Abstract
Objective
APOE ε4 status has been associated with greater cortical amyloid deposition whereas exercise has been associated with less in cognitively normal adults. The primary objective here was to examine whether physical exercise moderates the association between APOE genotype and amyloid deposition in cognitively normal adults.
Method
APOE genotyping and a questionnaire on physical exercise engagement over the last decade were obtained in conjunction with cerebrospinal fluid (CSF) samples and amyloid imaging with PET-PIB. Participants were classified as either low or high exercisers based on exercise guidelines of the American Heart Association.
Subjects
201 cognitively normal adults (135 females) aged 45–88 were recruited from the Knight Alzheimer Disease Research Center at Washington University. CSF samples were collected from 165 participants. Amyloid imaging was performed on 163 participants.
Results
APOE ε4 carriers evidenced higher PIB binding (p<.001) and lower CSF Aβ42 levels (p<.001) than non-carriers. Our previous findings of higher PIB binding (p=.005) and lower CSF Aβ42 levels (p=.009) in more sedentary individuals were replicated. Most importantly, we observed a novel interaction between APOE status and exercise engagement for PIB binding (p=.008) such that a more sedentary lifestyle was significantly associated with higher PIB binding for ε4 carriers (p=.013) but not for ε4 non-carriers (p=.208). All findings remained significant after controlling for age, gender, education, hypertension, body mass index, diabetes, heart problems, history of depression and interval between assessments.
Conclusion
Collectively, these results suggest that cognitively normal sedentary APOE ε4+ individuals may be at augmented risk for cerebral amyloid deposition.
INTRODUCTION
Presence of an APOE ε4 allele is the most established genetic risk factor for Alzheimer’s disease (AD), with a greater percentage of AD individuals having an ε4 allele in comparison to the general population (1,2). In addition, age of dementia onset is earlier (2,3) and rate of cognitive decline may be greater in AD ε4 carriers compared to non-carriers (e.g., 4–6, but see 7,8). Even in cognitively normal middle-aged and older adults, APOE ε4 status has been associated with reduced cognitive performance (9) and greater cognitive decline (10,11). More recently, it has been demonstrated that cognitively normal adults with an APOE ε4 allele evidence greater cortical amyloid deposition as indicated by increased binding of the amyloid imaging agent, Pittsburgh Compound B (PIB), and lowered Aβ42 in cerebrospinal fluid (CSF) (12–14).
Potentially modifiable lifestyle practices, such as engagement in physical exercise, may protect against cognitive decline. Mechanisms through which exercise may confer benefits include enhanced neurogenesis and angiogenesis, increased release of growth factors (e.g., brain-derived neurotrophic factor) that promote neuronal plasticity, and lowering of cardiovascular risk factors (16–18). An inverse association between physical activity and cognitive decline and dementia generally is supported, although there have been inconsistent findings (19,20). In addition, there have been mixed findings on the benefits of exercise in transgenic AD mouse models (21,22). We recently demonstrated, however, lower amyloid deposition, as estimated with PIB and CSF Aβ42, in cognitively normal individuals who exercised regularly (12).
It has been suggested that APOE status may modify associations between lifestyle factors such as exercise engagement and risk of cognitive decline and dementia (23). Several examinations of potential interactive effects of APOE status and physical activity on cognitive decline have yielded mixed findings, with reports of greater beneficial effects of exercise for ε4 carriers (24–29), no difference between ε4 carriers and non-carriers in exercise effects (30–32), and greater benefits for ε4 non-carriers (33). By contrast, interactive effects of APOE status and physical exercise on amyloid deposition have not been fully investigated. The goal of the current study was to assess whether exercise moderates the effects of APOE status on amyloid deposition in a cohort of cognitively normal older adults. The primary hypotheses were that a) APOE ε4 status would be associated with greater amyloid deposition; b) exercise engagement would be associated with lower amyloid deposition; and c) a sedentary lifestyle would have a greater effect on amyloid deposition in APOE ε4+ individuals.
MATERIALS AND METHODS
Participants
Middle-aged and older adults, age 45–88 years, were recruited from the Knight Alzheimer Disease Research Center (ADRC) at Washington University. A subsample was recruited as part of an ongoing study at the ADRC on adult children with parents who were diagnosed with AD (34). Based on the Washington University Clinical Dementia Rating (CDR), a validated and reliable interview-based measurement sensitive in detecting the earliest stages of dementia, all participants were classified as cognitively normal (CDR=0) (35,36). Clinical assessment included a health history that determined the presence or history of diabetes, hypertension, neurological illness or injury, depression or cardiovascular compromise (e.g., history of angioplasty, atrial fibrillation). Height and weight were also obtained and used to calculate body mass index. Exclusionary criteria were major neurologic illnesses or injury (e.g., stroke, cerebrovascular disease, Parkinson disease).
Cerebrospinal fluid (CSF) samples were collected from 165 participants. Amyloid imaging with PIB was obtained in 163 participants. An exercise engagement questionnaire was administered to all participants. All participants consented to participation in accordance with guidelines of the Washington University Human Research Protection Office. Exercise and structural data (n=52; 37) and amyloid data (n=69; 12) from some of these participants have been published previously.
Measurement of physical exercise engagement
Validity
A validated questionnaire assessing history of walking, running and jogging (WRJ) activity for the past 10 years was used to estimate exercise engagement (38). The measure was significantly correlated with cardiorespiratory fitness measured via treadmill test in a sample of 5063 individuals aged 18–80 years. Stable correlations were observed between retrospective self-report of activity for a particular year and aerobic fitness for that year across 10 1-year assessment periods, suggesting participants across examined age range were capable of relatively accurate self-report over extended time span. Correlations were similar with and without controlling for age, suggesting age was not a significant contributor to observed associations.
Procedure
The questionnaire was administered by telephone, and participants reported number of months/year, number of workouts/week, average number of miles/workout, and average time/mile for each year in which they engaged in WRJ activity for the preceding 10 years. A physical exercise engagement score was derived for each participant by estimating metabolic equivalent (MET) values using the compendium of physical activities (38). The index of exercise engagement was average MET hours/week over the past 10 years.
The distribution of exercise engagement scores was zero-inflated and heavily skewed. Transformations (e.g. logarithmic) could not resolve these distributional issues. Therefore, rather than treating exercise engagement score as a continuous variable, participants were categorized into low and high exercise engagement groups based on whether reported exercise levels were at or above 7.5 MET-hours/week (30 minutes of moderate exercise 5 days/week) recommended by the American Heart Association (AHA) (12).
Cerebrospinal fluid collection, processing, and biomarker measurement
CSF free from blood contamination was collected by lumbar puncture in polypropylene tubes at 8:00 AM after overnight fasting as described previously (39). Samples were gently inverted to avoid gradient effects, briefly centrifuged at low speed to pellet any cellular elements, and aliquoted (500µl) into polypropylene tubes before freezing at −84°C. Analyses for Aβ42 were completed using commercial enzyme-linked immunosorbant assay (INNOTEST; Innogenetics, Ghent, Belgium). Samples were continuously kept on ice with only a single thaw after initial freezing before assaying.
PET-PIB imaging
In vivo amyloid imaging via positron emission tomography (PET) with PIB ([N-methyl-[11C]]2-(4'-methylaminophenyl)-6-hydroxybenzothiazole) was performed as described previously (40). Approximately 12 mCi of [11C]PIB was administered intravenously simultaneous with initiation of a 60-minute dynamic PET scan in three-dimensional mode. Measured attenuation factors and a ramp filter were used to reconstruct dynamic PET images. Three-dimensional regions-of-interest were then created for each participant based on their individual MRI scans (T1-weighted 1×1×1.25mm MPRAGE). A binding potential (BP) for each region-of-interest was calculated to express regional binding values in a manner proportional to number of binding sites. BP values from prefrontal cortex, gyrus rectus, lateral temporal, and precuneus regions-of-interest were averaged to calculate a mean cortical binding potential (MCBP) value based on brain regions known to have high PIB uptake among participants with AD (40). This derived MCBP value has been shown to correlate inversely with CSF Aβ42 (39) and exercise engagement (12), predict progression from cognitively normal status to symptomatic AD (41), and be associated with disrupted functional connectivity of the default mode (42).
APOE genotyping
TaqMan assays (Applied Biosystems, Foster City, USA) for both rs429358 (ABI#C_3084793_20) and rs7412 (ABI#C_904973_10) were used for APOE genotyping. Allele calling was performed using the allelic discrimination analysis module of ABI Sequence Detection Software. Positive controls for each of six possible APOE genotypes were included on the genotyping plate. Individuals were then classified as ε4+ (44, 34, 24) or ε4− (33, 23, 22).
Timing of assessments
Clinical assessment was within ±6.0 months (SD=12.4) of the PET scan and ±2.9 months (SD=3.9) of the CSF assessment. The interval between clinical and exercise assessments was ±3.4 months (SD=7.7) for PIB sample and ±3.3 months (SD=7.6) for CSF sample. Individuals were CDR 0 at all assessments.
Exercise assessment was within ±0.92 years (SD=1.3) of the PET scan. For 105/163 individuals, the exercise assessment was subsequent to the PET scan and thus captured exercise behavior during the time of the PET scan. For the remaining 58 individuals exercise assessment was prior to the PET scan by an average of −.33 years (SD=.33). Thus, for these individuals reported exercise behavior would have occurred almost exclusively during a time period prior to the PET scan.
Exercise assessment was within ±1.6 years (SD=2.2) of CSF assessment. For 113/165 individuals, exercise assessment was subsequent to CSF assessment and thus captured exercise behavior during the time of CSF assessment. For the other 52 individuals exercise assessment was prior to CSF assessment by an average of −.28 years (SD=.34). Thus, for these individuals reported exercise behavior would have occurred almost exclusively during a time period prior to CSF assessment.
Statistical analyses
All analyses were conducted using SPSS/PASW 17.0 (SPSS Inc., Chicago, Illinois). We first tested for group differences (i.e., Exercise Group, APOE status) in demographic and health variables using Student’s t tests for continuous variables and Fisher's exact test for dichotomous variables (see Tables 1 and 2 for demographic and health data).
Table 1.
Participant characteristics for APOE groups.
| PET Sample | CSF Sample | |||
|---|---|---|---|---|
| APOE ε4+ | APOE ε4− | APOE ε4+ | APOE ε4− | |
| N | 52 | 111 | 56 | 109 |
| Mean age (SD), years | 65 (10) | 68 (10)* | 64 (9) | 68 (10)* |
| Gender (M/F) | 14/38 | 38/73 | 16/40 | 37/72 |
| Mean education (SD), years | 16 (3) | 16 (3) | 16 (3) | 16 (3) |
| Mean BMI (SD) | 28 (6) | 28 (6) | 27 (5) | 29 (6)* |
| Diabetes (−/+) | 49/3 | 100/11 | 52/4 | 97/12 |
| Mean MMSE (SD) | 29.1 (1.5) | 29.2 (.97) | 29.3 (1.3) | 29.1 (1.1) |
| Hypertension (−/+) | 35/17 | 61/50 | 40/16 | 58/51* |
| Heart problems (−/+) | 45/7 | 102/9 | 48/8 | 99/11 |
| Depression (−/+) | 43/9 | 97/14 | 47/9 | 96/13 |
| Mean GDS (SD) | 1.1 (1.4) | 1.1 (1.6) | 1.0 (1.4) | 1.1 (1.5) |
| AHA exercise group (−/+) | 39/13 | 86/25 | 43/13 | 87/22 |
| Mean Exercise Score (SD), Met-hrs/wk | 5.07 (7.06) | 5.21 (7.46) | 4.69 (6.45) | 4.52 (6.13) |
| Mean MCBP (SD) | .16 (.26) | .03 (.10)** | -- | -- |
| Mean Aβ42 (SD), pg/mL | -- | -- | 536 (181) | 692 (217)** |
p < .05;
p < .01.
MMSE=Mini-Mental State Exam; BMI=Body Mass Index; GDS=Geriatric Depression Scale. AHA exercise group represents whether individuals met the American Heart Association’s recommended exercise levels (see text for details) with + indicating those who met recommendations.
Table 2.
Participant characteristics for AHA exercise groups.
| PIB sample | CSF sample | |||
|---|---|---|---|---|
| Exercisers | Non-exercisers | Exercisers | Non-exercisers | |
| N | 38 | 125 | 35 | 130 |
| Mean age (SD), years | 65 (9) | 67 (10) | 66 (9) | 67 (10) |
| Gender (M/F) | 13/25 | 39/86 | 13/22 | 40/90 |
| Mean education (SD), years | 17 (3) | 16 (3)* | 16 (3) | 16 (3) |
| Mean BMI (SD) | 27 (5) | 29 (6) | 26 (4) | 29 (6)** |
| Mean MMSE (SD) | 29.4 (.94) | 29.1 (1.2) | 29.3 (.99) | 29.2 (1.2) |
| Diabetes (−/+) | 36/2 | 113/12 | 34/1 | 115/15 |
| Hypertension (−/+) | 27/11 | 69/56* | 26/9 | 72/58* |
| Heart problems (−/+) | 36/2 | 111/14 | 33/2 | 113/17 |
| Depression (−/+) | 34/4 | 106/19 | 30/5 | 113/17 |
| Mean GDS score (SD) | 1.1 (1.6) | 1.1 (1.5) | 1.3 (1.8) | 1.0 (1.5) |
| APOE ε4 (−/+) | 25/13 | 86/39 | 22/13 | 87/43 |
| Mean Exercise (SD), Met-hrs/wk | 14.95 (8.83) | 2.12 (2.28)** | 13.88 (7.97) | 2.14 (2.17)** |
| Mean MCBP (SD) | .01 (.06) | .09 (.20)** | -- | -- |
| Mean Aβ42 (SD), pg/mL | -- | -- | 710 (229) | 620 (212)** |
p < .05;
p<.01.
MMSE=Mini-Mental Status Exam; BMI=Body Mass Index; GDS=Geriatric Depression Scale; Exercise groups are based on the American Heart Association’s recommended exercise levels (see text for details).
We used hierarchical multiple regression (using ordinary least squares (OLS)) to examine the unique variance accounted for by the Exercise Group×APOE status interaction above and beyond the main effects (Exercise Group; APOE status) for each of the separate estimates of amyloid deposition. There were separate regression models for each complete cohort (i.e., MCBP and CSF Aβ42) with no missing data points. Exercise Group and APOE status main effects were entered in one step, and the Exercise Group×APOE status interaction term was entered in the next step. Importantly, a significant interaction indicates that Exercise Group exerts a moderating effect on the influence of APOE status on amyloid deposition above and beyond the influence of either Exercise Group or APOE status alone. All statistical significance tests were 1-tailed as we had a priori directional hypotheses regarding main and interactive effects, and α=0.05.
RESULTS
MCBP cohort
There were significant APOE group differences in age (p=.027) and a non-significant trend for a group difference in hypertension (p=.068). In addition, there were significant Exercise group differences in education (p=.043) and hypertension (p=.041) and a non-significant trend for body mass index (BMI) (p=.091). Lastly, there was a significant correlation between MCBP and age (p=.020). No other associations with demographic or health variables reached or approached significance (all ps>.145). Thus, age, education, BMI and hypertension were included as covariates in the first step in the model examining MCBP. As there was evidence of mild-to-moderate heteroscedasticity in the initial OLS model (White’s test=43.20, p=.033), robust regression was conducted and is reported below (results were equivalent for OLS and robust regression).
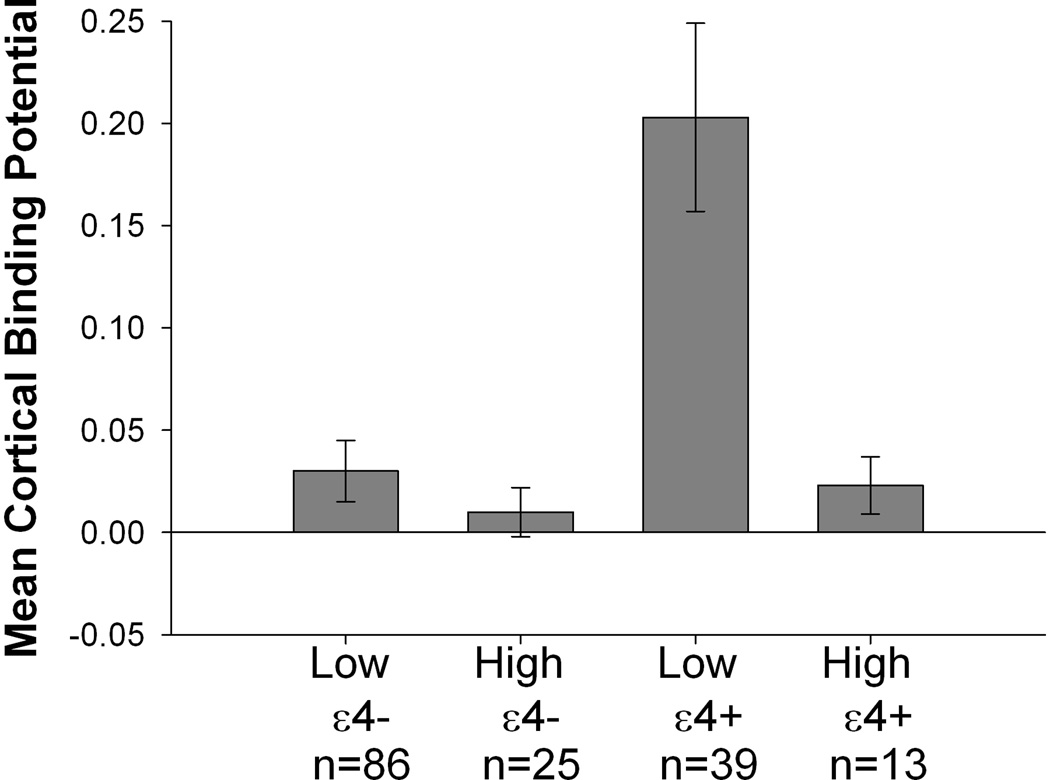
In the regression model examining MCBP (see Table 3), there were significant Exercise Group (p<.001) and APOE status (p<.001) differences. High exercise individuals evidenced lower MCBP compared to low exercise individuals (Mean difference=−.079; 95%CI=−.124; −.034) and ε4+ individuals evidenced higher MCBP compared to ε4− individuals (Mean difference=.141; 95%CI=.071;.211). There was a significant Exercise Group×APOE status interaction (p=.002; see Figure 1) that reflected a greater exercise effect on MCBP in ε4+ individuals (Mean difference between Exercise Groups=.183; 95%CI=−.308; −.059) compared to ε4− individuals (Mean difference between Exercise Groups=−.019; 95%CI=−.052;.014). Notably, results were similar when other potentially confounding demographic and health variables (i.e., gender, diabetes, heart problems, history of depression), and the delay between the PET scan and exercise assessment, were additionally included as covariates (Exercise Group: p<.001; APOE status: p<.001; Exercise Group×APOE status interaction: p=.004).
Table 3.
Regression results for MCBP cohort
| Effect | ΔR2 | F-value (DF) | Unstd B (95%CI) | Std B | p-value |
|---|---|---|---|---|---|
| Main Effects Step: | .167 | 16.707 (2, 156) | <.001 | ||
| AHA Exercise Group | −.079 (−.124; −.034) | −.188 | <.001 | ||
| APOE status | .141 (.071; .211) | .371 | <.001 | ||
| Interaction Step: | .029 | 6.021 (1, 155) | .002 | ||
| AHA Exercise Group × APOE status | −.154 (−.257; −.050) | −.236 | .002 |
Figure 1.
Association between APOE status and exercise engagement for MCBP. There was a significant APOE status × exercise engagement interaction such that a more sedentary lifestyle was significantly associated with higher PIB for ε4 carriers but not for ε4 non-carriers. See text for details.
CSF Aβ42 cohort
There were significant APOE group differences in age (p=.015), BMI (p=.014), and hypertension (p=.012) and significant Exercise group differences in BMI (p<.001) and hypertension (p=.022). No other associations approached or reached significance. Thus, age, BMI and hypertension were included as covariates in the first step in the model examining CSF Aβ42. There was no evidence of heteroscedasticity (White’s test=21.11, p=.391), so OLS regression results are reported.
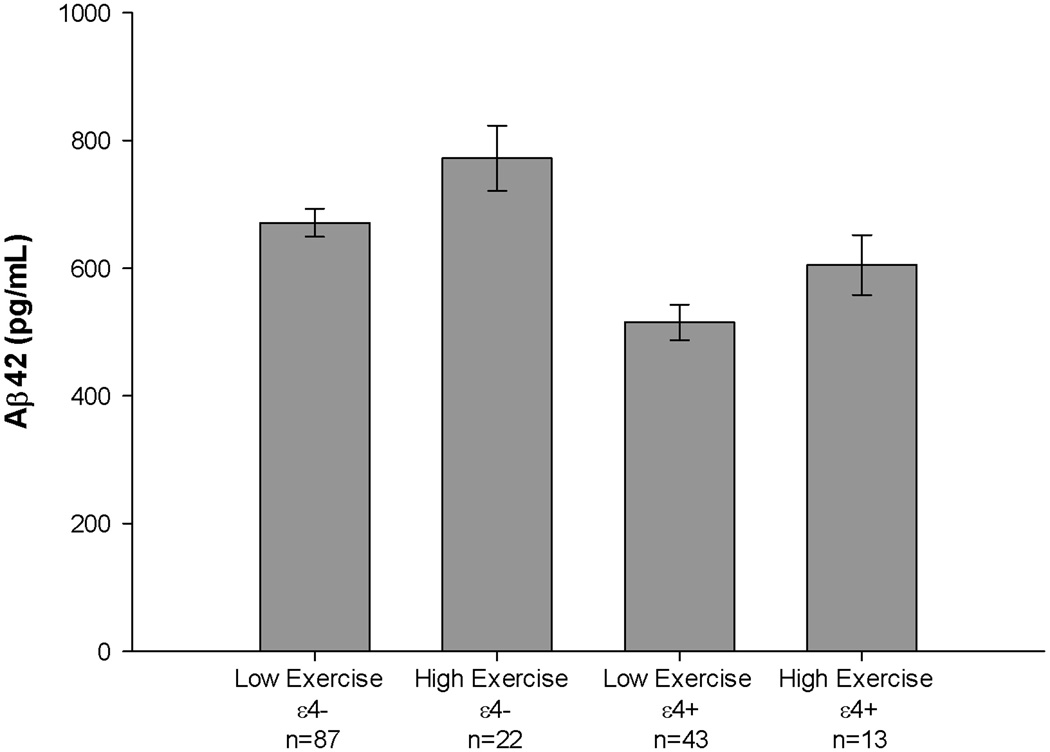
In the regression model examining CSF Aβ42 (see Table 4), there were significant Exercise Group (p=.005) and APOE status (p<.001) differences (see Figure 2). Low exercise individuals evidenced lower CSF Aβ42 compared to high exercise individuals (Mean difference=96.08; 95%CI=17.21; 174.95) and ε4+ individuals also evidenced lower CSF Aβ42 compared to ε4− individuals (Mean difference=−159.18; 95% CI=− 227.90; −90.45). However, the Exercise Group×APOE status interaction was not significant (p=.408). Thus, the exercise effect on CSF Aβ42 did not differ between ε4+ individuals (Mean difference between Exercise Groups=80.11; 95%CI=−−41.368;201.577) and ε4− individuals (Mean difference between Exercise Groups=102.48; 95%C =−2.354; 207.320). Notably, results were similar when other potentially confounding demographic and health variables (i.e., gender, education, diabetes, heart problems, history of depression), and the delay between the LP and exercise assessment, were included as covariates (Exercise Group: p=.011; APOE status: p<.001; Exercise Group×APOE status interaction: p=.365).
Table 4.
Regression results for CSF Aβ42 cohort
| Effect | ΔR2 | F-value (DF) | Unstd B (95%CI) | Std B | p- |
|---|---|---|---|---|---|
| Main Effects Step: | .144 | 13.460 (2, 158) | <.0001 | ||
| AHA Exercise Group | 96.079 (17.208; 174.951) | .181 | .008 | ||
| APOE status | −159.175 (−227.903; −90.448) | −.346 | <.0001 | ||
| Interaction Step: | .000 | .055 (1, 157) | .408 | ||
| AHA Exercise Group × APOE status | −19.078 (−179.724; 141.568) | −.024 | .408 |
Figure 2.
Association between APOE status and exercise engagement for CSF Aβ42. APOE ε4 carriers evidenced lower CSF Aβ42. Sedentary individuals evidenced lower CSF Aβ42. The APOE status × exercise engagement interaction was not significant. See text for details.
DISCUSSION
APOE status is associated with increased risk of cognitive decline and elevated amyloid deposition (4–6,10–15). In contrast, exercise engagement has been associated with reduced risk of cognitive decline (19,20) and lower levels of amyloid deposition (12). In the current investigation we sought to replicate effects of APOE genotype and exercise engagement on amyloid deposition and further examine whether exercise moderates effects of APOE genotype on amyloid deposition.
Consistent with several past findings (12–15), the presence of an APOE ε4 allele was associated with elevated amyloid deposition as assessed with PET-PIB. In addition, we observed lower PIB binding for individuals who exercised at or above levels recommended by AHA, similar to our previous study of 69 individuals (12). However, here we report the novel finding of a significant interaction between APOE and exercise engagement for cerebral amyloid burden. Specifically, a significant effect of exercise engagement was present for APOE ε4 carriers but not for non-carriers, with sedentary ε4+ individuals evidencing greater MCBP compared to active ε4+ individuals. In fact, post-hoc analyses indicate that the magnitude of MCBP was equivalent between active ε4+ individuals and all ε4− individuals (t=.07; p=.414), and between active ε4+ individuals and active ε4− individuals (t=−.722; p=.238). Collectively, these findings suggest that the combination of ε4+ status and a sedentary lifestyle may place individuals at augmented risk for amyloid deposition, as assessed via PET-PIB. This result remained robust after controlling for significant group differences in demographic and health variables, and for additional health variables that did not differ between groups but may have potentially contributed to observed findings.
A greater effect of exercise engagement in APOE ε4 carriers is consistent with and extends existing data demonstrating increased risk of cognitive decline and dementia in sedentary ε4+ individuals (24–29; but see 30–33). Greater exercise-related improvements in cognitive performance and markers of hippocampal plasticity in APOE ε4 transgenic mice is also supportive of differential benefits for ε4 carriers (43). APOE ε4 appears to be associated with reduced neuronal plasticity (44,45), and it has been argued that this inherent neurophysiological disadvantage makes beneficial lifestyle factors, such as exercise, preferentially important for ε4 carriers (28,43). The MCBP findings support the idea that a physically active lifestyle may allow ε4 carriers to experience brain amyloid levels equivalent to ε4− individuals. Although mechanisms through which exercise may influence amyloid deposition remain unclear, there may be both relatively direct effects on amyloid precursor protein metabolism (21,46,47) and indirect effects through influences on neurotrophic factors, neuroinflammation, cerebrovascular functioning or glucose metabolism (46–48).
In terms of CSF Aβ42, we again observed that the APOE ε4 allele had a negative influence, with ε4+ individuals evidencing lower CSF Aβ42, consistent with past reports (12–15). Exercise engagement was again associated with a more beneficial profile such that those who met AHA recommendations evidenced higher CSF Aβ42. However, there was no interaction between APOE status and exercise engagement for CSF Aβ42. Unlike for MCBP, sedentary ε4+ individuals did not evidence a significantly greater effect of exercise engagement on CSF Aβ42 compared to active ε4+ individuals.
The reason for the discrepancy between MCBP and CSF Aβ42 is uncertain. The two largely reflect complimentary estimates of the same process of amyloid plaque development in the brain and are strongly associated (e.g., 38,49). However, PET-PIB identifies only fibrillar Aβ whereas CSF Aβ42 levels may reflect nonfibrillar Aβ species as well (50–52). In addition, while CSF Aβ42 estimates could conceivably reflect amyloid deposition in various regions of the brain, the MCBP estimate represents select regions of high amyloid deposition, and this difference may contribute to our findings. It is also possible that the current sample size was insufficient to detect differences in exercise effects on APOE groups in terms of CSF Aβ42.
The current investigation provides support for an association between exercise engagement and amyloid deposition, with stronger associations in ε4+ individuals in terms of MCBP. However, several limitations must be considered. It is conceivable that confounds not assessed here (e.g., socioeconomic status, ability to engage in physical exercise, personality) may have influenced our results, and are thus relevant to examine in future investigations. Inferences about causal flow between exercise and amyloid deposition are not possible given the cross-sectional design. While it is possible that subclinical dementia may have subtly influenced exercise engagement or reporting of exercise in ε4+ individuals, neither the proportion of individuals meeting AHA-recommended levels nor mean exercise levels differed between APOE groups. Another potential concern is use of a self-report measure of exercise engagement and use of phone administration in contrast to the in-person interview used in the validation study (37). Furthermore, the measure is significantly, but not perfectly, correlated with cardiorespiratory fitness. Although the validation sample included older adults and the magnitude of association with cardiorespiratory fitness was similar with and without controlling for age, the measure may still be limited by older adults’ ability to accurately recall and report their exercise behavior over an extended time span.
In summary, our findings suggest that exercise at levels recommended by the AHA may be particularly beneficial for cognitively normal ε4+ individuals in reducing risk of brain amyloid deposition. Longitudinal investigations and intervention studies that incorporate measures through which exercise may influence amyloid deposition are warranted to address causality and mechanisms.
ACKNOWLEDGEMENTS
We thank our lumbar puncture physicians for obtaining CSF samples, Ms. Aarti Shah and Mr. Matt Amos for processing and analyzing the CSF samples, and Ms. Sushila Sathyan for scheduling the lumbar punctures. We thank the Knight Alzheimer Disease Research Center Genetics Core for APOE genotyping. This work was supported by NIH grants P50 AG05861, P01 AG03991 and P01 AG026276. J.M.B. was supported by NIA 5T32AG00030.
Footnotes
The authors and their institution have no conflicts of interest related to this work.
REFERENCES
- 1.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 2.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 3.Blacker D, Haines JL, Rodes L, et al. ApoE-4 and age at onset of Alzheimer's disease: the NIMH genetics initiative. Neurology. 1997;48:139–417. doi: 10.1212/wnl.48.1.139. [DOI] [PubMed] [Google Scholar]
- 4.Brayne C, Harrington CR, Wischik CM, et al. Apolipoprotein E genotype in the prediction of cognitive decline and dementia in a prospectively studied elderly population. Dementia. 1996;7:169–174. doi: 10.1159/000106873. [DOI] [PubMed] [Google Scholar]
- 5.Craft S, Teri L, Edland SD, et al. Accelerated decline in apolipoprotein E-epsilon4 homozygotes with Alzheimer's disease. Neurology. 1998;51:149–153. doi: 10.1212/wnl.51.1.149. [DOI] [PubMed] [Google Scholar]
- 6.Martins CA, Oulhaj A, de Jager CA, Williams JH. APOE alleles predict the rate of cognitive decline in Alzheimer disease: a nonlinear model. Neurology. 2005;65:1888–1893. doi: 10.1212/01.wnl.0000188871.74093.12. [DOI] [PubMed] [Google Scholar]
- 7.Hoyt BD, Massman PJ, Schatschneider C, Cooke N, Doody RS. Individual growth curve analysis of APOE epsilon 4-associated cognitive decline in Alzheimer disease. Arch Neurol. 2005;62:454–459. doi: 10.1001/archneur.62.3.454. [DOI] [PubMed] [Google Scholar]
- 8.Kleiman T, Zdanys K, Black B, et al. Apolipoprotein E epsilon4 allele is unrelated to cognitive or functional decline in Alzheimer's disease: retrospective and prospective analysis. Dement Geriatr Cogn Disord. 2006;22:73–82. doi: 10.1159/000093316. [DOI] [PubMed] [Google Scholar]
- 9.Small BJ, Rosnick CB, Fratiglioni L, Bäckman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging. 2004;19:592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- 10.Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361:255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knopman DS, Mosley TH, Catellier DJ, Coker LH. Atherosclerosis Risk in Communities Study Brain MRI Study. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dement. 2009:207–214. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Liang KY, Mintun MA, Fagan AM, et al. Exercise and Alzheimer's disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68:311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunderland T, Mirza N, Putnam KT, et al. Cerebrospinal fluid beta-amyloid1–42 and tau in control subjects at risk for Alzheimer's disease: the effect of APOE epsilon4 allele. Biol Psychiatry. 2004;56:670–676. doi: 10.1016/j.biopsych.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Vemuri P, Wiste HJ, Weigand SD, et al. Alzheimer's Disease Neuroimaging Initiative. Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol. 2010;67:308–316. doi: 10.1002/ana.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 18.van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y, Back JH, Kim J, et al. Systematic review of health behavioral risks and cognitive health in older adults. Int Psychogeriatr. 2010;22:174–187. doi: 10.1017/S1041610209991189. [DOI] [PubMed] [Google Scholar]
- 20.Plassman BL, Williams JW, Jr, Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010;153:182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- 21.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietropaolo S, Sun Y, Li R, Brana C, Feldon J, Yee BK. The impact of voluntary exercise on mental health in rodents: a neuroplasticity perspective. Behav Brain Res. 2008;192:42–60. doi: 10.1016/j.bbr.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Low LF, Yap MH, Brodaty H. Will testing for apolipoprotein E assist in tailoring dementia risk reduction? A review. Neurosci Biobehav Rev. 2010;34:408–437. doi: 10.1016/j.neubiorev.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Deeny SP, Poeppel D, Zimmerman JB, et al. Exercise, APOE, and working memory: MEG and behavioral evidence for benefit of exercise in epsilon4 carriers. Biol Psychol. 2008;78:179–187. doi: 10.1016/j.biopsycho.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Etnier JL, Caselli RJ, Reiman EM, Alexander GE, Sibley BA, Tessier D, McLemore EC. Cognitive performance in older women relative to ApoE-epsilon4 genotype and aerobic fitness. Med Sci Sports Exerc. 2007;39:199–207. doi: 10.1249/01.mss.0000239399.85955.5e. [DOI] [PubMed] [Google Scholar]
- 26.Kivipelto M, Rovio S, Ngandu T, et al. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med. 2008;12:2762–2771. doi: 10.1111/j.1582-4934.2008.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niti M, Yap KB, Kua EH, Tan CH, Ng TP. Physical, social and productive leisure activities, cognitive decline and interaction with APOE-epsilon 4 genotype in Chinese older adults. Int Psychogeriatr. 2008;20:237–251. doi: 10.1017/S1041610207006655. [DOI] [PubMed] [Google Scholar]
- 28.Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4:705–4711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 29.Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33:772–777. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 31.Sabia S, Kivimaki M, Kumari M, Shipley MJ, Singh-Manoux A. Effect of Apolipoprotein E epsilon4 on the association between health behaviors and cognitive function in late midlife. Mol Neurodegener. 2010;5:23–29. doi: 10.1186/1750-1326-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taaffe DR, Irie F, Masaki KH, et al. Physical activity, physical function, and incident dementia in elderly men: the Honolulu-Asia Aging Study. J Gerontol A Biol Sci Med Sci. 2008;63:529–535. doi: 10.1093/gerona/63.5.529. [DOI] [PubMed] [Google Scholar]
- 33.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 34.Xiong C, Roe C, Buckles V, et al. Family history of Alzheimer’s disease independently influences biomarker abnormalities in the Adult Children Study. Arch Neurol. doi: 10.1001/archneurol.2011.208. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 36.Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67:467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- 37.Bugg JM, Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowles HR, FitzGerald SJ, Morrow JR, Jr, Jackson AW, Blair SN. Construct validity of self-reported historical physical activity. Am J Epidemiol. 2004;160:279–286. doi: 10.1093/aje/kwh209. [DOI] [PubMed] [Google Scholar]
- 39.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 40.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 41.Morris JC, Roe CM, Grant EA, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66:1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheline YI, Raichle ME, Snyder AZ, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67:584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5:287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Y. Mechanisms linking apolipoprotein E isoforms with cardiovascular and neurological diseases. Curr Opin Lipidol. 2010;21:337–345. doi: 10.1097/MOL.0b013e32833af368. [DOI] [PubMed] [Google Scholar]
- 45.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 46.Nation DA, Hong S, Jak AJ, Delano-Wood L, Mills PJ, Bondi MW. Stress, exercise, and Alzheimer’s disease: A neurovascular pathway. Med Hypotheses. 2011;76:847–854. doi: 10.1016/j.mehy.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radak Z, Hart N, Sarga L, et al. Exercise plays a preventive role against Alzheimer’s disease. J Alzheimers Dis. 2010;20:777–783. doi: 10.3233/JAD-2010-091531. [DOI] [PubMed] [Google Scholar]
- 48.Um HS, Kang EB, Leem YH, et al. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer’s disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22:529–539. [PubMed] [Google Scholar]
- 49.Tolboom N, van der Flier WM, Yaqub M, et al. Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med. 2009;50:1464–1470. doi: 10.2967/jnumed.109.064360. [DOI] [PubMed] [Google Scholar]
- 50.Cairns NJ, Ikonomovic MD, Benzinger T, et al. Absence of Pittsburgh Compond B detection of cerebral amyloid beta in a patient with clinical, cognitive, and cerebrospinal fluid markers of Alzheimer disease: a case report. Arch Neurol. 2009;66:1557–1562. doi: 10.1001/archneurol.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fagan AM, Head D, Shah AR, et al. Decreased cerebrospinal fluid Abeta(42) correltes with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131:1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]