Abstract
The efficacy of insecticide-treated window curtains (ITCs) for dengue vector control was evaluated in Thailand in a cluster-randomized controlled trial. A total of 2,037 houses in 26 clusters was randomized to receive the intervention or act as control (no treatment). Entomological surveys measured Aedes infestations (Breteau index, house index, container index, and pupae per person index) and oviposition indices (mean numbers of eggs laid in oviposition traps) immediately before and after intervention, and at 3-month intervals over 12 months. There were no consistent statistically significant differences in entomological indices between intervention and control clusters, although oviposition indices were lower (P < 0.01) in ITC clusters during the wet season. It is possible that the open housing structures in the study reduced the likelihood of mosquitoes making contact with ITCs. ITCs deployed in a region where this house design is common may be unsuitable for dengue vector control.
Introduction
In recent decades, dengue fever (DF) and severe dengue (formerly termed dengue hemorrhagic fever [DHF]/dengue shock syndrome [DSS]) have become increasingly important worldwide. Today, an estimated 50 million infections occur annually, with 2.5 billion people at risk in virtually every country in the tropics; additionally, all four dengue serotypes now occur in most endemic regions worldwide, increasing the risk of DHF and DSS.1,2 There is no vaccine, and control of the main vectors, the peridomestic mosquitoes Aedes aegypti and Ae. albopictus, remains the only preventive measure.
Historically, most dengue vector control measures have targeted the immature mosquito stages that are found in water containers, mainly but not exclusively in the peridomestic environment. Usually, this approach is through a combination of community education, clean-up campaigns (removal of potential breeding sites), and attacking of the developing mosquito stages by either chemical or biological means (larviciding) with the intention of reducing vector breeding. Unlike interventions targeting adult mosquitoes, the control of immature stages primarily reduces vector density and does not impact on adult mosquito longevity, which limits its potential to reduce dengue transmission. Moreover, any impact on vector density is constrained by low levels of both adoption and sustained usage by target communities, leading to inadequate protection for those populations at risk.1,3
The only widely used adult dengue vector control intervention is insecticidal space spraying (delivered by fogging or ultra low-volume [ULV] techniques). The efficacy of space spraying is debatable, and it is not considered to be a viable or sustainable long-term control method but appropriate only as a response to outbreaks.4–7 As dengue continues to spread, the need for effective, long-term, and sustainable interventions targeting adult dengue vectors has become a major priority.
The importance of dengue in Southeast (SE) Asia predates the importance in the Pacific region or the Americas. Thailand first reported cases of DHF in 1949 and continues to record the highest numbers of cases annually in the SE Asian region, where an estimated 87% of the population is at risk of dengue infection.8,9 Like many other countries, routine dengue vector control in Thailand involves the treatment of potential breeding sites with the organophosphate insecticide Abate (1% Temephos) and peridomestic space spraying using either pyrethroid or organophosphate insecticides around houses reporting dengue cases (Thailand National Strategy for control of dengue fever). Despite widespread implementation of these interventions, dengue remains endemic in Thailand, and localized outbreaks still occur frequently. Here, as elsewhere, the challenge is to deliver appropriate tools that permit sustainable reductions in vector populations and dengue transmission.1,3
Initial cluster-randomized trials in Latin America showed that insecticide-treated materials (ITMs) deployed in households as curtains or water container covers could reduce dengue vector densities to low levels and potentially reduce dengue transmission.10 Insecticide-treated curtains (ITCs), in particular, reduced peridomestic Ae. aegypti infestations, sustained insecticidal effectiveness over periods of 18 months or longer, and required little behavioral change by target communities.10,11 These early results from Latin America clearly indicated the need for additional research to explore the potential of ITMs, including their efficacy in a range of communities in different geographic localities worldwide.
We report here on a trial of ITCs against dengue vectors in Thailand. ITMs have been investigated previously for dengue control in SE Asia in three small-scale studies of household-level use of permethrin-treated screens and curtains to control Ae. aegypti in Vietnam and the Philippines.12–16 Results suggested that the ITMs could reduce local dengue vector populations. Deltamethrin-treated water jar covers also reduced Ae. aegypti densities in a non-randomized trial in Cambodia.17 The study reported here is the first large-scale cluster-randomized controlled trial of ITM efficacy against dengue vectors carried out in SE Asia.
Methods
Ethical statement.
The study received approval from the research ethics committees at the Liverpool School of Tropical Medicine and the Faculty of Tropical Medicine at Mahidol University, and it is listed on the International Standard Randomized Controlled Trial Number Register (ISRCTN39952287).
Study site.
The study was undertaken over 12 months from October of 2007 to September of 2008 in two communities in the subdistricts of Krasom (8°22′ N, 98°26′ E) and Khok Kloi (8°15′ N, 98°18′ E) in Phang Nga province, Southern Thailand. Here, the wet season occurs typically from May to October, with an average annual rainfall of 3,855 mm; the average temperature is 27°C, with very little variation throughout the year, and relative humidity ranges from 80% in the dry season to 89% during the wet season (2007 and 2008 data from Takuapa District weather station, Phang Nga). The setting was periurban, with communities often distributed in hamlets or villages comprising 30–150 houses each.
Vector control activities being undertaken locally during the time of the study were limited to outdoor space spraying and larviciding within 100 m of recently diagnosed dengue cases.
Study design.
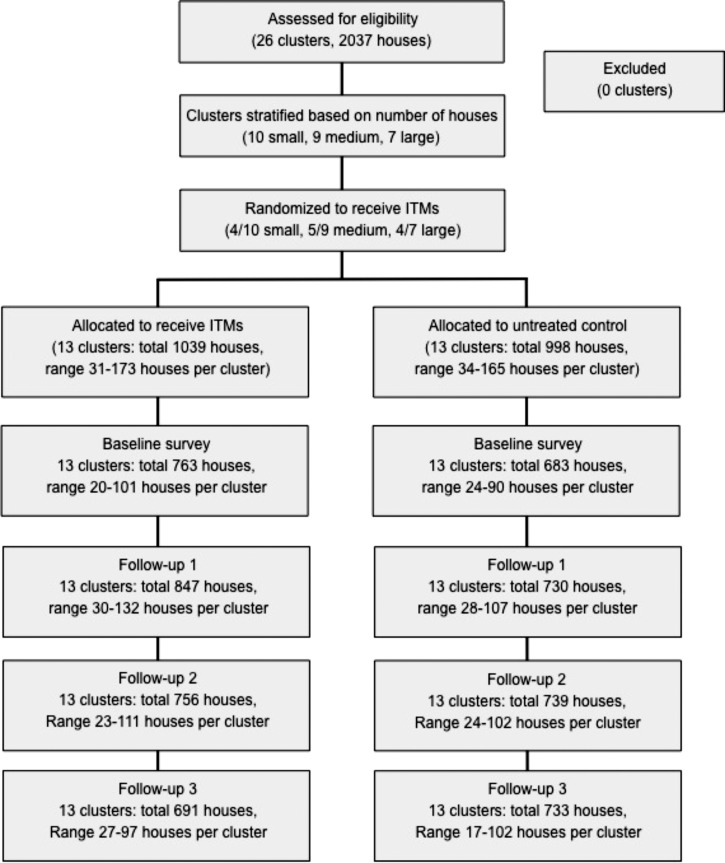
Because of the mobility of vectors and hence, the possibility of an effect of ITMs on neighboring houses, a cluster-randomized design was used (Figure 1). A sample size of 10 clusters per arm was initially planned based on earlier similar trials that used 9 clusters per arm.10 However, the density and distribution of households in the study site made it possible to increase this number to 13 clusters per arm for a total of 26 clusters. All occupied households were eligible (a total of 2,037 houses across all clusters). Clusters contained 31–173 houses each and were stratified as small (31–54 houses), medium (55–100 houses), or large (> 100 houses). Randomization was performed within these strata (by a lottery with A.L. and Y.T. and members of the research team), resulting in 4 of 10 small clusters, 5 of 9 medium clusters, and 4 of 7 large clusters being allocated to receive the ITCs, and the remaining clusters were allocated to the control arm (no intervention). This method maintained the investigators' ignorance of the upcoming assignment of each cluster, and therefore, although the allocation was not blinded, it was concealed effectively.
Figure 1.
Flow of households through the study.
The average minimum distance between clusters (defined as the minimum distance between any house in one cluster and the nearest one in another cluster) was 888 m (range = 70 m to 3.9 km; median = 495 m).
Intervention.
Intervention houses received ITCs made from PermaNet (Vestergaard-Frandsen, Lausanne, Switzerland), a polyester netting that has been factory-treated with a long-lasting deltamethrin formulation (55 mg/m2) coated with an unknown protectant (not disclosed by the manufacturer) to prevent degradation of the insecticide when exposed to ultraviolet (UV) light. PermaNet long-lasting insecticidal mosquito nets are recommended by the World Health Organization (WHO) for malaria prevention.18
A sufficient number of ITCs were provided to each house to hang in every window, regardless of the presence or absence of other window coverings. All were a standard white color and measured either 1- or 2-m wide by 1.5-m long. Houses in control clusters received no interventions, and therefore, the study was not blinded. The ITCs were distributed immediately after completion of the baseline entomological survey.
Entomological surveys.
After informed consent was obtained from the municipal health authorities and householders, baseline entomological surveys were conducted in all clusters. Surveys were undertaken in all households to inspect for the presence of Aedes breeding sites (both Ae. aegypti and Ae. albopictus were known to be present in the study area). All potential breeding sites inside and outside of houses were examined, and standard larval surveys were used to calculate the Breteau index (BI; number of containers with immature stages per 100 houses), house index (HI; number of houses containing immature stages per 100 houses), and container index (CI; number of containers with immature stages per 100 containers with water).19 Pupal surveys were used to count the total number of pupae per positive container and calculate the pupae per person index (PPI; number of pupae collected per human population in a cluster). All known potential household breeding sites were on or near ground level, and thus, they were relatively easy to access. In the cases of large positive containers, exhaustive sweeping with a fine-mesh colander was used to remove all pupae.
Larval samples were collected from positive containers and taken back to the laboratory for species identification. Oviposition traps were placed both inside and outside of 50% of the houses in each cluster, and they were left in situ for 1 week at baseline and during each follow-up survey. Follow-up entomological surveys were conducted at 3, 6, and 9 months post-intervention.
The primary outcome indicators were the cluster-level entomological indices: BI, HI, CI, and PPI.
Insecticide susceptibility.
Eggs collected in the oviposition traps were reared to adults in the insectary and screened for deltamethrin susceptibility using the standard WHO tube bioassay protocol.20
Data analysis.
The entomological indices were calculated for each cluster at baseline and each of the follow-up surveys. The follow-up values were summarized in terms of the area under the curve (AUC) of the index against time estimated by trapezium rule, taking each time point as the median survey date for each cluster.21 The AUC values were log-transformed, achieving a more normal distribution, and for each index, they were compared between arms by analysis of covariance, with the covariates being (1) stratum and (2) baseline value of the index. An additional analysis also included uptake of the intervention calculated as the AUC of the proportion of houses per cluster with curtains observed to still be in place. The analysis was by intention to treat. A two-sided P value of 0.05 was considered statistically significant. Oviposition data at each follow-up were analyzed by analysis of covariance using the study arm and the cluster size stratum as predictor variables. The baseline between-cluster coefficients of variation in BI and PPI were estimated using the method in the work by Hayes and Bennett22 for rates, and the baseline between-cluster coefficients of variation in HI and CI were estimated using their method for proportions.22
Results
A total of 2,037 houses in 26 clusters participated in the trial, of which 1,039 (51%) houses in 13 clusters were randomized to receive the ITCs and 998 (49%) houses in 13 clusters were untreated controls (Figure 1). Baseline data were collected from 1,446 (71.0%) houses; no one was at home in the remainder at the time of the survey. Follow-up data were available for 1,989 (97.6%) houses, with 1,500 (73.6%) houses participating in at least two follow-up surveys. The baseline between-cluster coefficients of variation for BI, HI, CI, and PPI were 0.52, 0.45, 0.65, and 1.36, respectively. No households refused entry, and none dropped out of the trial. No deviations were made from the protocol. Houses in which no one was home at the time of the surveys were revisited at least one time to try to maximize participation.
Mosquito species composition and breeding patterns.
The most common mosquito species found in intra- and peridomestic water containers were Ae. aegypti L. and Ae. albopictus Skuse, which accounted for nearly 90% of all mosquitoes identified. No other Aedes spp. were recorded. The other species were almost exclusively Culex spp., with Armigeres spp., Anopheles spp., and Toxorhynchites spp. also occasionally found.
At baseline, the majority of breeding sites were found outside houses (236/325, 72.6%), and the most common breeding sites were small (< 200-L capacity) water storage jars (N = 100; 30.8% of all breeding sites) and miscellaneous or discarded containers (N = 97; 29.8% of all breeding sites). The breeding sites responsible for producing the greatest number of pupae at baseline were miscellaneous or discarded containers that yielded 547 pupae (46.9% of all pupae collected). Other containers important in pupal production were small water storage jars, which yielded 337 pupae (28.9% of all pupae collected), and bathroom tanks (cement ground-level tanks of varying capacity located within bathrooms), which yielded 114 pupae (9.8%).
These breeding trends remained relatively consistent after the ITMs were introduced, and they were similar in both intervention and control clusters. Not surprisingly, the importance of outdoor discarded and miscellaneous containers as breeding sites increased during the rainy season (which coincided with the June and September entomological surveys).
Coverage of the intervention.
Approximately five ITCs per house were distributed to a total of 949 households in December of 2007. ITCs were initially accepted in 91.3% (949/1,039) of households in intervention clusters. After 3 months, 90.2% (803/890) of houses surveyed were still using at least one ITC; this number dropped to 79.9% (626/783) after 6 months and 70.5% (503/713) after 9 months. The average number of ITCs hanging in houses still using at least one ITC fell to 3.9 after 3 months, rose slightly to 4.1 after 6 months, and fell again to 3.9 after 9 months.
No adverse events or side effects associated with the intervention were detected at any time.
Impact on entomological indices.
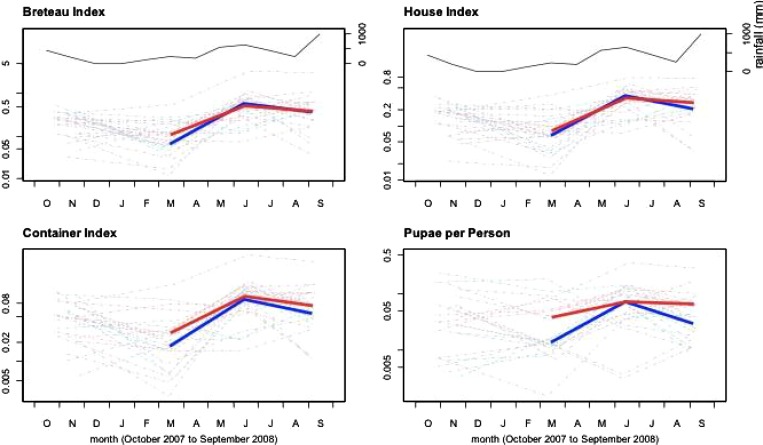
The baseline entomological survey coincided with the beginning of the dry season, and immature mosquito infestation levels were moderate to low throughout the study area (BI < 26.0, HI < 18.1, CI < 5.4, PPI < 0.27). Analyses of the AUC for all indices showed no statistically significant differences between arms and closely followed rainfall trends (Figure 2). For the primary endpoint of BI, the ratio of geometric mean AUC of ITC relative to control, adjusting for stratum and baseline, was 1.11 (95% confidence interval = 0.66 –1.87, P = 0.68), and for PPI, the ratio was 1.36 (0.78 –2.39, P = 0.26). For HI, the corresponding ratio was 1.02 (0.74 –1.43, P = 0.88), and for CI, the ratio was 1.34 (0.86 –2.09, P = 0.19). Adjusting for coverage (proportion of houses with ITCs still in place) did not change these findings.
Figure 2.
Entomological indices over time (one panel per index). The left vertical axis of each panel is the value of the corresponding index (logarithmic scale). The horizontal axis of each panel shows study month, from October (O) 2007 to September (S) 2008. In each panel, the thick lines show the geometric mean value of the index at the three follow-up surveys used to assess the impact of the intervention (gray, ITMs; black, control; red and blue respectively in online version). The thin lines show the values over time (including baseline) for each cluster. The upper two panels also have a right vertical axis, showing rainfall (in millimeters).
Oviposition rates (as measured by the mean number of eggs per trap) were not significantly different between control and ITC clusters, except at 6 months post-intervention, which was during the peak period of the wet season. At 6 months, the mean numbers of eggs recorded were 27.5 and 48.7 in indoor ovitraps (P < 0.05) and 47.5 and 94 in outdoor traps (P < 0.005) in ITC and control clusters, respectively.
Because of low hatch rates of eggs collected in the study site, it was not possible to conduct the bioassays to determine deltamethrin susceptibility at baseline or 3 months post-intervention. At 6 months post-intervention, the mortality rate after exposure to the insecticide as measured in the WHO bioassay was 84% for mosquitoes collected in the ITC clusters and 90% for mosquitoes collected in the control clusters. At 9 months post-intervention, the mortality rates were 92% in the ITC clusters and 92% in the control clusters.
Discussion
Dengue remains a serious public health problem in SE Asia, and the need for new and effective methods of protecting the human population is urgent. With ITCs showing an impact on Ae. aegypti populations in two studies in Latin America, it was disappointing to see no measurable effect on vector indices in treated houses at any stage throughout the study reported here.10,11 Precisely why this finding was the case is not immediately obvious from the data presented.
The Ae. aegypti population in the study site remained susceptible to deltamethrin, and therefore, it is unlikely that the results were caused by any reduction or failure of the ITCs in killing mosquitoes. A recent study found that ITCs identical to the ITCs used here retained nearly 100% efficacy, even after 12 months in the field.23 Also, acceptance and usage in our study were at high levels: more than 70% of households were still using at least one ITC after 9 months, which is well within the range recorded in previous studies where ITCs reduced vector populations.10,11
Previously, we compared entomological indices of Ae. aegypti abundance from a remote non-randomized external control site to check that the randomized internal controls were not simply tracking the intervention clusters as a consequence of a spillover of the ITC effect.10 We have not done so here for two reasons. First, the average minimum distance between clusters in this study was 888 m (exceeding the typical 100- to 200-m flight range of A. aegypti); this distance was considerably greater than the previous study, where clusters were adjacent, facilitating spillover.24,25 Second, seasonal vector abundance patterns in both intervention and control clusters were exactly as would be predicted by rainfall, which was unlike the previous study, where vector populations throughout the study remained low during the wet season as a consequence of the impact of the ITMs throughout the study site.9
We conclude, therefore, that other factors must be sought to explain the lack of impact reported here. We suspect that the housing style common throughout the study site may have been partly responsible. In Phang Nga, many traditional houses were open wood and thatch structures, often lacking an entire back and/or front wall. Moreover, many modern or recently constructed houses could open a large sliding door or screen during the daytime, effectively exposing the ground floor of the house to the exterior from floor to ceiling level. In such a setting, even with ITCs on windows, it seems certain that mosquitoes could enter and move through houses without ever coming into contact with insecticide.
Oviposition traps can be useful in areas of low infestation to detect both the presence of Aedes and provide some measure of the abundance of gravid females. Here, where breeding site abundance and immature infestation indices were similar between clusters, the differences detected in oviposition rates suggested that ITCs may have impacted on the adult mosquito population briefly at the onset of the rains but that any reductions in numbers were not sustained and were not pronounced enough to have achieved any detectable effect on the other entomological indices, which were the primary endpoints.
Despite the promising earlier results from Latin America, these results suggest that the potential of ITCs for dengue vector control may not prove to be universal.10,11 The types of housing seen in Phang Nga are common throughout SE Asia and in other locations worldwide. Should additional trials of ITCs in similar areas confirm these findings, other approaches to targeting adult mosquito populations will need to be found. Moreover, the potential of any alternative method may have to be examined in different contexts and locations, because acceptability or efficacy in one area may not be comparable elsewhere.17,26 Insecticide-treated water jar covers are unlikely to be suitable for Phang Nga as well, because the most productive containers recorded in this study were the smaller or discarded items that could not be targeted with water jar covers.
This finding highlights the highly variable nature of dengue vector control. Although ITMs may eventually be proven to have a useful role in dengue control, their success, like the success of all intervention tools, will be dependent on multiple factors, including those factors relating to the persistence of available insecticide on the material and their long-term acceptance by the community. The findings reported in this study indicate that housing design will have to be added to these considerations, regardless of whether ITMs are used alone or as one of a number of integrated strategies.
ACKNOWLEDGMENTS
We are grateful to the people, municipal authorities, and staff of the routine vector control programmes of Krasom and Khok Khloi, Phang Nga, Thailand for their participation, cooperation, and support throughout this study. The authors thank Vestergaard-Frandsen for their donation of the PermaNet curtains used in this study.
Disclaimer: Neither Vestergaard-Frandsen nor the funder had any role in the study design, analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: This research was undertaken within the DENCO (Dengue Control - Towards Successful Dengue Prevention and Control) project financed within the 6th Framework Programme of the European Commission (INCO-CT-2004-517708).
Authors' addresses: Audrey Lenhart, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: ajl8@cdc.gov. Yuwadee Trongtokit, Faculty of Science and Technology, Pibulsongkram Rajabhat University, Phitsanuloke Province, Thailand, E-mail: ytrongtokit@gmail.com. Neal Alexander, Department of Infectious Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK, E-mail: neal.alexander@lshtm.ac.uk. Chamnarn Apiwathnasorn, Department of Medical Entomology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mail: tmcaw@mahidol.ac.th. Wichai Satimai, Bureau of Vector-Borne Diseases, Department of Disease Control, Ministry of Public Health, Bangkok, Thailand, E-mail: wichaisatimai@yahoo.co.th. Veerle Vanlerberghe and Patrick Van der Stuyft, Epidemiology and Disease Control Unit, Institute of Tropical Medicine, Antwerp, Belgium, E-mails: vvanlerberghe@itg.be and pvdstuyft@itg.be. Philip J. McCall, Liverpool School of Tropical Medicine, Liverpool, UK, E-mail: mccall@liv.ac.uk.
References
- 1.WHO . Scientific Working Group Report on Dengue. Geneva: WHO; 2007. [Google Scholar]
- 2.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martínez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arunachalam N, Tana S, Espino F, Kittayapong P, Abeyewickreme W, Wai KT, Tyagi BK, Kroeger A, Sommerfeld J, Petzold M. Eco-bio-social determinants of dengue vector breeding: a multicountry study in urban and periurban Asia. Bull World Health Organ. 2010;88:173–184. doi: 10.2471/BLT.09.067892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiter P, Gubler DJ. Dengue and Dengue Haemorrhagic Fever. New York: CAB International; 1997. Surveillance and control of urban dengue vectors. [Google Scholar]
- 5.World Health Organization . Geneva: WHO; 2009. (Dengue: guidelines for diagnosis, treatment, prevention and control). [PubMed] [Google Scholar]
- 6.Esu E, Lenhart A, Smith L, Horstick O. Effectiveness of peridomestic space spraying with insecticide on dengue transmission; systematic review. Trop Med Int Health. 2010;15:619–631. doi: 10.1111/j.1365-3156.2010.02489.x. [DOI] [PubMed] [Google Scholar]
- 7.WHO . Space Spray Application of Insecticides for Vector and Public Health Pest Control: A Practitioner's Guide. Geneva: WHO; 2003. [Google Scholar]
- 8.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 9.WHO Reported Cases of DF/DHF in Selected Countries in SEA Region (1985–2005) 2007. http://www.searo.who.int/EN/Section10/Section332_1101.htm Available at.
- 10.Kroeger A, Lenhart A, Ochoa M, Villegas E, Levy M, Alexander N, McCall P. Effective control of dengue vectors with curtains and water container covers treated with insecticide in Mexico and Venezuela: cluster randomised trials. BMJ. 2006;332:1247–1252. doi: 10.1136/bmj.332.7552.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanlerberghe V, Villegas E, Oviedo M, Baly A, Lenhart A, McCall PJ, Van der Stuyft P. Evaluation of the effectiveness of insecticide treated materials for household level dengue vector control. PLoS Negl Trop Dis. 2011;5:e994. doi: 10.1371/journal.pntd.0000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nam VS, Nguyen HT, Tien TV, Niem TS, Hoa NT, Thao NT, Trung TQ, Yen NT, Ninh TU, Self LS. Permethrin-treated bamboo curtains for dengue vector control-field trial, Viet Nam. Dengue Newsletter. 1993;18:23–28. [Google Scholar]
- 13.Nguyen HT, Tien TV, Tien NC, Ninh TU, Hoa NT. The effect of Olyset net screen to control the vector of dengue fever in Viet Nam. Dengue Bull. 1996;20:87–92. [Google Scholar]
- 14.Itoh T, Okuno T. Evaluation of the polyethylene net incorporated with permethrin during manufacture of thread on efficacy against Aedes aegypti (Linnaeus) Med Entomol Zool. 1996;47:171–174. [Google Scholar]
- 15.Igarashi A. Impact of dengue virus infection and its control. FEMS Immunol Med Microbiol. 1997;18:291–300. doi: 10.1111/j.1574-695X.1997.tb01058.x. [DOI] [PubMed] [Google Scholar]
- 16.Madarieta SK, Salarda A, Benabaye MRS, Bacus MB, Tagle JR. Use of permethrin-treated curtains for control of Aedes aegypti in the Philippines. Dengue Bull. 1999;23:51–54. [Google Scholar]
- 17.Seng CM, Setha T, Nealon J, Chantha N, Socheat D, Nathan MB. The effect of long-lasting insecticidal water container covers on field populations of Aedes aegypti (L.) mosquitoes in Cambodia. J Vector Ecol. 2008;33:333–341. doi: 10.3376/1081-1710-33.2.333. [DOI] [PubMed] [Google Scholar]
- 18.WHO . Report of the Twelfth WHOPES Working Group Meeting. Geneva: WHO; 2008. [Google Scholar]
- 19.Focks D. A Review of Entomological Sampling Methods and Indicators for Dengue Vectors. Geneva: WHO; 2003. [Google Scholar]
- 20.WHO . Instructions for Determining the Susceptibility or Resistance of Adult Mosquitos to Organochlorine, Organophosphate and Carbamate Insecticides—Diagnostic Test. Geneva: WHO; 1981. [Google Scholar]
- 21.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol. 1999;28:319–326. doi: 10.1093/ije/28.2.319. [DOI] [PubMed] [Google Scholar]
- 23.Vanlerberghe V, Trongtokit Y, Cremonini L, Jirarojwatana S, Apiwathnasorn C, Van der Stuyft P. Residual insecticidal activity of long-lasting deltamethrin-treated curtains after 1 year of household use for dengue control. Trop Med Int Health. 2010;15:1067–1071. doi: 10.1111/j.1365-3156.2010.02582.x. [DOI] [PubMed] [Google Scholar]
- 24.Reiter P, Amador MA, Anderson RA, Clark GG. Short report: dispersal of Aedes aegypti in an urban area after blood feeding as demonstrated by rubidium-marked eggs. Am J Trop Med Hyg. 1995;52:177–179. doi: 10.4269/ajtmh.1995.52.177. [DOI] [PubMed] [Google Scholar]
- 25.Muir LE, Kay BH. Aedes aegypti survival and dispersal estimated by mark-release-recapture in northern Australia. Am J Trop Med Hyg. 1998;58:277–282. doi: 10.4269/ajtmh.1998.58.277. [DOI] [PubMed] [Google Scholar]
- 26.Vanlerberghe V, Villegas E, Jirarojwatana S, Santana N, Trongtorkit Y, Jirarojwatana R, Srisupap W, Lefèvre P, Van der Stuyft P. Determinants of uptake, short-term and continued use of insecticide-treated curtains and jar covers for dengue control. Trop Med Int Health. 2011;16:162–173. doi: 10.1111/j.1365-3156.2010.02668.x. [DOI] [PubMed] [Google Scholar]