Abstract
Cutaneous leishmaniasis is clinically widespread but lacks treatments that are effective and well tolerated. Because all present drugs have been grandfathered into clinical use, there are no examples of a pre-clinical product evaluation scheme that lead to new candidates for formal development. To provide oral agents for development targeting cutaneous leishmaniasis, we have implemented a discovery scheme that incorporates in vitro and in vivo testing of efficacy, toxicity, and pharmacokinetics/metabolism. Particular emphasis is placed on in vivo testing, progression from higher-throughput models to those with most clinical relevance, and efficient use of resources.
Introduction
Global incidence of symptomatic leishmaniasis is estimated at 2 million cases per year and is rapidly increasing, making leishmaniasis a top priority for the tropical disease program of the World Health Organization.1,2 Annual mortality is approximately 50,000 with a disease burden of 2.4 million disability-adjusted life years. Of the two million new cases of leishmaniasis that occur each year, approximately three-fourths are cases of cutaneous leishmaniasis (CL).1,2
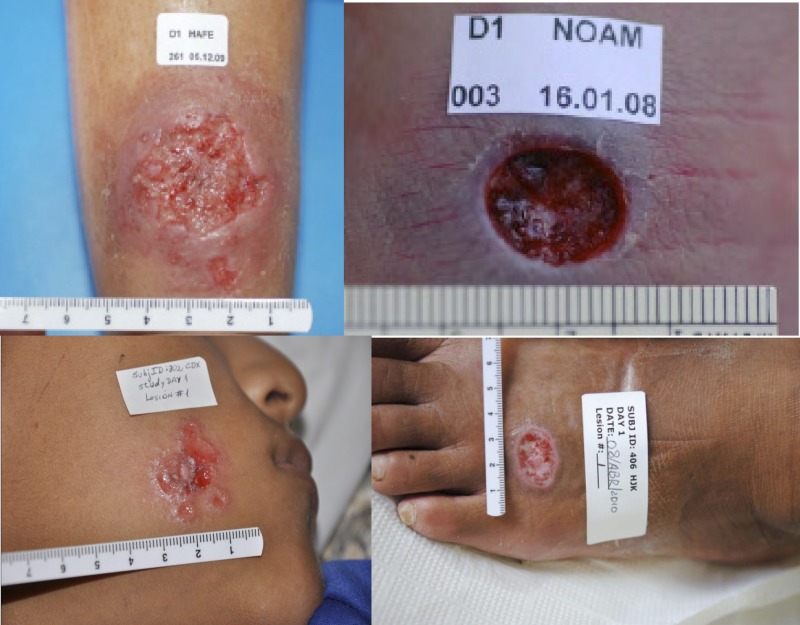
Current treatments for CL are poorly justified and have sub-optimal effectiveness. Treatment may be topical or systemic, but the infecting Leishmania species/strain and geographic region impact treatment efficacy. Few clinical trials have been appropriately designed and reported,3 especially when compared to the large number of possible combinations of treatments versus Leishmania species versus geographic regions. However, it may be said that because symptoms caused by L. major (Old World) and L. mexicana (New World) generally rapidly self-cure over several months, it is difficult to choose a drug with sufficiently low side effects to give a favorable risk-benefit ratio. In contrast, L. tropica (Old World) CL heals more slowly and is generally treated with local or systemic chemotherapy, and New World CL (non-L. mexicana) is treated either to speed healing or prevent dissemination to the oro-nasal mucosa (mucosal leishmaniasis).2–4 Thus, although these treatment strategies are used, each has uncertain cure rates and therapeutic indices. Several species of Leishmania that cause CL are shown in Figure 1.
Figure 1.
Cutaneous leishmaniasis lesions. Top left and right = Leishmania major; bottom left = L. peruviana; bottom right = L. panamensis.
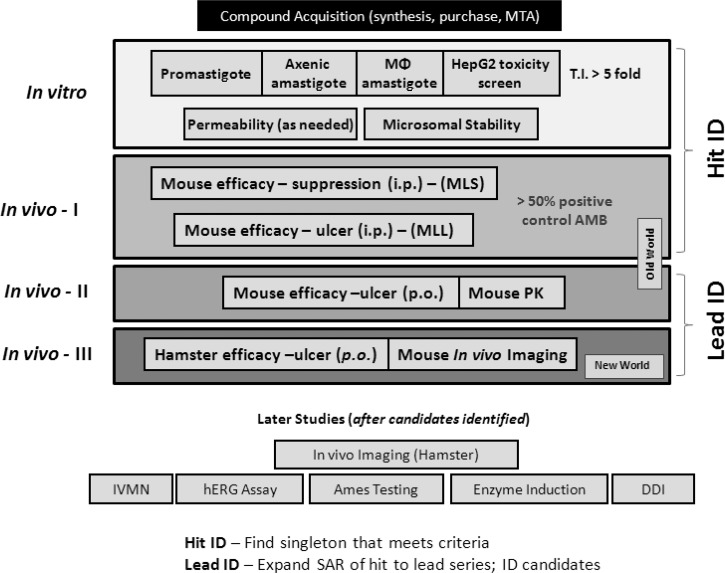
The overall need for CL that does not rapidly self-cure is an effective, well-tolerated, orally bioavailable agent that is active against several etiologic species, yields superior cosmetic results, and can be manufactured at low-cost and adapted for use in rural areas. Currently, the drugs used for CL are the classic agents pentavalent antimony, pentamidine, and amphotericin B; and the newer agents imidazoles, miltefosine, paromomycin (injectable and topical5), and liposomal amphotericin B. Because these agents were grandfathered in or developed for other diseases, there are no examples of a pre-clinical testing strategy that has lead to formal clinical development of anti-CL agents. Thus, we have implemented the following scheme (Figure 2) to provide oral agents for development against CL.
Figure 2.
Cutaneous leishmaniasis drug discovery scheme. MTA = material transfer agreement; T.I. = therapeutics initiative; i.p. = intraperitoneal; MLS = mouse leishmaniasis suppression; ID, identification; MLL = mouse leishmaniasis lesion; p.o. = per os; PK = pharmacokinetics; IVMN = in vitro micronucleus assay; hERG = human ether-à-go-go–related gene; DDI = drug-drug interaction; SAR = structure activity relationship.
Drug Discovery Algorithm
All experiments were performed in compliance with Accreditation of Laboratory Animal Care. The Walter Reed Army Institute of Research is an Accreditation of Laboratory Animal Care–accredited institution. Animal experimentation was approved by the Walter Reed animal ethics review body (Laboratory Animal Care and Use Committee).
In vitro evaluation of potential anti-CL agents.
Our algorithm for in vitro evaluation of compounds and prioritization of chemotypes for potential development was recently published.6 Our initial assay uses exponentially growing promastigotes in 384-well assay plate format suitable for high throughput screening (HTS). We initially evaluate compounds starting at a concentration of 10 μM using a single replicate, followed by the identification of compounds exhibiting at least 50% inhibition of signal, which are then further examined with multiple compound concentrations against promastigotes and axenic amastigotes to enable us to determine the concentration that produces 50% growth inhibition. Compound libraries that produce a significant number of active compounds (i.e., > 100) are prosecuted using cluster analysis of the actives, then we identify representative compounds from each structural cluster to reduce the number of compounds being evaluated to a more manageable number. With larger structural clusters (those that contain more > 10 compounds) we may select multiple representative compounds from the individual clusters. All compounds that remain unassigned to structural clusters (i.e., singletons) are evaluated when possible or available.
Compound evaluation has a series of internal validation procedures that align with industry HTS standards. Our HTS developmental and validation process has been described.7 In brief, for the assay development procedures, we optimize basic assay conditions, standardize experimental as well as automated procedures (i.e., culturing conditions, growth characteristics, seeding density), and perform a three-day variability assessment that determines the basic screenability of the assay. The HTS assay would then progress to dimethylsulfoxide validation, which consists of three sets of five 384-well microtiter assay plates and functions to help estimate false-positive results. Final compound validation step, which mimics an HTS campaign, uses the Library of Pharmaceutically Active Compounds 1280 (LOPAC) compound library. We screen the library in duplicate, determine the reproducibility of the duplicate assay evaluations (R2), and include the known inhibitors to confirm that the assay is functioning properly. Our criteria for assay progression is dependent on Z-factors, coefficients of variance, and signal to background, as well as low estimated rates of false-positive results and elimination of any automation issues. Once these steps are successfully completed, we undertake the actual HTS campaign with experimental compounds.
Once the compound evaluation is complete and we have 50% growth inhibition determinations, we then initially assess the pharmacokinetic properties and toxicity of compounds by using in silico methods. Select representative compounds or chemotypes are then evaluated using a MDR1-MDCK cell permeability assay, metabolic stability assays using mouse and human liver microsomes, and a HEPG2 cell toxicity assay. A minimum threshold for MDR1-MDCK permeability is 2 × 10−6 cm/sec and a microsomal stability in mouse and human liver microsomes of t½ > 20 minutes.
We define the in vitro therapeutic index as 50% toxic concentration to macrophages/50% efficacy concentration for parasites. Compounds with an in vitro therapeutic index ≥ 5, and metabolic stability > 20 minutes in mouse liver microsomes qualify as an in vitro hit and advancement to in vivo testing.
It is difficult to decide which form of the parasite to use in the calculation of therapeutic index. Promastigotes are simple to use but not clinically relevant; amastigotes have the opposite characteristics. We have determined the in vitro efficacy of all known anti-leishmanial drugs and several other chemical classes against promastigotes, axenic amastigotes, and within-macrophage amastigotes for 1–4 Leishmania species. We are currently comparing the results for these compounds with their observed in vivo efficacy. Until this in vitro/in vivo correlation is complete, we cannot specify the most appropriate in vitro efficacy model for a Leishmania species or a class of compounds. In this regard, we note that de Muylder and others have recently compared the frequency of in vitro hits by using promastigotes, axenic amastigotes, and within-macrophage amastigotes for 909 compounds,8 and a publication by Zhu and others is also now available.9 The in vitro experience of de Muylder and others suggested that screening against the promastigote stage of L. donovani, although more suitable for automation, fails to identify all active compounds and leads to numerous false-positive hits.8
In vivo evaluation of potential oral agents for CL agents.
In vivo screening and evaluation of chemical agents requires model systems that are efficient and represent the clinical situation (Table 1). Because these two requirements generally contradict each other, our in vivo testing paradigm starts with the most efficient model and proceeds to the most clinically comparable models.
Table 1.
In vivo efficacy models for cutaneous leishmaniasis drug discovery*
| Animal model (infected base tail) | Parasite | Initial drug dose schedule | Drug dose | Duration of therapy | Route and drug doses/week | Endpoint evaluated (day after infection) | Mean lesion size in negative controls, mm2 | Lesion size in positive controls, mm2 | % Suppression by positive control drug† |
|---|---|---|---|---|---|---|---|---|---|
| Tier 1a | |||||||||
| Mouse suppression: BALB/c mouse | L.m., L.p. | 3 days after infection | No prior in vivo: 40 MKD/160 MKD. Prior in vivo: ¼ LD50 | 1 qd × 10 d | IP = 22 | 28 (L.m.) 35 (L.p.) | 109 | AMB (25 MKD) IP = 8 | 93 |
| Tier 1b | |||||||||
| Mouse treatment: BALB/c mouse | L.m., L.p. | Lesion size = 2 mm in one dimension | Dose successful in mouse suppression test | 1 qd × 10 d | IP = 22 | 45 | 194 | AMB (25 MKD) IP = 8 | 92 |
| Tier 2 | |||||||||
| Mouse treatment: BALB/c mouse, PO drug | L.m., L.p. | Lesion size = 2 mm in one dimension | 1× and 4× dose successful in mouse treatment test with IP drug | 1 qd × 10 d | PO = 22 | 45 | 194 | AMB (25 MKD) IP = 8 | 92 |
| Tier 3 | |||||||||
| Hamster treatment: golden hamster, PO drug | L.p. | Lesion size = 70 mm2 | Full range of doses | 1 bid × 4 d | PO = 10 | 30 | 130 | Gluc (208 MKD) IM = 30 | 77 |
L.m. = Leishmania major; L.p. = L panamensis; MKD = mg/kg/day; LD50 = 50% lethal dose; qd = four times/day; d = day; IP = intraperitoneal; AMB = AmBiosome; PO = per os; Gluc = Glucantime; bid = two times/day.
% Suppression = 100 × [(negative control size − positive control size)/negative control size].
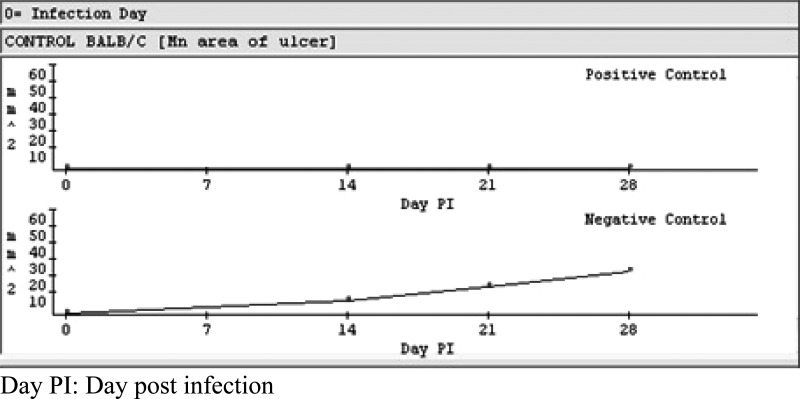
As a group, the animal models have undergone internal validation and are reproducible according to industry standards. The key parasite factors to ensure in vivo virulence are inherent parasite virulence, reproducibility of inoculum, and animal handling. The parasites are recent isolates from human ulcers that were selected for in vivo infectivity and typed by isoenzyme electrophoresis. To ensure reproducibility, the chosen strains were cloned, expanded, and used to generate a working seed. Parasites for infection are maintained in donor animals with a maximum of three passes in culture before infecting test animals. Animals are consistently infected with 1 × 106 metacyclic promastigotes. As a result, infection has been 100% and lesion appearance time and size (> 2 mm in one dimension) has been consistent at 14 days (L. major) (Figure 3) and 25 days (L. panamensis) in > 100 experiments. The injection site is shaved every other day so hair will not inhibit ulcer development. These measures have lead to consistent healing of the lesions by control drugs and thus good separation between lesion sizes in the negative and positive controls by day 28 for L. major (Figure 3) and day 35 for L. panamensis.
Figure 3.
Lesion sizes in Leishmania major–infected mice untreated (negative control) or treated with AmBisome (AMB) (positive control).
Tier 1a: in vitro mouse suppression test.
We first use a BALB/c mouse infected with L. major because it cannot mount an effective immune response to L. major.10 This step provides a stringent model that will solely evaluate efficacy of agents against the parasite. In the mouse suppression test, compounds are administered three days after parasite inoculation. The advantage of this model is that growth of the lesion can be evaluated in a relatively short time of less than 30 days. The throughput of this model is 22 compounds per week at one dose. This model can be scaled up as necessary.
Compounds are initially administered intraperitoneally as follows: compounds that are in vitro hits without prior in vivo data are administered as two dose groups (40 mg/kg/day and 160 mg/kg/day) and compounds with prior in vivo data (i.e., known drugs) are administered at one dose of ¼ of the established 50% lethal dose.
Efficacy is assessed by comparing the suppression of lesion size after 28 days in the drug treated group to that in positive controls administered Ambisome (25 mg/kg/day) for 10 days. Percent suppression is defined as {[(LS(-)C) − LS(drug)]/LS(-)C} × 100, where LS(-)C = lesion size in negative control and LS(drug) = lesion size in drug group. The threshold for success is percent suppression that is at least 50% that of the positive control Ambisome. For example, if the lesion size is 109 mm2 when treated with placebo and is 8 mm2 when treated with Ambisome (25 mg/kg/day), and the lesion size for a test compound is 50 mm2, % suppression (Ambisome) = 93% and % suppression (compound) = 54%. Thus, the compound is worth pursuing on the basis of the exhibited in vivo efficacy. If a compound is successful in the mouse suppression test (> 50% Ambisome index) and structural analogs are available, those analogs are also tested in this model at the same concentration as the initial compound. This step builds an initial structure activity relationship for the series. The most active compound of this series is then evaluated by intraperitoneal (IP) dosing, in the mouse treatment test. If structural analogs are not available, the initial compound itself is evaluated in the mouse treatment test.
Tiers 1b and 2: mouse treatment test.
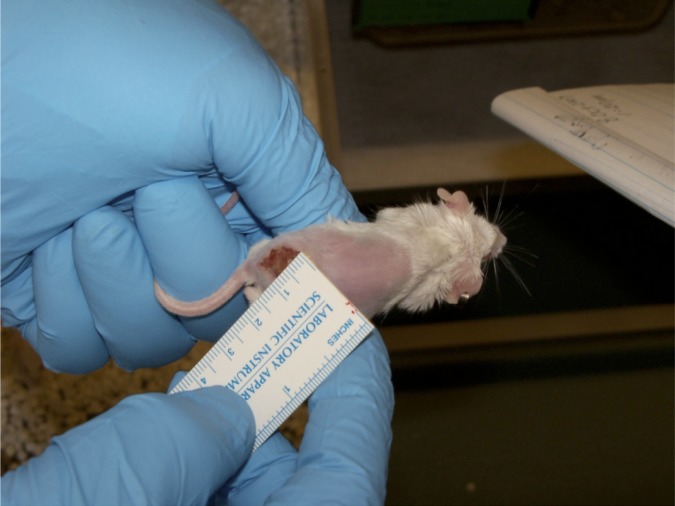
In the mouse treatment test, the test agent is administered when a lesion is no less than 2 mm in diameter (the minimal ulcer size that can be measured with a digital caliper consistently) (Figure 4). The advantage of this model is that it more closely resembles the clinical situation of treating a lesion, but lesion response requires evaluation over the longer period of 45 days.
Figure 4.
Shaved mouse with cutaneous leishmaniasis with lesion at base of the tail.
Tier 1b: in vivo mouse treatment test with intraperitoneal drug administration.
Intraperitoneal drug administration for the mouse treatment test uses the same dose that was successful in the mouse suppression test with IP drug administration. Success is similarly defined: % suppression that is at least 50% that of the positive control Ambisome. At this point, all easily acquired structural analogs will also be tested by IP administration, and all active compounds will progress to the mouse treatment test by using oral drug administration.
Tier 2: mouse treatment test with oral drug administration.
Because bioavailability is unlikely to be 100%, initial dosing in the mouse treatment model with oral drug administration uses two doses: the dose that was successful in the mouse treatment model using IP drug administration, and four times that dose. Success (% suppression that is at least 50% that of the positive control, Ambisome) with either dose leads to evaluation of a range of doses so that a 50% effective dose and toxicity parameters can be evaluated. Toxicity measures that we use are death, neurologic instability, or weight loss.
Pharmacokinetics is simultaneously performed when the compound moves into Tier 2 and evaluation of oral administration. At this point, we have started to accumulate enough data to identify a chemical series and its attributes. A medicinal chemistry campaign is initiated to design compounds to exploit any structure activity relationship that is evident and/or to mitigate any toxicity or pharmacokinetic issues. The ideal pharmacokinetic threshold criteria are oral bioavailability > 30%, clearance (projected human) < 10 mL/min/kg, and a half-life of at least 8 hours. The threshold for initial metabolism studies is a cytochrome P450 inhibition 50% inhibitory concentrations > 10 μM for CYP 3A4, 2D6, 1A2, and 2C19.
The therapeutic index for this in vivo model is defined as the dose that causes 10% toxicity/ED100. If the therapeutic index is ≥ 1 and the compound has attractive pharmacokinetic properties, the compound is evaluated in the hamster model.
Tier 3: in vivo hamster treatment test.
The final in vivo model uses a different host and parasite species, golden hamsters infected with L. panamensis. Leishmania panamensis ultimately self-cures in the hamster, but at the early period of three weeks after infection; treatment with the clinical agent pentavalent antimony reduces parasite numbers by 96%.11 Thus, agents previously shown to be orally active in the mouse treatment model are also evaluated by oral administration in this distinct treatment model that uses a different host, different parasite, and different immunity. Efficacy in two animal species is a routine regulatory requirement.
A full range of doses are evaluated in the hamster. The therapeutic index for this in vivo model is also defined as the dose that causes 10% toxicity/ED100. For this last evaluation of in vivo efficacy, the threshold for success is defined as a therapeutic index ≥ 2.
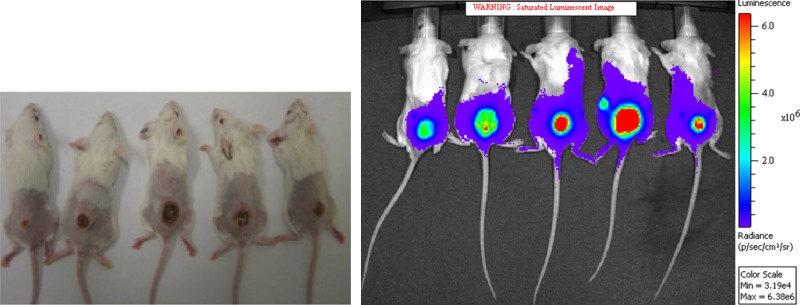
In vivo non-invasive imaging is performed in conjunction with the hamster treatment test. This imaging uses luciferase-tagged parasites in the mouse treatment model. Change in lesion light intensity with time reflects loss of parasites with time (Figure 5). Demonstration that the parasite number decreases post-drug administration, and that the decrease correlates with a change in lesion size, confirms the relationship between drug parasite killing activity and lesion cure. A discrepancy between diminution in lesion size and decrease in parasite number would suggest that the anti-leishmanial agent can modify host reactions in addition to directly killing the parasite.
Figure 5.
Shaved mice with Leishmania major lesions at base of tail at 35 days post infection (left) and as also seen in the in vivo non-invasive imaging system (right).
Transition to drug development.
Successful compounds in the mouse and hamster treatment tests will have demonstrated a favorable therapeutic index in two models and favorable pharmacokinetics. Further toxicity assessment is performed at this point by using the Ames test, in vitro micronucleus assay,12,13 and the hERG (human ether-à-go-go–related gene) patch-clamp assay.14 Exit criteria are negative results for the Ames test and the in vitro micronucleus assay, and an 50% inhibitory concentration > 100 times the efficacious dose in the hERG patch-clamp assay.14 If the compound has a favorable toxicity profile, displays efficacy against multiple strains of Old World and New World CL, and pharmacokinetics (see ideal criteria vide supra) are also attractive, the compound is considered a candidate for formal pre-clinical drug development.
Discussion
The clinical need for drugs for CL is the driver for discovery of potential anti-leishmanial agents. The key variables of efficacy, toxicity, and pharmacokinetics/metabolism are evaluated in our drug discovery scheme.
Our focus in this strategy is on in vivo data. Because there are no established threshold values for in vitro models of efficacy, we initiate in vivo evaluation as soon as possible. In fact, a compound with prior animal or human data will first be evaluated in the mouse suppression test rather than in in vitro tests.
We have optimized efficiency to minimize required resources and cycle time. For in vivo experimentation, only 1–2 doses of compound are evaluated in the initial IP models (Tier 1). The first resource intensive step is the mouse treatment model using oral administration (Tier 2) when a range of doses is evaluated. Time and compound are focused on a treatment situation evaluating efficacy and toxicity after oral administration. Initial use of a pathophysiologically stringent model with a short cycle time of 28 days also conserves resources. Time/resources are further conserved by limiting analog synthesis to compounds that can be prepared by using validated chemical methods and less than six chemical steps, thus addressing the cost of goods issue early.
We note that the BALB/c mouse can be infected with other Leishmania species. Infection with L. mexicana,15 L. amazonensis,16 L. panamensis,17 and L. braziliensis18 has been reported in the literature, and we have additionally been able to infect BALB/c mice with L. tropica, L. peruviana, and L. guyanensis (Grogl M and others, unpublished data). Hamsters also may be infected with L. braziliensis.19 Although demonstration of a favorable therapeutic index against L. major in BALB/c mice and L. panamensis in hamster may be sufficient, we would generally confirm the efficacy of the pre-clinical candidate against multiple Leishmania species in these animal models.
The ultimate transition to drug development involves evaluation of a full range of parameters: efficacy and toxicity after oral administration in two clinically-relevant treatment models; pharmacokinetics after oral administration; preliminary evaluation of metabolism, cardiotoxicity, and genetic toxicity. Overall, we present this drug discovery plan as a reasonable strategy to identify and evaluate potential agents for development for the oral treatment of CL.
ACKNOWLEDGMENTS
We thank Dr. K. Werbovetz for reviewing the manuscript.
Disclaimer: The views, opinions and/or findings contained in this presentation are those of the author(s) and do not necessarily reflect the views of the Department of Defense and should not be construed as an official DoD/Army position, policy or decision unless so designated by other documentation. No official endorsement should be made.
Footnotes
Authors' addresses: Max Grogl, Mark Hickman, William Ellis, Thomas Hudson, Jacob Johnson, Jonathan Berman, and Richard J. Sciotti, Division of Experimental Therapeutics, Walter Reed Army Institute of Research, Silver Spring, MD, E-mails: max.grogl1@us.army.mil, mark.r.hickman@us.army.mil, bill.ellis@us.army.mil, thomas.hudson@us.army.mil, jjohnson@wrp-ksm.org, jbe9320457@aol.com, and richard.sciotti@us.army.mil. John S. Lazo, Department of Pharmacology and Chemistry, University of Virginia, Charlottesville, VA, E-mail: jsl8f@hscmail.mcc.virginia.edu. Elizabeth R. Sharlow, Department of Pharmacology, University of Virginia, Charlottesville, VA, E-mail: ers7g@virginia.edu.
References
- 1.Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001;95:239–243. doi: 10.1016/s0035-9203(01)90223-8. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Control of the leishmaniases. World Health Organ Tech Rep Ser. 2010;949:1–186. [PubMed] [Google Scholar]
- 3.Gonzalez U, Pinart M, Reveiz L, Rengifo-Pardo M, Tweed J, Macaya A, Alvar J. Designing and reporting clinical trials on treatments for cutaneous leishmaniasis. Clin Infect Dis. 2010;51:409–419. doi: 10.1086/655134. [DOI] [PubMed] [Google Scholar]
- 4.Sharlow ER, Grögl M, Johnson J, Lazo JS. Anti-leishmanial drug discovery: rising to the challenges of a highly neglected disease. Mol Interv. 2010;10:72–75. doi: 10.1124/mi.10.2.4. [DOI] [PubMed] [Google Scholar]
- 5.Ben Salah A, Buffet PA, Morizot G, Ben Massoud N, Zâatour A, Ben Alaya N, Haj Hamida NB, El Ahmadi Z, Downs MT, Smith PL, Dellagi K, Grögl M. WR279,396, a third generation aminoglycoside ointment for the treatment of Leishmania major cutaneous leishmaniasis: a Phase 2, randomized, double blind, placebo controlled study. PLoS Negl Trop Dis. 2009;3:e432. doi: 10.1371/journal.pntd.0000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharlow ER, Close D, Shun T, Leimgruber S, Reed R, Mustata G, Wipf P, Johnson J, O'Neil M, Grögl M, Magill AJ, Lazo JS. Identification of potent chemotypes targeting Leishmania major using a high-throughput, low-stringency, computationally enhanced, small molecule screen. PLoS Negl Trop Dis. 2009;3:e540. doi: 10.1371/journal.pntd.0000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharlow ER, Leimgruber S, Yellow-Duke A, Barrett R, Wang QJ, Lazo JS. Development, validation and implementation of immobilized metal affinity for phosphochemicals (IMAP)-based high-throughput screening assays for low-molecular-weight compound libraries. Nat Protoc. 2008;3:1350–1363. doi: 10.1038/nprot.2008.111. [DOI] [PubMed] [Google Scholar]
- 8.De Muylder G, Ang KK, Chen S, Arkin MR, Engel JC, McKerrow JH. A screen against Leishmania intracellular amastigotes: comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl Trop Dis. 2011;5:e1253. doi: 10.1371/journal.pntd.0001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu X, Pandharkar T, Werbovetz K. Identification of new antileishmanial leads from hits obtained by high-throughput screening. Antimicrob Agents Chemother. 2012;56:1182–1189. doi: 10.1128/AAC.05412-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakthianandeswaren A, Foote SJ, Handman E. The role of host genetics in leishmaniasis. Trends Parasitol. 2009;25:383–391. doi: 10.1016/j.pt.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Hanson WL, Chapman WL, Waits VL, Lovelace JK. Development of Leishmania (Vianna) panamensis lesions and relationship of numbers of amastigotes to lesion areas on antimony-treated and untreated hamsters. J Parasitol. 1991;77:780–783. [PubMed] [Google Scholar]
- 12.Mortelmans K, Zeiger E. The Ames Salmonella/M microsome mutagenicity assay. Mutat Res. 2000;455:29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 13.Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 14.Sanguinetti M, Mitcheson J. Predicting drug-hERG channel interactions that cause acquired long QT syndrome. Trends Pharmacol Sci. 2005;26:119–124. doi: 10.1016/j.tips.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Serrano-Martín X, Payares G, De Lucca M, Martinez JC, Mendoza-León A, Benaim G. Amiodarone and miltefosine act synergistically against Leishmania mexicana and can induce parasitological cure in a murine model of cutaneous leishmaniasis. Antimicrob Agents Chemother. 2009;53:5108–5113. doi: 10.1128/AAC.00505-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva-Almeida M, Carvalho LO, Abreu-Silva AL, d'Escoffier LN, Calabrese KS. Leishmania (Leishmania) amazonensis infection: muscular involvement in BALB/c and C3H.HeN mice. Exp Parasitol. 2010;124:315–318. doi: 10.1016/j.exppara.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Castilho TM, Goldsmith-Pestana K, Lozano C, Valderrama L, Saravia NG, McMahon-Pratt D. Murine model of chronic L. (Viannia) panamensis infection: role of IL-13 in disease. Eur J Immunol. 2010;40:2816–2829. doi: 10.1002/eji.201040384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tonui W, Titus RG. Cross-protection against Leishmania donovani but not L. braziliensis caused by vaccination with L. major soluble promastigote exogenous antigens in BALB/C mice. Am J Trop Med Hyg. 2007;76:579–584. [PubMed] [Google Scholar]
- 19.Morais-Teixeira E, Carvalho AS, Costa J, Duarte SL, Mendonça JS, Boechat N, Rabello A. In vitro and in vivo activity of meglumine antimoniate produced at Farmanguinhos-Fiocruz, Brazil, against Leishmania (Leishmania) amazonensis, L. (L.) chagasi and L. (Viannia) braziliensis. Mem Inst Oswaldo Cruz. 2008;103:358–362. doi: 10.1590/s0074-02762008000400008. [DOI] [PubMed] [Google Scholar]