Abstract
Aims
Elevated levels of pro-inflammatory cytokine interleukin-17A (IL-17) are associated with hypertensive autoimmune diseases; however, the connection between IL-17 and hypertension is unknown. We hypothesized that IL-17 increases blood pressure by decreasing endothelial nitric oxide production.
Methods and results
Acute treatment of endothelial cells with IL-17 caused a significant increase in phosphorylation of the inhibitory endothelial nitric oxide (NO) synthase residue threonine 495 (eNOS Thr495). Of the kinases known to phosphorylate eNOS Thr495, only inhibition of Rho-kinase prevented the IL-17-induced increase. IL-17 caused a threefold increase in the Rho-kinase activator RhoA, and this was prevented by an IL-17 neutralizing antibody. In isolated mouse aortas, IL-17 significantly increased eNOS Thr495 phosphorylation, induced RhoA expression, and decreased NO-dependent relaxation responses, all of which were prevented by either an IL-17 neutralizing antibody or inhibition of Rho-kinase. In mice, IL-17 treatment for 1 week significantly increased systolic blood pressure and this was associated with decreased aortic NO-dependent relaxation responses, increased eNOS Thr495 phosphorylation, and increased RhoA expression. Inhibition of Rho-kinase prevented the hypertension caused by IL-17.
Conclusion
These data demonstrate that IL-17 activates RhoA/Rho-kinase leading to endothelial dysfunction and hypertension. Inhibitors of IL-17 or Rho-kinase may prove useful as anti-hypertensive drugs in IL-17-associated autoimmune diseases.
Keywords: Interleukin-17, Nitric oxide synthase, Endothelial dysfunction, Hypertension
1. Introduction
The interleukin-17 (IL-17) family of cytokines and their ubiquitous receptors play a major role in host defence against pathogens. Six known IL-17 family members (IL-17A–F), including the prototypical IL-17A (also known as IL-17), exert potent pro-inflammatory effects and are produced by a myriad of immune cells.1–3 Once thought to be generated only by a subset of CD4+ T cells (Th17 cells), IL-17 is now reportedly secreted by macrophages, dendritic cells, natural killer cells, natural killer T cells, and γδ T cells in response to immune system activation.3 Recent studies have implicated IL-17 in a variety of inflammatory autoimmune diseases, including rheumatoid arthritis, inflammatory bowel disease, and acute coronary syndrome.4–8 Additionally, elevated IL-17 levels are associated with hypertension and autoimmune diseases associated with hypertension including pre-eclampsia, systemic lupus erythematosus, and chronic allograft rejection.9–23 IL-17 augments the pro-inflammatory process in part by modulating target genes of secondary cytokines and chemokines; however, the molecular mechanisms by which IL-17 may contribute to the development of hypertension is largely unknown.
Endothelial function contributes greatly to vascular and blood pressure homeostasis. Endothelial cell production of nitric oxide (NO) is crucial for vasodilation, preventing leucocyte adhesion and extravasation, and regulating blood pressure. Vascular production of NO by endothelial NO synthase (eNOS) is tightly regulated by several post-translational modifications.24 Phosphorylation of eNOS modulates NO production and the hierarchal dephosphorylation of the inhibitory site Thr495 and subsequent phosphorylation of the stimulatory site Ser1177 optimizes eNOS activity.24–27 We and others have reported that increased eNOS Thr495 phosphorylation leads to decreased NO production and vasodilation and is associated with hypertension.28–32 Whether IL-17 affects eNOS Thr495 phosphorylation and endothelial function is unknown.
IL-17 can induce other pro-inflammatory cytokines, including IL-6, tumour necrosis factor-α, and IL-1β, all of which are also associated with endothelial dysfunction and hypertension.1,33–37 However, whether IL-17 directly exerts detrimental effects on eNOS and endothelial function is unknown. We hypothesized that IL-17 increases eNOS Thr495 phosphorylation leading to endothelial dysfunction and hypertension. To test this hypothesis we treated endothelial cells, isolated mouse aortas, as well as mice with IL-17 and determined the effects on eNOS Thr495 phosphorylation, endothelial function, and blood pressure.
2. Methods
2.1. Endothelial cell studies
Rat aortic endothelial cells were obtained from Cell Applications (San Diego, CA, USA) and cultured in complete DMEM (Invitrogen) containing endothelial cell growth supplement (Sigma), antibiotics (Invitrogen), and heparin (Sigma). All experiments were performed in cells at passage 6 or less. Immunoblotting using primary antibodies for anti-eNOS (BD Biosciences), anti-phospho-eNOS Thr495 (Cell Signaling), anti-phospho-eNOS Ser1177 (Millipore), anti-eNOS (BD Transduction Laboratories), anti-RhoA (Abcam), and anti-β-actin (Sigma) was performed as described previously, following a 1 h incubation with IL-17 (mouse rIL-17A, 1 µg/mL; eBioscience).28,29 Immunoblotting was also performed for these proteins in IL17-treated cells following co-treatment with an IL-17 neutralizing antibody (20 ng/mL for 30 min; BioLegend), the protein kinase C (PKC) inhibitor Gö6976 (1 µmol/L for 30 min; Sigma), the Rho-kinase inhibitor Y-27632 (50 µmol/L for 30 min; Cayman Chemical Co.), or appropriate vehicle. Secondary antibodies consisted of anti-rabbit and anti-mouse IgGs conjugated to Alexa-Fluor 680 and IR800Dye (LI-COR Biosciences), respectively. The blots were identified simultaneously using near-infrared visualization (Odyssey System, LI-COR Biosciences, Lincoln, NE, USA). Densitometry was performed using the Odyssey software.
2.2. Acute ex vivo and in vitro vascular studies
Male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME, USA) aged 10–12 weeks were anaesthetized by prolonged inhalation of 5% isoflurane, verified by lack of a response to toe pinch, followed by euthanization by cervical dislocation. Endothelium-intact aortas were isolated and treated as above. Aortic segments were used for immunoblotting as described above and for the determination of vascular nitrate levels while aortic rings were used to measure vascular reactivity as described previously.28,29 Immunoblotting in aortic homogenates was performed following a pull-down with eNOS as described in an available protocol (Cell Signaling); therefore, IgG was used as a loading control and corresponded directly with eNOS levels. Measurements of nitrate levels were performed using the Griess assay according to the manufacturer's instructions (Invitrogen). For measures of vascular reactivity, all aortic rings were treated with the cyclooxygenase inhibitor indomethacin (10 μmol/L, 45 min) in order to examine NO-mediated relaxation. Some endothelium-intact aortic rings were also co-incubated with cell permeable superoxide dismutase (PEG-SOD; 100 U/L, 45 min), an IL-17 neutralizing antibody, Gö6976, Y-27632, the more potent and specific Rho-kinase inhibitor H-1152P (1 μmol/L, 30 min), or appropriate vehicle as described above and/or the NOS inhibitor L-NAME (10 μmol/L, 60 min). Concentration–force curves were obtained in a cumulative fashion to acetylcholine (ACh), bradykinin, and sodium nitroprusside (SNP) following contraction to an EC70 concentration (1 µmol/L) of phenylephrine (PE) as well as to PE.
2.3. In vivo studies
Male C57BL/6 mice (Charles River) aged 10–12 weeks and housed on a 12:12 light/dark cycle were used in all experiments. Mouse recombinant IL-17A (1 µg/day, n = 9 mice) or diluent (saline and 7% ethanol, n = 9 mice) was given every day via i.p. injection of 150 µL volume as described previously.28,29 This dose of IL-17 was chosen based on previous reports and represents a medium to high level of serum IL-17 as seen in patients with inflammation.38,39 Some IL-17-treated mice were concurrently given Y-27632 at a dose that was shown not to affect blood pressure in control mice (10 mg/kg, daily i.p. injection).40 Tail-cuff systolic blood pressures (IITC, Inc.) were measured at baseline and prior to injections on Days 1, 4, and 7 of daily treatment. IL-17-treated mice had final blood pressure measurements taken on Day 7 and then on Day 8 all mice were anaesthetized with isoflurane and euthanized by exsanguination as described above followed by serum and tissue collection. Serum IL-17 and IL-6 levels were measured by ELISA (SA Biosciences Cytokine ELISArray-MEM-003A). Splenocytes were prepared and stained for CD3 and CD4 using the fluorescence-conjugated antibodies anti-mouse CD3e-PE-Cy7 and anti-mouse CD4-APC as described previously.41 Flow cytometry using a BD Canto II was performed and lymphocytes were gated in the forward by side scatter plots. Isotype controls were used to determine the appropriate gates for CD3+/CD4+ T cells and data analysis was performed using FlowJo software. Aortic eNOS Thr495 phosphorylation, eNOS and RhoA levels, and vascular reactivity were measured as described above. All procedures were approved by our Institutional Animal Care and Use Committee (#2007-018R) in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.4. Statistical analysis
Results are presented as mean ± SEM. The two-tailed Student's t-test was used to compare variables between two groups. For multiple comparisons, an analysis of variance was used followed by the Student'–Newman–Keuls post hoc test. The significance level was set at 0.05. All analyses were performed using the SigmaStat 3.5 software.
3. Results
3.1. Effects of IL-17 on eNOS Thr495 phosphorylation in endothelial cells
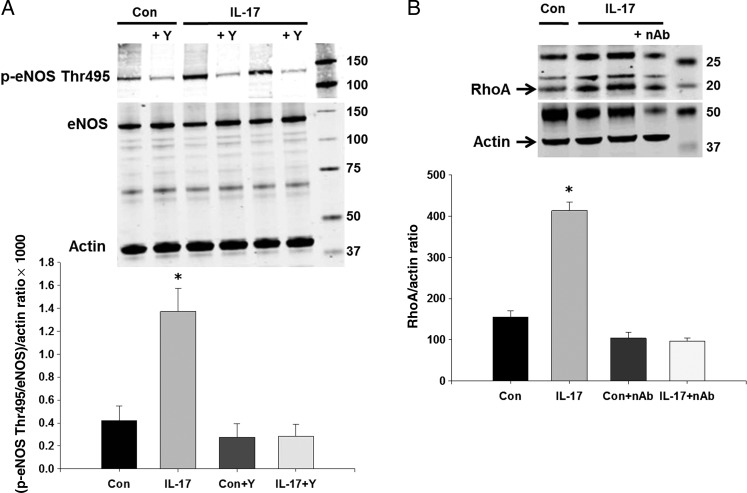
To examine the acute effects of IL-17 on endothelial cell eNOS activity, we measured a marker of eNOS activity, eNOS Thr495 phosphorylation. eNOS Thr495 phosphorylation inhibits NO production and dephosphorylation promotes NO production.27 Figure 1A demonstrates that treatment of endothelial cells with IL-17 for 1 h significantly increased eNOS Thr495 phosphorylation ∼3.5-fold compared with vehicle-treated controls while having no effects on total eNOS levels. PKC and Rho-kinase are known to phosphorylate eNOS Thr495; therefore, we tested whether inhibition of either of these could prevent the IL-17-induced increase in eNOS Thr495 phosphorylation. The PKC inhibitor Gö6976 had no effect (data not shown). However, pre-treatment with the Rho-kinase inhibitor Y-27632 prevented the increase in eNOS Thr495 phosphorylation induced by IL-17 (Figure 1A). To verify that Rho-kinase activity is increased in endothelial cells treated with IL-17, we measured expression of RhoA, an upstream activator of Rho-kinase. Figure 1B demonstrates that acute IL-17 treatment significantly increased endothelial cell RhoA levels ∼3-fold and this was prevented by an IL-17 neutralizing antibody. These findings support our hypothesis that IL-17 decreases eNOS activity, and that this is caused by increasing Rho-kinase-mediated phosphorylation of eNOS Thr495.
Figure 1.
Effects of IL-17 on eNOS phosphorylation and RhoA in endothelial cells. (A) Endothelial cells were treated with vehicle, mouse recombinant IL-17 (1 µg/mL), and/or the Rho-kinase inhibitor Y-27632 (50 µmol/L) for 1 h and eNOS Thr495 phosphorylation and eNOS were measured by western blot. (B) Endothelial cells were treated with vehicle, mouse recombinant IL-17, and/or an IL-17 neutralizing antibody (20 ng/mL) for 1 h and RhoA was measured by western blot. Representative western blots and densitometry quantification. Results are expressed as mean + SEM. *P < 0.05 vs. control. N = 3–5 independent experiments.
3.2. IL-17 causes endothelial dysfunction directly
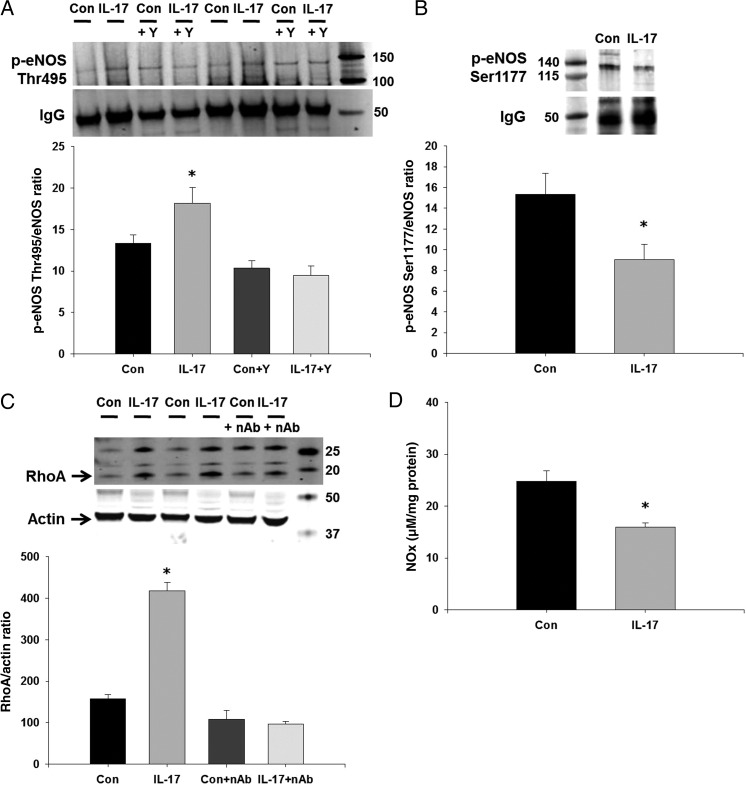
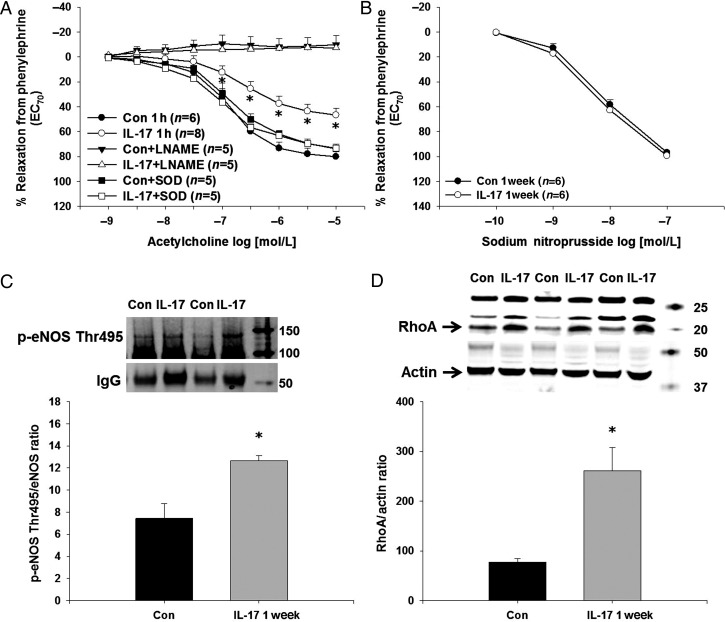
To examine the acute vascular effects of IL-17, we treated endothelium-intact aortas from control mice with IL-17 for 1 h and measured eNOS phosphorylation, RhoA levels, nitrate production, and endothelium-dependent relaxation and contraction responses. IL-17 significantly increased aortic eNOS Thr495 phosphorylation (Figure 2A), similar to that seen in endothelial cells. This could be prevented by pre-treatment with Y-27632 (Figure 2A). eNOS Ser1177 phosphorylation, a stimulatory eNOS phosphorylation site, was decreased significantly in IL-17-treated vessels (Figure 2B). IL-17 significantly increased aortic RhoA levels ∼3-fold, and this was prevented by an IL-17 neutralizing antibody (Figure 2C). To determine whether the IL-17-induced alteration in eNOS phosphorylation affected vascular NO production, we measured nitrate levels in IL-17- and vehicle-treated vessels. One hour incubation with IL-17 significantly decreased nitrate levels (Figure 2D).
Figure 2.
Effects of IL-17 on eNOS phosphorylation and RhoA in isolated mouse aortas. Isolated, endothelium-intact mouse aortas were treated with vehicle, mouse recombinant IL-17, and/or the Rho-kinase inhibitor Y-27632 for 1 h and (A) eNOS Thr495 and (B) eNOS Ser1177 phosphorylation was measured by western blot following a pull-down with anti-eNOS. IgG was used as a loading control and for normalization as this corresponded directly to eNOS levels. (C) Isolated, endothelium-intact mouse aortas were treated with vehicle, mouse recombinant IL-17, and/or an IL-17 neutralizing antibody for 1 h and RhoA was measured by western blot. Representative western blot and densitometry quantification. (D) Nitrate production was measured in vehicle-treated and IL-17-treated aortas using the Griess assay. Results are expressed as mean + SEM. *P < 0.05 vs. control. N = 3–6 independent experiments.
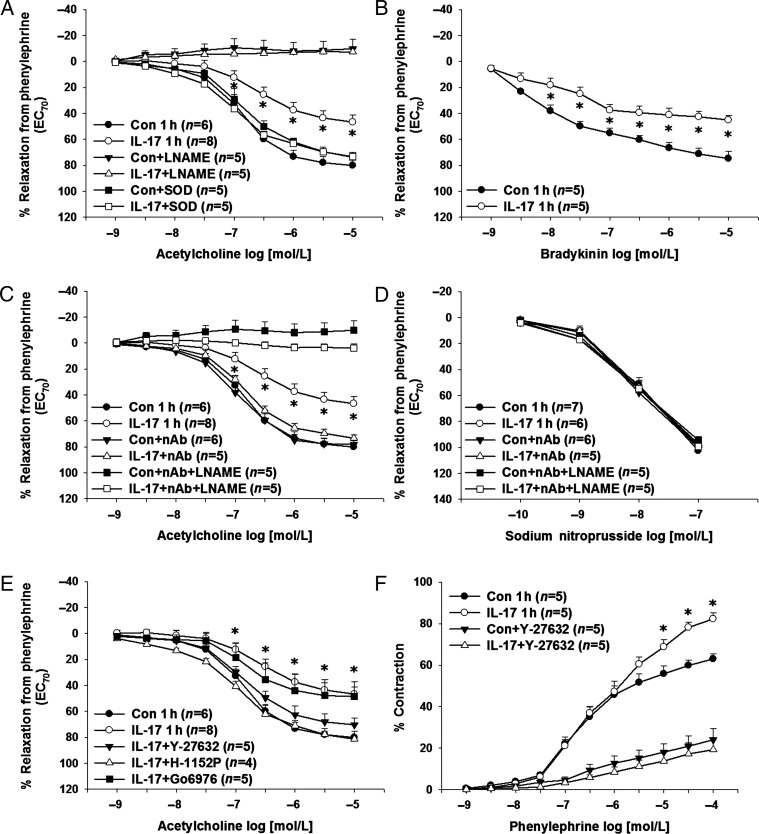
Next, we measured aortic endothelium-dependent and endothelium-independent relaxation responses as well as contraction responses following acute treatment with IL-17 or vehicle. IL-17 significantly decreased maximal ACh-induced relaxation responses compared with vehicle-treated controls (% relaxation from PE-induced contraction: controls: 80 ± 5% vs. IL-17: 47 ± 5%, P < 0.05; Figure 3A). The NOS inhibitor L-NAME (10 μmol/L) abolished relaxation responses to ACh in both groups (Figure 3A). Since both IL-17 and Rho-kinase are known to activate NADPH oxidase and increase superoxide production, we determined whether PEG-SOD could restore relaxation responses to ACh. Figure 3A demonstrates that PEG-SOD normalized relaxation responses in IL-17-treated vessels while having no effect in vehicle-treated vessels. Relaxation responses to another vasodilator, bradykinin, were also decreased in IL-17-treated aortas compared with controls (% relaxation from PE-induced contraction: controls: 75 ± 5% vs. IL-17: 45 ± 3%, P < 0.05; Figure 3B). The IL-17-mediated decrease in aortic relaxation responses was also normalized by an IL-17 neutralizing antibody which restored NO production as the improvement in relaxation was blocked by L-NAME (Figure 3C). IL-17, the IL-17 neutralizing antibody, or L-NAME had no effect on endothelium-independent relaxation responses to SNP (Figure 3D). The Rho-kinase inhibitors Y-27632 and H-1152P, the more specific and potent of the two, also restored aortic relaxation responses to that of controls while PKC inhibition with Gö6976 had no effect (Figure 3E). There were no differences in PE-induced contractions between IL-17-treated aortas and vehicle-treated aortas at the concentration used for the measurement of relaxation responses (contraction to PE 1 µmol/L in g: controls: 1.1 ± 0.2 vs. IL-17: 1.2 ± 0.2 g, P > 0.05). However, IL-17-treated aortas exhibited a significant increase in maximal PE-induced contraction consistent with decreased NO production (Figure 3F), and this was normalized by the Rho-kinase inhibitor Y-27632 (Figure 3F).
Figure 3.
Effects of IL-17 on endothelial function in isolated mouse aortas. (A) Isolated, endothelium-intact mouse aortas were treated with vehicle, mouse recombinant IL-17, the NOS inhibitor L-NAME, and/or the superoxide scavenger PEG-SOD for 1 h and relaxation responses to ACh were measured. (B) Relaxation responses to bradykinin were measured in vehicle-treated and IL-17-treated aortas. Aortas were treated with vehicle or mouse recombinant IL-17 in the absence and presence of an IL-17 neutralizing antibody with and without the NOS inhibitor L-NAME for 1 h and relaxation responses to ACh (C) and SNP (D) were measured. (E) Aortas were treated with vehicle or mouse recombinant IL-17 in the absence and presence of the Rho-kinase inhibitors Y-27632 or H-1152P or the cPKC inhibitor Gö6976 and relaxation responses to ACh were measured. (F) Isolated, endothelium-intact mouse aortas were treated with vehicle or mouse recombinant IL-17 in the absence and presence of the Rho-kinase inhibitor Y-27632 and contraction responses to PE were measured. Results are expressed as mean + SEM. *P < 0.05 vs. control. N for each group is given in parentheses.
3.3. Chronic in vivo treatment with IL-17 causes endothelial dysfunction and hypertension
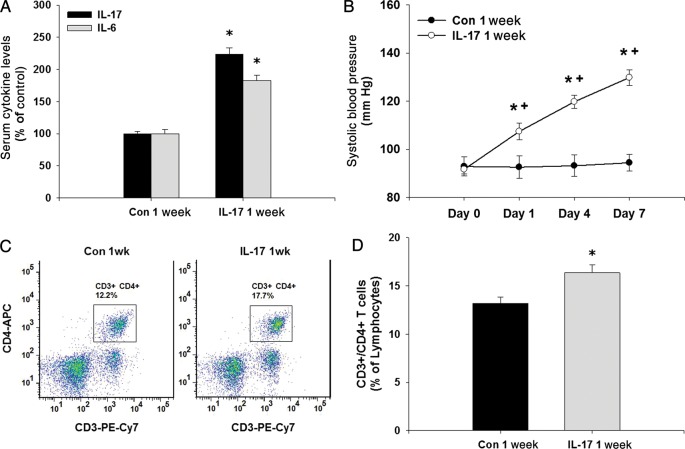
To test the in vivo effects of IL-17, we treated mice daily for 7 days with vehicle or IL-17 at a dose (1 µg/day) that was shown previously to activate an immune response and to induce IL-6 for 24 h.38,39 This was verified by measuring serum IL-17 and IL-6 levels after 7 days of treatment which were both significantly increased ∼2-fold in IL-17-treated mice (Figure 4A). After 1 day, IL-17 treatment increased systolic blood pressure mildly, but significantly, and blood pressure progressively increased throughout the treatment (Day 7 systolic blood pressure: controls: 94 ± 3 mmHg vs. IL-17-treated mice: 130 ± 3 mmHg, P < 0.05; Figure 4B). IL-17 treatment significantly increased the per cent of splenic CD3+/CD4+ T cells, confirming that an immune response was initiated by the daily IL-17 treatment (Figure 4C and D). Body weight was not different between vehicle-treated and IL-17-treated mice at baseline (controls: 25.8 ± 0.5 g vs. IL-17-treated: 26.8 ± 0.7 g, P > 0.05) or following the 1-week treatment regimen (controls: 26.6 ± 0.5 g vs. IL-17-treated: 27.2 ± 0.8 g, P > 0.05).
Figure 4.
Effects of daily IL-17 treatment on serum cytokines, blood pressure, and T cells in mice. (A) Serum levels of IL-17 and IL-6 were measured in mice treated with vehicle or mouse recombinant IL-17 daily for 1 week. (B) Tail-cuff systolic blood pressure was measured in mice at baseline and on Days 1, 4, and 7 of daily treatment with vehicle or mouse recombinant IL-17. (C) CD3+/CD4+ T cells were measured in the spleens of vehicle-treated and IL-17-treated mice using flow cytometry. (D) Quantification of splenic CD3+/CD4+ T cells as a per cent of lymphocytes in vehicle-treated and IL-17-treated mice. Results are expressed as mean ± SEM. *P < 0.05 vs. control, +P < 0.05 vs. previous time point. N = 9 mice for each.
Aortas from IL-17-treated mice had decreased L-NAME-sensitive, ACh-induced relaxation responses compared with aortas from vehicle-injected controls (Figure 5A). This could be prevented by in vitro treatment with PEG-SOD (Figure 5A). There were no differences in SNP-induced relaxation responses in aortas from IL-17-treated mice compared with vehicle-treated mice (Figure 5B). There were no differences in PE-induced contractions at the concentration used for relaxation measures between aortas from IL-17-treated and vehicle-treated mice (contraction to PE 1 µmol/L in g: controls: 1.3 ± 0.2 vs. IL-17-treated: 1.4 ± 0.2 g, P > 0.05). Consistent with the results in endothelial cells and isolated aortas, aortas from IL-17-treated mice exhibited a significant 2- to 3-fold increase in eNOS Thr495 phosphorylation (Figure 5C) and RhoA levels (Figure 5D) compared with aortas from vehicle-treated controls.
Figure 5.
Effects of daily IL-17 treatment in mice on aortic endothelial function, eNOS phosphorylation, and RhoA expression. (A) Relaxation responses to ACh were measured in endothelium-intact aortas isolated from vehicle-treated and IL-17-treated mice in the absence and presence of the NOS inhibitor L-NAME or the superoxide scavenger PEG-SOD. (B) Endothelium-independent relaxation responses to SNP were measured in endothelium-intact aortas isolated from vehicle-treated and IL-17-treated mice. (C) eNOS Thr495 phosphorylation was measured in endothelium-intact aortas isolated from vehicle-treated and IL-17-treated mice by western blot following a pull-down with anti-eNOS. IgG was used as a loading control and for normalization as this corresponded directly to eNOS levels. (D) RhoA was measured in endothelium-intact aortas isolated from vehicle-treated and IL-17-treated mice by western blot. Representative western blot and densitometry quantification. Results are expressed as mean + SEM. *P < 0.05 vs. control. N = 9 mice in each group.
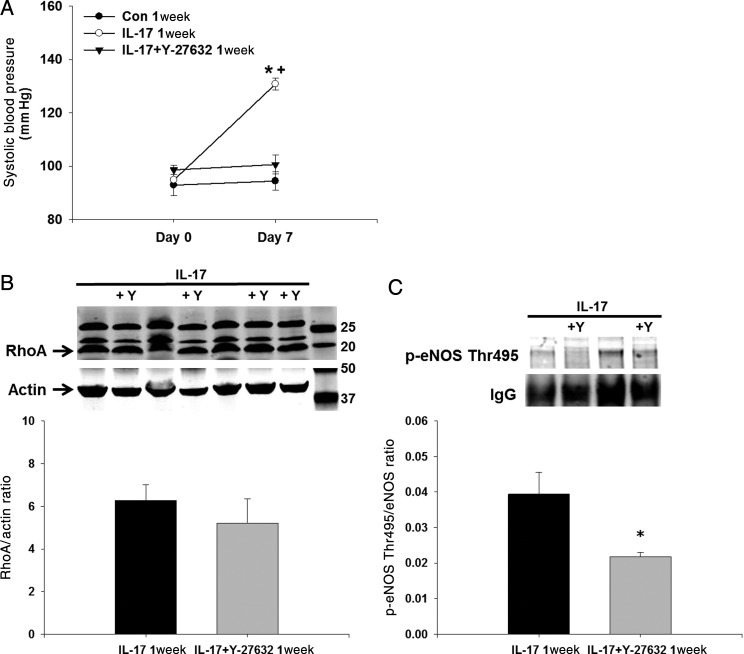
To determine whether inhibition of Rho-kinase could prevent the hypertension induced by IL-17, we administered Y-27632 at a dose that has no effect on blood pressure concurrently with IL-17 for 1 week. Figure 6A demonstrates that daily Y-27632 treatment prevented the significant increase in systolic blood pressure induced by IL-17 treatment. Co-treatment of IL-17 and Y-27632 had no effect on the IL-17-induced elevation in aortic RhoA levels as expected because Y-27632 inhibits Rho-kinase downstream of RhoA (Figure 6B). However, aortic eNOS Thr495 phosphorylation was decreased significantly in mice treated with both IL-17 and Y-27632 (Figure 6C).
Figure 6.
Effects of Rho-kinase inhibition on blood pressure and eNOS phosphorylation in daily IL-17-treated mice. (A) Tail-cuff systolic blood pressure was measured in mice at baseline and on Day 7 of daily treatment with vehicle, mouse recombinant IL-17, or IL-17 and the Rho-kinase inhibitor Y-27632. (B) RhoA was measured in endothelium-intact aortas isolated from IL-17-treated and IL-17 + Y-27632-treated mice by western blot. (C) eNOS Thr495 phosphorylation was measured in endothelium-intact aortas isolated from IL-17-treated and IL-17 + Y-27632-treated mice by western blot following a pull-down with anti-eNOS. IgG was used as a loading control and for normalization as this corresponded directly to eNOS levels. Representative western blot and densitometry quantification. Results are expressed as mean + SEM. *P < 0.05 vs. control. N = 9 mice for each.
4. Discussion
Given the importance of NO production and endothelial function in blood pressure maintenance, as well as the role of IL-17 in hypertension and hypertensive autoimmune diseases, we examined the direct, acute endothelial and vascular effects as well as chronic in vivo effects of IL-17 on eNOS phosphorylation, NO production, endothelial function, and blood pressure. Results from our studies demonstrate that IL-17 increases RhoA/Rho-kinase-mediated eNOS Thr495 phosphorylation leading to decreased NO-mediated vasodilation and hypertension in mice.
Although all cells contain IL-17 receptors, cells respond differently to IL-17. The most studied target cells of IL-17 include epithelial cells, mesenchymal cells, and immune cells. In the setting of an inflammatory response, one of the primary roles of IL-17 signalling is to enhance cytokine and chemokine production in an effort to propagate the pro-inflammatory response and to enable immune cell homing to the site of infection. Known target genes of IL-17 include IL-6 (which stabilizes IL-17-producing T cells), IL-8, IL-21, IL-23, and the immune cell chemoattractants CXCL1, CXCL2, CXCL5, CXCL9, CXCL10, CCL2 (monocyte chemoattractant protein 1, MCP-1), CCL7, CCL20, and granulocyte-macrophage colony-stimulating factor (GM-CSF).1–3,10 Endothelial cells have also been shown to respond to IL-17. IL-17 increases endothelial cell activation demonstrated by increased E-selectin, VCAM-1, and ICAM-1 in human lung microvascular cells.42 IL-17F, which has homology to IL-17, inhibits angiogenesis and causes endothelial cell production of IL-2, TGFβ, and MCP-1.43 The IL-17-induced increase in other pro-inflammatory cytokines and immune cell chemoattractants has been well documented, and the IL-17-mediated accumulation of immune cells also occurs in the vasculature. However, no one to date has examined whether IL-17 exerts direct, acute effects on vascular NO production. Our data show that an acute 1 h treatment of endothelial cells and isolated aortas with IL-17 increases RhoA/Rho-kinase-mediated eNOS Thr495 phosphorylation and decreases vasodilation. This rapid increase in RhoA activation is similar to that seen in response to TGFβ, hypoxia, and endothelin, all of which significantly increase RhoA protein expression within 1 h.44–46 Additionally, the rapid decrease in NO production is consistent with endothelial cell activation and increased eNOS phosphorylation at inhibitory sites. Supportive evidence from previous studies has shown that inhibition of Rho-kinase improves NO generation and endothelial-dependent dilation.47–49
Endothelial cells and IL-17-producing immune cells interact during pro-inflammatory responses and communicate with each other by regulating the cytokine milieu. Human microvascular HLA-DR+ endothelial cells were shown to activate and promote the expansion of Th17 cells via IL-6 production.50 We have recently reported that endothelial and haematopoietic cell TGFβ receptor activation increases endothelial cell activation, IL-6 production, Th17 cells, serum IL-17 levels, and blood pressure in mice. Interestingly, angiotensin II and the immunosuppressive drugs cyclosporine A and FK506 can all produce endothelial dysfunction and hypertension, and all increase TGFβ receptor activation, serum levels of IL-6 and IL-17, and IL-17-producing immune cells.11,41 Additionally, elevated circulating levels of IL-17 are present in patients with the autoimmune diseases pre-eclampsia, allograft rejection, and systemic lupus erythematosus, which are all associated with endothelial dysfunction and hypertension.15,19–22,51–53 Madhur et al. reported that Th17 cell infiltration of the aorta occurs in angiotensin II-induced hypertension and that hypertension was not maintained in IL-17 KO mice. While Rho-kinase inhibition can decrease blood pressure in various models of hypertension including angiotensin II-induced hypertension,54 it remains to be determined whether prevention of IL-17-induced increase in eNOS Thr495 phosphorylation and endothelial dysfunction contributes to the blood pressure-lowering effects.
Given the numerous effects of IL-17 on other pro-inflammatory cytokines, we cannot exclude that some of these may be playing a role in our findings, especially with respect to the in vivo studies. It is possible that in response to IL-17 endothelial cells rapidly produce IL-6, which is known to decrease endothelium-dependent dilation.55,56 Given the small influence of the aorta in blood pressure regulation, it also remains to be determined whether IL-17 increases RhoA/Rho-kinase-mediated eNOS Thr495 phosphorylation in resistance arteries and whether endothelial dysfunction in these vessels contributes to the hypertension caused by IL-17. In addition to the decreased NO production caused by altered eNOS phosphorylation, decreased NO bioavailability due to RhoA/Rho-kinase-mediated increases in superoxide production probably also contributes to the hypertension and endothelial dysfunction as superoxide quenching was able to restore endothelial function in our study.57,58 Additionally, IL-17 may activate vascular smooth muscle cell RhoA/Rho-kinase leading to increased myosin phosphatase phosphorylation, which may have contributed to the increased maximal vasoconstriction in IL-17-treated aortas. Chronic IL-17 exposure also induces genes in smooth muscle cells as Madhur et al. reported that although they found only one gene altered in human smooth muscle cells by IL-17 alone, IL-17 in combination with TNFα increased numerous genes that may impair vascular function.10 We acknowledge that these alternative mechanisms are likely contributing to the endothelial dysfunction and hypertension observed in our week long in vivo studies. Nonetheless, the increase in RhoA/Rho-kinase-mediated eNOS Thr495 phosphorylation in endothelial cells and subsequent decrease in vasodilation plays a major role and represents a novel, acute effect of IL-17 on the vasculature. Supportive evidence comes from our studies in which inhibition of Rho-kinase prevented the increase in eNOS Thr495 phosphorylation and hypertension induced by IL-17.
This study identifies a molecular mechanism by which the pro-inflammatory cytokine IL-17 decreases endothelial function which may contribute to the development of hypertension. In addition to its effects on augmenting other pro-inflammatory chemokines/cytokines, the binding of IL-17 to its receptor located on endothelial cells leads to RhoA/Rho-kinase-mediated eNOS Thr495 phosphorylation, endothelial dysfunction, and hypertension. Inhibition of RhoA/Rho-kinase may be beneficial in hypertensive disorders characterized by elevated levels of IL-17.
Conflict of interest: none declared.
Funding
This work was supported by the National Institutes of Health (grant number HL084299 to B.M.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the National Institutes of Health.
References
- 1.Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–407. doi: 10.1016/j.cyto.2008.07.017. doi:10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaffen SL, Kramer JM, Yu JJ, Shen F. The IL-17 cytokine family. Vitam Horm. 2006;74:255–282. doi: 10.1016/S0083-6729(06)74010-9. doi:10.1016/S0083-6729(06)74010-9. [DOI] [PubMed] [Google Scholar]
- 3.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. doi:10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chabaud M, Fossiez F, Taupin JL, Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol. 1998;161:409–414. [PubMed] [Google Scholar]
- 5.Hata K, Andoh A, Shimada M, Fujino S, Bamba S, Araki Y, et al. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1035–G1044. doi: 10.1152/ajpgi.00494.2001. [DOI] [PubMed] [Google Scholar]
- 6.Noronha AM. New insights into the role of IL-17 in inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:2180–2181. doi: 10.1002/ibd.21333. doi:10.1002/ibd.21333. [DOI] [PubMed] [Google Scholar]
- 7.Liang J, Zheng Z, Wang M, Han L, Peng J, Liu Z, et al. Myeloperoxidase (MPO) and interleukin-17 (IL-17) plasma levels are increased in patients with acute coronary syndromes. J Int Med Res. 2009;37:862–866. doi: 10.1177/147323000903700331. [DOI] [PubMed] [Google Scholar]
- 8.Csiszar A, Ungvari Z. Synergistic effects of vascular IL-17 and TNFalpha may promote coronary artery disease. Med Hypotheses. 2004;63:696–698. doi: 10.1016/j.mehy.2004.03.009. doi:10.1016/j.mehy.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Jafarzadeh A, Esmaeeli-Nadimi A, Nough H, Nemati M, Rezayati MT. Serum levels of interleukin (IL)-13, IL-17 and IL-18 in patients with ischemic heart disease. Anadolu Kardiyol Derg. 2009;9:75–83. [PubMed] [Google Scholar]
- 10.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. doi:10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. doi:10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison DG, Guzik TJ, Goronzy J, Weyand C. Is hypertension an immunologic disease? Curr Cardiol Rep. 2008;10:464–469. doi: 10.1007/s11886-008-0073-6. doi:10.1007/s11886-008-0073-6. [DOI] [PubMed] [Google Scholar]
- 13.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. doi:10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, et al. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. doi:10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, et al. Systemic increase in the ratio between FoxP3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183:7023–7030. doi: 10.4049/jimmunol.0901154. doi:10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- 16.Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9:589–593. doi: 10.1191/096120300678828703. doi:10.1191/096120300678828703. [DOI] [PubMed] [Google Scholar]
- 17.Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385–393. doi: 10.1016/j.clim.2008.01.019. doi:10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183:3160–3169. doi: 10.4049/jimmunol.0900385. doi:10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonysamy MA, Fanslow WC, Fu F, Li W, Qian S, Troutt AB, et al. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol. 1999;162:577–584. [PubMed] [Google Scholar]
- 20.Booth AJ, Bishop DK. TGF-beta, IL-6, IL-17 and CTGF direct multiple pathologies of chronic cardiac allograft rejection. Immunotherapy. 2010;2:511–520. doi: 10.2217/imt.10.33. doi:10.2217/imt.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoh S, Nakae S, Axtell RC, Velotta JB, Kimura N, Kajiwara N, et al. IL-17 contributes to the development of chronic rejection in a murine heart transplant model. J Clin Immunol. 2010;30:235–240. doi: 10.1007/s10875-009-9366-9. doi:10.1007/s10875-009-9366-9. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Ahmed E, Wang T, Wang Y, Ochando J, Chong AS, et al. TLR signals promote IL-6/IL-17-dependent transplant rejection. J Immunol. 2009;182:6217–6225. doi: 10.4049/jimmunol.0803842. doi:10.4049/jimmunol.0803842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiffrin EL. T lymphocytes: a role in hypertension? Curr Opin Nephrol Hypertens. 2010;19:181–186. doi: 10.1097/MNH.0b013e3283360a2e. doi:10.1097/MNH.0b013e3283360a2e. [DOI] [PubMed] [Google Scholar]
- 24.Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn’t calcium/calmodulin enough? J Pharmacol Exp Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 25.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42:271–279. doi: 10.1016/j.yjmcc.2006.05.023. doi:10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of THR(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001;88:E68–E75. doi: 10.1161/hh1101.092677. doi:10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 27.Lin MI, Fulton D, Babbitt R, Fleming I, Busse R, Pritchard KA, Jr, et al. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of L-arginine metabolism to efficient nitric oxide production. J Biol Chem. 2003;278:44719–44726. doi: 10.1074/jbc.M302836200. doi:10.1074/jbc.M302836200. [DOI] [PubMed] [Google Scholar]
- 28.Long C, Cook LG, Hamilton SL, Wu GY, Mitchell BM. FK506 binding protein 12/12.6 depletion increases endothelial nitric oxide synthase threonine 495 phosphorylation and blood pressure. Hypertension. 2007;49:569–576. doi: 10.1161/01.HYP.0000257914.80918.72. doi:10.1161/01.HYP.0000257914.80918.72. [DOI] [PubMed] [Google Scholar]
- 29.Long C, Cook LG, Wu GY, Mitchell BM. Removal of FKBP12/12.6 from endothelial ryanodine receptors leads to an intracellular calcium leak and endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2007;27:1580–1586. doi: 10.1161/ATVBAHA.107.144808. doi:10.1161/ATVBAHA.107.144808. [DOI] [PubMed] [Google Scholar]
- 30.Cook LG, Chiasson VL, Long C, Wu GY, Mitchell BM. Tacrolimus reduces nitric oxide synthase function by binding to FKBP rather than by its calcineurin effect. Kidney Int. 2009;75:719–726. doi: 10.1038/ki.2008.697. doi:10.1038/ki.2008.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gentile MT, Vecchione C, Maffei A, Aretini A, Marino G, Poulet R, et al. Mechanisms of soluble beta-amyloid impairment of endothelial function. J Biol Chem. 2004;279:48135–48142. doi: 10.1074/jbc.M407358200. doi:10.1074/jbc.M407358200. [DOI] [PubMed] [Google Scholar]
- 32.Jiang X, Yang F, Tan H, Liao D, Bryan RM, Jr, Randhawa JK, et al. Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler Thromb Vasc Biol. 2005;25:2515–2521. doi: 10.1161/01.ATV.0000189559.87328.e4. doi:10.1161/01.ATV.0000189559.87328.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (c-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005;19:149–154. doi: 10.1038/sj.jhh.1001785. doi:10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 34.Davis JR, Giardina JB, Green GM, Alexander BT, Granger JP, Khalil RA. Reduced endothelial NO-cGMP vascular relaxation pathway during TNF-alpha-induced hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R390–R399. doi: 10.1152/ajpregu.00270.2001. [DOI] [PubMed] [Google Scholar]
- 35.Gu JW, Tian N, Shparago M, Tan W, Bailey AP, Manning RD., Jr Renal NF-kappaB activation and TNF-alpha upregulation correlate with salt-sensitive hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1817–R1824. doi: 10.1152/ajpregu.00153.2006. doi:10.1152/ajpregu.00153.2006. [DOI] [PubMed] [Google Scholar]
- 36.Orshal JM, Khalil RA. Reduced endothelial NO-cGMP-mediated vascular relaxation and hypertension in IL-6-infused pregnant rats. Hypertension. 2004;43:434–444. doi: 10.1161/01.HYP.0000113044.46326.98. doi:10.1161/01.HYP.0000113044.46326.98. [DOI] [PubMed] [Google Scholar]
- 37.Lin RC, Morris BJ. Association analysis of polymorphisms at the interleukin-1 locus in essential hypertension. Am J Med Genet. 2002;107:311–316. doi: 10.1002/ajmg.10177. doi:10.1002/ajmg.10177. [DOI] [PubMed] [Google Scholar]
- 38.Jovcic G, Bugarski D, Petakov M, Krstic A, Vlaski M, Stojanovic N, et al. In vivo effects of interleukin-17 on haematopoietic cells and cytokine release in normal mice. Cell Prolif. 2004;37:401–412. doi: 10.1111/j.1365-2184.2004.00322.x. doi:10.1111/j.1365-2184.2004.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jovcic G, Bugarski D, Krstic A, Vlaski M, Petakov M, Mojsilovic S, et al. The effect of interleukin-17 on hematopoietic cells and cytokine release in mouse spleen. Physiol Res. 2007;56:331–339. doi: 10.33549/physiolres.930944. [DOI] [PubMed] [Google Scholar]
- 40.Wang S, Liang B, Viollet B, Zou MH. Inhibition of the AMP-activated protein kinase-alpha2 accentuates agonist-induced vascular smooth muscle contraction and high blood pressure in mice. Hypertension. 2011;57:1010–1017. doi: 10.1161/HYPERTENSIONAHA.110.168906. doi:10.1161/HYPERTENSIONAHA.110.168906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiasson VL, Talreja D, Young KJ, Chatterjee P, Banes-Berceli AK, Mitchell BM. FK506 binding protein 12 deficiency in endothelial and hematopoietic cells decreases regulatory T cells and causes hypertension. Hypertension. 2011;57:1167–1175. doi: 10.1161/HYPERTENSIONAHA.110.162917. doi:10.1161/HYPERTENSIONAHA.110.162917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roussel L, Houle F, Chan C, Yao Y, Berube J, Olivenstein R, et al. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J Immunol. 2010;184:4531–4537. doi: 10.4049/jimmunol.0903162. doi:10.4049/jimmunol.0903162. [DOI] [PubMed] [Google Scholar]
- 43.Starnes T, Robertson MJ, Sledge G, Kelich S, Nakshatri H, Broxmeyer HE, et al. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol. 2001;167:4137–4140. doi: 10.4049/jimmunol.167.8.4137. [DOI] [PubMed] [Google Scholar]
- 44.Kim JS, Kim JG, Moon MY, Jeon CY, Won HY, Kim HJ, et al. Transforming growth factor-beta1 regulates macrophage migration via RhoA. Blood. 2006;108:1821–1829. doi: 10.1182/blood-2005-10-009191. doi:10.1182/blood-2005-10-009191. [DOI] [PubMed] [Google Scholar]
- 45.Turcotte S, Desrosiers RR, Beliveau R. HIF-1alpha mRNA and protein upregulation involves Rho GTPase expression during hypoxia in renal cell carcinoma. J Cell Sci. 2003;116:2247–2260. doi: 10.1242/jcs.00427. doi:10.1242/jcs.00427. [DOI] [PubMed] [Google Scholar]
- 46.Marshall AK, Barrett OP, Cullingford TE, Shanmugasundram A, Sugden PH, Clerk A. ERK1/2 signaling dominates over RhoA signaling in regulating early changes in RNA expression induced by endothelin-1 in neonatal rat cardiomyocytes. PLoS One. 2010;5:e10027. doi: 10.1371/journal.pone.0010027. doi:10.1371/journal.pone.0010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah DI, Singh M. Effect of fasudil on macrovascular disorder-induced endothelial dysfunction. Can J Physiol Pharmacol. 2006;84:835–845. doi: 10.1139/y06-036. doi:10.1139/Y06-036. [DOI] [PubMed] [Google Scholar]
- 48.Bussemaker E, Pistrosch F, Forster S, Herbrig K, Gross P, Passauer J, et al. Rho kinase contributes to basal vascular tone in humans: role of endothelium-derived nitric oxide. Am J Physiol Heart Circ Physiol. 2007;293:H541–H547. doi: 10.1152/ajpheart.00770.2006. doi:10.1152/ajpheart.00770.2006. [DOI] [PubMed] [Google Scholar]
- 49.Versteilen AM, Korstjens IJ, Musters RJ, Groeneveld AB, Sipkema P. Rho kinase regulates renal blood flow by modulating eNOS activity in ischemia-reperfusion of the rat kidney. Am J Physiol Renal Physiol. 2006;291:F606–F611. doi: 10.1152/ajprenal.00434.2005. doi:10.1152/ajprenal.00434.2005. [DOI] [PubMed] [Google Scholar]
- 50.Taflin C, Favier B, Baudhuin J, Savenay A, Hemon P, Bensussan A, et al. Human endothelial cells generate Th17 and regulatory T cells under inflammatory conditions. Proc Natl Acad Sci USA. 2011;108:2891–2896. doi: 10.1073/pnas.1011811108. doi:10.1073/pnas.1011811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H, Wang W, Xie H, Xu X, Wu J, Jiang Z, et al. A pathogenic role of IL-17 at the early stage of corneal allograft rejection. Transpl Immunol. 2009;21:155–161. doi: 10.1016/j.trim.2009.03.006. doi:10.1016/j.trim.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Dong G, Ye R, Shi W, Liu S, Wang T, Yang X, et al. IL-17 induces autoantibody overproduction and peripheral blood mononuclear cell overexpression of IL-6 in lupus nephritis patients. Chin Med J (Engl) 2003;116:543–548. [PubMed] [Google Scholar]
- 53.Crispin JC, Tsokos GC. IL-17 in systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:943254. doi: 10.1155/2010/943254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doe C, Bentley R, Behm DJ, Lafferty R, Stavenger R, Jung D, et al. Novel Rho kinase inhibitors with anti-inflammatory and vasodilatory activities. J Pharmacol Exp Ther. 2007;320:89–98. doi: 10.1124/jpet.106.110635. doi:10.1124/jpet.106.110635. [DOI] [PubMed] [Google Scholar]
- 55.Myung SC, Han JH, Song KK, Kang GH, Lee SY, Kim TH, et al. The effects of interleukin-6 on the contraction and relaxation responses of the cavernous smooth muscle from rats. Eur J Pharmacol. 2008;589:228–232. doi: 10.1016/j.ejphar.2008.04.053. doi:10.1016/j.ejphar.2008.04.053. [DOI] [PubMed] [Google Scholar]
- 56.Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27:2576–2581. doi: 10.1161/ATVBAHA.107.153080. doi:10.1161/ATVBAHA.107.153080. [DOI] [PubMed] [Google Scholar]
- 57.Huang H, Kim HJ, Chang EJ, Lee ZH, Hwang SJ, Kim HM, et al. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: Implications for bone remodeling. Cell Death Differ. 2009;16:1332–1343. doi: 10.1038/cdd.2009.74. doi:10.1038/cdd.2009.74. [DOI] [PubMed] [Google Scholar]
- 58.Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010;24:1023–1034. doi: 10.1096/fj.09-141978. doi:10.1096/fj.09-141978. [DOI] [PubMed] [Google Scholar]