Abstract
Purpose
This study investigated finite marking (e.g., he walks, he walked) in boys with fragile X syndrome; the boys were grouped based on receptive vocabulary (i.e., borderline, or impaired vocabulary).
Method
Twenty-one boys with the full mutation of fragile X, between the ages of 8 to 16 years participated. The boys completed probes from the Test of Early Grammatical Impairment (Rice & Wexler, 2001), a language sample, a nonverbal IQ test (Leiter-R, Roid & Miller, 1997), a receptive vocabulary test (PPVT-IV Dunn & Dunn, 2007), and a measure of autistic symptoms (CARS; Schopler et al., 2002).
Results
There were group differences for finiteness responses on the third person singular probe; the group with impaired vocabulary omitted markers with greater frequency compared to borderline vocabulary group. There were not significant differences on the past tense probe, although boys with borderline and impaired vocabulary were delayed relative to language expectations. Nonverbal IQ was not correlated with the measures of finiteness marking.
Conclusion
Boys with FXS demonstrate delays in finiteness marking, in particular on past tense verbs. Boys with impaired vocabulary show a unique profile unlike children with SLI, in which their use of tense markers may exceed expectations benchmarked to clause length.
Keywords: fragile X syndrome, language phenotype, finiteness marking, receptive vocabulary
An objective of present research across various developmental disorders is the comparison of language phenotypes. One goal is to determine the extent to which there are unique profiles of strengths and weaknesses associated with different disorders, relative to explanations based primarily on the presence of an intellectual disability (Rice, Warren, & Betz, 2005; Rice & Warren, 2005; Warren & Abbeduto, 2007). Disorders with similar symptomology, such as autism and fragile X syndrome (FXS) are of particular interest. Given that FXS is a single-gene syndrome, it provides a unique opportunity to explore the effects of the genetic anomaly on language. Morphosyntax has been an area of interest in language disorders due to its important role in linguistic communication. Morphosyntax is the interplay between grammatical morphology and syntactic structure (Schütze, 2004). Finiteness is a property of morphosyntax involving verbal features of tense and agreement marking, such as past tense and third person singular –s, as in “she walks home.” Finiteness markers, also referred to as “grammatical tense markers”, have been identified as an area of weakness for children with Specific Language Impairment (SLI), and also for some children with autism (Rice, Wexler, & Cleave, 1995; Rice & Wexler, 1996; Roberts, Rice, & Tager-Flusberg, 2004).
The purpose of this study was to investigate finiteness markers in a group of boys with FXS. This study uses a bottom-up approach to investigate the language phenotype (Müller, 2004), beginning with the known gene defect to investigate phenotypes. Given that FXS is a single-gene syndrome, it provides an opportunity to explore the effects of the genetic defect on the language phenotype. This study builds on previous work focused on these same finiteness markers in other populations, namely autism and SLI (Roberts et al., 2004). The main research question is: Do children with FXS show the same pattern of impairments as the children in the Roberts et al. (2004) study using the same method of groups defined by levels of receptive vocabulary? Little is known about this area of linguistic development in FXS even though FXS and autism exhibit high comorbidity as well as similar language and behavioral phenotypes.
Fragile X is an X-linked disorder caused by a single gene identified in 1991 (Verkerk et al., 1991). The gene is located in the 5' untranslated region on the long arm of the X chromosome (locus Xq27.3). The gene is called FMR1, and it directs cells to produce the fragile X mental retardation protein (FMRP), which is believed to play an important role in typical brain development and functioning (Rogers, Wehner, & Hagerman, 2001). The FMR1 gene is made up of trinucleotide (CGG) repeats. A normal number of repeats ranges anywhere from 5 to 50. Fragile X syndrome occurs when an individual has an elevated number of CGG repeats (Hagerman, 2002). Trinucleotide repeats ranging from 50 to 200 signify a premutation carrier. Full mutation occurs when an individual has more than 200 repeats (Bailey et al., 2001; Hagerman, 2002). Fragile X is a dynamic gene mutation, meaning that it is unstable and will most likely expand through generations. Males are typically more affected compared to females, since it is an X-linked disorder; females have two X chromosomes, and FMRP is expressed by the unaffected X chromosome in females (Hagerman & Hagerman, 2002).
One reason FXS is of particular interest to researchers is the high co-morbidity with autism. This co-morbidity is not common among other types of intellectual disability, e.g. Down syndrome. An estimated 25–45% of males with FXS meet the criteria for a co-diagnosis of autism, and regardless of co-diagnosis, 50–90% of males are reported to display behaviors that are concurrent with autism symptomology including hand biting, hand flapping, perseveration in speech, tactile defensiveness, and poor eye contact (Bailey, Hatton, Mesibov, Ament, & Skinner, 2000; Hatton et al., 2006; Rogers et al., 2001). Males with both FXS and autism typically have more severe language and social impairments, as well as lower IQ scores compared with children with FXS and no autism (Bailey et al., 1998). Rogers and colleagues have completed a number of studies examining the impact of autism on FXS. They reported that children with comorbid FXS and autism have sensory impairments similar to children with autism and no FXS, and that imitation skills are more impaired in children with FXS and autism compared to FXS only (Rogers, Hepburn, Stackhouse, & Wehner, 2003; Rogers, Hepburn, & Wehner, 2003). Adaptive and problem behaviors also appeared to be more severe in boys with FXS and autism compared to boys with FXS only (Kau et al., 2004).
The Language Phenotype in FXS, SLI, and Autism
In addition to substantial cognitive and motor delays, individuals with FXS are reported to have a number of speech and language delays including late emergence of first spoken words, problems with speech intelligibility, and delays in both expressive and receptive morphosyntax (Abbeduto & Hagerman, 1997; Sterling & Warren, 2007). Boys with FXS show greater delays in expressive compared to receptive language (Roberts, Mirrett, & Burchinal, 2001). Research has indicated that children with FXS and autism generally have lower language skills compared to children with FXS and no autism (Bailey et al., 1998; Rogers et al., 2001). The work to date has not focused on linguistic skills above and beyond basic vocabulary, like morphosyntax. Although significant work has been completed on morphosyntactic development in children with Down syndrome and children with autism, research including children with FXS has been limited. The work that has been done for the most part has excluded children with comorbid FXS and autism, thereby ignoring this large and important subgroup of children.
Several studies of children with FXS focusing on grammar development have used mean length of utterance (MLU) and/or the Index of Productive Syntax as the primary measure (IPSyn; Scarborough, 1990; Price et al., 2008; Price et al., 2007, Roberts et al., 2007a). The IPSyn is a measure that yields a score representing emerging syntactic and morphological complexity based on spontaneous language sample data. In a series of studies in boys with FXS, Roberts, Price and colleagues analyzed IPSyn scores as well as MLU (Price et al., 2008; Price et al., 2007, Roberts et al., 2007a). Despite using many different nouns, pronouns, plurals and two-word noun phrases, boys with FXS used fewer complex noun phrases in a language sample. It is important to note that as an emergence measure the IPSyn score is not sensitive to possible group differences for affected children in the rate of acquisition of morphemes (Rice, Redmond, & Hoffman, 2006), given that it analyzes first uses of target morphemes (not the probability of use in obligatory contexts) in spontaneous language samples, and does not specifically elicit certain types of morphemes. An additional limitation of the studies of Roberts, Price and colleagues is that the boys with FXS had a mean MLU of 3.3, which was delayed compared to the control children (matched on developmental age) with typical development (mean MLU = 4.7).
Roberts et al. (2007) and Price et al. (2007, 2008) indicated that boys with FXS scored lower than nonverbal mental age expectations on expressive language, including grammatical complexity. However, their language samples were not designed to elicit specific syntactic structures, and the authors did not use standardized tests that captured performance in this area. The studies noted less complexity in children's spontaneous phrases, but did not report any information about the development of finiteness markers. In summary, these studies provide a general view of the grammatical abilities of children with FXS. Their results support the need for a deeper level of inquiry into the nature of specific aspects of grammatical development in children with FXS.
In contrast to work in FXS, morphosyntax, particularly finiteness marking, has been examined in depth in other clinical populations, including autism. Roberts et al. (2004) examined finiteness marking (third person singular, ex: he walks; past tense –ed, ex: he walked) in a large group of children (n=62) with autism between the ages of 5–15 years. The authors focused on the parallels between children with SLI and children with autism and language impairment. Finiteness/grammatical tense marking has been identified as a clinical marker for SLI in English-speaking children (Rice & Wexler, 1996; Rice, Wexler, & Cleave, 1995; Rice, Wexler, & Hershberger, 1998). Children with SLI frequently omit finiteness markers in everyday speech, including third person singular present tense (e.g., he walks), and past tense for both regular and irregular verbs (e.g., he walked, fell). Children with SLI also have difficulty producing these morphemes in experimental tasks (Leonard, Bortolini, Caselli, McGregor, & Sabbadini, 1992; Rice et al., 1995) and show protracted delays in acquisition that are apparent in production and in judgment tasks (Rice, Wexler & Hershberger, 1998; Rice, Wexler & Redmond, 1999). Recent genetics findings report a candidate gene for SLI, KIAA0319, also a candidate gene for reading impairments (Rice, Smith & Gayán, 2009), along with indications of a possible genetic contribution to finiteness marking.
Studies of children with autism provide important precedents for possible investigation of children with FXS. Roberts, Rice, and Tager-Flusberg (2004) studied three groups of children with autism, with groups defined by scores on a receptive vocabulary measure, the Peabody Picture Vocabulary Test-III (PPVT-III; Dunn & Dunn, 1997). The groups were: Normal language (standard scores above 85), borderline language (standard scores between 70 and 84), and impaired language (standard scores below 70). Children were given linguistic probes based on early forms (pre-publication) of the Test of Early Grammatical Impairment (TEGI; Rice & Wexler, 2001), as well as a nonverbal IQ test. The probes of interest elicited two kinds of finiteness markers, third person singular –s (e.g., he walks), and past tense –ed (e.g., he walked). The children with autism and language impairment made significantly fewer correct responses on the third person singular probe (36.8% correct) compared to both the children with normal (76.3%) and borderline language (61.3%). The same finding was reported for the past tense probe: children with autism and language impairment supplied significantly fewer correct responses (30.6% correct) compared to the other two groups (63.8% and 58.2% respectively). The children with autism in the normal language group performed well below age expectations on both probes.
Roberts et al. (2004) reported correlations between the two probes (percent correct in obligatory context for third person singular and past tense) and PPVT-III, chronological age in months and nonverbal IQ. Percent correct responses, collapsed across groups, were positively correlated with PPVT-III scores and chronological age. In terms of the past tense probes, the percent of correct responses was positively correlated with PPVT-III, chronological age, verbal and nonverbal IQ scores. The authors argued in their discussion that this study elucidated the lack of evidence linking finiteness marking and nonverbal intelligence. Based on the results, nonverbal IQ accounted for 6% of the variance in performance on the third person singular probe, and 13% of the variance on the past tense probes. They also noted that some children with high nonverbal IQ scored poorly on the probes, while some children with low nonverbal IQ scores performed quite well on the probes.
The Roberts et al. data suggest similarities between the linguistic profile of children with SLI and children with autism and language impairments. The authors noted the possibility of an SLI subgroup within children with autism. Given these results, a logical next step is to compare other groups of children with similar symptomology to determine whether they demonstrate similar or unique phenotypic patterns. Learning about the similarities and differences in language development will lead to hypotheses about the similarities and differences in causal pathways from genes to brain function to behavior across autism and FXS, and thereby more general neural constraints on language development. The similarities between FXS and autism, as well as the comorbidity between the two, support investigation of finiteness marking in this clinical group.
Benchmarks for Comparing FXS to Other Groups
Selecting an appropriate measure by which to categorize children's language abilities in developmental disabilities is an important issue. As stated previously, there is a wide range of abilities in terms of the language profile in FXS. Comparing at the diagnostic group level could hide important differences by assuming all children are performing at the same level. Roberts et al. (2004) recognized this in the study on children with autism. As with FXS, there is variability in the language phenotype of autism as well. The authors in the Roberts et al. study selected receptive vocabulary as the criteria for subgroups (PPVT-III; Dunn & Dunn, 1997). They selected this measure based on the findings from a previous study on autism by Kjelgaard and Tager-Flusberg (2001). Up until that manuscript, very little work had been done on characterizing the linguistic phenotype of autism.
Kjelgaard and Tager-Flusberg (2001) used a battery of standardized tests to characterize the heterogeneity of language abilities of a large sample of children with autism (n = 89). The authors selected measures commonly used in research of children with SLI and children with typical language development including measures of expressive and receptive vocabulary (EVT and PPVT), a test of articulation (Goldman & Fristoe, 1986), a measure of nonword repetition (Korkman, Kirk, & Kemp, 1998), and an omnibus language test (Semel, Wiig, & Secord, 1995). The authors determined that the measure of receptive vocabulary was the best characterization of the sample given that the majority of the children could complete the test (90% compared to only 49% completion for the omnibus test); among the 49% who did complete the omnibus language test the groups were the same regardless of whether they were grouped by the PPVT or the omnibus test. The authors argued that the appropriate measure for grouping was the measure of receptive vocabulary, given that it can be used with a larger group of children.
Receptive vocabulary is not the only measure/method used to characterize groups in studies of language and more specifically grammar. Mean length of utterance is a common variable used in studies of children with SLI (Leonard, 2002; Rice et al., 2010). It is a robust index of children's language acquisition, and can serve to benchmark samples within different groups of children with and without language impairments. However, given our research question, we selected the same grouping criterion as Roberts et al.
Another common reference point, particularly in the intellectual disability literature is that of nonverbal IQ. The impact of nonverbal IQ on morphosyntax has been examined in different clinical groups (e.g., SLI, children with intellectual impairments), to determine whether the delays seen in morphosyntax are a result of overall low cognitive skills, or whether aspects of morphosyntax are delayed regardless of cognitive abilities. A series of studies on children with SLI and typical development indicated that nonverbal intelligence was not a significant predictor of performance on measures of finiteness markers (Rice, Tomblin, Hoffman, Richman, & Marquis, 2004; Rice et al., 1998; Rice, Wexler, Marquis, & Hershberger, 2000). Roberts et al. (2004) reported that nonverbal IQ in a sample of children with autism and language impairment was not correlated with third person singular –s. However, it was significantly correlated with the past tense performance.
Data from several clinical groups, including children with SLI, autism and children with low cognitive scores and language skills in the normal range (Rice et al., 2004) indicate that nonverbal IQ is not a significant predictor of morphosyntax (in particular, finiteness markers). It seems that certain aspects of delayed morphosyntax represent a sort of deviant development, and not just a general delay associated with impaired cognition and language abilities. In other words low nonverbal IQ and delays in finiteness marking do not necessarily co-occur.
In sum, the literature to date indicates that certain children demonstrate deficits in morphosyntax development that are not explained by low cognition. The current study extends the research on this issue by investigating a group of children with FXS. The main research question is: Do children with FXS show the same pattern of impairments as the children with autism in the Roberts et al. (2004) study using the same method of participant grouping defined by receptive vocabulary level (i.e., borderline or impaired receptive vocabulary)? This is the first look at finiteness marking in children with FXS who are known to have overall deficits in grammar development and limited development of receptive vocabulary. The study addresses the issue of whether a deficit in expressive finiteness marking in children with FXS shows evidence of an overall delay in language and cognition, or if children with FXS, much like the children with autism and language impairments, demonstrate a linguistic profile of relative strengths and weaknesses (finiteness marking delayed relative to receptive vocabulary). If so, children with FXS would be expected to demonstrate a profile similar to that of children with SLI and children with autism and language impairments, and similar interventions used to target this aspect of language could be incorporated for children with FXS.
Method
Participants
The participants in this study included boys with the full mutation of FXS (n = 21). The diagnosis was confirmed by genetic testing for all participants. The children ranged in age from 8 to 16 years, with a mean age of 11 years 2 months. The boys were monolingual English speakers, as indicated by parent report. Participants were recruited from state-based support groups and a national parent listserv. This was a sample of convenience and the participants in the study were from 15 states spread across the country. All the children were white. All of the mothers had completed high school, with the majority of the mothers completing some college (mean years of education = 15). See Table 1 for additional participant information.
Table 1.
Participant Information
| Group | N | Age | PPVTa | NVIQb | CARSc | MLUd |
|---|---|---|---|---|---|---|
| Borderline Receptive Vocabulary | ||||||
| Mean | 10 | 9.84 | 76.70 | 55.90 | 26.50 | 3.18 |
| SD | 2.15 | 4.30 | 11.24 | 3.82 | .76 | |
| Range | 8.02–14.01 | 70–84 | 36–76 | 21.5–33.5 | 1.98–4.54 | |
| Impaired Receptive Vocabulary | ||||||
| Mean | 9 | 13.05 | 55.22 | 46.88 | 29.00 | 2.77 |
| SD | 2.07 | 8.53 | 13.52 | 3.79 | .72 | |
| Range | 10.02–16.04 | 40–66 | 36–79 | 22–32.5 | 1.52–3.85 |
Note. Participants divided into two groups based on criteria from past studies. One child did not complete language sample (from the borderline receptive vocabulary group).
Peabody Picture Vocabulary Test-IV (Dunn & Dunn, 2007) Standard Score (Mean = 100, SD = 15).
Nonverbal IQ assessed by Leiter Test of Nonverbal Intelligence (Leiter-R, Roid & Miller, 1997; Mean = 100, SD = 15).
Childhood Autism Rating Scale (CARS, Schopler, Reichler & Renner, 2002; < 30, non-autistic; 30–36.5, mildly-moderately autistic; >37, severely autistic).
Mean Length of Utterance in morphemes (MLU); MLU calculated using the SALT program (Miller & Chapman, 2000).
Procedures
The assessments were completed in the participants' home. All data were collected in the course of a single visit lasting 1 ½ to 3 hours. After obtaining informed consent from the legal guardian, and oral assent from the child, a number of different standardized tests as well as a language sample were completed. The assessment was videotaped using a digital video recorder mounted on a tripod. Participants were given breaks between testing as needed, and were rewarded for the completion of tasks with stickers. Each participant was given a ten-dollar gift card at the conclusion of the visit.
Dependent and Independent Variables
(See summary in Table 1).
Receptive vocabulary
The Peabody Picture Vocabulary Test Fourth Edition (PPVT-IV; Dunn & Dunn, 2007) was used to measure receptive vocabulary. Participants were asked to point to a visual representation of a word spoken by the examiner. The PPVT-IV is a norm-referenced test; age equivalent scores can be calculated based on results. The children were divided into one of three groups on the basis of their performance on the PPVT-IV: normal (standard scores 85 and above; n = 2), borderline (standard scores between 70–84; n = 10) or impaired receptive vocabulary (standard scores below 70; n = 9). This is based on previous studies on children with similar developmental disabilities; additionally this grouping criterion was selected in order to benchmark this sample within the literature (Kjeelgaard & Tager-Flusberg, 2001; Roberts et al., 2004). Roberts et al. (2004) labeled the groups normal, borderline or impaired language. We have used the same criterion. However, we have changed the labels to borderline or impaired vocabulary since the PPVT is a measure of vocabulary, not overall language. Of note, only two boys scored in the normal vocabulary range. Therefore we dropped them from the analyses. See pg. X for more information on these two boys.
Nonverbal intelligence
The Leiter Test of Nonverbal Intelligence (Leiter-R; Roid & Miller, 1997) served as the measure of nonverbal cognition. In order to compute a Brief IQ composite, four subtests were administered: Figure Ground, Form Completion, Sequential Order, and Repeated Patterns. Individuals were asked to find an item in a picture, choose the next item within a sequence, or arrange items in a pattern. The test took approximately 25 to 45 minutes to administer. This test was used to help characterize the sample, and benchmark the boys within the larger intellectual disabilities literature.
Autistic behaviors
The Childhood Autism Rating Scale (CARS; Schopler, Reichler, & Renner, 2002) was scored for all the children in the study. The CARS is a 15-item scale. The examiner completed this rating sheet after the assessment was completed. Each item ranges from one to four, with a score of one within normal limits, and four as severely abnormal for age. Total scores are based on the sum of the 15 items. A score below 30 is considered nonautistic, scores from 30 to 36.5 are considered mildly to moderately autistic, and scores above 37 are considered severely autistic. Although scores from the CARS do not serve as a diagnosis of autism, the test is often used for research purposes and has well documented reliability (Bailey, Hatton, Skinner, & Mesibov, 2001; Sevin, Matson, Coe, & Fee, 1991). Given the high comorbidity FXS has with autism, this measure was included in order to characterize the sample. The ratings were completed by a trained observer who had extensive experience completing the CARS with individuals with developmental disabilities of varying etiologies. In this research, the first author completed the ratings.
Mean length of utterance
A language sample was completed with each participant. The samples were concluded once the child reached a minimum of 100 non-imitative utterances (approximately 20–25 minutes depending on the child). The first author was trained in the second author's lab on “best practice guidelines” for sample collection, including following the child's conversational lead, engaging in parallel talk about conversation topics, and sharing personal anecdotes and experiences. The use of “yes/no” and wh-questions were kept to a minimum (see Rice et al., 2010 for more information). The examiner presented a standard set of conversational topics with questions drawn from the experimental interview protocol outlined by Evans and Craig (1992). Questions focused on three topics: family, school, and preferred after-school activities. Sample questions included “Tell me about your family”, “Let's talk about your school”, and “What types of things do you like to do when you are not in school?” The children were further prompted based on their answers. The examiner was trained to follow the child's conversational lead. All language samples were videotaped. Two children did not complete the language sample due to noncompliance.
Finiteness markers
The Test of Early Grammatical Impairment (TEGI; Rice & Wexler, 2001) was one of the dependent variables in this study. Three of the probes were given to each participant: articulation of word final consonants, third person singular probe, and the past tense probe. The past tense probe includes both regular and irregular forms of verbs. Children were first given the articulation probe as a screener to ensure each child could produce the target phonemes at the end of words. After the practice items, children were shown a picture and then asked to generate a sentence using the target structure (e.g., children shown a picture of a dentist; target answer: He cleans your teeth). Responses were scored online and then verified via audio recording by a trained research assistant. Following verification, criterion scores for each subscale were computed. The TEGI is a frequently used test in research involving children with SLI (Bishop, Adams, & Norbury, 2006; Redmond, Thompson, & Goldstein, 2011). It is sensitive and specific to the development of finiteness markers (see Rice & Wexler, 2001), and has been used for both children with normal and impaired cognition (Rice et al., 2000; Rice et al., 2004; Roberts et al., 2004).
The scores were computed based on the TEGI manual. Scores are presented in percentage forms; in other words, 80 represents 80% correct on the subscale of interest. It is important to note that the TEGI scores are based on responses to scorable items in obligatory contexts, and not necessarily all the items on the subscale. For example, the third person singular probe is comprised of 10 items. Children are prompted to give a response in obligatory context. In terms of the scoring, only verbs that can carry an overt third person singular marker are included in the score (e.g., correct: A dentist cleans your teeth; incorrect: A painter paint the fence). Children might provide a modal such as “can”, which does not have an overt tense marker. This would be considered an unscorable response. If the child had 7 responses including verbs that can carry overt tense markers, then the percentage would be calculated based on these 7 responses: 5 with finite marking in obligatory context, 2 incorrect would yield a score of 5/7 which is 71.4% correct. If a subject is not provided, the response is considered unscorable. The presence of the obligatory context is critical for the subscales.
Coding and reliability procedures
The standardized tests were scored on-line and then verified by a trained research assistant. Standardized scores were calculated and both raw and standardized scores were entered into SPSS. A second research assistant verified data entry. The language samples were transcribed, and analyzed using the Systematic Analysis of Language Transcripts (SALT; Miller & Chapman, 2000). Transcription guidelines were based on the conventions stated in the SALT manual, and following the conventions from the second author's research lab. Research assistants transcribed all language samples; the research assistants were undergraduate and graduate students in the Speech, Language and Hearing Sciences department at the University of Kansas. The samples were transcribed and coded for grammatical morphemes. Coding conventions and code entry were consistent with the SALT software (Miller & Chapman, 2002). Utterances were segmented according to terminal intonation contour and/or pauses of 2–3s, following the procedures of Miller (1981, p. 14), and those used in the second author's lab (Rice et al., 2010).
The reliability was monitored on a continuous basis. The examiners were trained to 85% agreement or better on five transcripts prior to beginning independent work. Transcripts were checked by a second transcriber, and the first author did a second reliability check on 50% of the transcripts. Disagreements were resolved through consensus. Reliability was set at 85%; average agreement for reliability was 87%.
Results
In order to answer our research question we completed separate Analysis of Variance (ANOVAs) to compare the performance in obligatory context on the third person singular and past tense probes of the TEGI between the borderline and impaired vocabulary groups. For the remainder of this section, we discuss the two probes on the TEGI separately. We first report on the overall characteristics of the sample, and we report a detailed analysis of the types of responses the participants reported.
Third Person Singular
Collapsing the two groups of boys, the overall average percent correct was 65.56% (SD = 31.94). The borderline receptive language group's average correct response was 81.78% (SD = 20.82), while the group with impaired receptive language had an average of 47.53% accuracy (SD = 47.53). An ANOVA was completed to compare group differences; the borderline group had correct responses at a significantly higher rate compared to the impaired group, F(1, 18) = 7.38, p = .015, d = 1.23 (borderline: M = 81.78, SD = 20.82; impaired: M = 47.53, SD = 33.36).
Following Roberts et al. (2004) we analyzed the types of responses provided by the participants, and recalculated percentages based on the type of response given. This level of analysis provides information about the ways in which the boys with FXS were generating unscorable as well as scorable responses on the probe. As noted above, if a child used a verb that did not allow for overt finiteness marking and therefore was not scorable for finiteness use (e.g., modal auxiliary), it would be classified as “other verb”. Another possible way for a child to react to the presentation of a probe item was for the child to listen and offer no response, to essentially ignore the item; the examiner would present the probe item twice and then with a cloze procedure for elicitation. If the child did not respond it was coded as “no response.” In this analysis, four possible responses comprised the denominator, because those responses accounted for almost all of the children's responses and conform to the analyses of Roberts et al. (2004). For example, a child with seven correct responses, two bare stem responses, and one other verb would have 70% correct responses, 20% bare stem, and 10% other verb. Percentages for types of responses for the boys with FXS on the third person singular –s probe are represented in Table 3. Bare stem (omission of the morpheme) responses were the most common error, followed by a response using an unscorable verb, or simply no response. This same pattern was consistent in the two vocabulary groups. The most common type of unscorable verb used by the boys in the borderline and impaired vocabulary groups was a modal (e.g., He can paint), followed by the present progressive form (e.g., He is throwing). Overall, 78% of the responses were scorable for the boys in this study.
Table 3.
Response Types per Total Number of Items Administered on Third Person Singular Probes
| Group | Finite Marking | Bare Stem/Omissions | Other Verb | No Response |
|---|---|---|---|---|
| Borderline | ||||
| Mean | 64.0 | 15.0 | 11.0 | 10.0 |
| SD | 25.9 | 16.5 | 8.8 | 24.9 |
| Impaired | ||||
| Mean | 41.1 | 35.6 | 12.2 | 11.1 |
| SD | 31.0 | 18.8 | 15.6 | 20.9 |
| Total | ||||
| Mean | 52.55 | 25.3 | 11.6 | 10.55 |
| SD | 28.45 | 17.65 | 12.2 | 22.9 |
Correlations with performance on the third person singular probe (based on scoring from TEGI manual) and additional measures are presented in Table 4. Correct responses were correlated with the language measures, PPVT scores and MLU. There was a significant negative correlation between PPVT and bare stem/omission responses.
Table 4.
Third Person Singular Probes: Correlations with PPVT, Age, NVIQ, and MLU
| PPVTa | Ageb | NVIQc | CARSd | MLUe | |
|---|---|---|---|---|---|
| Correct (%) | .641** | −.098 | .168 | −.384 | .560* |
| Bare Stem | −.623** | .351 | −.213 | .367 | −.337 |
| Other Verb | .104 | −.023 | −.003 | .139 | .025 |
| No Response | −.373 | −.181 | −.047 | .112 | −.443 |
Note.
Peabody Picture Vocabulary Test-IV (Dunn & Dunn, 2007) Standard Score (Mean = 100, SD = 15).
Age = Chronological age in months.
Nonverbal IQ assessed by Leiter Test of Nonverbal Intelligence (Leiter-R, Roid & Miller, 1997; Mean = 100, SD = 15).
Childhood Autism Rating Scale (CARS, Schopler, Reichler & Renner, 2002; < 30, non-autistic; 30–36.5, mildly-moderately autistic; >37, severely autistic).
Mean Length of Utterance in morphemes (MLU); MLU calculated using the SALT program (Miller & Chapman, 2000).
p < .05.
p < .01.
Past Tense Probes
Overall, the boys in this study, collapsed across receptive vocabulary groups, had a mean accuracy score of 44.59% (SD = 27.08) for the past tense probe. The boys' scorable responses to regular and irregular past tense verbs were summarized as a total score (based on the scoring from the TEGI manual), regular past tense percentage correct in obligatory contexts, irregular past tense, and irregular past tense adjusted for overregularizations (see Table 2). The borderline receptive vocabulary group had a mean for past tense finiteness marking of 52.68% (SD = 26.54), further subclassified as 52.53% (SD = 33.00) for regular past tense verbs. This group was 40.44% accurate on irregular past tense verbs, and when overregularization was counted as correct, their accuracy increased to 51.00%. Note the increases in accuracy when crediting overregularizations indicates progress toward mastery of past tense (see Rice et al., 2004 for details). The impaired receptive vocabulary group had a mean past tense finiteness score of 35.61% (SD = 26.17), subclassified as 42.22% correct (SD = 35.24) for regular past tense verbs, 27.25% (SD = 25.63) for irregular past tense, and 31.61% (SD = 29.30) for irregular past tense counting instances of overregularization. There was not a significant group difference for past tense based on the ANOVA; in other words, there were no significant differences between the borderline and impaired vocabulary groups on the past tense probe.
Table 2.
Percent Finite Marking in Obligatory context for Third Person Singular and Past Tense Probes
| Group | Third Person Singular Correct | Past Tense Total | Regular Past tense | Irregular Past Tense | Irregular Past tense + Overregularization |
|---|---|---|---|---|---|
| Borderline | |||||
| Mean | 81.78 | 52.68 | 52.53 | 40.44 | 50.97 |
| SD | 20.82 | 26.54 | 33.00 | 30.89 | 31.33 |
| Impaired | |||||
| Mean | 47.53 | 35.61 | 42.22 | 27.25 | 31.61 |
| SD | 33.36 | 26.17 | 35.24 | 25.63 | 29.30 |
| Total | |||||
| Mean | 64.66 | 44.15 | 47.38 | 33.85 | 41.29 |
| SD | 27.09 | 26.36 | 34.12 | 28.11 | 30.32 |
As with the third person singular –s, the two groups had a high percentage of scorable responses, with a mean of 84.3% for regular past tense verbs collapsed across the two groups, and 84.5% for irregular past tense verbs. Tables 5–6 provide detailed information about the types of responses for each probe. Tables 7–8 show correlations between the past tense probes and the independent variables. PPVT-IV scores were significantly correlated with correct performance on both the regular and irregular past tense verbs. CARS scores were significantly correlated with correct performance on the irregular past tense probe, and there was also a significant negative correlation between CARS scores and bare stem responses on the irregular past tense probe.
Table 5.
Response Types per Total Number of Items Administered on Regular Past Tense Probes
| Group | Correct | Bare Stem/Omissions | Other Verb | No Response |
|---|---|---|---|---|
| Borderline | ||||
| Mean | 51.6 | 38.4 | 3.3 | 6.7 |
| SD | 33.4 | 21.9 | 5.4 | 14.1 |
| Impaired | ||||
| Mean | 31.8 | 45.6 | 19.8 | 2.7 |
| SD | 27.2 | 25.1 | 19.9 | 5.6 |
| Total | ||||
| Mean | 41.7 | 42.0 | 11.55 | 4.7 |
| SD | 30.3 | 23.5 | 12.65 | 9.85 |
Table 6.
Response Types per Total Number of Items Administered on Irregular Past Tense Probes
| Group | Correct | Bare Stem | Other Verb | Over Regular | No Response |
|---|---|---|---|---|---|
| Borderline | |||||
| Mean | 38.3 | 42.7 | 3.5 | 9.9 | 5.7 |
| SD | 21.2 | 28.1 | 5.6 | 11.2 | 14.2 |
| Impaired | |||||
| Mean | 23.0 | 48.6 | 16.3 | 3.9 | 8.3 |
| SD | 22.6 | 20.2 | 17.1 | 5.9 | 20.0 |
| Total | |||||
| Mean | 30.65 | 45.65 | 9.9 | 6.9 | 7.0 |
| SD | 21.9 | 24.15 | 11.35 | 8.55 | 17.1 |
Table 7.
Past Tense Regular Verbs: Correlations with PPVT, Age, NVIQ, and MLU
| PPVTa | Ageb | NVIQc | CARSd | MLUe | |
|---|---|---|---|---|---|
| Correct (%) | .523* | .120 | −.202 | −.377 | .399 |
| Bare Stem | −.373 | −.151 | .324 | .285 | −.257 |
| Other Verb | −.415 | .157 | −.159 | .270 | −.119 |
| No Response | −.120 | −.260 | .119 | .094 | −.398 |
Note.
Peabody Picture Vocabulary Test-IV (Dunn & Dunn, 2007) Standard Score (Mean = 100, SD = 15).
Age = Chronological age in months.
Nonverbal IQ assessed by Leiter Test of Nonverbal Intelligence (Leiter-R, Roid & Miller, 1997; Mean = 100, SD = 15).
Childhood Autism Rating Scale (CARS, Schopler, Reichler & Renner, 2002; < 30, non-autistic; 30–36.5, mildly-moderately autistic; >37, severely autistic).
Mean Length of Utterance in morphemes (MLU); MLU calculated using the SALT program (Miller & Chapman, 2000).
p < .05.
p < .01.
Table 8.
Past Tense Irregular Verbs: Correlations with PPVT, Age, NVIQ, and MLU
| PPVTa | Ageb | NVIQc | CARSd | MLUe | |
|---|---|---|---|---|---|
| Correct (%) | .509* | .026 | −.288 | −.602** | .516* |
| Bare Stem | −.347 | .101 | .236 | .669** | −.360 |
| Overreg | .373 | −.206 | .024 | −.261 | −.111 |
| Other Verb | −.268 | .097 | .146 | −.024 | .039 |
| No Response | −.408 | −.172 | .020 | .221 | −.376 |
Note.
Peabody Picture Vocabulary Test-IV (Dunn & Dunn, 2007) Standard Score (Mean = 100, SD = 15).
Age = Chronological age in months.
Nonverbal IQ assessed by Leiter Test of Nonverbal Intelligence (Leiter-R, Roid & Miller, 1997; Mean = 100, SD = 15).
Childhood Autism Rating Scale (CARS, Schopler, Reichler & Renner, 2002; < 30, non-autistic; 30–36.5, mildly-moderately autistic; >37, severely autistic).
Mean Length of Utterance in morphemes (MLU); MLU calculated using the SALT program (Miller & Chapman, 2000).
p < .05.
p < .01.
Discussion
The main research question for the study was: Do children with FXS show the same pattern of impairments as the children in the Roberts et al. (2004) study using the same method of grouping defined by levels of receptive vocabulary? The Roberts et al. (2004) study divided the children with autism into three groups based on their receptive vocabulary scores (i.e., normal, borderline, and impaired). In order to benchmark this sample within the literature, comparisons were performed based on the same grouping criteria as the Roberts et al (2004) study. An ANOVA analysis indicated that there were group differences in responses for third person singular -s, and for scorable responses, boys in the language impaired group used bare stem responses with greater frequency compared to the boys in the borderline language group.
For the past tense probe, groups with borderline versus impaired vocabulary were not significantly different on past tense accuracy, with equal difficulty for both groups. The group with borderline vocabulary was 52.68 % correct in obligatory contexts overall, and used a bare stem response 47.32% of the time. The same pattern was true for the FXS group with impaired vocabulary. The boys in this group had a mean correct response of 35.61% and therefore used a bare stem response 64.39% of the time. Based on the percentages, it is clear that this was a challenging probe for the borderline and impaired groups.
Two boys with scores in the normal vocabulary range were identified in our sample, a number not sufficient for a separate group. As a result, we did not include their information in the analyses. However, it is noteworthy that in our limited sample of 21 boys with FXS, 9% of the sample (n=2) had scores in the normal range of vocabulary. Additionally, these two boys were not impaired in terms of their ability to use finite markers in obligatory context. The group with normal vocabulary was at ceiling for the third person singular –s probe, with both boys scoring at 100% accuracy. As with the third person singular, the group with normal receptive vocabulary performed well on the past tense probe, near ceiling (a mean of 96.45 for past tense total, SD = 5.02). Their IQ scores were 44 and 56; in other words, they were not at the high end for nonverbal IQ in this sample. For these two children with low nonverbal IQs, their language scores were much higher compared to the other boys in this sample. These two boys indicate the need to continue to examine the language phenotype in FXS, including finiteness markers, perhaps with a larger sample size more boys would be found who would qualify in the normal vocabulary score range, allowing for meaningful comparisons to be made.
Roberts et al. (2004) suggest that perhaps there is a subset of children with autism that display an SLI-like language profile. In this study the boys with lower receptive vocabulary scores had lower percentage correct scores in obligatory contexts. The data from this study indicate that boys with FXS show a pronounced deficit in finiteness marking, relative to receptive vocabulary. By the age of 5 years children should be, at minimum, 90% accurate on both the third person singular and past tense probes (Rice & Wexler, 2001; Roberts et al., 2004). The boys in this study scored around the age of 5 years 8 months developmentally in terms of their receptive vocabulary skills. However, their performance on all dependent measures for finiteness marking was well below 90%. At the same time, their responses showed creativity similar to that of young children with typical development, such as their tendency to adopt a modal verb such as “can” as a response to the third person singular probe thereby avoiding an obligatory finiteness marking grammatical context. Additionally their use of overregularizations of irregular past tense forms was a sign of their emerging generalization of morphophonological rules for past tense marking.
Further consideration of the response patterns of boys with FXS is needed in order to refine any possible parallels with the finiteness limitations of children with SLI. The extended optional infinitive account (EOI; Rice et al., 1995; Rice & Wexler, 1996) has been extensively studied as an account of the grammatical deficits of children with SLI. It has its basis in Wexler's optional infinitive (OI) account (Wexler, 1994), which is based on children with typical language development. In typical development, it is hypothesized that children go through a phase where they treat finiteness marking as optional, although it is obligatory in adult grammar. The basic premise of EOI is that children with SLI seem to get “stuck” in an optional infinitive stage, whereby they follow the same basic course of morphosyntactic development as children with typical language abilities, but their transition out of the OI period is protracted (Rice et al., 1995; Rice & Wexler, 1996).
As laid out by Rice (2003), there are two possible comparison groups: an age comparison group or a comparison to younger children at comparable levels of language acquisition. As noted above, the boys with FXS scored significantly below age expectations on receptive vocabulary and even further below age expectations on finiteness marking, benchmarked to the TEGI manual scores. In many of the studies of SLI, the younger comparison group is benchmarked to mean length of utterance (MLU). We can also benchmark the FXS groups based on their MLU scores to what has been reported for children with SLI and unaffected children in other reports. These comparisons are laid out in Table 9. For each of the groups with FXS, in the MLU column the mean MLU for the group with FXS is reported followed by mean MLU levels and mean ages for children with SLI and control children as reported by Rice et al. (2010). We can see that the normal vocabulary group with FXS, with an MLU of 3.79, has a level that aligns with children with SLI in the age level of 4;0–4;05, and with control children in the age level of 3;0–3;05; this indicates that for the children with FXS with normal levels of receptive vocabulary their clause length, indexed by MLU, is far below age expectations. Similarly, the borderline receptive vocabulary group's mean MLU aligns with averages reported by Rice et al. (2010) for children with SLI between the ages of 3;0 and 3;11, and for control children of 2;06–2;11. The impaired vocabulary group with FXS aligns, for MLU, with slightly younger children with SLI, and with control children younger than 2;06. Overall, the FXS children are notably deficient in clause length, even relative to their receptive vocabulary age equivalencies.
Table 9.
Benchmarking FXS Groups to SLI and Normative Groups
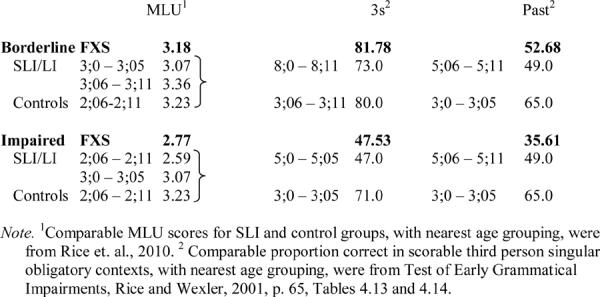
|
Also reported in Table 9 are data for performance on the third person singular and past tense probes of TEGI, drawn from the TEGI manual (Rice & Wexler, 2001, p. 65). Here we see that the performance on third person singular for the borderline vocabulary group with FXS is approximately 82% accurate in obligatory contexts; this is better than that of the group with SLI who show a mean of 73% reported in TEGI norms for children ages 8;0–8;11, and similar to control/typically developing children's mean of 80% for ages 3;06–3;11. For the past tense probe, the borderline vocabulary group's mean of 52.68% is roughly similar to that of an SLI group of 5;06–5;11 years, and lower than a control group of 3;0–3;5. Similarly, we can see from the table that the impaired vocabulary group's performance is similar to that of children with SLI ages 5;0–5;05 for third person singular and less than that of children with SLI ages 5;06–5;11 for past tense. This group's performance on both probe tasks is below what is expected for children with typical development at 3 years.
In summary, these age comparisons indicate that FXS groups are much more delayed in clause formation than the children with SLI. On the other hand, the age comparisons suggest that the groups with FXS, benchmarked by receptive vocabulary levels, seem to exceed the MLU expectations for accuracy on the third person singular and past tense probes. Thus, the pattern seems to be a profile unlike SLI, which is characterized as a delay-within-a-delay profile of SLI (Rice, 2003), wherein finiteness marking is below MLU expectations. For the children with FXS, they show a much delayed clause length, although third person singular and past tense usage is commensurate with older ages. The comparison to children with SLI or children with typical development does not align MLU to expected levels of performance on TEGI for the groups with FXS. Although further investigation is needed, the results indicate that boys with FXS who meet the criteria for inclusion used here may show a profile unlike children with SLI, in which their use of tense markers may exceed expectations benchmarked to clause length. In terms of clinical implications, understanding the nature of the language delay is important for targeting treatment; assessments and interventions for SLI might not be appropriate for children with FXS, given the differences in the profiles of language. Additionally, group comparisons indicate the need for multiple benchmarking in order to sort out the extent to which tense markers are relatively weak or strong in children with autism as compared to children with SLI and to children with FXS; this is necessary in order to develop targeted language interventions.
This study has several strengths and limitations. It represents the first detailed examination of finiteness markers in children with FXS, and benchmarks this sample within the broader literature of children with varying etiologies of language delays. Although the sample size is in line with studies of children with FXS, it is too small to allow for detailed analyses, and therefore can only be used as a preliminary look at the question of finiteness in FXS. Additionally, we were only able to recruit two boys in the normal receptive vocabulary group; therefore their data could not be used in the group analyses. Given the earlier literature indicating that boys with FXS are significantly delayed in all aspects of language development, the boys in the normal receptive vocabulary group demonstrate that boys with FXS are a heterogeneous group in terms of language development. The two boys in this group showed a similar pattern of finiteness marking use compared to the boys from Roberts et al. (2004). These preliminary findings indicate a need to recruit a larger sample of boys with FXS, in order to include more boys with higher scores on the PPVT. Further, comparison to MLU expectations suggests that the boys with borderline or impaired receptive vocabulary levels may be acquiring third person singular and past tense markers at lower levels of clause structure than expected.
Fragile X syndrome, as noted earlier, is a single gene disorder. Although there is still much to learn about how it impacts neural development, it is easier to determine the impact on neural functioning compared to disorders such as autism with unknown etiology. Studies such as this one indicate the need for more detailed studies of the acquisition of grammar in children with FXS. This study with its bottom-up approach adds to the foundation for specifying the language phenotype for FXS. Autistic symptoms were correlated with one of the measures in this study (past tense irregular verbs). Consequently, it provides the basis for future studies clarifying the additive effect of autism on FXS. Future studies should continue to explore the impact of autism on FXS, and the addition of the current diagnostic gold standard would allow group comparisons and a more rigorous design. Finally, the study has implications for how finiteness marking and other symptoms of language impairment can serve as phenotypes in studies of possible genetic effects, possibly involving multiple genes contributing to language impairments across different clinical groups.
Acknowledgements
This research is supported in part by grants 3 P30 HD003110-3, P30 HD002528-39, and T32 HD07489 from NICHD, as well as T32 DC000052 from NIDCD and a summer student fellowship from the National Fragile X Foundation. We would like to thank the children and families who participated in this research. We thank Len Abbeduto and Joanne Roberts for their input on the design of the study. We also thank Michaela Catlin, Emily Enright, Kara Knapp, and Holly Watson for their assistance with transcription and data entry, and Kandace Fleming for her assistance with the data analysis.
References
- Abbeduto L, Hagerman R. Language and communication in fragile X syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 1997;3:313–322. [Google Scholar]
- Bailey DB, Hatton DD, Mesibov G, Ament N, Skinner M. Early development, temperament, and functional impairment in autism and fragile X syndrome. Journal of Autism and Developmental Disorders. 2000;30(1):49–59. doi: 10.1023/a:1005412111706. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Hatton DD, Skinner M, Mesibov G. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. Journal of Autism and Developmental Disorders. 2001;31:165–174. doi: 10.1023/a:1010747131386. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Mesibov GB, Hatton DD, Clark RD, Roberts JE, Mayhew L. Autistic behavior in young boys with fragile X syndrome. Journal of Autism and Developmental Disorders. 1998;28:499–508. doi: 10.1023/a:1026048027397. [DOI] [PubMed] [Google Scholar]
- Brown R. A first language: The early stages. Harvard University Press; Cambridge, MA: 1973. [Google Scholar]
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test. fourth edition Pearson Education; Minneapolis, MN: 2007. [Google Scholar]
- Evans JL, Craig HK. Language sample collection and analysis: Interview compared to freeplay assessment contexts. Journal of Speech and Hearing Research. 1992;35:343–353. doi: 10.1044/jshr.3502.343. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ. The physical and behavioral phenotype. In: Hagerman RJ, Hagerman PJ, editors. Fragile X syndrome: Diagnosis, treatment, and research. 3rd Edition The Johns Hopkins University Press; Baltimore, MD: 2002. pp. 3–109. [Google Scholar]
- Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. Developmental and Behavioral Pediatrics. 2006;27:63–74. doi: 10.1097/00004703-200602000-00012. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Hagerman PJ, editors. Fragile X syndrome: Diagnosis, treatment, and research. 3rd Edition The Johns Hopkins University Press; Baltimore, MD: 2002. [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Roberts J, Mirrett P. Autistic behavior in children with fragile X syndrome: Prevalence, stability, and the impact of FMRP. American Journal of Medical Genetics Part A. 2006;140A(17):1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Kau A, Tierney E, Bukelis I, Stump MH, Kates WR, Trescher WH, Kaufmann WE. Social behavior profile in young males with fragile X syndrome: Characteristics and specificity. American Journal of Medical Genetics. 2004;126A:9–17. doi: 10.1002/ajmg.a.20218. [DOI] [PubMed] [Google Scholar]
- Kjelgaard MM, Tager-Flusberg H. An investigation of language impairment in autism: Implications for genetic subgroups. Language and Cognitive Processes. 2001;16:287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard L. Children with specific language impairment. MIT Press; Cambridge, MA: 2002. [Google Scholar]
- Leonard LB, Bortolini U, Caselli MC, McGregor KK, Sabbadini L. Morphological deficits in children with specific language impairment. The status of features in the underlying grammar. Language Acquisition. 1992;2:151–179. [Google Scholar]
- Miller J, Chapman R. Systematic Analysis of Language Transcripts. Research Version 6.1. Language Analysis Lab, University of Wisconsin; Madison: 2000. [Computer software] [Google Scholar]
- Müller RA. Genes, Language Disorders, and Developmental Archaeology: What role can neuroimaging play? In: Rice ML, Warren SF, editors. Developmental language disorders: From phenotypes to etiologies. Lawrence Erlbaum; Mahwah, NJ: 2004. pp. 291–328. [Google Scholar]
- Price JR, Roberts JE, Hennon EA, Berni MC, Anderson KL, Sideris J. Syntactic complexity during conversation of boys with fragile X syndrome and Down syndrome. Journal of Speech, Language, and Hearing Research. 2008;51:3–15. doi: 10.1044/1092-4388(2008/001). [DOI] [PubMed] [Google Scholar]
- Price J, Roberts J, Vandergrift N, Martin G. Language comprehension in boys with fragile X syndrome and boys with Down syndrome. Journal of Intellectual Disability Research. 2007;51:318–326. doi: 10.1111/j.1365-2788.2006.00881.x. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Dant CC. The behavioral neurogenetics of fragile X syndrome: Analyzing gene-brain-behavior relationships in child developmental psychopathologies. Development and Psychopathology. 2003;15:927–968. doi: 10.1017/s0954579403000464. [DOI] [PubMed] [Google Scholar]
- Rice ML. A Unified Model of specific and General Language Delay: Grammatical Tense as a Clinical Marker of Unexpected Variation. In: Levy Y, Schaeffer J, editors. Language Competence across Populations: Toward a Definition of Specific Language Impairment. Lawrence Erlbaum Associates; Mahwah, NJ: 2003. pp. 63–95. [Google Scholar]
- Rice ML, Redmond SM, Hoffman L. Mean length of utterance in children with specific language impairment and in younger control children shows concurrent validity and stable and parallel growth trajectories. Journal of Speech, Language, and Hearing Research. 2006;2006;49:793–808. doi: 10.1044/1092-4388(2006/056). [DOI] [PubMed] [Google Scholar]
- Rice ML, Smith SD, Gayan J. Convergent genetic linkage and associations to language, speech and reading measures in families of probands with specific language impairment. Journal of Neurodevelopmental Disorders. 2009;1:264–282. doi: 10.1007/s11689-009-9031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ML, Tomblin JB, Hoffman L, Richman WA, Marquis J. Grammatical tense deficits in children with SLI and nonspecific language impairment: Relationships with nonverbal IQ over time. Journal of Speech, Language, and Hearing Research. 2004;47:816–834. doi: 10.1044/1092-4388(2004/061). [DOI] [PubMed] [Google Scholar]
- Rice ML, Warren SF, Betz SK. Language symptoms of developmental language disorders: An overview of autism, Down syndrome, fragile X, specific language impairment, and Williams syndrome. Applied Psycholinguistics. 2005;26:7–27. [Google Scholar]
- Rice ML, Wexler K. Toward tense as a clinical marker of specific language impairment in English-speaking children. Journal of Speech and Hearing Research. 1996;39:239–257. doi: 10.1044/jshr.3906.1239. [DOI] [PubMed] [Google Scholar]
- Rice ML, Wexler K. Test of early grammatical impairment. Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Rice ML, Wexler K, Cleave PL. Specific language impairment as a period of extended optional infinitive. Journal of Speech and Hearing Research. 1995;38:850–863. doi: 10.1044/jshr.3804.850. [DOI] [PubMed] [Google Scholar]
- Rice ML, Wexler K, Hershberger S. Tense over time: The longitudinal course of tense acquisition in children with specific language impairment. Journal of Speech and Hearing Research. 1998;41:1412–1431. doi: 10.1044/jslhr.4106.1412. [DOI] [PubMed] [Google Scholar]
- Rice ML, Wexler K, Marquis J, Hershberger S. Acquisition of irregular past tense by children with SLI. Journal of Speech, Language, and Hearing Research. 2000;43:429–448. doi: 10.1044/jslhr.4305.1126. [DOI] [PubMed] [Google Scholar]
- Rice ML, Wexler K, Redmond SM. Grammaticality judgments of an extended optional infinitive grammar: Evidence from English-speaking children with specific language impairment. Journal of Speech, Language, and Hearing Research. 1999;42:943–961. doi: 10.1044/jslhr.4204.943. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Hennon EA, Price JR, Dear E, Anderson K, Vandergrift NA. Expressive language during conversational speech in boys with fragile X syndrome. American Journal on Mental Retardation. 2007a;112:1–17. doi: 10.1352/0895-8017(2007)112[1:ELDCSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Mirrett P, Burchinal MR. Receptive and expressive communication development of young males with fragile X syndrome. American Journal on Mental Retardation. 2001;106:216–230. doi: 10.1352/0895-8017(2001)106<0216:RAECDO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Roberts J, Price J, Barnes E, Nelson L, Burchinal M, Hennon EA, Moskowitz L, Edwards A, Malkin C, Anderson K, Misenheimer J, Hooper SR. Receptive vocabulary, expressive vocabulary, and speech production of boys with fragile X syndrome in comparison to boys with Down syndrome. American Journal on Mental Retardation. 2007b;112:177–193. doi: 10.1352/0895-8017(2007)112[177:RVEVAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Rice ML, Tager-Flusberg H. Tense marking in children with autism. Applied Psycholinguistics. 2004;25:429–448. [Google Scholar]
- Rogers SJ, Hepburn SL, Stackhouse T, Wehner E. Imitation performance in toddlers with autism and those with other developmental disorders. Journal of Child Psychology and Psychiatry. 2003;44:763–781. doi: 10.1111/1469-7610.00162. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, Wehner E. Parent report of sensory symptoms in toddlers with autism and those with other developmental disorders. Journal of Autism and Developmental Disorder. 2003;33:631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Wehner EA, Hagerman R. The behavioral phenotype in fragile X: Symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Developmental and Behavioral Pediatrics. 2001;22:409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Roid GH, Miller LJ. Leiter International Performance Scale-Revised. Stoelting; Wood Dale, IL: 1997. [Google Scholar]
- Scarborough HS. Index of productive syntax. Applied Psycholinguistics. 1990;11:1–22. [Google Scholar]
- Schopler E, Reichler J, Renner B. The Childhood Autism Rating Scale (CARS) Western Psychological Services; Los Angeles, CA: 1988. [Google Scholar]
- Schütze CT. Morphosyntax and syntax. In: Kent RD, editor. The MIT Encyclopedia of Communication Disorders. The MIT Press; Cambridge, Massachusetts: 2004. pp. 354–356. [Google Scholar]
- Sevin JA, Matson JL, Coe DA, Fee VE. A comparison and evaluation of three commonly used autism scales. Journal of Autism and Developmental Disorders. 1991;21:321–328. doi: 10.1007/BF02206868. [DOI] [PubMed] [Google Scholar]
- Smith N, Tsimpli I-M. The Mind of a Savant: Language Learning and Modularity. Blackwell; Oxford, UK: 1995. [Google Scholar]
- Sterling AM, Warren SF. Communication and language development in infants and toddlers with Down syndrome or fragile X syndrome. In: Roberts J, Chapman C, Warren S, editors. Communication and language intervention in fragile X and Down syndrome children. Brookes Publishing; Baltimore: 2007. [Google Scholar]
- Tassone F, Hagerman RJ, Chamberlain WD, Hagerman PJ. Transcription of the FMR1 gene in individuals with fragile X syndrome. American Journal of Medical Genetics. 2000;97:195–203. doi: 10.1002/1096-8628(200023)97:3<195::AID-AJMG1037>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. American Journal of Medical Genetics. 1996;64:196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Verkerk A, Pieretti M, Sutcliffe JS, Fu Y, Kuhl D, Pizzuti A, Reiner O, Richards S, Victoria M, Zhang F, Eussen B, van Ommen G, Blonden L, Riggins G, Chastain J, Kunst C, Galjaard H, Caskey CT, Nelson D, Oostra B, Warren S. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Warren SF, Abbeduto L. Introduction to communication and language development and intervention. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13(1):1–3. doi: 10.1002/mrdd.20158. [DOI] [PubMed] [Google Scholar]
- Wexler K. Optional infinities, head movement, and the economy of derivations. In: Lightfoot D, Hornstein N, editors. Verb movement. Cambridge University Press; Cambridge, England: 1994. pp. 305–350. [Google Scholar]


