Abstract
We evaluated a nurse-delivered adherence intervention in a preliminary randomized controlled trial among 70 HIV-positive outpatients initiating antiretroviral therapy (ART) in Beijing, China. In both arms, participants received a 30-min educational session, a pillbox, and a referral to a peer support group. In the enhanced arm, participants could choose an electronic reminder device, three sessions of counseling either alone or with a treatment adherence partner, or both reminder and counseling. Survey assessments and blood draws occurred at baseline, post-intervention (13 weeks), and follow-up (25 weeks). Primary outcomes were 7-day and 30-day adherence assessed by self-report and electronic drug monitoring (EDM), and secondary outcomes were HIV-1 RNA viral load and CD4 count. The intervention was feasible and well received. It led to some improvement in self-reported and EDM-assessed adherence but not the biological outcomes. Providing counseling and facilitating the use of electronic reminders to patients initiating ART merits further investigation as a culturally viable means of promoting adherence in China.
Keywords: HIV/AIDS, Adherence, Counseling, China, Nursing
Introduction
The HIV epidemic in China is in a critical phase, with increasing numbers of HIV-positive individuals in need of care [1]. Since the first case of HIV was reported in a tourist in 1985 [2], a total of 319,877 people have been registered as HIV-positive in China [3]. Although dire initial estimates of up to 10–20 million infections by 2010 have proved inflated [4], China’s HIV epidemic will quickly escalate if the rate of new infections continues unchecked. Many Chinese and international experts believe that the government must act now to contain the epidemic before it generalizes from rather isolated pockets to the population overall [5].
China recognizes its HIV/AIDS epidemic as a serious health, development, economic, and security challenge [6]. Accordingly, the Chinese government has taken several steps with the goal of limiting the infected population to less than 1.5 million this year. A key strategy of China’s response has been to aggressively pursue treatment. In 2003, China implemented a “Four Frees and One Care”, which committed the government to provide the following: (1) free antiretroviral medications to AIDS patients who are rural residents or who live in urban areas and are having financial difficulties; (2) free voluntary counseling and HIV screening tests; (3) free medications for HIV-infected pregnant women to prevent mother-to-child transmission, and HIV testing of newborn babies; and (4) free schooling for children orphaned by AIDS. One “care” refers to care and economic assistance for the households of people living with HIV/AIDS [7]. Although this policy stipulates increased access to ART, barriers to care remain, including stigma and discrimination, financial difficulties, fees for some medical tests (CD4 and VL), treatment for side effects, opportunistic infection treatment and hospitalizations, and costs associated with commuting to a healthcare facility [8-11].
The success of the treatment component of China’s AIDS policy depends on optimizing adherence to HIV medication, especially given the limited options for second-line regimens. However, among patients in the free medication programs, indicators of adherence problems already have begun to surface [12]. Since March, 2003, 5,289 Chinese patients have enrolled in the free medication program covering nine provinces. Six months after their initiation date, 20% were no longer refilling their prescriptions [13]. A separate report indicated that in June 2005, over 60% of treatment program participants dropped out or died within the first 3 months. The most common reasons for discontinuing treatment were side effects, patient request, difficulty with adherence, disease progression, and death [14]. Furthermore, a recent report of 31,070 patients indicated treatment failed for 25% of Chinese HIV patients (12.0 treatment failures per 100 person-years), with the cumulative treatment failure rate increasing to 50% at 5 years [6].
Two meta-analyses have indicated that various behavioral strategies can be efficacious in promoting adherence [15, 16] as well as improving clinical outcomes [16]. For example, successful interventions have employed couples-based counseling [17], modified directly observed therapy [18], and cognitive-behavioral approaches [19, 20]. Research on electronic reminders is more mixed, with one recent study noting no improvement with the use of programmable two-way pagers to alert participants to dosing times [21].
While much of the information on strategies to promote adherence has been generated in resource-rich settings, less is known about measuring and promoting adherence in resource-limited settings such as China. Indeed, we could find only one published report evaluating an adherence-promotion intervention in China. Specifically, Sabin et al. [22] found that counseling based on feedback from electronic drug monitors (EDM) led to improved average on-time adherence, greater likelihood of achieving 95% on-time adherence, and improved CD4 count (but not HIV-1 RNA viral load, VL) among 68 mainly former drug users in Yunnan Province. The effects appeared to be largely due to steep increases in adherence among intervention participants who were low adherers at baseline. In a separate report on the study, Gill et al. [23] reported associations between viral suppression and four measures of adherence: visual analog scale, pill count, EDM percentage adherence, and EDM percentage of on-time (±1 h) adherence. The authors noted ceiling effects and limited variation in the self-report measures and indicated only the EDM estimates, particularly the one incorporating dose timing, were significantly associated with undetectable VL.
The present study reports findings from a preliminary randomized controlled trial (RCT) of a counseling intervention and electronic reminders among HIV-positive patients in Beijing, China. A group of 70 patients initiating ART were assigned to a minimal or enhanced intervention arm to assess the feasibility and initial efficacy of the intervention. For the counseling, we adapted Safren et al.’s Life-Steps protocol [19] in which education, problem-solving, and rehearsal strategies are used to assist HIV-positive patients in developing better skills for adhering to HIV treatment. It has been tested with favorable results both in the United States [20] and India [24]. As part of our adaptation, we trained nurses to deliver the intervention. Chinese nurses spend most of their shifts in the wards and have extensive understanding of the HIV-positive patient’s condition; however, they are rarely involved in discussions or counseling about patient’s treatment planning [25]. We perceived nurses to be an untapped resource in China, knowledgeable about patients, medically informed, and with potentially more time than physicians to devote to ancillary services such as adherence counseling. Studies in the West have demonstrated that nurse counseling can improve HIV medication adherence [26-28]. Finally, we believed that offering individuals a choice of intervention strategies as part of an individualized approach might enhance motivation, retention, and efficacy. We thus also adapted the intervention to incorporate the option of counseling with a patient-selected treatment adherence partner present; this was in line with the strong emphasis on family relations in China and its collectivist culture. To further enhance choice, we incorporated the option of using a reminder device in addition to, or instead of, the counseling component.
Overall, the intervention addresses the four key components influencing medication adherence that we identified in a conceptual model based on the preliminary qualitative research from this project [11]. Namely, access to medications and treatment regimens (a structural component) is assured; accurate knowledge about the medications such as dosing and potential side-effects (a cognitive component) is provided; motivation to adhere to the medications (a psychological component) is presumably enhanced by attention to cultural factors in the counseling; and, an internal or external proximal cue to take the medication (a social/mechanical component) is delivered by the treatment partner or the electronic reminder.
Methods
Procedures
Data were collected from December 2006 to March 2008 at Beijing’s Ditan Hospital, the premier treatment center for infectious diseases in China. Eligible participants were Mandarin-speaking patients at Ditan Hospital who were at least 18 years of age, had CD4 counts lower than 350 cells/mm3, were otherwise eligible for ART, and were willing to and physically capable of attending follow-up visits at the hospital. Cognitively impaired or actively psychotic individuals were excluded.
Referrals came from the hospital-based HIV/AIDS support group (i.e., the Red Ribbon Society); providers at the clinic; and the patients themselves. Study staff presented detailed information about the scope of the trial to all referred patients. Those who were interested provided written informed consent before enrolling.
At the baseline appointment and before initiation of ART, all participants completed a 1-h interviewer–administered paper-and-pencil baseline survey. They then participated in a 30-min educational session facilitated with a flip-chart, which was designed to provide information regarding their treatment plans, likely side effects, and the importance of adherence. All patients also were given a daily medication schedule, a plastic pill box to organize daily doses, and a referral card to the hospital-based peer support group.
Patients initiating ART regimens at the clinic were generally prescribed three antiretroviral products on a non-nucleoside reverse transcriptase inhibitor-based regimen. The most common regimen was a combination of Lamivudine (Q.D.), Stavudine (B.I.D.), and Nevirapine (B.I.D), prescribed to 71% of participants in our study; Lamivudine (Q.D.), Stavudine (B.I.D.), and Efavirenz (B.I.D.) was the second most common regimen (13%).
An electronic drug monitoring device (EDM; i.e., the Medication Event Monitoring System or MEMS®; http://www.aardex.ch) was provided to all participants for the duration of the trial. EDM technology consists of a plastic pill vial and modified cap containing a microprocessor capable of recording the precise date and time of each vial opening as a presumptive dose. Each participant was given one EDM to use with Nevirapine or Efavirenz.
At the end of the baseline session, participants were randomized to either the minimal or enhanced 13-week intervention using sequentially numbered, opaque, sealed envelopes containing the intervention assignment, which the staff member opened at the moment of randomization. Participants assigned to the minimal intervention were to receive no further adherence-promotion intervention beyond the usual care at the clinic—which involved monthly medication pick-ups and any conversations patients initiated with their healthcare providers. All participants continued to receive medical care as usual at the clinic.
At baseline, post-intervention (13 weeks), and follow-up (25 weeks), the participants were given a survey, blood was drawn to determine VL and CD4 lymphocyte count, and EDM data were downloaded. The study coordinator conducted two brief telephone check-ins and adherence assessments at weeks 7 and 19. Participants were reimbursed 100 RMB ($12) for the baseline assessment, 120 RMB ($15) for the 13-week assessment, and 150 RMB ($18) for the 25-week assessment, with a 150 RMB ($18) bonus if they completed all three assessments and returned the EDM. Tracking efforts included unscripted reminder phone calls 1 week and 1 day before each scheduled assessment. Study staff administering surveys, but not the participants and intervention nurse, were generally blinded to study arm assignment.
Enhanced Intervention Arm
Participants assigned to the enhanced intervention could choose an alarm device, counseling, or both.
The reminder was a small battery-powered electronic alarm device. Enhanced intervention participants were first offered the choice of their cell phone as the electronic reminder. If the participant did not have a cell phone or preferred not to use it, then the study reminder device was introduced. In either case, project staff assisted participants in setting up a system in which an audible alarm would sound at pre-set dosing times.
The counseling intervention was delivered by one bachelor-level nurse who had been working at the clinic for 9 years. Hiring restrictions in China required the nurse interventionist be selected from the current team of nurse providers at the study hospital, all of whom participated in the 40 h of intensive training. The nurse intervener was supervised twice a month by telephone by study investigators to ensure fidelity to the intervention protocol, which included completion of a checklist and a progress note after each session. Note that the study nurse as well as the other nurses, all trained in the intervention, continued to see other patients at the hospital, including those in the minimal intervention arm. It was difficult, therefore, to limit the diffusion of the intervention.
In accordance with a manualized protocol, the counseling sessions involved a cognitive-behavioral and problem-solving approach to: (1) enhancing motivation for treatment continuation, (2) learning about antiretroviral medication and the prescribed regimen, (3) keeping appointments, (4) communicating with healthcare providers, (5) formulating a daily medication schedule, (6) storing medications, (7) setting reminder strategies, (8) coping with side effects, (9) securing family and social support, (10) handing adherence lapses, and (11) addressing additional barriers [19, 20].
Participants could invite a treatment partner to attend the counseling sessions with them. A “treatment partner” was defined as someone to whom the participant had already disclosed their HIV status and who was available to assist the participant on a daily basis. Most often, the treatment partner was a spouse/partner, parent, or sibling who lived with the participant. Treatment partners were fully integrated into a modified version of the counseling intervention, which involved joint counseling in the cognitive-behavioral and problem-solving approach as well as providing training specific to strengthening communication skills.
Participants who opted to have counseling, either individually or with a treatment adherence partner, had three counseling sessions of up to 1 h each with the study nurse. The first session occurred either at the enrollment interview or within the first two weeks of initiating ART; additional counseling sessions occurred at 5 and 9 weeks. If necessary, the later sessions were replaced with counseling provided over the telephone.
Primary and Secondary Outcome Measures
The primary outcome measures were self-reported and EDM-based medication adherence, using both 7-day and 30-day assessment intervals. Because all participants were initiating ART, no adherence data could be collected at the actual baseline; adherence outcomes were computed for 7 weeks (mid-intervention), 13 weeks (post-intervention), and 19 and 25 weeks (follow-ups). Self-reported 7-day adherence was based on a single item “How many of your HIV medication doses did you miss in the last 7 days?” for which we computed a dichotomous outcome of 100% versus less than 100% adherence. We also calculated a similar variable based on data covering a 30-day time frame. Based on EDM data, we computed two continuous adherence variables for each assessment point, defined as the number of bottle openings recorded by the EDM cap during the 7 days and the 30 days before each assessment date divided by the number of prescribed doses according to medical record review. We also computed similar estimates of on-time adherence for each assessment point. Since all EDM-medications were prescribed B.I.D., doses were ideally spaced 12 h apart. Therefore, only EDM openings 11–13 h after the previous dose were counted for the on-time adherence estimates.
The secondary outcomes were CD4 lymphocyte count and VL, which were assessed via blood draws at baseline, 13 weeks, and 25 weeks. As VL data were not normally distributed, we conducted a log10 transformation of VL data and used the transformed values in all analyses. CD4 and VL were analyzed as continuous variables to enhance power.
Statistical Methods
This preliminary RCT was undertaken to ascertain an approximate effect size and was not powered for statistical tests of efficacy. Interpretation focuses, therefore, on the magnitude and valence of the intervention effects, with P values provided for reference purposes. Also, the small N precluded an examination of the effects of specific intervention strategies (e.g., counseling vs. electronic reminders). Based on the assessment protocol, data on biological markers were available starting with the baseline interview and on adherence outcomes starting at the 7-week phone interview. Both cross-sectional and longitudinal intent-to-treat approaches were used to evaluate intervention efficacy.
The cross-sectional analyses evaluated the difference between minimal and enhanced intervention arm participant outcomes at each assessment point and were conducted using χ2 and t-tests for dichotomous and continuous outcomes, respectively. For these analyses, Fisher’s exact tests were applied to the χ2 statistics when data were sparse or cell counts were low in order to provide a more conservative analysis [29]. Only participants with non-missing data for the assessment point being evaluated were included in the cross-sectional analyses.
The longitudinal analyses evaluated the difference in average change over time between minimal and enhanced intervention arm participants. These were conducted using generalized estimating equations (GEE) [30], an approach that permits the retention of all participants, including those with some missing data. In the longitudinal GEE analyses, each outcome was regressed on arm (minimal vs. enhanced), time (weeks), and the arm × time interaction. The intervention effects were assessed with the arm × time interactions. A logit link function was utilized for the binary outcomes and Gaussian link function for continuous outcomes. An exchangeable working correlation structure and robust standard errors were specified to account for correlated outcome data across assessments.
Because there were differential rates of attrition, we did supplemental analyses accounting for incomplete follow-up data. We used a pattern-mixture approach to re-analyze the longitudinal models. In the pattern-mixture approach, the effect of missing data is incorporated as an explanatory variable in a longitudinal outcome analysis [30]. Accordingly, participants were classified into two patterns: those with complete versus incomplete follow-up data. The main effect of this missing data indicator and interactions with this indicator are then included in the analysis. This creates separate estimates of treatment effect for each missing data group. Each outcome was regressed on arm, time, the arm × time interaction, missing data group (completer vs. non-completer), and all interactions with the missing data group indicator. Treatment effects for completers and non-completers were averaged according to the relative frequency of individuals in each group. Findings were similar to those from the analyses not adjusting for missing data, so we report the unadjusted results below.
Results
Study Flow of Participants
As seen in Fig. 1, of the 89 patients screened for eligibility, 19 (21%) were excluded because they were not qualified to initiate ART, were too sick to participate, or refused. The reasons cited for refusal were living too far away to commute, being too busy, and having transportation difficulties. During the course of the trial, 6 of 34 participants enrolled in the minimal intervention arm dropped out due to moving to other provinces, ceasing ART because of severe side effects, losing interest in the study, or transferring to another institution for HIV care. None of the participants in the enhanced arm of the study dropped out. This difference was statistically significant (18% vs. 0%), Fisher’s exact χ2(1) = 6.95, P = 0.01. According to χ2 tests and one-way ANOVAs, participants across arms with complete self-report data (79%) did not differ from those who missed one or more assessments (21%) on any of the main socio-demographic, psychosocial, or biological variables as assessed at baseline. Furthermore, within the minimal intervention arm, attrition unrelated to any of the baseline psychosocial factors.
Fig. 1.
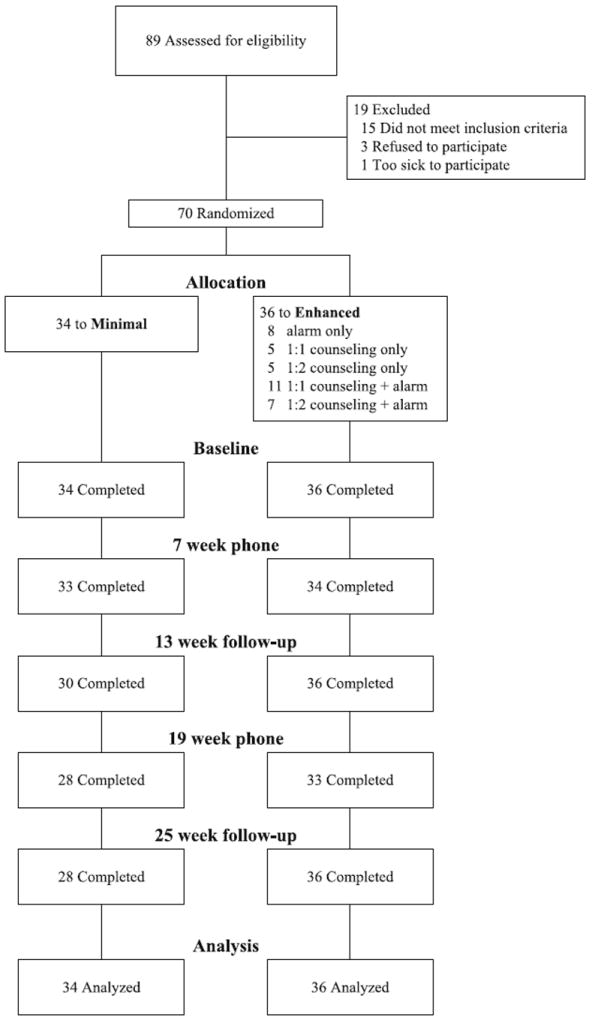
Flow chart of participants
Participants Description
The 70 participants enrolled in the trial ranged in age from 21 to 55 years (M = 36) at baseline and were mostly male (81%) and Han Chinese (96%). Participants were generally well educated, with 40% having a high school degree and 9%, a college degree; 47% were currently unemployed. Over half of the participants (61%) were married or had a steady partner. On average, participants had been diagnosed with HIV for 17 months. Comparison of participants in the two arms demonstrated that randomization was successful, with no statistically significant differences between them in any baseline measures of socio-demographics, regimen variables, or biomarkers.
Intervention Acceptability and Feasibility
Data in Fig. 1 on participants’ willingness to enter the study and minimal attrition attest to the intervention’s acceptability and feasibility. Furthermore, of the 28 participants opting for counseling, 75% completed 2 or 3 sessions. Seven participants choose to conduct one of the counseling sessions by phone due to various reasons, including being out of town or having transportation difficulties the day of the scheduled appointment. Twelve opted for counseling with a treatment adherence partner, in which they incorporated a spouse (n = 8), parent (n = 1), or same-sex partner (n = 3). The inclusion of a treatment adherence partner was an innovative aspect of the adaptation and should be of substantial interest to those working in regions where an adherence partner is a prerequisite for obtaining government-funded ART. Despite strong cultural prohibitions against divulging information that might bring shame to the family, counseling participants readily engaged in the intervention, often calling the study nurse for more support. Among the 26 participants opting for alarms, 7 decided to use their own cell phones instead due to set-up issues, loss of the study device, or personal preference. The remaining participants used the study alarm without problems throughout the study period.
Descriptive Data on Adherence and Clinical Outcomes
Descriptive statistics for average adherence are presented in Table 1. Data for self-reported dichotomous adherence and EDM continuous adherence were available at 7, 13, 19, and 25 weeks. Overall, self-reported 100% adherence was high and remained high throughout the study, ranging from 91 to 95% for the 7-day measure and 90 to 93% for the 30-day measure. Across arms, EDM adherence was lower than self-reported adherence and declined somewhat over time, from 78 to 67% for the 7-day measure and from 80 to 71% for the 30-day measure. On-time EDM adherence averaged approximately 11–18 percentage points lower than EDM adherence calculated without regard to dose timing. Clinical indicators demonstrated the efficacy of treatment. Across arms, 7.1% of the participants had undetectable VL at baseline, which increased to 78.1% over the 25 weeks of the trial. Immune functioning also improved, with average CD4 count rising during the trial from 147 to 263 cells/mm3.
Table 1.
Antiretroviral medication adherence and clinical outcomes by arm at each assessment point for HIV-positive outpatients participating in an adherence-promotion intervention in Beijing
| All participants (N = 70)
|
Minimal arm participants (n = 34)
|
Enhanced arm participants (n = 36)
|
χ2 | df | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||||
| Self-report 100% adherence (dichotomous) | |||||||||
| Past 7 days | |||||||||
| 7 weeks (telephone) | 67 | 91.0 | 33 | 93.9 | 34 | 88.2 | 0.67 | 1 | 0.67 |
| 13 weeks (in person) | 65 | 92.3 | 30 | 90.0 | 35 | 94.3 | 0.42 | 1 | 0.66 |
| 19 weeks (telephone) | 61 | 95.1 | 28 | 89.3 | 33 | 100.0 | 3.72 | 1 | 0.09 |
| 25 weeks (in person) | 64 | 93.8 | 28 | 92.9 | 36 | 94.4 | 0.07 | 1 | 0.99 |
| Past 30 days | |||||||||
| 7 weeks (telephone) | 67 | 89.6 | 33 | 93.9 | 34 | 85.3 | 1.34 | 1 | 0.43 |
| 13 weeks (in person) | 65 | 87.7 | 30 | 86.7 | 35 | 88.6 | 0.05 | 1 | 0.99 |
| 19 weeks (telephone) | 61 | 93.4 | 28 | 85.7 | 33 | 100.0 | 5.05 | 1 | 0.04 |
| 25 weeks (in person) | 64 | 90.6 | 28 | 85.7 | 36 | 94.4 | 1.41 | 1 | 0.39 |
| n | M (SD) | n | M (SD) | n | M (SD) | t | df | P | |
|
| |||||||||
| EDM % dose adherence (continuous) | |||||||||
| Past 7 days | |||||||||
| 7 weeks | 70 | 78.3 (36.8) | 34 | 74.6 (39.6) | 36 | 81.7 (34.2) | −0.81 | 68 | 0.42 |
| 13 weeks | 67 | 81.2 (34.2) | 31 | 77.2 (38.3) | 36 | 84.7 (30.3) | −0.90 | 65 | 0.37 |
| 19 weeks | 66 | 74.2 (36.8) | 30 | 70.2 (40.1) | 36 | 77.6 (34.0) | −0.80 | 64 | 0.42 |
| 25 weeks | 64 | 67.2 (40.7) | 28 | 67.1 (40.6) | 36 | 67.3 (41.3) | −0.02 | 62 | 0.99 |
| Past 30 days | |||||||||
| 7 weeks | 70 | 79.5 (34.0) | 34 | 76.0 (37.7) | 36 | 82.8 (30.4) | −0.84 | 68 | 0.40 |
| 13 weeks | 67 | 81.6 (33.1) | 31 | 77.4 (38.1) | 36 | 85.2 (28.3) | −0.97 | 65 | 0.34 |
| 19 weeks | 66 | 75.6 (35.0) | 30 | 72.6 (38.3) | 36 | 78.1 (32.3) | −0.64 | 64 | 0.52 |
| 25 weeks | 64 | 71.0 (38.5) | 28 | 70.1 (38.9) | 36 | 71.8 (38.7) | −0.17 | 62 | 0.86 |
| EDM % on-time adherence (continuous) | |||||||||
| Past 7 days | |||||||||
| 7 weeks | 70 | 67.4 (37.8) | 34 | 60.5 (40.1) | 36 | 74.0 (34.9) | −1.51 | 68 | 0.14 |
| 13 weeks | 67 | 68.2 (37.1) | 31 | 62.2 (39.2) | 36 | 73.4 (34.9) | −1.24 | 65 | 0.22 |
| 19 weeks | 66 | 59.6 (39.4) | 30 | 51.9 (39.2) | 36 | 66.1 (38.8) | −1.47 | 64 | 0.15 |
| 25 weeks | 64 | 52.3 (40.0) | 28 | 49.2 (39.4) | 36 | 54.8 (40.9) | −0.54 | 62 | 0.59 |
| Past 30 days | |||||||||
| 7 weeks | 70 | 64.9 (36.4) | 34 | 58.5 (39.1) | 36 | 70.9 (33.2) | −1.44 | 68 | 0.15 |
| 13 weeks | 67 | 67.6 (34.4) | 31 | 62.3 (37.5) | 36 | 72.3 (31.3) | −1.19 | 65 | 0.24 |
| 19 weeks | 66 | 57.3 (36.9) | 30 | 50.4 (36.0) | 36 | 63.0 (37.1) | −1.38 | 64 | 0.17 |
| 25 weeks | 64 | 53.8 (38.6) | 28 | 50.0 (39.5) | 36 | 56.7 (38.2) | −0.69 | 62 | 0.49 |
| Biomarkers | |||||||||
| HIV-1 RNA viral load (log10 copies/ml) | |||||||||
| Baseline | 70 | 4.5 (1.2) | 34 | 4.4 (1.3) | 36 | 4.6 (1.1) | −0.98 | 68 | 0.33 |
| 13 weeks | 67 | 2.2 (0.8) | 31 | 2.3 (0.9) | 36 | 2.2 (0.8) | 0.39 | 65 | 0.69 |
| 25 weeks | 64 | 1.9 (0.6) | 28 | 1.9 (0.5) | 36 | 2.0 (0.7) | −0.78 | 62 | 0.44 |
| CD4 count (cells/mm3) | |||||||||
| Baseline | 70 | 146.9 (101.3) | 34 | 143.2 (91.4) | 36 | 150.3 (111.1) | −0.29 | 68 | 0.77 |
| 13 weeks | 68 | 237.6 (123.6) | 32 | 229.4 (139.5) | 36 | 244.9 (109.1) | −0.51 | 66 | 0.61 |
| 25 weeks | 64 | 262.6 (135.9) | 28 | 255.8 (161.3) | 36 | 268.0 (114.5) | −0.35 | 62 | 0.72 |
Note: Baseline data were not available for the adherence measures because all participants were initiating antiretroviral therapy. Differences between minimal arm and enhanced arm participants at the designed time points were evaluated using χ2 and t-tests for dichotomous and continuous outcomes, respectively. These cross-sectional analyses include all participants with non-missing data
Evaluation of Intervention Effects
Cross-sectional Analyses
Cross-sectional tests comparing participants in the minimal and enhanced intervention arms at each assessment are reported in Table 1. The percentage of participants in the minimal intervention arm self-reporting 100% adherence in the 7 days prior to the assessment remained relatively stable from 7 to 25 weeks after baseline (ranging from 89 to 94%). However, enhanced intervention arm participants, despite being less likely than minimal intervention participants to report 100% adherence at 7 weeks (88% vs. 94%), were more likely to report 100% adherence at each of the next three assessment points. This difference was marginally significant at the 19-week assessment (100% vs. 89%), χ2(1) = 3.72, P = 0.09.
A similar pattern emerged for the self-reported 30-day dichotomous adherence outcome. The percentage of participants in the minimal intervention arm reporting 100% adherence in the 30 days prior to the assessment decreased over the course of the study (from 94 to 86%). However, enhanced intervention arm participants, despite being less likely than minimal intervention participants to report 100% adherence at 7 weeks (85% vs. 94%), reported higher levels of 100% adherence at each of the next three assessment points. This difference reached statistical significance at the 19-week assessment (100% vs. 86%), χ2(1) = 5.05, P = 0.04.
EDM 7-day adherence data indicated that participants in the enhanced intervention arm had greater average dose (82% vs. 75%) and on-time (74% vs. 61%) adherence than those in the minimal intervention arm by the 7-week assessment. They continued to have better adherence until the final follow-up assessment, with the greatest dose adherence difference occurring at the 13-week immediate post-intervention assessment (85% vs. 77%) and the greatest on-time adherence difference occurring at the 19-week assessment (66% vs. 52%). There were no statistically significant differences in EDM 7-day adherence between arms at any assessment point.
A very similar pattern emerged for the EDM 30-day dose adherence interval. Participants in the enhanced intervention had greater average 30-day dose (83% vs. 76%) and timing (71% vs. 59%) adherence than those in the minimal intervention by the 7-week assessment and they continued to have better adherence until the final follow-up assessment. There were no statistically significant differences in EDM 30-day adherence between arms at any assessment point.
With respect to the laboratory-based clinical outcome of VL, participants in both arms showed comparable improvement over time. CD4 count was slightly higher at baseline for enhanced intervention participants and remained higher throughout the study. There were no statistically significant differences between arms at any follow-up point for either clinical outcome.
Longitudinal Analyses
Recall that GEE analyses [31] were used to compare the average weekly change in each outcome for participants in the minimal versus enhanced intervention arms. Findings for the self-report adherence outcomes provided some evidence of superiority for the enhanced versus minimal intervention arm participants. With respect to the self-report dichotomous 7-day adherence outcome, the probability of 100% adherence remained stable in the minimal intervention arm, with 0.0 percentage point average weekly change. In contrast, the probability of 100% adherence increased by 0.6 percentage points per week in the enhanced intervention arm. This difference did not reach statistical significance, OR = 1.08, SE = 0.09, 95% CI = 0.92–1.28, P = 0.33. With respect to the self-report dichotomous 30-day adherence outcome, the probability of 100% adherence decreased by 0.3 percentage points per week in the minimal intervention arm but increased by 0.9 percentage points per week in the enhanced intervention arm. The weekly probability of 100% self-reported 30-day adherence for the enhanced versus minimal intervention arms, according to the longitudinal model, is plotted in Fig. 2. The arm × time interaction indicated significant improvement, OR = 1.14, SE = 0.07, 95% CI = 1.01–1.30, P = 0.04. The cumulative effect of these average weekly improvements between the initial 7-week assessment and 13-week post intervention was a more than twofold improvement in the odds of 100% adherence for the enhanced versus minimal intervention arm, OR = 2.23, SE = 0.85, 95% CI = 1.05–4.72, P = 0.04.
Fig. 2.
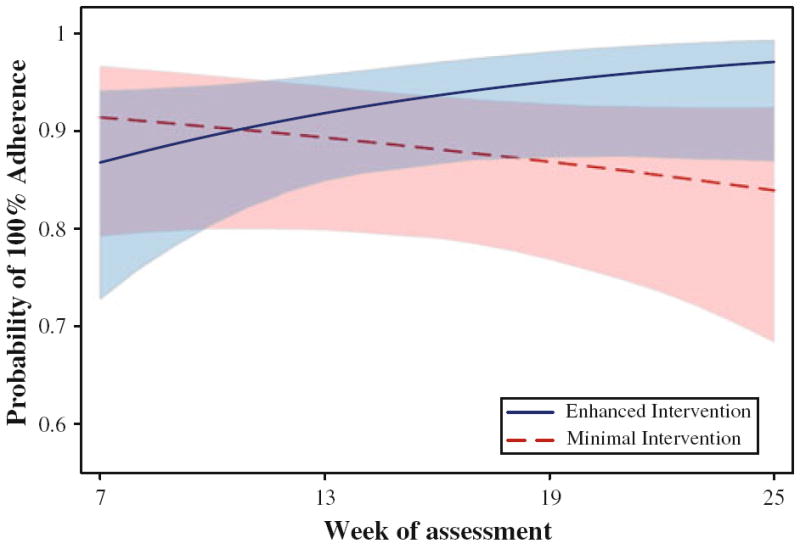
Self-reported 30-day adherence by study arm according to longitudinal model based on observed data at 7, 13, 19, and 25 weeks. Shaded regions are the 95% confidence intervals
GEE analyses on the EDM adherence outcomes, however, indicated little difference between arms. Specifically, EDM continuous 7-day adherence data indicated that dose adherence decreased by 0.7 percentage points each week in the minimal intervention arm and 0.8% each week in the enhanced intervention arm, a non-significant difference, Beta = −0.12, SE = 0.57, 95% CI = −1.24–0.99, P = 0.83. EDM 7-day on-time adherence decreased by 0.9 percentage points each week in the minimal intervention arm and 1.1 percentage points each week in the enhanced intervention arm, a non-significant difference, Beta = −0.15, SE = 0.55, 95% CI = −1.23–0.93, P = 0.79. Results were very similar with respect to the EDM continuous 30-day adherence outcome, with dose adherence decreasing by 0.6 percentage points each week in the minimal intervention arm and 0.7 percentage points each week in the enhanced intervention arm, also a non-significant difference, Beta = −0.05, SE = 0.51, 95% CI = −1.05–0.95, P = 0.92). EDM 30-day on-time adherence also decreased by 0.8 percentage points each week in the minimal intervention arm and 0.9 percentage points each week in the enhanced intervention arm, also a non-significant difference, Beta = −0.03, SE = 0.48, 95% CI = −0.97–0.90, P = 0.94.
With respect to the log10 VL outcome, longitudinal analyses indicated there was no difference between the arms, with average log10 VL over 25 weeks decreasing 0.1 log units per week within each, Beta = −0.005, SE = 0.01, 95% CI = −0.03–0.02, P = 0.75. Neither for the CD4 count outcome was there any difference between arms, with average immune functioning increasing 5 cells/mm3 per week within each (Beta = −0.10, SE = 1.12, 95% CI = −2.30–2.09, P = 0.93).
Discussion
This preliminary RCT examined a nurse-delivered anti-retroviral medication adherence counseling program targeting HIV-positive outpatients with or without a self-identified treatment adherence partner in Beijing, China. The option of using an alarm device—in addition to or instead of the counseling—also was provided. Feasibility data were promising, with minimal refusals, good attendance, and few drop outs in this preliminary trial. There were no adverse events in either arm.
Descriptive findings indicated a ceiling effect for self-reported adherence, with all but a handful of participants reporting 100% adherence to their medications in the 7- and 30-day periods before each of the five assessments. This finding mirrors other reports of Chinese patients’ high self-reported adherence. Specifically, among 69 mainly former injection drug users in Yunnan Province, self-reported adherence rates indicated almost no variability, with 95.6% of patients reporting at least 95% adherence during the 6 months of initial observation [23]. In a sample of 181 mainly former plasma donors in the provinces of Henan and Anhui, 81.8% reported adherence of 95% or greater adherence over the previous 3 days, with 49.7% claiming never to have missed a single dose of their medications [32]. Finally, among 308 individuals in south-central China, half of whom had been infected via plasma donation, 80% reported taking more than 90% of prescribed doses in the previous 7 days [12].
The Chinese participants in this and other studies appear to self-report higher adherence and less attenuation over time than participants in US-based studies. For example, 70% of 224 participants in a Seattle clinic-based study of participants initiating or switching regimens self-reported 100% adherence at baseline, which dropped to 49% at 9 months [21]. In fact, a pooled analysis of 31 North American studies, of which 71% used patient self-report to assess adherence, indicated 55% achieved “adequate” levels of adherence [33]. Adherence levels in the current study were closer to those first reported in sub-Saharan Africa; the pooled estimate of adequate adherence among 27 African studies, of which 66% assessed adherence with patient self-report, was 77% [33].
One possible explanation for the Chinese participants’ higher self-reported estimates is that individuals in this study were generally initiating ART at more advanced stages of HIV disease than were participants in comparable studies in the West. Their initial poor health might lead to enhanced motivation to adhere to their regimens at the outset, with drastic improvements fueling their maintenance of adherence over time. Alternatively, the self-reports of adherence may be inflated by participants responding in a socially desirable fashion. In China, a strong authoritarian tradition may enhance this tendency toward socially desirable responding. This would be particularly problematic if the enhanced intervention arm participants were more responsive to this bias more than those in the minimal intervention arm.
That EDM-based adherence was lower than self-reported adherence at baseline (as seen in US studies) [34] and dropped off during the 25 weeks of the trial lends further support to the possibility that the self-reported adherence data were overestimates of actual behavior. This also suggests a possible effect of treatment fatigue, indicating the need to evaluate the effect of ongoing support or booster sessions in future trials. Alternatively, participants may have been less likely to use their EDM device, especially over time, meaning EDM data were underestimates of adherence. This could explain why, despite the decreasing average EDM-based adherence levels, the bio-markers of VL and CD4 indicated increasingly robust response to the antiretroviral medication.
This preliminary RCT was not powered for statistical tests of efficacy (N = 70). However, both cross-sectional and longitudinal analyses indicated that the intervention arm was generally associated with positive effects on the primary outcome of adherence, with participants in the enhanced intervention arm reporting adherence levels up to 15 percentage points higher than those in the minimal intervention arm. Longitudinal analyses revealed that the enhanced intervention more than doubled a participant’s odds of attaining 100% self-reported adherence over the prior 30 days. The benefits were relatively consistent across assessment method (i.e., self-report or EDM); assessment period (i.e., 7 or 30 days); and type of adherence outcome (i.e., with or without the consideration of dose timing). The one exception was that effects on EDM-based adherence were more pronounced in the cross-sectional than longitudinal analyses, in part because these EDM data suggested adherence improvements achieved during the intervention were not maintained at follow-up. Biomarker levels (VL and CD4) were roughly equivalent between arms, suggesting any differences in adherence did not translate into measureable effects on disease progression. These findings are consistent with recent meta-analyses of US-based studies [15, 16] which indicated odds ratios of 1.50 [16] and 1.88 [15] for adherence outcomes and less robust findings for biomarkers (1.25) [16].
Replication efforts and future research might consider a more prolonged and intensive intervention, given the inconsistent improvements in adherence that tended to decay after the intervention phase. Additionally, targeting individuals potentially at risk for non-adherence may lead to stronger intervention effects, especially given the ceiling effects in adherence we noted. Indeed, in their meta-analysis, Amico and colleagues noted that interventions targeting participants with known or anticipated problems with ART adherence demonstrated larger effects on adherence than interventions that did not. [15].
Compared to the one other adherence intervention study we could locate that was conducted in China, our EDM-based estimates of average on-time adherence in the past 30 days were lower for intervention/control participants at initial assessment (87%/84% vs. 71%/59%) as well as at follow-up (97%/85% 26 weeks later vs. 57%/50% 18 weeks later) [22]. This difference is surprising as about half of the other study’s sample was infected through injection drug use and thus may have had other challenges that interfered with consistent adherence. Moreover, adherence in our urban-based study was apparently less amenable to intervention effects. The intervention in southwestern China was longer (6 months), but the counseling component was not highly specified, purposefully designed to accommodate each clinician’s style and encourage an individually tailored discussion. It is not clear, then, exactly what led to the improvement in that study—just as in our study we were not able to distinguish among the effects of the multiple components or determine which mediating variables were most related to improved adherence. Qualitative data, both from our work [11] and from the team in Yunnan [10, 22] suggest areas that might be worth specifically targeting in further adherence-promotion efforts in China, including the effects of acute HIV stigma in China, family relations, and mental health issues.
There are several limitations to the current study. Although self-reported adherence has been shown to correlate with VL [35], differential reporting from participants in intervention versus control arms of a trial, as mentioned earlier, could make it a less trustworthy indicator of treatment effects. These findings suggest the utility of incorporating multiple adherence assessment strategies in addition to or instead of relying solely on self-report in clinical trials. In the previously mentioned study by Gill et al., in China, the EDM was a more valid indicator of undetectable VL than self-report [23]. With their southwestern Chinese sample, half of whom had been infected via injection drug use, they also noted EDM-adherence based on dose timing was more sensitive to viral suppression than adherence assessed with EDM that did not consider the timing of doses. In our sample, EDM on-time adherence calculated based on a similar 1-h window led to lower estimates of adherence, not surprisingly, but they were not more likely to differ between intervention arms.
Another limitation is that participants were not randomly assigned to the different options within the enhanced treatment arm, so we were not able to determine their relative efficacy. In line with recent work by de Bruin and colleagues [36], we should note that the minimal intervention arm participants were supported in several ways for their adherence. They were given a pillbox and access to a peer support group because these are part of standard care at the clinic, and we provided them with the flip-chart education program as well. Diffusion of the intervention was possible, even likely, given that all nurses were trained in the intervention and all, including the study nurse intervener, continued to see patients in both study arms throughout the trial. Of course, any actual diffusion would mean our findings are conservative estimates of the actual effects. Thus, our intervention might prove even more beneficial if given to patients who otherwise get very little adherence support.
Finally, the analyses of the self-report dichotomous adherence outcomes require more cautious interpretation due to ceiling effects, particularly at the 19-week assessment. For the cross-sectional analyses, the use of Fisher’s exact test is generally deemed appropriate in sparse data situations [31]. In the longitudinal analyses, the effect of sparse data on statistical estimates was less clear, although the GEE approach has been shown to produce unbiased estimates with sparse dichotomous outcome data [37].
In conclusion, the HIV epidemic in China continues to provide a window of opportunity during which strict attention to adherence to life-sustaining medication regimens are urgently needed. Interventions such as the one studied here that capitalize on existing personnel resources (i.e., nurses), integrate easily with clinic care, address cultural values, incorporate family members and others who can provide social support, and offer choices and flexibility merit further attention.
Acknowledgments
This study was supported in part by MH074364 and MH074364-S1 from the US National Institute of Mental Health (Simoni, PI). We acknowledge Bu Huang for her assistance in obtaining funding for the study and Wei Qu for coordinating the study on-site.
Contributor Information
Jane M. Simoni, Email: jsimoni@uw.edu, Department of Psychology, University of Washington, 3909 Stevens Way NE, Campus Box 351525, Seattle, WA 98195-1525, USA.
Wei-Ti Chen, Department of Family and Child Nursing, School of Nursing, University of Washington, Seattle, WA, USA.
David Huh, Department of Psychology, University of Washington, 3909 Stevens Way NE, Campus Box 351525, Seattle, WA 98195-1525, USA.
Karen I. Fredriksen-Goldsen, School of Social Work, University of Washington, Seattle, WA, USA
Cynthia Pearson, School of Social Work, University of Washington, Seattle, WA, USA.
Hongxin Zhao, Institute of Infectious Diseases, Capital Medical University, Ditan Hospital, Beijing, China, zhao_hongxin66@yahoo.com.cn.
Cheng-Shi Shiu, School of Social Service Administration, University of Chicago, Chicago, IL, USA.
Xin Wang, Institute of Infectious Diseases, Capital Medical University, Ditan Hospital, Beijing, China.
Fujie Zhang, Division of Treatment and Care, National Center for AIDS/STD Control and Prevention, Beijing, China.
References
- 1.Gill B, Okie S. China and HIV—a window of opportunity. N Engl J Med. 2007;356(18):1801–5. doi: 10.1056/NEJMp078010. [DOI] [PubMed] [Google Scholar]
- 2.Wu Z, Sullivan SG, Wang Y, Rotheram-Borus MJ, Detels R. Evolution of China’s response to HIV/AIDS. Lancet. 2007;369(9562):679–90. doi: 10.1016/S0140-6736(07)60315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xinhua S. China’s health minister warns of HIV spread. China Daily. 2009 Nov 24; [Google Scholar]
- 4.Wu Z, Rou K, Cui H. The HIV/AIDS epidemic in China: history, current strategies and future challenges. AIDS Educ Prev. 2004;16(3 Suppl A):7–17. doi: 10.1521/aeap.16.3.5.7.35521. [DOI] [PubMed] [Google Scholar]
- 5.Ma Y, Zhao D, Yu L, Bulterys M, Robinson ML, Zhao Y, et al. Predictors of virologic failure in HIV-1-infected adults receiving first-line antiretroviral therapy in 8 provinces in China. Clin Infect Dis. 2010;50(2):264–71. doi: 10.1086/649215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang F, Dou Z, Ma Y, Zhao Y, Liu Z, Bulterys M, et al. Five-year outcomes of the China National Free Antiretroviral Treatment Program. Ann Intern Med. 2009;151(4):241-51–W-52. doi: 10.7326/0003-4819-151-4-200908180-00006. [DOI] [PubMed] [Google Scholar]
- 7.Zhang F, Chen RY, Lo SN, Ye M, editors. A decade of HAART: the development and global impact of highly active antiretroviral therapy. Chicago, IL: University of Chicago Press; 2008. [Google Scholar]
- 8.Chen WT, Starks H, Shiu CS, Fredriksen-Goldsen K, Simoni J, Zhang F, et al. Chinese HIV-positive patients and their healthcare providers: contrasting Confucian versus Western notions of secrecy and support. ANS Adv Nurs Sci. 2007;30(4):329–42. doi: 10.1097/01.ANS.0000300182.48854.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Sun S, Wu Z, Wu S, Lin C, Yan Z. Disclosure of HIV status is a family matter: field notes from China. J Fam Psychol. 2007;21(2):307–14. doi: 10.1037/0893-3200.21.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabin LL, Desilva MB, Hamer DH, Keyi X, Yue Y, Wen F, et al. Barriers to adherence to antiretroviral medications among patients living with HIV in southern China: a qualitative study. AIDS Care. 2008;20(10):1242–50. doi: 10.1080/09540120801918651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starks H, Simoni J, Zhao H, Huang B, Fredriksen-Goldsen K, Pearson C, et al. Conceptualizing antiretroviral adherence in Beijing, China. AIDS Care. 2008;20(6):607–14. doi: 10.1080/09540120701660379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, He G, Li X, Yang A, Chen X, Fennie KP, et al. Self-reported adherence to antiretroviral treatment among HIV-infected people in Central China. AIDS Patient Care STDS. 2008;22(1):71–80. doi: 10.1089/apc.2007.0047. [DOI] [PubMed] [Google Scholar]
- 13.Li H. San lian Life Weekly. Vol. 265. Chinese: 2003. HIV/AIDS and the promises of the government; pp. 22–5. [Google Scholar]
- 14.Zhang FJ, Pan J, Yu L, Wen Y, Zhao Y. Current progress of China’s free ART program. Cell Res. 2005;15(11–12):877–82. doi: 10.1038/sj.cr.7290362. [DOI] [PubMed] [Google Scholar]
- 15.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006;41(3):285–97. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- 16.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S23–35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Remien RH, Stirratt MJ, Dolezal C, Dognin JS, Wagner GJ, Carballo-Dieguez A, et al. Couple-focused support to improve HIV medication adherence: a randomized controlled trial. AIDS. 2005;19(8):807–14. doi: 10.1097/01.aids.0000168975.44219.45. [DOI] [PubMed] [Google Scholar]
- 18.Pearson CR, Micek MA, Simoni JM, Hoff PD, Matediana E, Martin DP, et al. Randomized control trial of peer-delivered, modified directly observed therapy for HAART in Mozambique. J Acquir Immune Defic Syndr. 2007;46(2):238–44. doi: 10.1097/QAI.0b013e318153f7ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safren SA, Otto MW, Worth JL. Life-steps: applying cognitive behavioral therapy to HIV medication adherence. Cogn Behav Pract. 1999;6:332–41. [Google Scholar]
- 20.Safren SA, Otto MW, Worth JL, Salomon E, Johnson W, Mayer K, et al. Two strategies to increase adherence to HIV antiretroviral medication: life-steps and medication monitoring. Behav Res Ther. 2001;39(10):1151–62. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 21.Simoni JM, Huh D, Frick PA, Pearson CR, Andrasik MP, Dunbar PJ, et al. Peer support and pager messaging to promote antiretroviral modifying therapy in Seattle: a randomized controlled trial. J Acquir Immune Defic Syndr. 2009;52(4):465–73. doi: 10.1097/qai.0b013e3181b9300c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabin LL, Desilva MB, Hamer DH, Xu K, Zhang J, Li T, et al. Using electronic drug monitor feedback to improve adherence to antiretroviral therapy among HIV-positive patients in China. AIDS Behav. 2010;14(3):580–9. doi: 10.1007/s10461-009-9615-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gill CJ, Sabin LL, Hamer DH, Keyi X, Jianbo Z, Li T, et al. Importance of dose timing to achieving undetectable viral loads. AIDS Behav. 2010;14(4):785–93. doi: 10.1007/s10461-009-9555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safren SA, Martin C, Menon S, Greer J, Solomon S, Mimiaga MJ, et al. A survey of MSM HIV prevention outreach workers in Chennai, India. AIDS Educ Prev. 2006;18(4):323–32. doi: 10.1521/aeap.2006.18.4.323. [DOI] [PubMed] [Google Scholar]
- 25.Chen W-T, Shiu C-S, Simoni J, Fredriksen-Goldsen K, Zhang F, Zhao H. Optimizing HIV care by expanding the nursing role in China: a qualitative study of patient and provider perspectives. J Adv Nurs. 2010;66(2):260–8. doi: 10.1111/j.1365-2648.2009.05165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook PF, McCabe MM, Emiliozzi S, Pointer L. Telephone nurse counseling improves HIV medication adherence: an effectiveness study. J Assoc Nurses AIDS Care. 2009;20(4):316–25. doi: 10.1016/j.jana.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Ramirez-Garcia P, Cote J. Development of a nursing intervention to facilitate optimal antiretroviral-treatment taking among people living with HIV. BMC Health Serv Res. 2009;9(1):113. doi: 10.1186/1472-6963-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams AB, Fennie KP, Bova CA, Burgess JD, Danvers KA, Dieckhaus KD. Home visits to improve adherence to highly active antiretroviral therapy: a randomized controlled trial. J Acquir Immune Defic Syndr. 2006;42(3):314–21. doi: 10.1097/01.qai.0000221681.60187.88. [DOI] [PubMed] [Google Scholar]
- 29.Agresti A. A survey of exact inference for contingency tables. Stat Sci. 1992;7:131–53. [Google Scholar]
- 30.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 31.Agresti A. Modelling patterns of agreement and disagreement. Stat Methods Med Res. 1992;1(2):201–18. doi: 10.1177/096228029200100205. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Wu Z. Factors associated with adherence to antiretroviral therapy among HIV/AIDS patients in rural China. AIDS. 2007;21(Suppl 8):S149–55. doi: 10.1097/01.aids.0000304711.87164.99. [DOI] [PubMed] [Google Scholar]
- 33.Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006;296(6):679–90. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 34.Arnsten JH, Li X, Mizuno Y, Knowlton AR, Gourevitch MN, Handley K, et al. Factors associated with antiretroviral therapy adherence and medication errors among HIV-infected injection drug users. J Acquir Immune Defic Syndr. 2007;46(Suppl 2):S64–71. doi: 10.1097/QAI.0b013e31815767d6. [DOI] [PubMed] [Google Scholar]
- 35.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10(3):227–45. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Bruin M, Viechtbauer W, Hospers HJ, Schaalma HP, Kok G. Standard care quality determines treatment outcomes in control groups of HAART-adherence intervention studies: implications for the interpretation and comparison of intervention effects. Health Psychol. 2009;28(6):668–74. doi: 10.1037/a0015989. [DOI] [PubMed] [Google Scholar]
- 37.Sharples K, Breslow N. Regression analysis of correlated binary data: some small sample results for the estimating equation approach. J Stat Comput Simul. 1992;42(1):1–20. [Google Scholar]


