Abstract
Objectives
Auto-antibodies were studied in a well-characterized cohort of children with chronic hepatitis C (CHC) during treatment with PEG-IFN and ribavirin to assess the relationship to treatment and development of autoimmune disease.
Methods
114 children (5–17 years), previously screened for the presence of high titer autoantibodies, were randomized to Peg-IFN with or without ribavirin. Anti-nuclear (ANA), anti-liver-kidney-microsomal (LKM), anti-thyroglobulin (TG), anti-thyroid peroxidase (TPO), insulin (IA2), anti-glutamic acid decarboxylase (GAD) antibodies were measured after trial completion using frozen sera.
Results
At baseline,19% had auto-antibodies: ANA (8%), LKM (4%), and GAD (4%). At 24 and 72 weeks (24 weeks after treatment completion), 23% and 26% had auto-antibodies (p=0.50, 0.48 compared to baseline). One child developed diabetes and two hypothyroidism during treatment; none developed autoimmune hepatitis. At 24 weeks, the incidence of flu-like symptoms, gastrointestinal symptoms, and headaches were 42%, 8% and 19% in those with auto-antibodies vs. 52%, 17%, and 26% in those without (p=0.18, 0.36, and 0.20, respectively). In children with negative HCV PCR at 24 weeks, there was no difference in the rate of early virologic response /sustained virologic response respectively in those with auto-antibodies 76%/69%, vs 58%/65% in those without (p=0.48).
Conclusions
Despite screening, we found autoantibodies commonly at baseline, during treatment for CHC and after. The presence of antibodies did not correlate with viral response, side effects, or autoimmune hepatitis. Neither screening nor archived samples assayed for thyroid and diabetes-related antibodies identified the 3 subjects who developed overt autoimmune disease, diabetes (1) and hypothyroidism (2).
Keywords: Pediatrics, Viral hepatitis, Therapy, Complications, Diabetes, Hypothyroid, Auto-immune
INTRODUCTION
The literature is replete with reports of de novo manifestations of autoimmune diseases occurring during the treatment of Chronic Hepatitis C (CHC) with interferon (IFN) based therapies. The most common diseases reported are thyroid disease1, type 1 insulin dependent diabetes2, and autoimmune hepatitis3. Rarer manifestations have included celiac disease4, autoimmune thrombocytopenia5, and autoimmune hemolytic anemia6. These complications are serious and, in some cases, permanent after cessation of therapy. Thyroid disease can occur in patients with CHC prior to treatment 7 and in as many as 15% of adults during IFN treatment 1, 7. A nearly 5% incidence has been reported for diabetes 2. These findings have implications for the risk and benefit of treating CHC with IFN-based therapies. To limit potential risk, some clinicians have suggested utilizing antibodies to screen and monitor the onset of disease. However, it is unclear if the presence of antibodies prior to treatment correlates with subsequent development of autoimmune disease 8.
Although combination therapy with PEG- IFN and ribavirin has become an established and effective therapy for CHC in children 9 little is known about the development of auto-antibodies and their predictive utility in children. The PEDS-C trial provided a unique opportunity to study a well characterized cohort of North American children regarding the impact of IFN on the development of auto-antibodies, the predictive value of antibody presence on the development of symptomatic complications, and the impact of baseline antibody on response to treatment 10. In this study, we applied a standardized approach to screening our population to minimize risk to our treatment population. All children entering the treatment portion of the clinical trial were excluded if they had increased titers of ANA, ASMA, LKM or TPO. In the 114 remaining children, ages 5 to 17 years, a population typical of those a clinician would treat in practice, we assessed auto-antibodies, using stored frozen sera obtained before, during, and after treatment in the PEDS-C trial of PEG-IFN alfa 2a with and without ribavirin. We evaluated the impact of auto-antibodies on response to therapy, tolerance of therapy, and development of de novo autoimmune disease.
MATERIALS AND METHODS
Subjects
Eligible children included treatment-naïve children from 5 to 17 years old with CHC infection documented by HCV RNA in serum on 2 occasions at least 6 months apart and a liver biopsy consistent with CHC. Details of the trial design and therapeutic outcomes were previously published10–11 and the study was registered at www.ClinicalTrials.gov, NCT00100659. Subjects were enrolled by investigators at 11 U.S. medical centers from December 2004 until May 2006. The present study was approved by institutional review boards at each site and was conducted in compliance with the Declaration of Helsinki, Good Clinical Practice Guidelines and local regulatory requirements. Children were excluded if there was evidence of decompensated liver disease or serological tests suggesting hepatitis A, hepatitis B, human immunodeficiency virus infection, or autoimmune hepatitis (ANA>1:160, ASMA >1:80, LKM >60 IU/ml, Covance labs)10–11. A total of 9 children were excluded from the study at screening: 5 with ANA, 1 with SMA, and 3 with LKM.
Treatment
Subjects were randomly assigned 1:1 to receive either PEG-IFN alfa-2a and RV or PEG-IFN alfa-2a and placebo10. PEG-IFN 2a was administered at a dose of 180 μg/1.73m2 body surface area (maximum 180 μg) subcutaneously once weekly. Ribavirin was administered at a dose of 15 mg/kg/day orally in 2 doses (maximum 1200 mg/day if ≥ 75 kg and 1000 mg if < 75 kg). Placebo was supplied in the same dosing regimen as RV. Patients without detectable HCV RNA at 24 weeks were continued on treatment for another 24 weeks, whereas those who had detectable HCV RNA at 24 weeks were considered treatment failures. Treatment was stopped in those subjects who had been given PEG-IFN alfa-2a with RV and who did not achieve viral clearance at 24 weeks. Patients who failed treatment with PEG-IFN alfa-2a plus placebo were offered “open-label” therapy with PEG-IFN alfa-2a plus RV for another 48 weeks (stopping after 24 weeks if HCV RNA remained positive). Further details of the study design and outcome results of the trial have been previously reported 10–11. Drug dose reductions were instituted for toxicities according to protocol.
Measurement
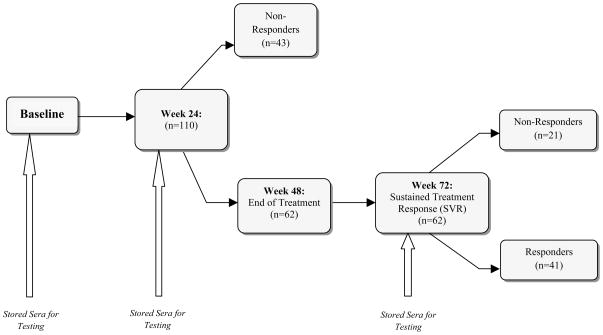
Auto-antibodies: All samples from weeks 0, 24 and 72 in the PEDS-C trial were stored at −80 degrees Celsius until analysis and were tested in a single lab simultaneously at the completion of the clinical trial. Figure 1 illustrates the times at which sera were obtained. Baseline and week 24 sera were tested from all subjects. Only those who were HCV negative at 24 weeks and continued on medication to complete a 48 week course of therapy were tested at the 72 week time point (24 weeks off therapy).
Figure 1.
The consort diagram shows treatment and decision points during the Peds-C Trial. At baseline, week 24 and week 72 serum was stored and frozen. The stored serum was used for analysis of serum auto-antibodies after completion of the clinical trial.
Auto-antibodies were measured by FDA-approved ELISA in a CLIA approved lab and reported in WHO international units. All tests were performed in the fully accredited Immune Disorders Laboratory at Johns Hopkins University (Clinical Laboratories Improvement Act CLIA #21D0709511). Positive cut-off values were all based on WHO standards established for each test. Thyroid-associated auto-antibodies included antibodies to TG (QUANTA Lite® ELISA) and TPO (QUANTA Lite® TPO ELISA) and were measured by commercially manufactured ELISA kits (Inova Diagnostics, San Diego, CA). Positive values were defined as levels exceeding the normal reference range of 100 units. Auto-antibodies LKM and ANA were measured utilizing Inova Diagnostics QUANTA Lite® ELISA assays. Positive results for LKM-1 auto-antibodies included values exceeding 25 units. A moderate positive result for ANA auto-antibodies fell within the range of 20–60 units; however, for the purpose of this study, only results exceeding 60 units (strong positive) were considered to be positive. Detection of F-actin antibodies was done via commercial QUANTA Lite® Actin IgG ELISA (Inova Diagnostics, San Diego, CA). Values exceeding 30 units (“moderate to strong positive”) were considered positive. GAD and IA-2 auto-antibodies associated with the presence of Type 1 diabetes, were measured using a GAD/IA-2 Auto-antibody Screen ELISA Kit (KRONUS, Inc., Star, ID). GAD positive values were defined as values exceeding 5 IU/ml. IA-2 positive values were defined as values exceeding 20 units.
Routine laboratory assessments were performed at Covance and included free T4, thyroid stimulating hormone, complete blood count and ALT among other tests that are fully described in Schwarz et al10–11.
Statistics
The generalized estimating equation (GEE) was utilized to analyze the study outcome by determining the correlation between autoantibody presence and treatment outcome. The presence of positive auto-antibodies at 24 and 72 weeks, treatment type, and visit time point functioned as covariates. All category variables were summarized using frequency and percentage. The comparison of adverse events between patients with and without auto-antibodies was performed using Fisher’s exact test. All continuous factors were reported as mean and standard deviation at each group. A two sided T-test or a Wilcoxon Rank-sum test was used to compare the significance of autoantibody presence and absence in relation to continuous variables (e.g., Knodell score). A P-value equal to or less than 0.05 was considered significant.
RESULTS
Antibody presence at baseline, 24 weeks and 72 weeks
114 treatment-naïve children with HCV entered the randomized trial. Mean age was 10.7 years, 55% were male, 75% Caucasian and the majority of children acquired their infection in the perinatal period10. Genotype 1 accounted for 81%. Auto-antibodies common to autoimmune hepatitis (ANA, LKM, anti-F-Actin), thyroid disease (TPO, TG) and diabetes (IA-2, GAD) were assessed (Table 1). At baseline 19 % of children had auto-antibodies. The most common auto-antibody was ANA (8%) followed by LKM (4%) and GAD (4%). Children with hepatitis-associated antibodies at baseline were younger, 7.8 vs 11.1 years (p=0.0007) and had slightly lower BMI Z-scores, −0.11 vs 0.70 (p=0.01) but did not differ from those without antibodies in terms of sex, genotype, viral load, Knodell inflammation or fibrosis scores (p= 1.00, 0.46, 0.13, 0.84 and 0.41 respectively).
Table 1.
Results of antibody testing on stored sera from baseline, week 24 and week 72. Last row provides the number of subjects who had any antibody positive.
| Autoantibody | Baseline (n=114) | Week 24 (n=108) | Week 72 (n=62) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| # Pos (%) | Mean | Range | # Pos (%) | Mean | Range | # Pos (%) | Mean | Range | |
| TPO (>100 units) | 3 (2.6) | 489 | 422–589 | 3 (2.8) | 444 | 353–497 | 2 (3.2) | 196.5 | 112–281 |
| TG (>100 units) | 0 (0) | 0 | 0 | 3 (2.8) | 257.7 | 222–287 | 4 (6.5) | 292 | 123–431 |
| LKM-1 (> 25 units) | 5 (4.4) | 37.1 | 25.8–44.5 | 4 (3.7) | 46.9 | 31.9–89.9 | 2 (3.2) | 26.1 | 26–26.1 |
| ANA (> 60 units) | 9 (7.9) | 104.5 | 60.1–157.7 | 9 (8.3) | 97.3 | 60.3–160.2 | 8 (12.9) | 94.2 | 60.6–189.2 |
| F-ACTIN (>30 units) | 1 (0.9) | 44.9 | 44.9 | 1 (0.9) | 33.8 | 33.8 | 1 (1.6) | 39.6 | 39.6 |
| GAD (>5IU/ml) | 5 (4.4) | 19.6 | 6.1–35.6 | 10 (9.3) | 23.7 | 8–45.2 | 1 (1.6) | 27.9 | 27.9 |
| AI-2 (>20 units) | 3 (2.6) | 46.7 | 22.6–85.2 | 2 (1.9) | 27.8 | 20.4–35.2 | 3 (4.8) | 44.3 | 22–64.7 |
|
| |||||||||
| ANY ANTIBODY | 22 (19) | 25 (23) | 16 (25) | ||||||
| # Pos (%) | |||||||||
After 24 weeks of PEG-IFN therapy, the overall prevalence of antibodies was 23% with ANA remaining the most common. At the 72 week time point, 26% of subjects had auto-antibodies. The antibody titers were not significantly different between time points before, during or 24 weeks after completion of therapy (P-value =0.58 for TPO, 0.53 for TG, 0.86 for F-Actin, 0.22 for ANA, 0.68 for IA-2). Mean antibody titers did not increase at 24 or at 72 weeks, whether assessed in all subjects or only in those with initial positive titers. The rate of antibodies becoming positive in those who were negative at baseline was up to 3% for liver-specific antibodies; among those who were positive for hepatitis-related autoantibodies at baseline, antibody subsequently became negative in 1 of 9 children with ANA, 2 of 5 children with LKM, and in the 1 child with F actin.
Relationship between antibody presence and EVR/ SVR
Of the 114 subjects in the trial, 61% achieved viral suppression at week 24. Baseline hepatitis-associated antibody positivity did not significantly affect subsequent EVR and SVR. No significant differences in response at 24 weeks were found when comparing those with any auto-antibodies and those without (Table 2). EVR in those with antibodies at week 24 was 76% (19/25), and in those without antibodies at week 24 was 58% (48/83). SVR among this group of children who had undetectable HCV-RNA at 24 weeks was 69% (11/16) in those with antibodies and 65% (30/ 46) in those without. (p=0.46) Additionally there was no difference in the rate of antibody positivity at week 72 in those who achieved SVR vs those who did not (27% vs 24%, p=0.50). The children who relapsed at week 72 were compared to those who achieved SVR. Again, there was no statistically significant association of auto-antibodies with SVR or relapse based on GEE analysis. When comparing the hepatitis-associated antibody positive and negative groups who had achieved SVR, there was no significant difference in the frequency of non-genotype I or initial combo therapy, parameters that might have influenced SVR.
Table 2.
Comparison of responders to non-responders at weeks 24 and 72. Of those achieving EVR, 28% had antibodies and of those achieving SVR, 27% had antibodies. EVR in those with antibodies was 19 out of 25 (76%), whereas EVR in those without antibodies was 48 out of 83 subjects (58%). SVR in those with anti-bodies was 11 of 16 (69%) and in those without 30 of 46 (65%).
| Autoantibody | Week 24 EVR | Week 72 SVR | ||||
|---|---|---|---|---|---|---|
| EVR+ (n=67) | EVR − (n=43) | Total (n=108) | SVR + (n=41) | SVR − (n=21) | Total (n=62) | |
| # Pos (%) | # Pos (%) | # Pos (%) | # Pos (%) | # Pos (%) | # Pos (%) | |
| TPO (>100 units) | 0 (0) | 3 (6.9) | 3 (2.8) | 2 (4.9) | 0 (0) | 2 (3.2) |
| TG (>100 units) | 1 (1.4) | 2 (4.6) | 3 (2.8) | 1 (2.4) | 3 (14.3) | 4 (6.5) |
| LKM-1 (> 25 units) | 3 (4.4) | 1 (2.3) | 4 (3.7) | 2 (4.9) | 0 (0) | 2 (3.2) |
| ANA (> 60 units) | 5 (7.5) | 4 (9.3) | 9 (8.3) | 6 (14.6) | 2 (9.5) | 8 (12.9) |
| F-ACTIN (>30 units) | 1 (1.4) | 0 (0) | 1 (0.9) | 0 (0) | 1 (4.7) | 1 (1.6) |
| GAD (>5IU/ml) | 8 (12) | 2 (4.7) | 10 (9.3) | 1 (2.4) | 0 (0) | 1 (1.6) |
| AI-2 (>20 units) | 2 (2.9) | 0 (0) | 2 (1.9) | 3 (7.3) | 0 (0) | 3 (4.8) |
|
| ||||||
| Any | 19 (28) | 6 (14) | 25 (23) | 11 (27) | 5 (24) | 16 (26) |
Auto-antibodies and end-organ disease
Three children developed significant autoimmune disease (Table 3). A 6 year old girl developed insulin dependent diabetes mellitus (IDDM) after 32 weeks of study drug. She developed severe hyperglycemia at 32 weeks of therapy. Study drug was discontinued at week 32 and she continued to require insulin. Study testing on stored sera revealed negative IA-2 antibody at each time point and negative GAD at baseline and week 24; GAD was positive at week 72. Two girls, ages 11 and 13, developed clinical hypothyroidism requiring treatment, neither child had positive TG or TPO at baseline. In the 11 year old at 24 weeks, thyroid stimulating hormone (TSH) was elevated to 45.33 IU/ml at week 24 and her TG increased from 19 units at baseline to 264 units at week 24. Subsequent TSH normalized on replacement therapy and she continued thyroid medication after study completion. The 13 year old girl developed elevated TSH 30 IU/ml and low free T4 0.6ng/dl at 36 weeks of study treatment; thyroid-specific auto-antibodies were not detected. She remained on thyroid hormone therapy with normal thyroid function tests after achieving SVR. Four children had elevated TPO and none of these children had abnormal T4 or TSH. Of the 7 with positive TG, only the one described above developed thyroid disease.
Table 3.
Genotype, antibody pattern and therapeutic response for the children who developed either insulin dependent diabetes or hypothryoidism
| Antibody (nl range) | 11 year old girl with hypothyroidism | 13 year old girl with hypothyroidism | 6 year old girl with IDDM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BL | 24 | 72 | BL | 24 | 72 | BL | 24 | 72 | |
| TPO (0–100) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TG (0–100) | 19 | 264 | 94 | 13 | 54 | 17 | 11 | 9 | 16 |
| LKM-1 (0–25) | 1.7 | 1.3 | 1.5 | 1.6 | 1.2 | 1.2 | 1.1 | 1.8 | 1.2 |
| ANA (0–20nl:20–60moderate:>60 high) | 10.4 | 14.9 | 10.6 | 23.6 | 25.1 | 26.9 | 8.2 | 7.1 | 11.2 |
| F-ACTIN (0–30) | 9.6 | 9.1 | 7.3 | 14.8 | 7.5 | 6.5 | 9.6 | 3.0 | 12.5 |
| GAD (0–5) | 1.9 | 0 | 1.3 | 0.3 | 0 | 0.8 | 1.0 | 2.7 | 27.9 |
| AI-2 (0–20) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.7 | 0.0 | 0.0 |
|
| |||||||||
| Treatment Group | Peg-IFN + Placebo | Peg-IFN + Placebo | Peg-IFN + RBV | ||||||
| SVR (yes/no) | Yes | Yes | Yes | ||||||
| Genotype | 1a | 1a | 1a | ||||||
Symptoms, ALT and auto-antibodies
ALT elevation was examined in those children with ANA, LKM or F-Actin. At baseline, week 24 and week 72, children with and without these antibodies had similar ALT values. Of those with autoimmune hepatitis-related antibodies the highest ALT was 126 U/L (Table 4). In contrast, the highest ALT values, those exceeding 5 times the upper limit of normal (ULN,) all occurred among those children without antibodies to ANA, LKM or F-Actin. The most common side effects of treatment were flu-like symptoms (67%), GI symptoms (33%) and headaches (24%). Comparing 24 week data for flu-like symptoms, GI symptoms and headaches, side effects were not more frequent in those with or without antibodies.
Table 4.
Association between ALT and presence of antibodies associated with autoimmune hepatitis. The top panel provides data for those subjects with positive antibodies to LKM-1, ANA or F-Actin. The bottom panel provides data for those subjects with no detectable antibodies. Among those with liver associated auto-antibodies, there was no increase risk of hepatic flare or hepatitis as assessed by ALT.
| Liver Specific Autoantibodies | Baseline (n=114) | Week 24 (n=108) | Week 72 (n=62) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| #Pos (%) | ALT (Mean) | ALT (Range) | #Pos (%) | ALT (Mean) | ALT (Range) | #Pos (%) | ALT (Mean) | ALT (Range) | |
| LKM-1 | 5 (4.4) | 64.4 | 26–117 | 4 (3.7) | 43.75 | 14–95 | 2 (3.2) | 17 | 14–20 |
| ANA | 9 (7.9) | 44.1 | 25–69 | 9 (8.3) | 49.89 | 14–126 | 8 (12.9) | 26.6 | 10–106 |
| F-Actin | 1 (0.9) | 54 | 54 | 1 (0.9) | 129 | 129 | 1 (1.6) | 106 | 106 |
| Liver Specific Autoantibodies | Baseline (n=114) | Week 24 (n=108) | Week 72 (n=60)* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| # Without (%) | ALT (Mean) | ALT (Range) | # Without (%) | ALT (Mean) | ALT (Range) | # Without (%) | ALT (Mean) | ALT (Range) | |
| LKM-1 | 109 (95.6) | 53.77 | 10–254 | 104 (96.3) | 50.52 | 7–316 | 58 (96.7) | 23.8 | 7–109 |
| ANA | 105 (92.1) | 55.1 | 10–254 | 99 (91.67) | 50.31 | 7–316 | 52 (86.7) | 23.3 | 7–109 |
| F-Actin | 113 (99.1) | 54.2 | 10–254 | 107 (99.1) | 49.7 | 7–316 | 59 (98.3) | 22.2 | 7–109 |
For week 72, two subjects were missing ALT levels. Therefore, the n=60.
DISCUSSION
This is the first study to prospectively and longitudinally assess the presence and development of auto-antibodies in children with CHC. Despite use of conventional screening techniques that excluded 9 children who had antibodies to ANA, LKM and SMA, we found that almost 20% of the enrolled subjects had baseline antibodies using an ELISA technique. We found thyroid-, diabetes-, and hepatitis-related antibodies to be common in these children. The frequency of auto-antibodies, however, did not increase significantly with treatment in this cohort, nor did it change significantly six months after completion of treatment. The presence of auto-antibodies did not impact response to therapy. Furthermore, despite screening, three children (3%), none of whom had organ-specific auto-antibodies at baseline, developed de novo clinical autoimmune disease, 2 with hypothyroidism and 1 with IDDM.
Previous studies have presented conflicting results about the importance of antibodies before and during treatment with interferon based therapies. This study has methodologic advantages. All samples were analyzed simultaneously on well-standardized FDA-approved and CLIA-certified (except for IA2) immunoassays carried out by ELISA. This technology allows large numbers of samples to be simultaneously and objectively measured in WHO units; the assays are quantitative and represent a continuous function, as opposed to immunofluorescence assays which are subjective and discontinuous measures not amenable to statistical analysis in an epidemiologic study. Since these tests are performed under the proficiency program of the College of American Pathologists, the results can be compared nationally from one clinical laboratory to another.
Auto-antibodies have been reported in both adults and children with CHC. Auto-antibodies are seen in 70% of adults with CHC; 41% are ANA positive, 9% are SMA positive 12. Gregorio et al screened for auto-antibodies in 51 Italian children with CHC and found 65% to be positive (90% of treated patients, 65% of untreated, p=0.12); SMA was the most common antibody (67%), followed by ANA (10%) and LKM (8%)13. Bortolotti reported positive SMA in 17.5%, LKM in 10% and ANA in 7.5% of 40 children with CHC 14–15. Jara found ANA in 13%, SMA in 3%, and LKM in 10% of 30 children, and 7 children developed ANA during treatment16. In our prospectively studied North American cohort, the frequency is less and at least in part explained by our exclusion of children with high titer AIH related antibodies. In this study, the frequency of F-actin antibody (the cytoskeletal target of SMA) was less than 2%, LKM less than 5%, and ANA at most 13%. Other potential contributors to the difference in antibody frequency between this study population and that reported by Bortolotti, include differences in the populations studied, differences in the laboratory techniques for measuring auto-antibodies, as well as, our use of a stringent standard of strong positivity as defined by the manufacturer for each assay, rather than designating moderate or weak results as positive. These methodologic and population differences may account for variability in frequencies reported among these different studies.
It is notable that no child in this study, with or without antibodies, developed evidence of autoimmune hepatitis. Although we excluded 9 children with AIH related antibodies, we still identified children who had moderate and strong positive antibody by ELISA in the archived samples. No child had an ALT flare during treatment that limited therapy, similar to the experience of Jara’s group, who did not exclude children with autoantibodies from treatment as long as they did not have features of autoimmune hepatitis16. In contrast Bertolotti et al. reported in a study of only 40 subjects, that 5 of 7 LKM-positive children, had a hepatitis flare during treatment14. Gregorio reported the need to discontinue treatment in several auto-antibody positive children who developed worsening hepatitis while on interferon13. New LKM antibodies during treatment and association with a hepatitis flare has been reported in one patient 17.
Like most adult studies, we did not detect a relationship between auto-antibody appearance and treatment outcome 8, 18–20. In our study, there was no significant difference in 72 week treatment outcomes (only those who were virus negative at 24 weeks were analyzed) between the auto-antibody positive and negative groups, with SVR of 70% and 65%, respectively.
Of particular note is that neither our screening antibodies nor our archived samples assayed for thyroid and islet cell antibodies identified the subjects who developed the most serious complications. As previously discussed, one child treated in this study developed IDDM at 32 weeks of treatment. She was negative for GAD and IA-2 antibodies at the start of treatment and at 24 weeks. GAD antibody was positive in the study week 72 sample tested. Diabetes has been reported to occur rarely in patients with CHC who are treated with IFN 2; among 11,000 adults with viral hepatitis treated with interferon in a retrospective European report, 10 (0.08%) developed diabetes. Several adult CHC patients with positive diabetes-antibodies who did not develop diabetes with IFN treatment have been reported 21, 22. Wirth reported one girl who developed diabetes mellitus while being treated with peg IFN and ribavirin for hepatitis C23. In the present study, up to 9.3% of subjects had positive diabetes related antibodies at some point, with the notable development of clinical diabetes in only one child, who was antibody negative at baseline and 24 weeks. For comparison, diabetes antibody positivity in the general pediatric population range from 0.5–2.9% % 24. Auto-antibodies were thus not helpful in identifying the child who would develop diabetes in this study, although the literature suggests that these autoantibodies are strong predictors of subsequent development of diabetes in non-hepatitis C patients 25.
The development of thyroid disease is common in adults with CHC, both with and without treatment. Most studies report a rate of 10% or below26,27 Auto-antibody positivity prior to treatment has been associated with an increased risk27 of clinical autoimmune disease and new auto-antibodies develop during treatment in 5–45% 28. In the pediatric literature, about 20% of children treated with interferon and ribavirin developed thyroid autoantibodies, and about 10% developed transient or permanent hypothyroidism requiring treatment 9, 16, 23, 29. In the present report, the prevalence of thyroid auto-antibodies was lower than in most series at around 3%, with the frequency of anti-TG trending up from 0% at baseline to 6.5% at week 72. It thus appears that thyroid auto-antibodies as well as clinical thyroid disease occur uncommonly in children treated with IFN and at risk children cannot be identified prospectively.
We found that auto-antibodies are commonly detected in children with CHC infection and de novo autoimmune disease still occurs despite screening at baseline. Even sensitive antibody measures did not predict subjects at risk. Three children developed clinical disease, two with hypothyroidism and one with IDDM. In all 3 cases, screening antibodies as well as baseline and on-treatment antibodies did not identify risk until the disease developed. These data affirm that a variety of autoantibodies are common in children with CHC before, during and after treatment with Peg-IFN based therapy, Our screening at baseline may have accounted for our lower frequency of complications compared to historic reports. Even with screening and subsequent autoantibody assays, we were not able to identify children who would develop autoimmune disease. We provide fresh insight into the limited clinical role for following these antibodies to predict complications when treating children with CHC.
Acknowledgments
Grant Support: Grant Supporting Project: This study is supported by a cooperative agreement between the National Institute of Diabetes and Digestive and Kidney Diseases and the Food and Drug Administration, contract number 1UO1DK067767-01.CRC: This project was supported in part by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780 and study sites: MO1- RR-00069- Children’s Hospital, Aurora CO; M01-RR-02172- Children’s Hospital, Boston MA; M01-RR-01271- University of California, San Francisco, CA; 5-M01-RR-020359-01- National Medical Center, Washington DC; M01-RR-00645- Columbia University Medical Center, New York, NY; M01-RR-00082- University of Florida, Gainesville FL; M01-RR-00037- University of Washington, Seattle, WA; 5-M01-RR-000240- Children’s Hospital of Philadelphia, University of Pennsylvania, Philadelphia PA; U01-DK-067767-02- Johns Hopkins Medical Center, Baltimore MD; M01-RR-08084- University of Cincinnati, Cincinnati OH; M01-RR-00750- Indiana University, Indianapolis, IN. American Autoimmune Related Disease Association and Mr. and Mrs. Joseph Scoby Foundation. Its contents are the authors’ sole responsibility and do not necessarily represent official NIH views. Additional support was provided by Hoffmann-La Roche for study medications and central laboratory costs.
Abbrevations
- ALT
Alanine Aminotransferase
- ANA
Anti-Nuclear Antibody
- ASMA
Anti-Smooth Muscle Antibody
- CHC
Chronic Hepatitis C
- EVR/SVR
Early Viral Response/ Sustained Viral Response
- GAD
Anti-Glutamic Acid Decarboxylase
- GEE
Generalized Estimating Equation
- HCV
Hepatitis C Virus
- HCV RNA
Hepatitis C Virus Ribonucleic Acid
- IA2
Insulinoma antigen
- IDDM
Insulin Dependent Diabetes Mellitus
- IFN
Interferon
- LKM
Anti-Liver-Kidney Microsomal
- LKM-1
Anti-Liver-Kidney Microsomal Type 1
- RV
Ribavirin
- SMA
Smooth Muscle Antibody
- TG
Anti-Thyroglobulin
- TPO
Anti-Thyroid Peroxidase
- TSH
Thyroid Stimulating Hormone
- VR
Viral Response
Contributor Information
Jean P. Molleston, Email: jpmolles@iupui.edu.
William Mellman, Email: wmellman@gmail.com.
Michael R. Narkewicz, Email: michael.narkewicz@childrenscolorado.org.
William F. Balistreri, Email: w.balistreri@cchmc.org.
Regino P. Gonzalez-Peralta, Email: regino@peds.ufl.edu.
Maureen M. Jonas, Email: Maureen.jonas@childrens.harvard.edu.
Steven J. Lobritto, Email: sjl12@columbia.edu.
Parvathi Mohan, Email: pmohan@cnmc.org.
Karen F. Murray, Email: karen.Murray@seattlechildrens.org.
Dolores Njoku, Email: dnjoku@jhmi.edu.
Philip Rosenthal, Email: prosenth@peds.ucsf.edu.
Bruce A. Barton, Email: bruce.barton@umassmed.edu.
Monica V. Talor, Email: mtalor@jhmi.edu.
Irene Cheng, Email: icheng@c-tasc.com.
Kathleen B. Schwarz, Email: kschwarz@jhmi.edu.
Barbara A. Haber, Email: Haber.barb@gmail.com.
References
- 1.Jamil KM, Leedman PJ, Kontorinis N, et al. Interferon-induced thyroid dysfunction in chronic hepatitis C. J Gastroenterol Hepatol. 2009 Jun;24(6):1017–1023. doi: 10.1111/j.1440-1746.2008.05690.x. [DOI] [PubMed] [Google Scholar]
- 2.Schreuder TC, Gelderblom HC, Weegink CJ, et al. High incidence of type 1 diabetes mellitus during or shortly after treatment with pegylated interferon alpha for chronic hepatitis C virus infection. Liver Int. 2008 Jan;28(1):39–46. doi: 10.1111/j.1478-3231.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- 3.Bayraktar Y, Bayraktar M, Gurakar A, Hassanein TI, Van Thiel DH. A comparison of the prevalence of autoantibodies in individuals with chronic hepatitis C and those with autoimmune hepatitis: the role of interferon in the development of autoimmune diseases. Hepatogastroenterology. 1997 Mar-Apr;44(14):417–425. [PubMed] [Google Scholar]
- 4.Durante-Mangoni E, Iardino P, Resse M, et al. Silent celiac disease in chronic hepatitis C: impact of interferon treatment on the disease onset and clinical outcome. J Clin Gastroenterol. 2004 Nov-Dec;38(10):901–905. doi: 10.1097/00004836-200411000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Elefsiniotis IS, Pantazis KD, Fotos NV, Moulakakis A, Mavrogiannis C. Late onset autoimmune thrombocytopenia associated with pegylated interferon-alpha-2b plus ribavirin treatment for chronic hepatitis C. J Gastroenterol Hepatol. 2006 Mar;21(3):622–623. doi: 10.1111/j.1440-1746.2006.03418.x. [DOI] [PubMed] [Google Scholar]
- 6.Sykia A, Gigi E, Sinakos E, Bibashi E, Bellou A, Raptopoulou-Gigi M. Severe autoimmune hemolytic anemia complicated with liver decompensation and invasive aspergillosis in a patient with chronic hepatitis C during treatment with peg-interferon-a and ribavirin. J Gastrointestin Liver Dis. 2009 Mar;18(1):118–119. [PubMed] [Google Scholar]
- 7.Vezali E, Elefsiniotis I, Mihas C, Konstantinou E, Saroglou G. Thyroid dysfunction in patients with chronic hepatitis C: virus- or therapy-related? J Gastroenterol Hepatol. 2009 Jun;24(6):1024–1029. doi: 10.1111/j.1440-1746.2009.05812.x. [DOI] [PubMed] [Google Scholar]
- 8.Dabrowska MM, Panasiuk A, Flisiak R. Thyroid dysfunction in antiviral therapy of chronic hepatitis C. Hepatogastroenterology. 2010 Jul-Aug;57(101):826–831. [PubMed] [Google Scholar]
- 9.Wirth S, Lang T, Gehring S, Gerner P. Recombinant alfa-interferon plus ribavirin therapy in children and adolescents with chronic hepatitis C. Hepatology. 2002 Nov;36(5):1280–1284. doi: 10.1053/jhep.2002.36495. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz KB, Gonzalez-Peralta RP, Murray KF, et al. The combination of ribavirin and peginterferon is superior to peginterferon and placebo for children and adolescents with chronic hepatitis C. Gastroenterology. 2011 Feb;140(2):450–458. e451. doi: 10.1053/j.gastro.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray KF, Rodrigue JR, Gonzalez-Peralta RP, et al. Design of the PEDS-C trial: pegylated interferon +/− ribavirin for children with chronic hepatitis C viral infection. Clin Trials. 2007;4(6):661–673. doi: 10.1177/1740774507085445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cacoub P, Renou C, Rosenthal E, et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d’Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l’Hepatite C. Medicine (Baltimore) 2000 Jan;79(1):47–56. doi: 10.1097/00005792-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Gregorio GV, Pensati P, Iorio R, Vegnente A, Mieli-Vergani G, Vergani D. Autoantibody prevalence in children with liver disease due to chronic hepatitis C virus (HCV) infection. Clin Exp Immunol. 1998 Jun;112(3):471–476. doi: 10.1046/j.1365-2249.1998.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bortolotti F, Muratori L, Jara P, et al. Hepatitis C virus infection associated with liver-kidney microsomal antibody type 1 (LKM1) autoantibodies in children. J Pediatr. 2003 Feb;142(2):185–190. doi: 10.1067/mpd.2003.45. [DOI] [PubMed] [Google Scholar]
- 15.Frenzel C, Herkel J, Luth S, Galle PR, Schramm C, Lohse AW. Evaluation of F-actin ELISA for the diagnosis of autoimmune hepatitis. Am J Gastroenterol. 2006 Dec;101(12):2731–2736. doi: 10.1111/j.1572-0241.2006.00830.x. [DOI] [PubMed] [Google Scholar]
- 16.Jara P, Hierro L, de la Vega A, et al. Efficacy and safety of peginterferon-alpha2b and ribavirin combination therapy in children with chronic hepatitis C infection. Pediatr Infect Dis J. 2008 Feb;27(2):142–148. doi: 10.1097/INF.0b013e318159836c. [DOI] [PubMed] [Google Scholar]
- 17.Bortolotti F, Vajro P, Balli F, et al. Non-organ specific autoantibodies in children with chronic hepatitis C. J Hepatol. 1996 Nov;25(5):614–620. doi: 10.1016/s0168-8278(96)80228-5. [DOI] [PubMed] [Google Scholar]
- 18.Cassani F, Cataleta M, Valentini P, et al. Serum autoantibodies in chronic hepatitis C: comparison with autoimmune hepatitis and impact on the disease profile. Hepatology. 1997 Sep;26(3):561–566. doi: 10.1002/hep.510260305. [DOI] [PubMed] [Google Scholar]
- 19.Clifford BD, Donahue D, Smith L, et al. High prevalence of serological markers of autoimmunity in patients with chronic hepatitis C. Hepatology. 1995 Mar;21(3):613–619. [PubMed] [Google Scholar]
- 20.Todros L, Saracco G, Durazzo M, et al. Efficacy and safety of interferon alfa therapy in chronic hepatitis C with autoantibodies to liver-kidney microsomes. Hepatology. 1995 Nov;22(5):1374–1378. [PubMed] [Google Scholar]
- 21.Piquer S, Hernandez C, Enriquez J, et al. Islet cell and thyroid antibody prevalence in patients with hepatitis C virus infection: effect of treatment with interferon. J Lab Clin Med. 2001 Jan;137(1):38–42. doi: 10.1067/mlc.2001.111515. [DOI] [PubMed] [Google Scholar]
- 22.Di Cesare E, Previti M, Russo F, et al. Interferon-alpha therapy may induce autoantibody development in patients with chronic viral hepatitis. Digestive Diseases and Sciences. 1996;41:1672–1677. doi: 10.1007/BF02087923. [DOI] [PubMed] [Google Scholar]
- 23.Wirth S, Pieper-Boustani H, Lang T, et al. Peginterferon alfa-2b plus ribavirin treatment in children and adolescents with chronic hepatitis C. Hepatology. 2005 May;41(5):1013–1018. doi: 10.1002/hep.20661. [DOI] [PubMed] [Google Scholar]
- 24.Kulmala P, Rahko J, Savola K, et al. Stability of autoantibodies and their relation to genetic and metabolic markers of Type I diabetes in initially unaffected schoolchildren. Diabetologia. 2000 Apr;43(4):457–464. doi: 10.1007/s001250051329. [DOI] [PubMed] [Google Scholar]
- 25.Winter WE, Schatz DA. Autoimmune markers in diabetes. Clin Chem. 2011 Feb;57(2):168–175. doi: 10.1373/clinchem.2010.148205. [DOI] [PubMed] [Google Scholar]
- 26.Chan WB, Chow CC, Cockram CS. Interferon alpha treatment and endocrine disease. J R Soc Med. 2003 Oct;96(10):481–485. doi: 10.1258/jrsm.96.10.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gelu-Simeon M, Burlaud A, Young J, Pelletier G, Buffet C. Evolution and predictive factors of thyroid disorder due to interferon alpha in the treatment of hepatitis C. World J Gastroenterol. 2009 Jan 21;15(3):328–333. doi: 10.3748/wjg.15.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deutsch M, Dourakis S, Manesis EK, et al. Thyroid abnormalities in chronic viral hepatitis and their relationship to interferon alfa therapy. Hepatology. 1997 Jul;26(1):206–210. doi: 10.1002/hep.510260127. [DOI] [PubMed] [Google Scholar]
- 29.Wirth S, Ribes-Koninckx C, Calzado MA, et al. High sustained virologic response rates in children with chronic hepatitis C receiving peginterferon alfa-2b plus ribavirin. J Hepatol. 2010 Apr;52(4):501–507. doi: 10.1016/j.jhep.2010.01.016. [DOI] [PubMed] [Google Scholar]