Abstract
Aims
Duchenne muscular dystrophy (DMD) is a muscle disease with serious cardiac complications. Changes in Ca2+ homeostasis and oxidative stress were recently associated with cardiac deterioration, but the cellular pathophysiological mechanisms remain elusive. We investigated whether the activity of ryanodine receptor (RyR) Ca2+ release channels is affected, whether changes in function are cause or consequence and which post-translational modifications drive disease progression.
Methods and results
Electrophysiological, imaging, and biochemical techniques were used to study RyRs in cardiomyocytes from mdx mice, an animal model of DMD. Young mdx mice show no changes in cardiac performance, but do so after ∼8 months. Nevertheless, myocytes from mdx pups exhibited exaggerated Ca2+ responses to mechanical stress and ‘hypersensitive’ excitation–contraction coupling, hallmarks of increased RyR Ca2+ sensitivity. Both were normalized by antioxidants, inhibitors of NAD(P)H oxidase and CaMKII, but not by NO synthases and PKA antagonists. Sarcoplasmic reticulum Ca2+ load and leak were unchanged in young mdx mice. However, by the age of 4–5 months and in senescence, leak was increased and load was reduced, indicating disease progression. By this age, all pharmacological interventions listed above normalized Ca2+ signals and corrected changes in ECC, Ca2+ load, and leak.
Conclusion
Our findings suggest that increased RyR Ca2+ sensitivity precedes and presumably drives the progression of dystrophic cardiomyopathy, with oxidative stress initiating its development. RyR oxidation followed by phosphorylation, first by CaMKII and later by PKA, synergistically contributes to cardiac deterioration.
Keywords: Dystrophic cardiomyopathy, Excitation–contraction coupling, Ryanodine receptor, Ca2+ signals
1. Introduction
Cytoskeletal remodelling often accompanies cardiac hypertrophy and failure.1 Dystrophinopathies represent a unique group of diseases in which the cytoskeletal disarray is not only a consequence but also the cause of the disease. They result from mutations in the dystrophin gene.2 Duchenne muscular dystrophy (DMD) is among the most severe forms of dystrophy. Cardiac manifestations of the disease are present in the majority of adolescent boys with DMD. About 20% of the patients suffer from ventricular dysfunctions and arrhythmias that ultimately lead to heart failure or sudden cardiac death.3 The most commonly used animal model of DMD is the mdx mouse, which lacks dystrophin. Mdx mice develop dilated cardiomyopathy over an 8-month period.4,5 By that age they also show electrocardiographic and magnetic resonance imaging abnormalities and develop sustained ventricular tachycardia when challenged with isoproterenol.6
The cellular pathology of the failing heart often shows impaired intracellular Ca2+ homeostasis, including abnormal excitation–contraction (EC) coupling.7 It is well described that at different stages of dystrophic cardiomyopathy its cellular phenotype exhibits augmented intracellular Ca2+ responses (i.e. a burst of Ca2+ sparks and waves) to mechanical stress,8–10 hypersensitive EC-coupling,11 increased Ca2+ leak from the sarcoplasmic reticulum (SR), and reduced SR Ca2+ load.6 These features clearly point to modifications in intracellular Ca2+ cycling and suggest elevated activity of Ca2+-induced Ca2+ release (CICR) from the SR via ryanodine receptor (RyR). As underlying causes, several and probably not mutually exclusive mechanisms of RyRs sensitization have been considered. They include oxidative9 and nitrosative6 post-translational modifications of RyRs as well as their phosphorylation by PKA.12 However, a causal links between these modifications and alterations of RyR function, development of cellular abnormalities and progression of dystrophic cardiomyopathy have not yet been established clearly.
The aim of this study was to define the interactions among several cellular pathomechanisms that converge to a common endpoint—they ultimately all sensitize or even hypersensitize the CICR machinery, in particular the RyRs. In addition, we wanted to determine whether RyR hypersensitivity to Ca2+ precedes and therefore possibly underlies the progression of this disease. We found that gradually developing and additive modifications of RyRs provoke the degradation of cardiomyocyte function that may ultimately result in cardiac failure in muscular dystrophy.
2. Methods
For additional information on methods, see Supplementary material online.
2.1. Cell isolation
All experiments conformed with the NIH Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH publication, 8th edition, 2011) and were approved by the Institutional Animal Care and Use Committee of the New Jersey Medical School, USA and by the State Veterinary Office of Bern, Switzerland. C57BL10 mice (wild-type, WT) and dystrophin-deficient mdx (C57BL/10ScSn-mdx) mice at the age of 1 (young), 3–4 (adult), and 12–15 months (senescent) were used in this study. Animals were purchased from the Jackson Laboratory. Ventricular myocytes were enzymatically isolated as previously described.13 Briefly, mice were heparinized (5000 U/kg), anaesthetized with sodium pentobarbital (100 mg/kg), and checked to ensure the absence of movement, flexor, and pedal reflexes. Hearts were then quickly removed, mounted on a Langendorff apparatus and perfused with a solution containing collagenase and protease. Ventricles were cut into small pieces from which cells dissociated with time. Myocytes were used for experiments within 5 h after isolation.
2.2. Cellular experiments
Changes in cytoplasmic [Ca2+] and production of reactive oxygen species (ROS) were monitored with fluorescent indicators fluo-3 AM (5 μM) and 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFD, 20 μM), respectively, and a laser-scanning confocal microscope (Bio-Rad, Radiance 2000) in XY-scan mode at the rate of 0.5 Hz. Dyes were excited with the 488 nm line of an Argon laser. The emitted light was collected above 500 nm. SR Ca2+ leak was estimated as described in Shannon et al.14 Cells were paced at 1 Hz in external control solution to obtain steady-state levels of SR Ca2+ load, and starting from the last five beats, [Ca2+]i was recorded in the line-scan mode (500 lines/s). After pacing, external solution was rapidly switched to Na+ and Ca2+-free solution for 15 s to stop Ca2+ influx and to inhibit Ca2+ removal via the NCX and thus, to avoid Ca2+ overload or Ca2+ unloading of the cells. Addition of tetracaine (1 mM, 15 s) eliminated RyR-mediated diastolic Ca2+ leak, leading to a rapid decrease in [Ca2+]i proportionally to the leak. The peak of the caffeine-induced Ca2+ transient measured after tetracaine washout (10 mM caffeine, 2 s) was used to estimate SR load. Membrane currents were recorded using the whole-cell patch-clamp technique with an Axopatch 200B amplifier (Axon Instruments). Cells were voltage clamped using low-resistance (1.5–3 MΩ) borosilicate glass micropipettes filled with pipette solution which contained (in mL): 120 CsAsp, 8 NaCl, 20 tetraethylammonium (TEA)-Cl, 5.5 MgCl2, 4 adenosine-triphosphate K salt, 5 HEPES, and 0.1 K5-fluo-3. pH was adjusted to 7.2 with CsOH and osmolarity was 305 mOsm. All experiments were carried out at room temperature, which may represent an experimental limitation of our studies.
2.3. Phosphorylation and oxidation of RyRs
Phosphorylation status of RyRs at Ser-2808 (PKA) and Ser-2814 (CaMKII) was assessed with antibodies purchased from Badrilla (UK) and CaMKII expression was evaluated with antibodies donated by Dr M.E. Anderson (University of Iowa), using standard procedures.15 Briefly, mouse ventricular tissue was collected and sonicated in the radio-immunoprecipitation assay (RIPA) lysis buffer. Forty micrograms of protein lysate per sample were denatured in 4× sodium dodecyl sulfate polyacrylamide gel electrophoresis sample loading buffer. Proteins were separated by electrophoresis in 4% (for RyR2) and 10% (for CaMKII), respectively, SDS–polyacrylamide gel, transferred to nitrocellulose membrane (Bio-Rad, CA) at 15 V for 16–18 h at 4°C (for RyR2) and at 90 V for 90 min at 4°C (for CaMKII), blocked with 1% bovine serum albumin in tris(hydroxymethyl)aminomethane (TRIS)-buffered saline Tween (TBS: 20 mM TRIS–HCl, 200 mM NaCl, 0.6% Tween 20, pH 7.5) for 1 h and probed for proteins of interest.
The content of free thiols was determined using a monobromobimane (mBB, Calbiochem, USA) assay.16 Isolated cardiomyocytes were permeabilized and incubated with 1 mM mBB for 1 h in a dark room. The proteins were separated in 4–15% TGX SDS-gels (Bio-Rad, CA, USA) and imaged with UV excitation (360 nm). Total RyR2 was determined from Coomassie blue staining of gels run in parallel and confirmed by western blotting with anti-RYR2 (Thermo Scientific, MA, USA) and mass spectrometry at UMDNJ core facility.
2.4. Statistics
Results are shown as mean ± standard error (SEM). All data sets contain results from a minimum of three mice (number on the figures indicates the number of cells studied with imaging and electrophysiological techniques or the number of tissue samples in biochemical experiments). Statistical significance was evaluated by Student's t-test. A P-value of <0.05 was considered significant.
3. Results
3.1. RyRs are already hypersensitive to activation by Ca2+ in myocytes from young mdx mice
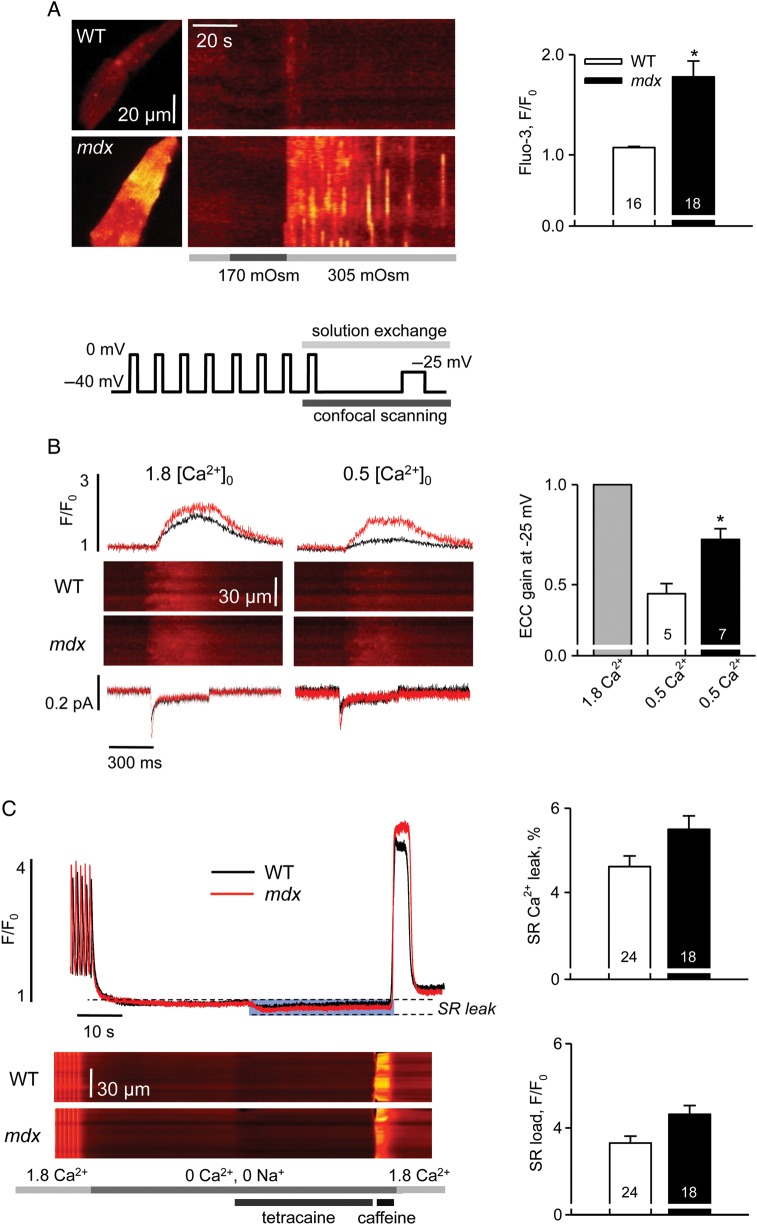
At the age of 1 month, dystrophic mice do not exhibit any signs of cardiac disease. However, 74% of apparently normal dystrophic cardiomyocytes exhibited exaggerated intracellular Ca2+ signals (Ca2+ waves and sparks) in response to a mechanical challenge applied as mild hypo-osmotic shock at rest. In contrast, intracellular Ca2+ transients in WT cells were minimal and detected only in 20% of myocytes (Figure 1A). Since the extent of Ca2+ influx is only slightly larger in mdx myocytes,9,17 this observation was taken as a strong indication of increased Ca2+ sensitivity of RyRs in young dystrophic hearts.
Figure 1.
Intracellular calcium homeostasis in cardiomyocytes from 1-month-old mice. (A) Intracellular Ca2+ responses to mild hypo-osmotic shock in WT and mdx cells. Left panels are XY images of cardiac myocytes after returning to isotonic solution and line-scan representations of series of images acquired from the cells on the left upon application of an osmotic challenge. Averaged fluorescence was determined within each cell and converted to a two-dimensional X,t image (as in Martins et al.32). Bars under the line-scans depict the protocol of extracellular solution changes. Right panel represents pooled data of mean values of normalized fluorescence during 60 s after the osmotic shock. The averaged response to the osmotic shock was extremely small in WT cells compared with mdx. (B) Left panels show representative traces of Ca2+ currents, line-scan images of Ca2+-related fluorescence and normalized cytosolic transients elicited by a 400 ms test pulse to −25 mV in WT and mdx cells superfused with either 1.8 or 0.5 mM Ca2+. Line plot on the top represents the voltage protocol used for the experiments. Right panel shows the statistical comparison of EC-coupling gain in 0.5 mM Ca2+ in WT and mdx cells. For each group, data were normalized to the value of the gain obtained in 1.8 mM Ca2+. SR Ca2+ release was much more resistant to the reduction in ICa trigger in mdx myocytes. (C) Left panels illustrate intracellular Ca2+ signals during the protocol designed to estimate SR Ca2+ leak (as in Shannon et al.14): line-scan images of fluo-3 fluorescence and normalized cytosolic transients. Bars on the bottom depict the protocol of extracellular solution changes. Averaged values of estimated SR Ca2+ leak and SR Ca2+ load in WT and mdx cells are shown at the right. The SR Ca2+ leak was determined as a reduction in the resting fluo-3 fluorescence following tetracaine application, expressed as a per cent of SR Ca2+ content estimated from the amplitude of the caffeine-induced SR Ca2+ transient. There was no significant difference in the values obtained in WT and mdx cells. See Supplementary material online, Table S1 for details.
In order to obtain more detailed insight into RyR function, we measured Ca2+ currents (ICa), corresponding Ca2+ transients, and determined the gain of EC coupling in patch-clamped cells. EC-gain reflects the efficiency of signal transduction between L-type Ca2+ channels and RyRs. The gain is calculated as the ratio of Ca2+ transient amplitude to the peak of corresponding ICa. When cells were superfused with a normal experimental solution, containing 1.8 mM Ca2+, we found no significant difference in the amplitudes of ICa, Ca2+ transients and values of EC-coupling gain between the two groups of cells within a range of test voltages (−25 to +50 mV). This observation was in agreement with our previous findings in myocytes from more mature animals.11 Then we repeated the analysis of Ca2+ transients and ICa at the lowest test voltage (−25 mV), but under conditions that challenge the fidelity of EC-coupling mechanisms—reduced Ca2+ concentration in the superfusion solution (0.5 mM instead of 1.8 mM).11,18 As a result, ICa and Ca2+ transient amplitudes were significantly decreased in all cardiomyocytes. However, the corresponding reduction in the amplitude in cytosolic Ca2+ transient was significantly less in mdx cells compared with WT, while the amplitude of ICa was affected to the same extent (Figure 1B, middle panes). This corresponds to a significantly smaller reduction in EC-coupling gain in dystrophic cells (Figure 1B, right panels). Thus, in contrast to young WT myocytes, SR Ca2+ release in mdx cells exhibited a surprising resistance to the reduction in ICa trigger, revealing a hypersensitivity in EC-coupling that is an additional indication of an increased sensitivity of RyRs to Ca2+.
An abnormal Ca2+ sensitivity of the RyRs could manifest itself as an elevated SR Ca2+ leak, resulting in a decreased Ca2+ content of the SR, reduced SR Ca2+ release, and impaired cellular contractility. Such changes were previously observed in 3- to 4-month-old mdx myocytes and were attributed to hyper-phosphorylation of RyRs by PKA.19 We compared the transient Ca2+ leak from the SR with the resting SR Ca2+ load using a method developed in Shannon et al.14 The SR of intact cardiomyocytes was loaded with Ca2+ by a train-of-field stimulations. Subsequently, the SR Ca2+ leak was estimated from the decrease in resting [Ca2+]i following the addition of the RyR blocker tetracaine. The peak of a caffeine-induced Ca2+ transient after tetracaine washout was used to estimate the SR Ca2+ load. No statistically significant increase in the transient SR Ca2+ leak was observed in myocytes from young mice, suggesting that the detection of such changes requires more severe modifications in RyRs functions (see below). The somewhat higher SR Ca2+ content in mdx myocytes may result from the slightly elevated trans-sarcolemmal Ca2+ influx in these cells.9,17,20
3.2. Hypersensitivity of RyRs is driven by oxidation in young mdx myocytes
There is a number of post-translational modifications that may contribute to the increased sensitivity of RyR activation by Ca2+. Oxidation, nitrosation, and phosphorylation are among them. Oxidative modification of RyRs was one of the first proposed mechanisms contributing to an impaired Ca2+ homeostasis of dystrophic cardiomyocytes.9,21 Moreover, an increased activity of plasmalemmal NAD(P)H oxidase (NOX) was identified to be a major source of oxidative stress in dystrophic cardiomyopathy.9,10,21 Here we tested whether oxidative stress modifies RyR functions already in very early stages of the disease. Four groups of experiments were performed: we (i) compared the extent of ROS production in WT and mdx cardiomyocytes with confocal microscopy and CM-H2DCFDA; (ii) assessed the oxidation and phosphorylation status of RyRs using mBB assays and phospho-specific antibodies against RyR PKA and CaMKII sites Ser-2808 and Ser-2814; (iii) evaluated the oxidative status of CaMKII with antiserum against oxidized Met281/282 residues; and (iv) tested whether scavengers of ROS, inhibitors of NOX, CaMKII, PKA, and various isoforms of nitric oxide synthase (eNOS, nNOS and iNOS) ‘normalize’ hypersensitive Ca2+ responses to osmotic shock and EC-coupling in dystrophic myocytes.
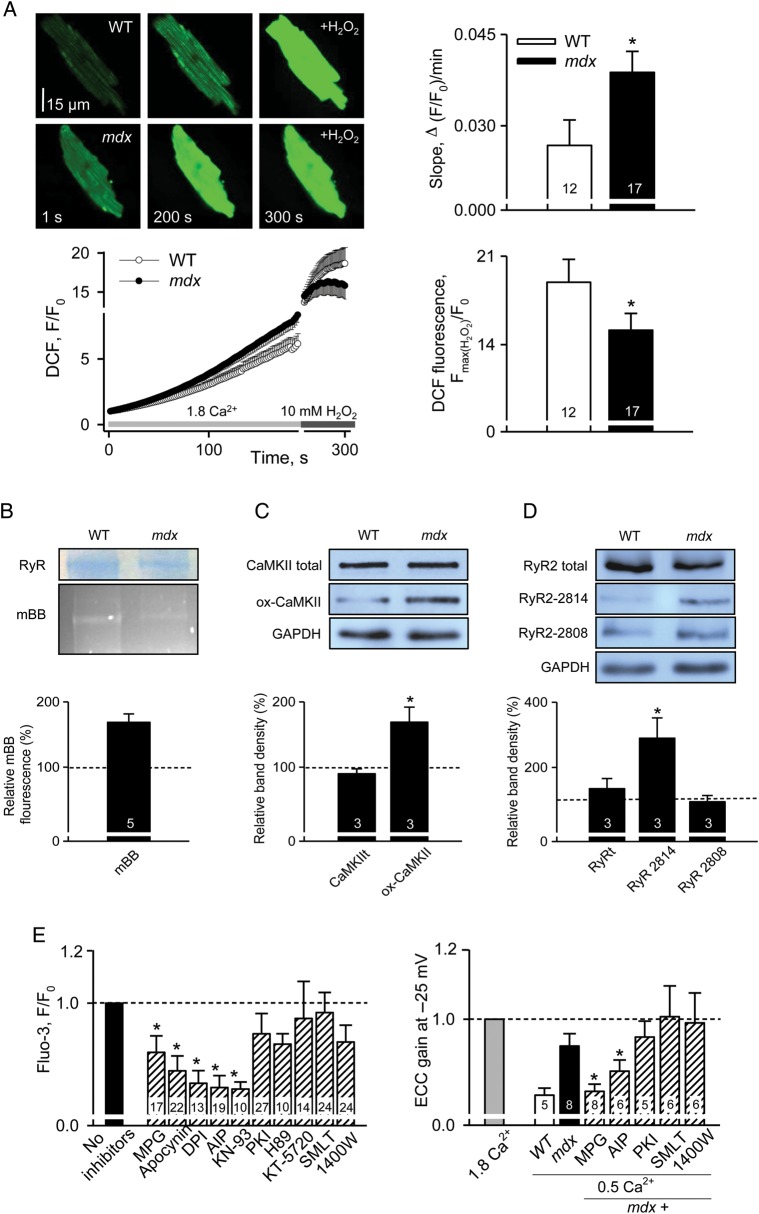
To assess oxidative stress, myocytes isolated from WT and mdx mice were identically loaded with CM-H2DCFDA. CM-H2DCFDA is hydrolyzed to DCFH in the cell, and DCFH is oxidized to form the highly fluorescent DCF compound in the presence of an appropriate oxidant. It should be noted that, as many other ROS indicators, DCF is not exclusively selective to detect ROS, but also some reactive nitrogen species (RNS). DCF-related fluorescence was followed for ∼3 min under resting conditions (Figure 2A). The slope of the averaged DCF signal largely reflects the rate of cellular ROS production. It was significantly greater in mdx cells. Another way to assess the redox state of the cytosol is to normalize resting DCF fluorescence (F0) to the signal after addition of 10 mM H2O2 (Fmax). The relative increase of fluorescence after the addition of H2O2 was significantly smaller in mdx myocytes, also indicating an increased resting ROS/RNS level in dystrophic hearts of young animals. Purified cardiac RyR tetramer contains multiple free cysteines22 which can be readily oxidized. The content of free thiols in RyRs was determined with the mBB technique. Relative content of free thiols in RyR was ∼70% greater in WT, suggesting that oxidative stress in dystrophic cells resulted in oxidation of RyRs (Figure 2B).
Figure 2.
Post-translational modifications of RyRs in cells from 1-month-old mice. (A) Representative images of DCF fluorescence in WT and mdx myocytes under resting conditions and after application of 10 mM H2O2. Graph at the bottom left illustrates changes in the averaged DCF signals. Bar graphs compare the rate of DCF oxidation (slope) at rest and normalized increases in DCF signals after application of H2O2 in WT and mdx cells. The values were 0.027 ± 0.004 and 0.039 ± 0.003 for the slope and 18.93 ± 1.81 and 15.14 ± 1.33 for F(max)/F0 in WT and mdx cells, respectively. Both groups of measurements indicate higher ROS production in mdx cells. (B) Representative Coomassie-stained gel and mBB fluorescence intensity blot in samples from WT and mdx hearts. Bar plot shows averaged data on free thiol content in RyRs from WT myocytes normalized to the corresponding value in mdx cells. The level of free thiols is significantly greater in WT hearts. (C) Immunoblots and summary of oxidized CaMKII. Band intensities recorded from mdx samples were normalized per GAPDH signals and expressed as a percentage of increase compared with values obtained in WT samples. The level of oxidized CaMKII are almost two-fold higher in mdx hearts. (D) Immunoblot and summary of phosphorylated RyR. Whereas normalized intensity of CaMKII-dependent immunoreactivity increased ∼3-fold in dystrophic tissue, the value did not change significantly for the PKA site. Numbers of samples studied are indicated on the bars. (E) Reduction in oxidative stress and CaMKII inhibition suppress exaggerated intracellular Ca2+ responses to osmotic shock (left panel) and prevent hypersensitivity of EC-coupling (right panel). In the experiments with osmotic shock, individual intracellular Ca2+ responses were averaged within each experimental group (e.g. each pharmacological intervention applied) and normalized to the averaged response under control conditions (no drug applied). In the EC-coupling gain experiments, averaged data within each experimental group were also normalized to the values obtained in control (no drug applied) conditions. Details are in Supplementary material online, Table S1.
RyRs are also predisposed to phosphorylation by both CaMKII and PKA. Moreover, CaMKII itself is susceptible to oxidative activation.23 Based on our data, the enzyme is expected to be more actively involved in mdx cardiomyocytes, as the amount of oxidized enzyme increased about 2-fold in dystrophic cells (Figure 2C). We assessed the phosphorylation status of RyRs with phospho-specific antibodies against Ser-2814 (putative phosphorylation site for CaMKII) and Ser-2808 (proposed site for PKA phosphorylation). These experiments indicate that CaMKII-, but not PKA-mediated phosphorylation, also contribute to the early cellular dysfunctions in dystrophic cardiomyopathy (Figure 2D).
To further evaluate the contribution of each particular post-translational RyR modification, a set of functional studies with various pharmacological tools was carried out. Figure 2E illustrates that the reducing agent 2-mercaptopropionyl glycine (MPG, 1 mM), inhibitors of NOX (apocynin, 0.5 mM and diphenylene iodonium, 10 µM) and CaMKII [K-93, 5 µM and autoctamide-2-related inhibitory peptide (AIP), 2.5 µM] reversibly inhibited intracellular Ca2+ responses to osmotic shock and normalized EC-coupling in mdx cells. In contrast, various PKA inhibitors (H89, 5 µM, KT5720, 2 µM, and PKA inhibitory peptide (PKI), 5 µM) as well as inhibitors of nNOS, eNOS, and iNOS (S-methyl-L-thiocitrulline, 10 µM and 1400 W, 100 nM, respectively) had no significant effect on the intracellular Ca2+ signals in mdx myocytes from young animals (see Supplementary material online, Table S1 for details). Note that after testing a broad range of inhibitors of PKA and CaMKII in the experiments with osmotic shock, we used only the more specific inhibitory peptides of kinases (AIP and PKI) in subsequent work. Right panel in Figure 2D shows that ‘hypersensitive’ gain of EC coupling in dystrophic cells was also normalized by reducing factors and inhibitors of NOX and CaMKII but was not affected by inhibitors of PKA or NOS. These results are in agreement with biochemical studies by us and others,6,19 which did not reveal changes in PKA-phosphorylation or nitrosative status of RyRs in very young mdx hearts. Taken together, the findings presented so far are consistent with the hypothesis that oxidative stress due to an increased production of ROS by NOX drives the cellular pathology of cardiac dystrophy at very early stages of the disease.
3.3. The progression of cellular pathology in cardiac dystrophy
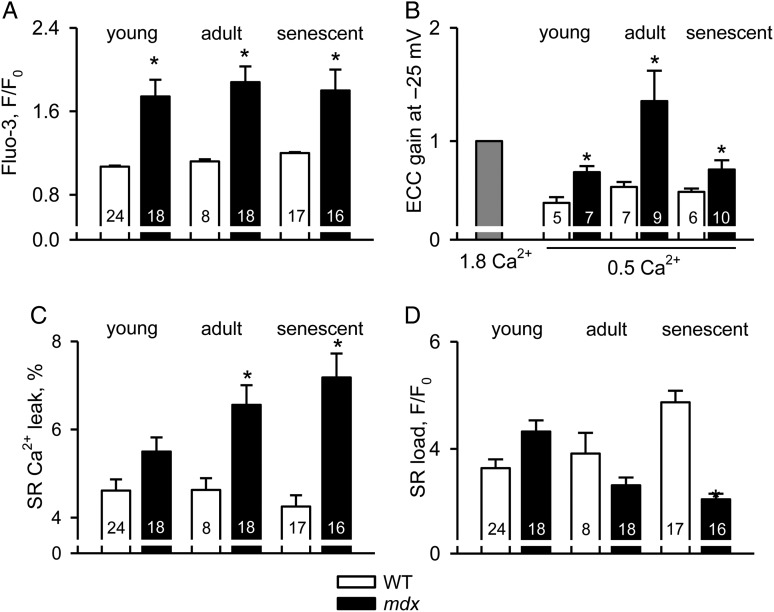
Data presented above suggest that cellular abnormalities in isolated dystrophic cardiomyocytes clearly precede the first clinical signs of cardiomyopathy.4,5 Here we followed up on the progression of the cellular phenotype of the disease to the age where heart dysfunction becomes prominent. Ventricular myocytes were isolated from 3–4- and 12–15-month-old animals. Similar to the experiments shown above, intracellular Ca2+ responses to mild osmotic shock, EC-coupling, SR Ca2+ leak, and load were examined. Data are summarized in Figure 3, which also includes some results from 1-month-old mice adapted from Figure 1 for comparison. With the progression of the disease, more mdx cardiomyocytes exhibited excessive intracellular Ca2+ responses to osmotic shock (74, 90, and 95% for the three age groups studied, respectively). EC coupling became extremely hypersensitive at the age of 3–4 months (just before the clinical onset of the disease), but sensitivity remained relatively high in the senescent animals. Contrary to cells from young mice, the transient SR Ca2+ leak significantly increased in mdx cells isolated from 3- to 4-month-old animals. This was associated with a tendency towards reduced SR Ca2+ content. However, a significant decrease in the SR Ca2+ load was only recorded in very old animals that had already developed cardiac myopathy. This decrease is at least partly responsible for a reduced resistance of the EC-coupling gain to challenges when compared with younger animals (i.e. reducing [Ca2+] in the external solution and applying a small test depolarization, Figure 3B).
Figure 3.
Gradual deterioration of intracellular Ca2+ homeostasis during the development of cardiac dystrophy. Intracellular Ca2+ responses to osmotic shock (A), gain of EC-coupling (B), intracellular Ca2+ leak (C), and SR Ca2+ content (D) in WT and mdx cardiomyocytes isolated from 1-, 3–4-, and 12–15-month-old mice. Note the gradually increased SR Ca2+ leak and decreased SR Ca2+ load in mdx cardiomyocytes from older mice. The corresponding values are listed in Supplementary material online, Table S2.
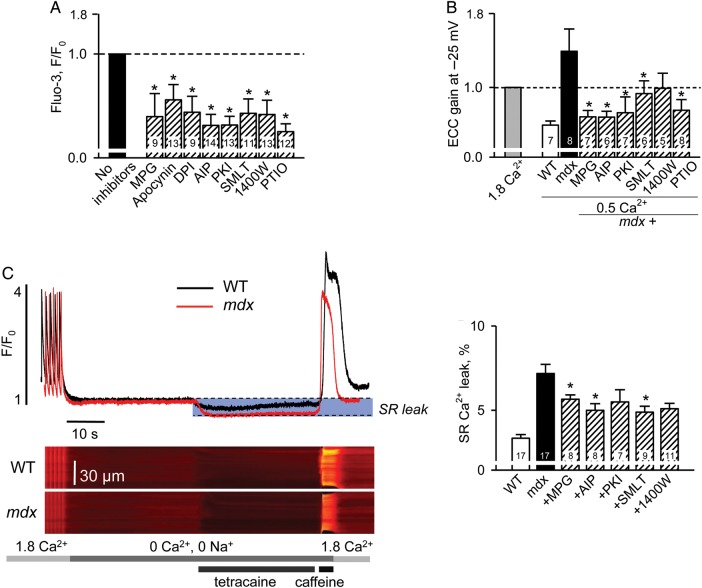
We also carried out experiments similar to those illustrated in Figure 2. It seems that by the age of 3–4 months (just before the appearance of clinical signs of cardiac dystrophy) several post-translational modifications additively enhance RyR function resulting in increased SR Ca2+ leak, reduced SR Ca2+ content, and consequently impaired force production by the individual myocytes. In cells from mdx mice older than 3 months, activation of NOS and PKA also seems to contribute to the hypersensitivity, possibly further aggravating the disease at later stages. A variety of pharmacological tools supports this conclusion as they not only normalized intracellular Ca2+ responses during mechanical stress and EC coupling in older animals (Figures 4A and B, and 5) but also significantly reduced intracellular Ca2+ leak in senescent mice (Figure 4C).
Figure 4.
Reduction in oxidative/nitrosative stress as well as CaMKII and PKA inhibition ameliorates excessive intracellular Ca2+ responses to osmotic shock and hypersensitivity of EC-coupling in mdx myocytes isolated from 3- to 4-month-old mice (A and B) and reduces SR Ca2+ leak in cells from mature 12- 15-month-old mdx mice (C). In the experiments with osmotic shock (A), individual intracellular Ca2+ responses were determined as mean values of fluorescence recorded within the cell during 60 s after the shock, then averaged within each experimental group (e.g. each pharmacological intervention applied) and normalized to the averaged response under control conditions (no drug applied). In the EC-coupling gain experiments (B), averaged data within each experimental group were normalized to the values obtained in control (no drug applied) conditions. (C) Typical intracellular Ca2+ signals measured during the ‘leak’ protocol and averaged values of estimated SR Ca2+ leak under control conditions in WT and mdx cells and in mdx myocytes pretreated with various pharmacological agents (dashed bars). All values are listed in Supplementary material online, Table S1.
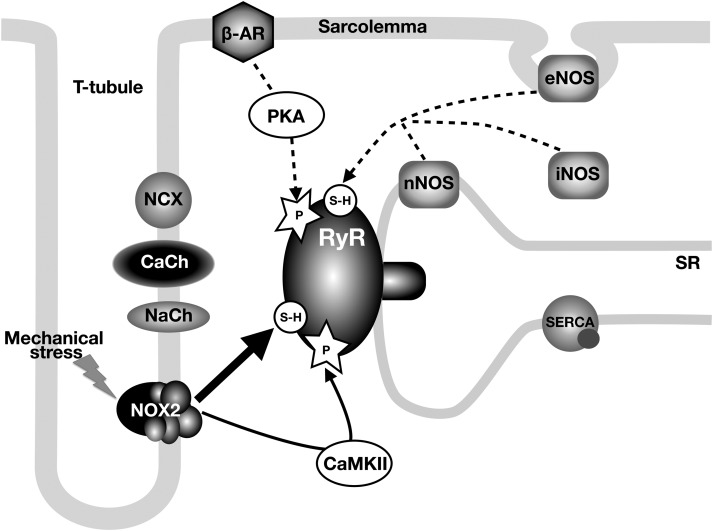
Figure 5.
Signalling pathways involved in regulation of RyR activity in dystrophic cardiomyocytes. The diagram shows the main molecules involved in Ca2+ signalling and EC-coupling. Solid lines depict primary (oxidation and CaMKII-phosphorylation) and dashed lines depict secondary (nitrosation and PKA phosphorylation) mechanisms resulting in hypersensitivity of RyRs. CaCh and NaCh refer to L-type Ca2+ channel and voltage-gated Na+ channel, respectively.
4. Discussion
Most cardiac diseases are accompanied by substantial remodelling of the heart tissue, also on cellular and molecular level.24 Depending on the cause of the illness and the particular pathophysiology, various adaptive remodelling mechanisms can initially compensate for the functional impairments. Unfortunately, some of these initially beneficial processes can become maladaptive with time and during progression of the illness, finally aggravating rather than improving the cardiac condition. This interplay between (i) defects causing a cardiac disease and (ii) the remodelling mechanisms that try to maintain function leads to a very complex situation. This complexity makes it extremely challenging to unravel the causal disease mechanisms and to comprehend the evolution of the disease. However, such an understanding would greatly facilitate the development of new therapies and the identification of new drug targets.
One possible strategy to gain insight into the interplay between causal and adaptive alterations of cardiac muscle is to follow the disease progression over longer periods of time. This approach is based on the rationale that causal changes can presumably be identified from the beginning of the disease, even before the pathology becomes manifest, while adaptive and maladaptive processes will develop at later stages only.
Based on this reasoning we carried out the present study, examining disease progression in dystrophic cardiomyopathy. Because of their slowly developing cardiac phenotype, mdx mice are a useful model for studies of the progression of DMD that can help to identify the cellular sequence of events leading from the genetic defect (lack of functional dystrophin) to the onset of cardiac disease. Hearts of 1- to 2-month-old mdx mice seem to have normal morphology, left ventricular function, and echocardiograms. First signs of cardiac disease, such as reduced left ventricular filling, ejection fraction, and ejection rate, are seen only in 3-month-old animals. These changes are more prominent and can be detected earlier in the right ventricle. By 8 months of age, hearts from mdx mice are dilated, hypertrophied, somewhat fibrotic, and poorly contracting.4,5,25 Ventricular cardiomyocytes isolated from senescent but not young mdx animals also show signs of dilated cardiomyopathy. The diameter of mdx myocytes from old animals is significantly smaller, and their length is significantly greater than that of WT cells [in μm: 22.2 ± 0.6 and 127.8 ± 2.4 (n = 57) vs. 24.7 ± 0.77 and 116.1 ± 2.5 (n = 65)], although cell capacitance is not changed [in pF: 239.0 ± 21.2 (n = 32) vs. 244.5 ± 20.6 (n = 32) in mdx and WT cells, respectively]. Tropnonin I was found to be degraded in dystrophic hearts.21 In addition, measurements in dystrophic mdx papillary muscles revealed that the myofibrillar Ca2+ sensitivity is reduced, a finding which contributes to the weak force, in addition to the smaller Ca2+ transients.26 Additionally, electrocardiographic (ECG) deviations such as diminished R- and S-wave amplitudes gradually become apparent in mdx mice. By the age of 6 months shortening of the PR interval and polymorphic R-waves suggest disturbances in the cardiac conduction system.27,28
While in the mdx mouse cardiomyopathy only becomes apparent in adulthood, the dystrophin protein is absent throughout the lifetime of the animal. Thus, functional consequences of the lack of dystrophin, such as stress sensitivity, would be expected to be present even in very young and apparently healthy mice. For our study, we focussed on the presently prevailing hypothesis to explain the damages caused by the lack of this protein, which links cytoskeletal proteins with the cell environment: the ‘Ca2+ hypothesis of muscular dystrophy’.29 This hypothesis proposes that the membrane fragility resulting from the lack of dystrophin leads to extra influx of Ca2+ via micro-ruptures and/or stretch activated channels, and that these Ca2+ signals are subsequently amplified by CICR and ultimately lead to activation of Ca2+ activated proteases, to mitochondrial damage and apoptotic or necrotic cell death. This hypothesis has recently been elaborated to contain another aggravating culprit, excessive oxidative stress.
Our results obtained using cardiomyocytes isolated from young animals now suggest that the oxidative stress is possibly among the very initial problems dystrophic myocytes encounter (Figure 5). In these cells, ROS production was already abnormally high. Furthermore, ROS scavengers and inhibitors of NAD(P)H oxidase could prevent most of the acute Ca2+ signalling alterations, suggesting NOX as the primary source of this oxidative stress. Why and how NOX becomes abnormally activated in dystrophic cells is not entirely clear and several possibilities exist. For example, NOX over-expression has been reported in dystrophic muscle.21,30Activation of NOX could occur indirectly after Ca2+ influx, via phosphorylation by Ca2+-sensitive PKC.31–33 PKC could also become activated via Angiotensin II receptor signalling, which seems to be activated by stretch, even in the absence of an agonist.34 An activation of NOX by stretch exerted via the intracellular tubulin network has also been suggested.10
NOX/ROS-mediated signalling has a significant downstream impact on Ca2+-signalling proteins, and presumably on many other proteins important for cell function. RyRs are not exquisitely susceptible to oxidative modulation. ROS can also turn on CaMKII in a Ca2+-independent way.23 This leads to further RyR sensitization by CaMKII-dependent phosphorylation, as observed here with antibodies and with pharmacological CaMKII inhibitors. Taken together, these mechanisms explain the susceptibility of dystrophic cardiomyocytes and their Ca2+-signalling system to stress at the very early subclinical phases of the disease.
Our studies further suggest that later during the progression of dystrophic cardiomyopathy, but before heart failure is observed, nitrosation and PKA-dependent phosphorylation contribute to the increased sensitivity of RyRs to Ca2+, in agreement with recently published data.6,19,36 At this stage of the disease, mdx mice and DMD patients often exhibit electrocardiographic abnormalities, such as premature ventricular beats. Moreover, during physical or emotional stress, CPVTs have also been reported in boys with DMDs.35 It is possible that under these conditions additional β-adrenergic input may lead to SERCA and ICa stimulation, followed by phosphorylation of the RyRs, culminating in a high arrhythmogenic potential due to overloaded SR and hypersensitive RyRs.19
Finally, our data indicate that at the terminal stages of cardiac dystrophy, several post-translational modifications together hypersensitize RyR to an extent where SR Ca2+ leak dramatically increases. This may subsequently contribute to cytosolic and mitochondrial Ca2+ overload,9,17 activation of necrotic and apoptotic processes, and loss of functional myocytes. The latter step usually precedes the development of cardiac fibrosis and reduction in heart contractility.4 In parallel, enhanced and uncompensated SR Ca2+ leak results in a reduction in SR Ca2+ load, which will in turn reduce the amplitude of beat-to-beat intracellular Ca2+ transients, thus resulting in reduced force production by the surviving cardiomyocytes. These two cellular processes could eventually impede cardiac contractility leading to cardiac failure.
5. Conclusions
The present study revealed the gradual time-dependent unfolding of prominent disease pathomechanisms in dystrophic hearts. Our major conclusion is that overall oxidative stress may be more important as one of the early steps of an initial cellular injury than previously thought. We suggest that the ‘Ca2+ hypothesis’ of cardiac dystrophy should be significantly modified as it was previously implemented for skeletal muscle disease.37 Obviously, this view should also be considered when developing new therapies for muscular dystrophy. One can speculate that therapies could even benefit from a combined approach, where oxidative stress and RyR hypersensitivity would be targeted simultaneously.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by NIH (HL093342 and AR053933 to N.Sh.), SNSF (31-132689 and 31-109693 to E.N.), Swiss Foundation for Research on Muscle Diseases (to E.N and N.Sh.) and Ambizione SNSF (PZ00P3_131987/1 to N.U.). Eva Polakova was recipient of a Postdoctoral Fellowship from AHA.
Supplementary Material
Acknowledgements
We are grateful to Drs Blackwell, Hidalgo, Terentyev and Zakharian for sharing with us their biochemical expertise.
Conflict of interest: none declared.
References
- 1.Hein S, Kostin S, Heling A, Maeno Y, Schaper J. The role of the cytoskeleton in heart failure. Cardiovasc Res. 2000;45:273–278. doi: 10.1016/s0008-6363(99)00268-0. [DOI] [PubMed] [Google Scholar]
- 2.Durbeej M, Campbell KP. Muscular dystrophies involving the dystrophin–glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev. 2002;12:349–361. doi: 10.1016/s0959-437x(02)00309-x. [DOI] [PubMed] [Google Scholar]
- 3.Finsterer J, Stöllberger C. The heart in human dystrophinopathies. Cardiology. 2003;99:1–19. doi: 10.1159/000068446. [DOI] [PubMed] [Google Scholar]
- 4.Quinlan JG, Hahn HS, Wong BL, Lorenz JN, Wenisch AS, Levin LS. Evolution of the mdx mouse cardiomyopathy: physiological and morphological findings. Neuromuscul Disord. 2004;14:491–496. doi: 10.1016/j.nmd.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Stuckey DJ, Carr CA, Camelliti P, Tyler DJ, Davies KE, Clarke K. In vivo MRI characterization of progressive cardiac dysfunction in the mdx mouse model of muscular dystrophy. PLoS One. 2012;7:e28569. doi: 10.1371/journal.pone.0028569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fauconnier J, Thireau J, Reiken S, Cassan C, Richard S, Matecki S, et al. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2010;107:1559–1564. doi: 10.1073/pnas.0908540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keurs ter HEDJ, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev. 2007;87:457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasuda S, Townsend D, Michele DE, Favre EG, Day SM, Metzger JM. Dystrophic heart failure blocked by membrane sealant poloxamer. Nat Cell Biol. 2005;436:1025–1029. doi: 10.1038/nature03844. [DOI] [PubMed] [Google Scholar]
- 9.Jung C, Martins AS, Niggli E, Shirokova N. Dystrophic cardiomyopathy: amplification of cellular damage by Ca2+ signalling and reactive oxygen species-generating pathways. Cardiovasc Res. 2007;77:766–773. doi: 10.1093/cvr/cvm089. [DOI] [PubMed] [Google Scholar]
- 10.Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 11.Ullrich ND, Fanchaouy M, Gusev K, Shirokova N, Niggli E. Hypersensitivity of excitation–contraction coupling in dystrophic cardiomyocytes. Am J Physiol Heart Circ Physiol. 2009;297:H1992–H2003. doi: 10.1152/ajpheart.00602.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wehrens XHT, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci USA. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolska BM, Solaro RJ. Method for isolation of adult mouse cardiac myocytes for studies of contraction and microfluorimetry. Am J Physiol. 1996;271:H1250–H1255. doi: 10.1152/ajpheart.1996.271.3.H1250. [DOI] [PubMed] [Google Scholar]
- 14.Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res. 2002;91:594–600. doi: 10.1161/01.res.0000036914.12686.28. [DOI] [PubMed] [Google Scholar]
- 15.Huke S, Bers DM. Ryanodine receptor phosphorylation at Serine 2030, 2808 and 2814 in rat cardiomyocytes. Biochem Biophys Res Commun. 2008;376:80–85. doi: 10.1016/j.bbrc.2008.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terentyev D, Gyorke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, et al. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res. 2008;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams IA, Allen DG. Intracellular calcium handling in ventricular myocytes from mdx mice. Am J Physiol Heart Circ Physiol. 2006;29:H846–H855. doi: 10.1152/ajpheart.00688.2006. [DOI] [PubMed] [Google Scholar]
- 18.McCall E, Ginsburg KS, Bassani RA, Shannon TR, Qi M, Samarel AM, et al. Ca flux, contractility, and excitation–contraction coupling in hypertrophic rat ventricular myocytes. Am J Physiol. 1998;274:H1348–H1360. doi: 10.1152/ajpheart.1998.274.4.H1348. [DOI] [PubMed] [Google Scholar]
- 19.Sarma S, Li N, van Oort RJ, Reynolds C, Skapura DG, Wehrens XHT. Genetic inhibition of PKA phosphorylation of RyR2 prevents dystrophic cardiomyopathy. Proc Natl Acad Sci USA. 2010;107:13165–13170. doi: 10.1073/pnas.1004509107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fanchaouy M, Polakova E, Jung C, Ogrodnik J, Shirokova N, Niggli E. Pathways of abnormal stress-induced Ca2+ influx into dystrophic mdx cardiomyocytes. Cell Calcium. 2009;46:114–121. doi: 10.1016/j.ceca.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams IA, Allen DG. The role of reactive oxygen species in the hearts of dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol. 2007;293:H1969–H1977. doi: 10.1152/ajpheart.00489.2007. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 23.Erickson JR, Joiner M-LA, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Hove ten M, Schneider JE, Stuckey DJ, Sebag-Montefiore L, Bia BL, et al. Abnormal cardiac morphology, function and energy metabolism in the dystrophic mdx mouse: an MRI and MRS study. J Mol Cell Cardiol. 2008;45:754–760. doi: 10.1016/j.yjmcc.2008.09.125. [DOI] [PubMed] [Google Scholar]
- 26.Wagner S, Knipp S, Weber C, Hein S, Schinkel S, Walther A, et al. The heart in Duchenne muscular dystrophy: early detection of contractile performance alteration. J Cell Mol Med. 2012;16:3028–36. doi: 10.1111/j.1582-4934.2012.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bia BL, Cassidy PJ, Young ME, Rafael JA, Leighton B, Davies KE, et al. Decreased myocardial nNOS, increased iNOS and abnormal ECGs in mouse models of Duchenne muscular dystrophy. J Mol Cell Cardiol. 1999;31:1857–1862. doi: 10.1006/jmcc.1999.1018. [DOI] [PubMed] [Google Scholar]
- 28.Chu V, Otero JM, Lopez O, Sullivan MF, Morgan JP, Amende I, et al. Electrocardiographic findings inmdx mice: a cardiac phenotype of Duchenne muscular dystrophy. Muscle Nerve. 2002;26:513–519. doi: 10.1002/mus.10223. [DOI] [PubMed] [Google Scholar]
- 29.Deconinck N, Dan B. Pathophysiology of duchenne muscular dystrophy: current hypotheses. Pediatr Neurol. 2007;36:1–7. doi: 10.1016/j.pediatrneurol.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Shkryl VM, Martins AS, Ullrich ND, Nowycky MC, Niggli E, Shirokova N. Reciprocal amplification of ROS and Ca2+ signals in stressed mdx dystrophic skeletal muscle fibers. Pflugers Arch. 2009;458:915–928. doi: 10.1007/s00424-009-0670-2. [DOI] [PubMed] [Google Scholar]
- 31.Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63:218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 32.Martins AS, Shkryl VM, Nowycky MC, Shirokova N. Reactive oxygen species contribute to Ca2+ signals produced by osmotic stress in mouse skeletal muscle fibres. J Physiol. 2008;586:197–210. doi: 10.1113/jphysiol.2007.146571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gul R, Shawl AI, Kim SH, Kim UH. Cooperative interaction between reactive oxygen species and Ca2+ signals contributes to angiotensin II-induced hypertrophy in adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2012;302:H901–H909. doi: 10.1152/ajpheart.00250.2011. [DOI] [PubMed] [Google Scholar]
- 34.Zhou C. Angiotensin II and stretch activate NADPH oxidase to destabilize cardiac Kv4.3 channel mRNA. Circ Res. 2006;98:1040–1047. doi: 10.1161/01.RES.0000218989.52072.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hermans MCE, Pinto YM, Merkies ISJ, de Die-Smulders CEM, Crijns HJGM, Faber CG. Hereditary muscular dystrophies and the heart. Neuromuscul Disord. 2010;20:479–492. doi: 10.1016/j.nmd.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Adamo CM, Dai D-F, Percival JM, Minamie E, Willis MS, Patrucco E, et al. Sildenafil reverses cardiac dysfunction in the mdx mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2010;107:19079–19083. doi: 10.1073/pnas.1013077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rando TA. Role of nitric oxide in the pathogenesis of muscular dystrophies: a ‘two hit’ hypothesis of the cause of muscle necrosis. Microsc Res Tech. 2001;55:223–235. doi: 10.1002/jemt.1172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.