Abstract
Objective
To investigate the expression and regulation of colony-stimulating factor 1 (CSF-1) and its receptor, C-FMS, in endometriosis.
Design
In vivo and vitro study.
Setting
University-based academic medical center.
Patient(s)
Reproductive-age women undergoing surgery for benign conditions.
Intervention(s)
Peritoneal and endometrial tissue samples were obtained.
Main Outcome Measure(s)
CSF-1 and C-FMS expression.
Result(s)
Significantly higher CSF-1 levels were found in peritoneal fluid of patients with endometriosis compared with control subjects. Ectopic endometriotic tissue had 3.5-fold and 1.7-fold increases in CSF-1 and C-FMS expression, respectively, compared with eutopic tissue. Coculture of endometrial cells from either established cell lines or patient samples with peritoneal mesothelial cells (PMCs) led to increased expression of CSF-1 and C-FMS. A higher but nonsignificant increase in levels of C-FMS and CSF-1 was found in cocultures of endometrial epithelial cells from patients with endometriosis compared with those without endometriosis.
Conclusion(s)
Increased CSF-1 levels may contribute to endometriosis lesion formation and progression. Elevation in CSF-1 after coculture of endometrial cells with PMCs suggests that endometrial tissue may be a source of peritoneal CSF-1. Increased C-FMS expression in endometrial cells from women with endometriosis cocultured with PMCs suggests that endometrial tissue involved in lesion formation is highly responsive to CSF-1 signaling. (Fertil Steril® 2012;97:1129-35. ©2012 by American Society for Reproductive Medicine.)
Keywords: Endometriosis, colony-stimulating factor 1, C-FMS, early lesion development, macrophage, cell culture, peritoneal fluid
Endometriosis affects 10% of reproductive-age women and is associated with infertility and pelvic pain (1, 2). The most widely accepted etiology is Sampson's theory of retrograde menstruation where shed endometrial tissue is refluxed through the fallopian tubes to attach and proliferate within the pelvis (1). Evidence supporting this theory is twofold: First, an increased incidence of endometriosis is seen in women with uterine outflow obstruction; second, placement of endometrial tissue in the peritoneal cavity results in endometriotic lesions in other animals (3). However, both women with and without endometriosis undergo retrograde menstruation, suggesting that other factors may mediate the formation of endometriotic lesions (4, 5). It has been postulated that susceptibility to endometriosis is due to enhanced endometrial cell adhesion to the peritoneum or poor clearance of refluxed endometrial cells by host immune responses. Increased peritoneal fluid leukocytes, cytokines and growth factors have been found in patients with endometriosis, suggesting that abnormalities in immune function may predispose patients to development of endometriosis (4).
Cytokines are synthesized by peritoneal macrophages, lymphocytes, and mesothelial cells as well as by endometriosis implants (6). Colony-stimulating factor 1 (CSF-1) is a cytokine produced by macrophages and has been implicated in the pathogenesis of endometriosis. Acting through its receptor, C-FMS, CSF-1 stimulates growth, recruitment, and differentiation of macrophages (7). Increased levels of CSF-1 and its receptor have been detected in gynecologic cancers and may contribute to malignant invasiveness. Cancer cells expressing C-FMS are responsive to growth stimulation by CSF-1 (8). Our previous studies have shown that CSF-1 stimulates endometrial epithelial cells via an autocrine mechanism (9). Knockdown of CSF-1 in endometrial epithelial cells results in decreased proliferation and transmesothelial invasion of endometrial epithelial cells (10). We have also demonstrated that endometrial tissue from CSF-1 knockout mice develop significantly fewer endometriotic lesions than control tissue in a syngeneic mouse model of endometrial tissue transplantation into the pelvic cavity (11).
It is unclear whether an increase in CSF-1 levels in the pelvic cavity of patients with endometriosis is due to inflammation-associated macrophages or whether increased CSF-1 is produced by endometriotic lesions themselves. Furthermore, studies on CSF-1 levels in the peritoneal fluid of women with endometriosis have been inconsistent. Fukaya et al. and Mettler et al. found an increased expression of CSF-1 and C-FMS in peritoneal fluid of patients with endometriosis, whereas Ueki et al. and Weinberg et al. noted no difference in levels of CSF-1 in peritoneal fluid of patients with endometriosis compared with those without (12–15). These inconclusive data on CSF-1 and C-FMS expression hamper our understanding of the physiologic role of CSF-1 signaling in endometriosis. To better define a physiologic context for CSF-1 signaling in endometriosis, we examined CSF-1 levels in the peritoneal fluid of women with endometriosis. Our data showed a significant increase in CSF-1 levels in women with endometriosis. We hypothesized that interaction of endometrial tissue with the mesothelial lining of the peritoneum induces CSF-1 and C-FMS expression in endometrial cells. Our data demonstrated that coculture with peritoneal mesothelial cells (PMCs) leads to elevated induction of CSF-1 and C-FMS expression in endometrial cells from women with endometriosis, suggesting that endometrial tissue may contribute to elevated CSF-1 levels in the pelvic cavity of women with endometriosis and that endometriotic lesions may exhibit heightened responsiveness to CSF-1 stimulation.
Materials and Methods
Patient Selection and Tissue Collection
Approval for this study was granted by the Institutional Review Boards of the University of Texas Health Science Center at San Antonio and Emory University. Patients aged ≥ 18 years with a history of pelvic laparoscopy were eligible. Menstrual-phase endometrium was obtained by effluent collection or aspiration biopsy using a Pipelle (Unimar; Prodimed).
Endometrial stroma cells (ESCs) and endometrial epithelial cells (EECs) were separated from the endometrial samples as previously described (16–18). Briefly, endometrium was enzymatically digested with 0.1% collagenase type 1 and 0.05% DNAse. EECs were separated from ESCs by gravity sedimentation. The stroma-rich supernatant was removed from the epithelial-rich pellet, and both fractions were washed, resuspended in Dynabead epithelial-enriched magnetic polystyrene beads coated with mouse IgG1 monoclonal antibody (mAb Ber-EP4) specific for two (34 and 39 kDa) glycopolypeptide membrane antigens expressed on most normal and neoplastic human epithelial tissues, and incubated for 30 minutes. EECs were isolated from the ESCs by placing the tubes on the magnet for 2 minutes.
The ESCs from supernatant were plated in equilibrated (37°C, in 5% CO2) culture flasks of Dulbecco Modified Eagle Medium (DMEM)/F12 (1:1) (Invitrogen Life Technologies), containing antibiotics/antimycotics, 5 ug/mL insulin, and 10% fetal bovine serum. Cells were allowed to adhere for 20 minutes then washed. Adherent ESCs were primary cultures of monolayers or passaged and frozen in aliquots of passage 1.
The pellets of EECs were plated in equilibrated culture flasks of enriched epithelium medium of MCDM 131/Medium 199/MEMα (40:40:20; Invitrogen) containing antibiotics/antimycotics, 5 ug/mL insulin, 300 ug/mL D-Glucose, and 10% fetal bovine serum. EECs were primary cultures of monolayers or passaged and frozen in aliquots of passage 1.
Flow cytometry was performed to quantitate separation of EECs from ESCs; 98.6% of purified ESCs expressed high levels of the stromal marker vimentin, whereas only 2% expressed the epithelial marker cytokeratin-18 (Fig. 1). More than 98% of purified EECs expressed cytokeratin-18. Our data are consistent with earlier studies showing high purification of EECs and ESCs using the methods developed by Irwin et al. (19).
Figure 1.
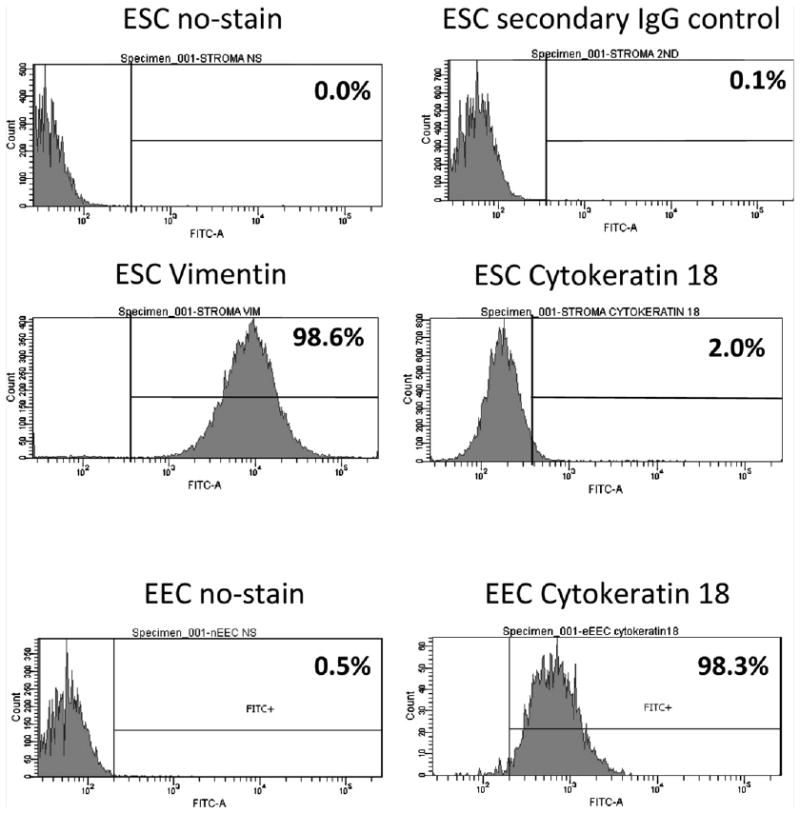
Purity of endometrial stromal (ESCs) and epithelial (EECs) cells. ESCs and EECs were separated with 98.3% and 98.6% purity by flow cytometry with EECs stained for cytokeratin-18 and ESCs stained for vimentin.
Paired Ectopic and Eutopic Tissue
Paired eutopic and ectopic endometrial tissue samples were obtained from patients (n = 7) aged ≥ 18 years undergoing scheduled surgery for endometriosis. Follicular-phase endometrium and peritoneal biopsies were collected at the time of laparoscopy. Tissue samples were immediately frozen in liquid nitrogen and stored in —80°C until used for Western blot analysis as described below.
Peritoneal Fluid Collection and CSF-1 ELISA
Patients undergoing laparoscopy for benign gynecologic conditions aged ≥ 18 years were eligible for enrollment. Peritoneal fluid was obtained from women with (n = 72) and without (n = 43) endometriosis and purified by Ficoll gradient purification. CSF-1 ELISA was performed on peritoneal fluid with the use of the R&D M-CSF Quantikine ELISA kit per the manufacturer's directions. CSF-1 levels were calculated in ng/mL.
Western Blot of CSF-1 and C-FMS in Endometrium and Endometriosis Tissues
Protein extracts from endometrial tissue were prepared by homogenizing the tissue in protein lysis buffer containing Triton-X and protease inhibitors. Equal amounts (generally 60–75 μg) of protein from each sample were separated on a denaturing polyacrylamide gel and transfered to nylon membrane. The protein-bound membranes were then incubated for ≥ 4 h at room temperature with Tris-buffered saline solution containing 0.05% Triton X-100 (TBST) and 5% nonfat dry milk to block nonspecific antibody binding. The membranes were then incubated with primary antibodies in TBST-milk overnight at 4°C, and specific binding was visualized by using species-specific IgG followed by enhanced chemiluminescent detection (ECL kit; Amersham) and exposure to ECL X-ray film. Densitometric analysis was used to estimate protein band densities from Western blot analyses. Protein expression was normalized to a housekeeping gene, actin, for comparison between eutopic and ectopic endometrial tissues and between samples.
Cell Coculture
Primary endometrial epithelial cells (EECs) and endometrial stromal cells (ESCs) were obtained and cocultured with LP9s, a commercially available peritoneal methosothelial cell line, as previously published (16–18). Briefly, EECs and ESCs were lifted from culture vessels using nonenzymatic cell dissociation buffer (Sigma-Aldrich). Cells were labeled with 10 μm thioreactive chloromethylfluoroscein diacetate (Celltracker Green; Invitrogen Molecular Probes), washed in phosphate-buffered saline solution, and divided into two aliquots of 0.5–1.0 × 106 cells each. One aliquot of each of the cells was pelleted and frozen at −70°C in 0.3 mL RLT Plus RNeasy lysis buffer (Qiagen Sciences) as pre-coculture control for subsequent RNA isolation and gene expression analysis. The other aliquot of cells (0.5 × 106 cells) was analyzed for baseline RNA expression and then cocultured with LP9 PMCsfor 16 hours. Sixteen hours was chosen for length of co-culture because previous experiments demonstrated transmethothelial invasion by endometrial cells by this time. After coculture, nonadherent cells were rinsed off and adherent cells lifted off the dishes nonenzymatically. Labeled EECs and ESCs were separated from their nonlabeled LP9 PMCs using flow cytometry based on fluorescence, pelleted, and frozen at −70°C in 0.3 mL RLT Plus RNeasy lysis buffer for reverse-transcription polymerase chain reaction (PCR) gene expression analysis. Because a limited number of cells were obtained after cell culture, we used RNA expression of cells immediately before culture as our control. Pre-coculture levels were similar to levels found in cells grown in culture for the duration of the coculture (16 hours) except in the case of C-FMS expression in EECs (Supplemental Fig. 1, available online at www.fertstert.org).
RNA Expression Studies
RNA was isolated using Qiagen RNeasy Plus with a genomic DNA removal step per the manufacturer's protocol. Reverse transcription was carried out using the Applied Biosystems kit. Real-time PCR and subsequent analyses were carried out with 0.25% Sybr Green in the Cepheid Smartcycler. Expression of CSF-1, C-FMS, and the housekeeping gene GAPDH was detected as described earlier (12). The CSF-1 and C-FMS PCR primer sets used in this study have been described previously (13). Melt curve analysis was performed after each real-time PCR to ascertain PCR product specificity. PCR reactions using CSF-1, C-FMS, and GAPDH primer sets gave unique melt peaks, indicative of discrete amplification products, at 87.7°C, 85.4°C, and 90.2°C, respectively. Relative expression was determined using the formula 2−ΔΔCt. Realtime PCR assays were performed in duplicate and repeated at least three times.
Statistics
CSF-1 concentrations were statistically described as mean ± SD. Relative expressions were calculated by dividing the post-coculture levels by the pre-coculture levels. Comparisons of quantitative variables were done using Student t test for independent samples. For paired analyses of quantitative variables, the paired Student t test was used. A χ2 test was performed to compare categoric data. A probability value (P value) of <.05 was considered to be statistically significant.
Results
CSF-1 Levels Are Elevated in Peritoneal Fluid from Women with Endometriosis
Previous studies on the levels of CSF-1 in the peritoneal fluid of women with endometriosis have been contradictory. Our data (Fig. 2) demonstrated that peritoneal fluid from women with endometriosis had significantly higher (3-fold increase) peritoneal fluid concentrations of CSF-1 than control subjects (P<.002). To determine whether CSF-1 and C-FMS expression are altered in endometriotic lesions, we examined protein levels in paired eutopic and ectopic endometrial samples from women with endometriosis (n = 7) with the use of Western blot analysis (Fig. 3). We observed a relative increase in CSF-1 (∼3-fold increase) and C-FMS (1.7-fold increase) expression in ectopic endometrial tissue compared with paired eutopic endometrium. This suggests that ectopic endometrium is a source of CSF-1 in the peritoneal environment.
Figure 2.
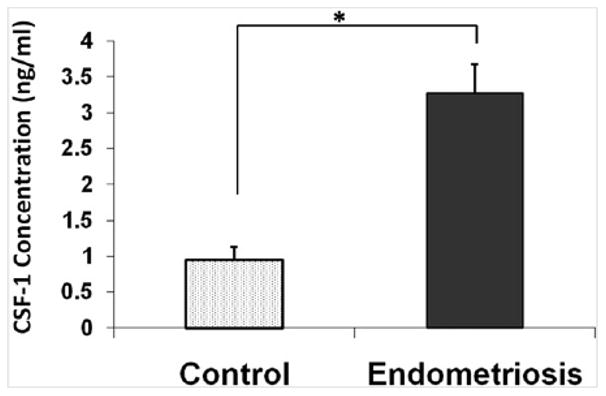
Peritoneal fluid concentration (ng/mL) of colony-stimulating factor 1 (CSF-1) in patients with endometriosis compared with patients without endometriosis (control). Peritoneal fluid concentration of CSF-1 is higher in patients with endometriosis. Values are expressed as mean ± SD. *P ≤.002.
Figure 3.
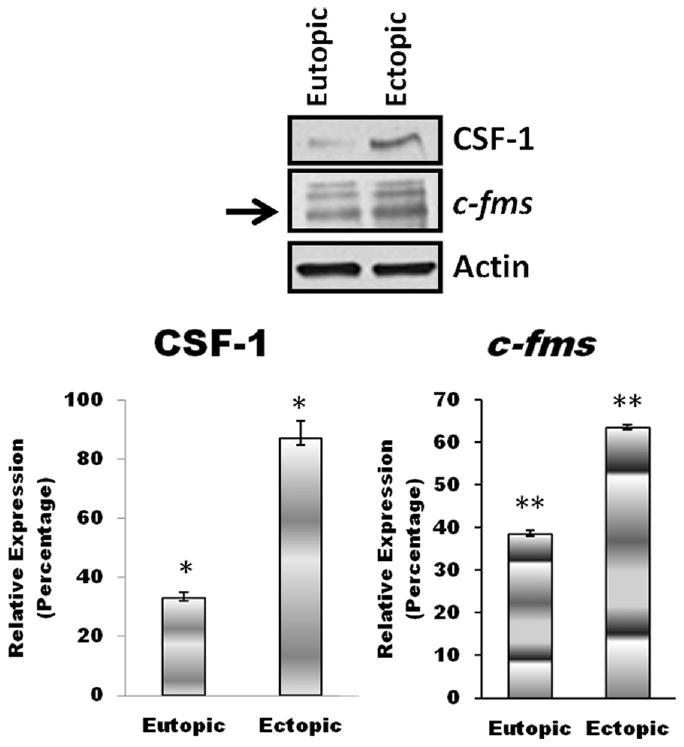
Colony-stimulating factor 1 (CSF-1) and its receptor, C-FMS, expression in ectopic tissue compared with paired eutopic tissue from women with endometriosis. Representative Western blot analysis (top) shows increased CSF-1 and C-FMS levels in ectopic lesions compared with paired eutopic endometrium. A relative increase in CSF-1 (∼3-fold increase) and C-FMS (∼1.7-fold increase) expression was seen in ectopic endometrial tissue compared with eutopic endometrial tissue samples. *P<.01; **P<.05.
CSF-1 and C-FMS in Endometrial Cells from Women with Endometriosis Are Induced in Coculture with PMCs
Because CSF-1 and C-FMS levels are elevated in endometriotic lesions, we examined whether interaction with PMCs leads to enhanced CSF-1 and C-FMS expression in endometrial cells from women with endometriosis. We investigated CSF-1 and C-FMS expression using an in vitro cell coculture model to simulate early endometriotic lesions. Eighteen patients with endometriosis and 13 without underwent menstrual-phase endometrial sampling. Endometrial stroma and epithelial cells were separated and then cocultured with LP9 mesothelial cells. Each pre-coculture sample served as the internal control for coculture expression. Thus, relative expression > 1 indicates increased expression. Coculture increased CSF-1 and C-FMS expression in all cells compared with pre-coculture levels. A nonsignificant induction of CSF-1 and C-FMS mRNA was observed in endometrial cells from women with endometriosis compared with women without endometriosis: respectively, CSF-1 6.85 ± 12.25 versus 3.72 ± 6.19 and C-FMS 4.35 ± 5.51 versus 2.84 ± 4.61 (Fig. 4A). Thus, EECs from patients with endometriosis had a 1.6- and 2-fold increase in C-FMS and CSF-1, respectively, compared with EECs from women without endometriosis (Fig. 4A). ESCs showed a small relative increase (1.3-fold) in C-FMS expression but not CSF-1 expression: CSF-1 2.99 ± 4.82 versus 3.90 ± 5.65, C-FMS 2.32 ± 2.4 versus 1.71 ± 1.54. (endometriosis vs. control, respectively; Fig. 4B). Increased time in culture did not lead to increased expression of either CSF-1 or C-FMS, except in the expression of C-FMS in EECs. This suggests that increased expression may be due to physicial contact with mesothelial cells and not from culture conditions themselves.
Figure 4.
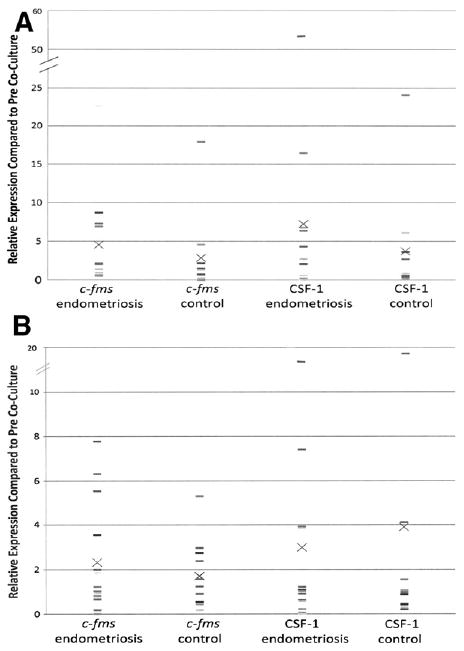
(A) Colony-stimulating factor 1 (CSF-1) and its receptor, C-FMS, expression in EECs from women with endometriosis compared with women without endometriosis (control) after coculture with peritoneal mesothelial cells (PMCs). Reverse-transcription polymerase chain reaction (RT-PCR) analysis was used to determine RNA expression of CSF-1 and C-FMS. Endometrial epithelial cells (EECs) that were not exposed to PMCs were used as baseline control. Individual data points are represented by dashes with the mean for each group indicated by an X. (B) CSF-1 and C-FMS expression in endometrial stroma cells (ESCs) from women with endometriosis compared to women without endometriosis (control) after coculture with PMCs. RT-PCR analysis was used to determine RNA expression of CSF-1 and C-FMS. ESCs that were not exposed to PMCs were used as baseline control. Individual data points are represented by dashes with the mean for each group indicated by an X.
Discussion
CSF-1 has been implicated in the immunomodulation of malignancy and inflammatory disease, with prominent effects on macrophage recruitment, survival, and differentiation (20). Increased macrophage number in the pelvic cavity of women with endometriosis is thought to contribute to chronic inflammation and increased levels of inflammatory cytokines. Earlier studies examined whether the levels of CSF-1 were altered in the pelvic cavity of women with endometriosis. In studies by Fukaya et al. and Mettler et al., CSF-1 levels were found to be increased in the peritoneal fluid of women with endometriosis compared with women without endometriosis; however, in other studies no difference in CSF-1 expression was detected (12–15). The present study confirms earlier reports that patients with endometriosis have elevated peritoneal CSF-1 levels and that CSF-1 and C-FMS are induced in ectopic endometriotic lesions compared with paired eutopic endometrium. The induction of C-FMS and CSF-1 suggests that endometrial cells within ectopic lesions are responsive to autocrine CSF-1 signaling. This is consistent with our previous data suggesting an autocrine mechanism of CSF-1 signaling in endometrial cell growth and that CSF-1 expressed by endometrial cells induces transmesothelial invasion (9). We also previously established, using transfer of endometrial cells from mice genetically ablated for CSF-1 into the peritoneum of syngeneic host, that CSF-1 induces early endometriosis lesion formation (11). Together, the evidence suggests that elevated CSF-1 signaling is a relevant factor during endometriotic lesion formation and that endometrial cells are responsive to CSF-1, leading to cellular growth and transmesothelial invasion.
It is not clear why CSF-1 and/or C-FMS levels are induced in endometriosis. Although increased macrophage number may account for elevated CSF-1 levels in the pelvic cavity of women of endometriosis, other cellular components may also contribute to CSF-1 expression. In our previous studies using the established EEC line EM42, we showed that coculture of EM42 cells with PMCs resulted in increased expression of CSF-1 and C-FMS; however, PMC-conditioned culture media did not affect CSF-1 or C-FMS expression. Those data suggested that direct interaction between endometrial cells and PMCs leads to induction of CSF-1 and its receptor (21). In the present study, we hypothesized that interaction of endometrial tissue with PMCs leads to increased CSF-1 expression in women who develop endometriosis compared with women without endometriosis. We have shown that coculture of primary endometrial cells with PMCs increased both CSF-1 and C-FMS expression. Increased expression was seen at higher levels in epithelial cells of women with endometriosis compared with women without endometriosis. Stromal cells in women with endometriosis showed higher levels of C-FMS, but not of CSF-1. A trend toward increased expression in our coculture data was noted. This may reflect the small number of subjects in our study and is a limitation of our data. Larger studies are required to confirm these results.
Another limitation of this research is the use of pre-coculture tissue rather than a 16-hour culture as control. This was chosen for two reasons. First, because pre-coculture samples are steady state, we were able to avoid variation in culture conditions which might have affected expression. Second, given the limited specimen size available, pre-coculture cells were used as control to increase the tissue available for reverse-transcription PCR studies, for which sample size is critical to obtaining accurate and precise results. Our results in Supplementary Figure 1 show that there were no differences in expression between pre-coculture and 16-hour culture in EECs and ESCs, except for the expression of C-FMS in EECs. In this case, the relative expression of C-FMS in the 16-hour culture and coculture were similar, suggesting that C-FMS expression in EECs may not be affected by co-culture with mesothelial cells. Again, larger sample sizes are needed to confirm these data.
Our data also suggest that endometriotic lesions may contribute to CSF-1 levels in the pelvic cavity of women with endometriosis. Therefore, therapeutic targeting of CSF-1 signaling in endometriosis would have a two-pronged effect: 1) down-modulating chronic macrophage infiltration; and 2) directly affecting growth and invasiveness of ectopic endometrial tissue. Imatinib (Gleevec; Novartis) is a commercially available orally active agent FDA approved for the treatment of myeloid leukemia. It has been shown to antagonize the action of both CSF-1 and its receptor. We have previously shown in a murine model that treatment with imatinib resulted in fewer lesions than in control mice (11). The present data suggest that drugs targeting CSF-1 signaling may be used therapeutically to treat endometriosis.
In conclusion, our data suggest that CSF-1 and its receptor, C-FMS, are involved in the genesis of early endometriotic lesions and that endometriotic lesions contribute to elevated CSF-1 levels in the peritoneal fluid of women with endometriosis. Direct interactions of endometrial tissue with PMCs during endometriotic lesion formation may lead to induction of CSF-1 and C-FMS. CSF-1 inhibitors, such as imatinib, may offer a novel therapeutic approach to the treatment and perhaps even prevention of endometriosis.
Supplementary Material
Colony-stimulating factor 1 (CSF-1) expression of endometrial cells is dependent on physical contact with mesothelial cells, not with time in culture. Endometrial epithelial cells (EECs) and endometrial stroma cells (ESCs) were separated from tissue culture. CSF-1 levels (A) and the CSF-1 receptor, C-FMS (B), were measured with the use of reverse-transcription polymerase chain reaction (RT-PCR) before coculture, after culture for 16 hours, and after coculture for 16 hours. CSF-1 and C-FMS levels were similar between the cultured samples and the pre-coculture samples.
Acknowledgments
The authors thank Drs. Ana Murphy and Nalini Santanam for peritoneal fluid and tissue collection at Emory University.
Supported in part by grants R01HD049637 and PO1HD35276 (to R.R.T.) from the National Institutes of Health/National Institute of Child Health and Human Development.
Footnotes
N.M.B. has nothing to disclose. H.B.N. has nothing to disclose. Y.-G.L. has nothing to disclose. N.B.K. has nothing to disclose. P.A.B. has nothing to disclose. S.K. has nothing to disclose. R.S.S. has nothing to disclose. R.R.T. has nothing to disclose.
References
- 1.Rogers PA, d'Hooghe TM, Fazleabas A, Gargett CE, Giudice LC, Montgomery GW, et al. Priorities for endometriosis research: recommendations from an international consensus workshop. Reprod Sci. 2009;16:335–46. doi: 10.1177/1933719108330568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–58. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- 3.Olive DL, Henderson DY. Endometriosis and mullerian anomalies. Obstet Gynecol. 1987;69:412–5. [PubMed] [Google Scholar]
- 4.D′Hooghe TM, Debrock S. Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Hum Reprod Update. 2002;8:84–8. doi: 10.1093/humupd/8.1.84. [DOI] [PubMed] [Google Scholar]
- 5.Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151–4. [PubMed] [Google Scholar]
- 6.Oral E, Olive DL, Arici A. The peritoneal environment in endometriosis. Hum Reprod Update. 1996;2:385–98. doi: 10.1093/humupd/2.5.385. [DOI] [PubMed] [Google Scholar]
- 7.Stanley ER. Colony-stimulating factor (CSF) radioimmunoassay: detection of a CSF subclass stimulating macrophage production. Proc Natl Acad Sci U S A. 1979;76:2969–73. doi: 10.1073/pnas.76.6.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baiocchi G, Kavanagh JJ, Talpaz M, Wharton JT, Gutterman JU, Kurzrock R. Expression of the macrophage colony-stimulating factor and its receptor in gynecologic malignancies. Cancer. 1991;67:990–6. doi: 10.1002/1097-0142(19910215)67:4<990::aid-cncr2820670422>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Gill K, Kirma N, Gunna VS, Santanam N, Parthasarathy S, Tekmal RR. Regulation of colony stimulating factor-1 (CSF-1) in endometrial cells: glucocorticoids and oxidative stress regulate the expression of CSF-1 and its receptor C-FMS in endometrial cells. Fertil Steril. 2001;76:1005–11. doi: 10.1016/s0015-0282(01)02735-2. [DOI] [PubMed] [Google Scholar]
- 10.Aligeti S, Kirma NB, Binkley PA, Schenken RS, Tekmal RR. Colony-stimulating factor-1 exerts direct effects on the proliferation and invasiveness of endometrial epithelial cells. Fertil Steril. 2011;95:2464–6. doi: 10.1016/j.fertnstert.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen JR, Witz CA, Schenken RS, Tekmal RR. A potential role for colony-stimulating factor 1 in the genesis of the early endometriotic lesion. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukaya T, Sugawara J, Yoshida H, Yajima A. The role of macrophage colony stimulating factor in the peritoneal fluid in infertile patients with endometriosis. Tohoku J Exp Med. 1994;172:221–6. doi: 10.1620/tjem.172.221. [DOI] [PubMed] [Google Scholar]
- 13.Mettler L, Schmutzler AG, Koch K, Schollmeyer T, Salmassi A. Identification of the M-CSF receptor in endometriosis by immunohistochemistry and RT-PCR. Am J Reprod Immunol. 2004;52:298–305. doi: 10.1111/j.1600-0897.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- 14.Ueki M, Tsurunaga T, Ushiroyama T, Ueda M. Macrophage activation factors and cytokines in peritoneal fluid from patients with endometriosis. Asia Oceania J Obstet Gynaecol. 1994;20:427–31. doi: 10.1111/j.1447-0756.1994.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 15.Weinberg JB, Haney AF, Xu FJ, Ramakrishnan S. Peritoneal fluid and plasma levels of human macrophage colony–stimulating factor in relation to peritoneal fluid macrophage content. Blood. 1991;78:513–6. [PubMed] [Google Scholar]
- 16.Dechaud H, Witz CA, Montoya-Rodriguez IA, Degraffenreid LA, Schenken RS. Mesothelial cell-associated hyaluronic acid promotes adhesion of endometrial cells to mesothelium. Fertil Steril. 2001;76:1012–8. doi: 10.1016/s0015-0282(01)02839-4. [DOI] [PubMed] [Google Scholar]
- 17.Kirk D, Irwin JC. Normal human endometrium in cell culture. Methods Cell Biol. 1980;21B:51–77. doi: 10.1016/s0091-679x(08)60678-0. [DOI] [PubMed] [Google Scholar]
- 18.Lucidi RS, Witz CA, Chrisco M, Binkley PA, Shain SA, Schenken RS. A novel in vitro model of the early endometriotic lesion demonstrates that attachment of endometrial cells to mesothelial cells is dependent on the source of endometrial cells. Fertil Steril. 2005;84:16–21. doi: 10.1016/j.fertnstert.2004.10.058. [DOI] [PubMed] [Google Scholar]
- 19.Irwin JC, Kirk D, King RJ, Quigley MM, Gwatkin RB. Hormonal regulation of human endometrial stromal cells in culture: an in vitro model for decidualization. Fertil Steril. 1989;52:761–8. doi: 10.1016/s0015-0282(16)61028-2. [DOI] [PubMed] [Google Scholar]
- 20.Patel S, Player MR. Colony-stimulating factor-1 receptor inhibitors for the treatment of cancer and inflammatory disease. Curr Top Med Chem. 2009;9:599–610. doi: 10.2174/156802609789007327. [DOI] [PubMed] [Google Scholar]
- 21.Nair AS, Nair HB, Lucidi RS, Kirchner AJ, Schenken RS, Tekmal RR, et al. Modeling the early endometriotic lesion: mesothelium-endometrial cell co-culture increases endometrial invasion and alters mesothelial and endometrial gene transcription. Fertil Steril. 2008;90:1487–95. doi: 10.1016/j.fertnstert.2007.09.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Colony-stimulating factor 1 (CSF-1) expression of endometrial cells is dependent on physical contact with mesothelial cells, not with time in culture. Endometrial epithelial cells (EECs) and endometrial stroma cells (ESCs) were separated from tissue culture. CSF-1 levels (A) and the CSF-1 receptor, C-FMS (B), were measured with the use of reverse-transcription polymerase chain reaction (RT-PCR) before coculture, after culture for 16 hours, and after coculture for 16 hours. CSF-1 and C-FMS levels were similar between the cultured samples and the pre-coculture samples.


