Abstract
Background and objective
It is unclear if physiologic measures are useful for assessing dyspnea. We examined the association among the subjective rating of dyspnea according to patients with advanced cancer, caregivers and nurses, and various physiologic measures.
Methods
We conducted a cross-sectional survey of patients with cancer hospitalized at MD Anderson Cancer Center. We asked patients, caregivers, and nurses to assess the patients' dyspnea at the time of study enrollment independently using a numeric rating scale (0=none, 10=worst). Edmonton Symptom Assessment Scale (ESAS) ratings, causes of dyspnea, vitals, and Respiratory Distress Observation Scale [RDOS] ratings were collected.
Results
A total of 299 patients were enrolled in the study: average age 62 (range 20–98), female 47%, lung cancer 37%, and oxygen use 57%. The median RDOS rating was 2/16 (interquartile range 1–3) and the number of potential causes was 3 (range 2–4), with pleural effusion (n=166, 56%), pneumonia (n=144, 48%), and lung metastasis (n=125, 42%) being the most common. The median intensity of patients' dyspnea at the time of assessment was 3 (interquartile range 0–6) for patients, 4 (interquartile range 1–6) for caregivers, and 2 (interquartile range 0–3) for bedside nurses. Patients' expression of dyspnea correlated moderately with caregivers' (r=0.68, p<0.001) and nurses' (r=0.50, p<0.001) assessments, and weakly with RDOS (r=0.35, p<0.001), oxygen level (r=0.32, p<0.001), and the number of potential causes (r=0.19, p=0.001). In multivariate analysis, patients' dyspnea was only independently associated with ESAS dyspnea (p=0.002) and dyspnea as assessed by caregivers (p<0.001).
Conclusion
Patients' level of dyspnea was weakly associated with physiologic measures. Caregivers' perception may be a useful surrogate for dyspnea assessment.
Introduction
Dyspnea is a subjective awareness of having difficulty breathing. It is one of the most common and most feared symptoms among cancer patients, occurring in approximately 20% to 40% of patients at the time of diagnosis of advanced disease,1 and increases up to 70% in the last 6 weeks of life.2,3
The pathophysiology of dyspnea has not been clearly elucidated, but is thought to be triggered by peripheral threats such as hypoxemia and increased respiratory effort, which activate various chemoreceptors, mechanoreceptors, baroreceptors, irritant receptors, and J receptors, ultimately resulting in the perception of dyspnea in the somatosensory cortex.4,5 Concurrent afferent signals to the thalamus and amygdala may also lead to increased sympathetic drive and various physiologic changes such as tachycardia, tachypnea, accessory muscle use, and paradoxical breathing.6
Dyspnea is one of the most under-researched symptoms.7,8 Few studies have examined whether pathophysiologic factors and physical signs can reliably assess dyspnea in cancer patients, and even fewer have examined whether caregivers' and nurses' impressions accurately reflect cancer patients' perception of dyspnea.2,9,10 A better understanding of the correlates of dyspnea would provide insights into the pathophysiology of this distressing symptom and allow us to better assess it. In this cross-sectional study, we examined the association between the subjective rating of dyspnea according to patients, caregivers and nurses, and various physiologic measures.
Methods
Study setting and eligibility criteria
Between July 1, 2011 and February 2, 2012, we enrolled patients with a diagnosis of advanced cancer (i.e., locally advanced, metastatic, or recurrent disease) who were 18 years of age or older, English speaking, and admitted to MD Anderson Cancer Center (MDACC), a National Cancer Institute designated comprehensive cancer center with more than 500 beds. Patients with delirium, pulmonary embolism, surgery within the past week, mechanical ventilation, or hemodynamic instability were excluded. As part of our enrichment strategy to identify patients most likely to have dyspnea, we screened all individuals newly initiated on supplemental oxygen and/or bronchodilator treatments by respiratory therapy while admitted, and received referrals from clinicians from the Departments of Thoracic Medicine, General Internal Medicine, and Palliative Care and Rehabilitation Medicine. The Institutional Review Board approved this study. All subjects who participated in this study provided verbal consent prior to enrollment.
Data collection
We collected baseline demographics including age, sex, and cancer diagnosis. A study physician also systematically reviewed the electronic chart including radiology report to examine potential causes of dyspnea, including the presence or absence of airway lesions, lung parenchymal metastases, lymphangitic carcinomatosis, pleural effusion, tamponade, pneumonia, pulmonary embolism, pulmonary fibrosis, heart failure, chronic obstructive pulmonary disease (COPD), anemia (hemoglobin <11g/L), metabolic acidosis, and other causes. There is currently no specific cutoff defining what level of hemoglobin is associated with dyspnea. A cutoff of 11g/L was chosen in this study because this level was associated with decreased survival in a previous study.11
We asked patients to “indicate the level of your shortness of breath now” using a validated, 11-point numeric rating scale where 0 represents no dyspnea and 10 represents worst possible.12 We also asked the self-identified primary caregiver and the bedside clinical nurse to “indicate the patient's level of shortness of breath now” using the same rating system. Patients, caregivers, and nurses each provided their assessment independently in the absence of the other parties and without knowledge of what answers the other parties provided. All assessments were done within 5 minutes of each other. At the time of study assessment, 135/299 (45%) caregivers and 216/299 (72%) nurses were available, and all provided a response.
Patients also completed the Edmonton Symptom Assessment Scale (ESAS), which measures 10 common symptoms in the past 24 hours (pain, fatigue, nausea, depression, anxiety, drowsiness, shortness of breath, appetite, sleep, and feeling of well-being) using numeric rating scales ranging from 0 (no symptom) to 10 (worst symptom).13 This questionnaire has been validated in cancer patients.14,15
Vital signs including heart rate, respiratory rate, oxygen saturation, and supplemental oxygen level at the time of study enrollment were collected. We also documented the oxygen delivery device.
We also collected physiologic changes associated with dyspnea using the validated Respiratory Distress Observation Scale (RDOS).16 RDOS is an ordinal scale based on heart rate, respiratory rate, accessory muscle use, paradoxical breathing pattern, restlessness, grunting at end-expiration, nasal flaring, and fearful facial display. Each variable is assigned a score between 0 and 2, with a total score of up to 16 points. Higher scores indicate more distress related to dyspnea. An earlier version codes tachycardia and tachypnea based on the magnitude of increase from baseline; the newer version only requires the absolute heart rate and respiratory rate at the time of assessment.16 These assessments were carried out by a research coordinator who has received 3 days of training under the principal investigator and longitudinal supervision to ensure the findings were consistent and accurate.
Statistical analysis
We summarized the baseline demographics using descriptive statistics, including means, medians, ranges, interquartile range, and frequencies. Correlation among patients', caregivers', and nurses' rating of dyspnea and various physiologic measures (number of potential causes, vitals, supplemental oxygen use, and RDOS) were examined using the Spearman test, in which coefficients of 0-0.19, 0.2-0.39, 0.4-0.59, 0.6-0.79, and 0.8-1.0 indicate very weak, weak, moderate, strong, and very strong correlations, respectively.
We used the Kappa statistic to examine inter-rater reliability on the intensity of dyspnea for patient-caregiver dyads and patient-nurse dyads. The Wilcoxon test was used to examine the differences in dyspnea ratings between patients' and caregivers' assessments, and also between those of patients and nurses.
We conducted nonparametric multivariate analysis to determine factors associated with patient's level of dyspnea at the time of study. We ranked patient's level of dyspnea at the time of study and designated it as a dependent variable in a linear regression model with backward selection. Variables included in the model were patient's age, sex, cancer (lung versus no lung), number of potential causes, heart rate, respiratory rate, oxygen saturation, supplemental oxygen level, RDOS score, all 10 ESAS symptoms, caregivers' rating, and nurses' rating. We repeated the above multivariate analysis with ESAS dyspnea removed from the model.
The Statistical Package for the Social Sciences (SPSS version 16.0, SPSS Inc., Chicago, IL) software was used for statistical analysis. A p value of <0.05 was considered significant. For the purpose of this study, a correlation coefficient of −0.19 is considered as very weak, 0.2-0.39 as weak, 0.40-0.59 as moderate, 0.6-0.79 as strong and 0.8-1 as very strong correlation.17
Results
Patient characteristics
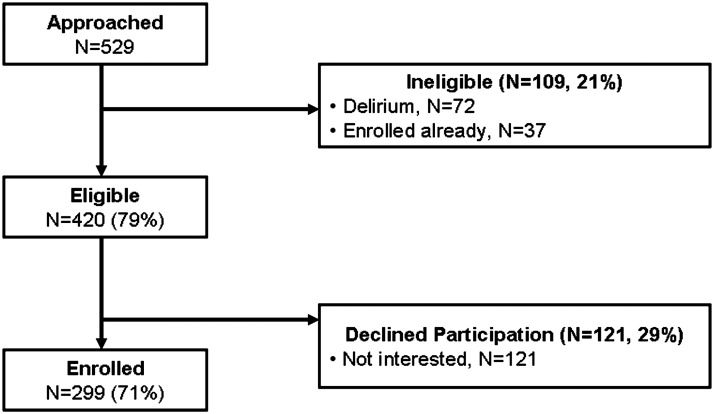
Among 529 patients approached for this study, 109 (21%) were either delirious or already enrolled (Fig. 1). Four hundred and twenty (79%) patients were eligible and 299 were subsequently enrolled, resulting in a recruitment rate of 71%.
FIG. 1.
Study flow diagram.
Table 1 illustrates the patient characteristics. One hundred and ten (37%) patients had lung malignancies. Pneumonia, pleural effusion, and lung parenchymal metastasis were commonly found. More than half were receiving supplemental oxygen. ESAS scores revealed significant symptom burden among these hospitalized patients, with an average dyspnea level of 5 (interquartile range 3–8) within the last 24 hours. The median number of potential causes of dyspnea was 3 (interquartile range 2–4).
Table 1.
Baseline Characteristics of 299 Patients with Advanced Cancer
| Characteristics | N (%)a |
|---|---|
| Age, average (range) | 62 (20–98) |
| Female sex | 141 (47) |
| Cancer diagnosis | |
| Breast | 32 (11) |
| Gastrointestinal | 43 (14) |
| Genitourinary | 33 (11) |
| Gynecologic | 14 (5) |
| Head and neck | 15 (5) |
| Hematologic | 25 (8) |
| Other | 27 (9) |
| Respiratory | 110 (37) |
| Edmonton Symptom Assessment Scale, median (interquartile range) | |
| Pain | 5 (2–7) |
| Fatigue | 6 (5–8) |
| Nausea | 0 (0–3) |
| Depression | 0 (0–5) |
| Anxiety | 3 (0–6) |
| Drowsiness | 5 (3–7) |
| Appetite | 5 (4–8) |
| Well-being | 5 (3–7) |
| Shortness of breath | 5 (3–8) |
| Sleep | 5 (3–7) |
| Potential causes of dyspneab | |
| Airway lesion | 24 (8) |
| Lung parenchymal metastasis | 125 (42) |
| Lymphangitic carcinomatosis | 25 (8) |
| Pulmonary embolism | 12 (4) |
| Pneumonia | 145 (48) |
| Pleural effusion | 166 (56) |
| Heart failure | 8 (3) |
| Tamponade | 2 (1) |
| Anemia (<11 g/L) | 232 (78) |
| Metabolic acidosis | 3 (1) |
| Atelectasis | 121 (41) |
| Pericardial effusion | 26 (9) |
| Chronic obstructive pulmonary disease | 29 (10) |
| Pneumothorax | 7 (2) |
| Pulmonary fibrosis | 7 (2) |
| Others | 9 (3) |
| Total number of potential causes, median (interquartile range) | 3 (2–4) |
Unless otherwise specified.
Based on clinical chart review by a physician, including blood work and radiology reports.
Correlation among patients' self-reported dyspnea and physiologic measures
The median RDOS was 2 (interquartile range 1–3) (Table 2). We found that higher heart rate, supplemental oxygen use, and RDOS score correlated weakly with patients' dyspnea at the time of assessment (Table 3). In contrast, respiratory rate and oxygen saturation did not have any significant correlation.
Table 2.
Physiologic Measures of Dyspnea in 299 Patients with Advanced Cancer
| Characteristics | N (%)a |
|---|---|
| Vitals, median (interquartile range) | |
| Heart rate/minute | 88 (77–100) |
| Respiratory rate /minute | 20 (18–20) |
| Oximetry, % | 96 (95–98) |
| Supplemental oxygen level, L/minute | 2 (0–3) |
| Oxygen device | |
| None | 129 (43) |
| Nasal cannula | 152 (51) |
| Nonrebreather mask | 6 (2) |
| Vapotherm | 11 (4) |
| Others | 1 (0.3) |
| Respiratory Distress Observation Scale | |
| Heart rate | |
| <90/minute | 164 (55) |
| 90–109/minute | 97 (32) |
| ≥110/minute | 38 (13) |
| Respiratory rate | |
| ≤18/minute | 108 (36) |
| 19–30/minute | 189 (63) |
| >30/minute | 2 (1) |
| Restlessness | |
| None | 246 (82) |
| Occasional, slight movements | 50 (17) |
| Frequent movements | 3 (1) |
| Paradoxical breathing pattern | 0 (0) |
| Accessory muscle use | |
| None | 271 (91) |
| Slight rise | 28 (9) |
| Pronounced rise | 0 (0) |
| Grunting at end-expiration | 3 (1) |
| Nasal flaring | 3 (1) |
| Look of fear | |
| None | 237 (79) |
| Eyes wide open, facial muscles tense, brow furrowed, mouth open, teeth together | 61 (21) |
| Total score, median (interquartile range) | 2 (1–3) |
Unless otherwise specified.
Table 3.
Association among Dyspnea Level as Perceived by Patients, ESAS Symptoms, and Physiologic Measures (n=299)
| Variables | N | Spearman Correlation Coefficient | P value |
|---|---|---|---|
| Edmonton Symptom Assessment Scale | |||
| Pain | 297 | 0.24 | <0.001 |
| Fatigue | 299 | 0.32 | <0.001 |
| Nausea | 299 | 0.07 | 0.26 |
| Depression | 297 | 0.32 | <0.001 |
| Anxiety | 297 | 0.36 | <0.001 |
| Drowsiness | 295 | 0.31 | <0.001 |
| Appetite | 292 | 0.24 | <0.001 |
| Well-being | 291 | 0.41 | <0.001 |
| Shortness of breath | 297 | 0.69 | <0.001 |
| Sleep | 294 | 0.44 | <0.001 |
| Number of potential causes | 299 | 0.19 | 0.001 |
| Heart rate | 299 | 0.19 | 0.001 |
| Respiratory rate | 299 | 0.05 | 0.36 |
| Oximetry | 299 | −0.07 | 0.20 |
| Supplemental oxygen level | 299 | 0.32 | <0.001 |
| Respiratory distress observation scale | 299 | 0.35 | <0.001 |
Correlation among patients' self-reported dyspnea and ESAS symptoms
As shown in Table 3, patients' dyspnea level at the time of assessment correlated strongly with ESAS dyspnea, moderately with sleep and well-being, and weakly with pain, fatigue, depression, anxiety, drowsiness, and anorexia.
Correlation among patients' self-reported dyspnea and caregivers' and nurses' ratings
The median level of dyspnea at the time of the study enrollment as perceived by patients was 3 (interquartile range 0–6). The patient's dyspnea level was overestimated by caregivers (median 4, interquartile range 1–6) and underestimated by nurses (median 2, interquartile range 0–3). The rating of dyspnea correlated strongly between patients and caregivers, and moderately between patients and nurses (Table 4). Caregivers overestimated dyspnea compared with patients, albeit this was not statistically significant (p=0.06). Nurses underrated dyspnea compared with patients (p<0.001).
Table 4.
Spearman Correlation among the Respiratory Distress Observation Scale and Dyspnea Level as Perceived by Patients, Caregivers, and Bedside Nurses Assessment
| Patient's perception | Caregiver perception | Bedside nurse perception | ||
|---|---|---|---|---|
| Patient's perception | Coefficient | - | - | - |
| P value | ||||
| N | ||||
| Caregiver perception | Coefficient | 0.68 | - | - |
| P value | <0.001 | |||
| N | 135 | |||
| Bedside nurse perception | Coefficient | 0.50 | 0.58 | - |
| P value | <0.001 | <0.001 | ||
| N | 216 | 107 | ||
| Respiratory Distress Observation Scale | Coefficient | 0.35 | 0.36 | 0.21 |
| P value | <0.001 | <0.001 | 0.002 | |
| N | 299 | 135 | 216 |
The inter-rater agreement was 0.23 (p<0.001, n=135) for patient-caregiver dyads, and 0.09 (p<0.001, n=216) for patient-nurse dyads. Eighty-three of one hundred and thirty-five (62%) and 106/135 (79%) of the patient-caregiver dyads were within 1 and 2 points of each other, respectively. For the patient-nurse dyads, 102/216 (47%) were within 1 point and 137/216 (63%) were within 2 points.
In multivariate analysis incorporating caregiver and nurse assessments, ESAS symptoms, and physiologic measures, we found that ESAS dyspnea (p=0.002) and dyspnea as assessed by caregivers (p<0.001) were independently associated with patients' dyspnea at the time of assessment, with an R2 of 0.53. With ESAS dyspnea removed from the model, dyspnea as assessed by caregivers (p<0.001) remains significant independent of RDOS (p=0.02), ESAS pain (p=0.03), and ESAS sleep (p=0.03), with an R2 of 0.50.
Discussion
In this study of hospitalized cancer patients, we found that patients' expression of dyspnea was strongly associated with caregivers' assessment and patients' own ESAS dyspnea level, moderately with various ESAS symptoms, and weakly with physiologic measures such as heart rate, RDOS, and supplemental oxygen level. Our findings suggest that physiologic measures cannot be used to assess patients' subjective rating, which by definition is the gold standard for dyspnea assessment. Further research is needed to determine if caregivers' perception could be a surrogate for dyspnea assessment.
The main assessment in this study is a validated numeric rating scale assessing cancer patients' self-reported dyspnea at the time of study,12 and is distinct from ESAS dyspnea, which measures patients' average level of dyspnea over the past 24 hours. By assessing patients' self-reported dyspnea, we were able to determine its association with other physiologic variables collected at the same time. Interestingly, ESAS dyspnea was higher compared with dyspnea at the time of assessment (median 5 versus 3). This is likely because ESAS dyspnea incorporates both dyspnea at rest and during exertion, whereas dyspnea at the time of study captures this symptom when patients were resting.
Our study reveals that vital signs had limited correlation with patients' expression of dyspnea. In addition, the number of potential causes of dyspnea and supplemental oxygen level only had a weak association. Previous studies in patients with asthma and COPD revealed poor correlation between dyspnea and spirometric measures.18,19 Bruera and colleagues also found limited association between dyspnea and various physiologic measures such as maximal inspiratory pressure, peak flow, and oxygen saturation.2 Our current study builds on the previous work and examines an additional set of physiologic variables (e.g., RDOS) as well as caregivers' and nurses' perception.
The RDOS is a behavioral instrument that assesses respiratory distress based on several physical signs, and is proposed as an assessment of dyspnea in patients who are unable to report the symptom themselves due to delirium and/or drowsiness.20 It was initially validated with a group of patients undergoing pulmonary rehabilitation, and compared with healthy volunteers and patients with postoperative pain.21 Several items in the RDOS, such as extent of accessory muscle use and look of fear, are dependent on the assessors' experience and judgment. A previous study of six patients with COPD showed that accessory muscle use was associated with high dyspnea level.22 In this study, we found only weak correlation between RDOS and self-reported dyspnea, which is consistent with the results of the previous validation study (r=0.39, p=0.001).21
We found correlation and some agreement between caregivers' and patients' assessment of dyspnea. Caregivers' impression remained significant in multivariate analysis, suggesting that caregivers may potentially be good surrogates for patients who have difficulties with self-report. Indeed, several studies have found that caregivers provide reasonable proxy for patients' symptoms.23,24 Because caregivers often overrate symptoms,25 further research is needed to identify ways to decrease the discrepancy between patient and caregiver assessment.26 Furthermore, caregivers' assessment of dyspnea in delirious or nonresponsive patients remains to be studied.
Patient self-reported dyspnea at the time of study was associated with many other ESAS symptoms, including shortness of breath, sleep, well-being, pain, fatigue, depression, anxiety, drowsiness and anorexia. Our findings are consistent with various other studies in cancer and noncancer patients.10,27–31 This observation may partly be related to elevated symptom expression as a result of confounders in hospitalized patients (e.g., psychological distress), and highlights the interrelatedness between dyspnea and other symptoms. The correlation between dyspnea and anxiety was statistically significant but weak (r=0.36, p<0.001), which is in line with the results from other studies by our group and others, including Bruera et al. (r=0.31, p=0.0003),2 Reddy et al. (r=0.32, p=0.008),27 Dudgeon et al. (r=0.26, p=0.03),32 and Chiu et al. (r=0.21, p<0.05)9 using different methodologies in different cancer populations. Taken together, our results suggest that anxiety is an important but not the sole contributor to this multidimensional construct of dyspnea.
Our study has potential implications for both the definition and assessment of dyspnea. Findings from this study are consistent with the definition put forth by the American Thoracic Society, in which dyspnea is defined as “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity. The experience derives from interactions among multiple physiological, psychological, social, and environmental factors, and may induce secondary physiological and behavioral responses.”5,33 The lack of correlation between dyspnea and physiologic measures in this study further challenges other proposed definitions that incorporate various physical signs, such as labored breathing, into their description.34 Our study also highlights the importance of patient-reported outcomes, although the optimal questionnaire to assess dyspnea remains to be determined.35,36
This study has several limitations. First, we only included hospitalized patients with advanced cancer, who are likely to have a different symptom burden compared with outpatients and those with noncancer diagnoses. Second, we approached patients seen by respiratory therapists or known to have dyspnea, which could bias our study sample. Because the objective of this study is to characterize the correlates of dyspnea rather than prevalence, we believe that an enriched population is justified. Third, caregivers were not present for approximately half of the assessments. Fourth, the Respiratory Distress Observation Scale was conducted by a research coordinator rather than a physician or nurse. We have provided extensive training to ensure quality assessments. Fifth, we recorded only the presence or absence of various potential causes of dyspnea based on chart review, and could not determine the extent to which each factor contributed to the overall dyspnea expression. Further studies are needed to characterize the etiology of dyspnea.
We conclude that physiologic measures cannot reliably inform about cancer patients' expression of dyspnea. At this time, subjective assessments remain the gold standard for assessing dyspnea. Caregivers' impression may be a potential proxy when patients are unable to rate their intensity of dyspnea. Further research is necessary to better characterize the major predictors of subjective dyspnea in cancer patients.
Acknowledgments
This study was supported in part by National Institutes of Health grants RO1NR010162-01A1, RO1CA122292-01, and RO1CA124481-01 (EB). This study was also supported by the MD Anderson Cancer Center Support Grant (CA 016672) and an institutional start-up grant, 18075582 (DH). The sponsor of the study had no role in study design, data collection, analysis, interpretation, or writing of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ripamonti C. Management of dyspnea in advanced cancer patients. Support Care Cancer. 1999;7:233–243. doi: 10.1007/s005200050255. [DOI] [PubMed] [Google Scholar]
- 2.Bruera E. Schmitz B. Pither J. Neumann CM. Hanson J. The frequency and correlates of dyspnea in patients with advanced cancer. J Pain Symptom Manage. 2000;19:357–362. doi: 10.1016/s0885-3924(00)00126-3. [DOI] [PubMed] [Google Scholar]
- 3.Solano JP. Gomes B. Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31:58–69. doi: 10.1016/j.jpainsymman.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Manning HL. Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med. 1995;333:1547–1553. doi: 10.1056/NEJM199512073332307. [DOI] [PubMed] [Google Scholar]
- 5.Parshall MB. Schwartzstein RM. Adams L. Banzett RB. Manning HL. Bourbeau J, et al. An Official American Thoracic Society Statement: Update on the Mechanisms, Assessment, and Management of Dyspnea. Am J Respir Crit Care Med. 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell ML. Terminal dyspnea and respiratory distress. Crit Care Clin. 2004;20:403–17. doi: 10.1016/j.ccc.2004.03.015. , viii–ix. [DOI] [PubMed] [Google Scholar]
- 7.Hui D. Parsons HA. Damani S. Fulton S. Liu J. Evans A, et al. Quantity, design, and scope of the palliative oncology literature. Oncologist. 2011;16:694–703. doi: 10.1634/theoncologist.2010-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorman S. Jolley C. Abernethy A. Currow D. Johnson M. Farquhar M, et al. Researching breathlessness in palliative care: Consensus statement of the National Cancer Research Institute Palliative Care Breathlessness Subgroup. Palliat Med. 2009;23:213–227. doi: 10.1177/0269216309102520. [DOI] [PubMed] [Google Scholar]
- 9.Chiu TY. Hu WY. Lue BH. Yao CA. Chen CY. Wakai S. Dyspnea and its correlates in Taiwanese patients with terminal cancer. J Pain Symptom Manage. 2004;28:123–132. doi: 10.1016/j.jpainsymman.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka K. Akechi T. Okuyama T. Nishiwaki Y. Uchitomi Y. Factors correlated with dyspnea in advanced lung cancer patients: Organic causes and what else? J Pain Symptom Manage. 2002;23:490–500. doi: 10.1016/s0885-3924(02)00400-1. [DOI] [PubMed] [Google Scholar]
- 11.Lima DF. Dela Coleta K. Tanni SE. Silveira LV. Godoy I. Potentially modifiable predictors of mortality in patients treated with long-term oxygen therapy. Respir Med. 2011;105:470–476. doi: 10.1016/j.rmed.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Gift AG. Narsavage G. Validity of the numeric rating scale as a measure of dyspnea. Am J Crit Care. 1998;7:200–204. [PubMed] [Google Scholar]
- 13.Bruera E. Kuehn N. Miller MJ. Selmser P. Macmillan K. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 14.Chang VT. Hwang SS. Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164–2171. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Nekolaichuk C. Watanabe S. Beaumont C. The Edmonton Symptom Assessment System: A 15-year retrospective review of validation studies (1991–2006) Palliat Med. 2008;22:111–122. doi: 10.1177/0269216307087659. [DOI] [PubMed] [Google Scholar]
- 16.Campbell ML. Templin T. Walch J. A Respiratory Distress Observation Scale for patients unable to self-report dyspnea. J Palliat Med. 2010;13:285–290. doi: 10.1089/jpm.2009.0229. [DOI] [PubMed] [Google Scholar]
- 17.Swinscow TDV (revised by Campbell MJ, University of Southampton) 9th. BMJ Publishing Group; 1997. [Jul 1;2012 ]. Correlation and regression. In: Statistics at Square One. [Google Scholar]
- 18.Wolkove N. Dajczman E. Colacone A. Kreisman H. The relationship between pulmonary function and dyspnea in obstructive lung disease. Chest. 1989;96:1247–1251. doi: 10.1378/chest.96.6.1247. [DOI] [PubMed] [Google Scholar]
- 19.Rosi E. Lanini B. Ronchi MC. Romagnoli I. Stendardi L. Bianchi R, et al. Dyspnea, respiratory function and sputum profile in asthmatic patients during exacerbations. Respir Med. 2002;96:745–750. doi: 10.1053/rmed.2002.1343. [DOI] [PubMed] [Google Scholar]
- 20.Campbell ML. Templin T. Walch J. Patients who are near death are frequently unable to self-report dyspnea. J Palliat Med. 2009;12:881–884. doi: 10.1089/jpm.2009.0082. [DOI] [PubMed] [Google Scholar]
- 21.Campbell ML. Psychometric testing of a respiratory distress observation scale. J Palliat Med. 2008;11:44–50. doi: 10.1089/jpm.2007.0090. [DOI] [PubMed] [Google Scholar]
- 22.Gift AG. Plaut SM. Jacox A. Psychologic and physiologic factors related to dyspnea in subjects with chronic obstructive pulmonary disease. Heart Lung. 1986;15:595–601. [PubMed] [Google Scholar]
- 23.Oi-Ling K. Man-Wah DT. Kam-Hung DN. Symptom distress as rated by advanced cancer patients, caregivers and physicians in the last week of life. Palliat Med. 2005;19:228–233. doi: 10.1191/0269216305pm1001oa. [DOI] [PubMed] [Google Scholar]
- 24.Lobchuk MM. Degner LF. Symptom experiences: Perceptual accuracy between advanced-stage cancer patients and family caregivers in the home care setting. J Clin Oncol. 2002;20:3495–507. doi: 10.1200/JCO.2002.01.153. [DOI] [PubMed] [Google Scholar]
- 25.Broberger E. Tishelman C. von Essen L. Discrepancies and similarities in how patients with lung cancer and their professional and family caregivers assess symptom occurrence and symptom distress. J Pain Symptom Manage. 2005;29:572–583. doi: 10.1016/j.jpainsymman.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Lobchuk MM. McClement SE. Daeninck PJ. Shay C. Elands H. Asking the right question of informal caregivers about patient symptom experiences: Multiple proxy perspectives and reducing interrater gap. J Pain Symptom Manage. 2007;33:130–145. doi: 10.1016/j.jpainsymman.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Reddy SK. Parsons HA. Elsayem A. Palmer JL. Bruera E. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med. 2009;12:29–36. doi: 10.1089/jpm.2008.0158. [DOI] [PubMed] [Google Scholar]
- 28.Gift AG. Psychologic and physiologic aspects of acute dyspnea in asthmatics. Nurs Res. 1991;40:196–199. [PubMed] [Google Scholar]
- 29.Gift AG. Cahill CA. Psychophysiologic aspects of dyspnea in chronic obstructive pulmonary disease: A pilot study. Heart Lung. 1990;19:252–257. [PubMed] [Google Scholar]
- 30.Kellner R. Samet J. Pathak D. Dyspnea, anxiety, and depression in chronic respiratory impairment. Gen Hosp Psychiatry. 1992;14:20–28. doi: 10.1016/0163-8343(92)90022-3. [DOI] [PubMed] [Google Scholar]
- 31.von Leupoldt A. Dahme B. Psychological aspects in the perception of dyspnea in obstructive pulmonary diseases. Respir Med. 2007;101:411–422. doi: 10.1016/j.rmed.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Dudgeon DJ. Lertzman M. Askew GR. Physiological changes and clinical correlations of dyspnea in cancer outpatients. J Pain Symptom Manage. 2001;21:373–379. doi: 10.1016/s0885-3924(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 33.Dyspnea. Mechanisms, assessment, and management: A consensus statement. American Thoracic Society. Am J Respir Crit Care Med. 1999;159:321–340. doi: 10.1164/ajrccm.159.1.ats898. [DOI] [PubMed] [Google Scholar]
- 34.Wright GW. Branscomb BV. The origin of the sensations of dyspnea. Trans Am Clin Climatol Assoc. 1954;66:116–125. [PMC free article] [PubMed] [Google Scholar]
- 35.Bausewein C. Farquhar M. Booth S. Gysels M. Higginson IJ. Measurement of breathlessness in advanced disease: A systematic review. Respir Med. 2007;101:399–410. doi: 10.1016/j.rmed.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Dorman S. Byrne A. Edwards A. Which measurement scales should we use to measure breathlessness in palliative care? A systematic review. Palliat Med. 2007;21:177–1791. doi: 10.1177/0269216307076398. [DOI] [PubMed] [Google Scholar]