Abstract
Over the past few years, genotypic methods based on the study of bacterial DNA polymorphism have shown high discriminatory power for strain differentiation and superiority over most phenotypic methods commonly available in the clinical microbiology laboratory. Some of the methods used, however, required either a high level of technology and sophisticated equipment (e.g., pulsed-field gel electrophoresis) or species-specific reagents of restricted availability (randomly cloned DNA probes or gene-specific probes). Because ribotyping uses a universal probe (rRNA) and is a rather simple technology, particularly since the advent of nonradioactive labelling systems, it has been widely used for strain differentiation of most bacterial species involved in nosocomial outbreaks. In vitro and in vivo stability of the markers studied has been demonstrated. Although there may be limitation to this approach, ribotyping was found to be highly discriminative, particularly for typing members of the family Enterobacteriaceae, Pseudomonas cepacia, and Xanthomonas maltophilia. In many cases, it has improved the understanding of the mechanism of nosocomial acquisition of organisms by allowing a distinction between endogenous and exogenous infections. Among exogenous infections, it has distinguished between individual and epidemic strains, thus differentiating cross-infection from independent acquisition.
Full text
PDF


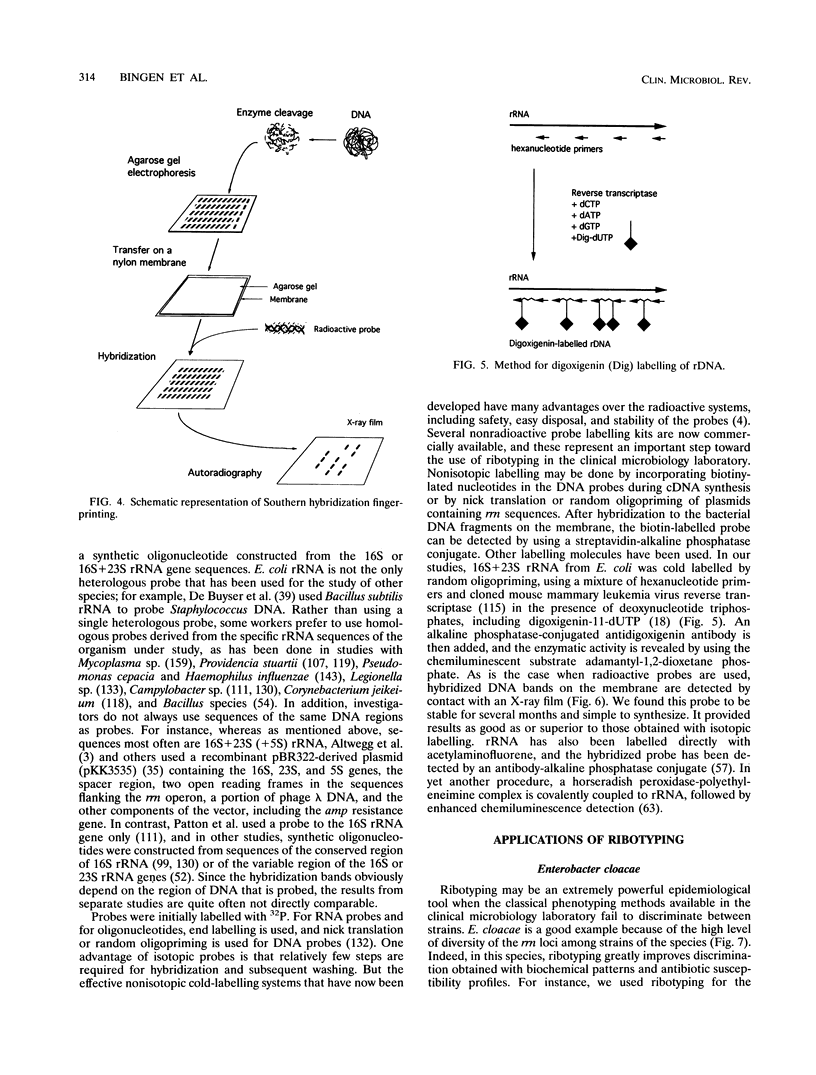












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardet-Servent A., Bouziges N., Carles-Nurit M. J., Bourg G., Gouby A., Ramuz M. Use of low-frequency-cleavage restriction endonucleases for DNA analysis in epidemiological investigations of nosocomial bacterial infections. J Clin Microbiol. 1989 Sep;27(9):2057–2061. doi: 10.1128/jcm.27.9.2057-2061.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alos J. I., Lambert T., Courvalin P. Comparison of two molecular methods for tracing nosocomial transmission of Escherichia coli K1 in a neonatal unit. J Clin Microbiol. 1993 Jul;31(7):1704–1709. doi: 10.1128/jcm.31.7.1704-1709.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altwegg M., Hickman-Brenner F. W., Farmer J. J., 3rd Ribosomal RNA gene restriction patterns provide increased sensitivity for typing Salmonella typhi strains. J Infect Dis. 1989 Jul;160(1):145–149. doi: 10.1093/infdis/160.1.145. [DOI] [PubMed] [Google Scholar]
- Altwegg M., Mayer L. W. Bacterial molecular epidemiology based on a nonradioactive probe complementary to ribosomal RNA. Res Microbiol. 1989 May-Jun;140(4-5):325–333. doi: 10.1016/0923-2508(89)90024-7. [DOI] [PubMed] [Google Scholar]
- Amikam D., Glaser G., Razin S. Mycoplasmas (Mollicutes) have a low number of rRNA genes. J Bacteriol. 1984 Apr;158(1):376–378. doi: 10.1128/jb.158.1.376-378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. J., Kuhns J. S., Vasil M. L., Gerding D. N., Janoff E. N. DNA fingerprinting by pulsed field gel electrophoresis and ribotyping to distinguish Pseudomonas cepacia isolates from a nosocomial outbreak. J Clin Microbiol. 1991 Mar;29(3):648–649. doi: 10.1128/jcm.29.3.648-649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. P., Roth J. R. Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- Arlet G., Sanson-le Pors M. J., Rouveau M., Fournier G., Marie O., Schlemmer B., Philippon A. Outbreak of nosocomial infections due to Klebsiella pneumoniae producing SHV-4 beta-lactamase. Eur J Clin Microbiol Infect Dis. 1990 Nov;9(11):797–803. doi: 10.1007/BF01967377. [DOI] [PubMed] [Google Scholar]
- Arthur M., Arbeit R. D., Kim C., Beltran P., Crowe H., Steinbach S., Campanelli C., Wilson R. A., Selander R. K., Goldstein R. Restriction fragment length polymorphisms among uropathogenic Escherichia coli isolates: pap-related sequences compared with rrn operons. Infect Immun. 1990 Feb;58(2):471–479. doi: 10.1128/iai.58.2.471-479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach S. B., McNeil M. M., Brown J. M., Lasker B. A., Jarvis W. R. Outbreak of pseudoinfection with Tsukamurella paurometabolum traced to laboratory contamination: efficacy of joint epidemiological and laboratory investigation. Clin Infect Dis. 1992 May;14(5):1015–1022. doi: 10.1093/clinids/14.5.1015. [DOI] [PubMed] [Google Scholar]
- Bacot C. M., Reeves R. H. Novel tRNA gene organization in the 16S-23S intergenic spacer of the Streptococcus pneumoniae rRNA gene cluster. J Bacteriol. 1991 Jul;173(13):4234–4236. doi: 10.1128/jb.173.13.4234-4236.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloga A. O., Harlander S. K. Comparison of methods for discrimination between strains of Listeria monocytogenes from epidemiological surveys. Appl Environ Microbiol. 1991 Aug;57(8):2324–2331. doi: 10.1128/aem.57.8.2324-2331.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril C., Richaud C., Baranton G., Saint Girons I. S. Linear chromosome of Borrelia burgdorferi. Res Microbiol. 1989 Oct;140(8):507–516. doi: 10.1016/0923-2508(89)90083-1. [DOI] [PubMed] [Google Scholar]
- Bercovier H., Kafri O., Sela S. Mycobacteria possess a surprisingly small number of ribosomal RNA genes in relation to the size of their genome. Biochem Biophys Res Commun. 1986 May 14;136(3):1136–1141. doi: 10.1016/0006-291x(86)90452-3. [DOI] [PubMed] [Google Scholar]
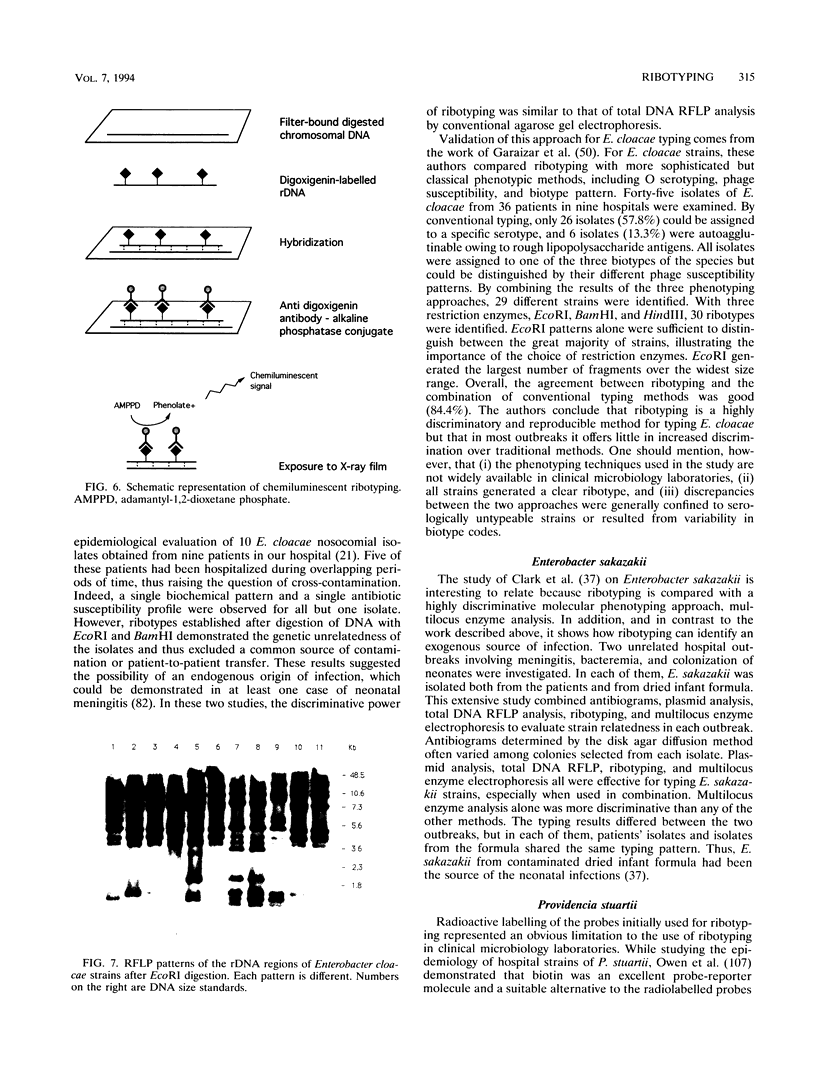
- Bialkowska-Hobrzanska H., Harry V., Jaskot D., Hammerberg O. Typing of coagulase-negative staphylococci by Southern hybridization of chromosomal DNA fingerprints using a ribosomal RNA probe. Eur J Clin Microbiol Infect Dis. 1990 Aug;9(8):588–594. doi: 10.1007/BF01967213. [DOI] [PubMed] [Google Scholar]
- Bingen E. H., Denamur E., Lambert-Zechovsky N. Y., Bourdois A., Mariani-Kurkdjian P., Cezard J. P., Navarro J., Elion J. DNA restriction fragment length polymorphism differentiates crossed from independent infections in nosocomial Xanthomonas maltophilia bacteremia. J Clin Microbiol. 1991 Jul;29(7):1348–1350. doi: 10.1128/jcm.29.7.1348-1350.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E. H., Denamur E., Lambert-Zechovsky N. Y., Elion J. Evidence for the genetic unrelatedness of nosocomial vancomycin-resistant Enterococcus faecium strains in a pediatric hospital. J Clin Microbiol. 1991 Sep;29(9):1888–1892. doi: 10.1128/jcm.29.9.1888-1892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E. H., Denamur E., Picard B., Goullet P., Lambert-Zechovsky N. Y., Brahimi N., Mercier J. C., Beaufils F., Elion J. Molecular epidemiology unravels the complexity of neonatal Escherichia coli acquisition in twins. J Clin Microbiol. 1992 Jul;30(7):1896–1898. doi: 10.1128/jcm.30.7.1896-1898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E. H., Desjardins P., Arlet G., Bourgeois F., Mariani-Kurkdjian P., Lambert-Zechovsky N. Y., Denamur E., Philippon A., Elion J. Molecular epidemiology of plasmid spread among extended broad-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates in a pediatric hospital. J Clin Microbiol. 1993 Feb;31(2):179–184. doi: 10.1128/jcm.31.2.179-184.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E. H., Mariani-Kurkdjian P., Lambert-Zechovsky N. Y., Desjardins P., Denamur E., Aujard Y., Vilmer E., Elion J. Ribotyping provides efficient differentiation of nosocomial Serratia marcescens isolates in a pediatric hospital. J Clin Microbiol. 1992 Aug;30(8):2088–2091. doi: 10.1128/jcm.30.8.2088-2091.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E. H., Weber M., Derelle J., Brahimi N., Lambert-Zechovsky N. Y., Vidailhet M., Navarro J., Elion J. Arbitrarily primed polymerase chain reaction as a rapid method to differentiate crossed from independent Pseudomonas cepacia infections in cystic fibrosis patients. J Clin Microbiol. 1993 Oct;31(10):2589–2593. doi: 10.1128/jcm.31.10.2589-2593.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E., Boissinot C., Desjardins P., Cave H., Brahimi N., Lambert-Zechovsky N., Denamur E., Blot P., Elion J. Arbitrarily primed polymerase chain reaction provides rapid differentiation of Proteus mirabilis isolates from a pediatric hospital. J Clin Microbiol. 1993 May;31(5):1055–1059. doi: 10.1128/jcm.31.5.1055-1059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E., Cavé H., Aujard Y., Lambert-Zechovsky N., Desjardins P., Elion J., Denamur E. Molecular analysis of multiply recurrent meningitis due to Escherichia coli K1 in an infant. Clin Infect Dis. 1993 Jan;16(1):82–85. doi: 10.1093/clinids/16.1.82. [DOI] [PubMed] [Google Scholar]
- Bingen E., Denamur E., Lambert-Zechovsky N., Aujard Y., Brahimi N., Geslin P., Elion J. Analysis of DNA restriction fragment length polymorphism extends the evidence for breast milk transmission in Streptococcus agalactiae late-onset neonatal infection. J Infect Dis. 1992 Mar;165(3):569–573. doi: 10.1093/infdis/165.3.569. [DOI] [PubMed] [Google Scholar]
- Bingen E., Denamur E., Lambert-Zechovsky N., Boissinot C., Brahimi N., Aujard Y., Blot P., Elion J. Mother-to-infant vertical transmission and cross-colonization of Streptococcus pyogenes confirmed by DNA restriction fragment length polymorphism analysis. J Infect Dis. 1992 Jan;165(1):147–150. doi: 10.1093/infdis/165.1.147. [DOI] [PubMed] [Google Scholar]
- Bingen E., Denamur E., Lambert-Zechovsky N., Brahimi N., el Lakany M., Elion J. Rapid genotyping shows the absence of cross-contamination in Enterobacter cloacae nosocomial infections. J Hosp Infect. 1992 Jun;21(2):95–101. doi: 10.1016/0195-6701(92)90028-k. [DOI] [PubMed] [Google Scholar]
- Bingen E., Denamur E., Picard B., Goullet P., Lambert-Zechovsky N., Foucaud P., Navarro J., Elion J. Molecular epidemiological analysis of Pseudomonas aeruginosa strains causing failure of antibiotic therapy in cystic fibrosis patients. Eur J Clin Microbiol Infect Dis. 1992 May;11(5):432–437. doi: 10.1007/BF01961858. [DOI] [PubMed] [Google Scholar]
- Blanc D. S., Siegrist H. H., Sahli R., Francioli P. Ribotyping of Pseudomonas aeruginosa: discriminatory power and usefulness as a tool for epidemiological studies. J Clin Microbiol. 1993 Jan;31(1):71–77. doi: 10.1128/jcm.31.1.71-77.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg H. M., Kiehlbauch J. A., Wachsmuth I. K. Molecular epidemiology of Yersinia enterocolitica O:3 infections: use of chromosomal DNA restriction fragment length polymorphisms of rRNA genes. J Clin Microbiol. 1991 Nov;29(11):2368–2374. doi: 10.1128/jcm.29.11.2368-2374.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg H. M., Rimland D., Kiehlbauch J. A., Terry P. M., Wachsmuth I. K. Epidemiologic typing of Staphylococcus aureus by DNA restriction fragment length polymorphisms of rRNA genes: elucidation of the clonal nature of a group of bacteriophage-nontypeable, ciprofloxacin-resistant, methicillin-susceptible S. aureus isolates. J Clin Microbiol. 1992 Feb;30(2):362–369. doi: 10.1128/jcm.30.2.362-369.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg H. M., Stephens D. S., Licitra C., Pigott N., Facklam R., Swaminathan B., Wachsmuth I. K. Molecular epidemiology of group B streptococcal infections: use of restriction endonuclease analysis of chromosomal DNA and DNA restriction fragment length polymorphisms of ribosomal RNA genes (ribotyping). J Infect Dis. 1992 Sep;166(3):574–579. doi: 10.1093/infdis/166.3.574. [DOI] [PubMed] [Google Scholar]
- Boros I., Kiss A., Venetianer P. Physical map of the seven ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1979;6(5):1817–1830. doi: 10.1093/nar/6.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukadida J., De Montalembert M., Lenoir G., Scheinmann P., Véron M., Berche P. Molecular epidemiology of chronic pulmonary colonisation by Pseudomonas aeruginosa in cystic fibrosis. J Med Microbiol. 1993 Jan;38(1):29–33. doi: 10.1099/00222615-38-1-29. [DOI] [PubMed] [Google Scholar]
- Charnock C., Bergan T. Multilocus enzyme electrophoresis of major O-antigen reference strains of Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 1992 Sep;11(9):810–816. doi: 10.1007/BF01960880. [DOI] [PubMed] [Google Scholar]
- Clark N. C., Hill B. C., O'Hara C. M., Steingrimsson O., Cooksey R. C. Epidemiologic typing of Enterobacter sakazakii in two neonatal nosocomial outbreaks. Diagn Microbiol Infect Dis. 1990 Nov-Dec;13(6):467–472. doi: 10.1016/0732-8893(90)90078-a. [DOI] [PubMed] [Google Scholar]
- Cookson B. D., Stapleton P., Ludlam H. Ribotyping of coagulase-negative staphylococci. J Med Microbiol. 1992 Jun;36(6):414–419. doi: 10.1099/00222615-36-6-414. [DOI] [PubMed] [Google Scholar]
- De Buyser M. L., Morvan A., Grimont F., el Solh N. Characterization of Staphylococcus species by ribosomal RNA gene restriction patterns. J Gen Microbiol. 1989 Apr;135(4):989–999. doi: 10.1099/00221287-135-4-989. [DOI] [PubMed] [Google Scholar]
- Denamur E., Picard-Pasquier N., Mura C., Picard B., Orfila J., Krishnamoorthy R. Comparison of molecular epidemiological tools for Branhamella catarrhalis typing. Res Microbiol. 1991 Jun;142(5):585–589. doi: 10.1016/0923-2508(91)90191-c. [DOI] [PubMed] [Google Scholar]
- Denamur E., Picard B., Goullet P., Bingen E., Lambert N., Elion J. Complexity of Pseudomonas aeruginosa infection in cystic fibrosis: combined results from esterase electrophoresis and rDNA restriction fragment length polymorphism analysis. Epidemiol Infect. 1991 Jun;106(3):531–539. doi: 10.1017/s0950268800067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning D. W., Baker C. J., Troup N. J., Tompkins L. S. Restriction endonuclease analysis of human and bovine group B streptococci for epidemiologic study. J Clin Microbiol. 1989 Jun;27(6):1352–1356. doi: 10.1128/jcm.27.6.1352-1356.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkshoorn L., Aucken H. M., Gerner-Smidt P., Kaufmann M. E., Ursing J., Pitt T. L. Correlation of typing methods for Acinetobacter isolates from hospital outbreaks. J Clin Microbiol. 1993 Mar;31(3):702–705. doi: 10.1128/jcm.31.3.702-705.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban E., Snipes K., Hird D., Kasten R., Kinde H. Use of ribotyping for characterization of Salmonella serotypes. J Clin Microbiol. 1993 Feb;31(2):233–237. doi: 10.1128/jcm.31.2.233-237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruque S. M., Haider K., Rahman M. M., Abdul Alim A. R., Ahmad Q. S., Albert M. J., Sack R. B. Differentiation of Shigella flexneri strains by rRNA gene restriction patterns. J Clin Microbiol. 1992 Nov;30(11):2996–2999. doi: 10.1128/jcm.30.11.2996-2999.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner P., Ebel J. P. Observations on the primary structure of the 23S ribosomal RNA from E. coli. Nature. 1970 Mar 21;225(5238):1131–1132. doi: 10.1038/2251131a0. [DOI] [PubMed] [Google Scholar]
- Ferdows M. S., Barbour A. G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes K. J., Bruce K. D., Jordens J. Z., Ball A., Pennington T. H. Rapid methods in bacterial DNA fingerprinting. J Gen Microbiol. 1991 Sep;137(9):2051–2058. doi: 10.1099/00221287-137-9-2051. [DOI] [PubMed] [Google Scholar]
- Garaizar J., Kaufmann M. E., Pitt T. L. Comparison of ribotyping with conventional methods for the type identification of Enterobacter cloacae. J Clin Microbiol. 1991 Jul;29(7):1303–1307. doi: 10.1128/jcm.29.7.1303-1307.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner-Smidt P. Ribotyping of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J Clin Microbiol. 1992 Oct;30(10):2680–2685. doi: 10.1128/jcm.30.10.2680-2685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordillo M. E., Singh K. V., Murray B. E. Comparison of ribotyping and pulsed-field gel electrophoresis for subspecies differentiation of strains of Enterococcus faecalis. J Clin Microbiol. 1993 Jun;31(6):1570–1574. doi: 10.1128/jcm.31.6.1570-1574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattard F., Gaudin O. G., Pozzetto B., Ros A., Mbida A. D. Genotypic homogeneity of nosocomial Pseudomonas aeruginosa O12 strains demonstrated by analysis of protein profiles, DNA fingerprints and rRNA gene restriction patterns. Eur J Clin Microbiol Infect Dis. 1993 Jan;12(1):57–61. doi: 10.1007/BF01997061. [DOI] [PubMed] [Google Scholar]
- Graves L. M., Swaminathan B., Reeves M. W., Wenger J. Ribosomal DNA fingerprinting of Listeria monocytogenes using a digoxigenin-labeled DNA probe. Eur J Epidemiol. 1991 Jan;7(1):77–82. doi: 10.1007/BF00221345. [DOI] [PubMed] [Google Scholar]
- Grimont F., Chevrier D., Grimont P. A., Lefevre M., Guesdon J. L. Acetylaminofluorene-labelled ribosomal RNA for use in molecular epidemiology and taxonomy. Res Microbiol. 1989 Sep;140(7):447–454. doi: 10.1016/0923-2508(89)90065-x. [DOI] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Grimont F., Lefèvre M., Ageron E., Grimont P. A. rRNA gene restriction patterns of Legionella species: a molecular identification system. Res Microbiol. 1989 Nov-Dec;140(9):615–626. doi: 10.1016/0923-2508(89)90193-9. [DOI] [PubMed] [Google Scholar]
- Grothues D., Koopmann U., von der Hardt H., Tümmler B. Genome fingerprinting of Pseudomonas aeruginosa indicates colonization of cystic fibrosis siblings with closely related strains. J Clin Microbiol. 1988 Oct;26(10):1973–1977. doi: 10.1128/jcm.26.10.1973-1977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner E., Kropec A., Huebner J., Altwegg M., Daschner F. Ribotyping of Pseudomonas aeruginosa strains isolated from surgical intensive care patients. J Infect Dis. 1993 May;167(5):1216–1220. doi: 10.1093/infdis/167.5.1216. [DOI] [PubMed] [Google Scholar]
- Gustaferro C. A., Persing D. H. Chemiluminescent universal probe for bacterial ribotyping. J Clin Microbiol. 1992 Apr;30(4):1039–1041. doi: 10.1128/jcm.30.4.1039-1041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel U. B., Geiser A., Stanbridge E. J. Oligonucleotide probes complementary to variable regions of ribosomal RNA discriminate between Mycoplasma species. J Gen Microbiol. 1987 Jul;133(7):1969–1974. doi: 10.1099/00221287-133-7-1969. [DOI] [PubMed] [Google Scholar]
- Hadorn K., Lenz W., Kayser F. H., Shalit I., Krasemann C. Use of a ribosomal RNA gene probe for the epidemiological study of methicillin and ciprofloxacin resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1990 Sep;9(9):649–653. doi: 10.1007/BF01964265. [DOI] [PubMed] [Google Scholar]
- Hall L. M., Duke B., Guiney M., Williams R. Typing of Enterococcus species by DNA restriction fragment analysis. J Clin Microbiol. 1992 Apr;30(4):915–919. doi: 10.1128/jcm.30.4.915-919.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidt A., Monteil H., Richard C. O and H serotyping of Pseudomonas cepacia. J Clin Microbiol. 1983 Sep;18(3):738–740. doi: 10.1128/jcm.18.3.738-740.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. W., Harnish B. W. Inversions between ribosomal RNA genes of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7069–7072. doi: 10.1073/pnas.78.11.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa-Ahumada M., Swaminathan B., Hunter S. B., Cameron D. N., Kiehlbauch J. A., Wachsmuth I. K., Strockbine N. A. Restriction fragment length polymorphisms in rRNA operons for subtyping Shigella sonnei. J Clin Microbiol. 1991 Nov;29(11):2380–2384. doi: 10.1128/jcm.29.11.2380-2384.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey B., Hall J. Hypervariable DNA fingerprinting in Escherichia coli: minisatellite probe from bacteriophage M13. J Bacteriol. 1989 May;171(5):2528–2532. doi: 10.1128/jb.171.5.2528-2532.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irino K., Grimont F., Casin I., Grimont P. A. rRNA gene restriction patterns of Haemophilus influenzae biogroup aegyptius strains associated with Brazilian purpuric fever. J Clin Microbiol. 1988 Aug;26(8):1535–1538. doi: 10.1128/jcm.26.8.1535-1538.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard N. C., Hächler H., Grehn M., Kayser F. H. Ribotyping of coagulase-negative staphylococci with special emphasis on intraspecific typing of Staphylococcus epidermidis. J Clin Microbiol. 1992 Apr;30(4):817–823. doi: 10.1128/jcm.30.4.817-823.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarlier V., Nicolas M. H., Fournier G., Philippon A. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988 Jul-Aug;10(4):867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- Jarry B., Rosset R. Localization of some 5s RNA cistrons on Escherichia coli chromosome. Mol Gen Genet. 1973 Mar 1;121(2):151–162. doi: 10.1007/BF00277529. [DOI] [PubMed] [Google Scholar]
- John J. F., Jr Molecular analysis of nosocomial epidemics. Infect Dis Clin North Am. 1989 Dec;3(4):683–700. [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M. Polymorphism of DNA sequence adjacent to human beta-globin structural gene: relationship to sickle mutation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5631–5635. doi: 10.1073/pnas.75.11.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl S. A., Avise J. C. Balancing selection at allozyme loci in oysters: implications from nuclear RFLPs. Science. 1992 Apr 3;256(5053):100–102. doi: 10.1126/science.1348870. [DOI] [PubMed] [Google Scholar]
- Kiehlbauch J. A., Plikaytis B. D., Swaminathan B., Cameron D. N., Wachsmuth I. K. Restriction fragment length polymorphisms in the ribosomal genes for species identification and subtyping of aerotolerant Campylobacter species. J Clin Microbiol. 1991 Aug;29(8):1670–1676. doi: 10.1128/jcm.29.8.1670-1676.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebe C., Nies B. A., Meyer J. F., Tolxdorff-Neutzling R. M., Wiedemann B. Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1985 Aug;28(2):302–307. doi: 10.1128/aac.28.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblavi S., Grimont F., Grimont P. A. Clonal diversity of Vibrio cholerae O1 evidenced by rRNA gene restriction patterns. Res Microbiol. 1990 Jul-Aug;141(6):645–657. doi: 10.1016/0923-2508(90)90059-y. [DOI] [PubMed] [Google Scholar]
- Kostman J. R., Edlind T. D., LiPuma J. J., Stull T. L. Molecular epidemiology of Pseudomonas cepacia determined by polymerase chain reaction ribotyping. J Clin Microbiol. 1992 Aug;30(8):2084–2087. doi: 10.1128/jcm.30.8.2084-2087.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B., Kornblum J., Arbeit R. D., Eisner W., Maslow J. N., McGeer A., Low D. E., Novick R. P. Evidence for a clonal origin of methicillin resistance in Staphylococcus aureus. Science. 1993 Jan 8;259(5092):227–230. doi: 10.1126/science.8093647. [DOI] [PubMed] [Google Scholar]
- Lambert-Zechovsky N., Bingen E., Denamur E., Brahimi N., Brun P., Mathieu H., Elion J. Molecular analysis provides evidence for the endogenous origin of bacteremia and meningitis due to Enterobacter cloacae in an infant. Clin Infect Dis. 1992 Jul;15(1):30–32. doi: 10.1093/clinids/15.1.30. [DOI] [PubMed] [Google Scholar]
- Lasker B. A., Brown J. M., McNeil M. M. Identification and epidemiological typing of clinical and environmental isolates of the genus Rhodococcus with use of a digoxigenin-labeled rDNA gene probe. Clin Infect Dis. 1992 Aug;15(2):223–233. doi: 10.1093/clinids/15.2.223. [DOI] [PubMed] [Google Scholar]
- Levin M. H., Weinstein R. A., Nathan C., Selander R. K., Ochman H., Kabins S. A. Association of infection caused by Pseudomonas aeruginosa serotype O11 with intravenous abuse of pentazocine mixed with tripelennamine. J Clin Microbiol. 1984 Oct;20(4):758–762. doi: 10.1128/jcm.20.4.758-762.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiPuma J. J., Dasen S. E., Nielson D. W., Stern R. C., Stull T. L. Person-to-person transmission of Pseudomonas cepacia between patients with cystic fibrosis. Lancet. 1990 Nov 3;336(8723):1094–1096. doi: 10.1016/0140-6736(90)92571-x. [DOI] [PubMed] [Google Scholar]
- LiPuma J. J., Fisher M. C., Dasen S. E., Mortensen J. E., Stull T. L. Ribotype stability of serial pulmonary isolates of Pseudomonas cepacia. J Infect Dis. 1991 Jul;164(1):133–136. doi: 10.1093/infdis/164.1.133. [DOI] [PubMed] [Google Scholar]
- LiPuma J. J., Mortensen J. E., Dasen S. E., Edlind T. D., Schidlow D. V., Burns J. L., Stull T. L. Ribotype analysis of Pseudomonas cepacia from cystic fibrosis treatment centers. J Pediatr. 1988 Nov;113(5):859–862. doi: 10.1016/s0022-3476(88)80018-0. [DOI] [PubMed] [Google Scholar]
- LiPuma J. J., Stull T. L., Dasen S. E., Pidcock K. A., Kaye D., Korzeniowski O. M. DNA polymorphisms among Escherichia coli isolated from bacteriuric women. J Infect Dis. 1989 Mar;159(3):526–532. doi: 10.1093/infdis/159.3.526. [DOI] [PubMed] [Google Scholar]
- Liu P. V. Changes in somatic antigens of Pseudomonas aeruginosa induced by bacteriophages. J Infect Dis. 1969 Mar;119(3):237–246. doi: 10.1093/infdis/119.3.237. [DOI] [PubMed] [Google Scholar]
- Loutit J. S., Tompkins L. S. Restriction enzyme and Southern hybridization analyses of Pseudomonas aeruginosa strains from patients with cystic fibrosis. J Clin Microbiol. 1991 Dec;29(12):2897–2900. doi: 10.1128/jcm.29.12.2897-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lányi B., Lantos J. Antigenic changes in Pseudomonas aeruginosa in vivo and after lysogenization in vitro. Acta Microbiol Acad Sci Hung. 1976;23(4):337–351. [PubMed] [Google Scholar]
- Mariani-Kurkdjian P., Denamur E., Milon A., Picard B., Cave H., Lambert-Zechovsky N., Loirat C., Goullet P., Sansonetti P. J., Elion J. Identification of a clone of Escherichia coli O103:H2 as a potential agent of hemolytic-uremic syndrome in France. J Clin Microbiol. 1993 Feb;31(2):296–301. doi: 10.1128/jcm.31.2.296-301.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz S. M., Veazey J. M., Jr, Macrina F. L., Mayhall C. G., Lamb V. A. Sequential outbreaks of infection due to Klebsiella pneumoniae in a neonatal intensive care unit: implication of a conjugative R plasmid. J Infect Dis. 1980 Jul;142(1):106–112. doi: 10.1093/infdis/142.1.106. [DOI] [PubMed] [Google Scholar]
- Martinetti G., Altwegg M. rRNA gene restriction patterns and plasmid analysis as a tool for typing Salmonella enteritidis. Res Microbiol. 1990 Nov-Dec;141(9):1151–1162. doi: 10.1016/0923-2508(90)90088-8. [DOI] [PubMed] [Google Scholar]
- Mayer L. W. Use of plasmid profiles in epidemiologic surveillance of disease outbreaks and in tracing the transmission of antibiotic resistance. Clin Microbiol Rev. 1988 Apr;1(2):228–243. doi: 10.1128/cmr.1.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurier S. I., Wernars K. Typing of Listeria strains by random amplification of polymorphic DNA. Res Microbiol. 1992 Jun;143(5):499–505. doi: 10.1016/0923-2508(92)90096-7. [DOI] [PubMed] [Google Scholar]
- Morgan E. A., Ikemura T., Lindahl L., Fallon A. M., Nomura M. Some rRNA operons in E. coli have tRNA genes at their distal ends. Cell. 1978 Feb;13(2):335–344. doi: 10.1016/0092-8674(78)90202-7. [DOI] [PubMed] [Google Scholar]
- Morgan E. A., Ikemura T., Nomura M. Identification of spacer tRNA genes in individual ribosomal RNA transcription units of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2710–2714. doi: 10.1073/pnas.74.7.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moureau P., Derclaye I., Gregoire D., Janssen M., Cornelis G. R. Campylobacter species identification based on polymorphism of DNA encoding rRNA. J Clin Microbiol. 1989 Jul;27(7):1514–1517. doi: 10.1128/jcm.27.7.1514-1517.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer N. P., Luccini G. M., Holcomb L. A., Hall N. H., Altwegg M. Application of ribotyping for differentiating aeromonads isolated from clinical and environmental sources. Appl Environ Microbiol. 1992 Jun;58(6):1940–1944. doi: 10.1128/aem.58.6.1940-1944.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastasi A., Mammina C., Villafrate M. R. rDNA fingerprinting as a tool in epidemiological analysis of Salmonella typhi infections. Epidemiol Infect. 1991 Dec;107(3):565–576. doi: 10.1017/s0950268800049268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Assembly of bacterial ribosomes. Science. 1973 Mar 2;179(4076):864–873. doi: 10.1126/science.179.4076.864. [DOI] [PubMed] [Google Scholar]
- O'Brien T. F., Ross D. G., Guzman M. A., Medeiros A. A., Hedges R. W., Botstein D. Dissemination of an antibiotic resistance plasmid in hospital patient flora. Antimicrob Agents Chemother. 1980 Apr;17(4):537–543. doi: 10.1128/aac.17.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle J. W., Janda J. M., Woods D. E., Vasil M. L. Characterization and use of a DNA probe as an epidemiological marker for Pseudomonas aeruginosa. J Infect Dis. 1987 Jan;155(1):119–126. doi: 10.1093/infdis/155.1.119. [DOI] [PubMed] [Google Scholar]
- Ojeniyi B., Baek L., Høiby N. Polyagglutinability due to loss of O-antigenic determinants in Pseudomonas aeruginosa strains isolated from cystic fibrosis patients. Acta Pathol Microbiol Immunol Scand B. 1985 Feb;93(1):7–13. doi: 10.1111/j.1699-0463.1985.tb02844.x. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Beck A., Dayal P. A., Dawson C. Detection of genomic variation in Providencia stuartii clinical isolates by analysis of DNA restriction fragment length polymorphisms containing rRNA cistrons. J Clin Microbiol. 1988 Oct;26(10):2161–2166. doi: 10.1128/jcm.26.10.2161-2166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J. Chromosomal DNA fingerprinting--a new method of species and strain identification applicable to microbial pathogens. J Med Microbiol. 1989 Oct;30(2):89–99. doi: 10.1099/00222615-30-2-89. [DOI] [PubMed] [Google Scholar]
- Pace N. R., Olsen G. J., Woese C. R. Ribosomal RNA phylogeny and the primary lines of evolutionary descent. Cell. 1986 May 9;45(3):325–326. doi: 10.1016/0092-8674(86)90315-6. [DOI] [PubMed] [Google Scholar]
- Panlilio A. L., Beck-Sague C. M., Siegel J. D., Anderson R. L., Yetts S. Y., Clark N. C., Duer P. N., Thomassen K. A., Vess R. W., Hill B. C. Infections and pseudoinfections due to povidone-iodine solution contaminated with Pseudomonas cepacia. Clin Infect Dis. 1992 May;14(5):1078–1083. doi: 10.1093/clinids/14.5.1078. [DOI] [PubMed] [Google Scholar]
- Patterson T. F., Patterson J. E., Masecar B. L., Barden G. E., Hierholzer W. J., Jr, Zervos M. J. A nosocomial outbreak of Branhamella catarrhalis confirmed by restriction endonuclease analysis. J Infect Dis. 1988 May;157(5):996–1001. doi: 10.1093/infdis/157.5.996. [DOI] [PubMed] [Google Scholar]
- Patton C. M., Wachsmuth I. K., Evins G. M., Kiehlbauch J. A., Plikaytis B. D., Troup N., Tompkins L., Lior H. Evaluation of 10 methods to distinguish epidemic-associated Campylobacter strains. J Clin Microbiol. 1991 Apr;29(4):680–688. doi: 10.1128/jcm.29.4.680-688.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegues D. A., Carson L. A., Anderson R. L., Norgard M. J., Argent T. A., Jarvis W. R., Woernle C. H. Outbreak of Pseudomonas cepacia bacteremia in oncology patients. Clin Infect Dis. 1993 Mar;16(3):407–411. doi: 10.1093/clind/16.3.407. [DOI] [PubMed] [Google Scholar]
- Picard-Pasquier N., Ouagued M., Picard B., Goullet P., Krishnamoorthy R. A simple, sensitive method of analyzing bacterial ribosomal DNA polymorphism. Electrophoresis. 1989 Mar;10(3):186–189. doi: 10.1002/elps.1150100306. [DOI] [PubMed] [Google Scholar]
- Picard-Pasquier N., Picard B., Heeralal S., Krishnamoorthy R., Goullet P. Correlation between ribosomal DNA polymorphism and electrophoretic enzyme polymorphism in Yersinia. J Gen Microbiol. 1990 Aug;136(8):1655–1666. doi: 10.1099/00221287-136-8-1655. [DOI] [PubMed] [Google Scholar]
- Picard B., Picard-Pasquier N., Krishnamoorthy R., Goullet P. Characterization of highly virulent Escherichia coli strains by ribosomal DNA restriction fragment length polymorphism. FEMS Microbiol Lett. 1991 Aug 1;66(2):183–188. doi: 10.1016/0378-1097(91)90330-d. [DOI] [PubMed] [Google Scholar]
- Pignato S., Giammanco G., Grimont F., Grimont P. A. Molecular typing of Salmonella enterica subsp. enterica serovar Wien by rRNA gene restriction patterns. Res Microbiol. 1992 Sep;143(7):703–709. doi: 10.1016/0923-2508(92)90065-v. [DOI] [PubMed] [Google Scholar]
- Pitcher D., Johnson A., Allerberger F., Woodford N., George R. An investigation of nosocomial infection with Corynebacterium jeikeium in surgical patients using a ribosomal RNA gene probe. Eur J Clin Microbiol Infect Dis. 1990 Sep;9(9):643–648. doi: 10.1007/BF01964264. [DOI] [PubMed] [Google Scholar]
- Pitt T. L. Epidemiological typing of Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 1988 Apr;7(2):238–247. doi: 10.1007/BF01963095. [DOI] [PubMed] [Google Scholar]
- Pitt T. L., Livermore D. M., Pitcher D., Vatopoulos A. C., Legakis N. J. Multiresistant serotype O 12 Pseudomonas aeruginosa: evidence for a common strain in Europe. Epidemiol Infect. 1989 Dec;103(3):565–576. doi: 10.1017/s095026880003096x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh C. L., Yeo C. C., Tay L. Genome fingerprinting by pulsed-field gel electrophoresis and ribotyping to differentiate Pseudomonas aeruginosa serotype O11 strains. Eur J Clin Microbiol Infect Dis. 1992 Sep;11(9):817–822. doi: 10.1007/BF01960881. [DOI] [PubMed] [Google Scholar]
- Postic D., Edlinger C., Richaud C., Grimont F., Dufresne Y., Perolat P., Baranton G., Grimont P. A. Two genomic species in Borrelia burgdorferi. Res Microbiol. 1990 May;141(4):465–475. doi: 10.1016/0923-2508(90)90072-x. [DOI] [PubMed] [Google Scholar]
- Preheim L., Pitcher D., Owen R., Cookson B. Typing of methicillin resistant and susceptible Staphylococcus aureus strains by ribosomal RNA gene restriction patterns using a biotinylated probe. Eur J Clin Microbiol Infect Dis. 1991 May;10(5):428–436. doi: 10.1007/BF01968023. [DOI] [PubMed] [Google Scholar]
- Prevost G., Jaulhac B., Piemont Y. DNA fingerprinting by pulsed-field gel electrophoresis is more effective than ribotyping in distinguishing among methicillin-resistant Staphylococcus aureus isolates. J Clin Microbiol. 1992 Apr;30(4):967–973. doi: 10.1128/jcm.30.4.967-973.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérolat P., Grimont F., Regnault B., Grimont P. A., Fournié E., Thevenet H., Baranton G. rRNA gene restriction patterns of Leptospira: a molecular typing system. Res Microbiol. 1990 Feb;141(2):159–171. doi: 10.1016/0923-2508(90)90025-l. [DOI] [PubMed] [Google Scholar]
- Rabkin C. S., Jarvis W. R., Anderson R. L., Govan J., Klinger J., LiPuma J., Martone W. J., Monteil H., Richard C., Shigeta S. Pseudomonas cepacia typing systems: collaborative study to assess their potential in epidemiologic investigations. Rev Infect Dis. 1989 Jul-Aug;11(4):600–607. doi: 10.1093/clinids/11.4.600. [DOI] [PubMed] [Google Scholar]
- Richard C., Monteil H., Mégraud F., Chatelain R., Laurent B. Caractères phénotypiques de 100 souches de Pseudomonas cepacia. Proposition d'un schéma de biovars. Ann Biol Clin (Paris) 1981;39(1):9–15. [PubMed] [Google Scholar]
- Richet H. M., Craven P. C., Brown J. M., Lasker B. A., Cox C. D., McNeil M. M., Tice A. D., Jarvis W. R., Tablan O. C. A cluster of Rhodococcus (Gordona) Bronchialis sternal-wound infections after coronary-artery bypass surgery. N Engl J Med. 1991 Jan 10;324(2):104–109. doi: 10.1056/NEJM199101103240206. [DOI] [PubMed] [Google Scholar]
- Samadpour M., Moseley S. L., Lory S. Biotinylated DNA probes for exotoxin A and pilin genes in the differentiation of Pseudomonas aeruginosa strains. J Clin Microbiol. 1988 Nov;26(11):2319–2323. doi: 10.1128/jcm.26.11.2319-2323.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders N. A., Harrison T. G., Kachwalla N., Taylor A. G. Identification of species of the genus Legionella using a cloned rRNA gene from Legionella pneumophila. J Gen Microbiol. 1988 Aug;134(8):2363–2374. doi: 10.1099/00221287-134-8-2363. [DOI] [PubMed] [Google Scholar]
- Schoonmaker D., Heimberger T., Birkhead G. Comparison of ribotyping and restriction enzyme analysis using pulsed-field gel electrophoresis for distinguishing Legionella pneumophila isolates obtained during a nosocomial outbreak. J Clin Microbiol. 1992 Jun;30(6):1491–1498. doi: 10.1128/jcm.30.6.1491-1498.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot D., Sirot J., Labia R., Morand A., Courvalin P., Darfeuille-Michaud A., Perroux R., Cluzel R. Transferable resistance to third-generation cephalosporins in clinical isolates of Klebsiella pneumoniae: identification of CTX-1, a novel beta-lactamase. J Antimicrob Chemother. 1987 Sep;20(3):323–334. doi: 10.1093/jac/20.3.323. [DOI] [PubMed] [Google Scholar]
- Soto A., Pitcher D. G., Soriano F. A numerical analysis of ribosomal RNA gene patterns for typing clinical isolates of Corynebacterium group D2. Epidemiol Infect. 1991 Oct;107(2):263–272. doi: 10.1017/s0950268800048913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Campbell M. E., Farmer S. W., Volpel K., Joffe A. M., Paranchych W. Use of a pilin gene probe to study molecular epidemiology of Pseudomonas aeruginosa. J Clin Microbiol. 1989 Nov;27(11):2589–2593. doi: 10.1128/jcm.27.11.2589-2593.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreadbury C. L., Bainbridge B. W., Cohen J. Restriction fragment length polymorphisms in isolates of Aspergillus fumigatus probed with part of the intergenic spacer region from the ribosomal RNA gene complex of Aspergillus nidulans. J Gen Microbiol. 1990 Oct;136(10):1991–1994. doi: 10.1099/00221287-136-10-1991. [DOI] [PubMed] [Google Scholar]
- Strockbine N. A., Parsonnet J., Greene K., Kiehlbauch J. A., Wachsmuth I. K. Molecular epidemiologic techniques in analysis of epidemic and endemic Shigella dysenteriae type 1 strains. J Infect Dis. 1991 Feb;163(2):406–409. doi: 10.1093/infdis/163.2.406. [DOI] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Tablan O. C., Chorba T. L., Schidlow D. V., White J. W., Hardy K. A., Gilligan P. H., Morgan W. M., Carson L. A., Martone W. J., Jason J. M. Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors and clinical outcome. J Pediatr. 1985 Sep;107(3):382–387. doi: 10.1016/s0022-3476(85)80511-4. [DOI] [PubMed] [Google Scholar]
- Taylor R. F., Dalla Costa L., Kaufmann M. E., Pitt T. L., Hodson M. E. Pseudomonas cepacia pulmonary infection in adults with cystic fibrosis: is nosocomial acquisition occurring? J Hosp Infect. 1992 Jul;21(3):199–204. doi: 10.1016/0195-6701(92)90076-x. [DOI] [PubMed] [Google Scholar]
- Thomson-Carter F. M., Carter P. E., Pennington T. H. Differentiation of staphylococcal species and strains by ribosomal RNA gene restriction patterns. J Gen Microbiol. 1989 Jul;135(7):2093–2097. doi: 10.1099/00221287-135-7-2093. [DOI] [PubMed] [Google Scholar]
- Tompkins L. S. The use of molecular methods in infectious diseases. N Engl J Med. 1992 Oct 29;327(18):1290–1297. doi: 10.1056/NEJM199210293271808. [DOI] [PubMed] [Google Scholar]
- Tram C., Simonet M., Nicolas M. H., Offredo C., Grimont F., Lefevre M., Ageron E., Debure A., Grimont P. A. Molecular typing of nosocomial isolates of Legionella pneumophila serogroup 3. J Clin Microbiol. 1990 Feb;28(2):242–245. doi: 10.1128/jcm.28.2.242-245.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tümmler B., Koopmann U., Grothues D., Weissbrodt H., Steinkamp G., von der Hardt H. Nosocomial acquisition of Pseudomonas aeruginosa by cystic fibrosis patients. J Clin Microbiol. 1991 Jun;29(6):1265–1267. doi: 10.1128/jcm.29.6.1265-1267.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M. Q., Groth D. M., Mendis A. H., Sampson J., Wetherall J. D., Grubb W. B. Typing of methicillin-resistant Staphylococcus aureus with an M13 repeat probe. J Hosp Infect. 1992 Apr;20(4):233–245. doi: 10.1016/0195-6701(92)90002-4. [DOI] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990 Dec 25;18(24):7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990 Nov 25;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton J., Jung K., Vedin I., Aronsson B., Flock J. I. Comparative evaluation of a new molecular method for typing Staphylococcus epidermidis. Eur J Clin Microbiol Infect Dis. 1992 Jun;11(6):515–521. doi: 10.1007/BF01960806. [DOI] [PubMed] [Google Scholar]
- Wolz C., Kiosz G., Ogle J. W., Vasil M. L., Schaad U., Botzenhart K., Döring G. Pseudomonas aeruginosa cross-colonization and persistence in patients with cystic fibrosis. Use of a DNA probe. Epidemiol Infect. 1989 Apr;102(2):205–214. doi: 10.1017/s0950268800029873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford N., Morrison D., Johnson A. P., Briant V., George R. C., Cookson B. Application of DNA probes for rRNA and vanA genes to investigation of a nosocomial cluster of vancomycin-resistant enterococci. J Clin Microbiol. 1993 Mar;31(3):653–658. doi: 10.1128/jcm.31.3.653-658.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods T. C., Helsel L. O., Swaminathan B., Bibb W. F., Pinner R. W., Gellin B. G., Collin S. F., Waterman S. H., Reeves M. W., Brenner D. J. Characterization of Neisseria meningitidis serogroup C by multilocus enzyme electrophoresis and ribosomal DNA restriction profiles (ribotyping). J Clin Microbiol. 1992 Jan;30(1):132–137. doi: 10.1128/jcm.30.1.132-137.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev D., Halachmi D., Kenny G. E., Razin S. Distinction of species and strains of mycoplasmas (mollicutes) by genomic DNA fingerprints with an rRNA gene probe. J Clin Microbiol. 1988 Jun;26(6):1198–1201. doi: 10.1128/jcm.26.6.1198-1201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ketel R. J., de Wever B. Genetic typing in a cluster of Legionella pneumophila infections. J Clin Microbiol. 1989 May;27(5):1105–1107. doi: 10.1128/jcm.27.5.1105-1107.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]