Abstract
Tubal factor infertility (TFI) represents 36% of female infertility and genital infection by Chlamydia trachomatis (C. trachomatis) is a major cause. Although TFI is associated with host inflammatory responses to bacterial components, the molecular pathogenesis of Chlamydia-induced infertility remains poorly understood. We investigated the hypothesis that activation of specific cysteine proteases, the caspases, during C. trachomatis genital infection causes the disruption of key fertility-promoting molecules required for embryo development and implantation. We analyzed the effect of caspase inhibition on infertility and the integrity of Dicer, a caspase-sensitive, fertility-promoting ribonuclease III enzyme, and key micro-RNAs in the reproductive system. Genital infection with the inflammation- and caspase-inducing, wild-type C. trachomatis serovar L2 led to infertility, but the noninflammation-inducing, plasmid-free strain did not. We confirmed that caspase-mediated apoptotic tissue destruction may contribute to chlamydial pathogenesis. Caspase-1 or -3 deficiency, or local administration of the pan caspase inhibitor, Z-VAD-FMK into normal mice protected against Chlamydia-induced infertility. Finally, the oviducts of infected infertile mice showed evidence of caspase-mediated cleavage inactivation of Dicer and alteration in critical miRNAs that regulate growth, differentiation, and development, including mir-21. These results provide new insight into the molecular pathogenesis of TFI with significant implications for new strategies for treatment and prevention of chlamydial complications.
Keywords: Chlamydia, Dicer, caspase, miRNAs, inflammasome, infertility and pathogenesis
Genital infection by Chlamydia trachomatis causes severe complications, including pelvic inflammatory disease (PID), ectopic pregnancy and tubal factor infertility (TFI) [1]. TFI accounts for 36% of female infertility [2] and the emerging antibiotic resistant strains [3] and absence of a vaccine require a better understanding of the pathogenesis of specific chlamydial diseases. The pathogenesis of Chlamydia-induced infertility remains poorly understood. Clinical and experimental evidence of immunopathogenic basis for chlamydial complications implicates both acute and chronic inflammation in the pathologic process of tubal scarring and damage, causing obstruction or dysfunction sometimes associated with TFI [4]. Thus, Chlamydia-induced infertility is traditionally studied by measuring nonspecific inflammatory responses (ie, proinflammatory cytokines, cellular activation signaling, fibrosis), specific immune effectors, and immunoreactive microbial virulence factors [5, 6]. In summary, secreted chlamydial and plasmid components [7], candidate substrate effectors for the type III secretion apparatus, interact with host's Nod-like receptors (NLRs) to induce the inflammasomes, caspase-1 activation and proinflammatory cytokines [8, 9]. Tumor necrosis factor α (TNF-α)-mediated pathologies [10] involve cellular signaling for caspase activation, initiation of fibrosis [11] and apoptotic tissue destruction [12], such as oviductal interstitial cells of Cajal (ICCs) that may lead to ectopic pregnancy [13].
Besides, the requirement for caspase activation and the cryptic plasmid in chlamydial inflammatory pathologies [6, 14–16] suggests that disruption of pro-fertility biologic processes in the reproductive tract is a potential mechanism of Chlamydia-induced infertility. This may involve the modulation and functional alteration of key determinants of fertility in the reproductive system, including Dicer, estrogen receptors, the cannabinoid receptor-1 (CB-1) and ICCs [13, 17]. Dicer (encoded by Dicer1) is a multidomain ribonuclease III enzyme required for the biogenesis of the small noncoding regulatory RNAs, microRNAs (miRNAs), and small interfering RNAs (siRNAs), key regulators of posttranscriptional gene expression [18]. Dicer is necessary for the normal development and physiological function of several tissues and organ systems, the reproductive system, and cellular processes such as apoptosis and other responses to external stimuli including infection [19]. The loss or abnormality of Dicer expression and function in the reproductive system results in the abnormal development and dysfunction of the oviducts, cyst formation, ectopic pregnancy, non-implantation, neoplasia, premature death of oocytes, zygote and embryos, and infertility [17, 19–21]. The oocytes and zygotes contain 10–15-fold higher Dicer transcripts than any other cells and tissues (BioGPS; http://biogps.gnf.org/) [22], and its expression is regulated by key factors such as estrogen and the receptors [23]. Thus, abnormality in Dicer- or miRNAs-dependent hormonal and physiological functions in the reproductive system caused infertility [23], prominently exerted during postfertilization zygotic development [24]. Interestingly, Dicer is the target of certain caspases for cleavage inactivation [25]; therefore, cellular insults, stimuli, or signaling events that activate the caspases (eg, infections, inflammasome, apoptosis, and proinflammatory cytokines) cause Dicer inactivation and negatively impact Dicer-dependent processes [25, 26]. Intriguingly, the adverse reproductive consequences and pathologic features of Dicer deficiency in female animals bear striking similarities to Chlamydia-induced tubal abnormalities, especially the infertility associated ectopic pregnancy, poor survival rate of fertilized oocytes and early embryos, cyst formation, and oviduct inflammation and dysfunction [20]. Taken together, we hypothesized that the pathogenesis of Chlamydia-induced infertility involves Chlamydia-encoded gene products acting as pathogenic ligands, inducing the caspases, deleterious inflammatory and cellular processes and dysfunction of key determinants of fertility, including Dicer, in the reproductive system. The present study focuses on caspase and its target molecule Dicer and miRNAs in the oviducts.
MATERIALS AND METHODS
Chlamydia trachomatis Strains, Animal Infection and Assessment of Infertility
Stocks of C. trachomatis serovar L2 and the plasmid-free L2 (L2R) mutant, the original L2 (25667R) isolate [27], kindly provided by Dr Luis de la Maza (University of California, Irvine, CA), were propagated in HeLa cells. The purified elementary bodies were titered as inclusion-forming units per milliliter (IFU/mL) by standard procedure [28]. Female C57BL/6 mice, 5–8 weeks old, including specific caspase gene-knockouts and strain controls, were obtained from either Taconic Farms, Inc (Hudson, NY) or Jackson Laboratory (Bar Harbor, MA) and infected intravaginally with 1 × 105 IFU per mouse with each chlamydial strain while under the long-acting anesthetic sodium pentobarbital (30 µg/body weight; Sigma-Aldrich, St Louis, MO) approximately 5 days after intramuscular administration of 2.5 µg/mouse Depo Provera (medroxy-progesterone Acetate; Pfizer Inc, NY). These conditions are a key factor to a successful mouse model of chlamydial genital infection using the human chlamydial strains [29]. Control mice were sham infected. The course of the infection was monitored by periodic (every 3 days) cervicovaginal swabbing of individual mouse and tissue culture isolation and enumeration of chlamydial inclusions by a standard immunofluorescence method [28]. To assess Chlamydia-induced infertility, mice were infected twice at 4–6-week intervals to ensure repeated infection that enhanced infertility [30] and mated with proven fertile males, followed by monitoring daily weight-gain for approximately 21 days. A consistent 3 days of weight gain by mice was considered evidence of pregnancy. The numbers of pregnant mice and the mean number of pups in the different groups were enumerated and calculated. In addition to fertility assessment, animals were visually and microscopically inspected and scored for uni- and bilateral hydrosalpinx or cysts, and presence of other abnormalities in the reproductive system [30, 31]. The cell permeable, irreversible pan-caspase inhibitor, N-benzyloxy-cabonyl-Val-Ala-Asp- fluoromethyl ketone (Z-VAD-FMK; R&D Systems, Inc, Minneapolis, MN) was applied intravaginally by administering 0.02 mL of a 50 µM solution in phosphate-buffered saline per mouse under anesthesia. Control animals were treated with the prescribed inhibitor control, Z-FA-FMK, at the same concentration.
Western Blotting
Western immunoblotting was used to determine the expression levels of specific proteins in oviduct lysates. Briefly, lysates of oviduct tissues stored at −80°C were prepared by homogenization in RIPA lysis buffer supplemented with 1 mmol/L phenylmethylsulfonyl fluoride and protease inhibitor cocktail on ice. Equal amounts of total proteins from each sample were loaded onto a 4%–20% SDS-PAGE gradient gel, electrophoresed, and transferred on to nitrocellulose membranes. The blots were incubated with a primary antibody (against Dicer or the pro- or activated caspases) at 4°C overnight. A primary antibody against glyceraldehyde 3 phosphate dehydrogenase (GAPDH) was used as the housekeeping gene expression control. The secondary antibody was detected by Western ECL-enhanced luminol reagent (PerkinElmer Inc, Morrisville, NC).
Caspase-Dependent Apoptosis
Infection-induced apoptosis was measured by the Millipore TUNEL apoptosis detection kit (Temecula, CA) according to the manufacturer's protocol [32]. Briefly, HeLa cells were grown on 8-well chamber-slides, treated with 50 µM of either Z-VAD-FMK or the control Z-FA-FMK (R&D Systems, Inc. Minneapolis, MN) for 90 minutes and then infected for 72 hours with C. trachomatis at a multiplicity of infection (MOI) of 1. Bovine DNase I at 5 µg/mL and 500 nM of Staurosporine (Streptomyces staurospores; R&D Systems, Inc, Minneapolis, MN) were used as positive controls, whereas negative controls were stained in the absence of terminal deoxynucleotidyl transferase enzyme. Following post-treatment, the cells were fixed with 4% paraformaldehyde in 0.1 M NaH2PO4, pH 7.4 and incubated with a reaction mix containing biotin-dUTP and terminal deoxynucleotidyl transferase for 60 minutes. Avidin-FITC was applied to the infected cells, which were then incubated in the dark for 30 minutes. In situ detection of fluorescein-labeled fragmented DNA were visualized and photographed on a Nikon Eclipse 80i fluorescence microscope. The total number of TUNEL-positive cells were counted under the 20 × objective in 17 randomly selected microscopic fields and normalized to the total number of cells per slide. Data were analyzed as the mean (+SEM) of TUNEL-positive cells.
Microarray and Real Time Polymerase Chain Reaction
For miRNA microarray analysis, the Signosis’ miRNA Array III service was used according to the company's standard procedure (Signosis, Inc, Sunnyvale, CA). Briefly, total RNA was isolated from homogenized oviduct tissues and 5 µg from each sample incubated with and annealed to a biotin-UTP labeled oligonucleotide probe mixture corresponding to 140 randomly selected miRNAs, which have been reported to play a role in inflammation, apoptosis and cancer by literature search [33]. After hybridization of biotin-UTP labeled probes, the miRNA expression arrays were detected by Streptavidin-HRP chemiluminescence. The chemiluminescent signals were acquired using the Alpha innotech FluorChem FC2 imaging system. With the array assay, the expression of 140 miRNAs was profiled in the samples. Quantitative real-time polymerase chain reaction (PCR) was used to validate the microarray data for 7 selected miRNAs based on a minimum of 2-fold increase among the experimental groups observed in the microarrays. There was a focus on miRNAs showing a decrease in the oviducts from infected infertile mice. For real-time miRNA PCR, the miRNA-specific oligo mix was used instead of the random oligomix. The ligated products were eluted, heated, and mixed with miRNA specific quantitative PCR primers and SYBR miRNA PCR buffer mix. The real-time PCR was conducted on the ABI 7700 system using TaqMan small RNA assays (Applied Biosystems) and subjected to 35 PCR cycles: 95°C 15 seconds and 50 seconds. The small RNA U6 was used as an internal (endogenous) control, and so the relative expression of miRNAs was normalized to U6 miRNA expression. Real-time PCR data were analyzed using the ddCT method by the standard procedure [33]. Information about miRNA target mRNA genes was obtained from several Web-accessible miRNA database searching programs, including http://www.microrna.org, http://www.miRBase.org, http://www.targetscan.org, and published reports [20]. The complete microarray data are available to any requestor and provided as Supplemental file 1. Results presented in Table 1 were derived from oviducts harvested at 2, 4, or 6 weeks after infection. Experiments were repeated 2 times with at least 6 oviducts in each group.
Table 1.
Integrity of Select MicroRNAs (miRNAs) in the Oviducts of Infertile and Fertile Mice
| miRNA Category | miRNA IDs | Fold Change (Decrease in Expression) | Functional/Biological Significance |
|---|---|---|---|
| Decrease in infertile (WT-L2-infected) as compared to fertile uninfected or PF-L2 infected mice | mir-21 | 2.52** | Target genes are involved in cell proliferation, growth & differentiation; apoptosis, cancer, inflammation |
| mir-103 | 2.6** | Target genes: Hoxa10, Fzd1, Mapk7, Wnt4 | |
| mm-mir-107 | 3.2** | Target genes: Hoxa10, Fzd1, Wnt4 | |
| mm-let-7i | 2.2* | Target genes: Cald1, Hoxa9, Tagin | |
| mm-mir-92b | 2.0* | Target genes: Hoxa9, Fzd1 |
Only miRNAs showing decreased expression are shown. Changes are statistically significant with P value range from .02 to .001.
* Slightly significant.
** Significant.
Statistical Analysis
The data derived from different experiments were analyzed and compared by performing a 1- or 2-tailed t test, and the relationship between different experimental groupings was assessed by analysis of variance (ANOVA). Statistical significance was judged at P < .05.
RESULTS
Chlamydia-Induced Infertility Requires an Intact Chlamydial Cryptic Plasmid.
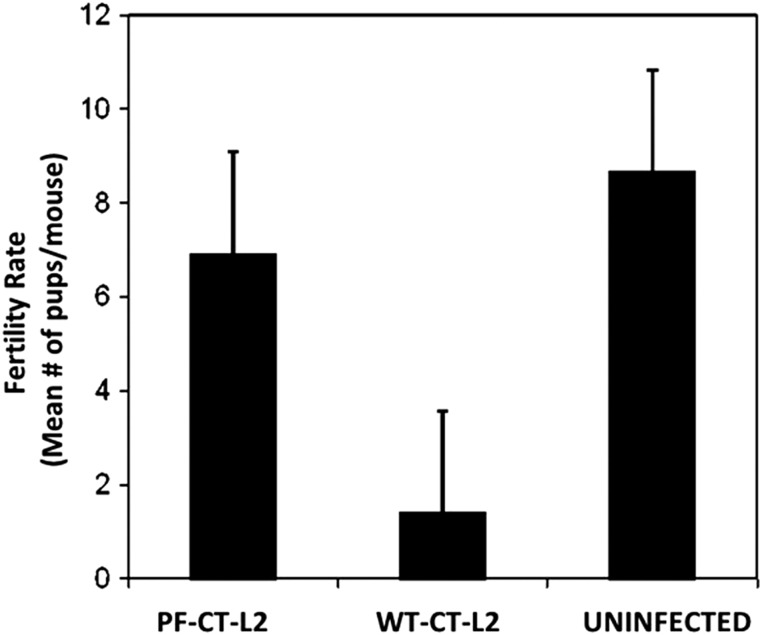
The female mouse model of chlamydial genital disease exhibits vital aspects of human infection [34, 35], thus providing a reliable experimental system to analyze the molecular basis of Chlamydia-induced infertility. To better define the host and pathogen factors and conditions for development of infertility, we initially compared the fertility of female mice after genital infection with the wild-type C. trachomatis L2 (WT-L2) and the plasmid-free C. trachomatis L2 (PF-L2). The results presented in Figure 1 revealed that WT-L2 infection caused significant infertility in the mice with approximately 80 percent reduction in the mean number of viable embryos or pups per mouse (P < .002). However, mice infected with PF-L2 retained fertility that was comparable to noninfected mice in both number of mice pregnant and average pups per mouse. As all animals were productively infected by both the WT-L2 and PF-L2, these results showed that the chlamydial cryptic plasmid is required for Chlamydia-induced infertility. Because caspase-1 deficiency also prevented the onset of tubal inflammation after chlamydial genital infection [6, 36], we next analyzed the role of caspases in Chlamydia-induced pathologies in vitro and in vivo.
Figure 1.
Fertility of mice infected with wild-type Chlamydia trachomatis L2 (WT-CT-L2) and the plasmid-free C. trachomatis (PF-CT-L2). Groups of C57BL/6 female mice were infected intravaginally with either WT-L2 or PF-L2, mated and scored for pregnancy and no. of pups, as described in the Materials and Methods section. The plotted data are the means (SEM) for groups from 4 independent experiments with 6 mice per experimental group.
Caspase Activation Is Required for Chlamydia-Induced Apoptosis at the Late Stage of Infection
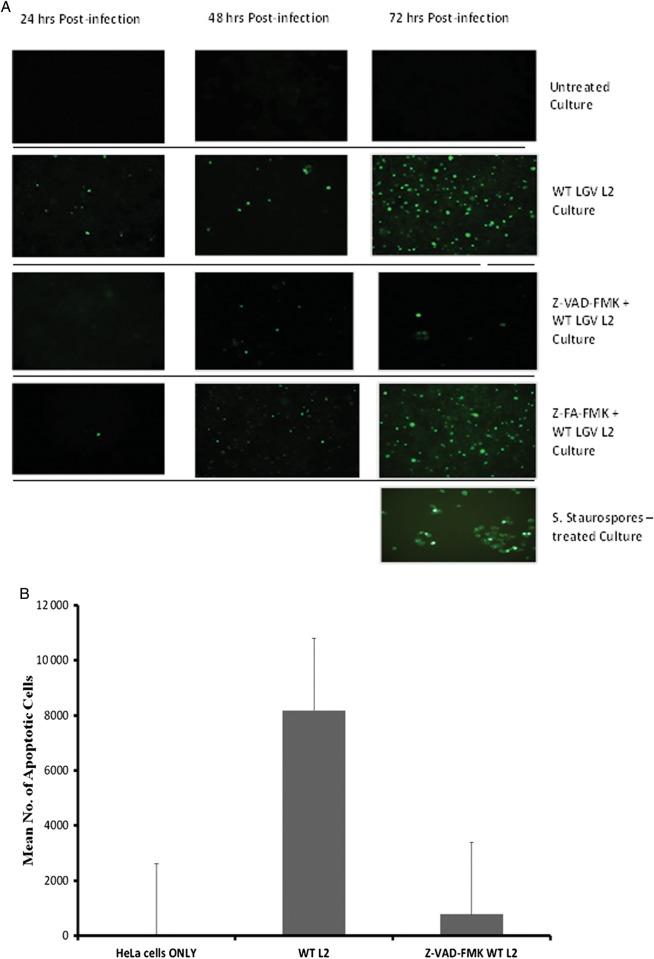
Chlamydiae resist apoptosis of infected cells during the early stages of infection to ensure successful establishment of infection and inclusion growth [37]; however, caspase-mediated forms of apoptosis contribute to the release of elementary bodies and inflammatory tissue destruction at the later stages of infection [38]. To demonstrate the involvement of caspase activation in Chlamydia-mediated tissue destruction via apoptosis during infection, we chemically inhibited the caspases during an in vitro infection and measured the effect on apoptosis. Results presented in Figure 2 show that treatment of infected cells with the pan-caspase inhibitor Z-VAD-FMK abrogated Chlamydia-induced apoptosis. Also, we showed that Chlamydia-induced apoptosis was minimal during the early stages of infection (24 and 48 hours) but became pronounced during the later stages (Figure 2a), which confirmed previous reports that Chlamydia inhibits apoptosis during the early stages of the infection. Thus, we focused our subsequent studies on the involvement of the caspases in Chlamydia-induced infertility.
Figure 2.
Caspase-dependent processes during Chlamydia infection. HeLa cells were treated with 50 µM of either Z-VAD-FMK or the control Z-FA-FMK for 90 min and then infected for 72 h with Chlamydia trachomatis at a MOI of 1. (A) Cells were fixed, incubated with a reaction mix containing biotin-dUTP, terminal deoxynucleotidyl transferase and avidin-FITC, as described in the Materials and Methods. Fluorescein-labeled fragmented DNA were visualized and photographed on a Nikon Eclipse 80i fluorescence microscope. (B) For quantification, the total number of TUNEL-positive cells were counted under in 17 randomly selected microscopic fields and normalized to the total number of cells per slide. Data were analyzed as the mean (+ SEM) of TUNEL-positive cells. The experiment was repeated 3 times. Bovine DNase I at 5 µg/mL and 500 nM of Staurosporine (Streptomyces staurospores) were used as positive controls.
Caspase 1 and 3 Are Involved in Chlamydia-Induced Infertility
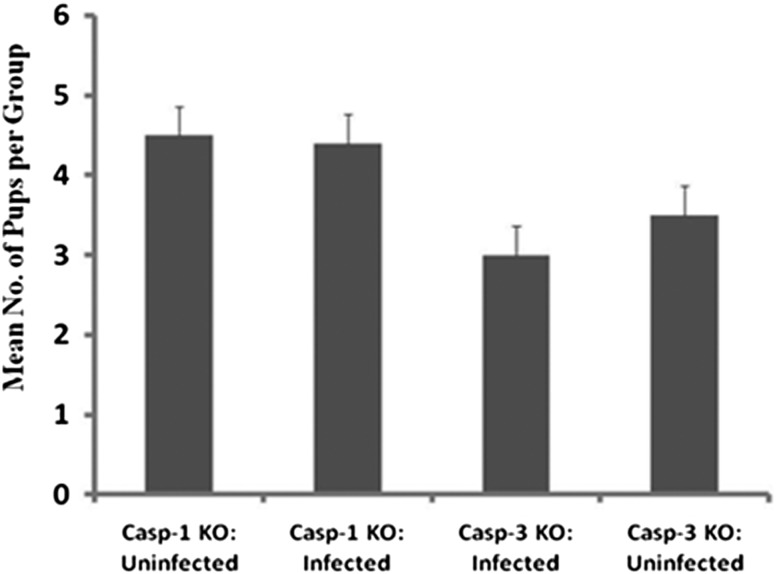
Activation of caspase-1 (required for the maturation of interleukin 1β, interleukin 18, and interleukin 33) has been shown to be necessary for Chlamydia-induced inflammatory pathologies in the upper genital tract of mice; thus mice deficient in caspase-1 failed to develop severe pathologies in the upper genital tract although were susceptible to chlamydial infection [6]. To determine whether caspase-1 and caspase-3, which are centrally located on the caspase cascade pathway, are involved in mediating Chlamydia-induced infertility, caspase-1 and caspase-3 knockout (KO) mice were genitally infected with wild-type C. trachomatis (WT-L2) and evaluated for infertility by mating. As shown in Figure 3, although caspase-1 and -3 KO mice as well as the control mice were productively infectable with WT-L2, the fertility of infected and noninfected animals was indistinguishable. As shown in Figure 1, infected normal (caspase-1- and -3-positive) mice exhibited approximately 80% infertility after a similar genital infection. The results suggest that the activation of the host's caspase-1 and -3 are required for chlamydial induction of infertility.
Figure 3.
Caspase-1 and -3 knockout mice are fertile. Groups of caspase-1 and -3 knockout female mice were infected intravaginally with wild-type C. trachomatis L2 (WT-L2), as described in the Materials and Methods section. Infected and noninfected control mice were mated and scored for pregnancy and no. of pups. The plotted data are the means (SEM) for groups from 2 independent experiments with 6 mice per experimental group.
Inhibition of Local Caspase Activation Prevented Chlamydia-Induced Infertility
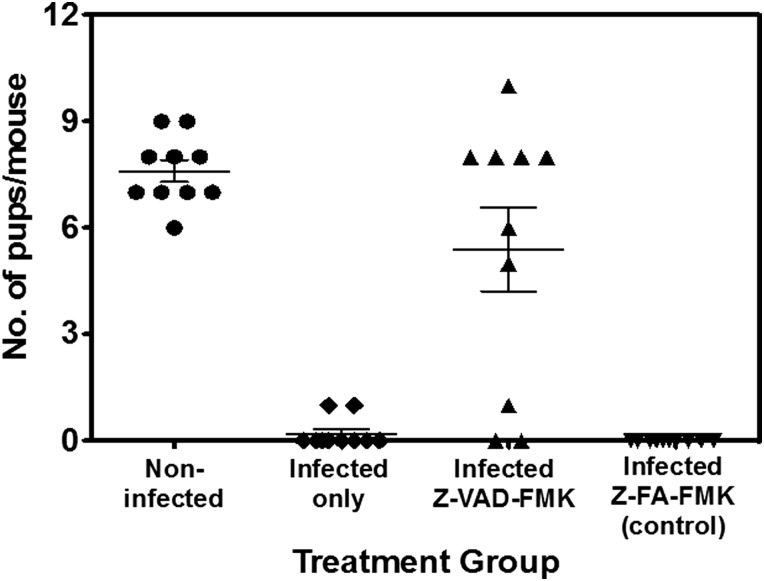
If activation of caspases is necessary for chlamydial infections to induce pathologies, we evaluated the possible benefit of pharmacological inhibition of caspases as a component of an antichlamydial treatment regimen to specifically prevent infertility. We tested the effect of local treatment with the pan-caspase inhibitor (Z-VAD-FMK) on Chlamydia-induced infertility. As shown in Figure 4, the local intravaginal treatment of mice with the pan-caspase inhibitor, Z-VAD-FMK significantly prevented infertility in infected animals (P > .004); however, shedding of chlamydiae was not affected, confirming previous reports that the ability to activate the caspases is a requirement for chlamydial infections to induce tubal pathologies.
Figure 4.
Prevention of Chlamydia-induced infertility by local administration of a pan-caspase inhibitor. Groups of C57BL/6 female mice were treated intravaginally with 0.02 ML of a 50 µM solution of the pan-caspase inhibitor, N-benzyloxy-cabonyl-Val-Ala-Asp-fluoromethyl ketone (Z-VAD-FMK) or the inhibitor control, Z-FA-FMK in phosphate-buffered saline before and after intravaginal infection with the wild-type Chlamydia trachomatis L2 (WT-L2), then mated to assess infertility, as described in the Materials and Methods. The experiments were repeated at 4 times with 6 mice per experimental group.
Caspase Activation and Dicer Cleavage in the Oviducts of Chlamydia-Infected Infertile Mice
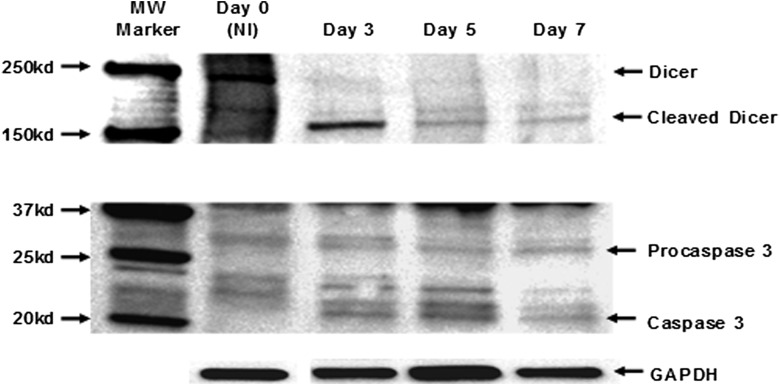
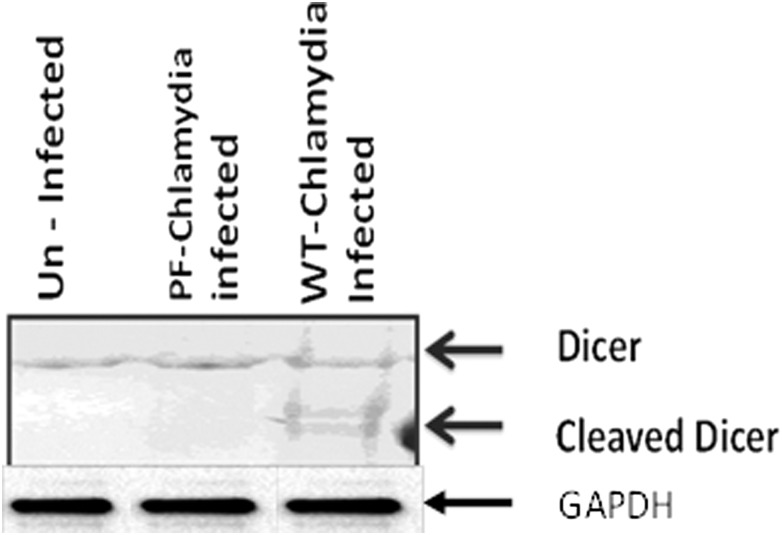
To explored the possibility that Chlamydia-induced infertility could occur via caspase-mediated disruption of vital fertility-promoting biological process(es) in the reproductive system, we focused on Dicer. Dicer is a caspase-sensitive, key fertility promoting enzyme whose targeted genetic deletion from reproductive tissues produces a female mouse that exhibits a reproductive infertility profile essentially identical to that in Chlamydia-infected, infertile female mice [17, 19–21]. Specifically, caspase-3, and to a lesser extent caspase-1, can mitigate Dicer-dependent biological functions by cleavage inactivation that converts Dicer from the 220 kDa protein into a major N-terminal 150–190 kDa and minor C-terminal fragments [25, 26]. Upon analysis of the integrity of Dicer and caspase-3 in the oviducts of Chlamydia-infected infertile mice, Figure 5 shows that the appearance of the ∼20 kDa activated caspase-3 from day 3 postinfection coincided with the generation of a lower sized caspase-3-cleaved Dicer (a 180 kDa N-terminal fragment) in the oviducts of infected, infertile mice. In addition, Dicer is cleaved in the oviducts from infertile (WT-L2-infected) but not in fertile (PF-L2 infected or noninfected) mice (Figure 6). The results indicated that caspase-mediated cleavage of Dicer occurred after genital chlamydial infection, and its inadequacy possibly causes infertility, similar to the infertility in Dicer-deficient mice [20].
Figure 5.
Western blotting analysis of the expression of Dicer and activated caspase-3 in the oviducts after genital chlamydial infection. Oviducts from infected and noninfected mice were homogenized, and equal amounts of total protein from each sample were loaded onto a 4%–20% SDS-PAGE gradient gel, electrophoresed and transferred on to nitrocellulose membranes. The blots were probed with primary antibodies against Dicer, the pro- or activated caspases). A primary antibody against Glyceraldehyde 3 phosphate dehydrogenase (GAPDH) was used as the housekeeping gene expression control. The secondary antibody was detected by Western ECL-enhanced luminol reagent (PerkinElmer Inc, Morrisville, NC). The figure shown is a representative of 4 independent experiments showing identical results.
Figure 6.
Western blotting analysis of expression of Dicer in the oviducts after genital chlamydial infection with wild-type Chlamydia trachomatis L2 (WT-CT-L2) or the plasmid-free C. trachomatis (PF-CT-L2). Oviducts from mice were homogenized and equal amounts of total protein from each sample were loaded onto a 4%–20% SDS-PAGE gradient gel, electrophoresed and transferred on to nitrocellulose membranes. The blots were probed with a primary antibody against Dicer. A primary antibody against Glyceraldehyde 3 phosphate dehydrogenase (GAPDH) was used as the housekeeping gene expression control. The secondary antibody was detected by Western ECL-enhanced luminol reagent (PerkinElmer Inc, Morrisville, NC). The figure shown is a representative of 4 independent experiments showing identical results.
Evidence of Alteration in Critical Developmental miRNAs in the Oviducts of Chlamydia-Infected Infertile Mice
The molecular and biochemical basis of infertility caused by chlamydial activation of the caspases and cleavage inactivation of Dicer was further probed by analyzing the miRNA expression pattern in the oviducts of infected infertile mice. As shown in Table 1, a combination of microarray and quantitative real-time PCR identified among others mir-21 and Mir-103 to be significantly decreased in the oviducts from wild-type C. trachomatis-infected, infertile mice, as compared to oviducts from fertile (PF-infected or uninfected) mice. This would suggest that target genes controlled by these miRNAs, including those involved in cell growth, differentiation, tissue development, and inflammation are affected by chlamydial infection.
DISCUSSION
The molecular basis of Chlamydia-induced infertility has remained unresolved. Dicer is a key molecular determinant of reproductive fertility [17, 19–21]; its susceptibility to cleavage inactivation by the caspases inducible by chlamydial infection [25] led us to investigate whether caspase-mediated processes, including the cleavage inactivation of Dicer and miRNA alteration, play a role in Chlamydia-induced infertility. Such evidence may have implications for TFI caused by other agents of sexually transmitted diseases, including Neisseria gonorrheae, Mycoplasma genitalium, and Trichomonas vaginalis. Our results corroborate previous studies that the chlamydial cryptic plasmid and caspase activation are required for chlamydial tubal or ocular inflammatory pathologies [6, 14–16] and suggest that the plasmid-free chlamydial strain would not activate the caspases. This conclusion was partly confirmed by results showing that the local application of the pan-caspase inhibitor Z-VAD-FMK prevented the Chlamydia-induced infertility. The finding suggests that safe, human-compatible anticaspase agents, used individually or in combination with antichlamydial antibiotics, may prevent TFI and possibly PID, offering a potentially new treatment against a major chlamydial sequeale in control and prevention programs. The prospect for clinical use of caspase inhibitors to prevent caspase-induced apoptosis was demonstrated for islet cell transplant in mice [39].
Mechanistically, the molecular basis of caspase-dependent Chlamydia-induced infertility could include caspase activation via TNF-α signaling, leading to apoptotic destruction of the ICCs and triggering ectopic pregnancy and infertility due to lack of electrical waves for fallopian tube smooth muscle contraction that transports fertilized embryos to the uterus for implantation [13]. The destruction of ICCs does occur after genital chlamydial infection, although they were regenerated [40]; however, the extent of recovery of the molecules that conduct, transmit, and regulate the electrical waves on the smooth muscles of the reproductive tract, including β adrenergic and CB-1 receptors, and ion channels such as Ano-1 [41, 42] was not investigated. Second, Dicer is the target of caspases, especially caspase-3 and to some extent caspase-1 [25, 26], which are activated during chlamydial infection [38]; and the adverse reproductive consequences and pathologic features of Dicer deficiency in female animals bear striking similarities to Chlamydia-induced tubal abnormalities in mice [20]. Therefore, there may be a pathobiological link in the reproductive disease outcomes associated with genital chlamydial infection and Dicer deficiency. Our present study revealed that the integrity of Dicer and select miRNAs in the oviducts of infected infertile mice was impaired, with evidence of caspase-mediated Dicer cleavage in the oviducts from Chlamydia-infected infertile mice. Among the prominent differentially expressed miRNAs identified was mir-21, which plays a key regulatory role in cell proliferation, differentiation, and growth, such that its overexpression in certain cancers serves as a diagnostic and therapeutic target [43]. Mir-21 will likely be vital in early embryonic development, and its reduction may compromise zygote and embryonic development following fertilization. Other differentially expressed miRNAs identified in the oviducts of infection-induced infertile mice include mir-103, mir-107, Let-7i, and mir-92b, which regulate expression of members of the wingless family of secreted glycoproteins (Wnt4, Wnt5a, Wnt7a) and the homeobox transcription factors encoded by the Hox genes that have major functions in reproductive tissues, implantation and fertility, as well as cell signaling pathways that involve the β-catennin during host immune response and inflammation [44–46]. Therefore, caspase activation and Dicer cleavage inactivation may affect the generation and function of miRNAs required for regulating gene expression during postfertilization embryonic development as well as local inflammation and immune reactivity in the female reproductive system. Fertilized Dicer-deficient oocytes are arrested at the early stages of embryonic due to limitation of de novo synthesis of critical miRNAs; they were fragmented with reduced and disorganized spindles and chromosomes [24]. Moreover, the single copy of Dicer1 in the mouse genome for the biosynthesis of both miRNAs and siRNAs [47] implies that any alterations in Dicer expression or the state of the enzyme in reproductive tissues will drastically affect differentiation and physiological processes during reproduction. Thus, the loss of Dicer in mouse oviducts resulted in major reproductive abnormalities, including loss of the smooth muscle layer and disorganization of the epithelium, particularly the isthmus, retention of fertilized embryos in the oviduct (ie, ectopic pregnancy), poor smooth muscle contractility, and developmentally delayed embryos, which culminated in infertility [19].
Finally, the proposed caspase-Dicer-miRNA pathway to Chlamydia-induced TFI would not necessarily invalidate the role of inflammation-induced scarring, fibrosis, and obstruction. In fact, it is likely that both processes occur concurrently. In addition, relevant questions for further investigation include how caspase activation, Dicer cleavage inactivation, and other downstream processes leading to onset of infertility are sustained after host clearance of chlamydial infections or whether recurrent or chronic infection is required to drive the pathologic process. Inadequate Dicer cellular recovery will be required to validate any sustained deficiency without reinfection to reactive the destructive caspases. Furthermore, a recent report revealed that activation of the caspases modulates Dicer expression in at least 2 ways; first, caspase cleavage of Dicer inactivates its miRNA generation function [25, 26]. Second, caspase cleavage of Dicer potentiates cellular apoptotic processes, through the conversion of Dicer (an RNase) into a proapoptotic DNase represented by the N-terminal fragment [48]. Chlamydia-induced caspase-mediated cleavage of Dicer may therefore increase cellular apoptosis in the reproductive tract tissues, leading to cellular fragmentation, embryonic demise, and depletion of ICCs. Consequently, we have nonsurvival of embryos and inability of surviving zygotes or early embryos to transit the oviduct to the uterus for implantation. Finally, alteration in expression of certain miRNAs is a potential biomarker for diagnosing or monitoring the onset and evolution of tubal pathologies into PID or infertility. Furthermore, with relevant technology, there are future prospects for replacement therapies of Dicer gene or enzyme therapy, and miRNA targeting.
Supplementary Material
Notes
Acknowledgments. We thank Dr Chryssa Kanellopoulou of the National Institutes of Health (NIH), Bethesda, Maryland, for providing us antimouse Dicer antisera for comparison with the commercially available anti-Dicer antibodies.
Financial support. This work was supported by the Centers for Disease Control and Prevention (CDC) and PHS grants (AI41231, GM 08248, RR03034, and 1SC1GM098197) from the NIH.
The conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Detels R, Green A, Klausner J, et al. The incidence and correlates of symptomatic and asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae infections in selected populations in five countries. Sex Transm Dis. 2011;38:503–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Healy D, Trounson A, Andersen A. Female infertility: causes and treatment Lancet. 1994;343:1539–44. doi: 10.1016/s0140-6736(94)92941-6. [DOI] [PubMed] [Google Scholar]
- 3.Ison C. Antimicrobial resistance in sexually transmitted infections in the developed world: implications for rational treatment. Curr Opin Infect Dis. 2012;25:73–8. doi: 10.1097/QCO.0b013e32834e9a6a. [DOI] [PubMed] [Google Scholar]
- 4.Soper D. Pelvic inflammatory disease. Obstet Gynecol. 2010;116:419–28. doi: 10.1097/AOG.0b013e3181e92c54. [DOI] [PubMed] [Google Scholar]
- 5.Darville T, O'Niell JM, Andrews CW, Jr, Nagarajan UM, Ojcius DM. Toll-like receptor-2, but not toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol. 2003;171:6187–97. doi: 10.4049/jimmunol.171.11.6187. [DOI] [PubMed] [Google Scholar]
- 6.Cheng W, Shivshankar P, Li Z, Chen L, Yeh I, Zhong G. Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection. Infect Immun. 2008;76:515–22. doi: 10.1128/IAI.01064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Chen D, Zhong Y, Wang S, Zhong G. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect Immun. 2008;76:3415–28. doi: 10.1128/IAI.01377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchholz K, Stephens R. The cytosolic pattern recognition receptor NOD1 induces inflammatory interleukin-8 during Chlamydia trachomatis infection. Infect Immun. 2008;76:3150–5. doi: 10.1128/IAI.00104-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdul-Sater A, Said-Sadier N, Ojcius DM, Yilmaz O, Kelly KA. Inflammasomes bridge signaling between pathogen identification and the immune response. Drugs Today. 2009;45:105–12. [PMC free article] [PubMed] [Google Scholar]
- 10.Murthy A, Li W, Chaganty B, et al. Tumor necrosis factor alpha production from CD8+ T cells mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection. Infect Immun. 2011;79:2928–35. doi: 10.1128/IAI.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wynn T. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–50. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melloul D. Role of NF-κB in β-cell death. Biochem Soc Trans. 2008;36:334–9. doi: 10.1042/BST0360334. [DOI] [PubMed] [Google Scholar]
- 13.Dixon R, Hwang S, Hennig G, et al. Chlamydia infection causes loss of pacemaker cells and inhibits oocyte transport in the mouse oviduct. Biol Reprod. 2009;80:665–73. doi: 10.1095/biolreprod.108.073833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Connell C, Ingalls R, Andrews CJ, Scurlock A, Darville T. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol. 2007;179:4027–34. doi: 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 15.Olivares-Zavaleta N, Whitmire W, Gardner D, Caldwell H. Immunization with the attenuated plasmidless Chlamydia trachomatis L2(25667R) strain provides partial protection in a murine model of female genitourinary tract infection. Vaccine. 2009;28:1454–62. doi: 10.1016/j.vaccine.2009.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kari L, Whitmire W, Olivares-Zavaleta N, et al. A live-attenuated chlamydial vaccine protects against trachoma in nonhuman primates. J Exp Med. 2011;208:2217–23. doi: 10.1084/jem.20111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao R. Understanding the mechanisms of human tubal ectopic pregnancies: new evidence from knockout mouse models. Hum Reprod. 2010;25:584–7. doi: 10.1093/humrep/dep438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams A. Functional aspects of animal microRNAs. Cell Mol Life Sc. 2008;65:545–62. doi: 10.1007/s00018-007-7355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luense L, Carletti M, Christenson L. Role of Dicer in female fertility. Trends Endocrinol Metab. 2009;20:265–72. doi: 10.1016/j.tem.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagaraja A, Andreu-Vieyra C, Franco H, et al. Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol. 2008;22:2336–52. doi: 10.1210/me.2008-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carletti M, Christenson L. MicroRNA in the ovary and female reproductive tract. J Anim Sci. 2009;87:E29–38. doi: 10.2527/jas.2008-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su A, Cooke M, Ching K, et al. Large-scale analysis of the human and mouse transcriptomes. PNAS USA. 2002;99:4465–70. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhat-Nakshatri P, Wang G, Collins N, et al. Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Research. 2009;37:4850–61. doi: 10.1093/nar/gkp500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang F, Kaneda M, O'Carroll D, et al. Maternal microRNAs are essential for mouse zygotic development. Genes Dev. 2007;21:644–8. doi: 10.1101/gad.418707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matskevich A, Moelling K. Stimuli-dependent cleavage of Dicer during apoptosis. Biochem J. 2008;412:527–34. doi: 10.1042/BJ20071461. [DOI] [PubMed] [Google Scholar]
- 26.Ghodgaonkar M, Shah R, Kandan-Kulangara F, et al. Abrogation of DNA vector-based RNAi during apoptosis in mammalian cells due to caspase-mediated cleavage and inactivation of Dicer-1. Cell Death Differ. 2009;16:858–68. doi: 10.1038/cdd.2009.15. [DOI] [PubMed] [Google Scholar]
- 27.Peterson EM, Markoff BA, Schachter J, de la Maza LM. The 7.5-kb plasmid present in Chlamydia trachomatis is not essential for the growth of this microorganism. Plasmid. 1990;23:144–8. doi: 10.1016/0147-619x(90)90033-9. [DOI] [PubMed] [Google Scholar]
- 28.Igietseme J, He Q, Joseph K, et al. Role of T lymphocytes in the pathogenesis of Chlamydia disease. J Infect Dis. 2009;200:926–34. doi: 10.1086/605411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuffrey M, Taylor-Robinson D. Progesterone as a key factor in the development of a mouse model for genital-tract infection with Chlamydia trachomatis. FEMS Microbiol Let. 1981;12:111–5. [Google Scholar]
- 30.Tuffrey M, Alexander F, Woods C, Taylor-Robinson D. Genetic susceptibility to chlamydial salpingitis and subsequent infertility in mice. J Reprod Fertil. 1992;95:31–8. doi: 10.1530/jrf.0.0950031. [DOI] [PubMed] [Google Scholar]
- 31.Pal S, Hui W, Peterson EM, de la Maza LM. Factors influencing the induction of infertility in a mouse model of Chlamydia trachomatis ascending genital infection. J Med Microbiol. 1998;47:599–605. doi: 10.1099/00222615-47-7-599. [DOI] [PubMed] [Google Scholar]
- 32.Muppidi J, Porter M, Siegel R. Measurement of apoptosis and other forms of cell death. Curr Protoc Immunol. 2004;3 doi: 10.1002/0471142735.im0317s59. Unit 3.17. [DOI] [PubMed] [Google Scholar]
- 33.Chang C, Zhang Q, Liu Z, Clynes R, Suciu-Foca N, Vlad G. Downregulation of inflammatory microRNAs by Ig-like transcript 3 is essential for the differentiation of human CD8(+) T suppressor cells. J Immunol. 2012;188:3042–52. doi: 10.4049/jimmunol.1102899. [DOI] [PubMed] [Google Scholar]
- 34.Rank RG. Animal models of urogenital infections. Methods Enzymol. 1994;235:83–93. doi: 10.1016/0076-6879(94)35133-3. [DOI] [PubMed] [Google Scholar]
- 35.Johansson M, Schon K, Ward M, Lycke N. Studies in knockout mice reveal that anti-chlamydial protection requires TH1 cells producing IFN-gamma: is this true for human? Scand J Immunol. 1997;46:546–52. doi: 10.1046/j.1365-3083.1997.d01-167.x. [DOI] [PubMed] [Google Scholar]
- 36.Prantner D, Darville T, Sikes J, et al. Critical role for interleukin-1beta (IL-1β) during Chlamydia muridarum genital infection and bacterial replication-independent secretion of IL-1β in mouse macrophages. Infect Immun. 2009;77:5334–46. doi: 10.1128/IAI.00883-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong G. Chlamydia trachomatis secretion of proteases for manipulating host signaling pathways. Front Microbiol. 2011;2:14. doi: 10.3389/fmicb.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ying S, Pettengill M, Ojcius D, Häcker G. Host-cell survival and death during Chlamydia infection. Curr Immunol Rev. 2007;3:31–40. doi: 10.2174/157339507779802179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emamaullee J, Davis J, Pawlick R, et al. The caspase selective inhibitor EP1013 augments human islet graft function and longevity in marginal mass islet transplantation in mice. Diabetes. 2008;57:1556–66. doi: 10.2337/db07-1452. [DOI] [PubMed] [Google Scholar]
- 40.Dixon R, Ramsey K, Schripsema J, Sanders K, Ward S. Time-dependent disruption of oviduct pacemaker cells by Chlamydia infection in mice. Biol Reprod. 2010;83:2244–253. doi: 10.1095/biolreprod.110.083808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dixon R, Hennig G, Baker S, et al. Electrical slow waves in the mouse oviduct are dependent upon a calcium activated chloride conductance encoded by Tmem16a. Biol Reprod. 2012;86:1–7. doi: 10.1095/biolreprod.111.095554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez-Pinilla P, Gibbons S, Bardsley M, et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–81. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonci D. MicroRNA-21 as therapeutic target in cancer and cardiovascular disease. Recent Pat Cardiovasc Drug Discov. 2010;5:156–61. doi: 10.2174/157489010793351962. [DOI] [PubMed] [Google Scholar]
- 44.Vitiello D, Kodaman P, Taylor H. HOX genes in implantation. Semin Reprod Med. 2007;25:431–6. doi: 10.1055/s-2007-991040. [DOI] [PubMed] [Google Scholar]
- 45.Miller J. The Wnts. Genome Biol. 2002;3:3001.1–15. doi: 10.1186/gb-2001-3-1-reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mellman I, Clausen B. B-Catenin balances immunity. Science. 2010;329:767–9. doi: 10.1126/science.1194185. [DOI] [PubMed] [Google Scholar]
- 47.Harfe B, McManus M, Mansfield J, Hornstein E, Tabin C. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci U S A. 2005;102:10898–903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakagawa A, Shi Y, Kage-Nakadai E, Mitani S, Xue D. Caspase-dependent conversion of dicer ribonuclease into a death-promoting deoxyribonuclease. Science. 2010;328:327–34. doi: 10.1126/science.1182374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.