Abstract
Lassa fever is an acute and sometimes severe viral hemorrhagic illness endemic in West Africa. One important question regarding Lassa fever is the duration of immunoglobulin G (IgG) antibody after infection. We were able to locate three persons who worked in Nigeria dating back to the 1940s, two of whom were integrally involved in the early outbreaks and investigations of Lassa fever in the late 1960s, including the person from whom Lassa virus was first isolated. Two persons had high titers of Lassa virus-specific IgG antibody over 40 years after infection, indicating the potential for long-term duration of these antibodies. One person was likely infected in 1952, 17 years before the first recognized outbreak. We briefly recount the fascinating stories of these three pioneers and their important contribution to our understanding of Lassa fever.
Lassa fever (LF) is an acute and sometimes severe viral hemorrhagic illness endemic in West Africa.1 The disease was first recognized in Nigeria in 1969.2 Humans contract Lassa virus (LASV) primarily through contact with contaminated excreta of the rodent Mastomys natalensis, which is the natural reservoir.1 Secondary transmission between humans occurs through direct contact with infected blood or bodily secretions.1 Nosocomial outbreaks have been described in endemic areas.1 The onset of illness of LF typically comprises non-specific signs and symptoms difficult to distinguish from many other febrile diseases. Some patients progress to severe vascular instability and multiorgan system failure, with case fatality ratios in hospitalized cases of about 20%.1 Enzyme-linked immunosorbent assay (ELISA) is the mainstay of diagnosis.3 The immunofluoresent antibody assay may also be used, although problems of sensitivity, specificity, and subjectivity in interpretation of the test have been reported.3–5 The antiviral drug ribavirin is effective therapy, especially when given within 6 days of the onset of illness.1
Many questions remain regarding the pathogenesis and natural history of LF. One important question is the duration of IgG antibody after infection. Anecdotal reports suggest that the IgG antibody response persists for years, but few survivors of LF have been tested more than 2 years after acute disease.5,6 Knowing the duration of the antibody response is important in estimating the true incidence of infection and interpreting seroprevalence data, especially considering that a seroreversion rate of IgG antibody (i.e., from positive to negative) measured by the immunofluoresent antibody assay of 6.4% per year has been reported.7 If IgG antibody seroreversion frequently and rapidly occurs after infection, secondary infections may be mistaken for primary ones, resulting in overestimation of the rate of mild and asymptomatic disease. Indeed, existing dogma on LF is that most LASV infections are asymptomatic or result in mild illness.7 The prevalence of past infection in the community as determined by IgG antibodies could also be significantly underestimated if seroreversion occurs rapidly.
The duration of IgG antibody after a single infection is almost impossible to discern in populations living in endemic areas because reexposure to LASV can rarely be definitively ruled out. To provide insight into the duration of IgG antibody after LASV infection, we were able to locate and perform testing for LASV-specific IgG antibodies on three people who worked in Nigeria dating back to the 1940s, two of whom were integrally involved in the early outbreaks and investigations of LF in the late 1960s. The study was approved by the Institutional Review Board of Tulane University. In addition to the scientific knowledge to be gleaned from investigation of these individuals, we found the stories of these three pioneers to be a fascinating and important contribution to science and felt that they should be presented in the scientific literature on LF. Although respect for patient confidentially would normally preclude the use of names, the names of the persons involved in these early outbreaks of LF were widely publicized in the popular press as well as scientific literature. Furthermore, all three subjects, or their families, have provided written permission for the use of their names in this publication. A brief description of the three subjects is provided here.
-
(1)
Subject 1 is Ms. Lily (Penny) Pinneo, who was the first documented case of LF and from whom the first LASV was isolated in 1969.2 Her story was well-documented in the scientific and popular press, including by Ms. Pinneo herself.8,9 Ms. Pinneo was infected while working as a nurse at Jos Mission Hospital in Jos, Nigeria, and she recovered from her infection after being medically evacuated to the United States. She returned to Jos in 1970 with a small supply of her own serum intended to treat her colleague Dr. Jeanette Troup, who was infected with LASV while performing an autopsy.2 Unfortunately, Ms. Pinneo arrived 10 days after Dr. Troup's death. Ms. Pinneo continued working in Jos as a nurse, anesthetist, and midwife until she retired in 1985, with two 1-year furloughs to the United States during that span. In 1983, she also went briefly to Zorzor and Phoebe Hospitals in Liberia to assist with research on LF, collecting blood from patients and hospital staff. Ms. Pinneo denies any illness consistent with LF or noted exposure to LASV after 1969. For more details on Ms. Pinneo, see the video “Penny's Story” at www.vimeo.com/calmdog/pennystory.
-
(2)
Subject 2 was Mr. Harry Elyea, who also worked as a missionary nurse in Nigeria from 1945 to 1968. He never returned to West Africa after leaving Nigeria. According to both Ms. Pinneo and Mr. Elyea, he was one of the missionaries who was tested and found positive for LASV IgG by complement fixation antibody testing in a study by Frame10 performed in 1972. Furthermore, Mr. Elyea reported having a disease consistent with LF in 1952, with fever, headache, and sore throat, and he was told by the clinicians taking care of him that he “was not going to make it.” Nevertheless, he eventually recovered to continue his work in Nigeria. Sadly, Mr. Elyea died 9 months after we conducted this study.
-
(3)
Subject 3 is Dr. Hal White, a physician who worked at Jos Mission Hospital in Nigeria from 1968 to 1975, during which time he was involved in the investigation of numerous cases and outbreaks of LF.9,11–13 He returned to Nigeria for a few weeks, primarily for administrative reasons, in 1976. Dr. White denies having had LF himself, although he worked in an area and hospital where nosocomial transmission of Lassa virus was repeatedly seen; therefore, he was obviously at risk. Dr. White did have an illness consistent with dengue fever during his time in Africa.
We visited and obtained serum from Ms. Pinneo, Mr. Elyea, and Dr. White in December of 2010, approximately 42 years after Ms. Pinneo's confirmed episode of LF and 58 years after Mr. Elyea's febrile illness that he attributed to LASV. We tested all three subjects for LASV-specific IgG antibodies using a recombinant antigen-based ELISA assay.14 The antigens used were recombinant LASV (Josiah strain originally isolated from Sierra Leone) glycoproteins (GPs) and nucleoproteins (NPs) kindly provided by Dr. Erica Ollmann Saphire of the Scripps Institute, La Jolla, CA. Briefly, antigen was diluted in 0.1 M sodium bicarbonate and coated onto 96-well ELISA plates at 2 μg/mL. After a block step, serum diluted in Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS), 10% goat serum, and 0.5% Tween was added, followed by horseradish peroxidase (HRP)-goat anti-human IgG as the secondary antibody and tetramethylbenzidine-peroxide substrate. Positive controls consisted of convalescent serum from three cases of LF from Sierra Leone confirmed by ELISA antigen assay.3,15 Negative controls were sera from 2 persons from Sierra Leone without histories of LF as well as 10 normal healthy persons in New Orleans without histories of LF or travel to West Africa. All steps were performed at room temperature with 1-hour incubations. Assays were run in triplicate.
We initially screened each serum sample for evidence of antibody to LASV at a single dilution of 1:300. Samples with optical densities greater than 2 SDs above the average for the negative controls (> 0.422 and 0.640 for GP and NP, respectively) were considered positive and further tested along with a negative control with threefold dilutions beginning at 1:50. Samples on this second-round ELISA were considered positive at a specific dilution if the optical density exceeded 2.5 times the normal control. In addition, because of Mr. White's history of reported dengue fever, we tested all three subjects for IgG antibody to dengue virus serotypes 1 and 2 using an antigen capture ELISA.16
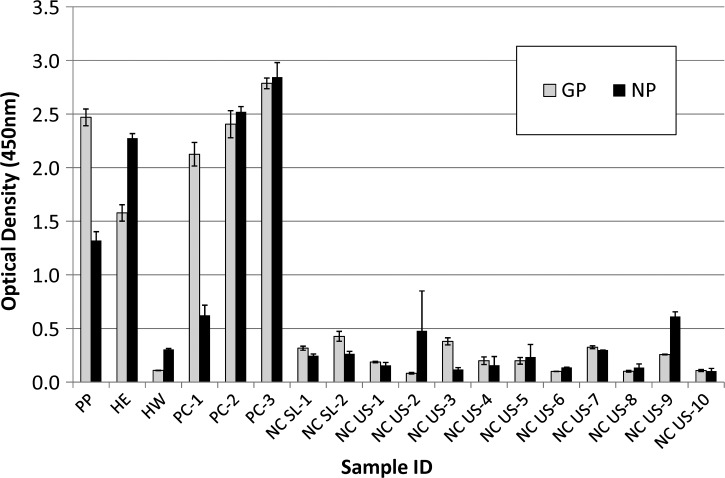
Ms. Pinneo and Mr. Elyea both had strong IgG antibody responses on initial screening for LASV, with optical densities against GP of 2.469 and 1.578 and against NP of 1.320 and 2.275, respectively (Figure 1). For GP, Ms. Pinneo's serum was positive to a dilution of 1:12,150, and Mr. Elyea's serum was positive to a dilution of 1:4,050. Interestingly, Ms. Pinneo's response to NP was much weaker than Mr. Elyea's response (1:450 versus 1:4,050, respectively). Mr. White's serum had readings well below the cutoffs for both LASV antigens (0.109 for GP and 0.305 for NP) but was positive for exposure to dengue virus (titer of 1:1,350), confirming his reported history of dengue fever. Ms. Pinneo and Mr. Elyea were negative for dengue fever (data not shown). These results confirm the potential for long-term duration of IgG antibody against LASV (in this case, over 40 years after disease).
Figure 1.
Optical density results of Lassa virus-specific IgG antibody testing by ELISA on heat-inactivated serum tested at a dilution of 1:300. Assays were run in triplicate. Error bars represent ± 1 SD. HE = Harry Elyea; HW = Hal White; NCSL = negative controls from Sierra Leone; NCUS = negative controls from the United States; PC = positive controls from Sierra Leone; PP = Lily Pinneo.
We recognize various limitations to our study. We cannot say whether the presence of antibodies in these persons indicates protective immunity. Immunity after LASV infection is presumed to be lifelong, a conclusion supported by studies in non-human primates, although no data are available in humans.17 In humans, surreptitiously noted sudden increases in IgG antibody titer have been interpreted as reinfection.7,17 We did not have access to a biosafety level 4 laboratory to perform studies with live virus as required to test for neutralizing antibody. Furthermore, the cellular response is usually considered to be the most important in LF immunity.1
We cannot definitively exclude the possibility that the two antibody-positive persons were reexposed to LASV, with a consequent boost in antibody response, including its duration. However, they reported no high-risk exposures after their initial disease, and calculating from the dates of their last departures from West Africa, Ms. Pinneo's and Mr. Elyea's last possible exposures to LASV were 25 and 42 years ago, respectively. Also, Dr. White was not exposed, despite sharing similar risks (although physicians are generally at lower risk than nurses). Subsequent exposure to arenaviruses found in the United States is theoretically possible but highly unlikely considering that human exposure to these viruses is rare, especially in persons who do not have regular contact with rodents.18 Lastly, because the subjects tested here were infected in Nigeria and there is significant strain variation in LASV, perhaps translating to antigenic variability, the testing would ideally use an ELISA based on antigens from Nigerian LASVs.19 Unfortunately, to our knowledge, no such assay exists. Nevertheless, it is reasonable to surmise that signals produced on an ELISA assay with a closer antigen–antibody match would be even stronger than those signals noted here and lead to the same conclusion.
Antibody testing of larger numbers of people over a range of years after infection would help determine the true duration of antibody to LASV after infection, but such a study could only be conducted in countries endemic for LF, where the continued possibility of reexposure would inevitably cloud interpretation, or possibly in populations that have emigrated from the endemic area. No systematic study of antibody responses and durations has been conducted for any arenavirus, and it is difficult to extrapolate from data on other virus families, for which the pathogenesis and immune response may differ greatly. Although anecdotal, our findings nevertheless shed some light on the potential for long-term antibody duration in LF. Lastly, given the prior report of Mr. Elyea's positive antibody status, confirmed many years later by our findings, we have no reason to doubt that his illness in 1952 was LF. Assuming that he had LF, he could be considered the first confirmed case of LF, although LASV was almost certainly present in Nigeria and infected many native Africans before recognized infections in the expatriate missionary population.
Summary of Conclusions
The potential for long-term immunoglobulin G (IgG) antibody duration after Lassa fever was shown by finding high antibody titers in two persons over 40 years after infection, one of whom was likely infected in 1952, 17 years before the first recognized outbreak.
ACKNOWLEDGMENTS
The authors thank Penny Pinneo, Harry Elyea, Hal White, and all the members of the Sudan Interior Mission in Sebring, Florida, for their friendship and support in conducting this study. We also thank Kathryn Hastie and Erica Ollmann Saphire for providing reagents, John Humphrey for logistical support, and Lee Fritz for filming and assisting with subject interviews.
Footnotes
Financial support: Financial support for this study came from the Tulane School of Public Health and Tropical Medicine and the National Institutes of Health.
Authors' addresses: Nell Bond, Lina M. Moses, and Andrew J. Bennett, Tulane School of Public Health and Tropical Medicine, Department of Tropical Medicine, New Orleans, LA, E-mails: nbond@tulane.edu, lmoses2@tulane.edu, and andrew.j.bennett@gmail.com. John S. Schieffelin, Tulane School of Medicine, Pediatrics Department, Section of Infectious Diseases, New Orleans, LA, E-mail: jschieff@tulane.edu. Daniel G. Bausch, Tulane School of Public Health and Tropical Medicine, Department of Tropical Medicine and Tulane School of Medicine, Internal Medicine Department, Section of Infectious Diseases, New Orleans, LA, E-mail: dbausch@tulane.edu.
References
- 1.Enria DA, Mills JN, Bausch D, Shieh W, Peters CJ. Arenavirus infections. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical Infectious Diseases: Principles, Pathogens, and Practice. 3rd ed. Philadelphia, PA: Churchill Livingstone; 2011. pp. 449–461. [Google Scholar]
- 2.Frame JD, Baldwin JM, Jr, Gocke DJ, Troup JM. Lassa fever, a new virus disease of man from West Africa. I. Clinical description and pathological findings. Am J Trop Med Hyg. 1970;19:670–676. doi: 10.4269/ajtmh.1970.19.670. [DOI] [PubMed] [Google Scholar]
- 3.Bausch DG, Rollin PE, Demby AH, Coulibaly M, Kanu J, Conteh AS, Wagoner KD, McMullan LK, Bowen MD, Peters CJ, Ksiazek TG. Diagnosis and clinical virology of Lassa fever as evaluated by enzyme-linked immunosorbent assay, indirect fluorescent-antibody test, and virus isolation. J Clin Microbiol. 2000;38:2670–2677. doi: 10.1128/jcm.38.7.2670-2677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wulff H, Lange JV. Indirect immunofluorescence for the diagnosis of Lassa fever infection. Bull World Health Organ. 1975;52:429–436. [PMC free article] [PubMed] [Google Scholar]
- 5.Van der Waals FW, Pomeroy KL, Goudsmit J, Asher DM, Gajdusek DC. Hemorrhagic fever virus infections in an isolated rainforest area of central Liberia. Limitations of the indirect immunofluorescence slide test for antibody screening in Africa. Trop Geogr Med. 1986;38:209–214. [PubMed] [Google Scholar]
- 6.Fabiyi A. Lassa fever (arenaviruses) as a public health problem. Bull Pan Am Health Organ. 1976;10:335–337. [PubMed] [Google Scholar]
- 7.McCormick JB, Webb PA, Krebs JW, Johnson KM, Smith ES. A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis. 1987;155:437–444. doi: 10.1093/infdis/155.3.437. [DOI] [PubMed] [Google Scholar]
- 8.Pinneo L, Pinneo R. Mystery virus from Lassa. Am J Nurs. 1971;71:1352–1355. [PubMed] [Google Scholar]
- 9.Carey DE, Kemp GE, White HA, Pinneo L, Addy RF, Fom AL, Stroh G, Casals J, Henderson BE. Lassa fever. Epidemiological aspects of the 1970 epidemic, Jos, Nigeria. Trans R Soc Trop Med Hyg. 1972;66:402–408. doi: 10.1016/0035-9203(72)90271-4. [DOI] [PubMed] [Google Scholar]
- 10.Frame JD. Surveillance of Lassa fever in missionaries stationed in West Africa. Bull World Health Organ. 1975;52:593–598. [PMC free article] [PubMed] [Google Scholar]
- 11.Troup JM, White HA, Fom AL, Carey DE. An outbreak of Lassa fever on the Jos plateau, Nigeria, in January–February 1970. A preliminary report. Am J Trop Med Hyg. 1970;19:695–696. doi: 10.4269/ajtmh.1970.19.695. [DOI] [PubMed] [Google Scholar]
- 12.White HA. Lassa fever. A study of 23 hospital cases. Trans R Soc Trop Med Hyg. 1972;66:390–401. doi: 10.1016/0035-9203(72)90269-6. [DOI] [PubMed] [Google Scholar]
- 13.Edington GM, White HA. The pathology of Lassa fever. Trans R Soc Trop Med Hyg. 1972;66:381–389. doi: 10.1016/0035-9203(72)90268-4. [DOI] [PubMed] [Google Scholar]
- 14.Hastie KM, Liu T, Li S, King LB, Ngo N, Zandonatti MA, Woods VL, Jr, de la Torre JC, Saphire EO. Crystal structure of the Lassa virus nucleoprotein-RNA complex reveals a gating mechanism for RNA binding. Proc Natl Acad Sci USA. 2011;108:19365–19370. doi: 10.1073/pnas.1108515108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grove JN, Branco LM, Boisen ML, Muncy IJ, Henderson LA, Schieffellin JS, Robinson JE, Bangura JJ, Fonnie M, Schoepp RJ, Hensley LE, Seisay A, Fair JN, Garry RF. Capacity building permitting comprehensive monitoring of a severe case of Lassa hemorrhagic fever in Sierra Leone with a positive outcome: case report. Virol J. 2011;8:314. doi: 10.1186/1743-422X-8-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schieffelin JS, Costin JM, Nicholson CO, Orgeron NM, Fontaine KA, Isern S, Michael SF, Robinson JE. Neutralizing and non-neutralizing monoclonal antibodies against dengue virus E protein derived from a naturally infected patient. Virol J. 2010;7:28. doi: 10.1186/1743-422X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher-Hoch SP, McCormick JB. Towards a human Lassa fever vaccine. Rev Med Virol. 2001;11:331–341. doi: 10.1002/rmv.329. [DOI] [PubMed] [Google Scholar]
- 18.Fulhorst CF, Milazzo ML, Armstrong LR, Childs JE, Rollin PE, Khabbaz R, Peters CJ, Ksiazek TG. Hantavirus and arenavirus antibodies in persons with occupational rodent exposure. Emerg Infect Dis. 2007;13:532–538. doi: 10.3201/eid1304.061509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowen MD, Rollin PE, Ksiazek TG, Hustad HL, Bausch DG, Demby AH, Bajani MD, Peters CJ, Nichols ST. Genetic diversity among Lassa virus strains. J Virol. 2000;74:6992–7004. doi: 10.1128/jvi.74.15.6992-7004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]