Abstract
A novel approach that preserved most mesenchymal stem cell (MSC) characteristics was developed using MSC encapsulation in a hydrogel based on hyaluronic acid (HA). An optimized HA–hydrogel composition, whose characteristics were assessed by scanning electron microscopy and viscoelastic property analyses, as well as the more favorable MSC seeding density, was established. These optimal three-dimensional MSC culture conditions allowed morphological cell remodeling, maintained the expression of stem cell markers over 28 days of culture, and preserved MSC differentiation plasticity. In addition, MSCs in HA–hydrogel submitted for 7 days to mechanical constraint that aimed at mimicking in vivo cardiac beat displayed enhanced cell survival by more than 40% compared to static culture conditions. Thus, the optimized HA-based hydrogel provides a niche for MSCs, which preserves their properties and opens ways for cell therapy, in particular in aortic repair medicine.
Introduction
The greatest challenge in regenerative medicine is to replace or to repair injured native tissue with functional engineered tissue. The use of mesenchymal stem cells (MSCs) in cellular therapies is attractive due to their ability to extensively self-replicate and differentiate into a multitude of cell lineages.1 Indeed, MSCs present in bone marrow exhibit interesting characteristics that provide them with a great potential to treat a variety of diseases. In an appropriate environment, they can differentiate into several cell types, including adipocytes, osteocytes, myoblasts, chondrocytes, or neurons.1 Nevertheless, MSCs can respond directly to the mechanical stiffness or structure of their environment by entering into a differentiation process.2,3 Therefore, it is necessary to specify which conditions would favor their survival and growth and also to preserve their stem cell properties before using them for therapeutic purposes.
Several studies have shown that, contrary to traditional two-dimensional (2D) cultures, a three-dimensional (3D) system mimics more physiological microenvironments4,5 directly affecting cell behavior and phenotype. Several biomaterials have been proposed as artificial niches for engineering stem cells5–7 that aim at better understanding stem cell biology under 3D biomimetic conditions and to develop new strategies for efficient long-term maintenance, and also direct the differentiation of these cells into various therapeutic lineages. A recent study2 has demonstrated the usefulness of thixotropic gels for 3D cell culture studies, as well as the use of liquefaction stress as an effective measure of matrix stiffness that could be correlated to MSC differentiation. Similarly, it has been shown that MSCs cultured in moderate stiffness gel exhibit a myogenic phenotype, whereas a rigid matrix can induce osteogenic differentiation.3
Among the biomaterials that can serve as a 3D matrix, those based on hyaluronic acid (HA) are of special interest.6,8–11 HA, a nonsulfated highly hydrated glycosaminoglycan, is a natural extracellular matrix (ECM) component of many tissues. Through the CD44 receptor, HA permits cell adhesion, and regulates proliferation, motility, and angiogenesis.12 It also plays an important role during the early stage of human embryogenesis, when it is highly accumulated before cell differentiation.13 Nonimmunogenic, nontoxic physicochemical features confer to HA interesting biomaterial properties for tissue engineering and clinical applications.8,14 HA-based 3D matrices have been used in many studies.8–11 However, major limitations of this natural polymer are fast enzymatic degradation and poor mechanical properties. Used alone and without chemical modification, HA cannot create 3D scaffolds.8,9 However, such scaffolds can be achieved by adding chemically modified gelatin (gelin) to HA in the presence of a crosslinker. Gelatin is a natural polymer derived from collagen. It has been widely used for hydrogel cell encapsulation in tissue engineering due to its biocompatibility, biodegradability, and cell adhesion.15,16
The aim of this study was to investigate the behavior of MSCs cultured in such HA-based hydrogels. We present here the optimal concentrations of HA and gelin in which MSCs were encapsulated and retained their stem cell properties. In addition, it was shown that a cyclic mechanical constraint applied on such MSC 3D cultures enhanced cell survival, indicating that the proposed 3D HA hydrogel is a promising biomaterial for MSC cardiac therapy.
Materials and Methods
Hydrogel preparation
Hydrogels were prepared according to the manufacturer's instructions. In brief, gels were prepared aseptically by combining varying concentrations of thiolated HA and gelin in water at the final concentrations of (40:60)%, (50:50)%, or (60:40)%, respectively (Table 1). The extralinker polyethylene glycol diacrylate was then added to the mixtures at volume ratios 1:4, 1:6, or 1:8 (Table 1). Immediately after, 0.25 mL gel/cm2 was poured in 24- or 6-well plates that were then incubated at 37°C under 5% CO2 until hydrogel gelation. Then, gels were hydrated in phosphate-buffered saline (PBS) or in an appropriate culture medium when hydrogels were used to encapsulate cells. All products were purchased from Tebu Bio, Le Perray en Yvelines.
Table 1.
Different Compositions of Tested Hydrogels
| (A) Hydrogels were Prepared by Combining Varying Concentrations of Thiolated Hyaluronic Acid and Gelin in Water at the Final Concentrations of (40:60), (50:50), or (60:40)%, Respectively: the Extralinker Polyethylene Glycol Diacrylate was then Added to the Mixtures at Volume Ratios 1:4, 1:6, or 1:8 | |||
|---|---|---|---|
| Conditions | |||
| HA and gelin concentration (%) | HA: gelin (40:60) | HA: gelin (50:50) | HA: gelin (60:40) |
| Extralinker (PEGDA): HA and gelin mix (v/v) | r 1:4 r 1:6 r 1:8 | ||
| (B) Nine Hydrogel Formulations Obtained Based on Combination of Different Extralinker Concentrations (1:4, 1:6, or 1:8) and Different Hyaluronic Acid:Gelin Concentration | |||
|---|---|---|---|
| |
r [Extralinker (PEGDA): HA and gelin mix] (v/v) |
||
| 1:4 | 1:6 | 1:8 | |
| HA and gelin concentration (%) | |||
| HA: gelin (40:60) | HA: gelin (40:60), r 1:4 | HA: gelin (40:60), r 1:6 | HA: gelin (40:60), r 1:8 |
| HA: gelin (50:50) | HA: gelin (50:50), r 1:4 | HA: gelin (50:50), r 1:6 | HA: gelin (50:50), r 1:8 |
| HA: gelin (60:40) | HA: gelin (60:40), r 1:4 | HA: gelin (60:40), r 1:6 | HA: gelin (60:40), r 1:8 |
Here, r: designated extralinker ratio.
PEGDA, polyethylene glycol diacrylate; HA, hyaluronic acid.
Scanning electron microscopy
The porosity and pore size of the optimal hydrogel type (HA:gelin [40:60], extralinker ratio 1:8) were analyzed. As previously described in,17 the hydrogel was dehydrated in serial ethanol baths (70%, 85%, 95%, and 100%) for 30 min each. The gel was immersed in 1,1,1,3,3,3-hexamethyldisilazane for 30 min and then dried. The samples were imaged using scanning electron microscopy (SEM) at 15 kV, after gold coating. All images were analyzed by ImageJ software. Porosity of hydrogel was determined by evaluating the empty space appearing in black on images.
Rheological analysis of hydrogel
The viscoelastic properties of hydrogel were assessed by small-amplitude oscillatory shear experiments using a rotational rheometer BOHLIN CVOR (Malvern). The experiments were performed at 37°C (Peltier thermostatic system) with oscillation frequency ranging from 0.1 up to 5 Hz, and a strain amplitude at which viscoelastity is monitored to ensure that it is linear (data not shown). The shear storage or elastic modulus (G′), as well as the shear loss or viscous modulus (G′′), was thus measured as a function of time. The gap was set to maintain a positive normal force on the sample. For all tests, the samples were placed into a chamber properly designed to avoid evaporation.
Cell culture
MSCs were isolated from Fisher rat bone marrow (Charles River, St Germain Sur L'arbresle) according to18 and cultured in an alpha-minimum essential medium (α-MEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS), 1% penicillin–streptomycin (Gibco), and 250 μg Fungizone (Gibco). After 3 days of culture, the unattached cells were removed, and the medium was renewed three times a week. For cell encapsulation, MSCs at second passage (80% confluency) were harvested with 0.25% trypsin 1 mM EDTA (Invitrogen) and seeded into hydrogels before adding the extralinker. The hydrogel formation was performed as described above. Three cell densities were tested: 1×105, 2×105, and 4×105 cells/mL hydrogel.
To assess the influence of cell density and hydrogel reticulation on 3D MSC organization and morphologies, a Zeiss Primo Vert inverted microscope fitted with 10×/0.25 Ph1 objective lens, and a Q Imaging Camera were used.
Cell viability
To investigate MSC viability within hydrogel, Alamar blue test (Invitrogen) was used at 1, 7, 14, 21, and 28 days after cell encapsulation. At each culture time, Alamar blue was added in a culture medium at a volume ratio 1:10 and incubated for 3 h. The fluorescence was measured using a plate reader (VICTOR™ X3 Multilabel Plate Reader, PerkinElmer) according to the instructions. Results were expressed as fluorescence intensity of Alamar blue (excitation 555 nm and emission 590 nm).
Cell characterization
For immunocytochemical characterization, MSCs were cultivated in hydrogels in glass Lab-Tek chamber slides (Dutcher). Cells in gels were fixed with 4% neutral buffered paraformaldehyde and blocked for 1 h at room temperature (RT) in 3% bovine serum albumin (BSA; Sigma Aldrich). Then, cells were incubated for 1 h in the presence of 1:100 (v/v) primary antibody: CD11b (Santa Cruz), CD31, CD44, CD45, CD73, or CD90 (all obtained from BD Biosciences). The absence of first antibody served as a negative control. Cells were rinsed three times in 1× PBS, and stained for 1 h with an antibody: Alexa Fluor-647 rabbit anti-goat (Invitrogen) or Alexa Fluor-647 goat anti-mouse (Invitrogen). Images were acquired using a confocal microscope (Zeiss LSM 510 META).
Fluorescence-activated cell-sorting analysis
After 3 weeks of culture, the MSCs encapsulated in hydrogel were released by hydrogel degradation in the presence of 300 U/mL collagenase and 100 U/mL hyaluronidase (STEMCELL Technologies) for 16 h. Cells were washed by centrifugation and suspended in a blocking solution (3% BSA in 1× PBS). After nonspecific site saturation in the blocking solution for 30 min at RT, each sample containing 5.105 cells was incubated for 15 min at RT with 2.5 g/mL of an antibody (CD11b-PE, CD31-PE, CD44, CD45-FITC, CD73, or CD90) or control Immunoglobulin G (IgG) (mouse IgG1-PE, mouse IgG1-FITC, or mouse IgG2aK-PE). All antibodies and control IgG were obtained from BD Biosciences. Cell pellets, obtained after centrifugation for 5 min at 500 g, were rinsed twice with a blocking buffer. Secondary antibodies were added to the tubes containing the primary uncoupled antibodies CD44, CD73, and CD90. After three washes in the blocking solution, the cells were fixed in paraformaldehyde (PFA) 1%. The fluorescence was then analyzed by a Cyan flow cytometer (Beckman Coulter).
Induction of MSC differentiation
To investigate the multipotency of MSCs, the osteogenic, the adipogenic, and the smooth muscle lineages were induced for 3 weeks in the presence of an appropriate medium. The MSCs cultured in a basal medium (α-MEM, 10% FBS, and antibiotics) were used as negative control.
Osteogenic differentiation
The osteogenic medium was the basal medium containing 0.1 μM dexamethasone (Sigma Aldrich), in which 50 μM ascorbic acid (Sigma Aldrich) and 10 mM ß glycerophosphate (Sigma Aldrich) were added. The osteogenesis was assessed using von Kossa staining. Cells were fixed with 4% PFA and incubated for 1 h with 1% silver nitrate solution (Sigma-Aldrich) under 60-Watt light. Cells were rinsed three times in distilled water before 5-min incubation in 5% sodium thiosulfate (Sigma-Aldrich). After being washed twice with distilled water, cultures were air-dried and then observed and photographed under a light microscope.
Adipogenic differentiation
Adipogenic differentiation was induced using a basal medium containing 0.1 μM dexamethasone, 0.2 μM indomethacin, 0.5 mM 3-isobutyl-1-methylxanthine, and 10 μg/mL insulin (all purchased from Sigma-Aldrich). Adipogenesis was assessed by Oil Red staining. After cell fixation in 4% PFA, the cells were stained by Oil Red solution (0.3% Oil Red in 60% isopropanol). Oil Red was removed and cultures were rinsed three times in distillated water. The lipid drops were visualized, and images were taken with light microscopy.
Smooth muscle differentiation
The smooth muscle lineage differentiation was induced by the addition of 25 ng/mL transforming growth factor (TGF)-β (Sigma-Aldrich) in the culture medium.19 After 21 days in this medium, the smooth muscle cell (SMC) phenotype was analyzed by immunostaining using mouse anti-rat smooth muscle myosin heavy chain as a primary antibody (1:500, Abcam) and Alexa Fluor-647 goat anti-mouse (Invitrogen) as a secondary antibody.
Mechanical test
Using the optimal hydrogel preparation and optimal cellular density, the effects of mechanical stimuli on 3D MSC cultures were investigated. The MSCs mixed with hydrogel solution were deposited on silicone membrane wells of BioFlex culture plates, and then the culture medium was added after gel gelation. A soft gel was formed directly on the silicone membrane bonded with foam circular anchor for improved sample attachment. After 24 h of 3D culture, cells were subjected to an equibiaxial strain. However, before that we had ensured that the chosen mechanical stress preserved the gel integrity. Then, the cultures were exposed to a cyclic strain of 300 cycles/min (5 Hz and 5% biaxial strain) by applying negative vacuum maximum pressure fixed at 140 mmHg (the minimum pressure was 80 mmHg) delivered by a computer-controlled Flexcell FX-5000T strain unit (Flexcell international). These conditions aimed at mimicking the cardiac cycle. This constraint was applied for 7 days. Using Alamar blue test, MSC viability under dynamic condition was compared to static conditions.
Statistical analysis
For statistical analysis, two-tailed and unpaired Student's t-tests were used with a significance level 0.05. All quantitative results were given as mean±standard error of the mean.
Results
Search for an optimal HA–hydrogel composition
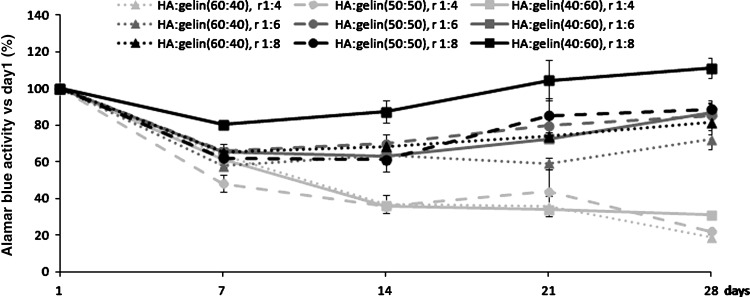
To determine the optimal HA–hydrogel composition allowing the best viability of encapsulated cells, several concentrations of HA, gelin, and extralinker were tested. Cells were seeded at 4×105 cells/mL hydrogel. Alamar blue tests were performed in each gel conditions at 1, 7, 14, 21, and 28 days of 3D cell culture. Since Alamar blue is a nontoxic substance that can be used repeatedly, the cell viability of each dish could be followed over the 4 weeks (Fig. 1). Cell viability at each time was compared to the viability at day 1 after seeding. Whatever the hydrogel composition, viability was reduced at 7 days of culture. Compared to day 1, cellular viability diminished from 15% to 50% depending on the hydrogel composition. During the following days, the decay in cell survival continued in hydrogels where the ratio 1:4 of extralinker was used. However, when the crosslinker ratio decreased, cells viability increased after day 7 of culture regardless of the HA:gelin ratio. An enhanced gelin percentage preserved or increased cell viability, especially when the 1:8 extralinker ratio was used. Altogether, it clearly appeared that the following hydrogel composition HA:gelin (40:60), extralinker ratio 1:8, represents the optimal hydrogel for MSC viability.
FIG. 1.
Mesenchymal stem cell (MSC) viability and proliferation depend on hydrogel reticulation and hyaluronic acid concentration. MSCs were cultivated for 28 days at 4×105 cell/mL, in hydrogels of different compositions of hyaluronic acid (HA):gelin (40:60)%, (50:50)%, or (60:40)%, respectively, containing three different extralinker concentrations (1:4, 1:6, or 1:8). Cell viability was examined using Alamar blue test. Values are represented as (mean±standard error of the mean) normalized per day one results (n=4).
Search for an optimal density of encapsulated MSCs
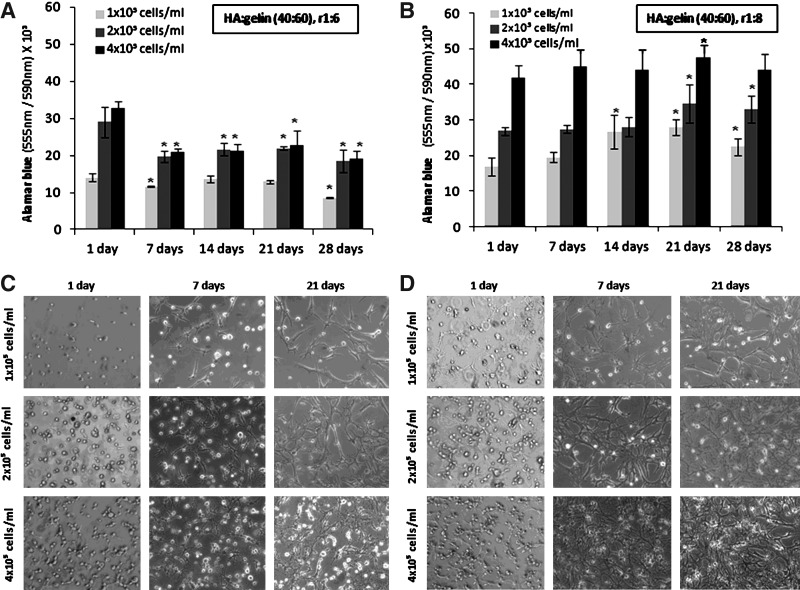
Cells at either 1×105, 2×105, or 4×105 cells/mL of hydrogel were encapsulated in HA:gelin (40:60) in the presence of varying reticulation conditions (extralinker ratios 1:6 or 1:8). The viability tests were performed after 1, 7, 14, 21, and 28 days of cell encapsulation (Fig. 2A, B). When a 1:4 extralinker ratio was applied, cell viability was not well preserved, and this type of hydrogel was not further used. In 1:6 extralinker hydrogel conditions, cell viability was reduced by about 30% after 7 days of culture with the highest cell concentration used. Then, cell viability remained at about the same level during the following days of culture. With 1×105 cells/mL, cell survival was not altered except at 28 days of culture when it was reduced by about 40% (Fig. 2A). By contrast, the use of the 1:8 extralinker ratio preserved cell viability whatever the cell concentrations over 28 days of culture. While the highest cell concentration allowed only a 10% cell increase, with 2×105 cells/mL and 1×105 cells/mL, cell viability increased by about 25% during the first 3 weeks of culture.
FIG. 2.
Cellular density and hydrogel reticulation affect MSC proliferation and morphology. Viability and phenotype of MSCs cultivated within (HA: gelin [40:60], extralinker ratio 1:8) at different cellular concentrations (1×105, 2×105 or 4×105 cells/mL), and two hydrogel crosslinking ratios 1:6 (A, C) and 1:8 (B, D) for 1, 7, 14, 21, and 28 days. Viable MSCs in a hydrogel containing extralinker ratio 1:6 (A) or extralinker ratio 1:8 (B); reticulation reagents were evaluated using Alamar blue test. Results are given as means±standard error of the mean. Light microscope shows encapsulated MSC morphology and network formation, at various cell densities and crosslinking concentration 1:6 (C) and 1:8 (D) over 21 days in culture (magnification×10). (*) corresponds to p<0.05.
Hydrogel reticulation and cell density also influenced MSC morphology. The first days of culture, MSCs uniformly distributed in hydrogels displayed a round shape. With a 1:4 extralinker ratio, the MSCs remained round, and were unable to interact with each other (data not shown). In both 1:6, 1:8 extralinker ratios, at 7 days of culture, cells showed elongated morphology whatever the cell densities are. However, more cellular connections were observed when the density was the highest. A cellular network was formed more rapidly in 1:8 reticulation conditions compared to 1:6 conditions, when cell density increased (Fig. 2C, D). However, at 28 days of culture, the cellular network seemed to dissociate. Altogether, results showed that the optimal condition of MSC seeding in hydrogels containing HA:gelin (40:60), extralinker ratio 1:8, is 4×105 cells/mL. These experimental conditions were used in the following experiments.
Properties of the selected HA–hydrogel
Porosity analysis
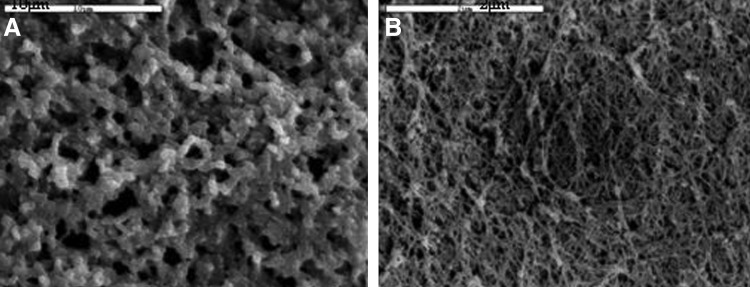
Hydrogel prepared with the optimal composition was analyzed using SEM images to investigate the hydrogel microstructural architecture (Fig. 3). These images showed a complex porous network, composed of micropores of about 6 μm. Image analysis showed a percent porosity of about 70%. However, compared to hydrated condition of hydrogel use, this observation of hydrogel under dehydrated conditions might probably underestimate the pore size.
FIG. 3.
Scanning electron microscopy pictures of selected hydrogel; HA:gelin (40:60) and 1:8 crosslinking. Images A and B show hydrogel architecture and microporosity. Scale bar is (A) 10 μm and (B) 2 μm.
Rheological analysis
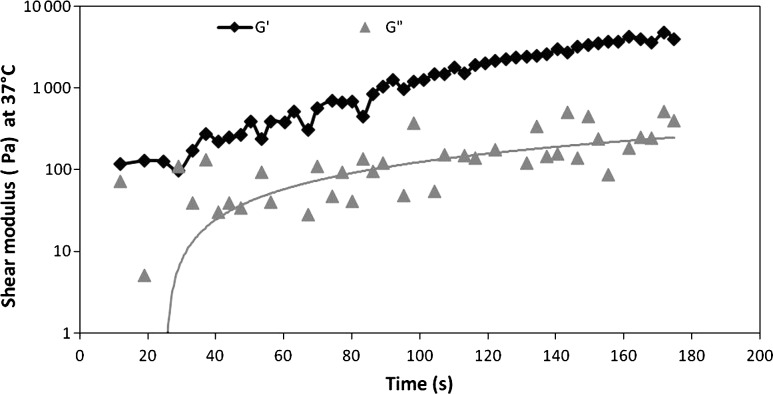
Hydrogel rheological properties were evaluated by shear oscillatory measurements. Evolution of storage G′ and loss G′′ moduli of gel over time are reported in Figure 4. The selected hydrogel has an elastic modulus G′ (storage modulus) higher than the viscous modulus G′′ (loss modulus). These rheological characteristics allow a stable 3D structure formation. Furthermore, the low rigidity of the matrix allows cell elongation, and contacts both between the cells and of cells and their environment.
FIG. 4.
Rheological test of the selected HA–hydrogel. Elastic modulus (G′) and viscous modulus (G′′) measured as a function of time. The mechanical properties of the hydrogel were evaluated by shear oscillatory measurements with oscillation frequency ranging from 0.1 up to 5 Hz.
HA–hydrogel maintained MSC phenotype
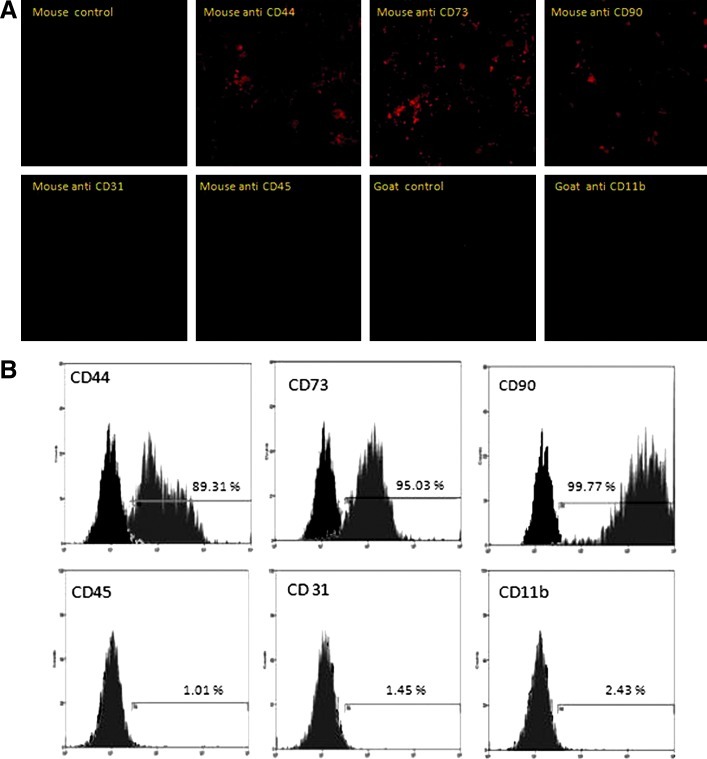
The MSCs encapsulated within hydrogel preserved their characteristic markers. This was shown by immunocytochemistry and flow cytometry analyses. After 21 days of 3D culture, cells still expressed the CD44 (89.31%), CD73 (95.03%) and CD90 (99.77%) markers, which characterize MSCs. The absence of the hematopoietic markers CD11b (2.43%) and CD45 (1.01%) or the endothelial marker CD31 (1.45%) proved that culture conditions preserved the MSC phenotype (Fig. 5).
FIG. 5.
Hydrogel encapsulation preserves MSC phenotype. Immunofluorescence analysis of MSCs encapsulated within hydrogel (HA:gelin [40:60], extralinker ratio 1:8) (A) shows expression of MSC markers (CD44, CD73, and CD90), and the absence of either hematopoietic marker (CD11b and CD45) or endothelial marker (CD31). Images were analyzed using a confocal microscope Zeiss Axio Observer Z.1. Fluorescence-activated cell-sorting analysis exhibits same expression profiles as immunofluorescence staining (B). Gray area indicated MSC signal, while black-shaded area represents isotype controls. Color images available online at www.liebertpub.com/tec
HA–hydrogel maintained the differentiation potential of MSCs
To investigate the multipotency of MSCs that were cultures in HA–hydrogels, cells were incubated for 21 days in the presence of a proper medium to induce the osteogenic, adipogenic, or SMC lineages. Osteogenesis was demonstrated by a calcium deposition in cells stained by the von Kossa technique (Fig. 6A). Oil drops were visualized in MSCs grown in an adipocyte differentiation medium (Fig. 6B). In the presence of TGFβ1, MSCs differentiated toward SMCs (Fig. 6C). Altogether, this proved that 3D HA–hydrogel culture preserved the differentiation potential of MSCs.
FIG. 6.
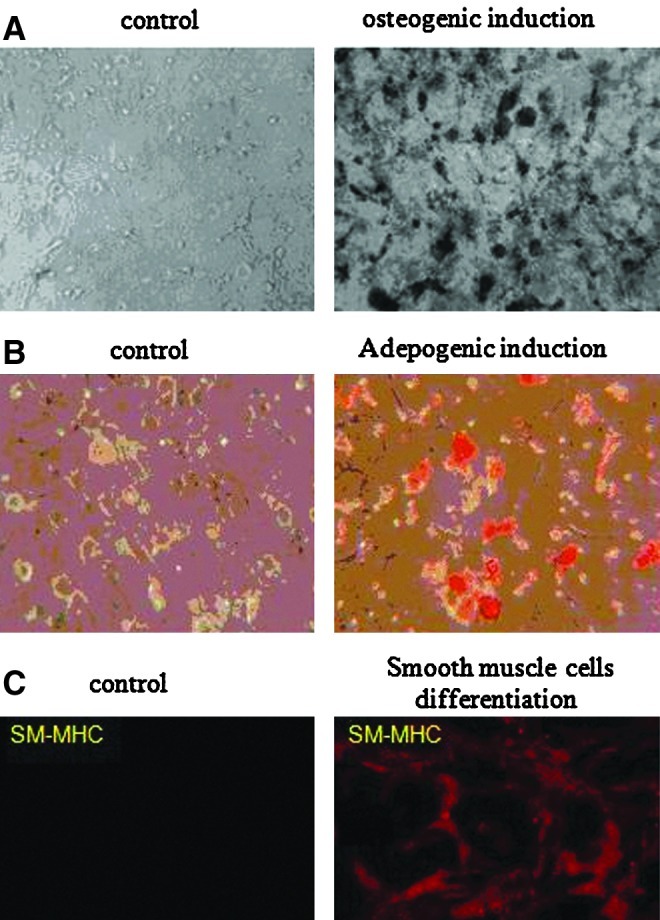
HA–hydrogel preserves the differentiation potential of encapsulated MSCs. Osteogenic (A), adipogenic (B), and smooth muscle cell (C) differentiation was induced for 21 days in MSCs cultivated within (HA:gelin [40:60], extralinker ratio 1:8) hydrogel. von Kossa staining was used to detect mineral in osteogenic differentiation (A). Lipid drops were detected in adipogenic differentiation by 0.3% Oil Red staining (B). Expression SM marker smooth-muscle myosin heavy chain (SM-MHC) analyzed using a confocal microscope Zeiss Axio Observer Z.1 (C). Encapsulated MSCs cultivated in a basal medium were used as negative control. Images A and B were taken using light microscopy at×10 magnification. Color images available online at www.liebertpub.com/tec
Dynamic culture condition better preserved MSC viability in HA–hydrogel
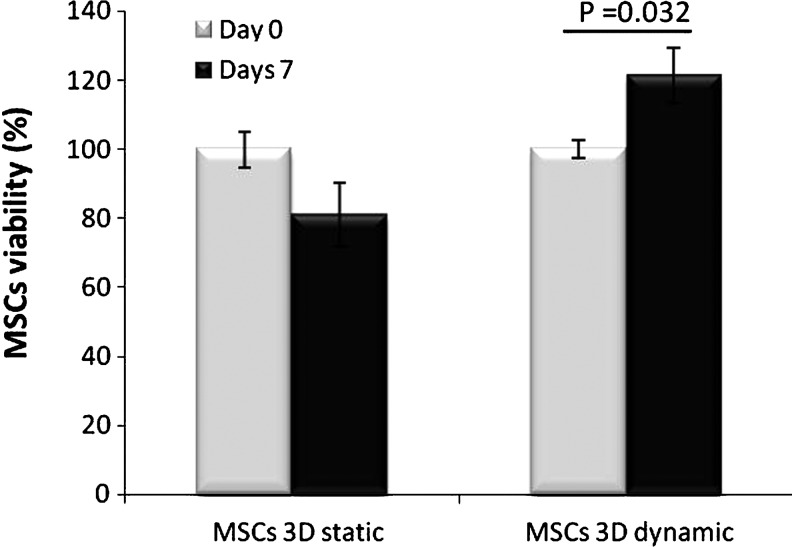
Since MSCs grown in HA–hydrogel might be used in vivo in particular for cardiac or aortic therapy, it was interesting to test their viability under experimental conditions close to a physiological environment. In particular, cells would be submitted in vivo to heart beating. Therefore, encapsulated cells in HA hydrogel were submitted to mechanical constraints using a Flexcell device that mimics such specific physiological conditions. It was observed that dynamic conditions increased cellular viability by more than 40%, compared to static conditions after 7 days of dynamic conditions (Fig. 7).
FIG. 7.
Culture in dynamic condition improves MSC viability in HA–hydrogel. The MSCs viability was tested using Alamar blue test after 0 day or 7 days of hydrogel culture in a static or dynamic condition (Flexcell). Values of fluorescence intensity obtained at day 0 were used as reference (100% cell viability). n=3, values are mean±standard error of the mean.
Discussion
The number of studies in the field of MSCs in cell therapy has grown continuously in recent years. It appears that long-term preservation of stem cell properties and ability to differentiate in different lineages are a challenge. It is clear that monolayer culture does not reproduce the MSC in vivo environment.4,5 This type of culture could alter their phenotype and limit the possibility of their use in regenerative medicine. Three-dimensional cultures are closer to in vivo cellular life condition; however, only a few scaffolds preserving the MSCs are available, which demonstrates a need for novel 3D devices, particularly for vascular disease repair. Therefore, we hypothesized that a hydrogel containing both acid hyaluronic and gelatin could preserve the MSC undifferentiated state and furthermore could improve their capacity to differentiate. For this purpose, we optimized a hydrogel containing chemically modified HA and gelin that provides a 3D structure suitable for MSCs. This hybrid structure of HA–gelin hydrogel could overcome the shortcomings of each component taken in their natural state.8,20 First, we were interested in finding the best HA–gelin hydrogel composition and its degree of crosslinking. We also tested different cell densities in hydrogels. We have demonstrated that (HA:gelin [40:60], extralinker ratio 1:8) hydrogel in which 4×105 cells/mL were encapsulated is the optimal condition for 3D MSC culture. It was noticed that the quality of gelin, a chemically modified gelatin, could differ among batches. However, whatever the gelin quality was, the selected hydrogel composition remained the best. In the proposed optimal hydrogel formulation, cell death did not exceed 15%. This low mortality rate of MSCs encapsulated in the hydrogel is probably due to an anoikis mechanism.21–23 Nevertheless, more than 85% of the encapsulated cells survived in the selected hydrogel composition where cells were able to adapt to their new environment by establishing cell–cell contacts and cell–matrix interactions necessary to resist to anoikis and to enter into the cell cycle. In fact, cell–matrix interactions via transmembrane receptors mediate and regulate many biological processes, including cell attachment, cell phenotype, cell morphology, viability, proliferation, differentiation, and migration.24 Using naturally derived materials such as collagen and HA in 3D scaffolds is very attractive, because it can facilitate the cell communication with their environment. Here, we clearly show that MSC survival increases with increased gelatin component that promotes cell adhesion and proliferation of various types of cells through integrin receptors.24 Due to their basic residues, a gelatin is positively charged, which may improve attachment of negatively charged cells.25 However, contrary to gelatin, HA poorly supports cell adhesion because of its anionic chain and hydrophilic behavior.26,27 It has been reported that fibroblasts cultured in HA-only hydrogel slightly adhere and do not survive.28 In the aim to enhance cell attachment and spreading in HA hydrogel, many approaches have been used, such as addition RGD peptides or ECM proteins such as collagen, gelatin, and fibronectin to HA–hydrogel increase cell adhesion,27,29–31 and cell proliferation could also be facilitated in these hybrid scaffolds28,32 Also, a high cell density required to support MSC survival in hydrogel could probably be explained by cell–cell interactions involving several surface receptors, autocrine, and paracrine factor synthesis, as it has previously been reported for other stem cells.33,34
The morphology of MSCs encapsulated in the 3D scaffold was dependent on the hydrogel composition. We show that the lowest concentration of the extralinker (1:8) favors the elongation of cells, thus allowing the formation of a network. Cells show an elongated shape, and network takes place more rapidly when gelin amount is increased. This is in accordance with,31,35 who suggests that the presence of high concentrations of gelin in the cell environment is positive for cell adhesion and survival. However, HA may provide significant benefits; particularly thiol-modified HA inhibits excessively collagen hydrogel contraction as reported previously.36 To better understand the relationship between MSCs and this hydrogel matrix, it will be important to identify mechanisms involving MSCs' adaptation to their new environment. Thus, it will be interesting to investigate implication of metalloproteinases synthesis and cell migration processes in MSC network formation within a 3D hydrogel matrix.
Scaffold design and hydrogel properties may drastically affect cell behavior in 3D culture. It is largely admitted in literature that pore size, pore interconnectivity, and porosity control several parameters, including nutrient diffusion, gas exchange, cell, and ECM degradation, and that they can also affect growth factor transport, which modulates cell signaling and growth.4,35,37,38 Pore size may alter the distribution of the ECM components synthesized by cells, as shown in studies where osteoblasts and chondrocytes were cultured in PEG hydrogels.21 In the present study, scaffold features were controlled not only by varying HA and gelin amounts, but also by linker concentration. In agreement with previously published data,21,35 our results indicate that cell viability in hydrogel indeed depends on reticulation concentration. The design parameters of the selected hydrogel (HA:gelin [40:60], extralinker ratio 1:8) were analyzed using SEM. Our results show that the porosity and pore size within this optimal hydrogel of about 6 μm provide cell adhesion, viability, and proliferation (Figs. 1 and 2B). Furthermore, the hydrogel complexity microarchitecture promotes MSC organization in the network (Fig. 2C, D). The matrix's mechanical properties are extremely important in 3D environments, especially for MSCs whose proliferation and differentiation are highly influenced by scaffold stiffness.2,3,6,35 It has been demonstrated that low elastic modulus environments favor neurogenic differentiation, whereas stiff scaffolds permit osteogenic differentiation.2,3 Matrix rigidity affects cell behavior through integrin–ligand signalization with transducer mechanical stimuli to cellular cytoskeleton.5,39,40 Here, the selected hydrogel displayed an elastic storage modulus (G′) higher than viscous loss modulus (G′′), confirming the hydrogel elastic behavior and 3D network formation and stability.41 The low elasticity matrix stiffness allowed cell spreading, elongation through this hydrogel, and proliferation.40 In addition, these mechanical properties may preserve stem cell renewal as reported by others.39,42
Finally, our study demonstrates that the chosen HA–hydrogel preserves MSC phenotype and differentiation potential, underscoring the importance of the presence of HA in the hydrogel, as suggested elsewhere.10,43
Bone marrow MSCs are undifferentiated cells that have multilineage differentiation potential, including adipogenic, osteogenic, chondrogenic, skeletal, tendon, and SMCs.1 Previous studies have demonstrated that MSCs differentiate into one targeted cell type, such as osteogenic or chondrocytic lineages, depending on the scaffold used.7,9 In our hands, the optimized HA–gelin hydrogel was shown not only to preserve MSC stem cell markers (CD44+, CD73+, CD90+, CD11b−, CD31−, and CD45−), but also to keep their plasticity. MSCs were able to generate adipogenic, osteogenic, or SMCs in adequate media. Similar results have been obtained by others who used embryonic stem cells cultivated in another HA hydrogel.10
Whether undifferentiated MSCs are more interesting than differentiated ones in cell therapy strategy is still debated. It has generally been observed that MSCs do not extensively differentiate in injured sites where they have been grafted, but rather act through paracrine pathways on host cells and tissue.44,45 The HA–hydrogel used here, which maintained the encapsulated MSCs in an undifferentiated stem cell state for at least 21 days, could permit better repair and regeneration of tissue damaged in vivo.
One possible application of MSC cultures in HA hydrogel is abdominal aortic aneurysm (AAA) therapy. A rat xenograft model of AAA had previously been developed in our laboratory.46,47 Therefore, it was important to ascertain MSC viability in dynamic culture conditions that aimed at reproducing the in vivo conditions of cells submitted to heart beat. Interestingly, Flexcell technology applied to this end revealed here that the dynamic in vitro 3D microenvironment favored MSC viability better than the static conditions. After 7 days of culture in the dynamic Flexcell device, there were 40% more cells than in static conditions (Fig. 7). It has been suggested that dynamic culture conditions may improve oxygenation and molecule diffusion within the scaffolds that could improve cell survival.48–50 Nevertheless, the MSC proliferation (40%) cannot only be explained by hydrogel oxygenation, but this implies biological mechanisms as cytokine synthesis, which may be stimulated by mechanical stimulus. As suggested by,51 the growth in dynamic conditions also may stimulate cytokine synthesis by MSCs, which may in turn favor cell survival and proliferation. Other studies have reported that application of equibiaxial strain mimicking aneurysmal wall strain to cells cultivated in collagen hydrogel improves cell viability.52 The cellular response has not been obtained with Flexcell system but using another mechanical loading device. Therefore, our results are consistent with literature and show a mechanical stimulus effect on cells viability and proliferation in a 3D hydrogel matrix.
Conclusion
Developments in stem cell biology open new directions in cell therapy. This study shows that the HA biomaterial used as an artificial niche for engineering MSCs is a promising device both for the purpose of better understanding their biology under 3D conditions and for the development of new strategies for efficient long-term maintenance undifferentiated state and directed differentiation of stem cells into various therapeutic lineages.
Appendix
Supplementary Materials and Methods
Inflammation test
All the animal laboratory procedures and experimental techniques were conducted in compliance with ethics approval. This study was carried out in strict accordance to the French Ministry of Agriculture's authorization.
To test the possible inflammation effect of hydrogel, three male Fisher 344 rats (250—300 g, Charles-Rivers, St Germain Sur L'arbresle, France) were injected intraperitoneally with sodium pentobarbital (50 mg/kg), and then 2 mL of hydrogel was injected in skin and abdominal muscle located in one abdomen side of rat. Saline (0.9% NaCl) was injected in the other side. Two weeks after injection, rats were euthanatized by pentobarbital solution. A sample of each injected tissue was collected and fixed in 4% paraformaldehyde, dehydrated in ethanol then in toluene, and embedded in paraffin. The inflammatory cells were visualized in obtained tissues using anti-rat CD68 (AbD Serotec, Colmar, France) and Vector DAB substrate. Images were obtained using a Zeiss microscope (Zeiss LSM 510 META).
Potential inflammatory effect
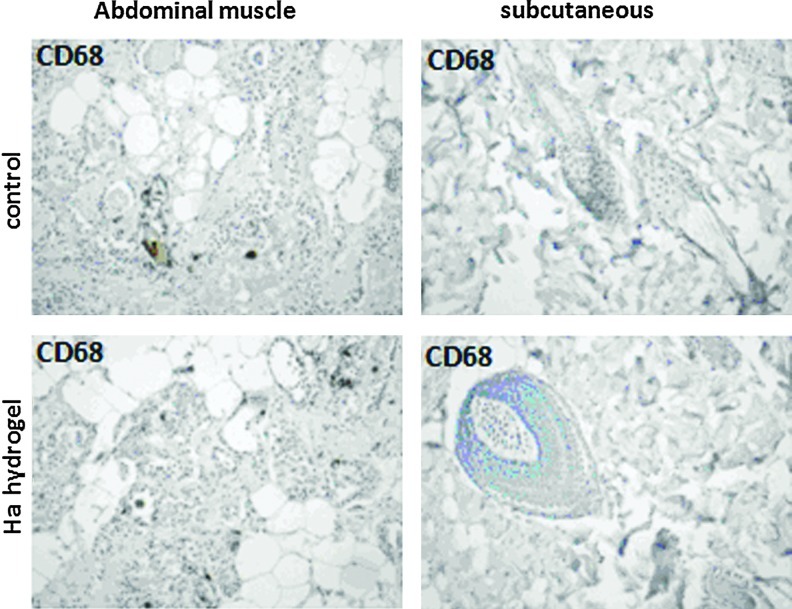
Since 3D cultures of mesenchymal stem cells are supposed to be used for further cell therapy, the optimal hyaluronic acid (HA)–hydrogel was checked for its potential inflammatory effect in vivo. Hydrogel injected either in abdominal muscle or subcutaneously did not induce any inflammatory effect as controlled by the presence of CD68-positive cells (Appendix Fig. A1). Our data indicated that there was no increase in cells bearing the immunohistochemical markers between hydrogel injection and control. In addition, we never noticed edema or any other signs of general inflammation in rats. This verified that HA–hydrogel had no adverse effect when used in vivo.
Appendix FIG. A1.
Analysis of the inflammatory response induced by hydrogel in rat. Two weeks after of intramuscular or subcutaneous hydrogel (hyaluronic acid:gelin [40:60], extralinker ratio 1:8) injections, the inflammatory response was examined by immunochemistry. The injection of saline was used as control. The tissues were labeled with antibody against CD68 (anti-inflammatory macrophages) and reveled with Vector DAB (brown). Hematoxylin–eosin counterstained (blue). Optical microscopy images with magnification (× 10). Color images available online at www.liebertpub.com/tec
Acknowledgments
The authors would like to acknowledge the Institut Mondor de Recherche Biomédicale and LISA laboratory, UPEC, Créteil, France, for the use of their platforms. Efficient technical support in obtaining data from rheological tests and helpful discussions with Dr Anissa Eddhahak Ouni, Arts et Métiers ParisTech (ENSAM-ESTP), Cachan, France, are greatly appreciated. This work was supported by an European Union Grant (FP7-Health-200647, Fighting Aneurysmal Diseases).
Disclosure Statement
No competing financial interests exist.
References
- 1.Bobis S. Jarocha D. Majka M. Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol. 2006;44:215. [PubMed] [Google Scholar]
- 2.Pek Y.S. Wan A.C. Ying J.Y. The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials. 2010;31:385. doi: 10.1016/j.biomaterials.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 3.Engler A.J. Sen S. Sweeney H.L. Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Pampaloni F. Reynaud E.G. Stelzer E.H. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 5.Saha K. Pollock J.F. Schaffer D.V. Healy K.E. Designing synthetic materials to control stem cell phenotype. Curr Opin Chem Biol. 2007;11:381. doi: 10.1016/j.cbpa.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson E. Mapili G. Erickson K. Taqvi S. Roy K. Biomaterials for stem cell differentiation. Adv Drug Deliv Rev. 2008;60:215. doi: 10.1016/j.addr.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Facchini A. Lisignoli G. Cristino S. Roseti L. De Franceschi L. Marconi E., et al. Human chondrocytes and mesenchymal stem cells grown onto engineered scaffold. Biorheology. 2006;43:471. [PubMed] [Google Scholar]
- 8.Garcia-Fuentes M. Meinel A.J. Hilbe M. Meinel L. Merkle H.P. Silk fibroin/hyaluronan scaffolds for human mesenchymal stem cell culture in tissue engineering. Biomaterials. 2009;30:5068. doi: 10.1016/j.biomaterials.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Cristino S. Grassi F. Toneguzzi S. Piacentini A. Grigolo B. Santi S., et al. Analysis of mesenchymal stem cells grown on a three-dimensional HYAFF 11-based prototype ligament scaffold. J Biomed Mater Res A. 2005;73:275. doi: 10.1002/jbm.a.30261. [DOI] [PubMed] [Google Scholar]
- 10.Gerecht S. Burdick J.A. Ferreira L.S. Townsend S.A. Langer R. Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:11298. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutty J.K. Cho E. Soo Lee J. Vyavahare N.R. Webb K. The effect of hyaluronic acid incorporation on fibroblast spreading and proliferation within PEG-diacrylate based semi-interpenetrating networks. Biomaterials. 2007;28:4928. doi: 10.1016/j.biomaterials.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Slevin M. Kumar S. Gaffney J. Angiogenic oligosaccharides of hyaluronan induce multiple signaling pathways affecting vascular endothelial cell mitogenic and wound healing responses. J Biol Chem. 2002;277:41046. doi: 10.1074/jbc.M109443200. [DOI] [PubMed] [Google Scholar]
- 13.Toole B.P. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001;12:79. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 14.Nehrer S. Domayer S. Dorotka R. Schatz K. Bindreiter U. Kotz R. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur J Radiol. 2006;57:3. doi: 10.1016/j.ejrad.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Fu Y. Xu K. Zheng X. Giacomin A.J. Mix A.W. Kao W.J. 3D cell entrapment in crosslinked thiolated gelatin-poly(ethylene glycol) diacrylate hydrogels. Biomaterials. 2012;33:48. doi: 10.1016/j.biomaterials.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunt N.C. Grover L.M. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol Lett. 2010;32:733. doi: 10.1007/s10529-010-0221-0. [DOI] [PubMed] [Google Scholar]
- 17.Woerly S. Pinet E. De Robertis L. Bousmina M. Laroche G. Roitback T., et al. Heterogeneous PHPMA hydrogels for tissue repair and axonal regeneration in the injured spinal cord. J Biomater Sci Polym Ed. 1998;9:681. doi: 10.1163/156856298x00091. [DOI] [PubMed] [Google Scholar]
- 18.Dobson K.R. Reading L. Haberey M. Marine X. Scutt A. Centrifugal isolation of bone marrow from bone: an improved method for the recovery and quantitation of bone marrow osteoprogenitor cells from rat tibiae and femurae. Calcif Tissue Int. 1999;65:411. doi: 10.1007/s002239900723. [DOI] [PubMed] [Google Scholar]
- 19.Jeon E.S. Moon H.J. Lee M.J. Song H.Y. Kim Y.M. Bae Y.C., et al. Sphingosylphosphorylcholine induces differentiation of human mesenchymal stem cells into smooth-muscle-like cells through a TGF-beta-dependent mechanism. J Cell Sci. 2006;119:4994. doi: 10.1242/jcs.03281. [DOI] [PubMed] [Google Scholar]
- 20.Li X. Feng Q. Wang W. Cui F. Chemical characteristics and cytocompatibility of collagen-based scaffold reinforced by chitin fibers for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2006;77:219. doi: 10.1002/jbm.b.30425. [DOI] [PubMed] [Google Scholar]
- 21.Mahoney M.J. Anseth K.S. Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials. 2006;27:2265. doi: 10.1016/j.biomaterials.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Weber L.M. Hayda K.N. Anseth K.S. Cell-matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue Eng Part A. 2008;14:1959. doi: 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frith J.E. Thomson B. Genever P.G. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16:735. doi: 10.1089/ten.TEC.2009.0432. [DOI] [PubMed] [Google Scholar]
- 24.Gumbiner B.M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 25.Lee S.B. Jeon H.W. Lee Y.W. Cho S.K. Lee Y.M. Song K.W. Hwa Hong M.H.P. Artificial dermis composed of gelatin, hyaluronic acid and (1–3),(1–6)-b-glucan. Macromol Res. 2003;11:368. [Google Scholar]
- 26.Liu Y. Zheng Shu X. Prestwich G.D. Biocompatibility and stability of disulfide-crosslinked hyaluronan films. Biomaterials. 2005;26:4737. doi: 10.1016/j.biomaterials.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Park Y.D. Tirelli N. Hubbell J.A. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials. 2003;24:893. doi: 10.1016/s0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 28.Prestwich G.D. Liu Y. Yu B. Shu X.Z. Scott A. 3-D culture in synthetic extracellular matrices: new tissue models for drug toxicology and cancer drug discovery. Adv Enzyme Regul. 2007;47:196. doi: 10.1016/j.advenzreg.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh K. Ren X.D. Shu X.Z. Prestwich G.D. Clark R.A. Fibronectin functional domains coupled to hyaluronan stimulate adult human dermal fibroblast responses critical for wound healing. Tissue Eng. 2006;12:601. doi: 10.1089/ten.2006.12.601. [DOI] [PubMed] [Google Scholar]
- 30.Shu X.Z. Ahmad S. Liu Y. Prestwich G.D. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J Biomed Mater Res A. 2006;79:902. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- 31.Shu X.Z. Liu Y. Palumbo F. Prestwich G.D. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: a covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials. 2003;24:3825. doi: 10.1016/s0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 32.Zhang F. He C. Cao L. Feng W. Wang H. Mo X., et al. Fabrication of gelatin-hyaluronic acid hybrid scaffolds with tunable porous structures for soft tissue engineering. Int J Biol Macromol. 2011;48:474. doi: 10.1016/j.ijbiomac.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe K. Nakamura M. Okano H. Toyama Y. Establishment of three-dimensional culture of neural stem/progenitor cells in collagen type-1 gel. Restor Neurol Neurosci. 2007;25:109. [PubMed] [Google Scholar]
- 34.Taupin P. Ray J. Fischer W.H. Suhr S.T. Hakansson K. Grubb A., et al. FGF-2-responsive neural stem cell proliferation requires CCg, a novel autocrine/paracrine cofactor. Neuron. 2000;28:385. doi: 10.1016/s0896-6273(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 35.Salinas C.N. Anseth K.S. Mesenchymal stem cells for craniofacial tissue regeneration: designing hydrogel delivery vehicles. J Dent Res. 2009;88:681. doi: 10.1177/0022034509341553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehra T.D. Ghosh K. Shu X.Z. Prestwich G.D. Clark R.A. Molecular stenting with a crosslinked hyaluronan derivative inhibits collagen gel contraction. J Invest Dermatol. 2006;126:2202. doi: 10.1038/sj.jid.5700380. [DOI] [PubMed] [Google Scholar]
- 37.Park J.H. Chung B.G. Lee W.G. Kim J. Brigham M.D. Shim J., et al. Microporous cell-laden hydrogels for engineered tissue constructs. Biotechnol Bioeng. 2010;106:138. doi: 10.1002/bit.22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee M. Wu B.M. Dunn J.C. Effect of scaffold architecture and pore size on smooth muscle cell growth. J Biomed Mater Res A. 2008;87:1010. doi: 10.1002/jbm.a.31816. [DOI] [PubMed] [Google Scholar]
- 39.Lee S.T. Yun J.I. Jo Y.S. Mochizuki M. van der Vlies A.J. Kontos S., et al. Engineering integrin signaling for promoting embryonic stem cell self-renewal in a precisely defined niche. Biomaterials. 2010;31:1219. doi: 10.1016/j.biomaterials.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 40.Ghosh K. Pan Z. Guan E. Ge S. Liu Y. Nakamura T., et al. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials. 2007;28:671. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weng L. Chen X. Chen W. Rheological characterization of in situ crosslinkable hydrogels formulated from oxidized dextran and N-carboxyethyl chitosan. Biomacromolecules. 2007;8:1109. doi: 10.1021/bm0610065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y.J. Chung E.H. Rodriguez R.T. Firpo M.T. Healy K.E. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J Biomed Mater Res A. 2006;79:1. doi: 10.1002/jbm.a.30732. [DOI] [PubMed] [Google Scholar]
- 43.Flynn L. Prestwich G.D. Semple J.L. Woodhouse K.A. Adipose tissue engineering with naturally derived scaffolds and adipose-derived stem cells. Biomaterials. 2007;28:3834. doi: 10.1016/j.biomaterials.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Copland I.B. Mesenchymal stromal cells for cardiovascular disease. J Cardiovasc Dis Res. 2011;2:3. doi: 10.4103/0975-3583.78581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gnecchi M. Zhang Z. Ni A. Dzau V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohand-Kaci F. Eddhahak Ouni A. Dai J. Allaire E. Zidi M. Stochastic modelling of wall stresses in abdominal aortic aneurysms treated by a gene therapy. Comput Methods Biomech Biomed Engin. 2012;15:435. doi: 10.1080/10255842.2010.540759. [DOI] [PubMed] [Google Scholar]
- 47.Allaire E. Muscatelli-Groux B. Mandet C. Guinault A.M. Bruneval P. Desgranges P., et al. Paracrine effect of vascular smooth muscle cells in the prevention of aortic aneurysm formation. J Vasc Surg. 2002;36:1018. doi: 10.1067/mva.2002.127347. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X. Wang X. Keshav V. Johanas J.T. Leisk G.G. Kaplan D.L. Dynamic culture conditions to generate silk-based tissue-engineered vascular grafts. Biomaterials. 2009;30:3213. doi: 10.1016/j.biomaterials.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cimetta E. Flaibani M. Mella M. Serena E. Boldrin L. De Coppi P., et al. Enhancement of viability of muscle precursor cells on 3D scaffold in a perfusion bioreactor. Int J Artif Organs. 2007;30:415. doi: 10.1177/039139880703000509. [DOI] [PubMed] [Google Scholar]
- 50.Tiitu V. Pulkkinen H.J. Valonen P. Pulliainen O. Kellomaki M. Lammi M.J., et al. Bioreactor improves the growth and viability of chondrocytes in the knitted poly-L,D-lactide scaffold. Biorheology. 2008;45:539. [PubMed] [Google Scholar]
- 51.Li Y. Zhao Z. Song J. Feng Y. Wang Y. Li X., et al. Cyclic force upregulates mechano-growth factor and elevates cell proliferation in 3D cultured skeletal myoblasts. Arch Biochem Biophys. 2009;490:171. doi: 10.1016/j.abb.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 52.Butcher J.T. Barrett B.C. Nerem R.M. Equibiaxial strain stimulates fibroblastic phenotype shift in smooth muscle cells in an engineered tissue model of the aortic wall. Biomaterials. 2006;27:5252. doi: 10.1016/j.biomaterials.2006.05.040. [DOI] [PubMed] [Google Scholar]