian Society for Exercise Physiology; 2003. [Google Scholar]
- Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary] CMAJ. 2007;7:S1–13. doi: 10.1503/cmaj.061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;7:307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;7:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;7:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macera CA, Hootman JM, Sniezek JE. Major public health benefits of physical activity. Arthritis Rheum. 2003;7:122–128. doi: 10.1002/art.10907. [DOI] [PubMed] [Google Scholar]
- Macera CA, Powell KE. Population attributable risk: implications of physical activity dose. Med Sci Sports Exerc. 2001;7:S635–639. doi: 10.1097/00005768-200106001-00032. discussion 640-631. [DOI] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report. Washington, DC: U.S. Department of Health and Human Services; 2008. p. 683. [Google Scholar]
- Myers J, Kaykha A, George S, Abella J, Zaheer N, Lear S, Yamazaki T, Froelicher V. Fitness versus physical activity patterns in predicting mortality in men. Am J Med. 2004;7:912–918. doi: 10.1016/j.amjmed.2004.06.047. [DOI] [PubMed] [Google Scholar]
- Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Med Sci Sports Exerc. 2001;7:754–761. doi: 10.1097/00005768-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikssen G. Physical fitness and changes in mortality: the survival of the fittest. Sports Med. 2001;7:571–576. doi: 10.2165/00007256-200131080-00001. [DOI] [PubMed] [Google Scholar]
- Erikssen G, Liestol K, Bjornholt J, Thaulow E, Sandvik L, Erikssen J. Changes in physical fitness and changes in mortality. Lancet. 1998;7:759–762. doi: 10.1016/S0140-6736(98)02268-5. [DOI] [PubMed] [Google Scholar]
- Blair SN, Kohl HW, Barlow CE, Paffenbarger RS Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;7:1093–1098. doi: 10.1001/jama.273.14.1093. [DOI] [PubMed] [Google Scholar]
- Bijnen FC, Feskens EJ, Caspersen CJ, Nagelkerke N, Mosterd WL, Kromhout D. Baseline and previous physical activity in relation to mortality in elderly men: the Zutphen Elderly Study. Am J Epidemiol. 1999;7:1289–1296. doi: 10.1093/oxfordjournals.aje.a009960. [DOI] [PubMed] [Google Scholar]
- Johansson SE, Sundquist J. Change in lifestyle factors and their influence on health status and all-cause mortality. Int J Epidemiol. 1999;7:1073–1080. doi: 10.1093/ije/28.6.1073. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Cauley JA, Stone K, Thompson TJ, Bauer DC, Cummings SR, Ensrud KE. Relationship of changes in physical activity and mortality among older women. JAMA. 2003;7:2379–2386. doi: 10.1001/jama.289.18.2379. [DOI] [PubMed] [Google Scholar]
- Wannamethee SG, Shaper AG, Walker M. Changes in physical activity, mortality, and incidence of coronary heart disease in older men. Lancet. 1998;7:1603–1608. doi: 10.1016/S0140-6736(97)12355-8. [DOI] [PubMed] [Google Scholar]
- Kujala UM, Kaprio J, Koskenvuo M. Modifiable risk factors as predictors of all-cause mortality: the roles of genetics and childhood environment. Am J Epidemiol. 2002;7:985–993. doi: 10.1093/aje/kwf151. [DOI] [PubMed] [Google Scholar]
- Wessel TR, Arant CB, Olson MB, Johnson BD, Reis SE, Sharaf BL, Shaw LJ, Handberg E, Sopko G, Kelsey SF, Pepine CJ, Merz NB. Relationship of physical fitness vs body mass index with coronary artery disease and cardiovascular events in women. JAMA. 2004;7:1179–1187. doi: 10.1001/jama.292.10.1179. [DOI] [PubMed] [Google Scholar]
- Katzmarzyk PT, Church TS, Blair SN. Cardiorespiratory fitness attenuates the effects of the metabolic syndrome on all-cause and cardiovascular disease mortality in men. Arch Intern Med. 2004;7:1092–1097. doi: 10.1001/archinte.164.10.1092. [DOI] [PubMed] [Google Scholar]
- Morris JN, Heady JA. Mortality in relation to the physical activity of work: a preliminary note on experience in middle age. Br J Ind Med. 1953;7:245–254. doi: 10.1136/oem.10.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW. Coronary heart-disease and physical activity of work. Lancet. 1953;7:1111–1120. doi: 10.1016/S0140-6736(53)91495-0. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS Jr, Brand RJ, Sholtz RI, Jung DL. Energy expenditure, cigarette smoking, and blood pressure level as related to death from specific diseases. Am J Epidemiol. 1978;7:12–18. [PubMed] [Google Scholar]
- Paffenbarger RS, Hale WE. Work activity and coronary heart mortality. N Engl J Med. 1975;7:545–550. doi: 10.1056/NEJM197503132921101. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. Physical activity and health: a report of the Surgeon General. Atlanta, G.A.: Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
- Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol. 2000;7:774–787. doi: 10.1152/jappl.2000.88.2.774. [DOI] [PubMed] [Google Scholar]
- Katzmarzyk PT, Gledhill N, Shephard RJ. The economic burden of physical inactivity in Canada. CMAJ. 2000;7:1435–1440. [PMC free article] [PubMed] [Google Scholar]
- Oguma Y, Sesso HD, Paffenbarger RS Jr, Lee IM. Physical activity and all cause mortality in women: a review of the evidence. Br J Sports Med. 2002;7:162–172. doi: 10.1136/bjsm.36.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmarzyk P. In: ACSM's Resource Manual for Guidelines for Exercise Testing and Prescription. 5. Kohl HW, editor. Philadelphia: Lippincott, Williams and Wilkins; 2005. Physical Activity Status and Chronic Diseases; pp. 122–135. [Google Scholar]
- Statistics Canada. Deaths, 2002. Ottawa: Statistics Canada; 2004. http://www.statcan.ca/Daily/English/040927/d040927a.htm [Google Scholar]
- World Health Organization. The World Health Report: Reducing risks, promoting healthy life. Geneva: World Health Organization; 2002. [Google Scholar]
- Emberson JR, Whincup PH, Morris RW, Wannamethee SG, Shaper AG. Lifestyle and cardiovascular disease in middle-aged British men: the effect of adjusting for within-person variation. Eur Heart J. 2005;7:1774–1782. doi: 10.1093/eurheartj/ehi224. [DOI] [PubMed] [Google Scholar]
- Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;7:716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- Oguma Y, Shinoda-Tagawa T. Physical activity decreases cardiovascular disease risk in women: review and meta-analysis. Am J Prev Med. 2004;7:407–418. doi: 10.1016/j.amepre.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Wisloff U, Nilsen TI, Droyvold WB, Morkved S, Slordahl SA, Vatten LJ. A single weekly bout of exercise may reduce cardiovascular mortality: how little pain for cardiac gain? 'The HUNT study, Norway'. Eur J Cardiovasc Prev Rehabil. 2006;7:798–804. doi: 10.1097/01.hjr.0000216548.84560.ac. [DOI] [PubMed] [Google Scholar]
- Lee IM, Rexrode KM, Cook NR, Manson JE, Buring JE. Physical activity and coronary heart disease in women: is "no pain, no gain" passe? JAMA. 2001;7:1447–1454. doi: 10.1001/jama.285.11.1447. [DOI] [PubMed] [Google Scholar]
- Berlin JA, Colditz GA. A meta-analysis of physical activity in the prevention of coronary heart disease. Am J Epidemiol. 1990;7:612–628. doi: 10.1093/oxfordjournals.aje.a115704. [DOI] [PubMed] [Google Scholar]
- Bauman AE. Updating the evidence that physical activity is good for health: an epidemiological review 2000-2003. J Sci Med Sport. 2004;7:6–19. doi: 10.1016/S1440-2440(04)80273-1. [DOI] [PubMed] [Google Scholar]
- Kohl HW. Physical activity and cardiovascular disease: evidence for a dose response. Med Sci Sports Exerc. 2001;7:S472–483. doi: 10.1097/00005768-200105001-01015. discussion S493-474. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;7:605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- Schnohr P, Scharling H, Jensen JS. Intensity versus duration of walking, impact on mortality: the Copenhagen City Heart Study. Eur J Cardiovasc Prev Rehabil. 2007;7:72–78. doi: 10.1097/HJR.0b013e3280144470. [DOI] [PubMed] [Google Scholar]
- Kushi LH, Fee RM, Folsom AR, Mink PJ, Anderson KE, Sellers TA. Physical activity and mortality in postmenopausal women. JAMA. 1997;7:1287–1292. doi: 10.1001/jama.277.16.1287. [DOI] [PubMed] [Google Scholar]
- Leon AS, Connett J, Jacobs DR Jr, Rauramaa R. Leisure-time physical activity levels and risk of coronary heart disease and death. The Multiple Risk Factor Intervention Trial. JAMA. 1987;7:2388–2395. doi: 10.1001/jama.258.17.2388. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;7:538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- Heart and Stroke Foundation of Canada. Press Releases - Canadian leads effort to raise awareness of stroke as a global health epidemic. Ottawa: Heart and Stroke Foundation of Canada; 2007. [Google Scholar]
- Abbott RD, Curb JD, Rodriguez BL, Masaki KH, Popper JS, Ross GW, Petrovitch H. Age-related changes in risk factor effects on the incidence of thromboembolic and hemorrhagic stroke. J Clin Epidemiol. 2003;7:479–486. doi: 10.1016/S0895-4356(02)00611-X. [DOI] [PubMed] [Google Scholar]
- Gillum RF, Mussolino ME, Ingram DD. Physical activity and stroke incidence in women and men. The NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1996;7:860–869. doi: 10.1093/oxfordjournals.aje.a008829. [DOI] [PubMed] [Google Scholar]
- Lee CD, Blair SN. Cardiorespiratory fitness and stroke mortality in men. Med Sci Sports Exerc. 2002;7:592–595. doi: 10.1097/00005768-200205001-01747. [DOI] [PubMed] [Google Scholar]
- Hu FB, Stampfer MJ, Colditz GA, Ascherio A, Rexrode KM, Willett WC, Manson JE. Physical activity and risk of stroke in women. JAMA. 2000;7:2961–2967. doi: 10.1001/jama.283.22.2961. [DOI] [PubMed] [Google Scholar]
- Lee IM, Paffenbarger RS Jr. Physical activity and stroke incidence: the Harvard Alumni Health Study. Stroke. 1998;7:2049–2054. doi: 10.1161/01.str.29.10.2049. [DOI] [PubMed] [Google Scholar]
- Lee IM, Hennekens CH, Berger K, Buring JE, Manson JE. Exercise and risk of stroke in male physicians. Stroke. 1999;7:1–6. doi: 10.1161/01.str.30.1.1. [DOI] [PubMed] [Google Scholar]
- Rankinen T, Church TS, Rice T, Bouchard C, Blair SN. Cardiorespiratory fitness, BMI, and risk of hypertension: the HYPGENE study. Med Sci Sports Exerc. 2007;7:1687–1692. doi: 10.1249/mss.0b013e31812e527f. [DOI] [PubMed] [Google Scholar]
- Pereira MA, Folsom AR, McGovern PG, Carpenter M, Arnett DK, Liao D, Szklo M, Hutchinson RG. Physical activity and incident hypertension in black and white adults: the Atherosclerosis Risk in Communities Study. Prev Med. 1999;7:304–312. doi: 10.1006/pmed.1998.0431. [DOI] [PubMed] [Google Scholar]
- Haapanen N, Miilunpalo S, Vuori I, Oja P, Pasanen M. Association of leisure time physical activity with the risk of coronary heart disease, hypertension and diabetes in middle-aged men and women. Int J Epidemiol. 1997;7:739–747. doi: 10.1093/ije/26.4.739. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS Jr, Wing AL, Hyde RT, Jung DL. Physical activity and incidence of hypertension in college alumni. Am J Epidemiol. 1983;7:245–257. doi: 10.1093/oxfordjournals.aje.a113537. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS Jr, Lee IM. Intensity of physical activity related to incidence of hypertension and all-cause mortality: an epidemiological view. Blood Press Monit. 1997;7:115–123. [PubMed] [Google Scholar]
- Hernelahti M, Kujala UM, Kaprio J, Sarna S. Long-term vigorous training in young adulthood and later physical activity as predictors of hypertension in middle-aged and older men. Int J Sports Med. 2002;7:178–182. doi: 10.1055/s-2002-23176. [DOI] [PubMed] [Google Scholar]
- Hu G, Barengo NC, Tuomilehto J, Lakka TA, Nissinen A, Jousilahti P. Relationship of physical activity and body mass index to the risk of hypertension: a prospective study in Finland. Hypertension. 2004;7:25–30. doi: 10.1161/01.HYP.0000107400.72456.19. [DOI] [PubMed] [Google Scholar]
- Gu D, Wildman RP, Wu X, Reynolds K, Huang J, Chen CS, He J. Incidence and predictors of hypertension over 8 years among Chinese men and women. J Hypertens. 2007;7:517–523. doi: 10.1097/HJH.0b013e328013e7f4. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Tsumura K, Suematsu C, Okada K, Fujii S, Endo G. Walking to work and the risk for hypertension in men: the Osaka Health Survey. Ann Intern Med. 1999;7:21–26. doi: 10.7326/0003-4819-131-1-199907060-00005. [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Suzuki K. Daily life activity and the risk of developing hypertension in middle-aged Japanese men. Arch Intern Med. 2005;7:214–220. doi: 10.1001/archinte.165.2.214. [DOI] [PubMed] [Google Scholar]
- Foy CG, Foley KL, D'Agostino RB Jr, Goff DC Jr, Mayer-Davis E, Wagenknecht LE. Physical activity, insulin sensitivity, and hypertension among US adults: findings from the Insulin Resistance Atherosclerosis Study. Am J Epidemiol. 2006;7:921–928. doi: 10.1093/aje/kwj113. [DOI] [PubMed] [Google Scholar]
- Haennel RG, Lemire F. Physical activity to prevent cardiovascular disease. How much is enough? Can Fam Physician. 2002;7:65–71. [PMC free article] [PubMed] [Google Scholar]
- Fagard RH. Exercise characteristics and the blood pressure response to dynamic physical training. Med Sci Sports Exerc. 2001;7:S484–492. doi: 10.1097/00005768-200106001-00018. discussion S493-484. [DOI] [PubMed] [Google Scholar]
- Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;7:533–553. doi: 10.1249/01.MSS.0000115224.88514.3A. [DOI] [PubMed] [Google Scholar]
- Cornelissen VA, Fagard RH. Effect of resistance training on resting blood pressure: a meta-analysis of randomized controlled trials. J Hypertens. 2005;7:251–259. doi: 10.1097/00004872-200502000-00003. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Fast Stats: Hypertension. Hyattsville, MD: Centers for Disease Control and Prevention; 2008. [Google Scholar]
- Statistics Canada. CANSIM. Ottawa: Statistics Canada; 2007. [Google Scholar]
- McAlister FA, Wooltorton E, Campbell NR. The Canadian Hypertension Education Program (CHEP) recommendations: launching a new series. CMAJ. 2005;7:508–509. doi: 10.1503/cmaj.050737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffres MR, Ghadirian P, Fodor JG, Petrasovits A, Chockalingam A, Hamet P. Awareness, treatment, and control of hypertension in Canada. Am J Hypertens. 1997;7:1097–1102. doi: 10.1016/S0895-7061(97)00224-0. [DOI] [PubMed] [Google Scholar]
- Fagard RH. Physical activity in the prevention and treatment of hypertension in the obese. Med Sci Sports Exerc. 1999;7:S624–630. doi: 10.1097/00005768-199911001-00022. [DOI] [PubMed] [Google Scholar]
- Kelley GA. Aerobic exercise and resting blood pressure among women: a metaanalysis. Prev Med. 1999;7:264–275. doi: 10.1006/pmed.1998.0417. [DOI] [PubMed] [Google Scholar]
- Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;7:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- Dickinson HO, Mason JM, Nicolson DJ, Campbell F, Beyer FR, Cook JV, Williams B, Ford GA. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;7:215–233. doi: 10.1097/01.hjh.0000199800.72563.26. [DOI] [PubMed] [Google Scholar]
- Hamer M, Taylor A, Steptoe A. The effect of acute aerobic exercise on stress related blood pressure responses: a systematic review and meta-analysis. Biol Psychol. 2006;7:183–190. doi: 10.1016/j.biopsycho.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Fagard RH, Cornelissen VA. Effect of exercise on blood pressure control in hypertensive patients. Eur J Cardiovasc Prev Rehabil. 2007;7:12–17. doi: 10.1097/HJR.0b013e3280128bbb. [DOI] [PubMed] [Google Scholar]
- Fagard RH. Exercise is good for your blood pressure: effects of endurance training and resistance training. Clin Exp Pharmacol Physiol. 2006;7:853–856. doi: 10.1111/j.1440-1681.2006.04453.x. [DOI] [PubMed] [Google Scholar]
- Fagard RH. Effects of exercise, diet and their combination on blood pressure. J Hum Hypertens. 2005;7(Suppl 3):S20–24. doi: 10.1038/sj.jhh.1001956. [DOI] [PubMed] [Google Scholar]
- Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;7:667–675. doi: 10.1161/01.HYP.0000184225.05629.51. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Crouse SF, Goodpaster B, Kelley D, Moyna N, Pescatello L. The acute versus the chronic response to exercise. Med Sci Sports Exerc. 2001;7:S438–445. doi: 10.1097/00005768-200106001-00012. discussion S452-433. [DOI] [PubMed] [Google Scholar]
- Monninkhof EM, Elias SG, Vlems FA, Tweel I van der, Schuit AJ, Voskuil DW, van Leeuwen FE. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;7:137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- Lee IM. Physical activity and cancer prevention--data from epidemiologic studies. Med Sci Sports Exerc. 2003;7:1823–1827. doi: 10.1249/01.MSS.0000093620.27893.23. [DOI] [PubMed] [Google Scholar]
- Rockhill B, Willett WC, Hunter DJ, Manson JE, Hankinson SE, Colditz GA. A prospective study of recreational physical activity and breast cancer risk. Arch Intern Med. 1999;7:2290–2296. doi: 10.1001/archinte.159.19.2290. [DOI] [PubMed] [Google Scholar]
- Sesso HD, Paffenbarger RS Jr, Lee IM. Physical activity and breast cancer risk in the College Alumni Health Study (United States) Cancer Causes Control. 1998;7:433–439. doi: 10.1023/A:1008827903302. [DOI] [PubMed] [Google Scholar]
- Shephard RJ, Futcher R. Physical activity and cancer: how may protection be maximized? Crit Rev Oncog. 1997;7:219–272. doi: 10.1615/critrevoncog.v8.i2-3.40. [DOI] [PubMed] [Google Scholar]
- Thune I, Furberg AS. Physical activity and cancer risk: dose-response and cancer, all sites and site-specific. Med Sci Sports Exerc. 2001;7:S530–550. doi: 10.1097/00005768-200106001-00025. discussion S609-510. [DOI] [PubMed] [Google Scholar]
- Cust AE, Armstrong BK, Friedenreich CM, Slimani N, Bauman A. Physical activity and endometrial cancer risk: a review of the current evidence, biologic mechanisms and the quality of physical activity assessment methods. Cancer Causes Control. 2007;7:243–58. doi: 10.1007/s10552-006-0094-7. [DOI] [PubMed] [Google Scholar]
- Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;7:1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH. A prospective study of exercise and incidence of diabetes among US male physicians. JAMA. 1992;7:63–67. doi: 10.1001/jama.268.1.63. [DOI] [PubMed] [Google Scholar]
- Lynch J, Helmrich SP, Lakka TA, Kaplan GA, Cohen RD, Salonen R, Salonen JT. Moderately intense physical activities and high levels of cardiorespiratory fitness reduce the risk of non-insulin-dependent diabetes mellitus in middle-aged men. Arch Intern Med. 1996;7:1307–1314. doi: 10.1001/archinte.156.12.1307. [DOI] [PubMed] [Google Scholar]
- Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;7:790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- Schulze MB, Hu FB. Primary prevention of diabetes: what can be done and how much can be prevented? Annu Rev Public Health. 2005;7:445–467. doi: 10.1146/annurev.publhealth.26.021304.144532. [DOI] [PubMed] [Google Scholar]
- Sato KK, Hayashi T, Kambe H, Nakamura Y, Harita N, Endo G, Yoneda T. Walking to work is an independent predictor of incidence of type 2 diabetes in Japanese men: the Kansai Healthcare Study. Diabetes Care. 2007;7:2296–2298. doi: 10.2337/dc07-0090. [DOI] [PubMed] [Google Scholar]
- Hu G, Qiao Q, Silventoinen K, Eriksson JG, Jousilahti P, Lindstrom J, Valle TT, Nissinen A, Tuomilehto J. Occupational, commuting, and leisure-time physical activity in relation to risk for Type 2 diabetes in middle-aged Finnish men and women. Diabetologia. 2003;7:322–329. doi: 10.1007/s00125-003-1031-x. [DOI] [PubMed] [Google Scholar]
- Hsia J, Wu L, Allen C, Oberman A, Lawson WE, Torrens J, Safford M, Limacher MC, Howard BV. Physical activity and diabetes risk in postmenopausal women. Am J Prev Med. 2005;7:19–25. doi: 10.1016/j.amepre.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Folsom AR, Kushi LH, Hong CP. Physical activity and incident diabetes mellitus in postmenopausal women. Am J Public Health. 2000;7:134–138. doi: 10.2105/AJPH.90.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamethee SG, Shaper AG, Alberti KG. Physical activity, metabolic factors, and the incidence of coronary heart disease and type 2 diabetes. Arch Intern Med. 2000;7:2108–2116. doi: 10.1001/archinte.160.14.2108. [DOI] [PubMed] [Google Scholar]
- Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, Rosner B, Hennekens CH, Speizer FE. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;7:774–778. doi: 10.1016/0140-6736(91)90664-B. [DOI] [PubMed] [Google Scholar]
- Helmrich SP, Ragland DR, Paffenbarger RS Jr. Prevention of non-insulin-dependent diabetes mellitus with physical activity. Med Sci Sports Exerc. 1994;7:824–830. [PubMed] [Google Scholar]
- Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS Jr. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med. 1991;7:147–152. doi: 10.1056/NEJM199107183250302. [DOI] [PubMed] [Google Scholar]
- Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med. 1999;7:89–96. doi: 10.7326/0003-4819-130-2-199901190-00002. [DOI] [PubMed] [Google Scholar]
- Eriksson J, Tuominen J, Valle T, Sundberg S, Sovijarvi A, Lindholm H, Tuomilehto J, Koivisto V. Aerobic endurance exercise or circuit-type resistance training for individuals with impaired glucose tolerance? Horm Metab Res. 1998;7:37–41. doi: 10.1055/s-2007-978828. [DOI] [PubMed] [Google Scholar]
- Katzmarzyk PT, Craig CL, Gauvin L. Adiposity, physical fitness and incident diabetes: the physical activity longitudinal study. Diabetologia. 2007;7:538–544. doi: 10.1007/s00125-006-0554-3. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;7:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;7:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Williamson DF, Vinicor F, Bowman BA. Primary prevention of type 2 diabetes mellitus by lifestyle intervention: implications for health policy. Ann Intern Med. 2004;7:951–957. doi: 10.7326/0003-4819-140-11-200406010-00036. [DOI] [PubMed] [Google Scholar]
- Wolff I, van Croonenborg JJ, Kemper HC, Kostense PJ, Twisk JW. The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int. 1999;7:1–12. doi: 10.1007/s001980050109. [DOI] [PubMed] [Google Scholar]
- Berard A, Bravo G, Gauthier P. Meta-analysis of the effectiveness of physical activity for the prevention of bone loss in postmenopausal women. Osteoporos Int. 1997;7:331–337. doi: 10.1007/BF01623773. [DOI] [PubMed] [Google Scholar]
- Kelley GA. Exercise and regional bone mineral density in postmenopausal women: a meta-analytic review of randomized trials. Am J Phys Med Rehabil. 1998;7:76–87. doi: 10.1097/00002060-199801000-00018. [DOI] [PubMed] [Google Scholar]
- Kelley GA. Aerobic exercise and bone density at the hip in postmenopausal women: a meta-analysis. Prev Med. 1998;7:798–807. doi: 10.1006/pmed.1998.0360. [DOI] [PubMed] [Google Scholar]
- Kelley GA, Kelley KS. Efficacy of resistance exercise on lumbar spine and femoral neck bone mineral density in premenopausal women: a meta-analysis of individual patient data. J Womens Health (Larchmt) 2004;7:293–300. doi: 10.1089/154099904323016455. [DOI] [PubMed] [Google Scholar]
- Bonaiuti D, Shea B, Iovine R, Negrini S, Robinson V, Kemper HC, Wells G, Tugwell P, Cranney A. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2002. p. CD000333. [DOI] [PubMed]
- Brown JP, Josse RG. 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ. 2002;7:S1–34. [PMC free article] [PubMed] [Google Scholar]
- Wallace BA, Cumming RG. Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif Tissue Int. 2000;7:10–18. doi: 10.1007/s00223001089. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Baker DI, McAvay G, Claus EB, Garrett P, Gottschalk M, Koch ML, Trainor K, Horwitz RI. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med. 1994;7:821–827. doi: 10.1056/NEJM199409293311301. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Barnhart HX, Kutner NG, McNeely E, Coogler C, Xu T. Reducing frailty and falls in older persons: an investigation of Tai Chi and computerized balance training. Atlanta FICSIT Group. Frailty and Injuries: Cooperative Studies of Intervention Techniques. J Am Geriatr Soc. 1996;7:489–497. doi: 10.1111/j.1532-5415.1996.tb01432.x. [DOI] [PubMed] [Google Scholar]
- Carter ND, Khan KM, Petit MA, Heinonen A, Waterman C, Donaldson MG, Janssen PA, Mallinson A, Riddell L, Kruse K, Prior JC, Flicker L, McKay HA. Results of a 10 week community based strength and balance training programme to reduce fall risk factors: a randomised controlled trial in 65-75 year old women with osteoporosis. Br J Sports Med. 2001;7:348–351. doi: 10.1136/bjsm.35.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Ambrose T, Khan KM, Eng JJ, Janssen PA, Lord SR, McKay HA. Resistance and agility training reduce fall risk in women aged 75 to 85 with low bone mass: a 6- month randomized, controlled trial. J Am Geriatr Soc. 2004;7:657–665. doi: 10.1111/j.1532-5415.2004.52200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JM, Snow CM. Weighted vest exercise improves indices of fall risk in older women. J Gerontol A Biol Sci Med Sci. 1998;7:M53–58. doi: 10.1093/gerona/53a.1.m53. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Pereira MA, Caspersen CJ. Physical activity, falls, and fractures among older adults: a review of the epidemiologic evidence. J Am Geriatr Soc. 2000;7:883–893. doi: 10.1111/j.1532-5415.2000.tb06884.x. [DOI] [PubMed] [Google Scholar]
- Stevens JA, Powell KE, Smith SM, Wingo PA, Sattin RW. Physical activity, functional limitations, and the risk of fall-related fractures in community-dwelling elderly. Ann Epidemiol. 1997;7:54–61. doi: 10.1016/S1047-2797(96)00110-X. [DOI] [PubMed] [Google Scholar]
- Carter ND, Kannus P, Khan KM. Exercise in the prevention of falls in older people: a systematic literature review examining the rationale and the evidence. Sports Med. 2001;7:427–438. doi: 10.2165/00007256-200131060-00003. [DOI] [PubMed] [Google Scholar]
- Boyce WJ, Vessey MP. Habitual physical inertia and other factors in relation to risk of fracture of the proximal femur. Age Ageing. 1988;7:319–327. doi: 10.1093/ageing/17.5.319. [DOI] [PubMed] [Google Scholar]
- Kujala UM, Kaprio J, Kannus P, Sarna S, Koskenvuo M. Physical activity and osteoporotic hip fracture risk in men. Arch Intern Med. 2000;7:705–708. doi: 10.1001/archinte.160.5.705. [DOI] [PubMed] [Google Scholar]
- Joakimsen RM, Fonnebo V, Magnus JH, Stormer J, Tollan A, Sogaard AJ. The Tromso Study: physical activity and the incidence of fractures in a middle-aged population. J Bone Miner Res. 1998;7:1149–1157. doi: 10.1359/jbmr.1998.13.7.1149. [DOI] [PubMed] [Google Scholar]
- Feskanich D, Willett W, Colditz G. Walking and leisure-time activity and risk of hip fracture in postmenopausal women. JAMA. 2002;7:2300–2306. doi: 10.1001/jama.288.18.2300. [DOI] [PubMed] [Google Scholar]
- Robitaille J, Yoon PW, Moore CA, Liu T, Irizarry-Delacruz M, Looker AC, Khoury MJ. Prevalence, family history, and prevention of reported osteoporosis in U.S. women. Am J Prev Med. 2008;7:47–54. doi: 10.1016/j.amepre.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Keramat A, Patwardhan B, Larijani B, Chopra A, Mithal A, Chakravarty D, Adibi H, Khosravi A. The assessment of osteoporosis risk factors in Iranian women compared with Indian women. BMC Musculoskelet Disord. 2008;7:28. doi: 10.1186/1471-2474-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr D, Morton A, Dick I, Prince R. Exercise effects on bone mass in postmenopausal women are site-specific and load-dependent. J Bone Miner Res. 1996;7:218–225. doi: 10.1002/jbmr.5650110211. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik JM. Grip strength changes over 27 yr in Japanese-American men. J Appl Physiol. 1998;7:2047–2053. doi: 10.1152/jappl.1998.85.6.2047. [DOI] [PubMed] [Google Scholar]
- Katzmarzyk PT, Craig CL. Musculoskeletal fitness and risk of mortality. Med Sci Sports Exerc. 2002;7:740–744. doi: 10.1097/00005768-200205001-01269. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;7:B359–365. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Nakamura Y, Hiraoka J, Kobayashi K, Sakata K, Nagai M, Yanagawa H. Physical-strength tests and mortality among visitors to health- promotion centers in Japan. J Clin Epidemiol. 1995;7:1349–1359. doi: 10.1016/0895-4356(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Brill PA, Macera CA, Davis DR, Blair SN, Gordon N. Muscular strength and physical function. Med Sci Sports Exerc. 2000;7:412–416. doi: 10.1097/00005768-200002000-00023. [DOI] [PubMed] [Google Scholar]
- Mason C, Brien SE, Craig CL, Gauvin L, Katzmarzyk PT. Musculoskeletal fitness and weight gain in Canada. Med Sci Sports Exerc. 2007;7:38–43. doi: 10.1249/01.mss.0000240325.46523.cf. [DOI] [PubMed] [Google Scholar]
- Blair SN, LaMonte MJ, Nichaman MZ. The evolution of physical activity recommendations: how much is enough? Am J Clin Nutr. 2004;7:913S–920S. doi: 10.1093/ajcn/79.5.913S. [DOI] [PubMed] [Google Scholar]
- Paterson DH, Warburton DER. Physical activity and functional limitations in older adults: a systematic review related to Canada's Physical Activity Guidelines. Int J Behav Nutr Phys Act. 2010;7:38. doi: 10.1186/1479-5868-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson JB, Armstrong A, Smith T, Bellew B. The costs of illness attributable to physical inactivity in Australia: A preliminary study. Commonwealth Department of Health and Aged Care and the Australian Sports Commission. 2000. http://www.health.gov.au/internet/main/Publishing.nsf/Content/health-pubhlth-publicatdocument- phys_costofillness-cnt.htm/$FILE/phys_costofillness.pdf
- Colditz GA. Economic costs of obesity and inactivity. Med Sci Sports Exerc. 1999;7:S663–667. doi: 10.1097/00005768-199911001-00026. [DOI] [PubMed] [Google Scholar]
- Andersen LB, Schnohr P, Schroll M, Hein HO. All-cause mortality associated with physical activity during leisure time, work, sports, and cycling to work. Arch Intern Med. 2000;7:1621–1628. doi: 10.1001/archinte.160.11.1621. [DOI] [PubMed] [Google Scholar]
- Barengo NC, Hu G, Lakka TA, Pekkarinen H, Nissinen A, Tuomilehto J. Low physical activity as a predictor for total and cardiovascular disease mortality in middle-aged men and women in Finland. Eur Heart J. 2004;7:2204–2211. doi: 10.1016/j.ehj.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Bath PA. Differences between older men and women in the self-rated healthmortality relationship. Gerontologist. 2003;7:387–395. doi: 10.1093/geront/43.3.387. discussion 372-385. [DOI] [PubMed] [Google Scholar]
- Bijnen FC, Caspersen CJ, Feskens EJ, Saris WH, Mosterd WL, Kromhout D. Physical activity and 10-year mortality from cardiovascular diseases and all causes: The Zutphen Elderly Study. Arch Intern Med. 1998;7:1499–1505. doi: 10.1001/archinte.158.14.1499. [DOI] [PubMed] [Google Scholar]
- Blair SN, Kohl HW, Barlow CE. Physical activity, physical fitness, and all-cause mortality in women: do women need to be active? J Am Coll Nut. 1993;7:368–371. doi: 10.1080/07315724.1993.10718324. [DOI] [PubMed] [Google Scholar]
- Blair SN, Kampert JB, Kohl HW, Barlow CE, Macera CA, Paffenbarger RS Jr, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;7:205–210. doi: 10.1001/jama.276.3.205. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Buchman AS, Wilson RS, Bienias JL, Bennett DA. Physical activity is associated with incident disability in community-based older persons. J Am Geriatr Soc. 2007;7:195–201. doi: 10.1111/j.1532-5415.2007.01038.x. [DOI] [PubMed] [Google Scholar]
- Bucksch J. Physical activity of moderate intensity in leisure time and the risk of all cause mortality. Br J Sports Med. 2005;7:632–638. doi: 10.1136/bjsm.2004.015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucksch J, Helmert U. Leisure time sports activity and all-cause-mortality in West-Germany (1984-1998) Z Gesundheitswiss. 2004;7:351–358. doi: 10.1007/s10389-004-0069-7. [DOI] [Google Scholar]
- Carlsson S, Andersson T, Wolk A, Ahlbom A. Low physical activity and mortality in women: baseline lifestyle and health as alternative explanations. Scand J Public Health. 2006;7:480–487. doi: 10.1080/14034940600551293. [DOI] [PubMed] [Google Scholar]
- Crespo CJ, Palmieri MR, Perdomo RP, McGee DL, Smit E, Sempos CT, Lee IM, Sorlie PD. The relationship of physical activity and body weight with all-cause mortality: results from the Puerto Rico Heart Health Program. Ann Epidemiol. 2002;7:543–552. doi: 10.1016/S1047-2797(01)00296-4. [DOI] [PubMed] [Google Scholar]
- Davey Smith G, Shipley MJ, Batty GD, Morris JN, Marmot M. Physical activity and cause-specific mortality in the Whitehall study. Public Health. 2000;7:308–315. doi: 10.1038/sj.ph.1900675. [DOI] [PubMed] [Google Scholar]
- Eaton CB, Medalie JH, Flocke SA, Zyzanski SJ, Yaari S, Goldbourt U. Self-reported physical activity predicts long-term coronary heart disease and all-cause mortalities. Twenty-one-year follow-up of the Israeli Ischemic Heart Disease Study. Arch Fam Med. 1995;7:323–329. doi: 10.1001/archfami.4.4.323. [DOI] [PubMed] [Google Scholar]
- Fang J, Wylie-Rosett J, Alderman MH. Exercise and cardiovascular outcomes by hypertensive status: NHANES I epidemiological follow-up study, 1971-1992. Am J Hypertens. 2005;7:751–758. doi: 10.1016/j.amjhyper.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, Robbins JA, Gardin JM. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;7:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- Fujita K, Takahashi H, Miura C, Ohkubo T, Sato Y, Ugajin T, Kurashima K, Tsubono Y, Tsuji I, Fukao A, Hisamichi S. Walking and mortality in Japan: the Miyagi Cohort Study. J Epidemiol. 2004;7(Suppl 1):S26–32. doi: 10.2188/jea.14.S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass TA, de Leon CM, Marottoli RA, Berkman LF. Population based study of social and productive activities as predictors of survival among elderly Americans. BMJ. 1999;7:478–483. doi: 10.1136/bmj.319.7208.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH, Al-Hani AJ, Black HR. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation. 2003;7:1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- Haapanen N, Miilunpalo S, Vuori I, Oja P, Pasanen M. Characteristics of leisure time physical activity associated with decreased risk of premature all-cause and cardiovascular disease mortality in middle-aged men. Am J Epidemiol. 1996;7:870–880. doi: 10.1093/oxfordjournals.aje.a008830. [DOI] [PubMed] [Google Scholar]
- Hakim AA, Petrovitch H, Burchfiel CM, Ross GW, Rodriguez BL, White LR, Yano K, Curb JD, Abbott RD. Effects of walking on mortality among nonsmoking retired men. N Engl J Med. 1998;7:94–99. doi: 10.1056/NEJM199801083380204. [DOI] [PubMed] [Google Scholar]
- Hillsdon M, Thorogood M, Murphy M, Jones L. Can a simple measure of vigorous physical activity predict future mortality? Results from the OXCHECK study. Public Health Nutr. 2004;7:557–562. doi: 10.1079/PHN2003548. [DOI] [PubMed] [Google Scholar]
- Hu G, Tuomilehto J, Silventoinen K, Barengo NC, Peltonen M, Jousilahti P. The effects of physical activity and body mass index on cardiovascular, cancer and all-cause mortality among 47 212 middle-aged Finnish men and women. Int J Obes (Lond) 2005;7:894–902. doi: 10.1038/sj.ijo.0802870. [DOI] [PubMed] [Google Scholar]
- Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;7:2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- Kampert JB, Blair SN, Barlow CE, Kohl HW. Physical activity, physical fitness, and all-cause and cancer mortality: a prospective study of men and women. Ann Epidemiol. 1996;7:452–457. doi: 10.1016/S1047-2797(96)00059-2. [DOI] [PubMed] [Google Scholar]
- Kaplan GA, Strawbridge WJ, Cohen RD, Hungerford LR. Natural history of leisuretime physical activity and its correlates: associations with mortality from all causes and cardiovascular disease over 28 years. Am J Epidemiol. 1996;7:793–797. doi: 10.1093/oxfordjournals.aje.a009003. [DOI] [PubMed] [Google Scholar]
- Khaw KT, Jakes R, Bingham S, Welch A, Luben R, Day N, Wareham N. Work and leisure time physical activity assessed using a simple, pragmatic, validated questionnaire and incident cardiovascular disease and all-cause mortality in men and women: The European Prospective Investigation into Cancer in Norfolk prospective population study. Int J Epidemiol. 2006;7:1034–1043. doi: 10.1093/ije/dyl079. [DOI] [PubMed] [Google Scholar]
- Kohl HW, Nichaman MZ, Frankowski RF, Blair SN. Maximal exercise hemodynamics and risk of mortality in apparently healthy men and women. Med Sci Sports Exerc. 1996;7:601–609. doi: 10.1097/00005768-199605000-00011. [DOI] [PubMed] [Google Scholar]
- Kujala UM, Kaprio J, Sarna S, Koskenvuo M. Relationship of leisure-time physical activity and mortality: the Finnish twin cohort. JAMA. 1998;7:440–444. doi: 10.1001/jama.279.6.440. [DOI] [PubMed] [Google Scholar]
- LaCroix AZ, Leveille SG, Hecht JA, Grothaus LC, Wagner EH. Does walking decrease the risk of cardiovascular disease hospitalizations and death in older adults? J Am Geriatr Soc. 1996;7:113–120. doi: 10.1111/j.1532-5415.1996.tb02425.x. [DOI] [PubMed] [Google Scholar]
- Lam TH, Ho SY, Hedley AJ, Mak KH, Leung GM. Leisure time physical activity and mortality in Hong Kong: case-control study of all adult deaths in 1998. Ann Epidemiol. 2004;7:391–398. doi: 10.1016/j.annepidem.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Lan TY, Chang HY, Tai TY. Relationship between components of leisure physical activity and mortality in Taiwanese older adults. Prev Med. 2006;7:36–41. doi: 10.1016/j.ypmed.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Laukkanen JA, Lakka TA, Rauramaa R, Kuhanen R, Venalainen JM, Salonen R, Salonen JT. Cardiovascular fitness as a predictor of mortality in men. Arch Intern Med. 2001;7:825–831. doi: 10.1001/archinte.161.6.825. [DOI] [PubMed] [Google Scholar]
- Lee IM, Paffenbarger RS Jr. Associations of light, moderate, and vigorous intensity physical activity with longevity. The Harvard Alumni Health Study. Am J Epidemiol. 2000;7:293–299. doi: 10.1093/oxfordjournals.aje.a010205. [DOI] [PubMed] [Google Scholar]
- Lee IM, Hsieh CC, Paffenbarger RS Jr. Exercise intensity and longevity in men. The Harvard Alumni Health Study. JAMA. 1995;7:1179–1184. doi: 10.1001/jama.273.15.1179. [DOI] [PubMed] [Google Scholar]
- Lee IM, Sesso HD, Oguma Y, Paffenbarger RS Jr. The "weekend warrior" and risk of mortality. Am J Epidemiol. 2004;7:636–641. doi: 10.1093/aje/kwh274. [DOI] [PubMed] [Google Scholar]
- Leitzmann MF, Park Y, Blair A, Ballard-Barbash R, Mouw T, Hollenbeck AR, Schatzkin A. Physical activity recommendations and decreased risk of mortality. Arch Intern Med. 2007;7:2453–2460. doi: 10.1001/archinte.167.22.2453. [DOI] [PubMed] [Google Scholar]
- Leon AS, Myers MJ, Connett J. Leisure time physical activity and the 16-year risks of mortality from coronary heart disease and all-causes in the Multiple Risk Factor Intervention Trial (MRFIT) Int J Sports Med. 1997;7(Suppl 3):S208–215. doi: 10.1055/s-2007-972717. [DOI] [PubMed] [Google Scholar]
- Lissner L, Bengtsson C, Bjorkelund C, Wedel H. Physical activity levels and changes in relation to longevity. A prospective study of Swedish women. Am J Epidemiol. 1996;7:54–62. doi: 10.1093/oxfordjournals.aje.a008657. [DOI] [PubMed] [Google Scholar]
- Manini TM, Everhart JE, Patel KV, Schoeller DA, Colbert LH, Visser M, Tylavsky F, Bauer DC, Goodpaster BH, Harris TB. Daily activity energy expenditure and mortality among older adults. JAMA. 2006;7:171–179. doi: 10.1001/jama.296.2.171. [DOI] [PubMed] [Google Scholar]
- Matthews CE, Jurj AL, Shu XO, Li HL, Yang G, Li Q, Gao YT, Zheng W. Influence of exercise, walking, cycling, and overall nonexercise physical activity on mortality in Chinese women. Am J Epidemiol. 2007;7:1343–1350. doi: 10.1093/aje/kwm088. [DOI] [PubMed] [Google Scholar]
- Menotti A, Seccareccia F. Physical activity at work and job responsibility as risk factors for fatal coronary heart disease and other causes of death. J Epidemiol Community Health. 1985;7:325–329. doi: 10.1136/jech.39.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensink GB, Deketh M, Mul MD, Schuit AJ, Hoffmeister H. Physical activity and its association with cardiovascular risk factors and mortality. Epidemiology. 1996;7:391–397. doi: 10.1097/00001648-199607000-00009. [DOI] [PubMed] [Google Scholar]
- Morgan K, Clarke D. Customary physical activity and survival in later life: a study in Nottingham, UK. J Epidemiol Community Health. 1997;7:490–493. doi: 10.1136/jech.51.5.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;7:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- Ostbye T, Taylor DH, Jung SH. A longitudinal study of the effects of tobacco smoking and other modifiable risk factors on ill health in middle-aged and old Americans: results from the Health and Retirement Study and Asset and Health Dynamics among the Oldest Old survey. Prev Med. 2002;7:334–345. doi: 10.1006/pmed.2001.0991. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS Jr, Kampert JB, Lee IM, Hyde RT, Leung RW, Wing AL. Changes in physical activity and other lifeway patterns influencing longevity. Med Sci Sports Exerc. 1994;7:857–865. [PubMed] [Google Scholar]
- Richardson CR, Kriska AM, Lantz PM, Hayward RA. Physical activity and mortality across cardiovascular disease risk groups. Med Sci Sports Exerc. 2004;7:1923–1929. doi: 10.1249/01.MSS.0000145443.02568.7A. [DOI] [PubMed] [Google Scholar]
- Rockhill B, Willett WC, Manson JE, Leitzmann MF, Stampfer MJ, Hunter DJ, Colditz GA. Physical activity and mortality: a prospective study among women. Am J Public Health. 2001;7:578–583. doi: 10.2105/AJPH.91.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren A, Wilhelmsen L. Physical activity protects against coronary death and deaths from all causes in middle-aged men. Evidence from a 20-year follow-up of the primary prevention study in Goteborg. Ann Epidemiol. 1997;7:69–75. doi: 10.1016/S1047-2797(96)00106-8. [DOI] [PubMed] [Google Scholar]
- Schnohr P, Scharling H, Jensen JS. Changes in leisure-time physical activity and risk of death: an observational study of 7,000 men and women. Am J Epidemiol. 2003;7:639–644. doi: 10.1093/aje/kwg207. [DOI] [PubMed] [Google Scholar]
- Schnohr C, Hojbjerre L, Riegels M, Ledet L, Larsen T, Schultz-Larsen K, Petersen L, Prescott E, Gronbaek M. Does educational level influence the effects of smoking, alcohol, physical activity, and obesity on mortality? A prospective population study. Scand J Public Health. 2004;7:250–256. doi: 10.1080/14034940310019489. [DOI] [PubMed] [Google Scholar]
- Schnohr P, Lange P, Scharling H, Jensen JS. Long-term physical activity in leisure time and mortality from coronary heart disease, stroke, respiratory diseases, and cancer. The Copenhagen City Heart Study. Eur J Cardiovasc Prev Rehabil. 2006;7:173–179. doi: 10.1097/01.hjr.0000198923.80555.b7. [DOI] [PubMed] [Google Scholar]
- Schooling CM, Lam TH, Li ZB, Ho SY, Chan WM, Ho KS, Tham MK, Cowling BJ, Leung GM. Obesity, physical activity, and mortality in a prospective chinese elderly cohort. Arch Intern Med. 2006;7:1498–1504. doi: 10.1001/archinte.166.14.1498. [DOI] [PubMed] [Google Scholar]
- Sundquist K, Qvist J, Sundquist J, Johansson SE. Frequent and occasional physical activity in the elderly: a 12-year follow-up study of mortality. Am J Prev Med. 2004;7:22–27. doi: 10.1016/j.amepre.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Talbot LA, Morrell CH, Fleg JL, Metter EJ. Changes in leisure time physical activity and risk of all-cause mortality in men and women: the Baltimore Longitudinal Study of Aging. Prev Med. 2007;7:169–176. doi: 10.1016/j.ypmed.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Trolle-Lagerros Y, Mucci LA, Kumle M, Braaten T, Weiderpass E, Hsieh CC, Sandin S, Lagiou P, Trichopoulos D, Lund E, Adami HO. Physical activity as a determinant of mortality in women. Epidemiology. 2005;7:780–785. doi: 10.1097/01.ede.0000181312.35964.22. [DOI] [PubMed] [Google Scholar]
- Villeneuve PJ, Morrison HI, Craig CL, Schaubel DE. Physical activity, physical fitness, and risk of dying. Epidemiology. 1998;7:626–631. doi: 10.1097/00001648-199811000-00011. [DOI] [PubMed] [Google Scholar]
- Weller I, Corey P. The impact of excluding non-leisure energy expenditure on the relation between physical activity and mortality in women. Epidemiology. 1998;7:632–635. doi: 10.1097/00001648-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Yu S, Yarnell JW, Sweetnam PM, Murray L. What level of physical activity protects against premature cardiovascular death? The Caerphilly study. Heart. 2003;7:502–506. doi: 10.1136/heart.89.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri A, Tavani A, Gallus S, La Vecchia C. Occupational and leisure time physical activity and the risk of nonfatal acute myocardial infarction in Italy. Ann Epidemiol. 2004;7:461–466. doi: 10.1016/j.annepidem.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Batty GD, Shipley MJ, Marmot MG, Smith GD. Leisure time physical activity and disease-specific mortality among men with chronic bronchitis: evidence from the Whitehall study. Am J Public Health. 2003;7:817–821. doi: 10.2105/AJPH.93.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Millar WJ. Health effects of physical activity. Health Rep. 1999;7 21-30(Eng); 21-31(Fre) [PubMed] [Google Scholar]
- Conroy MB, Cook NR, Manson JE, Buring JE, Lee IM. Past physical activity, current physical activity, and risk of coronary heart disease. Med Sci Sports Exerc. 2005;7:1251–1256. doi: 10.1249/01.mss.0000174882.60971.7f. [DOI] [PubMed] [Google Scholar]
- Dorn JP, Cerny FJ, Epstein LH, Naughton J, Vena JE, Winkelstein W Jr, Schisterman E, Trevisan M. Work and leisure time physical activity and mortality in men and women from a general population sample. Ann Epidemiol. 1999;7:366–373. doi: 10.1016/S1047-2797(99)00025-3. [DOI] [PubMed] [Google Scholar]
- Folsom AR, Arnett DK, Hutchinson RG, Liao F, Clegg LX, Cooper LS. Physical activity and incidence of coronary heart disease in middle-aged women and men. Med Sci Sports Exerc. 1997;7:901–909. doi: 10.1097/00005768-199707000-00009. [DOI] [PubMed] [Google Scholar]
- Fransson E, de Faire U, Ahlbom A, Reuterwall C, Hallqvist J, Alfredsson L. The risk of acute myocardial infarction: interactions of types of physical activity. Epidemiology. 2004;7:573–582. doi: 10.1097/01.ede.0000134865.74261.fe. [DOI] [PubMed] [Google Scholar]
- Fransson E, de Faire U, Ahlbom A, Reuterwall C, Hallqvist J, Alfredsson L. The effect of leisure-time physical activity on the risk of acute myocardial infarction depending on body mass index: a population-based case-control study. BMC Public Health. 2006;7:296. doi: 10.1186/1471-2458-6-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapanen-Niemi N, Miilunpalo S, Pasanen M, Vuori I, Oja P, Malmberg J. Body mass index, physical inactivity and low level of physical fitness as determinants of all-cause and cardiovascular disease mortality--16 y follow-up of middle-aged and elderly men and women. Int J Obes Relat Metab Disord. 2000;7:1465–1474. doi: 10.1038/sj.ijo.0801426. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Belanger A, D'Agostino R, Israel I. Physical activity and physical demand on the job and risk of cardiovascular disease and death: the Framingham Study. Am Heart J. 1986;7:820–825. doi: 10.1016/0002-8703(86)90480-1. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Kujala UM, Koskenvuo M, Sarna S. Physical activity and other risk factors in male twin-pairs discordant for coronary heart disease. Atherosclerosis. 2000;7:193–200. doi: 10.1016/S0021-9150(99)00368-8. [DOI] [PubMed] [Google Scholar]
- Lakka TA, Venalainen JM, Rauramaa R, Salonen R, Tuomilehto J, Salonen JT. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N Engl J Med. 1994;7:1549–1554. doi: 10.1056/NEJM199406023302201. [DOI] [PubMed] [Google Scholar]
- Laukkanen JA, Kurl S, Salonen R, Rauramaa R, Salonen JT. The predictive value of cardiorespiratory fitness for cardiovascular events in men with various risk profiles: a prospective population-based cohort study. Eur Heart J. 2004;7:1428–1437. doi: 10.1016/j.ehj.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Lee IM, Sesso HD, Paffenbarger RS Jr. Physical activity and coronary heart disease risk in men: does the duration of exercise episodes predict risk? Circulation. 2000;7:981–986. doi: 10.1161/01.cir.102.9.981. [DOI] [PubMed] [Google Scholar]
- Lee IM, Sesso HD, Oguma Y, Paffenbarger RS Jr. Relative intensity of physical activity and risk of coronary heart disease. Circulation. 2003;7:1110–1116. doi: 10.1161/01.CIR.0000052626.63602.58. [DOI] [PubMed] [Google Scholar]
- Lemaitre RN, Siscovick DS, Raghunathan TE, Weinmann S, Arbogast P, Lin DY. Leisure-time physical activity and the risk of primary cardiac arrest. Arch Intern Med. 1999;7:686–690. doi: 10.1001/archinte.159.7.686. [DOI] [PubMed] [Google Scholar]
- Lemaitre RN, Heckbert SR, Psaty BM, Siscovick DS. Leisure-time physical activity and the risk of nonfatal myocardial infarction in postmenopausal women. Arch Intern Med. 1995;7:2302–2308. doi: 10.1001/archinte.155.21.2302. [DOI] [PubMed] [Google Scholar]
- Li TY, Rana JS, Manson JE, Willett WC, Stampfer MJ, Colditz GA, Rexrode KM, Hu FB. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation. 2006;7:499–506. doi: 10.1161/CIRCULATIONAHA.105.574087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes C, Santos AC, Azevedo A, Maciel MJ, Barros H. Physical activity and risk of myocardial infarction after the fourth decade of life. Rev Port Cardiol. 2005;7:1191–1207. [PubMed] [Google Scholar]
- Lovasi GS, Lemaitre RN, Siscovick DS, Dublin S, Bis JC, Lumley T, Heckbert SR, Smith NL, Psaty BM. Amount of leisure-time physical activity and risk of nonfatal myocardial infarction. Ann Epidemiol. 2007;7:410–416. doi: 10.1016/j.annepidem.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Speizer FE, Hennekens CH. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;7:650–658. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;7:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor GT, Hennekens CH, Willett WC, Goldhaber SZ, Paffenbarger RS Jr, Breslow JL, Lee IM, Buring JE. Physical exercise and reduced risk of nonfatal myocardial infarction. Am J Epidemiol. 1995;7:1147–1156. doi: 10.1093/oxfordjournals.aje.a117573. [DOI] [PubMed] [Google Scholar]
- Rastogi T, Vaz M, Spiegelman D, Reddy KS, Bharathi AV, Stampfer MJ, Willett WC, Ascherio A. Physical activity and risk of coronary heart disease in India. Int J Epidemiol. 2004;7:759–767. doi: 10.1093/ije/dyh042. [DOI] [PubMed] [Google Scholar]
- Rodriguez BL, Curb JD, Burchfiel CM, Abbott RD, Petrovitch H, Masaki K, Chiu D. Physical activity and 23-year incidence of coronary heart disease morbidity and mortality among middle-aged men. The Honolulu Heart Program. Circulation. 1994;7:2540–2544. doi: 10.1161/01.cir.89.6.2540. [DOI] [PubMed] [Google Scholar]
- Rothenbacher D, Hoffmeister A, Brenner H, Koenig W. Physical activity, coronary heart disease, and inflammatory response. Arch Intern Med. 2003;7:1200–1205. doi: 10.1001/archinte.163.10.1200. [DOI] [PubMed] [Google Scholar]
- Seccareccia F, Menotti A. Physical activity, physical fitness and mortality in a sample of middle aged men followed-up 25 years. J Sports Med Phys Fitness. 1992;7:206–213. [PubMed] [Google Scholar]
- Sesso HD, Paffenbarger RS Jr, Lee IM. Physical activity and coronary heart disease in men: The Harvard Alumni Health Study. Circulation. 2000;7:975–980. doi: 10.1161/01.cir.102.9.975. [DOI] [PubMed] [Google Scholar]
- Sundquist K, Qvist J, Johansson SE, Sundquist J. The long-term effect of physical activity on incidence of coronary heart disease: a 12-year follow-up study. Prev Med. 2005;7:219–225. doi: 10.1016/j.ypmed.2004.09.043. [DOI] [PubMed] [Google Scholar]
- Talbot LA, Morrell CH, Metter EJ, Fleg JL. Comparison of cardiorespiratory fitness versus leisure time physical activity as predictors of coronary events in men aged < or = 65 years and > 65 years. Am J Cardiol. 2002;7:1187–1192. doi: 10.1016/S0002-9149(02)02302-0. [DOI] [PubMed] [Google Scholar]
- Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;7:1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- Vatten LJ, Nilsen TI, Romundstad PR, Droyvold WB, Holmen J. Adiposity and physical activity as predictors of cardiovascular mortality. Eur J Cardiovasc Prev Rehabil. 2006;7:909–915. doi: 10.1097/01.hjr.0000239463.80390.52. [DOI] [PubMed] [Google Scholar]
- Wagner A, Simon C, Evans A, Ferrieres J, Montaye M, Ducimetiere P, Arveiler D. Physical activity and coronary event incidence in Northern Ireland and France: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2002;7:2247–2252. doi: 10.1161/01.CIR.0000016345.58696.4F. [DOI] [PubMed] [Google Scholar]
- Agnarsson U, Thorgeirsson G, Sigvaldason H, Sigfusson N. Effects of leisure-time physical activity and ventilatory function on risk for stroke in men: the Reykjavik Study. Ann Intern Med. 1999;7:987–990. doi: 10.7326/0003-4819-130-12-199906150-00006. [DOI] [PubMed] [Google Scholar]
- Ellekjaer H, Holmen J, Ellekjaer E, Vatten L. Physical activity and stroke mortality in women. Ten-year follow-up of the Nord-Trondelag health survey, 1984-1986. Stroke. 2000;7:14–18. doi: 10.1161/01.str.31.1.14. [DOI] [PubMed] [Google Scholar]
- Evenson KR, Rosamond WD, Cai J, Toole JF, Hutchinson RG, Shahar E, Folsom AR. Physical activity and ischemic stroke risk. The atherosclerosis risk in communities study. Stroke. 1999;7:1333–1339. doi: 10.1161/01.str.30.7.1333. [DOI] [PubMed] [Google Scholar]
- Haheim LL, Holme I, Hjermann I, Leren P. Risk factors of stroke incidence and mortality. A 12-year follow-up of the Oslo Study. Stroke. 1993;7:1484–1489. doi: 10.1161/01.str.24.10.1484. [DOI] [PubMed] [Google Scholar]
- Hu G, Sarti C, Jousilahti P, Silventoinen K, Barengo NC, Tuomilehto J. Leisure time, occupational, and commuting physical activity and the risk of stroke. Stroke. 2005;7:1994–1999. doi: 10.1161/01.STR.0000177868.89946.0c. [DOI] [PubMed] [Google Scholar]
- Kiely DK, Wolf PA, Cupples LA, Beiser AS, Kannel WB. Physical activity and stroke risk: the Framingham Study. Am J Epidemiol. 1994;7:608–620. doi: 10.1093/oxfordjournals.aje.a117298. [DOI] [PubMed] [Google Scholar]
- Krarup LH, Truelsen T, Pedersen A, Lerke H, Lindahl M, Hansen L, Schnohr P, Boysen G. Level of physical activity in the week preceding an ischemic stroke. Cerebrovasc Dis. 2007;7:296–300. doi: 10.1159/000105683. [DOI] [PubMed] [Google Scholar]
- Kurl S, Laukkanen JA, Rauramaa R, Lakka TA, Sivenius J, Salonen JT. Cardiorespiratory fitness and the risk for stroke in men. Arch Intern Med. 2003;7:1682–1688. doi: 10.1001/archinte.163.14.1682. [DOI] [PubMed] [Google Scholar]
- Myint PK, Luben RN, Wareham NJ, Welch AA, Bingham SA, Day NE, Khaw KT. Combined work and leisure physical activity and risk of stroke in men and women in the European prospective investigation into Cancer-Norfolk Prospective Population Study. Neuroepidemiology. 2006;7:122–129. doi: 10.1159/000095551. [DOI] [PubMed] [Google Scholar]
- Noda H, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, Koizumi A, Kondo T, Watanabe Y, Wada Y, Inaba Y, Tamakoshi A. Walking and sports participation and mortality from coronary heart disease and stroke. J Am Coll Cardiol. 2005;7:1761–1767. doi: 10.1016/j.jacc.2005.07.038. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Perez Barreto M. Stroke risk in older men and women: aspirin, estrogen, exercise, vitamins, and other factors. J Gend Specif Med. 2001;7:18–28. [PubMed] [Google Scholar]
- Pitsavos C, Panagiotakos DB, Chrysohoou C, Kokkinos P, Menotti A, Singh S, Dontas A. Physical activity decreases the risk of stroke in middle-age men with left ventricular hypertrophy: 40-year follow-up (1961-2001) of the Seven Countries Study (the Corfu cohort) J Hum Hypertens. 2004;7:495–501. doi: 10.1038/sj.jhh.1001692. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Gan R, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke. 1998;7:380–387. doi: 10.1161/01.str.29.2.380. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, Lafferty ME, Phillips CL, Mendes de Leon CF, Kasl SV, Seeman TE, Fillenbaum G, Hebert P, Lemke JH. Risk due to inactivity in physically capable older adults. Am J Public Health. 1993;7:1443–1450. doi: 10.2105/AJPH.83.10.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrift AG, Donnan GA, McNeil JJ. Reduced risk of intracerebral hemorrhage with dynamic recreational exercise but not with heavy work activity. Stroke. 2002;7:559–564. doi: 10.1161/hs0202.102878. [DOI] [PubMed] [Google Scholar]
- Folsom AR, Prineas RJ, Kaye SA, Munger RG. Incidence of hypertension and stroke in relation to body fat distribution and other risk factors in older women. Stroke. 1990;7:701–706. doi: 10.1161/01.str.21.5.701. [DOI] [PubMed] [Google Scholar]
- Levenstein S, Smith MW, Kaplan GA. Psychosocial predictors of hypertension in men and women. Arch Intern Med. 2001;7:1341–1346. doi: 10.1001/archinte.161.10.1341. [DOI] [PubMed] [Google Scholar]
- Hou L, Ji BT, Blair A, Dai Q, Gao YT, Chow WH. Commuting physical activity and risk of colon cancer in Shanghai, China. Am J Epidemiol. 2004;7:860–867. doi: 10.1093/aje/kwh301. [DOI] [PubMed] [Google Scholar]
- Boutron-Ruault MC, Senesse P, Meance S, Belghiti C, Faivre J. Energy intake, body mass index, physical activity, and the colorectal adenoma-carcinoma sequence. Nutr Cancer. 2001;7:50–57. doi: 10.1207/S15327914nc391_7. [DOI] [PubMed] [Google Scholar]
- Brownson RC, Chang JC, Davis JR, Smith CA. Physical activity on the job and cancer in Missouri. Am J Public Health. 1991;7:639–642. doi: 10.2105/AJPH.81.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton BA, Lacey JV Jr, Schatzkin A, Schairer C, Colbert LH, Albanes D, Leitzmann MF. Physical activity and the risk of colon cancer among women: a prospective cohort study (United States) Int J Cancer. 2006;7:385–391. doi: 10.1002/ijc.21840. [DOI] [PubMed] [Google Scholar]
- Chao A, Connell CJ, Jacobs EJ, McCullough ML, Patel AV, Calle EE, Cokkinides VE, Thun MJ. Amount, type, and timing of recreational physical activity in relation to colon and rectal cancer in older adults: the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2004;7:2187–2195. [PubMed] [Google Scholar]
- Colbert LH, Hartman TJ, Malila N, Limburg PJ, Pietinen P, Virtamo J, Taylor PR, Albanes D. Physical activity in relation to cancer of the colon and rectum in a cohort of male smokers. Cancer Epidemiol Biomarkers Prev. 2001;7:265–268. [PubMed] [Google Scholar]
- Dosemeci M, Hayes RB, Vetter R, Hoover RN, Tucker M, Engin K, Unsal M, Blair A. Occupational physical activity, socioeconomic status, and risks of 15 cancer sites in Turkey. Cancer Causes Control. 1993;7:313–321. doi: 10.1007/BF00051333. [DOI] [PubMed] [Google Scholar]
- Friedenreich C, Norat T, Steindorf K, Boutron-Ruault MC, Pischon T, Mazuir M, Clavel-Chapelon F, Linseisen J, Boeing H, Bergman M, Johnsen NF, Tjonneland A, Overvad K, Mendez M, Quiros JR, Martinez C, Dorronsoro M, Navarro C, Gurrea AB, Bingham S, Khaw KT, Allen N, Key T, Trichopoulou A, Trichopoulos D, Orfanou N, Krogh V, Palli D, Tumino R, Panico S, Vineis P, Bueno-de-Mesquita HB, Peeters PH, Monninkhof E, Berglund G, Manjer J, Ferrari P, Slimani N, Kaaks R, Riboli E. Physical activity and risk of colon and rectal cancers: the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2006;7:2398–2407. doi: 10.1158/1055-9965.EPI-06-0595. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;7:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- Isomura K, Kono S, Moore MA, Toyomura K, Nagano J, Mizoue T, Mibu R, Tanaka M, Kakeji Y, Maehara Y, Okamura T, Ikejiri K, Futami K, Yasunami Y, Maekawa T, Takenaka K, Ichimiya H, Imaizumi N. Physical activity and colorectal cancer: the Fukuoka Colorectal Cancer Study. Cancer Sci. 2006;7:1099–1104. doi: 10.1111/j.1349-7006.2006.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen NF, Christensen J, Thomsen BL, Olsen A, Loft S, Overvad K, Tjonneland A. Physical activity and risk of colon cancer in a cohort of Danish middle-aged men and women. Eur J Epidemiol. 2006;7:877–884. doi: 10.1007/s10654-006-9076-z. [DOI] [PubMed] [Google Scholar]
- Larsen IK, Grotmol T, Almendingen K, Hoff G. Lifestyle as a predictor for colonic neoplasia in asymptomatic individuals. BMC Gastroenterol. 2006;7:5. doi: 10.1186/1471-230X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson SC, Rutegard J, Bergkvist L, Wolk A. Physical activity, obesity, and risk of colon and rectal cancer in a cohort of Swedish men. Eur J Cancer. 2006;7:2590–2597. doi: 10.1016/j.ejca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Lee IM, Paffenbarger RS Jr. Physical activity and its relation to cancer risk: a prospective study of college alumni. Med Sci Sports Exerc. 1994;7:831–837. [PubMed] [Google Scholar]
- Lee IM, Manson JE, Ajani U, Paffenbarger RS Jr, Hennekens CH, Buring JE. Physical activity and risk of colon cancer: the Physicians' Health Study (United States) Cancer Causes Control. 1997;7:568–574. doi: 10.1023/A:1018438228410. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S. Physical activity and risk of colorectal cancer in Japanese men and women: the Japan Public Health Center-based prospective study. Cancer Causes Control. 2007;7:199–209. doi: 10.1007/s10552-006-0098-3. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Gerhardsson le Verdier M, Frumkin H, Carpenter C. A case-control study of physical activity in relation to risk of cancer of the right colon and rectum in men. Int J Epidemiol. 1995;7:42–50. doi: 10.1093/ije/24.1.42. [DOI] [PubMed] [Google Scholar]
- Mai PL, Sullivan-Halley J, Ursin G, Stram DO, Deapen D, Villaluna D, Horn-Ross PL, Clarke CA, Reynolds P, Ross RK, West DW, Anton-Culver H, Ziogas A, Bernstein L. Physical activity and colon cancer risk among women in the California Teachers Study. Cancer Epidemiol Biomarkers Prev. 2007;7:517–525. doi: 10.1158/1055-9965.EPI-06-0747. [DOI] [PubMed] [Google Scholar]
- Martinez ME, Giovannucci E, Spiegelman D, Hunter DJ, Willett WC, Colditz GA. Leisure-time physical activity, body size, and colon cancer in women. Nurses' Health Study Research Group. J Natl Cancer Inst. 1997;7:948–955. doi: 10.1093/jnci/89.13.948. [DOI] [PubMed] [Google Scholar]
- Nilsen TI, Romundstad PR, Petersen H, Gunnell D, Vatten LJ. Recreational physical activity and cancer risk in subsites of the colon (the Nord-Trondelag Health Study) Cancer Epidemiol Biomarkers Prev. 2008;7:183–188. doi: 10.1158/1055-9965.EPI-07-0746. [DOI] [PubMed] [Google Scholar]
- Schnohr P, Gronbaek M, Petersen L, Hein HO, Sorensen TI. Physical activity in leisure-time and risk of cancer: 14-year follow-up of 28,000 Danish men and women. Scand J Public Health. 2005;7:244–249. doi: 10.1080/14034940510005752. [DOI] [PubMed] [Google Scholar]
- Slattery ML, Schumacher MC, Smith KR, West DW, Abd-Elghany N. Physical activity, diet, and risk of colon cancer in Utah. Am J Epidemiol. 1988;7:989–999. doi: 10.1093/oxfordjournals.aje.a115072. [DOI] [PubMed] [Google Scholar]
- Slattery ML, Edwards SL, Ma KN, Friedman GD, Potter JD. Physical activity and colon cancer: a public health perspective. Ann Epidemiol. 1997;7:137–145. doi: 10.1016/S1047-2797(96)00129-9. [DOI] [PubMed] [Google Scholar]
- Slattery ML, Potter J, Caan B, Edwards S, Coates A, Ma KN, Berry TD. Energy balance and colon cancer--beyond physical activity. Cancer Res. 1997;7:75–80. [PubMed] [Google Scholar]
- Takahashi H, Kuriyama S, Tsubono Y, Nakaya N, Fujita K, Nishino Y, Shibuya D, Tsuji I. Time spent walking and risk of colorectal cancer in Japan: the Miyagi Cohort study. Eur J Cancer Prev. 2007;7:403–408. doi: 10.1097/01.cej.0000236249.63489.05. [DOI] [PubMed] [Google Scholar]
- Tang R, Wang JY, Lo SK, Hsieh LL. Physical activity, water intake and risk of colorectal cancer in Taiwan: a hospital-based case-control study. Int J Cancer. 1999;7:484–489. doi: 10.1002/(SICI)1097-0215(19990812)82:4<484::AID-IJC3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tavani A, Braga C, La Vecchia C, Conti E, Filiberti R, Montella M, Amadori D, Russo A, Franceschi S. Physical activity and risk of cancers of the colon and rectum: an Italian case-control study. Br J Cancer. 1999;7:1912–1916. doi: 10.1038/sj.bjc.6690304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thune I, Lund E. Physical activity and risk of colorectal cancer in men and women. Br J Cancer. 1996;7:1134–1140. doi: 10.1038/bjc.1996.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vena JE, Graham S, Zielezny M, Swanson MK, Barnes RE, Nolan J. Lifetime occupational exercise and colon cancer. Am J Epidemiol. 1985;7:357–365. doi: 10.1093/oxfordjournals.aje.a114116. [DOI] [PubMed] [Google Scholar]
- Vetter R, Dosemeci M, Blair A, Wacholder S, Unsal M, Engin K, Fraumeni JF Jr. Occupational physical activity and colon cancer risk in Turkey. Eur J Epidemiol. 1992;7:845–850. doi: 10.1007/BF00145330. [DOI] [PubMed] [Google Scholar]
- White E, Jacobs EJ, Daling JR. Physical activity in relation to colon cancer in middle-aged men and women. Am J Epidemiol. 1996;7:42–50. doi: 10.1093/oxfordjournals.aje.a008853. [DOI] [PubMed] [Google Scholar]
- Wolin KY, Lee IM, Colditz GA, Glynn RJ, Fuchs C, Giovannucci E. Leisure-time physical activity patterns and risk of colon cancer in women. Int J Cancer. 2007;7:2776–2781. doi: 10.1002/ijc.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cantor KP, Dosemeci M, Lynch CF, Zhu Y, Zheng T. Occupational and leisure-time physical activity and risk of colon cancer by subsite. J Occup Environ Med. 2006;7:236–243. doi: 10.1097/01.jom.0000199521.72764.26. [DOI] [PubMed] [Google Scholar]
- Bernstein L, Henderson BE, Hanisch R, Sullivan-Halley J, Ross RK. Physical exercise and reduced risk of breast cancer in young women. J Natl Cancer Inst. 1994;7:1403–1408. doi: 10.1093/jnci/86.18.1403. [DOI] [PubMed] [Google Scholar]
- Bernstein L, Patel AV, Ursin G, Sullivan-Halley J, Press MF, Deapen D, Berlin JA, Daling JR, McDonald JA, Norman SA, Malone KE, Strom BL, Liff J, Folger SG, Simon MS, Burkman RT, Marchbanks PA, Weiss LK, Spirtas R. Lifetime recreational exercise activity and breast cancer risk among black women and white women. J Natl Cancer Inst. 2005;7:1671–1679. doi: 10.1093/jnci/dji374. [DOI] [PubMed] [Google Scholar]
- Carpenter CL, Ross RK, Paganini-Hill A, Bernstein L. Lifetime exercise activity and breast cancer risk among post-menopausal women. Br J Cancer. 1999;7:1852–1858. doi: 10.1038/sj.bjc.6690610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter CL, Ross RK, Paganini-Hill A, Bernstein L. Effect of family history, obesity and exercise on breast cancer risk among postmenopausal women. Int J Cancer. 2003;7:96–102. doi: 10.1002/ijc.11186. [DOI] [PubMed] [Google Scholar]
- Chang SC, Ziegler RG, Dunn B, Stolzenberg-Solomon R, Lacey JV Jr, Huang WY, Schatzkin A, Reding D, Hoover RN, Hartge P, Leitzmann MF. Association of energy intake and energy balance with postmenopausal breast cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2006;7:334–341. doi: 10.1158/1055-9965.EPI-05-0479. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Feskanich D, Chen WY, Hunter DJ, Willett WC. Physical activity and risk of breast cancer in premenopausal women. Br J Cancer. 2003;7:847–851. doi: 10.1038/sj.bjc.6601175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan PF, Newcomb PA, Clapp RW, Trentham-Dietz A, Baron JA, Longnecker MP. Physical activity in usual occupation and risk of breast cancer (United States) Cancer Causes Control. 1997;7:626–631. doi: 10.1023/A:1018402615206. [DOI] [PubMed] [Google Scholar]
- Coogan PF, Aschengrau A. Occupational physical activity and breast cancer risk in the upper Cape Cod cancer incidence study. Am J Ind Med. 1999;7:279–285. doi: 10.1002/(SICI)1097-0274(199908)36:2<279::AID-AJIM7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Dallal CM, Sullivan-Halley J, Ross RK, Wang Y, Deapen D, Horn-Ross PL, Reynolds P, Stram DO, Clarke CA, Anton-Culver H, Ziogas A, Peel D, West DW, Wright W, Bernstein L. Long-term recreational physical activity and risk of invasive and in situ breast cancer: the California teachers study. Arch Intern Med. 2007;7:408–415. doi: 10.1001/archinte.167.4.408. [DOI] [PubMed] [Google Scholar]
- Dirx MJ, Voorrips LE, Goldbohm RA, Brandt PA van den. Baseline recreational physical activity, history of sports participation, and postmenopausal breast carcinoma risk in the Netherlands Cohort Study. Cancer. 2001;7:1638–1649. doi: 10.1002/1097-0142(20010915)92:6<1638::AID-CNCR1490>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Dorn J, Vena J, Brasure J, Freudenheim J, Graham S. Lifetime physical activity and breast cancer risk in pre- and postmenopausal women. Med Sci Sports Exerc. 2003;7:278–285. doi: 10.1249/01.MSS.0000048835.59454.8D. [DOI] [PubMed] [Google Scholar]
- Drake DA. A longitudinal study of physical activity and breast cancer prediction. Cancer Nurs. 2001;7:371–377. doi: 10.1097/00002820-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM, Bryant HE, Courneya KS. Case-control study of lifetime physical activity and breast cancer risk. Am J Epidemiol. 2001;7:336–347. doi: 10.1093/aje/154.4.336. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM, Courneya KS, Bryant HE. Relation between intensity of physical activity and breast cancer risk reduction. Med Sci Sports Exerc. 2001;7:1538–1545. doi: 10.1097/00005768-200109000-00018. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM, Rohan TE. Physical activity and risk of breast cancer. Eur J Cancer Prev. 1995;7:145–151. doi: 10.1097/00008469-199504000-00004. [DOI] [PubMed] [Google Scholar]
- Gammon MD, Schoenberg JB, Britton JA, Kelsey JL, Coates RJ, Brogan D, Potischman N, Swanson CA, Daling JR, Stanford JL, Brinton LA. Recreational physical activity and breast cancer risk among women under age 45 years. Am J Epidemiol. 1998;7:273–280. doi: 10.1093/oxfordjournals.aje.a009447. [DOI] [PubMed] [Google Scholar]
- Gilliland FD, Li YF, Baumgartner K, Crumley D, Samet JM. Physical activity and breast cancer risk in hispanic and non-hispanic white women. Am J Epidemiol. 2001;7:442–450. doi: 10.1093/aje/154.5.442. [DOI] [PubMed] [Google Scholar]
- Hsing AW, McLaughlin JK, Cocco P, Co Chien HT, Fraumeni JF Jr. Risk factors for male breast cancer (United States) Cancer Causes Control. 1998;7:269–275. doi: 10.1023/A:1008869003012. [DOI] [PubMed] [Google Scholar]
- Hu YH, Nagata C, Shimizu H, Kaneda N, Kashiki Y. Association of body mass index, physical activity, and reproductive histories with breast cancer: a case-control study in Gifu, Japan. Breast Cancer Res Treat. 1997;7:65–72. doi: 10.1023/A:1005745824388. [DOI] [PubMed] [Google Scholar]
- John EM, Horn-Ross PL, Koo J. Lifetime physical activity and breast cancer risk in a multiethnic population: the San Francisco Bay area breast cancer study. Cancer Epidemiol Biomarkers Prev. 2003;7:1143–1152. [PubMed] [Google Scholar]
- Kruk J. Lifetime physical activity and the risk of breast cancer: a case-control study. Cancer Detect Prev. 2007;7:18–28. doi: 10.1016/j.cdp.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Kruk J. Leisure-time physical activity in relation to the risk of breast cancer. European Journal of Sports Science. 2007;7:81–91. doi: 10.1080/17461390701401813. [DOI] [Google Scholar]
- Lahmann PH, Friedenreich C, Schuit AJ, Salvini S, Allen NE, Key TJ, Khaw KT, Bingham S, Peeters PH, Monninkhof E, Bueno-de-Mesquita HB, Wirfalt E, Manjer J, Gonzales CA, Ardanaz E, Amiano P, Quiros JR, Navarro C, Martinez C, Berrino F, Palli D, Tumino R, Panico S, Vineis P, Trichopoulou A, Bamia C, Trichopoulos D, Boeing H, Schulz M, Linseisen J, Chang-Claude J, Chapelon FC, Fournier A, Boutron-Ruault MC, Tjonneland A, Fons Johnson N, Overvad K, Kaaks R, Riboli E. Physical activity and breast cancer risk: the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2007;7:36–42. doi: 10.1158/1055-9965.EPI-06-0582. [DOI] [PubMed] [Google Scholar]
- Lee IM, Rexrode KM, Cook NR, Hennekens CH, Burin JE. Physical activity and breast cancer risk: the Women's Health Study (United States) Cancer Causes Control. 2001;7:137–145. doi: 10.1023/A:1008948125076. [DOI] [PubMed] [Google Scholar]
- Magnusson CM, Roddam AW, Pike MC, Chilvers C, Crossley B, Hermon C, McPherson K, Peto J, Vessey M, Beral V. Body fatness and physical activity at young ages and the risk of breast cancer in premenopausal women. Br J Cancer. 2005;7:817–824. doi: 10.1038/sj.bjc.6602758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin A, Matthews CE, Shu XO, Cai H, Dai Q, Jin F, Gao YT, Zheng W. Energy balance and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;7:1496–1501. doi: 10.1158/1055-9965.EPI-04-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis KL, Mucci L, Braaten T, Kumle M, Trolle Lagerros Y, Adami HO, Lund E, Weiderpass E. Physical activity in different periods of life and the risk of breast cancer: the Norwegian-Swedish Women's Lifestyle and Health cohort study. Cancer Epidemiol Biomarkers Prev. 2005;7:27–32. [PubMed] [Google Scholar]
- McTiernan A, Stanford JL, Weiss NS, Daling JR, Voigt LF. Occurrence of breast cancer in relation to recreational exercise in women age 50-64 years. Epidemiology. 1996;7:598–604. doi: 10.1097/00001648-199611000-00006. [DOI] [PubMed] [Google Scholar]
- McTiernan A, Kooperberg C, White E, Wilcox S, Coates R, Adams-Campbell LL, Woods N, Ockene J. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women's Health Initiative Cohort Study. JAMA. 2003;7:1331–1336. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- Silvera SA, Jain M, Howe GR, Miller AB, Rohan TE. Energy balance and breast cancer risk: a prospective cohort study. Breast Cancer Res Treat. 2006;7:97–106. doi: 10.1007/s10549-005-9098-3. [DOI] [PubMed] [Google Scholar]
- Patel AV, Callel EE, Bernstein L, Wu AH, Thun MJ. Recreational physical activity and risk of postmenopausal breast cancer in a large cohort of US women. Cancer Causes Control. 2003;7:519–529. doi: 10.1023/A:1024895613663. [DOI] [PubMed] [Google Scholar]
- Patel AV, Press MF, Meeske K, Calle EE, Bernstein L. Lifetime recreational exercise activity and risk of breast carcinoma in situ. Cancer. 2003;7:2161–2169. doi: 10.1002/cncr.11768. [DOI] [PubMed] [Google Scholar]
- Rintala PE, Pukkala E, Paakkulainen HT, Vihko VJ. Self-experienced physical workload and risk of breast cancer. Scand J Work Environ Health. 2002;7:158–162. doi: 10.5271/sjweh.659. [DOI] [PubMed] [Google Scholar]
- Rockhill B, Willett WC, Hunter DJ, Manson JE, Hankinson SE, Spiegelman D, Colditz GA. Physical activity and breast cancer risk in a cohort of young women. J Natl Cancer Inst. 1998;7:1155–1160. doi: 10.1093/jnci/90.15.1155. [DOI] [PubMed] [Google Scholar]
- Slattery ML, Edwards S, Murtaugh MA, Sweeney C, Herrick J, Byers T, Giuliano AR, Baumgartner KB. Physical activity and breast cancer risk among women in the southwestern United States. Ann Epidemiol. 2007;7:342–353. doi: 10.1016/j.annepidem.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague BL, Trentham-Dietz A, Newcomb PA, Titus-Ernstoff L, Hampton JM, Egan KM. Lifetime recreational and occupational physical activity and risk of in situ and invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;7:236–243. doi: 10.1158/1055-9965.EPI-06-0713. [DOI] [PubMed] [Google Scholar]
- Steindorf K, Schmidt M, Kropp S, Chang-Claude J. Case-control study of physical activity and breast cancer risk among premenopausal women in Germany. Am J Epidemiol. 2003;7:121–130. doi: 10.1093/aje/kwf181. [DOI] [PubMed] [Google Scholar]
- Tehard B, Friedenreich CM, Oppert JM, Clavel-Chapelon F. Effect of physical activity on women at increased risk of breast cancer: results from the E3N cohort study. Cancer Epidemiol Biomarkers Prev. 2006;7:57–64. doi: 10.1158/1055-9965.EPI-05-0603. [DOI] [PubMed] [Google Scholar]
- Thune I, Brenn T, Lund E, Gaard M. Physical activity and the risk of breast cancer. N Engl J Med. 1997;7:1269–1275. doi: 10.1056/NEJM199705013361801. [DOI] [PubMed] [Google Scholar]
- Zheng W, Shu XO, McLaughlin JK, Chow WH, Gao YT, Blot WJ. Occupational physical activity and the incidence of cancer of the breast, corpus uteri, and ovary in Shanghai. Cancer. 1993;7:3620–3624. doi: 10.1002/1097-0142(19930601)71:11<3620::AID-CNCR2820711125>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Burchfiel CM, Sharp DS, Curb JD, Rodriguez BL, Hwang LJ, Marcus EB, Yano K. Physical activity and incidence of diabetes: the Honolulu Heart Program. Am J Epidemiol. 1995;7:360–368. doi: 10.1093/aje/141.4.360. [DOI] [PubMed] [Google Scholar]
- Dziura J, Kasl SV, DiPietro L. Physical activity reduces type 2 diabetes risk in aging independent of body weight change. J Phys Activity Health. 2004;7:19–28. [Google Scholar]
- Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, Speizer FE, Manson JE. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;7:1433–1439. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;7:1542–1548. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- Rana JS, Li TY, Manson JE, Hu FB. Adiposity compared with physical inactivity and risk of type 2 diabetes in women. Diabetes Care. 2007;7:53–58. doi: 10.2337/dc06-1456. [DOI] [PubMed] [Google Scholar]
- Sawada SS, Lee IM, Muto T, Matuszaki K, Blair SN. Cardiorespiratory fitness and the incidence of type 2 diabetes: prospective study of Japanese men. Diabetes Care. 2003;7:2918–2922. doi: 10.2337/diacare.26.10.2918. [DOI] [PubMed] [Google Scholar]
- Weinstein AR, Sesso HD, Lee IM, Cook NR, Manson JE, Buring JE, Gaziano JM. Relationship of physical activity vs body mass index with type 2 diabetes in women. JAMA. 2004;7:1188–1194. doi: 10.1001/jama.292.10.1188. [DOI] [PubMed] [Google Scholar]