Abstract
Cell therapies are potential alternatives to organ transplantation for liver failure or dysfunction but are compromised by inefficient engraftment, cell dispersal to ectopic sites, and emboli formation. Grafting strategies have been devised for transplantation of human hepatic stem cells (hHpSCs) embedded into a mix of soluble signals and extracellular matrix biomaterials (hyaluronans, type III collagen, laminin) found in stem cell niches. The hHpSCs maintain a stable stem cell phenotype under the graft conditions. The grafts were transplanted into the livers of immuno-compromised murine hosts with and without carbon tetrachloride treatment to assess the effects of quiescent versus injured liver conditions. Grafted cells remained localized to the livers resulting in a larger bolus of engrafted cells in the host livers under quiescent conditions and with potential for more rapid expansion under injured liver conditions. By contrast, transplantation by direct injection or via a vascular route resulted in inefficient engraftment and cell dispersal to ectopic sites. Transplantation by grafting is proposed as a preferred strategy for cell therapies for solid organs such as liver.
Keywords: Hepatic Stem Cells, Cell Transplantation, Hyaluronans, Grafting, Regeneration
Patients with severe liver disease are currently managed by orthotopic liver transplantation (OLT). Due to the paucity of available liver donors and morbidity associated with this surgical procedure, alternative therapies are under investigation. These include liver cell therapies in which liver cell suspensions are transplanted by a vascular route or by direct injection into the liver with hopes of restoring liver functions in the recipient patients (1, 2). Mature liver cell transplantation is emerging as an alternative “bridge” support for patients waiting for a donor organ (3, 4). However, mature hepatocytes are limited by short-term survival, proliferate poorly both in vitro and in vivo, and are difficult to cryopreserve (5–8). To date, clinical trials of liver cell therapies have employed primarily freshly isolated, mature hepatocytes. Despite clinical improvements in patients, significant problems have arisen due to (i) inefficient engraftment, (ii) death or ectopic distribution of cells that didn’t engraft in the target tissue, (iii) emboli formation, (iv) immunological rejection, and (v) transient effects of transplanted cells.
Transplantation of stem and progenitor cell populations, rather than mature hepatocytes, has been recently investigated, exploiting their known proliferative potential. Stem/progenitor cells are minimally immunogenic and readily cryopreserved, but are small and engraft with lower efficiency than the larger mature cells when injected by a vascular route (6, 9, 10). Engraftment is improved if cells are injected directly into the tissue or via the hepatic artery, resulting in up to ~20–30% engrafting efficiency(11). Clinical trials with transplantation of fetal liver-derived cells expressing epithelial cell adhesion molecule (EpCAM), comprising both human hepatic stem cells (hHpSCs) and their descendents, hepatoblasts (hHBs), have revealed no evidence of emboli formation, no need for immunosuppression, improved MELD scores, much longer survival of seriously ill patients, and improved liver functions in all transplanted patients (11).
We propose that clinical programs of liver cell therapies will be best accomplished using stem/progenitor cells in combination with a strategy that optimizes delivery and retention of the cells in the target tissue. A way of achieving cell retention in cell therapies involves grafting strategies by embedding cells in biomaterials that concentrate cells in the target tissue and provide a microenvironment conducive to survival, proliferation, vascularization, and integration into the tissue (12). Optimized grafts for human hepatic stem/progenitors are most likely those using extracellular matrix components and soluble factors found in the liver’s stem cell niches, the canals of Hering (13).
Hyaluronic acid, or hyaluronan (HA), is a non-sulfated glycosaminoglycan (GAG) that makes up a significant portion of the extracellular matrix chemistry of stem cell niches. In these niches, HA is non-covalently associated with laminins, collagens dominant in fetal tissue (e.g. type III collagen), and forms of chondroitin sulfate proteoglycans (CS-PGs) that have minimal sulfation (14, 15). HA is more abundant during cellular expansion/proliferation events such as embryogenesis, wound repair, and organ regeneration (16–18), including hepatic regeneration (19). The chemical structure of HA is conserved across all species, is biocompatible and does not elicit inflammatory, immunologic or toxic responses, making HA an attractive biomaterial in grafting strategies to deliver and retain cells in a regenerative niche graft (20–22). Other matrix components and soluble signals needed for such a graft have been defined in multiple investigations assessing the effects on expansion and differentiation of hHpSCs and hHBs, and include type III collagens and laminins (14, 23, 24).
There are numerous reports about isolation, purification, and characterization of hHpSCs and hHBs found within human livers of all donor ages (13, 25). These two subpopulations of multipotent cells have overlapping but distinguishable antigenic profiles. Both express EpCAM, cytokeratins (CK) 8, 18 and 19, CD133 (prominin), Hedgehog proteins (Indian and Sonic), CXCR4, SOX 17, and SOX 9. The hHpSCs express neural cell adhesion molecule (NCAM) that is absent in hHBs; hHBs express intercellular adhesion molecule (ICAM-1), α-fetoprotein (AFP) and cytochrome P450 A7, all being absent in hHpSCs (13, 14, 25–27). We have established completely defined ex vivo conditions to maintain hHpSCs in culture as self-replicating cells versus lineage restriction to hHBs or to hepatocytic or cholangiocytic phenotypes (14, 24, 28, 29).
In this study, we corroborate the findings in our prior studies that hHpSCs can be cultured and expanded in HA using combinations of appropriate matrix biomaterials and soluble signals that mimic the liver’s stem cell niche. We also show that HA–based grafts containing hHpSCs can be transplanted into hosts, remain localized with minimal or no distribution to ectopic sites, and dramatically improve engraftment efficiency in the target organ over current cell transplantation approaches.
Methods
Hepatic Stem Cell Culture Conditions
Fetal human liver cells were suspended into a serum-free, hormonally defined medium, Kubota’s medium (KM), tailored for stem/progenitors from endodermal tissues (23). Freshly isolated fetal liver cells were plated at 4,000–8,000 cells/cm2 on tissue culture plastic (Becton-Dickinson, Franklin Lakes, N.J.). These culture conditions are not conducive to survival of mature parenchymal or mature mesenchymal cells but only of stem/progenitors from both parenchymal and mesenchymal cell lineages. Cells were plated with KM with 10% fetal bovine serum (FBS) for up to 24 hrs to facilitate attachment. Use of serum-free conditions was essential to keep the hHpSCs and their mesenchymal cell partners, the angioblasts, stable and with the requisite paracrine signaling (14) enabling them to self-replicate. Serum-free KM was changed every 3–4 days. Typical plates have single cells and small clusters of cell that adhere after the initial 24 hrs. Colonies began to appear after 1–2 weeks.
Preparation of Hyaluronans with and without other matrix components
All hyaluronan materials are from Glycosan Biosciences (Salt Lake City, UT; now a subdivision of BioTime, Alameda, CA), and consist of thiol-modified carboxymethyl HA (or CMHA-S), a chemically modified HA derivative with disulfide bridges for cross-linking. The cross-linking is initiated by a PEGDA crosslinker and the level of crosslinking activity and stiffness can be regulated by the amount of PEGDA added(20, 21, 24, 30–33), proven to be a variable in regulating the stem cell fate. The hydrogel substrata were constructed by dissolving dry reagents in KM to give a 2.0% solution (weight/volume) for the HA gels, and the PEGDA crosslinker was dissolved in KM to give a 4.0% weight/volume solution, and allowed to incubate at 37°C to dissolve. Collagen III and laminin from Sigma (St. Louis, MO) were used at a concentration of 1.0 mg/ml. A ratio of 1:4 was applied to blend the cross-linker and hyaluronans.
Cell matrix culture conditions
After three weeks in culture, stem cell colonies, approximating 3000–5,000 cells/colony, were picked and put into suspension. Cell suspensions of 200,000 cells were then combined with hyaluronan-matrix mix. PEGDA cross-linker was added, and the cell matrix material immediately added to wells in a 4-well chamber slide. Once the gel set, an equal amount of Kubota’s Medium was added to the top of the well. Cultures were then maintained for a period of 21 days, with medium changes every 48 hrs. Multiple runs were performed with different liver samples to ensure consistency.
In vivo engraftment with direct injection strategies
Athymic nude, male mice, aged 8–12 weeks, were bred in house at the UNC Animal Care Facility. Animals received care according to the Division of Laboratory Animal Medicine, UNC-CH guidelines, approved by AALAC. All protocols regarding animal care and use were approved by IACUC. Freshly isolated hepatic progenitors were infected for 4 hrs at 37°C with a luciferase-expressing adenoviral vector at 50 POI (Ad-CMV-Luc, Vector Biolabs, Philadelphia, PA). The vector provides intense expression of luciferase for at least 48 hrs and up to 72 hrs. By 72 hrs or soon thereafter, its expression is terminated by silencing mechanisms involving methylation of the promoter (34).
Mice (8–12 weeks) were anesthetized using Ketamine (90–120 mg/kg, Bioniche Pharma, Lake Forrest IL), and Xylazine (10mg/kg, Akorn, Decatur, IL). Survival surgery was performed, opening the abdomen and slowly injecting 1.5 × 106 cells directly into the liver lobe, via cell suspension or grafted using HA hydrogels crosslinked with PEGDA, injected intrahepatically into the front liver lobe. The incision site was closed, and animals were given 0.1 mg/kg Buprenorphine (Reckitt Benckiser Pharmaceuticals, Richmond, VA) every 12 hrs for 48 hrs. Past studies have indicated that transplanting mice with hHpSCs first and then establishing liver failure results in survival of all the transplanted mice, whereas the reverse results in significant loss of mice (25). For liver injury models, a one-time dose of carbon tetrachloride (CCl4, Sigma-Aldrich, St Louis, MO) was administered IP at 0.6 μl/g.
Statistical analysis
Experiments were repeated at least 3 times with duplicate or triplicate samples for each condition. Data from representative experiments are presented where similar trends were seen in multiple trials. Results were presented as mean ± standard error of the mean. Statistical analysis of data was performed by a one-way ANOVA. Significant findings were followed with pair-wise t-tests corrected for multiple comparisons using the step-down Bonferroni method.
Additional methods can be found in the online supplementary information.
Results
Hyaluronan biomaterials are permissive for survival and phenotypic stability of hHpSCs and hHBs
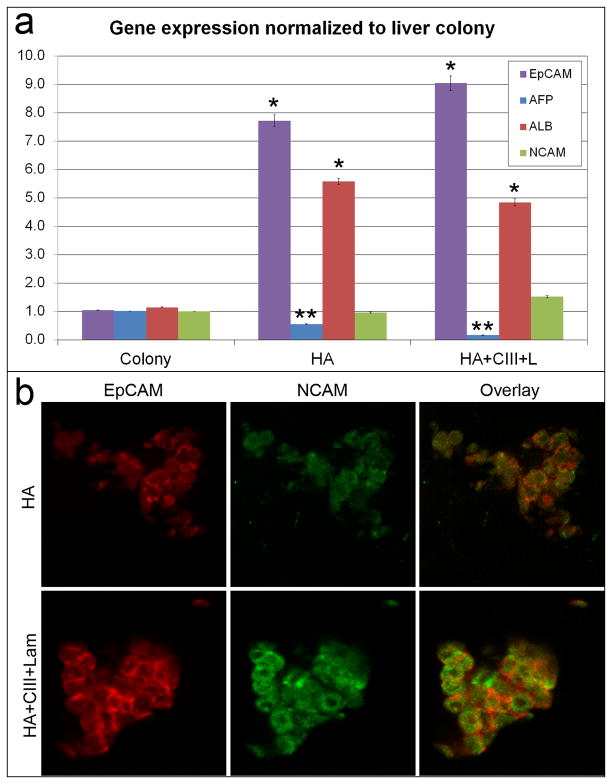
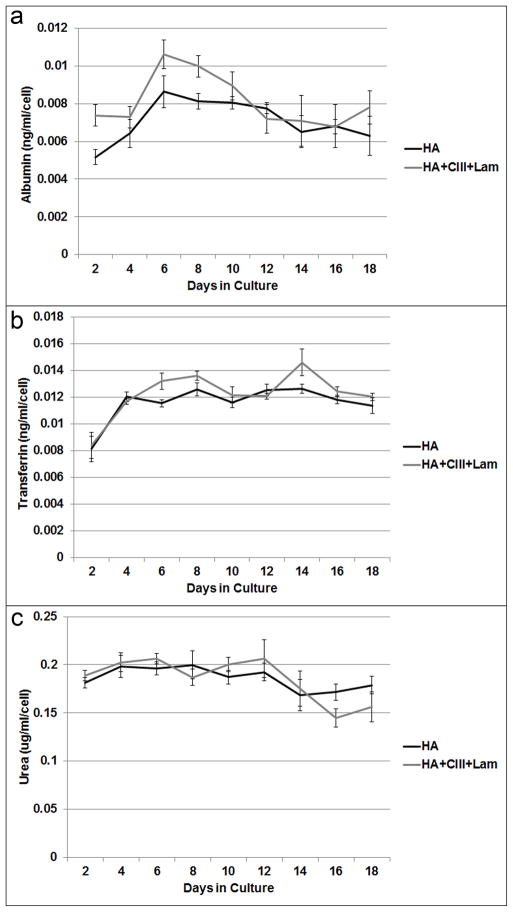
The hHpSCs survived and maintained a stable stem cell phenotype for more than 3 weeks in cultures fed Kubota’s Medium and when embedded within composite matrix biomaterials (HA, type III collagen, laminin), conditions found in stem cell niches. In both forms of hyaluronan hydrogels used (HA versus HA + Collagen III + laminin), mRNA expression levels (Figure 1A) show a significant (p<0.05) fold-increase in EpCAM (7.72 ± 1.42, 9.04 ± 1.82) and albumin (5.57 ± 0.73, 4.84 ± 0.84) when compared to cells on plastic. There was also a significant decrease in AFP (0.55 ± 0.11, 0.17 ± 0.03), and an increase in NCAM, the combination of which indicates that cells maintain a stem cell phenotype. When the hHpSCs lineage restricted to hepatoblasts (hHBs), they lost NCAM, and dramatically turned on expression of AFP (14). At the protein expression level, cells in hyaluronans with or without other matrix components, demonstrated co-expressed EpCAM and NCAM (Figure 1B). In hydrogels supplemented with type III collagen and laminin, the EpCAM signal was the strongest as compared to that in HA hydrogel alone. Immunosorbent assays on regularly collected media showed that normalized albumin, transferrin, and urea concentrations were stably synthesized by cells in both HA hydrogel conditions (Figure 2).
Figure 1. Stability of hepatic stem cell phenotype in cells cultured in biomaterials comprised of matrix factors found in the liver’s stem cell niches.
a) Gene expression in stem cell colonies cultured in Kubota’s Medium (KM) and on culture plastic versus in cells cultured three-dimensionally (3-D) in hyaluronans or in HA supplemented with type III collagen and laminin, all components known to be in the liver’s stem cell niche. Expression is normalized to GAPDH, and fold changes are normalized to those in hHpSC colonies on plastic. A significance level of p<0.05% (*) is seen that in cultures on plastic versus those in hyaluronan conditions. That comparing the expression in HA alone versus HA with collagen III and laminin are denoted by (**) b) Expression of EpCAM and NCAM in 3-D HA colonies. Higher levels of EpCAM and NCAM are seen in HA supplemented with collagen III and laminin.
Figure 2. Hepatic Functional Assays over 18 days of 3-D cultures in Kubota’s Medium and in matrix components found in human hepatic stem cell niches.
Levels of albumin (a), transferrin (b), and urea (c) production in 3-D cultures in hyaluronans. The levels are normalized per cell. The cells remain stable and functional over weeks under these conditions.
Grafting improves transplantation of stem cells and with restricted localization to the target tissue
We used Luciferin-marked cells, transplanted into livers of immuno-compromised mice, and used bioluminescent signal acquisition to test cell localization and engraftment efficiency with grafting versus other transplantation strategies. The marking was achieved using an adenoviral vector, Ad-CMV-Luc, that does not integrate in the genome and provides intense but only transient expression enabling whole animal imaging. The expression is terminated at a time point between 48 and 72 hrs or soon thereafter due to silencing of the promoter by methylation mechanisms (34). Long-term studies required cells permanently marked using vectors that integrated into the genome. We used lentiviral constructs coupled either to Green Florescent Protein (GFP) or to thymidine kinase and studied long-term, stable localization of cells using immunofluorescence (for evaluation at histological/tissue levels) or Positron Emission Tomography (for whole body imaging). Further descriptions of the studies with long-term marking are provided in the online supplement.
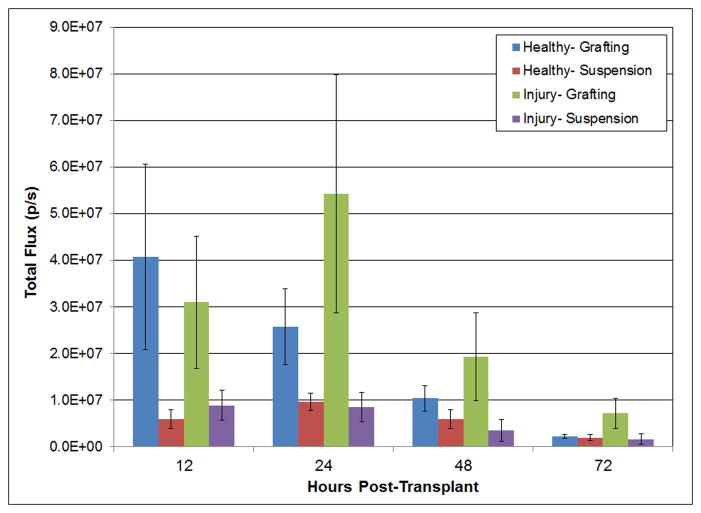
Figure 3 shows the total flux (photons per second) detected in animals within a consistent region of interest (ROI) containing the liver where cells were injected, either by the grafting method or by direct injection of cells. In both healthy animals and those injured with carbon tetrachloride (CCl4), there was a noticeable difference at 12 and 48 hrs in animals transplanted by grafting methods versus by direct injection. The grafted animals have transplanted cells restricted to the liver and yielding higher bioluminescent flux signals. This can be shown through the localization and distribution of bioluminescent signals (Figures 4 and 5). By contrast, those transplanted by direct injection have transplanted cells in the liver and with significantly lower flux signals. The data are consistent with our hypothesis that transplantation of cells with hyaluronans enhances engraftment in the target organ. Direct injection without hyaluronans results in loss of cells either due to distribution to ectopic sites and/or due to loss of cell viability.
Figure 3. Total flux signal captured by Luciferin-expressing, transplanted cells in grafts of cells in hyaluronans versus injected as a cell suspension in healthy and CCl4 -induced liver injury models.
Flux readings are normalized to those in control animals receiving no cell transplant. Much higher flux signals are observed in grafted cells, both in the healthy and injured models, most noticeably in the first 12 and 24 hrs post-transplant. By 72 hrs, the signal is lost in all cases due to silencing mechanisms involving methylation of the CMV promoter in the adenoviral vector.
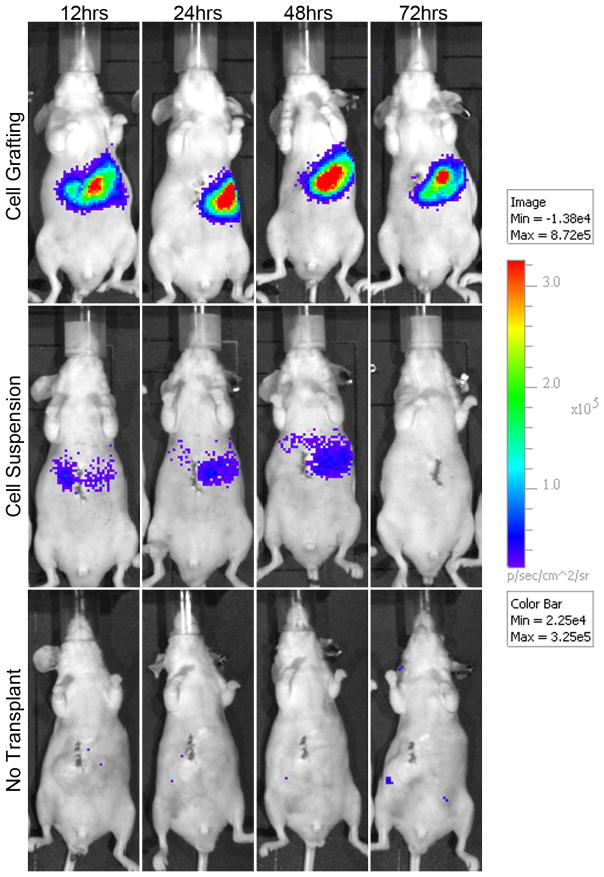
Figure 4. In vivo real time imaging of luminescent signal produced by luciferin-expressing cells in hyaluronan grafts versus injected as a cell suspension.
The signals seen in animals that were grafted are found entirely in the liver, whereas those that were transplanted without grafting materials yielded a weaker, dispersed signal throughout the abdominal cavity.
Figure 5. In vivo real time imaging of luminescent signal produced by luciferin-express cells in hyaluronan grafts versus injected as a cell suspension.
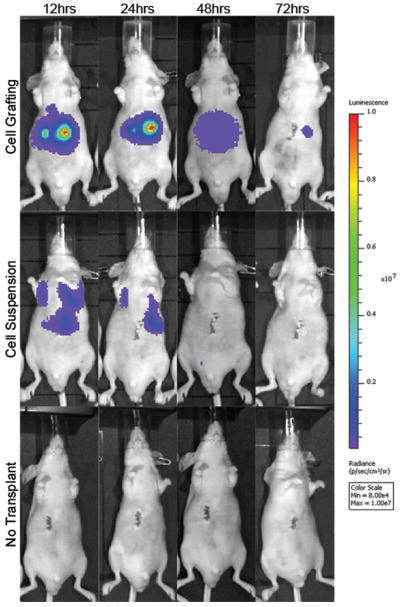
Shown is a replicate experiment from that demonstrated in Figure 4. The distribution of cells to ectopic sites, such as lungs, is evident in animals with direct injection. The loss of signal at 72 hrs has been shown by others (34) to be due to silencing of the CMV promoter by methylation.
Of importance is that grafting reduced significantly the extent of ectopic cell distribution. Cells grafted to the liver using HA hydrogels were specifically localized to the injected liver tissue. Cells that were directly injected intra-hepatically were observed to spread throughout the abdomen, giving a weaker signal and were not localized to the initial transplanted area of the liver.
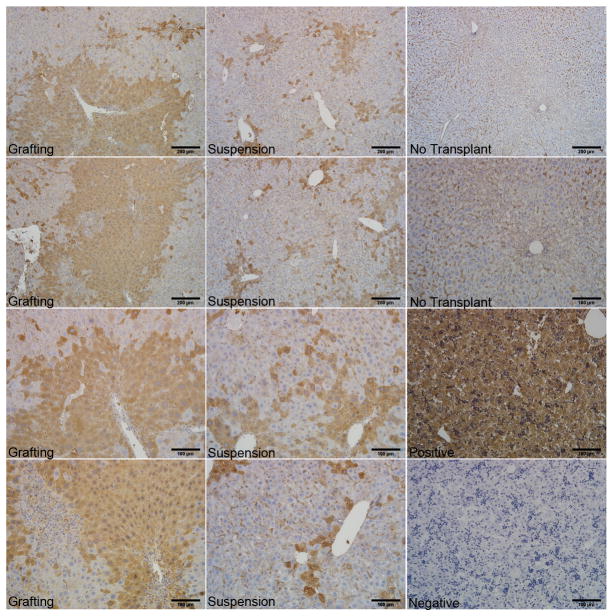
At day 7, tissues in CCl4-treated mice were removed and fixed for histology (Figure 6). In mice with HA-grafts of hHpSC transplants (first panel), cells formed large masses of transplanted cells in the areas of injection and transplantation, indicating a substantial area of humanized liver (50% or more positive staining in the field of view). Cells transplanted via direct injection of a cell suspension (middle panel) resulted in small aggregates dispersed throughout the liver in the area of transplantation, and with the extent of humanization (~10–20% in the field of view) comparable to that reported by others as summarized in a recent review (35). Sham, positive and isotype controls for albumin expression are also displayed (third panel).
Figure 6. Humanization of host livers is facilitated by grafting strategies.
Grafting of transplanted cells in biomaterials such as hyaluronans, key components in the liver’s stem cell niche, results in localization of the transplanted cells to the target organ and more rapid humanization of that organ. By contrast, transplantation of cell suspensions without grafting biomaterials results in smaller numbers of human cells within the target organ and also in human cells found at ectopic sites such as lung (data not shown). Identification of the human cells is by immunohistochemistry for human albumin. Controls include livers from animals that were not transplanted and human fetal liver with and without primary antibody staining.
Complementary studies with marked cells that were transplanted by a vascular route are described further in the online supplement. These cells were transfected with a lentiviral construct derived from Herpes Simplex Virus (HCV) and expressing thymidine kinase (TK). (Supplementary Figures 1 and 2). They survived in culture in Kubota’s Medium for more than 6 months (Supplementary Figure 3). Cells were transplanted via the spleen and, therefore via the portal vein, into the livers of mice. Post-transplantation, tissues were imaged using positron emission tomography (PET). Findings are shown after 22 and 85 days with signals present in the liver, lung, spleen, and kidney (Supplementary Figure 4). Therefore, the transplanted cells survived in these ectopic sites for at least 3 months.
Discussion
Grafting protocols are compelling alternative strategies for transplantation of cells from solid organs. Our studies dramatically demonstrate that engraftment is improved and dispersal to ectopic sites is negligible by use of grafting, as opposed to direct injection or delivery of cells by a vascular route. Cells transplanted by a vascular route or by direct injection have a propensity to aggregate during intravascular administration and can result in emboli that can be life threatening.
The advantages of using grafting strategies are especially important in transplantation of stem cells (7–10 μm), readily lost to ectopic sites if transplanted by vascular routes, especially if by the portal vein. Moreover, they require a distinct microenvironment for survival, expansion and integration into the target tissue as compared to that for the mature cells. Ongoing clinical trials of hepatic stem cell therapies (35) demonstrated that the engraftment efficiency of mature liver cells is only ~20–30%, small stem/progenitor cells is less than 5% when transplanted into the liver via the portal vein. Others have shown that the efficiency can be improved to a level of ~20–30%, if the stem/progenitors are transplanted into the hepatic artery as opposed to the portal vein (11). Even with this improvement, the majority of the cells escape to vascular beds at ectopic sites (36). This results both in a concern for the fate of the cells in these ectopic sites and also in a need for many more donor cells for the transplantations, since so many are lost due to the dispersal to sites other than the target tissue. Our findings of stem cells marked with thymidine kinase and then monitored by PET indicate that the cells at ectopic sites can survive for months.
Grafting strategies overcome these concerns by using factors and matrix biomaterials that can be gelled into place, and thereby restricted to the desired target tissue. Moreover, the grafts can be tailored to optimize the microenvironment for the cells, to facilitate vascularization and also to increase the speed of regeneration within the tissue. In vivo luminescent imaging confirmed the enhanced localization of the cells to liver by grafting strategies. We measured luminescent signals in both suspension and grafting methods and showed that cells are, in fact, present within the animal in both cases, with the highest signals occurring in grafting methods for both healthy and liver injury models. Luminescent images enhance localization of the cells within the mouse abdomen following transplantation into the liver. While a signal is produced by both transplantation methods, it is clear that the cells are specifically localized to the liver lobe in grafting but dispersed in part when injected via a vascular route.
The histology of host tissue further exemplified the consistent localization of transplanted hHpSCs if via a grafting strategy and yielded striking evidence of potentially rapid humanization of the livers of the immunocompromised hosts. In hosts transplanted by grafting strategies, cells formed large masses of cells, and cells remained localized to the liver tissue where injected. Significant humanization of the target organ does not occur in animals transplanted with stem cells or mature cells by direct injection or by vascular route unless the hosts have been subjected to an injury process with major loss of pericentral cells (4–8, 37). We found that stem cells injected by a vascular route or direct injection resulted in smaller, more dispersed groups of cells in the host livers and accounting for less than 5% if by vascular route(25) or up to 10% if by direct injection. This is consistent with the findings of others testing engrafting with stem/progenitors and who have reported 2–3% engraftment (6). The combination of in vivo imaging and tissue histology gives a macro and micro image wholly supporting the need for grafting methods as strategies for cell transplant therapies for cells from solid organs.
The grafting biomaterials we chose are ones that elicit optimal survival and growth of the transplanted cells along with rapid and efficient vascularization of the graft. Ideal grafting biomaterials for hHpSCs and hHBs include HA, type III collagen and laminin, components found in the stem cell niche (13, 14, 25). The hHpSCs and hHBs seeded into the HA hydrogels were found to retain their viability, their ability to divide for weeks, and their stable stem cell and progenitor phenotypes ex vivo, facilitating the long-term survival, proliferation and maintenance. Previous studies have shown the same hepatic functions of cells in vivo of transplanted hHpSCs in long-term studies (25). Thus, it is anticipated that with localized grafting of cells, cell functions will increase also over time in vivo.
Gene expression in the cells cultured in the grafting biomaterials was comparable to that of the stem/progenitors with some interesting distinctions to the findings in cultures on plastic. There was an increased overall expression of EpCAM(25), and at levels much higher than that for colonies on culture plastic. Similarly, the albumin expression of cells in both HA hydrogel conditions was higher than for cells on plastic and reflects increased functions of hHpSCs in a 3-D environment. Thus, some patterns of gene expression were influenced primarily by the 3D culture conditions.
Preservation of the stem/progenitor cell phenotype was the net result of the cell response to the 3-D micro-environmental conditions used in the graft. AFP is not expressed in hHpSCs at all, either in culture or in vivo, but is characteristic of hHBs, and its expression gradually fades with maturation to later lineage stages (25, 26, 37). In this study, the significant decrease in AFP expression in both HA hydrogel conditions studied can be interpreted either that the cells remained as hHpSCs, or that they matured to later lineage stages at which AFP is not expressed. The maintenance of NCAM, a marker of stem cells, but not mature cells, implicates that the former interpretation is correct. This suggests that the matrix chemistry has an influence in maintaining the hHpSC phenotype, as cultures supplemented with collagen III and laminin in addition to the 3D hyaluronan structure also underwent decreased AFP expression.
The rigidity of the HA hydrogels can be adjusted easily and has been shown to be a critical variable in defining the phenotype of the cells (24, 38, 39). For these studies, the formulation of the HA gels used was that shown to be optimal for the stem cell phenotype (26) and in which the hydrogel rigidity was at 25 Pa, well below the rigidity level of 200 Pa found needed to induce differentiation via mechanical forces. The interaction of the cells within the HA hydrogels was accompanied by gradual breakdown of the gels and with some material loss in the media changes. This biodegradation is extremely beneficial for cells being transplanted, in that the initial microenvironment is replaced with matrix components and soluble factors from the host tissue, allowing the graft to transition to conditions for integration into the host tissue and then differentiation, responses needed to promote regeneration.
Matrix components have long been known to be primary determinants of attachment, survival, differentiation, cytoskeletal organization, and stabilization of requisite cell surface receptors that prime the cells for responses to specific signals. Grafting of cells using injectable biomaterials has been successful for therapies other than liver cell therapies as discussed here. Studies involving in situ engineered tissue, including studies of injectable Matrigel with embryonic stem (ES) cells (40) or of skeletal myoblasts in fibrin (41) have shown restoration of cardiac function and geometry after cardiac injury. Injectable materials solidify in vivo and retain the geometry of the injured tissue. The matrix chemistry changes with maturational stages, with host age, and with disease states (13). Therefore, graft biomaterials should mimic the matrix chemistry of the particular lineage stages desired for the graft and be biocompatible for humans. Many of the biomaterials such as Matrigel can only be used for experimental but not clinical studies. Others, such as alginate gels, are used for walling off cells in order to protect them from immune reactions. The use of HA hydrogels as an injectable material for tissue engineering is possibly the most optimal of any considered due to its neutrality with respect to effects on cells embedded into it, to its long-lasting effect, while maintaining biocompatibility (42) and ability to minimize patient discomfort, lower risk of infection, scar formation, and overall costs (43). Cross-linking methods also maintain the material biocompatibility, ensure retention of the cells in the relevant target tissue and minimize escape of cells to ectopic sites.
Grafts have proven highly successful for transplantation, with localization of hHpSCs within the target organ, and with dramatically enhanced potential for humanization of host livers. These methods translate readily to clinical programs, since the biomaterials of these grafts are available. Moreover, use of hyaluronans clinically is done routinely for diverse procedures (mostly orthopedic) and found safe in those tried to date. The method can be used for diverse liver diseases except for endstage cirrhosis with bleeding disorders necessitating patch grafts on the liver surface, ones now under development. Translation to clinical programs is now underway and will be attempted in clinical trials within approximately a year. We assume that grafting strategies will comprise diverse forms in the near future.
Supplementary Material
Acknowledgments
Financial Support:
Funding derived from sponsored research grants from Vesta Therapeutics (Bethesda, MD), NC TraCS (UNC CTSA) Grant (#2KR50905), a grant from the North Carolina Biotechnology Center (NCBC), an NIH Center grant (CA016086), and a DOE grant (03-SC-DOE-1017). PET studies were performed at the Duke Center for In Vivo Microscopy, an NIH/NCRR Biomedical Technology Resource Center (P41 RR005959) and Small Animal Imaging Resource Program (U24 CA092656).
We acknowledge our research collaborator and friend, Dr. Claire Barbier, and recognize posthumously her invaluable contributions to the performance of these studies. Technical core services were provided by the Nucleic Acids, Histology, and Functional Genomics core facilities.
List of Abbreviations
- AFP
α-fetoprotein
- ALB
Albumin
- CCl4
Carbon tetracholoride
- EpCAM
Epithelial cell adhesion molecule
- HA
Hyaluronic Acid(hyaluronans)
- HBs
Hepatoblasts
- HpSCs
Hepatic stem cells
- ICAM
Intercellular adhesion molecule
- KM
Kubota’s Medium
- Lam
Laminin
- NCAM
Neural cell adhesion molecule
Footnotes
Disclosures:
The authors declare no conflicts of interests. A patent on the grafting strategies has been filed; is owned by UNC; and has been licensed to Vesta Therapeutics for eventual use in clinical programs. None of the authors have equity or a position in Vesta, and none are paid consulting fees by the company.
Author Contributions: The original ideas for the project were by LM Reid and RA Turner. LM Reid supervised all aspects of human cells, matrix chemistry and biology, and biological assays. D Gerber supervised all aspects of surgical transplantation in animal models. E Wauthier and R Turner did preparations to isolate hepatic stem cells and hepatoblasts. E Wauthier and C Barbier also assisted with miscellaneous aspects of the project as needed. OA Lozoya assisted with RT-PCR primer design, validation, and analysis. E Wauthier designed the lentiviral constructs with GFP or with TK, did all marking studies, and helped with the transplantation of cells by a vascular route. R McClelland, J Bowsher, and E Hsu did the PET imaging. GD Prestwich supplied hyaluronan materials for the experiment, provided counseling on the hyaluronan graft chemistry, and did editing of the manuscript. RA Turner performed all HA experiments, assays, imaging, data processing, created figures and tables, and wrote the initial drafts of the manuscript. RA Turner and LM Reid did the final edits leading to manuscript submission.
References
- 1.Strom S, Fisher RA, Thompson MT, Sanyal AJ, Cole PE, Ham JM, Posner MT. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63:559–569. doi: 10.1097/00007890-199702270-00014. [DOI] [PubMed] [Google Scholar]
- 2.Fox IJ, Chowdhury JR. Hepatocyte transplantation. Am J Transplant. 2004;4 (Suppl 6):7–13. doi: 10.1111/j.1600-6135.2004.0340.x. [DOI] [PubMed] [Google Scholar]
- 3.Najimi M, Sokal E. Liver cell transplantation. Minerva Pediatr. 2005;57:243–257. [PubMed] [Google Scholar]
- 4.Stephenne X, Najimi M, Sibille C, Nassogne MC, Smets F, Sokal EM. Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology. 2006;130:1317–1323. doi: 10.1053/j.gastro.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S, Bhargava KK, Novikoff PM. Mechanisms of cell engraftment during liver repopulation with hepatocyte transplantation. Seminars in Liver Disease. 1999;19:15–26. doi: 10.1055/s-2007-1007094. [DOI] [PubMed] [Google Scholar]
- 6.Weber A, Groyer-Picard MT, Franco D, Dagher I. Hepatocyte transplantation in animal models. Liver Transpl. 2009;15:7–14. doi: 10.1002/lt.21670. [DOI] [PubMed] [Google Scholar]
- 7.Dagher I, Boudechiche L, Branger J, Coulomb-Lhermine A, Parouchev A, Sentilhes L, Lin T, et al. Efficient hepatocyte engraftment in a nonhuman primate model after partial portal vein embolization. Transplantation. 2006;82:1067–1073. doi: 10.1097/01.tp.0000236103.99456.8f. [DOI] [PubMed] [Google Scholar]
- 8.Ambrosino G, Varotto S, Basso SM, Cecchetto A, Carraro P, Naso A, De Silvestro G, et al. Hepatocyte transplantation in the treatment of acute liver failure: microencapsulated hepatocytes versus hepatocytes attached to an autologous biomatrix. Cell Transplant. 2003;12:43–49. doi: 10.3727/000000003783985124. [DOI] [PubMed] [Google Scholar]
- 9.Susick R, Moss N, Kubota H, LeCluyse E, Hamilton G, Luntz T, Ludlow J, et al. Hepatic progenitors and strategies for liver cell therapies. Annals of the New York Academy of Science. 2002;943:398–419. doi: 10.1111/j.1749-6632.2001.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 10.Turner R, Mendel G, Wauthier E, Barbier C, Reid L. Hyaluronan-Supplemented Buffers Preserve Adhesion Mechanisms Facilitating Cryopreservation of Human Hepatic Stem/Progenitor Cells. [accepted November 2011.];Cell Transplantation. doi: 10.3727/096368912X637000. [DOI] [PubMed] [Google Scholar]
- 11.Khan AA, Shaik MV, Parveen N, Rajendraprasad A, Aleem MA, Habeeb MA, Srinivas G, et al. Human Fetal Liver-Derived Stem Cell Transplantation as Supportive Modality in the Management of End-Stage Decompensated Liver Cirrhosis. Cell Transplantation. 2010;19:1–10. doi: 10.3727/096368910X498241. [DOI] [PubMed] [Google Scholar]
- 12.Turner R, Gerber D, Reid L. The future of cell transplant therapies: a need for tissue grafting. Transplantation. 2010;90:807–810. doi: 10.1097/TP.0b013e3181f24ea2. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Theise N, Chua M, Reid LM. The stem cell niche of human livers: symmetry between development and regeneration. Hepatology. 2008;48:1598–1607. doi: 10.1002/hep.22516. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Yao H-L, Cui C-B, Wauthier E, Barbier C, Costello MJ, Moss N, et al. Paracrine signals from mesenchymal cell populations govern the expansion and differentiation of human hepatic stem cells to adult liver fates. Hepatology. 2010;52:1443–1454. doi: 10.1002/hep.23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes A, Tudor D, Nowell M, Caterson B, Hughes C. Unique forms of chondroitin sulfate proteoglycans in stem cell niches. Journal of Histochemistry and Cytochemistry. 2007;56:125–138. doi: 10.1369/jhc.7A7320.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Y, Kirker KR, Prestwich GD. Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery. J Control Release. 2000;69:169–184. doi: 10.1016/s0168-3659(00)00300-x. [DOI] [PubMed] [Google Scholar]
- 17.Shepard S, Becker H, Hartmann JX. Using hyaluronic acid to create a fetal-like environment in vitro. Ann Plast Surg. 1996;36:65–69. doi: 10.1097/00000637-199601000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Vrochides D, Papanikolaou V, Pertoft H, Antoniades AA, Heldin P. Biosynthesis and degradation of hyaluronan by nonparenchymal liver cells during liver regeneration. Hepatology. 1996;23:1650–1655. doi: 10.1002/hep.510230648. [DOI] [PubMed] [Google Scholar]
- 19.Ogata T, Okuda K, Ueno T, Saito N, Aoyagi S. Serum hyaluronan as a predictor of hepatic regeneration after hepatectomy in humans. Eur J Clin Invest. 1999;29:780–785. doi: 10.1046/j.1365-2362.1999.00513.x. [DOI] [PubMed] [Google Scholar]
- 20.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3:1304–1311. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 21.Prestwich GD. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J Control Release. 2011;155:193–199. doi: 10.1016/j.jconrel.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23:H41–56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubota H, Reid LM. Clonogenic hepatoblasts, common precursors for hepatocytic and biliary lineages, are lacking classical major histocompatibility complex class I antigen. Proc Natl Acad Sci U S A. 2000;97:12132–12137. doi: 10.1073/pnas.97.22.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lozoya OA, Wauthier E, Turner RA, Barbier C, Prestwich GD, Guilak F, Superfine R, et al. Regulation of hepatic stem/progenitor phenotype by microenvironment stiffness in hydrogel models of the human liver stem cell niche. Biomaterials. 2011;32:7389–7402. doi: 10.1016/j.biomaterials.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, Moss N, et al. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204:1973–1987. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem Cells. 2006;24:1852–1858. doi: 10.1634/stemcells.2006-0036. [DOI] [PubMed] [Google Scholar]
- 27.Schmelzer E, Reid LM. Telomerase activity in human hepatic stem cells, hepatoblasts and hepatocytes from neonatal, pediatric, adult and geriatric donors. European Journal of Hepatology and Gastroenterology. 2009;21:1191–1198. doi: 10.1097/MEG.0b013e32832973fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner WS, Schmelzer E, McClelland R, Wauthier E, Chen W, Reid LM. Human hepatoblast phenotype maintained by hyaluronan hydrogels. J Biomed Mater Res B Appl Biomater. 2007;82:156–168. doi: 10.1002/jbm.b.30717. [DOI] [PubMed] [Google Scholar]
- 29.Turner WS, Seagle C, Galanko JA, Favorov O, Prestwich GD, Macdonald JM, Reid LM. Nuclear magnetic resonance metabolomic footprinting of human hepatic stem cells and hepatoblasts cultured in hyaluronan-matrix hydrogels. Stem Cells. 2008;26:1547–1555. doi: 10.1634/stemcells.2007-0863. [DOI] [PubMed] [Google Scholar]
- 30.Shu XZ, Ahmad S, Liu Y, Prestwich GD. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J Biomed Mater Res A. 2006;79:902–912. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- 31.Shu XZ, Liu Y, Palumbo F, Luo Y, Prestwich GD. In situ crosslinkable glycosaminoglycan hydrogels for tissue engineering. Biomaterials. 2004;25:1339–1348. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Vanderhooft JL, Alcoutlabi M, Magda JJ, Prestwich GD. Rheological properties of cross-linked hyaluronan-gelatin hydrogels for tissue engineering. Macromolecular Bioscience. 2009;9:20–28. doi: 10.1002/mabi.200800141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai S, Liu Y, Zheng Shu X, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26:6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. Epub 2005 Apr 6022. [DOI] [PubMed] [Google Scholar]
- 34.Brooks AR, Harkins RN, Wang P, Qian HS, Liu P, Rubanyi GM. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J Gene Med. 2004;6:395–404. doi: 10.1002/jgm.516. [DOI] [PubMed] [Google Scholar]
- 35.Puppi J, Strom SJ, Hughes RD, Bansal S, Castell JV, Dagher I, Ellis ECS, et al. Improving the Techniques for Human Hepatocyte Transplantation: Report from a Consensus Meeting in London/ Cell Transplantation. 2011 doi: 10.3727/096368911X566208. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Hoppo T, Komori J, Manohar R, Stolz DB, Lagasse E. Rescue of lethal hepatic failure by hepatized lymph nodes in mice. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.11.006. [DOI] [PMC free article] [PubMed]
- 37.Turner R, Lozoya O, Wang Y, Cardinale V, Gaudio E, Alpini G, Mendel G, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology. 2011;53:1035–1045. doi: 10.1002/hep.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 39.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 40.Kofidis T, Lebl DR, Martinez EC, Hoyt G, Tanaka M, Robbins RC. Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation. 2005;112:I173–177. doi: 10.1161/CIRCULATIONAHA.104.526178. [DOI] [PubMed] [Google Scholar]
- 41.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 42.Gutowska A, Jeong B, Jasionowski M. Injectable gels for tissue engineering. Anat Rec. 2001;263:342–349. doi: 10.1002/ar.1115. [DOI] [PubMed] [Google Scholar]
- 43.Hong Y, Gao C, Shen J. Research development of injectable scaffolds for tissue regeneration. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2007;24:463–465. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.