Abstract
Deficiencies in folate lead to increased serum concentrations of homocysteine (Hcy), which is known as hyperhomocysteinemia (HHcy), is associated with bone disorders. Although, homocysteine (Hcy) accumulates collagen in bone and contribute to decrease in bone strength. The mechanism of Hcy induced bone loss and remodeling is unclear. Therefore, the present study was aimed to determine the role of folic acid in genetically HHcy associated decrease in bone blood flow and remodeling. Wildtype (WT) and cystathionine-β-synthase heterozygous (CBS+/−) mice were used in this study and supplemented with or without folic acid (FA 300 mg/kg, Hcy reducing agent) in drinking water for 6 weeks. The tibial bone blood flow was measured by laser Doppler and ultrasonic flow probe method. The tibial bone density was assessed by dual energy X-ray absorptiometry. The bone homogenates were analyzed for oxidative stress,NOX-4 as oxidative marker and thioredoxin-1 (Trx-1) as anti-oxidant marker, bone remodeling (MMP-9) and bio-availability of nitric oxide (eNOS/iNOS/NO) by Western blot method. The results suggested that there was decrease in tibial blood flow in CBS+/− mice. The bone density was also reduced in CBS+/− mice. There was an increase in NOX-4, iNOS, MMP -9 protein as well as MMP-9 activity in CBS+/− mice and decrease in Trx-1, eNOS protein levels, in part by decreasing NO bio-availability in CBS+/− mice. Interestingly, these effects were ameliorated by folic acid and suggested that folic acid supplementation may have therapeutic potential against genetically HHcy induced bone loss.
Keywords: Methionine, Osteoporosis, MMP-9, Nitric Oxide, eNOS, iNOS
INTRODUCTION
Homocysteine is a sulfur-containing amino acid formed in the metabolic pathway between methionine and cysteine (30). Elevated levels of homocysteine known as hyperhomocysteinemia (HHcy) are associated with various bone abnormalities, such as osteopenia and osteoporosis (1; 30). The thiol groups in Hcy undergo auto-oxidation, thus triggering oxidative stress and causing the production of reactive oxygen species (ROS). Oxidative stress subsequently induces the synthesis of matrix metalloproteinases (MMPs). The MMPs are membrane-bound, zinc-dependent endoproteinases (27). MMP family consists of three major subgroups, the interstitial collagenases, the gelatinases and the stromelysins; these are zinc-dependent endopeptidases, are involved in extracellular matrix degradation and bone remodeling. Both collagenases (i.e., MMP-1 and MMP-13) and gelatinases A (MMP-2) and B (MMP-9) have been considered the principal MMPs in the digestion of bone collagen by osteoblasts (16; 20). Homocysteine mediated remodeling also results in increased super oxide anion. Super oxide anion may react with nitric oxide to produce peroxynitrite with reduced NO bio-availability (2), which may impair the osteoblast-osteoclast balance with unpredictable consequences on bone remodeling. An increase in oxidative stress, leads to endothelial dysfunction (35), decreased bone blood flow and eventually to osteoporosis (28). Decreased bone blood flow would intuitively result in bone pathology from absence of nutrient delivery to bone. Folic acid (FA) is also known as vitamin B12, a naturally occurring dietary component that reduces the levels of Hcy by increasing the rate of recycling of Hcy to methionine (36). A lack of dietary folic acid leads to a folate deficiency. It is known that Hcy lowering therapy can favorably influence the course in osteoporotic patients (17). In addition, HHcy and vitamin B12 deficiencies are related to elevated bone turnover markers in humans (9). However, these investigations do not demonstrate the causality between HHcy and changes in bone blood flow. Moreover, they do not provide any data regarding the mechanisms behind their observations. This study investigates the homocysteine related alterations in bone remodeling i.e bone blood flow, density and matrix composition in the bone. To do this, we examined the change in bone blood flow, density, expression level of MMP-9.oxidative stress and nitric oxide bio-availability in bone.
MATERIALS AND METHODS
Animals
Breeding pair of CBS+/− mice were obtained from Jackson Laboratories (Bar Harbor, ME). The mice were grouped: (wild type (WT), WT+ FA, CBS (+/−) and CBS (+/−) + FA) and housed in the animal care facility at the University of Louisville. All animal procedures were carried out in accordance with the National Institute of Health Guidelines for animal research. The Institutional Animal Care and Use Committee of the University of Louisville, School of Medicine approved this protocol. All the mice were treated with 0.03 g/L folic acid (FA) for 6 weeks in drinking water. Every alternate day drinking waters was supplemented with FA.
Measurement of plasma Hcy
At the time of sacrifice, blood samples were collected into tubes, placed on ice immediately and plasma was isolated by centrifugation at 2300g for 15 min at 4 °C and stored at −80 °C until use. Total plasma homocysteine was assayed as described earlier by High Pressure Liquid Chromatography.
In vivo blood flow measurement
Mice were anesthetized with Pentabarbitol (100mg/kg). Body temperature was maintained between 37- 39 °C using a heating pad. Blood flow in tibial artery was measured by using LASER DOPPLER IMAGING flow meter. Tibial artery was cannulated, drug were injected through a syringe. Drugs were made up in 0.9% saline and three or four drugs were administered in random order in each experiment. Ample time was allowed for recovery between injections. The drugs used were Phenylephrine hydrochloride, Acetylcholine, Sodium Nitroprusside and were procured from Sigma Aldrich.
Measurement of bone density (BD)
The apparent bone density was determined by Archimedes principle to decide if there was a change in bone mechanical properties.
Angiography
Barium sulfate has been used as good contrast for visualizing gastro intestinal tract in pediatric population. Barium sulfate is toxic if enters in to circulation system. We used barium sulfate for tibia imaging of mice vasculature (15). All images were taken with Kodak 4000 MM image station. Dissected animals were placed in the x-ray chamber and angiogram images were captured with high penetrative phosphorous screen by 31 KVP X-ray exposure for 4 minutes using high resolution phosphorous screen and aperture settings of approximately 4.0, f-stop-12 and zoom of 40 mm.
Nitrite assay
NO metabolites such as nitrite production was measured fluorimetricaly using commercially available kit from Biovision. Results expressed as nanomole of nitrite produced per mg of protein (25).
Western Blot Assay
Western blotting was performed as described previously (13).
Gelatin gel zymography
MMP-9 activities were measured using gelatin gel zymography as described previously (26).
Statistical analysis
Values are reported as mean ± SEM in all four groups. Differences between groups were tested by two-way ANOVA. Tukey’s multiple comparison test was used to compare group means and were considered significant if p < 0.05.
RESULTS
To phenotypically characterize CBS+/− mice, in comparison with WT mice, we measured the total, plasma Hcy concentration. We found that the plasma levels of total homocysteine were significantly higher in CBS+/− mice (19.0 μmol/L) in compared to WT (5 μmol/L). There was no change in the plasma Hcy levels in the group supplemented with folic Acid.
To identify the nutrient artery branch that fed to the tibia, we performed x-ray angiography (Fig. 1). The arrows in Figure 1 indicate the artery and the tibial bone. The bone density was decreased in the CBS+/− mice as compared to WT. Interestingly; the treatment with FA elevated bone density in CBS+/− mice. To determine whether the decrease in bone density in CBS+/− mice is due, in part, to the decrease in nutrients and blood flow to the tibia, we examined blood flow response to acetylcholine (an endothelial dependent vasodilator), nitroprusside (an endothelial-independent vasodilator) and phenylephrine (an alpha-adrenergic vasoconstrictor). There was significant decline in tibial artery flux in CBS+/− mice when compared to WT mice (Fig. 2A). Administration of phenylephrine resulted in decrease tibial artery volumetric flow (flux) in all groups (Fig. 2B) . Also, administration of sodium nitroprusside (SNP) caused no significant changes in tibial artery flux in all the groups (Fig. 2C). The results suggest that Hcy attenuates the vasodilatation and vasoconstriction of tibial bone nutrient artery (Fig. 2). To determine whether the Hcy induces bone specific oxidative stress, we measured NOX-4 as the oxidative stress marker and Trx-1 as the anti-oxidant marker. We found significant increase in NOX-4 and decrease in Trx-1 levels in CBS+/− tibial extract. Interestingly, the treatment with FA ameliorated these effects (Fig. 3) compared with WT group.
Figure 1.
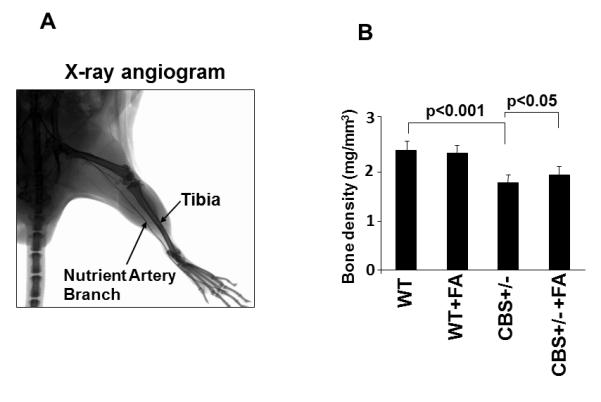
A: Representative, a typical barium-contrast x-ray angiogram of tibia bone of mice. The arrows indicate tibia bone and the nutrient artery to the tibia bone. B: Effect of folic acid on tibia bone density. The bone density was measured using KODAK MM-4000 whole body x-ray image scanner and bone density software. n=5 in each group. *P < 0.05 compared with WT; #P < 0.05 compared with CBS+/−. n = 5 for all groups.
Figure 2.
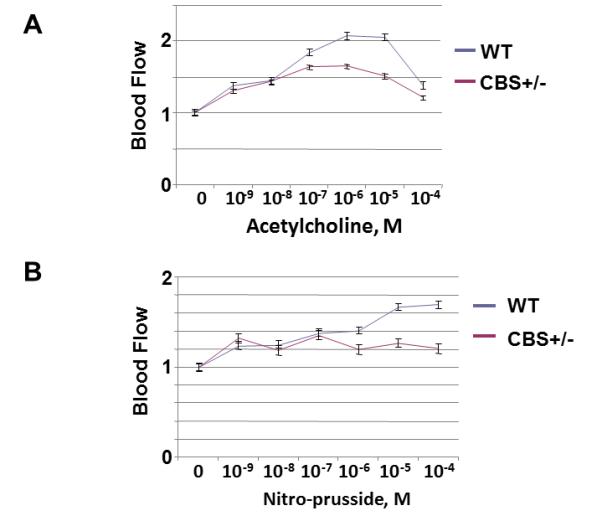
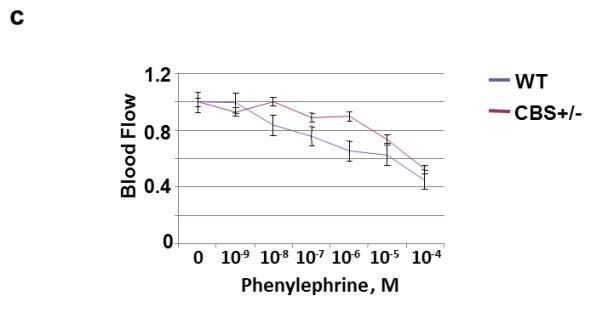
A: Effect of Hcy on acetylcholine and nitroprusside-mediated tibia nutrient artery blood flow (laser Doppler flow in arbitrary unit) in WT and CBS+/− mice. B: Effect of Hcy on phenylephrine-mediated tibia nutrient artery blood flow (laser Doppler flow in arbitrary unit) in WT and CBS+/− mice.
Figure 3.
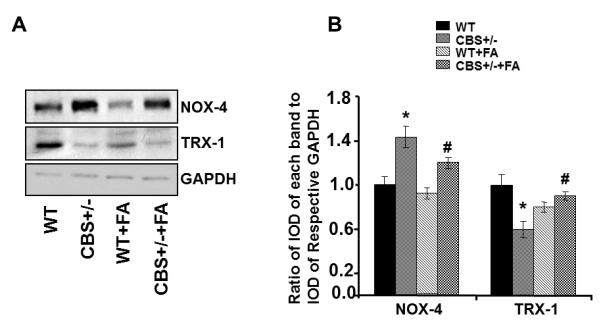
Effect of Folic acid on oxidative stress: A: Western blot analysis for NOX-4 and Trx-1protein levels in tibia tissue of WT, CBS+/−, WT+ FA, and CBS (+/−) +FA. GAPDH was used as control (gel panels). B: Relative protein expression is reported as a ratio of IOD of each band to the IOD of the respective GAPD H band. *P < 0.05 vs. WT. #P<0.05 vs. CBS+/−. n = 5 for all groups.
To elucidate the role of homocysteine in NO bio-availability, we measured iNOS and eNOS protein levels. In CBS+/− mice as shown in Fig. 4A, B, the protein expression of eNOS was significantly decreased whereas protein expression of iNOS was significantly increased in compared to WT group. Interestingly, CBS+/− mice supplemented with FA showed a significant decrease in iNOS and increase in eNOS protein level (Fig. 4A, B). These results suggested that Hcy decreased NO bioavailability by antagonizing the FA. To elucidate the role of homocysteine in NO bio-availability, the authors also measured NO metabolite nitrite levels in plasma. The graph (Fig. 4C) shows the decreased NO metabolite levels in plasma samples of CBS+/− mice when compared with WT group. Treatment with FA restores these levels.
Figure 4.
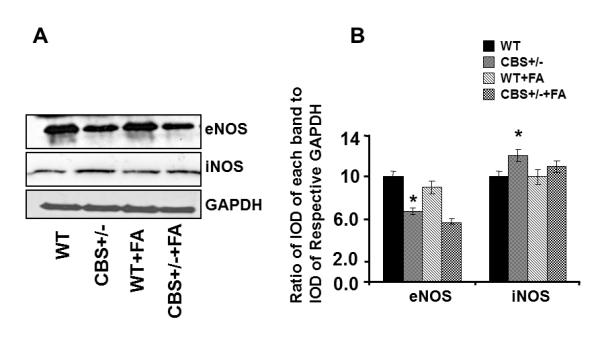
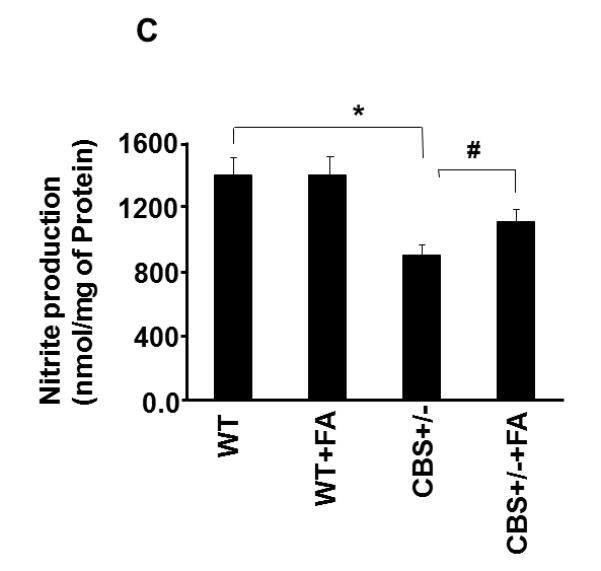
Effect of Folic acid on NO bio-availability. A: Western blot analysis of eNOS and iNOS protein levels in tibia tissue of WT, CBS+/−, WT+ FA, and CBS (+/−) +FA. GAPDH was using as loading control (gel panels). B: Relative protein expression is reported as a ratio of IOD of each band to the IOD of the respective GAPDH band. *P < 0.05 vs. WT. #P<0.05 vs. CBS+/−. n = 5 for all groups. C: Nitrite measurement using fluorometric assay (nmole/mg of protein). *P < 0.05 vs. WT. #P<0.05 vs. n = 5 in each group.
MMP- 9 was critically involved in the bone matrix. Therefore, we examined the MMP -9 protein levels and activity (Fig. 5). We observed an increase in MMP-9 activity in CBS+/− as compared to WT group (Fig. 5 A, B). In addition of activity, protein level of MMP-9 was also significantly higher in CBS+/− as compared to WT group (Fig. 5 C, D). Importantly, FA supplementation almost normalized MMP-9 protein levels as well as activity in CBS+/− FA mice as compared to CBS+/− mice. These results suggested that MMP-9 play an important role in genetically HHcy associated bone remodeling.
Figure 5.
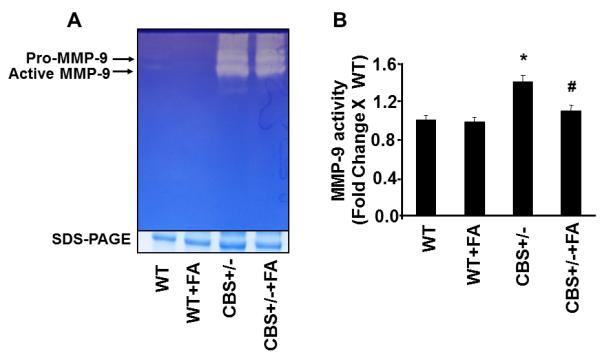
Effect of Folic acid on MMPs expression and activity. A: In-gel gelatin zymography for MMP-9 activity and SDS-PAGE used a loading control in tibia tissue extract of WT, WT+ FA, CBS (+/−) and CBS (+/−) +FA (gel panel). B: Densitometric analysis was performed and plotted as a bar graph as fold change to WT. *P < 0.05 compared with WT; #P < 0.05 compared with CBS+/−. n = 3 C: Western blot analysis of MMP-9 protein levels in tibia tissue extract of WT, WT+ FA, CBS( +/−) and CBS( +/−)+ FA and GAPDH was using as loading control (gel panels). D: Relative protein expression is reported as a ratio of IOD of each band to the IOD of the respective GAPDH band. *P < 0.05 compared with WT; #P < 0.05 compared with CBS+/−. n = 5 for all groups
DISCUSSION
The present study clearly demonstrated that tibial bone density as well as tibial artery blood flow decreased in CBS (+/−) mice (Fig.1, 2) in comparisons to WT. The increased MMP-9 protein level was associated with the increased oxidative stress (31), which has been shown to be associated with HHcy (Fig.6). In addition, the increased iNOS protein level and decreased eNOS protein level were corroborated with the bone damage, as evidenced by decreased NO bio-availability. Interestingly; the extent of HHcy-associated bone damage was ameliorated by FA supplementation. Evrengul et.al showed a correlation with high Hcy and coronary flow, and a possible role in the pathogenesis of impaired flow (12). We tested whether HHcy had an effect on bone blood flow. We examined blood flow response to three different drugs acetylcholine (an endothelial dependent vasodilator), nitroprusside (an endothelial-independent vasodilator) and phenylephrine (an alpha-adrenergic vasoconstrictor). There was significant decline in tibial artery flux in CBS+/− mice when compared to WT mice (Fig.2). A decrease in bone blood flow is a potential cause of compromised bone mechanical properties (14). Our results have provided another context and model by which Hcy alters bone blood flows. The pathological consequences of this remain to be elucidated. We proposed that there would be a decrease in bone density in CBS+/− mice. However, we did not find any significant changes (Fig.1). Bone density measurement is a basic tool for the diagnosis of osteoporosis. Dhonukshe-Rutten etal. studied 1267 patients in the Netherlands and found that the presence of hyperhomocysteinemia has increased risk of fracture rate but there were no correlation with bone mineral density (BMD) (8). Herrman etal also showed no relation between hyperhomocysteinemia and BMD (18). It seems that there is a correlation between increased level of homocysteine and fracture rate, but not such a correlation between homocysteine and BMD. In some studies attention has been paid to folic acid and other group of B vitamins. It has been shown that the source of fractures in some patients was not homocysteine itself but other nutrient deficiencies such as folic acid and group B vitamins. Serum level of homocysteine could be an index of such nutrients deficiencies (4; 5; 7; 23). Since folic acid and vitamins are necessary for Hcy clearance, and their absence result in hyperhomocysteinemia, we expected a decreased bone density in our experiment as a consequence of elevated Hcy (Fig.1B). Many studies have noted bone quality as a major risk factor for fracture; however, bone density is not the only clinically important value in determining susceptibility to fracture (21; 22). Therefore, there was not a significant decrease in bone density; there could possibly have been a change in bone quality. However, we did not perform these measurements, and this is warranted for future study. In tissue and cells, NADPH oxidase is a primary source of ROS generation (10). ROS are widely considered to be a causal factor in bone disease. Their role in bone metabolism is dual, considering their effects under physiological condition, the production of ROS by osteoclasts helps accelerate destruction of calcified tissue, thus assisting in bone remodeling. In pathological conditions, radical generation is remarkably high e.g. when a bone fractures. Although, the increases in osteoclastic activity and ROS production are linked in many skeletal pathologies, it remains to be clarified whether increased ROS production overwhelm anti-oxidant defenses, leaving the individual open to hyperoxidant stress (19). Heterozygous CBS mice generated more super-oxides in aortic tissue compared to controls (11). Similarly, endothelial cell culture incubated with Hcy enhances ROS production (33). The results of the present study show that Hcy increased NOX-4 and decreased expression of Trx-1, while FA reversed these effects of Hcy (Fig.3A, B). These data suggest a role of FA in modulating the expression of the redox enzymes (Trx-1 /NOX-4) disrupted by increased levels of Hcy. These results also suggest that FA alleviates the oxidative stress. In vivo studies showed that mild and severe HHcy are associated with impaired endothelium-dependent relaxation and blood flow (3; 29). These findings are consistent with impairment in NO bioavailability. However, recent research on Hcy affecting NO production and bioavailability is conflicting. Some studies show that high concentrations ofHcy increase NO production (34), whereas other studies show that Hcy decreases NO production (6; 37). Our present results show that Hcy induced oxidative stress by inducing iNOS and decreasing eNOS expression in CBS+/− in comparison to WT (Fig.3A,B and 4A,B). Furthermore, there was decreased NO metabolite levels in plasma of CBS+/− mice in comparison to WT (Fig.4C). Previously, we showed latent matrix metalloproteinases (MMPs) in the basement membranes of microvascular endothelial cells (MVEC) (32), and these latent MMPs are activated by serine proteinases as well as by Hcy-mediated oxidative stress (24). However, there may be no “cause-and-effect” relation between MMP. However, the role of FA in activation of MMP-9 was not defined. In the present study, for the first time, we demonstrated a significant increase in the MMP-9 protein expression and MMP-9 activity in CBS+/− mice (Fig.5). MMP-9 activity was suppressed in CBS+/− mice treated with FA (Fig.5 A,B). These results we further confirmed at protein level. MMP-9 protein was significantly up regulated in CBS+/− mice in comparison to WT and treatment of FA restore these level (Fig.5C,D). Others have suggested that the level of MMP-9 mRNA expression in osteoporotic bone tissue was significantly higher than that in normal control group. It was suggested that MMP-9 play a key role in the development of bone loss in osteoporosis (38). Our observation defined a novel role of folic acid in HHcy induced bone remodeling and supported the hypothesis presented in Fig.6. In summary, we have shown that elevation of plasma Hcy level decreased blood flow in tibial artery, causing dis-balance of NOX-4/TRX-1 enzymes leads to increased production of ROS and activation of MMP-9. Enhanced production of ROS may cause decrease in eNOS and increase in iNOS protein levels, which may lead a decreased bioavailability of NO. Thus, our study unequivocally underscores the essential role of folic acid in HHcy-induced alterations leading to the bone remodeling.
Figure 6.
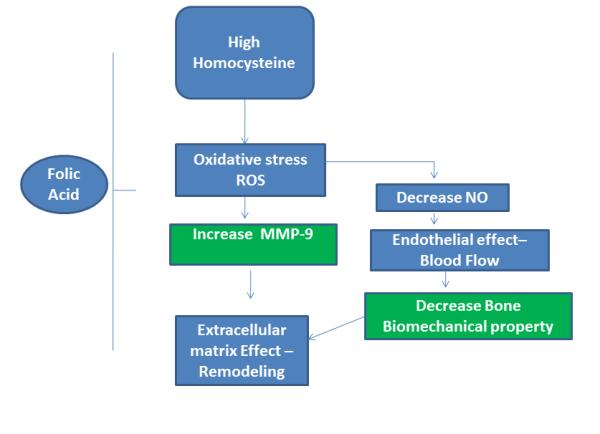
Schematic representation of the proposed hypothesis for HHcy-induced bone remodeling in mouse.
ACKNOWLEDGEMENTS
A part of this study was supported by NIH grants NS-51568 and HL-71010.
REFERENCE LIST
- 1.Alley RA, Chen EL, Beyer TD, Prinz RA. Does homocysteine contribute to bone disease in hyperparathyroidism? Am J Surg. 2008;195:374–377. doi: 10.1016/j.amjsurg.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Banfi G, Iorio EL, Corsi MM. Oxidative stress, free radicals and bone remodeling. Clin Chem Lab Med. 2008;46:1550–1555. doi: 10.1515/CCLM.2008.302. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy MF, McDowell IF, Ramsey MW, Brownlee M, Bones C, Newcombe RG, Lewis MJ. Hyperhomocysteinemia after an oral methionine load acutely impairs endothelial function in healthy adults. Circulation. 1998;98:1848–1852. doi: 10.1161/01.cir.98.18.1848. [DOI] [PubMed] [Google Scholar]
- 4.Browner WS, Malinow MR. Homocyst(e)inaemia and bone density in elderly women. Lancet. 1991;338:1470. doi: 10.1016/0140-6736(91)92782-w. [DOI] [PubMed] [Google Scholar]
- 5.Cagnacci A, Baldassari F, Rivolta G, Arangino S, Volpe A. Relation of homocysteine, folate, and vitamin B12 to bone mineral density of postmenopausal women. Bone. 2003;33:956–959. doi: 10.1016/j.bone.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Chow K, Cheung F, Lao TT, O K. Effect of homocysteine on the production of nitric oxide in endothelial cells. Clin Exp Pharmacol Physiol. 1999;26:817–818. doi: 10.1046/j.1440-1681.1999.03133.x. [DOI] [PubMed] [Google Scholar]
- 7.Claes L, Schmalenbach J, Herrmann M, Olku I, Garcia P, Histing T, Obeid R, Schorr H, Herrmann W, Pohlemann T, Menger MD, Holstein JH. Hyperhomocysteinemia is associated with impaired fracture healing in mice. Calcif Tissue Int. 2009;85:17–21. doi: 10.1007/s00223-009-9262-6. [DOI] [PubMed] [Google Scholar]
- 8.Dhonukshe-Rutten RA, Lips M, de JN, Chin APM, Hiddink GJ, van DM, de Groot LC, van Staveren WA. Vitamin B-12 status is associated with bone mineral content and bone mineral density in frail elderly women but not in men. J Nutr. 2003;133:801–807. doi: 10.1093/jn/133.3.801. [DOI] [PubMed] [Google Scholar]
- 9.Dhonukshe-Rutten RA, Pluijm SM, de Groot LC, Lips P, Smit JH, van Staveren WA. Homocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation, and fractures in healthy elderly people. J Bone Miner Res. 2005;20:921–929. doi: 10.1359/JBMR.050202. [DOI] [PubMed] [Google Scholar]
- 10.Dworakowski R, Anilkumar N, Zhang M, Shah AM. Redox signalling involving NADPH oxidase-derived reactive oxygen species. Biochem Soc Trans. 2006;34:960–964. doi: 10.1042/BST0340960. [DOI] [PubMed] [Google Scholar]
- 11.Eberhardt RT, Forgione MA, Cap A, Leopold JA, Rudd MA, Trolliet M, Heydrick S, Stark R, Klings ES, Moldovan NI, Yaghoubi M, Goldschmidt-Clermont PJ, Farber HW, Cohen R, Loscalzo J. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest. 2000;106:483–491. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evrengul H, Tanriverdi H, Kuru O, Enli Y, Yuksel D, Kilic A, Kaftan A, Kirac S, Kilic M. Elevated homocysteine levels in patients with slow coronary flow: relationship with Helicobacter pylori infection. Helicobacter. 2007;12:298–305. doi: 10.1111/j.1523-5378.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 13.Fajardo M, Liu CJ, Ilalov K, Egol KA. Matrix metalloproteinases that associate with and cleave bone morphogenetic protein-2 in vitro are elevated in hypertrophic fracture nonunion tissue. J Orthop Trauma. 2010;24:557–563. doi: 10.1097/BOT.0b013e3181ed294c. [DOI] [PubMed] [Google Scholar]
- 14.Fleming JT, Barati MT, Beck DJ, Dodds JC, Malkani AL, Parameswaran D, Soukhova GK, Voor MJ, Feitelson JB. Bone blood flow and vascular reactivity. Cells Tissues Organs. 2001;169:279–284. doi: 10.1159/000047892. [DOI] [PubMed] [Google Scholar]
- 15.Givvimani S, Sen U, Tyagi N, Munjal C, Tyagi SC. X-ray imaging of differential vascular density in MMP-9−/−, PAR-1−/+, hyperhomocysteinemic (CBS−/+) and diabetic (Ins2−/+) mice*. Arch Physiol Biochem. 2010 doi: 10.3109/13813455.2010.512042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heath JK, Atkinson SJ, Meikle MC, Reynolds JJ. Mouse osteoblasts synthesize collagenase in response to bone resorbing agents. Biochim Biophys Acta. 1984;802:151–154. doi: 10.1016/0304-4165(84)90046-1. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann M, Taban-Shomal O, Muller S, Gunter L, Hubner U, Bohm M, Herrmann W. Hyperhomocysteinemia--the biochemical link between a weak heart and brittle bones? Clin Lab. 2006;52:137–147. [PubMed] [Google Scholar]
- 18.Herrmann M, Widmann T, Herrmann W. Homocysteine--a newly recognised risk factor for osteoporosis. Clin Chem Lab Med. 2005;43:1111–1117. doi: 10.1515/CCLM.2005.194. [DOI] [PubMed] [Google Scholar]
- 19.Koh JM, Lee YS, Kim YS, Kim DJ, Kim HH, Park JY, Lee KU, Kim GS. Homocysteine enhances bone resorption by stimulation of osteoclast formation and activity through increased intracellular ROS generation. J Bone Miner Res. 2006;21:1003–1011. doi: 10.1359/jbmr.060406. [DOI] [PubMed] [Google Scholar]
- 20.Kusano K, Miyaura C, Inada M, Tamura T, Ito A, Nagase H, Kamoi K, Suda T. Regulation of matrix metalloproteinases (MMP-2, -3, -9, and -13) by interleukin-1 and interleukin-6 in mouse calvaria: association of MMP induction with bone resorption. Endocrinology. 1998;139:1338–1345. doi: 10.1210/endo.139.3.5818. [DOI] [PubMed] [Google Scholar]
- 21.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 22.McCreadie BR, Goldstein SA. Biomechanics of fracture: is bone mineral density sufficient to assess risk? J Bone Miner Res. 2000;15:2305–2308. doi: 10.1359/jbmr.2000.15.12.2305. [DOI] [PubMed] [Google Scholar]
- 23.McLean RR, Jacques PF, Selhub J, Tucker KL, Samelson EJ, Broe KE, Hannan MT, Cupples LA, Kiel DP. Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med. 2004;350:2042–2049. doi: 10.1056/NEJMoa032739. [DOI] [PubMed] [Google Scholar]
- 24.Mujumdar VS, Aru GM, Tyagi SC. Induction of oxidative stress by homocyst(e)ine impairs endothelial function. J Cell Biochem. 2001;82:491–500. doi: 10.1002/jcb.1175. [DOI] [PubMed] [Google Scholar]
- 25.Munjal C, Givvimani S, Qipshidze N, Tyagi N, Falcone JC, Tyagi SC. Mesenteric vascular remodeling in hyperhomocysteinemia. Mol Cell Biochem. 2011;348:99–108. doi: 10.1007/s11010-010-0643-y. [DOI] [PubMed] [Google Scholar]
- 26.Ohkubo K, Shimokawa H, Ogawa T, Suzuki S, Fukada K, Ohya K, Ohyama K. Immunohistochemical localization of matrix metalloproteinase 13 (MMP-13) in mouse mandibular condylar cartilage. J Med Dent Sci. 2003;50:203–211. [PubMed] [Google Scholar]
- 27.Renaud S, Leppert D. Matrix metalloproteinases in neuromuscular disease. Muscle Nerve. 2007;36:1–13. doi: 10.1002/mus.20772. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Rodriguez MA, Ruiz-Ramos M, Correa-Munoz E, Mendoza-Nunez VM. Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymes. BMC Musculoskelet Disord. 2007;8:124. doi: 10.1186/1471-2474-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tawakol A, Omland T, Gerhard M, Wu JT, Creager MA. Hyperhomocyst(e)inemia is associated with impaired endothelium-dependent vasodilation in humans. Circulation. 1997;95:1119–1121. doi: 10.1161/01.cir.95.5.1119. [DOI] [PubMed] [Google Scholar]
- 30.Tyagi N, Gillespie W, Vacek JC, Sen U, Tyagi SC, Lominadze D. Activation of GABA-A receptor ameliorates homocysteine-induced MMP-9 activation by ERK pathway. J Cell Physiol. 2009;220:257–266. doi: 10.1002/jcp.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tyagi N, Gillespie W, Vacek JC, Sen U, Tyagi SC, Lominadze D. Activation of GABA-A receptor ameliorates homocysteine-induced MMP-9 activation by ERK pathway. J Cell Physiol. 2009;220:257–266. doi: 10.1002/jcp.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyagi N, Givvimani S, Qipshidze N, Kundu S, Kapoor S, Vacek JC, Tyagi SC. Hydrogen sulfide mitigates matrix metalloproteinase-9 activity and neurovascular permeability in hyperhomocysteinemic mice. Neurochem Int. 2010;56:301–307. doi: 10.1016/j.neuint.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:H2649–H2656. doi: 10.1152/ajpheart.00548.2005. [DOI] [PubMed] [Google Scholar]
- 34.Upchurch GR, Jr., Welch GN, Fabian AJ, Pigazzi A, Keaney JF, Jr., Loscalzo J. Stimulation of endothelial nitric oxide production by homocyst(e)ine. Atherosclerosis. 1997;132:177–185. doi: 10.1016/s0021-9150(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 35.Weiss N, Heydrick S, Zhang YY, Bierl C, Cap A, Loscalzo J. Cellular redox state and endothelial dysfunction in mildly hyperhomocysteinemic cystathionine beta-synthase-deficient mice. Arterioscler Thromb Vasc Biol. 2002;22:34–41. doi: 10.1161/hq1201.100456. [DOI] [PubMed] [Google Scholar]
- 36.Woo KS, Chook P, Lolin YI, Sanderson JE, Metreweli C, Celermajer DS. Folic acid improves arterial endothelial function in adults with hyperhomocystinemia. J Am Coll Cardiol. 1999;34:2002–2006. doi: 10.1016/s0735-1097(99)00469-6. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Li H, Jin H, Ebin Z, Brodsky S, Goligorsky MS. Effects of homocysteine on endothelial nitric oxide production. Am J Physiol Renal Physiol. 2000;279:F671–F678. doi: 10.1152/ajprenal.2000.279.4.F671. [DOI] [PubMed] [Google Scholar]
- 38.Zhao H, Cai G, Du J, Xia Z, Wang L, Zhu T. Expression of matrix metalloproteinase-9 mRNA in osteoporotic bone tissues. J Tongji Med Univ. 1997;17:28–31. doi: 10.1007/BF02887998. [DOI] [PubMed] [Google Scholar]


