Abstract
Many studies have shown that hydrogen can play important roles on the antioxidant, anti-inflammatory and other protective effects. Ohsawa et al have proved that hydrogen can electively and directly scavenge hydroxyl radical. But this mechanism cannot explain more new experimental results. In this article, the hypothesis, which is inspired by H2 could bind to the metal as a ligand, come up to explain its extensive biology effect: Hydrogen could regulate particular metalloproteins by bonding (M–H2 interaction) it. And then it could affect the metabolization of ROS and signal transduction. Metalloproteins may be ones of the target molecules of H2 action. Metal ions may be appropriate role sites for H2 molecules. The hypothesis pointed out a new direction to clarify its mechanisms.
Keywords: Hypothesis, Hydrogen, Mechanism, Ligand
Background
Basal cellular metabolism continuously produces reactive oxygen species (ROS). O2 may generate successively superoxide (O2−), hydrogen peroxide (H2O2) and hydroxyl radical (OH·). ROS are able to oxidize biological macromolecules such as DNA, protein and lipids [1,2]. Some enzymic systems detoxify ROS, however, catalase dismutates H2O2, and SOD eliminates O2− (but generates H2O2). Excess oxygen can react with H2O2 to produce hydroxyl radicals by the Fenton reaction [3] (Figure 1). Ohsawa et al provide evidence that hydrogen could reach subcellular compartments such as the nucleus and mitochondria, biochemical experiments using fluorescent probes and electron paramagnetic resonance spectroscopy spin traps indicated that hydrogen gas may selectively scavenge the hydroxyl radical [4]; They were the first to show the ability of H2 to suppress oxidation in vivo. So far, many researches have proved the central role of hydrogen on the antioxidant, anti-inflammatory and other protective effects.
Figure 1.
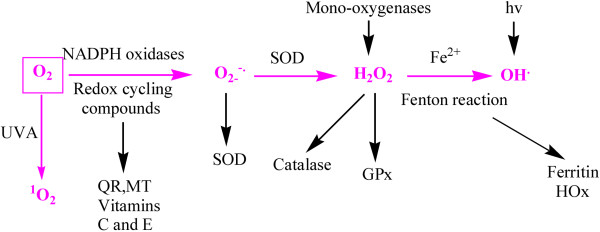
ROS generation and detoxification. Various chemical reactions, with or without enzymic catalysis, generate ROS. The dioxygen molecule undergoes successive reductions which yield the superoxide radical anion (O2−), hydrogen peroxide (H2O2) and the hydroxyl radical (OH·). Antioxidant systems act as ROS scavengers to maintain the intracellular redox status. Quinone reductase (QR) detoxifies quinone compounds, metallothionein (MT) traps (heavy) metal cations, and vitamins C and E trap free radicals. SOD and catalase respectively dismutate superoxide (into oxygen and hydrogen peroxide) and hydrogen peroxide (into oxygen and water). Glutathione peroxidase (GPx) acts like catalase on various peroxide compounds, including H2O2[5].
Much evidence has shown that hydrogen exert beneficial effects in animal models of a number of diseases mainly associated with oxidative stress; However, the findings don’t cover plants. Moreover, the mechanism of the effect of hydrogen remains unclear, the most acceptable mechanism is that the hydrogen can electively and directly scavenge hydroxyl radical while preserving other reactive oxygen and nitrogen species important in signaling [4]. There is some question, however, the published rate constant for the reaction of ·OH with H2 to form H2O and H· is drastically slower than most radical-radical reactions [6]. Furthermore, it can’t explain some new experimental results. For example, Tomohiro Itoh proved that hydrogen exerts its beneficial effect by modulating some signaling pathways. Experimenters found that oral intake of hydrogen-rich water abolishes an immediate-type allergic reaction in mice. The results indicated that hydrogen attenuates phosphorylation of the FcεRI-associated Lyn and its downstream signal transduction, which subsequently inhibits the NADPH oxidase activity and reduces the generation of hydrogen peroxide. they also found that inhibition of NADPH oxidase attenuates phosphorylation of Lyn in mast cells, indicating the presence of a feed-forward loop that potentiates the allergic responses. Hydrogen accordingly inhibits all tested signaling molecule(s) in the loop. The results imply that effects of hydrogen in some diseases are possibly mediated by modulation of yet unidentified signaling pathways [7].
Hypothesis and discussion
Although the beneficial effect of hydrogen is generally accepted, the mechanism is not still clear. There can be little doubt that biology function of H2 depends on the physical and chemical interaction of other molecules with it , so what would it be like?
In organometallic and inorganic chemistry, for some metal complexes, the "arrested" addition product can be isolated–the dihydrogen complex is obtained as a stable species that can be put in a bottle: [8] (Scheme 1).
Scheme 1.
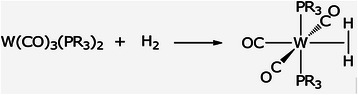
Formation of a dihydrogen complex.
Traditional ligands use lone pairs of electrons in their bonding, but in dihydrogen complexes, the bonding to the metal comes from donation of electron density from the nonpolar H-H σ bond to d orbitals on the metal (Figure 2). We hold that hydrogenase is a typical case in point. Hydrogenases catalyse the reversible oxidation of molecular hydrogen (H2). The active site domain of the Fe hydrogenases contains an unusual Fe-S centre termed the H-cluster. H-cluster consists of the [Fe4S4 subcluster bridged via the Cys thiolate to the [Fe2 (binuclear iron) subcluster (Figure 3) [9-12]. Iron sulfur clusters are found at the active sites of numerous enzymes where they commonly facilitate electron transfer and substrate transformations (Table 1) [13]. It is infered that M–H2 interaction also exists in these metalloproteins. By extension, it also should exist in non-cluster metalloproteins. All this suggestes that metal ions may be the site where H2 interacted with metalloproteins.
Figure 2.
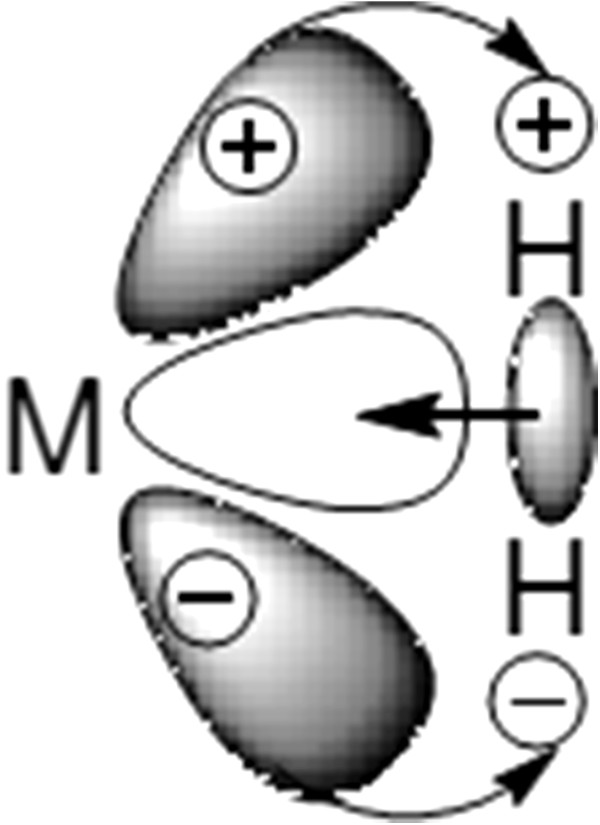
Schematic bonding model between molecular hydrogen and a metal [14].
Figure 3.
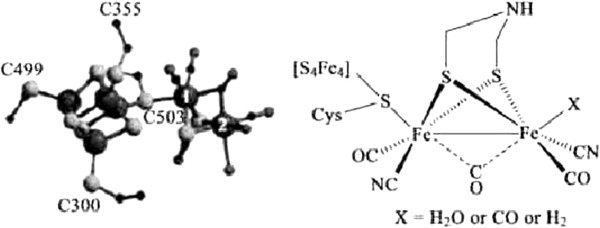
Stereo view of the CpI H-cluster and coordinating Cys ligands located at the boundary of the two lobes of the active site domain [15].
Table 1.
Iron-sulphur proteins in cells
| Function | Protein class |
|---|---|
| Catalysis |
Bacterial nitrate reductase, Formate dehydrogenase, Fumarate reductase, Glutamine PRPP amidotransferase, Hydrogenase, Methane monooxygenase, NADH:ubiquinone reductase, Phthalate dioxygenase reductase, Succinate dehydrogenase, Sulphite reductase, Xanthine dehydrogenase, Aconitase (TCA cycle) |
| Electron transfer | Ferredoxins, Rieske proteins, Rubredoxins, NADH Dehydrogenase, Succinate-CoQ Reductase, CoQ-cyt c Reductase (respiratory chain complexes) |
It is proved from experiments that molecules containing a metallic cation may promote O2− formation because they have the ability to store and easily give an electron to molecular dioxygen [16]. Free radicals arise through the autoxidation catalyzed by metalloproteins, this mainly occurs within the mitochondria. Some experimental results proved that hydrogen can permeate into mitochondria and prevent superoxide formation [17]. It indicates that H2 can affect the metabolization of ROS by way of superoxie formation. And it must have a big impact on the content of H2O2. Besides, free radical production can also happen in other cellular compartments, such as NADPH oxidase. ROS production can interfere with signal transduction pathways [18-20]. ROS, in particular H2O2, are indeed second messengers for many physiological stimuli, some stimuli have been proven to induce mitochondrial H2O2 release [21].
On the basis of the mentioned analysis, we evaluate that metal ions could be appropriate role sites for H2 molecules. We propose that hydrogen can permeate into mitochondria and concentrate at mitochondrial membrane to regulate activity of metalloproteins (complexesI,II,III) by M–H2 interaction. The same may be true of NADPH oxidase. This could reduce the production of superoxide and prevent synthesis of the hydroxyl from the source. Hydrogen, on the one hand, maybe regulate the content of H2O2 by affecting the metabolization of ROS. And then it disturbs signaling pathway. On the other hand, hydrogen maybe directly influence signal transduction by regulating particular metalloproteins of signaling pathways. It may be the mechanism of its extensive biology effect.
Conclusions
We propose that metalloproteins may be ones of the target molecules of H2 action. Metal ions may be appropriate role sites for H2 molecules. And in this way can we explain its extensive biology effect. Although some details remain murky, the hypothesis pointed out a new direction for the continuation. It is a good inspiration to clarify the mechanism of the effect of hydrogen. We predict that hydrogen can affect many metalloproteins activities.Therefore, more studies will be necessary to test the hypothesis.
Contributor Information
Penghui Shi, Email: shiph150@sina.cn.
Wancang Sun, Email: wangcangsun@yahoo.com.cn.
Pengzhong Shi, Email: spz150@163.com.
Acknowledgment
We wish to sincerely thank Xuejun Sun (Second Military Medical University, Shanghai, China) for advice about the manuscript.
Funding
There is no source of support in the form of grants.
References
- Vaughan M. Oxidative modification of macromolecules minireview series. J Biol Chem. 1997;272:18513. doi: 10.1074/jbc.272.30.18513. [DOI] [Google Scholar]
- Hagen TM, Ingersoll RT, Lykkesfeldt J, Liu J, Wehr CM, Vinarsky V, Bartholomew JC, Ames AB. (R)-alpha-lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J. 1999;13:411–418. doi: 10.1096/fasebj.13.2.411. [DOI] [PubMed] [Google Scholar]
- Woo ES, Lazo JS. Nucleocytoplasmic functionality of metallothionein. Cancer Res. 1997;57:4236–4241. [PubMed] [Google Scholar]
- Ohsawa I, Ishikawa M, Takahashi K. et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- Morel Y, Barouki R. Repression of gene expression by oxidative stress. Biochem J. 1999;342:481–496. doi: 10.1042/0264-6021:3420481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KC, Gladwin MT. The hydrogen highway to reperfusion therapy. Nat Med. 2007;13:673–674. doi: 10.1038/nm0607-673. [DOI] [PubMed] [Google Scholar]
- Itoh T, Fujita Y, Ito M. et al. Molecular hydrogen suppresses FcRI-mediated signal transduction and prevents degranulation of mast cells. Biochem Biophys Res Commun. 2009;389:651–656. doi: 10.1016/j.bbrc.2009.09.047. [DOI] [PubMed] [Google Scholar]
- Kubas GJ, Ryan RR, Swanson BI, Vergamini PJ, Wasserman HJ. J Am Chem Soc. 1984. pp. 451–452. [DOI]
- Darensbourg MY, Lyon EJ, Smee JJ. The bio-organometallic chemistry of active site iron in hydrogenases. Coord Chem Rev. 2000;219/221:533–561. [Google Scholar]
- Marr AC, Spencer DJE, Schurder M. Structural mimics for the active site of [NiFe] hydrogenase. Coord Chem Rev. 2001;219/221:1055–1074. [Google Scholar]
- Nicolet Y, de Lacey AL, Vernde X. et al. Crystallographic and FTIR Spectroscopic Evidence of Changes in Fe Coordination Upon Reduction of the Active Site of the Fe-Only Hydrogenase from Desulfovibrio desulfuricans. J Am Chem Soc. 2001;123:1596–1601. doi: 10.1021/ja0020963. [DOI] [PubMed] [Google Scholar]
- Lawrence JD, Li HX, Rauchfuss TB, Angew Chem Int Ed. 2001. pp. 1768–1771. [DOI] [PubMed]
- Cowan JA. Inorganic Biochemistry: An Introduction. New York: VCH Publishers; 1993. [Google Scholar]
- Martin HG. Prechtl. WiKu: Publisher; 2007. [Google Scholar]
- He Chengjiang WangMei, Li Minna, Sun Licheng. Advance in chemical mimic of fe-only hydrogenase. Progress in chemistry. 2004;16(2):250–255. [Google Scholar]
- Kagan VE, Tyurina YY. Recycling and redox cycling of phenolic antioxidants. Ann N Y Acad Sci. 1998;854:425–434. doi: 10.1111/j.1749-6632.1998.tb09921.x. [DOI] [PubMed] [Google Scholar]
- Sato Y, Kajiyama S, Amano A. et al. Hydrogen-rich pure water prevents superoxide formation in brain slices of vitamin C-depleted SMP30/GNL knockout mice. Biochem Biophys Res Commun. 2008;375:346–350. doi: 10.1016/j.bbrc.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/S0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11:118–124. [PubMed] [Google Scholar]
- Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radical Biol. Med. 1997;22:269–285. doi: 10.1016/S0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- Quillet-Mary A, Jaffrezou JP, Mansat V, Bordier C, Naval J, Laurent G. Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. J Biol Chem. 1997;272:21388–21395. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]


