To the Editor—A recent article by Redd et al [1] reported preferential transmission of human immunodeficiency virus type 1 (HIV-1) most closely related to strains sampled at least 2 years prior to transmission (ancestral), compared with strains sampled just after transmission (contemporary), in heterosexual couples in Rakai, Uganda. Characterization of the viral population in the recipient is essential when determining both the source of the transmitted virus in the donor and establishing the viral characteristics that may favor successful transmission. We contend that the data do not support the conclusion by Redd et al because of a common misinterpretation of relatedness in an evolutionary framework [2] and statistical issues associated with computing genetic distances between short, highly similar sequences.
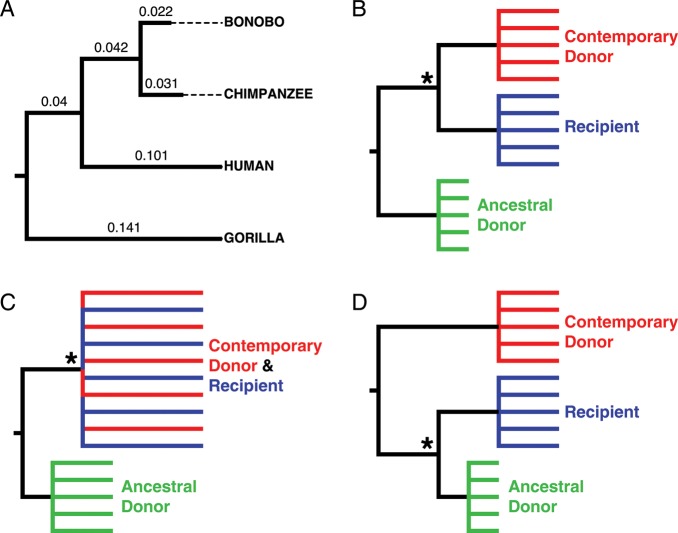
The closest relative of a given taxon—whether it be nucleotide sequence, strain, or species—is the taxon (or taxa) with which it shares a most recent common ancestor. Once a phylogeny has been inferred, genetic distance is inconsequential when determining relatedness. For example, humans are more closely related to both chimpanzees and bonobos than we are to gorillas, because we share a common ancestor with chimpanzees and bonobos more recently than we share a common ancestor with gorillas (Figure 1A) [3]. Furthermore, even though the genetic distance separating gorillas and chimpanzees (0.245 substitutions/site) is shorter than the distance between gorillas and humans (0.282 substitutions/site), gorillas are equally related to both humans and chimpanzees.
Figure 1.
Depictions of phylogenetic relationships. (A) Phylogeny of African apes inferred from the coding region of mitochondrial genomes. Nucleotide substitutions per site are shown on each branch. (B) Idealized phylogeny in which recipient viruses are monophyletic and more closely related to contemporary donor sequences than ancestral donor sequences. (C) Idealized phylogeny in which recipient viruses are intermixed with contemporary donor sequences with whom they share a common ancestor more recently than with ancestral donor sequences. (D) Idealized phylogeny in which recipient viruses are more closely related to ancestral donor sequences than contemporary donor sequences. An asterisk indicates the node whose support is relevant to inference; paraphyletic relationships between donor populations and recipient viruses would yield the same conclusions.
When using phylogenetic analysis to determine the source of HIV-1 transmission, these points are essential. The viral source population in the donor (ancestral vs contemporary) can be identified by determining which donor viruses share the most recent common ancestor with virus in the recipient. We reanalyzed viral sequences from the 9 couples subjected to 454 next-generation sequencing (Figures 3 and 4 in the article by Redd et al [1]), before and after transmission, using a molecular clock in a Bayesian Markov chain Monte Carlo framework [4].
In 4 couples (couples 8–11), viruses from the recipient were monophyletic and shared a most recent common ancestor with contemporary viruses from the donor (posterior probability, ≥ .98; Figure 1B). In 3 couples, (couples 1, 12, and 14) viruses from the recipient were intermixed with contemporary donor viruses and were therefore more closely related to contemporary donor viruses than ancestral donor viruses (posterior probability, ≥ .99; Figure 1C). In 2 couples (couples 6 and 15), the phylogenies were poorly resolved because of insufficient differentiation between the ancestral and contemporary donor viruses. In no couple was there phylogenetic evidence to support recipient viruses being the closest relatives of ancestral donor viruses (Figure 1D). Therefore, our analysis finds it unlikely that ancestral viruses were preferentially transmitted from a sequestered, long-lived reservoir or from a population persisting at low levels in the serum, as suggested by Redd et al [1].
In Figure 1 in the article by Redd et al [1], similarity between ancestral donor and recipient sequences was illustrated by plotting point estimates of genetic distances between donor sequences, sampled at different times, and recipient sequences. Genetic distances between 2 sequences are estimated using statistical procedures, and distances inferred from relatively short nucleotide sequences are imprecise and should be compared with care. For example, in 1 couple (F04331 and H50053), the recipient sequence had 1 nucleotide difference from the ancestral donor sequence and 3 nucleotide differences from the contemporary donor sequence. These differences yielded distance estimates (Tamura-Nei 93 [5]) of 0.25% (95% bootstrap confidence interval [CI], 0.0%–1.25%) and 0.76% (95% bootstrap CI, 0.23%–1.8%), respectively. Because of wide overlapping bootstrap CIs, one cannot determine which donor sequence is more genetically similar to the recipient sequence (posterior probability, .51, by the likelihood ratio test [6]). Indeed, we found sufficient signal to assert that the recipient sequence is genetically more similar to the ancestral donor sequence in only 1 of 22 total couples (posterior probability, ≤.05; no correction for multiple testing).
It remains an open question whether viruses in some recipients are genetically more similar to the ancestral donor population, compared with the contemporary donor population. An increase in the evolutionary rate in donor virus later in infection or a slowdown in the rate of viral evolution in the recipient, due to changes in natural selection or demography (ie, bottleneck followed by population expansion), would account for this phenomenon. Alternatively, convergent evolution (ie, reversion to an ancestral genotype/phenotype) is a possible explanation for decreased genetic distance separating recipient sequences from the ancestral donor viruses [7–8]. Nevertheless, none of these scenarios point to the preferential transmission of ancestral viruses.
Notes
Financial support. This work was supported in part by the National Institutes of Health (grants AI43638, AI47745, GM093939, AI100665 AI74621, AI090970, and AI36214).
Potential conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Redd AD, Collinson-Streng AN, Chatziandreou N, et al. Previously transmitted HIV-1 strains are preferentially selected during subsequent sexual transmissions. J Infect Dis. 2012;206:1433–42. doi: 10.1093/infdis/jis503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baum DA, Smith SD, Donovan SS. The tree-thinking challenge. Science. 2005;310:979. doi: 10.1126/science.1117727. [DOI] [PubMed] [Google Scholar]
- 3.Horai S, Satta Y, Hayasaka K, et al. Man's place in Hominoidea revealed by mitochondrial DNA genealogy. J Mol Evol. 1992;35:32. doi: 10.1007/BF00160258. [DOI] [PubMed] [Google Scholar]
- 4.Drummond AJ, Suchard MA, Xie D, et al. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012;29:1969. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 6.Muse SV, Weir BS. Testing for equality of evolutionary rates. Genetics. 1992;132:269. doi: 10.1093/genetics/132.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbeck JT, Nickle DC, Learn GH, et al. Human immunodeficiency virus type 1 env evolves toward ancestral states upon transmission to a new host. J Virol. 2006;80:1637. doi: 10.1128/JVI.80.4.1637-1644.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lythgoe KA, Fraser C. New insights into the evolutionary rate of HIV-1 at the within-host and epidemiological levels. Proc Biol Sci. 2012;279:3367. doi: 10.1098/rspb.2012.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]