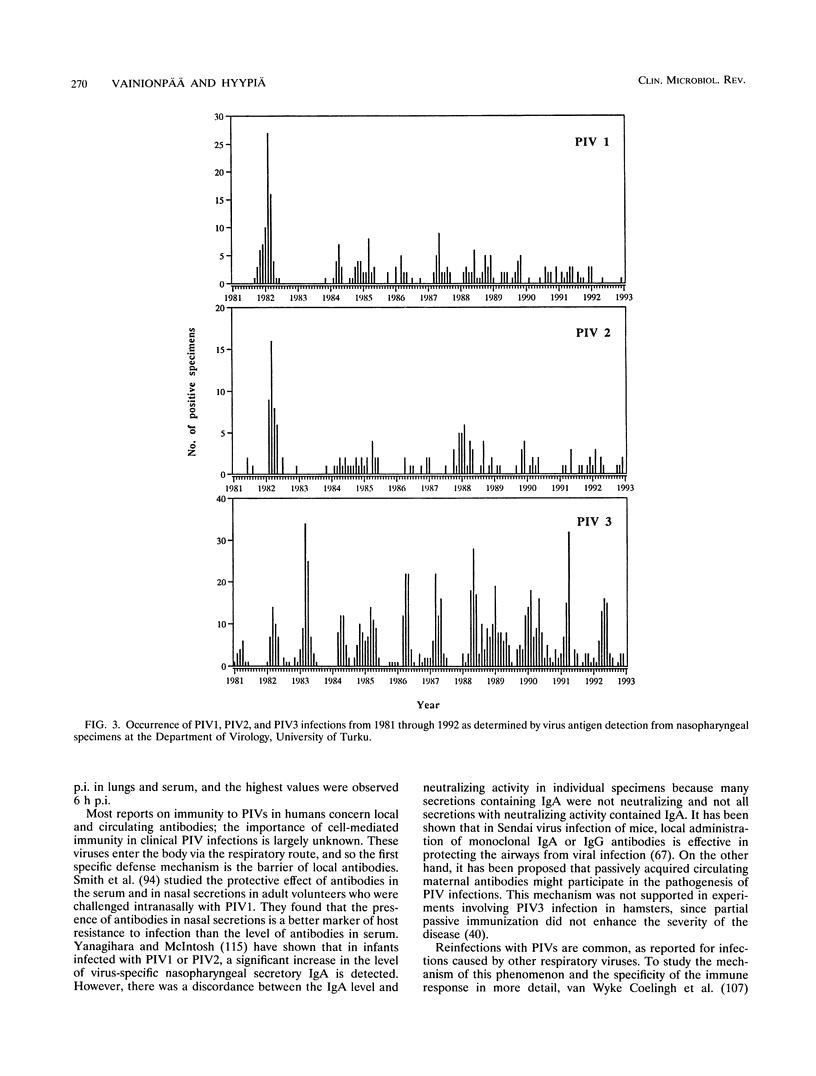
Abstract
Parainfluenza virus types 1 to 4 (PIV1 to PIV4) are important human pathogens that cause upper and lower respiratory tract infections, especially in infants and children. PIV1, PIV2, and PIV3 are second only to respiratory syncytial virus as a cause of croup in young children. Although some clinical symptoms are typical of PIVs, etiologic diagnosis always requires detection of infectious virus, viral components, or an antibody response. PIVs are typical paramyxoviruses, causing a syncytial cytopathic effect in cell cultures; virus growth can be confirmed either by hemadsorption or by using immunological reagents. Currently, PIV is most often diagnosed by demonstrating viral antigens in clinical specimens by rapid and highly sensitive immunoassays. More recently, PCR has been used for the detection of PIVs. Serological diagnosis is made by detecting a rising titer of immunoglobulin G or by demonstrating immunoglobulin M antibodies. PIVs infect species other than humans, and animal models are used to study the pathogenesis of PIV infections and to test candidate vaccines. Accumulating knowledge on the molecular structure and mechanisms of replication of PIVs has accelerated research on prevention and treatment. Several strategies for vaccine development, such as the use of live attenuated, inactivated, recombinant, and subunit vaccines, have been investigated, and it may become possible to prevent PIV infections in the near future.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose M. W., Wyde P. R., Ewasyshyn M., Bonneau A. M., Caplan B., Meyer H. L., Klein M. Evaluation of the immunogenicity and protective efficacy of a candidate parainfluenza virus type 3 subunit vaccine in cotton rats. Vaccine. 1991 Jul;9(7):505–511. doi: 10.1016/0264-410x(91)90037-7. [DOI] [PubMed] [Google Scholar]
- Arguedas A., Stutman H. R., Blanding J. G. Parainfluenza type 3 meningitis. Report of two cases and review of the literature. Clin Pediatr (Phila) 1990 Mar;29(3):175–178. doi: 10.1177/000992289002900307. [DOI] [PubMed] [Google Scholar]
- Belshe R. B., Karron R. A., Newman F. K., Anderson E. L., Nugent S. L., Steinhoff M., Clements M. L., Wilson M. H., Hall S. L., Tierney E. L. Evaluation of a live attenuated, cold-adapted parainfluenza virus type 3 vaccine in children. J Clin Microbiol. 1992 Aug;30(8):2064–2070. doi: 10.1128/jcm.30.8.2064-2070.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Rose K., Kolakofsky D. Sequence determination of the Sendai virus fusion protein gene. J Gen Virol. 1985 Feb;66(Pt 2):317–331. doi: 10.1099/0022-1317-66-2-317. [DOI] [PubMed] [Google Scholar]
- Blumberg B., Giorgi C., Roux L., Raju R., Dowling P., Chollet A., Kolakofsky D. Sequence determination of the Sendai virus HN gene and its comparison to the influenza virus glycoproteins. Cell. 1985 May;41(1):269–278. doi: 10.1016/0092-8674(85)90080-7. [DOI] [PubMed] [Google Scholar]
- Bohn W., Rutter G., Hohenberg H., Mannweiler K., Nobis P. Involvement of actin filaments in budding of measles virus: studies on cytoskeletons of infected cells. Virology. 1986 Feb;149(1):91–106. doi: 10.1016/0042-6822(86)90090-5. [DOI] [PubMed] [Google Scholar]
- Brideau R. J., Oien N. L., Lehman D. J., Homa F. L., Wathen M. W. Protection of cotton rats against human parainfluenza virus type 3 by vaccination with a chimeric FHN subunit glycoprotein. J Gen Virol. 1993 Mar;74(Pt 3):471–477. doi: 10.1099/0022-1317-74-3-471. [DOI] [PubMed] [Google Scholar]
- Castells E., George V. G., Hierholzer J. C. NCI-H292 as an alternative cell line for the isolation and propagation of the human paramyxoviruses. Arch Virol. 1990;115(3-4):277–288. doi: 10.1007/BF01310536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Kaelin K., Baczko K., Billeter M. A. Measles virus editing provides an additional cysteine-rich protein. Cell. 1989 Mar 10;56(5):759–764. doi: 10.1016/0092-8674(89)90679-x. [DOI] [PubMed] [Google Scholar]
- Chambers P., Millar N. S., Emmerson P. T. Nucleotide sequence of the gene encoding the fusion glycoprotein of Newcastle disease virus. J Gen Virol. 1986 Dec;67(Pt 12):2685–2694. doi: 10.1099/0022-1317-67-12-2685. [DOI] [PubMed] [Google Scholar]
- Clements M. L., Belshe R. B., King J., Newman F., Westblom T. U., Tierney E. L., London W. T., Murphy B. R. Evaluation of bovine, cold-adapted human, and wild-type human parainfluenza type 3 viruses in adult volunteers and in chimpanzees. J Clin Microbiol. 1991 Jun;29(6):1175–1182. doi: 10.1128/jcm.29.6.1175-1182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelingh K. J., Winter C. C., Murphy B. R., Rice J. M., Kimball P. C., Olmsted R. A., Collins P. L. Conserved epitopes on the hemagglutinin-neuraminidase proteins of human and bovine parainfluenza type 3 viruses: nucleotide sequence analysis of variants selected with monoclonal antibodies. J Virol. 1986 Oct;60(1):90–96. doi: 10.1128/jvi.60.1.90-96.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelingh K. V., Winter C. C. Naturally occurring human parainfluenza type 3 viruses exhibit divergence in amino acid sequence of their fusion protein neutralization epitopes and cleavage sites. J Virol. 1990 Mar;64(3):1329–1334. doi: 10.1128/jvi.64.3.1329-1334.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Mottet G. Homooligomerization of the hemagglutinin-neuraminidase glycoprotein of human parainfluenza virus type 3 occurs before the acquisition of correct intramolecular disulfide bonds and mature immunoreactivity. J Virol. 1991 May;65(5):2362–2371. doi: 10.1128/jvi.65.5.2362-2371.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman P. M., Hoyne P. A., Lawrence M. C. Sequence and structure alignment of paramyxovirus hemagglutinin-neuraminidase with influenza virus neuraminidase. J Virol. 1993 Jun;67(6):2972–2980. doi: 10.1128/jvi.67.6.2972-2980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté M. J., Storey D. G., Kang C. Y., Dimock K. Nucleotide sequence of the coding and flanking regions of the human parainfluenza virus type 3 fusion glycoprotein gene. J Gen Virol. 1987 Apr;68(Pt 4):1003–1010. doi: 10.1099/0022-1317-68-4-1003. [DOI] [PubMed] [Google Scholar]
- Delage G., Brochu P., Pelletier M., Jasmin G., Lapointe N. Giant-cell pneumonia caused by parainfluenza virus. J Pediatr. 1979 Mar;94(3):426–429. doi: 10.1016/s0022-3476(79)80591-0. [DOI] [PubMed] [Google Scholar]
- Dhib-Jalbut S., McFarlin D. E., McFarland H. F. Measles virus-polypeptide specificity of the cytotoxic T-lymphocyte response in multiple sclerosis. J Neuroimmunol. 1989 Feb;21(2-3):205–212. doi: 10.1016/0165-5728(89)90176-8. [DOI] [PubMed] [Google Scholar]
- Dimock K., Rud E. W., Kang C. Y. 3'-Terminal sequence of human parainfluenza virus 3 genomic RNA. Nucleic Acids Res. 1986 Jun 11;14(11):4694–4694. doi: 10.1093/nar/14.11.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A. J., Eglin R. P. Epidemiology of parainfluenza virus type 3 in England and Wales over a ten-year period. Epidemiol Infect. 1989 Jun;102(3):531–535. doi: 10.1017/s0950268800030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebata S. N., Côté M. J., Kang C. Y., Dimock K. The fusion and hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus 3 are both required for fusion. Virology. 1991 Jul;183(1):437–441. doi: 10.1016/0042-6822(91)90162-5. [DOI] [PubMed] [Google Scholar]
- Elango N., Coligan J. E., Jambou R. C., Venkatesan S. Human parainfluenza type 3 virus hemagglutinin-neuraminidase glycoprotein: nucleotide sequence of mRNA and limited amino acid sequence of the purified protein. J Virol. 1986 Feb;57(2):481–489. doi: 10.1128/jvi.57.2.481-489.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris J. A., Aherne W. A., Locke W. S., McQuillin J., Gardner P. S. Sudden and unexpected deaths in infants: histology and virology. Br Med J. 1973 May 26;2(5864):439–442. doi: 10.1136/bmj.2.5864.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank A. L., Couch R. B., Griffis C. A., Baxter B. D. Comparison of different tissue cultures for isolation and quantitation of influenza and parainfluenza viruses. J Clin Microbiol. 1979 Jul;10(1):32–36. doi: 10.1128/jcm.10.1.32-36.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulginiti V. A., Amer J., Eller J. J., Joyner J. W., Sr, Askin P. Parainfluenza virus immunization. IV. Simultaneous immunization with parainfluenza types 1, 2, and 3 aqueous vaccines. Am J Dis Child. 1967 Jul;114(1):26–28. doi: 10.1001/archpedi.1967.02090220032005. [DOI] [PubMed] [Google Scholar]
- Galinski M. S., Mink M. A., Lambert D. M., Wechsler S. L., Pons M. W. Molecular cloning and sequence analysis of the human parainfluenza 3 virus RNA encoding the nucleocapsid protein. Virology. 1986 Mar;149(2):139–151. doi: 10.1016/0042-6822(86)90116-9. [DOI] [PubMed] [Google Scholar]
- Galinski M. S., Mink M. A., Lambert D. M., Wechsler S. L., Pons M. W. Molecular cloning and sequence analysis of the human parainfluenza 3 virus gene encoding the matrix protein. Virology. 1987 Mar;157(1):24–30. doi: 10.1016/0042-6822(87)90309-6. [DOI] [PubMed] [Google Scholar]
- Galinski M. S., Mink M. A., Lambert D. M., Wechsler S. L., Pons M. W. Molecular cloning and sequence analysis of the human parainfluenza 3 virus mRNA encoding the P and C proteins. Virology. 1986 Nov;155(1):46–60. doi: 10.1016/0042-6822(86)90167-4. [DOI] [PubMed] [Google Scholar]
- Galinski M. S., Mink M. A., Pons M. W. Molecular cloning and sequence analysis of the human parainfluenza 3 virus gene encoding the L protein. Virology. 1988 Aug;165(2):499–510. doi: 10.1016/0042-6822(88)90594-6. [DOI] [PubMed] [Google Scholar]
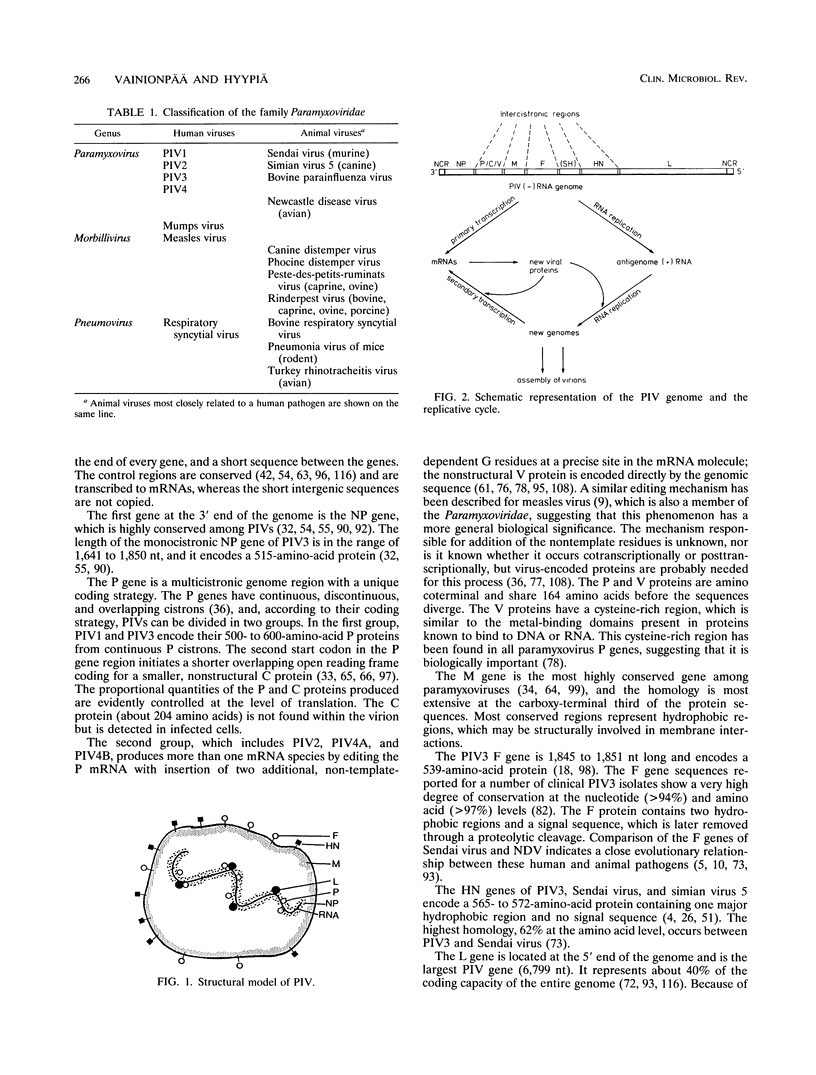
- Galinski M. S. Paramyxoviridae: transcription and replication. Adv Virus Res. 1991;39:129–162. doi: 10.1016/s0065-3527(08)60794-0. [DOI] [PubMed] [Google Scholar]
- Galinski M. S., Troy R. M., Banerjee A. K. RNA editing in the phosphoprotein gene of the human parainfluenza virus type 3. Virology. 1992 Feb;186(2):543–550. doi: 10.1016/0042-6822(92)90020-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlinghouse L. E., Jr, Van Hoosier G. L., Jr, Giddens W. E., Jr Experimental Sendai virus infection in laboratory rats. I. Virus replication and immune response. Lab Anim Sci. 1987 Aug;37(4):437–441. [PubMed] [Google Scholar]
- Giddens W. E., Jr, Van Hoosier G. L., Jr, Garlinghouse L. E., Jr Experimental Sendai virus infection in laboratory rats. II. Pathology and immunohistochemistry. Lab Anim Sci. 1987 Aug;37(4):442–448. [PubMed] [Google Scholar]
- Giuffre R. M., Tovell D. R., Kay C. M., Tyrrell D. L. Evidence for an interaction between the membrane protein of a paramyxovirus and actin. J Virol. 1982 Jun;42(3):963–968. doi: 10.1128/jvi.42.3.963-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen W. P., Fernald G. W. Effect of passive antibody on parainfluenza virus type 3 pneumonia in hamsters. Infect Immun. 1976 Jul;14(1):212–216. doi: 10.1128/iai.14.1.212-216.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen W. P., Frank A. L., Taber L. H., Kasel J. A. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J Infect Dis. 1984 Dec;150(6):851–857. doi: 10.1093/infdis/150.6.851. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Kingsbury D. W. Complete sequences of the intergenic and mRNA start signals in the Sendai virus genome: homologies with the genome of vesicular stomatitis virus. Nucleic Acids Res. 1984 May 11;12(9):3829–3841. doi: 10.1093/nar/12.9.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. B., Douglas R. G., Jr, Simons R. L., Geiman J. M. Interferon production in children with respiratory syncytial, influenza, and parainfluenza virus infections. J Pediatr. 1978 Jul;93(1):28–32. doi: 10.1016/s0022-3476(78)80594-0. [DOI] [PubMed] [Google Scholar]
- Hall S. L., Murphy B. R., van Wyke Coelingh K. L. Protection of cotton rats by immunization with the human parainfluenza virus type 3 fusion (F) glycoprotein expressed on the surface of insect cells infected with a recombinant baculovirus. Vaccine. 1991 Sep;9(9):659–667. doi: 10.1016/0264-410x(91)90192-9. [DOI] [PubMed] [Google Scholar]
- Hall S. L., Stokes A., Tierney E. L., London W. T., Belshe R. B., Newman F. C., Murphy B. R. Cold-passaged human parainfluenza type 3 viruses contain ts and non-ts mutations leading to attenuation in rhesus monkeys. Virus Res. 1992 Mar;22(3):173–184. doi: 10.1016/0168-1702(92)90049-f. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M., Yoshida T., Nishikawa K., Naruse H., Nagai Y. Transcriptive complex of Newcastle disease virus. I. Both L and P proteins are required to constitute an active complex. Virology. 1983 Jul 15;128(1):105–117. doi: 10.1016/0042-6822(83)90322-7. [DOI] [PubMed] [Google Scholar]
- Heilman C. A. From the National Institute of Allergy and Infectious Diseases and the World Health Organization. Respiratory syncytial and parainfluenza viruses. J Infect Dis. 1990 Mar;161(3):402–406. doi: 10.1093/infdis/161.3.402. [DOI] [PubMed] [Google Scholar]
- Henrickson K. J. Monoclonal antibodies to human parainfluenza virus type 1 detect major antigenic changes in clinical isolates. J Infect Dis. 1991 Dec;164(6):1128–1134. doi: 10.1093/infdis/164.6.1128. [DOI] [PubMed] [Google Scholar]
- Henrickson K. J., Savatski L. L. Genetic variation and evolution of human parainfluenza virus type 1 hemagglutinin neuraminidase: analysis of 12 clinical isolates. J Infect Dis. 1992 Nov;166(5):995–1005. doi: 10.1093/infdis/166.5.995. [DOI] [PubMed] [Google Scholar]
- Hiebert S. W., Paterson R. G., Lamb R. A. Hemagglutinin-neuraminidase protein of the paramyxovirus simian virus 5: nucleotide sequence of the mRNA predicts an N-terminal membrane anchor. J Virol. 1985 Apr;54(1):1–6. doi: 10.1128/jvi.54.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio R. M., Bratt M. A. Monoclonal antibodies as functional probes of the HN glycoprotein of Newcastle disease virus: antigenic separation of the hemagglutinating and neuraminidase sites. J Immunol. 1984 Oct;133(4):2215–2219. [PubMed] [Google Scholar]
- Iorio R. M., Bratt M. A. Neutralization of Newcastle disease virus by monoclonal antibodies to the hemagglutinin-neuraminidase glycoprotein: requirement for antibodies to four sites for complete neutralization. J Virol. 1984 Aug;51(2):445–451. doi: 10.1128/jvi.51.2.445-451.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N., Taira H., Omata T., Mizumoto K., Hattori S., Iwasaki K., Kawakita M. Sequence of 2,617 nucleotides from the 3' end of Newcastle disease virus genome RNA and the predicted amino acid sequence of viral NP protein. Nucleic Acids Res. 1986 Aug 26;14(16):6551–6564. doi: 10.1093/nar/14.16.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambou R. C., Elango N., Venkatesan S., Collins P. L. Complete sequence of the major nucleocapsid protein gene of human parainfluenza type 3 virus: comparison with other negative strand viruses. J Gen Virol. 1986 Nov;67(Pt 11):2543–2548. doi: 10.1099/0022-1317-67-11-2543. [DOI] [PubMed] [Google Scholar]
- Josephs S., Kim H. W., Brandt C. D., Parrott R. H. Parainfluenza 3 virus and other common respiratory pathogens in children with human immunodeficiency virus infection. Pediatr Infect Dis J. 1988 Mar;7(3):207–209. [PubMed] [Google Scholar]
- Julkunen I., Hovi T., Seppälä I., Mäkelä O. Immunoglobulin G subclass antibody responses in influenza A and parainfluenza type 1 virus infections. Clin Exp Immunol. 1985 Apr;60(1):130–138. [PMC free article] [PubMed] [Google Scholar]
- Karron R. A., O'Brien K. L., Froehlich J. L., Brown V. A. Molecular epidemiology of a parainfluenza type 3 virus outbreak on a pediatric ward. J Infect Dis. 1993 Jun;167(6):1441–1445. doi: 10.1093/infdis/167.6.1441. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Kondo K., Bando H., Tsurudome M., Kawano M., Nishio M., Ito Y. Sequence analysis of the phosphoprotein (P) genes of human parainfluenza type 4A and 4B viruses and RNA editing at transcript of the P genes: the number of G residues added is imprecise. Virology. 1990 Sep;178(1):321–326. doi: 10.1016/0042-6822(90)90413-l. [DOI] [PubMed] [Google Scholar]
- Korppi M., Leinonen M., Mäkelä P. H., Launiala K. Bacterial involvement in parainfluenza virus infection in children. Scand J Infect Dis. 1990;22(3):307–312. doi: 10.3109/00365549009027052. [DOI] [PubMed] [Google Scholar]
- Kurilla M. G., Stone H. O., Keene J. D. RNA sequence and transcriptional properties of the 3' end of the Newcastle disease virus genome. Virology. 1985 Sep;145(2):203–212. doi: 10.1016/0042-6822(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Luk D., Masters P. S., Sánchez A., Banerjee A. K. Complete nucleotide sequence of the matrix protein mRNA and three intergenic junctions of human parainfluenza virus type 3. Virology. 1987 Jan;156(1):189–192. doi: 10.1016/0042-6822(87)90453-3. [DOI] [PubMed] [Google Scholar]
- Luk D., Sánchez A., Banerjee A. K. Messenger RNA encoding the phosphoprotein (P) gene of human parainfluenza virus 3 is bicistronic. Virology. 1986 Sep;153(2):318–325. doi: 10.1016/0042-6822(86)90036-x. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y., Curran J., Pelet T., Kolakofsky D., Ray R., Compans R. W. The P gene of human parainfluenza virus type 1 encodes P and C proteins but not a cysteine-rich V protein. J Virol. 1991 Jun;65(6):3406–3410. doi: 10.1128/jvi.65.6.3406-3410.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazanec M. B., Lamm M. E., Lyn D., Portner A., Nedrud J. G. Comparison of IgA versus IgG monoclonal antibodies for passive immunization of the murine respiratory tract. Virus Res. 1992 Apr;23(1-2):1–12. doi: 10.1016/0168-1702(92)90063-f. [DOI] [PubMed] [Google Scholar]
- McGinnes L. W., Morrison T. G. The nucleotide sequence of the gene encoding the Newcastle disease virus membrane protein and comparisons of membrane protein sequences. Virology. 1987 Feb;156(2):221–228. doi: 10.1016/0042-6822(87)90401-6. [DOI] [PubMed] [Google Scholar]
- McIntosh K. Pathogenesis of severe acute respiratory infections in the developing world: respiratory syncytial virus and parainfluenza viruses. Rev Infect Dis. 1991 May-Jun;13 (Suppl 6):S492–S500. doi: 10.1093/clinids/13.supplement_6.s492. [DOI] [PubMed] [Google Scholar]
- Millar N. S., Chambers P., Emmerson P. T. Nucleotide sequence analysis of the haemagglutinin-neuraminidase gene of Newcastle disease virus. J Gen Virol. 1986 Sep;67(Pt 9):1917–1927. doi: 10.1099/0022-1317-67-9-1917. [DOI] [PubMed] [Google Scholar]
- Monto A. S., Koopman J. S., Bryan E. R. The Tecumseh Study of Illness. XIV. Occurrence of respiratory viruses, 1976-1981. Am J Epidemiol. 1986 Sep;124(3):359–367. doi: 10.1093/oxfordjournals.aje.a114406. [DOI] [PubMed] [Google Scholar]
- Morgan E. M., Rakestraw K. M. Sequence of the Sendai virus L gene: open reading frames upstream of the main coding region suggest that the gene may be polycistronic. Virology. 1986 Oct 15;154(1):31–40. doi: 10.1016/0042-6822(86)90427-7. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Prince G. A., Collins P. L., Van Wyke Coelingh K., Olmsted R. A., Spriggs M. K., Parrott R. H., Kim H. W., Brandt C. D., Chanock R. M. Current approaches to the development of vaccines effective against parainfluenza and respiratory syncytial viruses. Virus Res. 1988 Aug;11(1):1–15. doi: 10.1016/0168-1702(88)90063-9. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Morishima T., Toyoda T., Miyadai T., Yokochi T., Yoshida T., Nagai Y. Topological and operational delineation of antigenic sites on the HN glycoprotein of Newcastle disease virus and their structural requirements. J Virol. 1986 Dec;60(3):987–993. doi: 10.1128/jvi.60.3.987-993.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgimoto S., Bando H., Kawano M., Okamoto K., Kondo K., Tsurudome M., Nishio M., Ito Y. Sequence analysis of P gene of human parainfluenza type 2 virus: P and cysteine-rich proteins are translated by two mRNAs that differ by two nontemplated G residues. Virology. 1990 Jul;177(1):116–123. doi: 10.1016/0042-6822(90)90465-4. [DOI] [PubMed] [Google Scholar]
- Park K. H., Krystal M. In vivo model for pseudo-templated transcription in Sendai virus. J Virol. 1992 Dec;66(12):7033–7039. doi: 10.1128/jvi.66.12.7033-7039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Scroggs R. A., Metzger D. W. Distinct functions of antigenic sites of the HN glycoprotein of Sendai virus. Virology. 1987 May;158(1):61–68. doi: 10.1016/0042-6822(87)90238-8. [DOI] [PubMed] [Google Scholar]
- Prince G. A., Jenson A. B., Hemming V. G., Murphy B. R., Walsh E. E., Horswood R. L., Chanock R. M. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactiva ted virus. J Virol. 1986 Mar;57(3):721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinoski K., Côté M. J., Kang C. Y., Dimock K. Evolution of the fusion protein gene of human parainfluenza virus 3. Virus Res. 1992 Jan;22(1):55–69. doi: 10.1016/0168-1702(92)90089-r. [DOI] [PubMed] [Google Scholar]
- Prinoski K., Côté M. J., Kang C. Y., Dimock K. Nucleotide sequence of the human parainfluenza virus 3 matrix protein gene. Nucleic Acids Res. 1987 Apr 10;15(7):3182–3182. doi: 10.1093/nar/15.7.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putto A., Ruuskanen O., Meurman O. Fever in respiratory virus infections. Am J Dis Child. 1986 Nov;140(11):1159–1163. doi: 10.1001/archpedi.1986.02140250085040. [DOI] [PubMed] [Google Scholar]
- Rocchi G., Arangio-Ruiz G., Giannini V., Jemolo A. M., Andreoni G., Archetti I. Detection of viraemia in acute respiratory disease of man. Acta Virol. 1970 Sep;14(5):405–407. [PubMed] [Google Scholar]
- Ruuskanen O., Putto A., Sarkkinen H., Meurman O., Irjala K. C-reactive protein in respiratory virus infections. J Pediatr. 1985 Jul;107(1):97–100. doi: 10.1016/s0022-3476(85)80624-7. [DOI] [PubMed] [Google Scholar]
- Ruutu P., Halonen P., Meurman O., Torres C., Paladin F., Yamaoka K., Tupasi T. E. Viral lower respiratory tract infections in Filipino children. J Infect Dis. 1990 Feb;161(2):175–179. doi: 10.1093/infdis/161.2.175. [DOI] [PubMed] [Google Scholar]
- Rydbeck R., Löve A., Norrby E. Protective effects of monoclonal antibodies against parainfluenza virus type 3-induced brain infection in hamsters. J Gen Virol. 1988 May;69(Pt 5):1019–1024. doi: 10.1099/0022-1317-69-5-1019. [DOI] [PubMed] [Google Scholar]
- Rydbeck R., Löve A., Orvell C., Norrby E. Antigenic analysis of human and bovine parainfluenza virus type 3 strains with monoclonal antibodies. J Gen Virol. 1987 Aug;68(Pt 8):2153–2160. doi: 10.1099/0022-1317-68-8-2153. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T., Takao S., Kiyotani K., Fujii Y., Nakayama T., Yoshida T. Expression of the HN, F, NP and M proteins of Sendai virus by recombinant vaccinia viruses and their contribution to protective immunity against Sendai virus infections in mice. J Gen Virol. 1993 Mar;74(Pt 3):479–484. doi: 10.1099/0022-1317-74-3-479. [DOI] [PubMed] [Google Scholar]
- Sarkkinen H. K., Halonen P. E., Salmi A. A. Type-specific detection of parainfluenza viruses by enzyme-immunoassay and radioimmunoassay in nasopharyngeal specimens of patients with acute respiratory disease. J Gen Virol. 1981 Sep;56(Pt 1):49–57. doi: 10.1099/0022-1317-56-1-49. [DOI] [PubMed] [Google Scholar]
- Shioda T., Hidaka Y., Kanda T., Shibuta H., Nomoto A., Iwasaki K. Sequence of 3,687 nucleotides from the 3' end of Sendai virus genome RNA and the predicted amino acid sequences of viral NP, P and C proteins. Nucleic Acids Res. 1983 Nov 11;11(21):7317–7330. doi: 10.1093/nar/11.21.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Iwasaki K., Shibuta H. Determination of the complete nucleotide sequence of the Sendai virus genome RNA and the predicted amino acid sequences of the F, HN and L proteins. Nucleic Acids Res. 1986 Feb 25;14(4):1545–1563. doi: 10.1093/nar/14.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. B., Purcell R. H., Bellanti J. A., Chanock R. M. Protective effect of antibody to parainfluenza type 1 virus. N Engl J Med. 1966 Nov 24;275(21):1145–1152. doi: 10.1056/NEJM196611242752101. [DOI] [PubMed] [Google Scholar]
- Southern J. A., Precious B., Randall R. E. Two nontemplated nucleotide additions are required to generate the P mRNA of parainfluenza virus type 2 since the RNA genome encodes protein V. Virology. 1990 Jul;177(1):388–390. doi: 10.1016/0042-6822(90)90497-f. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Collins P. L. Human parainfluenza virus type 3: messenger RNAs, polypeptide coding assignments, intergenic sequences, and genetic map. J Virol. 1986 Sep;59(3):646–654. doi: 10.1128/jvi.59.3.646-654.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs M. K., Collins P. L. Sequence analysis of the P and C protein genes of human parainfluenza virus type 3: patterns of amino acid sequence homology among paramyxovirus proteins. J Gen Virol. 1986 Dec;67(Pt 12):2705–2719. doi: 10.1099/0022-1317-67-12-2705. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Collins P. L., Tierney E., London W. T., Murphy B. R. Immunization with vaccinia virus recombinants that express the surface glycoproteins of human parainfluenza virus type 3 (PIV3) protects patas monkeys against PIV3 infection. J Virol. 1988 Apr;62(4):1293–1296. doi: 10.1128/jvi.62.4.1293-1296.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs M. K., Johnson P. R., Collins P. L. Sequence analysis of the matrix protein gene of human parainfluenza virus type 3: extensive sequence homology among paramyxoviruses. J Gen Virol. 1987 May;68(Pt 5):1491–1497. doi: 10.1099/0022-1317-68-5-1491. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Murphy B. R., Prince G. A., Olmsted R. A., Collins P. L. Expression of the F and HN glycoproteins of human parainfluenza virus type 3 by recombinant vaccinia viruses: contributions of the individual proteins to host immunity. J Virol. 1987 Nov;61(11):3416–3423. doi: 10.1128/jvi.61.11.3416-3423.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs M. K., Olmsted R. A., Venkatesan S., Coligan J. E., Collins P. L. Fusion glycoprotein of human parainfluenza virus type 3: nucleotide sequence of the gene, direct identification of the cleavage-activation site, and comparison with other paramyxoviruses. Virology. 1986 Jul 15;152(1):241–251. doi: 10.1016/0042-6822(86)90388-0. [DOI] [PubMed] [Google Scholar]
- Stallcup K. C., Raine C. S., Fields B. N. Cytochalasin B inhibits the maturation of measles virus. Virology. 1983 Jan 15;124(1):59–74. doi: 10.1016/0042-6822(83)90290-8. [DOI] [PubMed] [Google Scholar]
- Sánchez A., Banerjee A. K., Furuichi Y., Richardson M. A. Conserved structures among the nucleocapsid proteins of the paramyxoviridae: complete nucleotide sequence of human parainfluenza virus type 3 NP mRNA. Virology. 1986 Jul 15;152(1):171–180. doi: 10.1016/0042-6822(86)90382-x. [DOI] [PubMed] [Google Scholar]
- Tashiro M., Homma M. Protection of mice from wild-type Sendai virus infection by a trypsin-resistant mutant, TR-2. J Virol. 1985 Jan;53(1):228–234. doi: 10.1128/jvi.53.1.228-234.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Curran J., Kolakofsky D. Editing of the Sendai virus P/C mRNA by G insertion occurs during mRNA synthesis via a virus-encoded activity. J Virol. 1990 Jan;64(1):239–246. doi: 10.1128/jvi.64.1.239-246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Kolakofsky D. Modified model for the switch from Sendai virus transcription to replication. J Virol. 1989 May;63(5):1951–1958. doi: 10.1128/jvi.63.5.1951-1958.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorinen T., Meurman O. Enzyme immunoassays for detection of IgG and IgM antibodies to parainfluenza types 1, 2 and 3. J Virol Methods. 1989 Jan;23(1):63–70. doi: 10.1016/0166-0934(89)90090-6. [DOI] [PubMed] [Google Scholar]
- Waris M., Ziegler T., Kivivirta M., Ruuskanen O. Rapid detection of respiratory syncytial virus and influenza A virus in cell cultures by immunoperoxidase staining with monoclonal antibodies. J Clin Microbiol. 1990 Jun;28(6):1159–1162. doi: 10.1128/jcm.28.6.1159-1162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt C. H., Weisdorf D. J., Jordan M. C., Balfour H. H., Jr, Hertz M. I. Parainfluenza virus respiratory infection after bone marrow transplantation. N Engl J Med. 1992 Apr 2;326(14):921–926. doi: 10.1056/NEJM199204023261404. [DOI] [PubMed] [Google Scholar]
- Yanagihara R., McIntosh K. Secretory immunological response in infants and children to parainfluenza virus types 1 and 2. Infect Immun. 1980 Oct;30(1):23–28. doi: 10.1128/iai.30.1.23-28.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusoff K., Millar N. S., Chambers P., Emmerson P. T. Nucleotide sequence analysis of the L gene of Newcastle disease virus: homologies with Sendai and vesicular stomatitis viruses. Nucleic Acids Res. 1987 May 26;15(10):3961–3976. doi: 10.1093/nar/15.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinserling A. Pecularities of lesions in viral and mycoplasma infections of the respiratory tract. Virchows Arch A Pathol Pathol Anat. 1972;356(3):259–273. doi: 10.1007/BF00543159. [DOI] [PubMed] [Google Scholar]
- de Arruda E., Hayden F. G., McAuliffe J. F., de Sousa M. A., Mota S. B., McAuliffe M. I., Geist F. C., Carvalho E. P., Fernandes M. C., Guerrant R. L. Acute respiratory viral infections in ambulatory children of urban northeast Brazil. J Infect Dis. 1991 Aug;164(2):252–258. doi: 10.1093/infdis/164.2.252. [DOI] [PubMed] [Google Scholar]
- de Silva L. M., Cloonan M. J. Brief report: parainfluenza virus type 3 infections: findings in Sydney and some observations on variations in seasonality world-wide. J Med Virol. 1991 Sep;35(1):19–21. doi: 10.1002/jmv.1890350105. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C. C., Jorgensen E. D., Murphy B. R. Antigenic and structural properties of the hemagglutinin-neuraminidase glycoprotein of human parainfluenza virus type 3: sequence analysis of variants selected with monoclonal antibodies which inhibit infectivity, hemagglutination, and neuraminidase activities. J Virol. 1987 May;61(5):1473–1477. doi: 10.1128/jvi.61.5.1473-1477.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C. C., Tierney E. L., Hall S. L., London W. T., Kim H. W., Chanock R. M., Murphy B. R. Antibody responses of humans and nonhuman primates to individual antigenic sites of the hemagglutinin-neuraminidase and fusion glycoproteins after primary infection or reinfection with parainfluenza type 3 virus. J Virol. 1990 Aug;64(8):3833–3843. doi: 10.1128/jvi.64.8.3833-3843.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C., Murphy B. R. Antigenic variation in the hemagglutinin-neuraminidase protein of human parainfluenza type 3 virus. Virology. 1985 Jun;143(2):569–582. doi: 10.1016/0042-6822(85)90395-2. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K., Tierney E. L. Antigenic and functional organization of human parainfluenza virus type 3 fusion glycoprotein. J Virol. 1989 Jan;63(1):375–382. doi: 10.1128/jvi.63.1.375-382.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


