Abstract
Purpose
To explore surface-immobilized and suspended modalities of the hydrophobic polycation N,N-dodecyl, methyl-polyethylenimine (DMPEI) for the ability to reduce viral infectivity in aqueous solutions containing herpes simplex viruses (HSVs) 1 and 2.
Methods
Surface-immobilized (coated onto surfaces) and suspended DMPEI were incubated with aqueous solutions containing HSV-1 or -2 to measure the antiviral effect of the hydrophobic polycation’s formulations on HSVs.
Results
DMPEI coated on either polyethylene slides or male latex condoms dramatically decreases infectivity in solutions containing HSV-1 or -2. Moreover, DMPEI suspended in aqueous solution markedly reduces the infectious titer of these HSVs.
Conclusion
Our results suggest potential uses of DMPEI for both prophylaxis (in the form of coated condoms) and treatment (as a topical suspension) for HSV infections.
Keywords: antiviral, herpes simplex viruses, polyethylenimine, prophylaxis, therapy
INTRODUCTION
Herpes simplex viruses (HSVs) 1 and 2 are enveloped DNA viruses that can cause oral and genital lesions in humans. While typically HSV-1 causes oral lesions and HSV-2 genital ones, both are capable of infecting either region (1). These communicable viruses are ubiquitous and a serious public health concern because of acute and lifelong manifestations of herpes in infected individuals (2). HSV-2 is particularly prevalent, with a 16% reported frequency in people aged between 15 and 49 worldwide (3,4). HSVs also have high recurrence rates of 33–90% per year in infected individuals (5). Infectious viral particles are excreted from the lesions caused by HSVs and can be transmitted through such personal contact as kissing and sexual intercourse. Furthermore, individuals infected with HSV-2 have an increased risk of infection by human immunodeficiency virus (6). HSV transmission can be reduced by avoiding direct contact during symptomatic outbreaks, but asymptomatic shedding of the virus is still possible resulting in transmission. Although such antiviral drugs as acyclovir reduce outbreaks and wane symptoms during them (7), acyclovir-resistant HSV strains have been reported (8). There is no cure or vaccine available for either HSV-1 or HSV-2; thus developing a strategy that directly prevents viral transmission is important to reduce its spread.
Certain long-chained hydrophobic polycationic polymers have been shown both in vitro and in vivo to inactivate a wide variety of microbial pathogens, including Escherichia coli, Staphylococcus aureus and epidermides, and Pseudomonas aeruginosa bacteria, Candida albicans yeast, and human and avian strains of enveloped influenza viruses, as well as non-enveloped poliovirus and rotavirus (9–21). In the present work, we demonstrate that the hydrophobic polycation N,N-dodecyl, methyl-polyethylenimine (DMPEI) (previously determined to be maximally antimicrobial when used as a non-covalent coating (10,12,13,20)) can also decrease the infectivity of HSV-1 or -2. Based on this finding, we have explored DMPEI for potential therapeutic and prophylactic uses.
MATERIALS AND METHODS
Materials
All chemicals and reagents were from Sigma-Aldrich Chemical Co. (St. Louis, MO) and used without further purification. Polyethylene sheets, from McMaster Carr (Atlanta, GA), were cut into 2.5×2.5-cm slides. Non-lubricated male latex condoms (Trojan®), distributed by Church & Dwight Co. (Princeton, NJ) and obtained from a local drug store, were used in this study as the simplest type of a condom uncomplicated by possible effects of the lubricant. They were rinsed thoroughly in double-distilled (dd) H2O and cut into 3×4-cm rectangles for further use. The MTS assay kit (CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay) was purchased from Promega (Madison, WI). Dulbecco’s Modified Eagle Medium (DMEM) was from Cellgro (Manassas, VA) and supplemented with 5% fetal bovine serum (FBS), 5% bovine calf serum (BCS), 1% penicillin, 1% streptomycin, 4.5 g/L glucose, and 2 mM L-glutamine for cell culture. Human immunoglobulin (IgG) (cat# NDC 0944-2700-02) was from Baxter (Deerfield, IL) and used to supplement DMEM in the plaque assay. Giemsa cell stain utilized in plaque assays was from Sigma-Aldrich.
Syntheses of Polycations
DMPEI was synthesized from linear 217-kDa polyethylenimine (PEI) as previously described (15). Per-methylated PEI (PMPEI) was synthesized similarly to a reported procedure (22). Briefly, 1 g of 750-kDa branched PEI was dissolved in 15 mL of anhydrous methanol, and an excess (17.2 mL) of iodomethane was added to the solution at room temperature. The mixture was refluxed at 65°C for 2 h, followed by cooling to room temperature, addition of 928 mg of NaOH, and heating for an additional 12 h. The solvent was then removed by rotary evaporation, and the resultant solid was dissolved in ddH2O and dialyzed against it four times, followed by lyophilization to obtain solid PMPEI. The structure of PMPEI was verified by elemental analysis (C, 24.7%; H, 5.58%; N, 9.14%) and NMR (1H NMR (D2O) δ (400 mHz) for PMPEI: 2.8–4.6 (CH2, m, CH3, s)).
Preparation of Slides and Coated Condoms
Coated slides were prepared by painting one side of a 2.5× 2.5-cm polyethylene slide with a 50 mg/mL solution of DMPEI in chloroform, followed by solvent evaporation; this painting was performed in quadruplicate (15). Coated condoms were prepared similarly, except that DMPEI was dissolved in butanol (23).
Cells and Viruses
HSV-1 KOS strain and HSV-2 186/syn+-1 strain were originally obtained from Priscilla A. Schaffer (24,25). The viruses were diluted with an aqueous buffer (phosphate-buffered saline (PBS) supplemented with 1% BCS, 0.01% glucose). Vero cells were from the American Type Culture Collection (#CCL-81, Manassas, VA) and maintained in supplemented cell culture DMEM (described above) at 37°C in a 5% CO2 atmosphere. They were grown to confluent monolayers in 6-well plates or T25 flasks for use in plaque assays.
Antiviral Assay for DMPEI-coated Polyethylene Slides
To determine the antiviral effect of DMPEI against HSV-1 and HSV-2, a DMPEI-coated polyethylene slide was placed in a polystyrene Petri dish (6.0×1.5-cm), and a 10-μL droplet of either HSV-1 ((4.4±3.9)×105 plaque forming units (PFU)) or HSV-2 ((5.0±0.69)×104 PFU) was added to the middle of the coated region. The droplet was sandwiched with a plain polyethylene slide to increase surface contact of the virus with the coated slide. After 15 min at room temperature, the slides were separated and washed thoroughly with 0.99 mL of the supplemented PBS buffer. The washings were collected, serially diluted, and analyzed in the plaque assay as described below. The infectious viral titer from the above experiments was compared to those of a control with two uncoated slides and a control with the virus never in contact with slides to determine the relative reduction in viral titer upon incubation with DMPEI-coated slides (9,12,15).
Infectivity Reduction Assay
Ten-fold serial dilutions of the washings from the antiviral assays were tested in the infectivity assay to calculate infectious viral titers. Specifically, cell growth medium was removed from confluent monolayers of Vero cells in a 6-well plate and replaced with 0.5 mL of diluted virus in each well. The plate was gently shaken for 1 h at 37°C to initiate infection, after which the virus solutions were removed and replaced with 2.5 mL of DMEM supplemented with 1% BCS and 0.16% human IgG (for HSV-2, DMEM with 1% BCS and 0.32% human IgG was used). The cells were incubated for 2 days at 37°C and 3 days at 34°C for HSV-1 and HSV-2, respectively, then fixed with ice-cold methanol for 8–10 min and stained with Giemsa dye. Plaques were counted to determine the viral titer of the solutions.
Antiviral Assay with PEI and PMPEI
HSV-1 (10 μL containing (7.8±0.40)×107 PFU) was incubated with 0.99 mL of either branched 750-kDa PEI (2 mg/mL) or PMPEI (1 mg/mL) in the supplemented PBS buffer for 30 min on a rotating arm. Serial dilutions of the resultant mixtures were tested with the plaque assay and compared to HSV-1 incubated with the supplemented PBS buffer alone to determine the antiviral effect of the polycations.
Preparation of DMPEI Suspensions
DMPEI was dissolved in dimethylsulfoxide at 10 mg/mL and added to 3 volumes of ddH2O with vigorous stirring. The resultant mixture was lyophilized and resuspended in ddH2O with vigorous stirring and sonication until the suspension was devoid of visible chunks. The stock DMPEI suspension was diluted in PBS for further use.
A DMPEI suspension thickened with hydroxyethyl-cellulose (HEC) was prepared by making a 1.5% solution of HEC in 2.5 mL of PBS containing the desired concentration of DMPEI. The mixture was heated gently and stirred until the HEC dissolved and the solution thickened.
MTS Assay
A 3 mg/mL DMPEI suspension in PBS was 10-fold serially diluted, followed by incubation of 100 μL thereof for 1 h with confluent monolayers of Vero cells seeded in a 96-well plate. After 1 h, the serial dilutions of DMPEI suspension were removed, and the cells were washed twice with PBS and replenished with 100 μL of fresh DMEM. The MTS assay was carried out according to the manufacturer’s instructions.
Antiviral Assays with DMPEI Suspensions
To quantify the antiviral effect of a DMPEI suspension, 10 μL of a HSV-1 solution ((8.7±2.8)×107 PFU) was incubated with 0.99 mL of a DMPEI suspension (0.3 mg/mL) for 30 min on a rotating arm. Thereafter, the virus and suspension mixture was filtered with a 0.45-μm cut-off filter (Pall Life Sciences), and the filtrate was 10-fold serially diluted and used in an infectivity assay to determine the reduction in viral titer. Controls in which HSV-1 was incubated either in the supplemented PBS buffer and not filtered, or in the supplemented PBS buffer and subsequently filtered, or with a 0.3 mg/mL DMPEI suspension solution filtrate were performed in parallel.
The antiviral effect of a DMPEI suspension present during infection was determined by incubating different concentrations of the suspension (or just the supplemented PBS buffer as a control) with HSV-1 during infection of a confluent Vero cell monolayer in a T25 flask. Specifically, 0.5 mL of a DMPEI suspension (or the supplemented PBS buffer) and 0.5 mL containing 210±10 PFU of HSV-1 were mixed in a T25 flask and gently agitated for 1 h at 37°C. Then the virus and DMPEI suspension mixture was removed and replaced with 5 mL of DMEM containing 1% BCS and 0.16% human IgG. The T25 flasks were incubated, fixed, and stained as described above to determine the viral titer with and without the DMPEI suspension treatment.
For antiviral assays with various concentrations of DMPEI suspensions containing 1.5% HEC, 25 μL of either HSV-1 ((3.8±0.20)×105 PFU) or HSV-2 ((2.5±0.90)×104 PFU) was added to 2.5 mL of HEC-thickened DMPEI suspensions in 20-mL scintillation vials containing 1-cm long magnetic stir bars (to aid in mixing). Vials were placed on a rotating arm for 30 min, after which time 0.5 mL of the solution was carefully withdrawn by a pipette and diluted for the subsequent infectivity assay. Controls containing 2.5 mL of plain PBS, as well as a 1.5% HEC solution in the absence of DMPEI, were also carried out.
Antiviral Assay for DMPEI-coated Condoms
To assess the antiviral activity of the coated latex condoms, the above-described procedure for polyethylene slides was followed, except that a piece of coated condom replaced the coated slide. In addition, to better mimic the intended real-life use of a condom, we also tested a piece of coated condom that had been stretched both horizontally and vertically ten times after painting (denoted henceforth as a stretched latex condom) to determine the effect of stretching and handling on antiviral activity. After a 15-min incubation with HSV-1 ((8.8±0.80)×105 PFU) or HSV-2 ((4.8±0.1)× 104 PFU), the plain slide was separated from the condom piece, and both were submerged in a 50-mL falcon tube containing 10 mL of the supplemented PBS buffer. This 10-mL washing was serially diluted and tested in the infectivity assay. The same experiment was performed with a piece of uncoated latex condom and also without one as controls.
RESULTS AND DISCUSSION
To ascertain whether immobilized DMPEI can reduce infectivity of aqueous solutions containing HSV-1 or HSV-2, we placed a virus-containing solution on either a DMPEI-coated or uncoated polyethylene slide and measured the change in infectivity in the solution by comparing viral titers of the resultant washings (9,16). Exposure to a DMPEI-coated polyethylene slide reduced the viral titer to below the limit of detection of the plaque assay, thus yielding at least a 5-log reduction in viral titer for HSV-1 and at least a 4-log reduction for HSV-2 compared to minimal reduction for the uncoated polyethylene slide (Fig. 1). These observations encouraged us to further explore this phenomenon and its potential applications.
Fig 1.
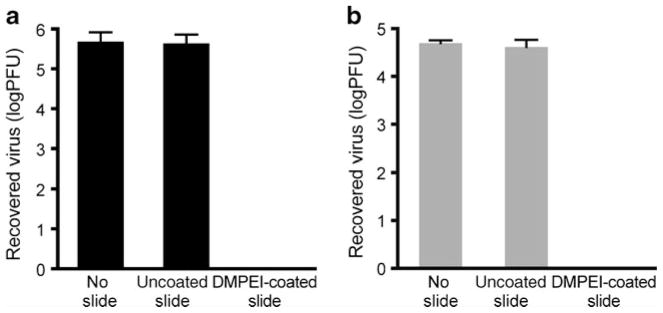
Reduction of viral titers of HSV-1 (a) and HSV-2 (b) incubated for 15 min at room temperature in a buffered aqueous solution (the left set of bars), in that in the presence of an uncoated polyethylene slide (the middle set of bars), and in that in the presence of a DMPEI-coated polyethylene slide (the right set of bars). In the “No slide” sample, a virus solution was incubated in buffer only. In the “Uncoated slide” sample, a virus solution was incubated between two uncoated polyethylene slides for 15 min to account for any non-specific adsorption of the virus to the slide. In the “DMPEI-coated slide” sample, one of the two polyethylene slides was painted with the hydrophobic polycation. The limit of detection for the assay is approximately 1 PFU. The heights of the bars are mean values, and the error bars are standard deviations.
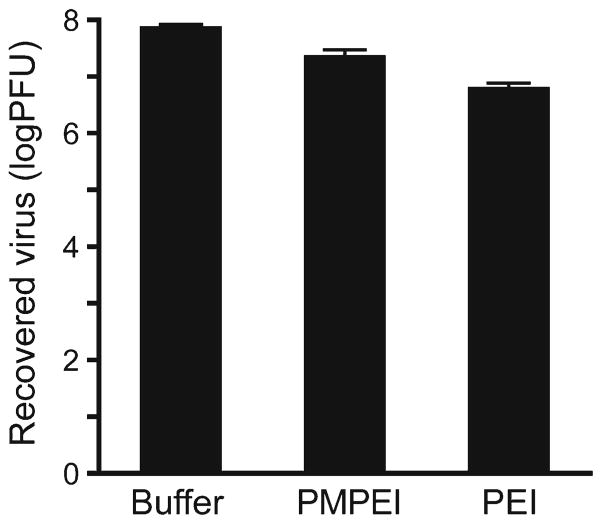
Since HSVs are transmitted by direct contact with viral lesions, an antiviral formulation should ideally be available in a form that can intimately interact with infected tissues. Because DMPEI was deliberately designed as a non-leaching surface coating and it is insoluble in aqueous solution (10), we explored whether its water-soluble homolog, per-methylated PEI (PMPEI), or even an underivatized PEI were capable of inactivating HSV-1 (as a soluble form of the polymer could be easily administered to a lesion). To this end, we incubated PMPEI or PEI with an aqueous solution containing HSV-1 and determined the consequent loss of infectivity. Not only was the polycation PMPEI incapable of lowering the HSV-1 titer by more than a single log in aqueous solution, but also it was less potent than the unalkylated PEI (Fig. 2). We concluded, therefore, that the polycationic nature was not the sole, or even the main, determinant of anti-HSV activity and substantial hydrophobicity was required as well.
Fig 2.
Reduction of viral titer of HSV-1 incubated for 30 min at room temperature in a buffered aqueous solution (the left bar), in that in the presence of PMPEI (1 mg/mL) (the middle bar), and in that in the presence of PEI (2 mg/mL) (the right bar). The heights of the bars are mean values, and the error bars are standard deviations.
We reasoned that a suspension of the hydrophobic DMPEI in aqueous buffer could circumvent the solubility issues (as well as the use of organic solvents), while still allowing a rather intimate contact with the virus and hence possibly an antiviral effect. To test this hypothesis, we prepared an aqueous suspension of DMPEI and investigated whether it was capable of inactivating HSV-1 and HSV-2. Although surface-immobilized DMPEI showed little toxicity to mammalian cells (21,23,26), we found greater toxicity of DMPEI in suspension. Based on an MTS assay, we observed a 50% cell cytotoxicity (CC50) at a 120-μg/mL polycation concentration. To determine what causes the toxicity, we subjected a 0.3-mg/mL suspension of DMPEI to filtration through a 0.45-μm filter and compared the filtrate’s toxicity in Vero cells to that of the unfiltered suspension. Since the DMPEI filtrate exhibited no visible toxicity (results not shown), we concluded that toxicity must result from particles larger than approximately 0.45-μm. Therefore, to eliminate artifacts caused by large particles during plaque assay, we modified the assay protocol: following the incubation of HSV-1 with the DMPEI suspension, we filtered the mixture through a 0.45-μm filter, which should remove the corresponding polycation particles but not the virus (which is 0.2-μm diameter (27)).
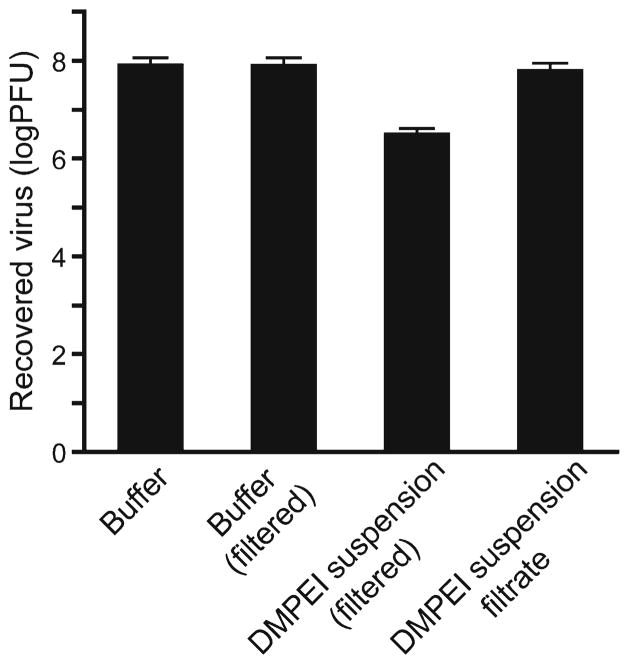
Using these conditions in a modified assay, we observed that incubating a 0.3-mg/mL DMPEI suspension with HSV-1 lowered the viral titer by more than 1.5 logs (Fig. 3). Moreover, most of the observed antiviral activity was due to the DMPEI particles larger than 0.45 μm because incubation with DMPEI suspension filtrate did not have a marked anti-HSV effect (Fig. 3). Lastly, filtering itself did not contribute to a reduction of viral titer (second bar, Fig. 3). The lower antiviral activity detected for the DMPEI suspension, as compared to DMPEI-coated polyethylene slides (Fig. 1), was likely caused by the inherent differences in surface contact between the virus and DMPEI in the two assays.
Fig 3.
Reduction of viral titer of HSV-1 incubated for 30 min at room temperature in a buffered aqueous solution (the left bar), in that subsequently filtered through a 0.45-μm filter (the middle left bar), in that containing 0.3 mg/mL of DMPEI suspension and subsequently filtered through a 0.45-μm filter (the middle right bar), and in that of a 0.3 mg/mL DMPEI suspension filtrate (the right bar). The heights of the bars are mean values, and the error bars are standard deviations.
If used as a therapeutic, DMPEI would presumably be applied topically in the presence of both the virus and host cells. Therefore, we next examined a scenario where a DMPEI suspension was incubated with HSV-1 during the infection of Vero cells. During this experiment, we observed a dose-dependent response for the DMPEI suspension with regard to both inhibition of HSV-1 infection and toxicity. For example, when the virus encountered a 30-μg/mL DMPEI suspension, a 41% drop in viral titer was observed with no apparent toxicity. As the concentration of the DMPEI in the suspension was increased to 0.1, 0.3, and 0.5 mg/mL, we observed a 85%, 95%, and 100% decrease in viral titer, respectively (Table I). These marked drops in infectivity were accompanied by increasing toxicity. From these data, the half-maximal inhibitory concentration (IC50) was calculated to be 24 μg/mL. From our previous MTS assay toxicity data, we conclude that the therapeutic index (CC50/IC50) of our DMPEI suspension is 5.
Table I.
Antiviral Activity Against HSV-1 (Assessed by Infectivity Reduction Assay) and Toxicity Toward Vero Cells (Assessed Visually After Staining the Cells) of DMPEI Suspensions
| Concentration of DMPEI (mg/mL) | % reductiona | Cell viabilityb |
|---|---|---|
| 0 | 0 ± 3 | +++ |
| 0.03 | 41 ± 6 | +++ |
| 0.1 | 85 ± 2 | ++ |
| 0.3 | 95 ± 8 | + |
| 0.5 | 100 ± 0 | + |
Reduction is compared to an incubation of HSV-1 without DMPEI under otherwise the same conditions. Experiments were carried out in triplicate, with the mean and standard deviation values presented in the table. See Materials and Methods for experimental details
Score based on visual appearance of the cells: three pluses denote all healthy cells, two pluses denote some visible toxicity, and a single plus denotes marked visible toxicity
For a DMPEI suspension to be used as a topical therapeutic, it should be “pharmaceutically elegant”, i.e., in the form of a homogeneous cream or lotion. To achieve the corresponding no-drip consistency, we thickened a DMPEI suspension by adding the common pharmaceutical excipient hydroxyethyl-cellulose (HEC) (28). As with the non-thickened suspensions above, we observed a dose-dependent antiviral response; for example, a 1.5-mg/mL aqueous DMPEI suspension containing 1.5% HEC elicited over a 2-log reduction in viral titer for both HSV-1 and HSV-2, while less concentrated suspensions of DMPEI resulted in a reduction in viral titers between one and two logs (Fig. 4). Note that a control aqueous solution containing the same concentration of HEC alone had no appreciable influence on viral titer.
Fig 4.
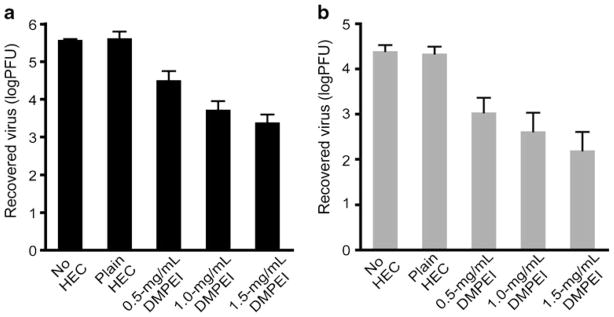
Reduction of viral titers of HSV-1 (a) and HSV-2 (b) incubated for 30 min at room temperature in an aqueous PBS buffer (the left set of bars), in that thickened with 1.5% HEC (the 2nd set of bars), and in that thickened with 1.5% HEC in which various concentrations of DMPEI were suspended (the 3rd, 4th, and 5th sets of bars). The heights of the bars are mean values, and the error bars are standard deviations.
To explore whether DMPEI could be used in a prophylactic mode, we painted the outside surface of a male latex condom with a 50 mg/mL solution of DMPEI in butanol and, following evaporation of solvent, tested its ability to disinfect aqueous solutions of HSV-1 and HSV-2. As seen in Fig. 5, more than a 3-log reduction in infectivity for both viruses compared to the uncoated latex condom was observed.
Fig 5.
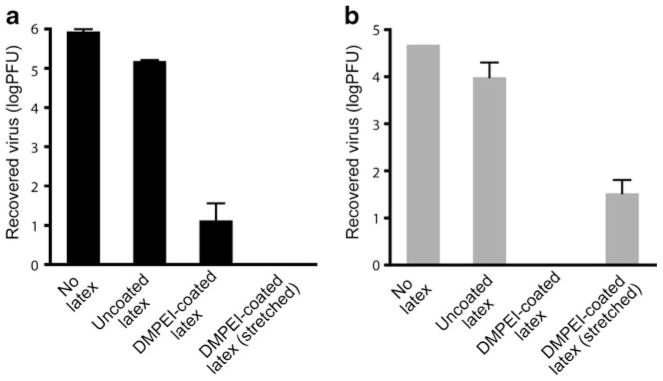
Reduction of viral titers of HSV-1 (a) and HSV-2 (b) incubated for 15 min at room temperature in a buffered aqueous solution (the left set of bars), in that sandwiched between an uncoated polyethylene slide and an uncoated piece of latex condom (the middle left set of bars), in that sandwiched between a DMPEI-coated latex condom and an uncoated polyethylene slide (the middle right set of bars), and in that sandwiched between a DMPEI-coated latex condom that had been stretched extensively and an uncoated polyethylene slide (the right set of bars). In the “No latex” sample, a virus solution was incubated in buffer only. In the “Uncoated latex” sample, a virus solution was incubated between a piece of uncoated polyethylene slide and of uncoated latex for 15 min to account for a non-specific adsorption of the virus to the slide and/or latex. In the “DMPEI-coated latex” sample, a piece of latex was painted with the hydrophobic polycation. In the “DMPEI-coated latex (stretched) ” sample, a piece of latex was painted with the hydrophobic polycation, allowed to dry, and stretched 10-times horizontally and vertically to imitate real-life use of condoms, after which time virus solution was incubated with it. The limit of detection for the assay is approximately 1 PFU. The heights of the bars are mean values, and the error bars are standard deviations.
We also examined whether handling and extensive stretching of DMPEI-coated condoms (imitating the conditions likely to be encountered during their intended use) affected their ability to disinfect viral solutions. More than a 3-log reduction was observed with a stretched DMPEI-coated latex condom for HSV-1 and more than a 2-log reduction for HSV-2 (Fig. 5). The reduced inactivation observed for the coated latex condoms, as compared to DMPEI-coated polyethylene slides (see Fig. 1), could be due to a lower rigidity of latex than of polyethylene resulting in less uniform coatings. In addition, stretching may produce cracks in the DMPEI coating where HSVs can remain unmolested. It was also noted that some of the DMPEI flaked off into the supplemented PBS buffer during the washing step; clearly, coating of the condoms must be optimized further.
CONCLUSIONS
We have demonstrated herein that the hydrophobic polycationic material DMPEI could be employed both in a prophylactic modality (as a coating on latex condoms) and in a therapeutic modality (as a suspension) to inactivate HSVs. A drawback of the latter application is the uncovered toxicity toward mammalian cells. Although we previously observed no appreciable acute toxicity, either in vitro (23) or in vivo (21,26) for DMPEI coatings, a suspension presumably results in a more intimate contact between the cells and the hydrophobic polycation and thus greater toxicity. Future studies need to address whether the antiviral activity and toxicity are coupled and, if so, whether structural changes to the polycation can uncouple them. Once this is done, one can progress to in vivo studies to investigate a topical application of DMPEI in animal HSV models. As to a possible prophylactic use, both optimized noncovalent painting of, and covalent attachment of hydrophobic polycations to, latex (polyisoprene) condoms should be explored and the resultant coated condoms tested in terms of their long-term stability and safety.
Footnotes
DISCLOSURES
This research was supported by the U.S. Army Research Office under contract W911NF-07-D-0004 (to AMK) and NIH grant AI057552 (to DMK). AML is a recipient of a Martin Family graduate fellowship.
Contributor Information
Alyssa M. Larson, Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA
Hyung Suk Oh, Department of Microbiology and Immunobiology, Harvard Medical School, 200 Longwood Avenue, Boston, Massachusetts 02115, USA.
David M. Knipe, Email: david_knipe@hms.harvard.edu, Department of Microbiology and Immunobiology, Harvard Medical School, 200 Longwood Avenue, Boston, Massachusetts 02115, USA
Alexander M. Klibanov, Email: klibanov@mit.edu, Departments of Chemistry and Biological Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, USA
References
- 1.Lookerand KJ, Garnett GP. A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sex Transm Infect. 2005;81:103–7. doi: 10.1136/sti.2004.012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao P, Hong Thanh P, Kulkarni A, Yang Y, Liu X, Knipe DM, et al. Herpes simplex virus 1 glycoprotein B and US3 collaborate to inhibit CD1d antigen presentation and NKT cell function. J Virol. 2011;85:8093–104. doi: 10.1128/JVI.02689-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease and Prevention. Seroprevalence of herpes simplex virus type 2 among persons aged 14–49 years—United States, 2005–2008. MMWR Morbidity and mortality weekly report. 2010;59:456–459. [PubMed] [Google Scholar]
- 4.Looker KJ, Gamett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organization. 2008;86:805–12. doi: 10.2471/BLT.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields virology. 5. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 2501–2601. [Google Scholar]
- 6.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 7.Obrienand JJ, Campolirichards DM. Acyclovir- an updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1989;37:233–309. doi: 10.2165/00003495-198937030-00002. [DOI] [PubMed] [Google Scholar]
- 8.Piretand J, Boivin G. Resistance of Herpes Simplex Viruses to Nucleoside Analogues: Mechanisms, Prevalence, and Management. Antimicrob Agents Chemother. 2011;55:459–72. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haldar J, An D, de Cienfuegos LA, Chen J, Klibanov AM. Polymeric coatings that inactivate both influenza virus and pathogenic bacteria. Proc Natl Acad Sci USA. 2006;103:17667–71. doi: 10.1073/pnas.0608803103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klibanov AM. Permanently microbicidal materials coatings. J Mater Chem. 2007;17:2479–82. [Google Scholar]
- 11.Lin J, Qiu SY, Lewis K, Klibanov AM. Mechanism of bactericidal and fungicidal activities of textiles covalently modified with alkylated polyethylenimine. Biotechnol Bioeng. 2003;83:168–72. doi: 10.1002/bit.10651. [DOI] [PubMed] [Google Scholar]
- 12.Larson AM, Hsu BB, Rautaray D, Haldar J, Chen J, Klibanov AM. Hydrophobic polycationic coatings disinfect poliovirus and rotavirus solutions. Biotechnol Bioeng. 2011;108:720–3. doi: 10.1002/bit.22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haldar J, Chen J, Tumpey TM, Gubareva LV, Klibanov AM. Hydrophobic polycationic coatings inactivate wild-type and zanamivir- and/or oseltamivir-resistant human and avian influenza viruses. Biotechnol Lett. 2008;30:475–9. doi: 10.1007/s10529-007-9565-5. [DOI] [PubMed] [Google Scholar]
- 14.Haldar J, Weight AK, Klibanov AM. Preparation, application and testing of permanent antibacterial and antiviral coatings. Nat Protoc. 2007;2:2412–7. doi: 10.1038/nprot.2007.353. [DOI] [PubMed] [Google Scholar]
- 15.Hsu BB, Ouyang J, Wong SY, Hammond PT, Klibanov AM. On structural damage incurred by bacteria upon exposure to hydrophobic polycationic coatings. Biotechnol Lett. 2011;33:411–6. doi: 10.1007/s10529-010-0419-1. [DOI] [PubMed] [Google Scholar]
- 16.Hsu BB, Wong SY, Hammond PT, Chen J, Klibanov AM. Mechanism of inactivation of influenza viruses by immobilized hydrophobic polycations. Proc Natl Acad Sci USA. 2011;108:61–6. doi: 10.1073/pnas.1017012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin J, Murthy SK, Olsen BD, Gleason KK, Klibanov AM. Making thin polymeric materials, including fabrics, microbicidal and also water-repellent. Biotechnol Lett. 2003;25:1661–5. doi: 10.1023/a:1025613814588. [DOI] [PubMed] [Google Scholar]
- 18.Lin J, Qiu SY, Lewis K, Klibanov AM. Bactericidal properties of flat surfaces and nanoparticles derivatized with alkylated polyethylenimines. Biotechnol Prog. 2002;18:1082–6. doi: 10.1021/bp025597w. [DOI] [PubMed] [Google Scholar]
- 19.Milovic NM, Wang J, Lewis K, Klibanov AM. Immobilized N-alkylated polyethylenimine avidly kills bacteria by rupturing cell membranes with no resistance developed. Biotechnol Bioeng. 2005;90:715–22. doi: 10.1002/bit.20454. [DOI] [PubMed] [Google Scholar]
- 20.Park D, Wang J, Klibanov AM. One-step, painting-like coating procedures to make surfaces highly and permanently bactericidal. Biotechnol Prog. 2006;22:584–9. doi: 10.1021/bp0503383. [DOI] [PubMed] [Google Scholar]
- 21.Behlau I, Mukherjee K, Todani A, Tisdale AS, Cade F, Wang L, et al. Biocompatibility and biofilm inhibition of N, N-hexyl, methyl-polyethylenimine bonded to Boston Keratoprosthesis materials. Biomaterials. 2011;32:8783–96. doi: 10.1016/j.biomaterials.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas M, Klibanov AM. Enhancing polyethylenimine’s delivery of plasmid DNA into mammalian cells. Proc Natl Acad Sci USA. 2002;99:14640–5. doi: 10.1073/pnas.192581499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukherjee K, Rivera JJ, Klibanov AM. Practical aspects of hydrophobic polycationic bactericidal “paints”. Appl Biochem Biotechnol. 2008;151:61–70. doi: 10.1007/s12010-008-8151-1. [DOI] [PubMed] [Google Scholar]
- 24.Schaffer P, Vonka V, Lewis R, Benyeshm M. Temperature-senisitive mutants of herpes simplev virus. Virology. 1970;42:1144–6. doi: 10.1016/0042-6822(70)90364-8. [DOI] [PubMed] [Google Scholar]
- 25.Spang AE, Godowski PJ, Knipe DM. Characterization of herpes-simplex virus-2 temperature-sensitive mutants whose lesions map in or near the coding sequences for the major DNA-binding protein. J Virol. 1983;45:332–42. doi: 10.1128/jvi.45.1.332-342.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaer TP, Stewart S, Hsu BB, Klibanov AM. Hydrophobic polycationic coatings that inhibit biofilms and support bone healing during infection. Biomaterials. 2012;33:1245–54. doi: 10.1016/j.biomaterials.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 27.Grunewald K, Desai P, Winkler DC, Heymann JB, Belnap DM, Baumeister W, et al. Three-dimensional structure of herpes simplex virus from cryo-electron tomography. Science. 2003;302:1396–8. doi: 10.1126/science.1090284. [DOI] [PubMed] [Google Scholar]
- 28.Nel AM, Smythe SC, Habibi S, Kaptur PE, Romano JW. Pharmacokinetics of 2 dapivirine vaginal microbicide gels and their safety vs. hydroxyethyl cellulose-based universal placebo gel. Jaids-J Acquired Immune Defic Syndromes. 2010;55:161–9. doi: 10.1097/QAI.0b013e3181e3293a. [DOI] [PubMed] [Google Scholar]