Abstract
We investigated the effectiveness of routine preventive measures for anemia in Beninese pregnant women during pregnancy. Anemia (hemoglobin < 110 g/L) was common: 68.3% at first antenatal visit (ANV1), 64.7% at second antenatal visit (ANV2), and 40.6% at delivery. Parasitic infections and nutritional deficiencies were the most preventable causes. After intermittent preventive treatment (IPTp) and antihelminthic treatments, malaria prevalence decreased from 15.1% (ANV1) to 4.0% (ANV2) and increased again to 9.6% at delivery. Helminth infections dropped from 11.1% (ANV1) to 7.2% (ANV2) and 2.4% at delivery. Malaria was associated with lower mean hemoglobin on ANV1 and delivery, and iron deficiency was associated with lower mean hemoglobin on ANV1 and ANV2. IPTp and antihelminthic treatments were efficacious to clear parasitic infections and improve hematologic status, whereas the effectiveness of daily iron and folic acid supplements to correct iron and folate deficiencies and decrease anemia was less marked, possibly because of lack of compliance.
Introduction
Gestational anemia is common in developing countries, where it affects more than 57% of pregnancies1,2 and adversely impacts the course of gestation and its outcomes.3 In Benin, a previous study showed that over 60% of women experience anemia during gestation.4 The causes of maternal anemia are complex, including infections (malaria and helminth infestations), nutrient deficiencies (iron, folic acid, and vitamin B12), and genetic factors (hemoglobinopathies).5–7 In a preceding article, we had found that potentially preventable causes, such as micronutrient deficiencies and parasitic diseases, were the main factors associated with anemia in Beninese pregnant women in early pregnancy.8
Because of hemodilution and increasing needs of iron and other nutrients for both the mother and the fetus, hemoglobin (Hb) levels decrease progressively in pregnancy, whereas in the third trimester, hemoconcentration results in higher Hb levels.9,10 To prevent the consequences of gestational anemia on mother's health and pregnancy outcomes, several measures have been recommended by the World Health Organization (WHO), including the administration of a daily iron and folic acid supplement in pregnant women11 and the preventive treatment of malaria and intestinal helminths with sulfadoxine-pyrimethamine intermittent preventive treatment (SP-IPTp)12 and mebendazole (or albendazole), all administered at antenatal visits (ANVs).13
Although widely implemented, the effectiveness of such preventive measures in sub-Saharan Africa still needs to be documented, because the information is incomplete and sometimes conflicting.14,15 After our first study in Benin, which was conducted before the administration of any treatment or supplement, we followed a cohort of pregnant women included in a clinical trial of IPTp, aiming to assess the effectiveness of routine antimalarials, antihelminthic treatments, and hematinics on the main etiologies that we had found and their global effectiveness on maternal anemia at different time points of gestation.
Materials and Methods
Study design.
We followed a cohort of 1,005 pregnant women participating in Malaria in Pregnancy Preventive Alternative Drugs (MiPPAD; http://clinicaltrials.gov/ct2/show/NCT00811421), a randomized trial of IPTp with either SP or mefloquine (MQ), from early pregnancy until the time of delivery.
Study site and population.
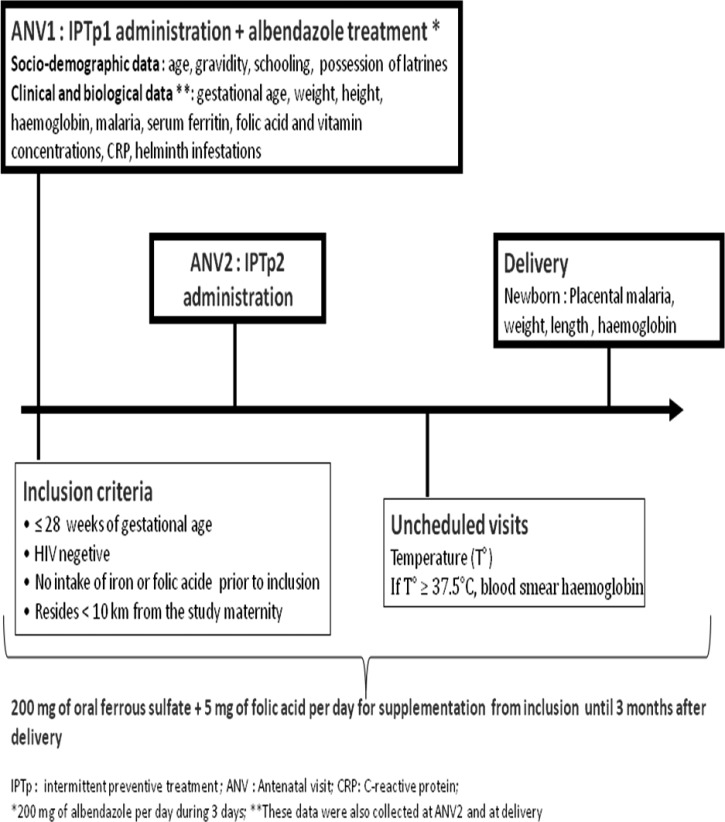
The study site and population have been described elsewhere.8 Briefly, the study was conducted in the district of Allada, a semirural area located in southern Benin. Malaria is perennial, and Plasmodium falciparum is the most common species. There are two high transmission peaks: from April to July and from October to November. The MiPPAD study population was composed of human immunodeficiency virus (HIV) -negative pregnant women of less than or equal to 28 weeks gestational age who attended one of three study maternity clinics of the area for the first time between January of 2010 and May of 2011. The eligibility criteria included no intake of IPTp, iron, folic acid, vitamin B12, or antihelminthic treatment, which are part of the ANV package in Benin, since the beginning of the pregnancy. Two doses of IPTp (1,500/75 mg SP per dose or 15 mg/kg MQ per dose) were administered on ANVs. The second dose of IPTp was given at least 1 month apart from the administration of the first dose. On the day of inclusion, each woman received a long-lasting insecticide-treated net that was replaced in case of damage or loss during the follow-up. Women were also systematically given 600 mg albendazole to be taken at home (100 mg two times per day for 3 days) according to the guidelines of the Beninese Ministry of Health. In addition, supplements of oral ferrous sulfate (200 mg per day) and folic acid (5 mg per day) were given to the women for home treatment (Figure 1). Pregnant women found to have a Hb concentration below 110 g/L were treated according to the severity of anemia (i.e., 200 mg oral ferrous sulfate two times per day for mild or moderate anemia when Hb was between 70 and 110 g/L) and referred to the tertiary hospital of the district in case of severe anemia (Hb < 70 g/L). All the medications prescribed to the women during their participation in the study were free of charge.
Figure 1.
Study procedures.
Study procedures.
Sociodemographic data collection.
At enrolment (ANV1), all pregnant women who attended any of the maternity clinics for ANV were approached to participate in the study. They were screened for inclusion and exclusion criteria, and sociodemographic data, such as age, parity, area of residence, marital status, level of education, occupation, and socioeconomic characteristics (sanitation in the house, personal means of transportation, possession of fridge or television, and connection to electricity), were recorded. ANV1 was also the occasion to administer antiparasitic treatments and nutritional supplements to the women.
Clinical data collection.
At ANV1, the woman was examined, and parity, gestational age, middle upper arm circumference, weight, and height were recorded. Medical history, including history of previous pregnancies, history of any known disease (such as high blood pressure, diabetes, or asthma), and information on previous children (birth weight, gestational age at delivery, and notion of anemia during previous pregnancy), was also recorded.
At the time of ANV2, at delivery, and during the unscheduled visits, gestational age, middle upper arm circumference, weight, and height were measured again. The second intake of IPTp was also given on ANV2. Weights were measured to the nearest 0.1 kg using an electronic scale (SECA France, Semur-en Auxois, France), and heights were measured to the nearest 0.1 cm with a SECA bodymeter device (SECA France).
Blood and stool samples collection.
At ANV1, ANV2, and delivery, 8 mL venous blood were collected from each participant; 4 mL were dispensed into a dipotassium (ethylenedinitrilo)tetraacetic acid (EDTA) tube, and 4 mL were dispersed into a dry iron-free tube. A container was also given to the woman to collect stools in search of intestinal helminths. These containers were collected the next day by the study nurses within the first 6 hours after emission.
At delivery, a placental blood smear was performed to look for placental malaria (Figure 1).
Laboratory tests.
The study sample examination techniques have been described elsewhere.8 Hb rate was measured with a Hemo_Control photometer (EKF Diagnostics, Magdeburg, Germany) on 10 μL blood.
Hb genotypes were determined by alkaline electrophoresis on cellulose acetate (Helena Laboratories, Mount Waverley, Victoria, Australia) on 50 μL blood.
Serum ferritin and vitamin B12 concentrations were measured using a microparticle enzyme immunoassay (MEIA) method. A fluorescence polarization immunoassay (FPIA) technique was used to determine folic acid concentrations with an AxSym Immuno-Assay Analyzer (Abbott Diagnostics, Frankfurt, Germany). C-reactive protein (CRP) concentrations were determined with a rapid slide test (Cypress Diagnostics, Langdorp, Belgium).
HIV detection is part of the first ANV package in Benin. Determine Kit HIV 1 and 2 package insert (Alere Orgenics, Paris, France) and SD Bioline Kit HIV 1 and 2 3.0 package insert (Umhlanga, Kwazulu Natal, South Africa) rapid tests were used to detect HIV infections with a serial testing algorithm.
Lambaréné technique was used to assess malarial infection. It consists of spreading 10 μL blood on a slide's rectangular area of 1.8 cm2 (1.8 × 1 cm). The slide is stained with Giemsa and read at 100× oil immersion. To assess parasite density, a multiplication factor is applied to the average parasitemia per field to get a number of parasites per microliter. Lambaréné method detection threshold has been estimated to 5 parasites/μL.
Infestations by helminths were assessed using the Kato–Katz concentration method (Vestergaard Frandsen, New Delhi, India).
Definitions.
Anemia.
Anemia was defined as Hb below 110 g/L. Severe, moderate, and mild anemia were defined as Hb concentrations less than 80 g/L, between 80 and 99 g/L, and between 100 and 109 g/L, respectively.
Iron deficiency and iron deficiency anemia.
Iron deficiency (ID) was defined as serum ferritin < 12 μg/L or serum ferritin between 12 and 70 μg/L in the context of inflammation defined as a positive CRP (i.e., CRP concentration > 5 mg/mL). Iron deficiency anemia (IDA) was defined as Hb < 110 g/L with ID.
Folic acid and vitamin B12 deficiencies.
Folic acid deficiency was defined as a serum concentration below 6 ng/mL. Vitamin B12 deficiency was defined as a serum concentration below 150 pg/mL.
Helminth infestations.
Intestinal helminth infestations were diagnosed by the presence of intestinal helminth eggs in the stool sample. Eggs were counted as number of eggs per 1 g stool.
Statistical analysis.
Data were entered and analyzed with ACCESS 2003 and STATA 11.0 Softwares for Windows (Stata Corp, College Station, TX). We first described the baseline and general characteristics of the women at each IPTp administration and delivery. The variations of mean Hb between ANV1, ANV2, and delivery were assessed by a Kruskal–Wallis test. We compared the variation of the proportions between ANV1 and ANV2 or between ANV1 and delivery with a McNemar test.
The effectiveness of preventive measures was assessed at ANV2 and delivery by studying the variations of the risk factors found at ANV1 before any prevention. At each time point (ANV1, ANV2, and delivery), we estimated the association of the risk factors with the Hb concentration. Means were compared with Student or Mann–Whitney non-parametric tests as appropriate. All variables with P values less than 0.2 were then included in a multilinear regression. The impact of preventive measures on the risk of maternal anemia was appreciated by using a univariate logistic regression. Thereafter, all variables with P values below 0.2 were included in a multivariate logistic regression for each ANV.
The previous analyses investigated the association between different risk factors and Hb concentrations on each visit and at the time of delivery, but they did not take into account the evolution of Hb with time throughout pregnancy. Assuming that successive Hb measurements in the same individual are correlated and dependent on gestational age, the data presented a hierarchical two-level structure, where Hb measurements (level 1) were clustered within women (level 2). We then analyzed our data using a linear mixed model with a random intercept and a random slope, which is specified in the equation
Hemoglobin (ij) is the ith Hb measurement of woman j. β0 is the intercept. Xqj is the q explicative variables of woman j with their associated coefficients βq. μ0j is the random intercept corresponding to the woman-to-woman variation in Hb level [μ0j − N (0, π00)]. μ1j is the random slope corresponding to the variation in Hb level throughout time (gestational age in weeks), and ∈(ij) is the residual variation [∈(ij) − N (0, σ2)]. We assumed that random effects [μ0j and ∈(ij)] were independent. Fixed effects parameters were estimated using the maximum likelihood method, and variance components were estimated using the restricted maximum likelihood method. All variables with P < 0.20 in the univariate analyses were included in the model. Statistical significance was set at P < 0.05.
Ethical considerations.
This study was approved by the Ethics Committee of the Faculty of Medicine of Cotonou, Benin. Before each inclusion, the study was explained in the local language to the participant, and her voluntary consent was obtained. In the case that the woman could not read, an impartial witness was involved in the process. In addition to the assent of minors, consent was obtained from the parents or legal guardians. Women were free to interrupt their participation at any time in the study.
Results
Study profile and description of the study population.
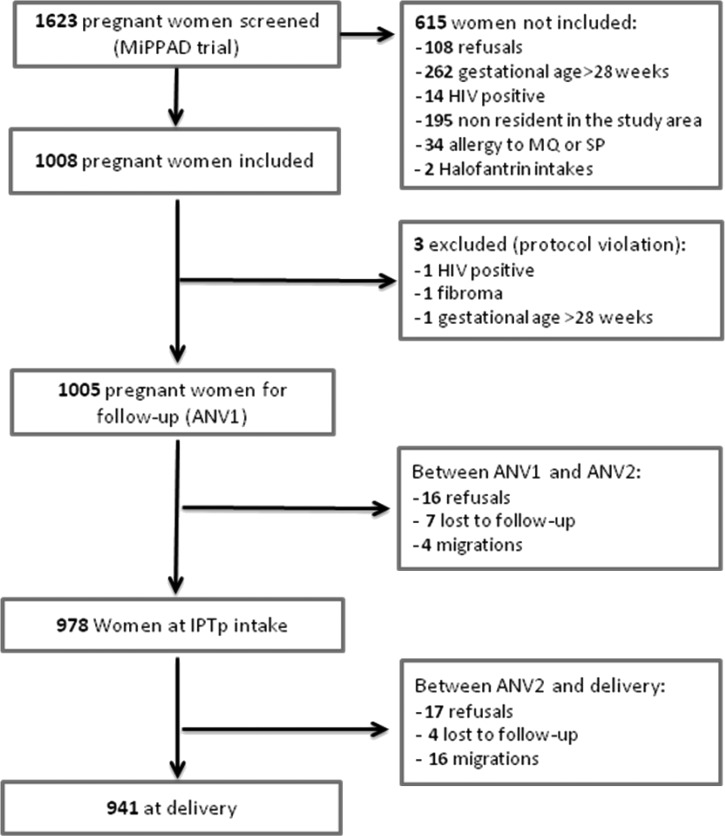
From January of 2010 to May of 2011, 1,623 pregnant women were screened for inclusion in the study; 618 women were not included, either because they refused (108 women) or did not fulfill the inclusion and exclusion criteria (510 women). Three women were excluded, because they had been inappropriately enrolled (HIV infection [1 woman], uterus fibroma [1 woman], and gestational age over 28 weeks [1 woman]). Among the 1,005 remaining women, 978 were followed up until the second IPTp intake (ANV2), and 941 were followed until delivery. The proportions lost to follow-up were 0.7% (7 of 1,005) and 0.4% (4 of 978) between the first IPTp administration (ANV1) and ANV2 and between ANV2 and delivery, respectively (Figure 2). Hb was assessed in 100% (1,005 of 1,005) and 99.7% (944 of 947) of women at the time of ANV1 and ANV2. It was assessed in 91.2% (865 of 941) of women at delivery.
Figure 2.
Study profile.
Variation of maternal Hb over time.
The mean gestational ages at the first, second, and third Hb assessments were 22.1, 28.9, and 39.3 weeks gestation, respectively. The mean duration between Hb assessments was 44.8 days between ANV1 and ANV2 and 84.5 days between ANV2 and delivery. Overall, the proportions of women with Hb < 80 g/L and Hb < 110 g/L were 3.4% and 68.3% at ANV1, 1.7% and 64.7% at ANV2, and 2.3% and 40.6% at delivery, respectively. The mean concentrations of Hb at ANV1 and ANV2 and at delivery differed significantly (103.2 g/L, 95% confidence interval [CI] = [102.4–103.9]; 105.1 g/L, 95% CI = [104.4–105.8]; and 111.4 g/L, 95% CI = [110.5–112.4], respectively; Kruskal–Wallis test; P = 0.0001) (Table 1).
Table 1.
General characteristics of pregnant women in Allada at the time of intermittent preventive treatment administrations (ANV1 and ANV2) and delivery
| Characteristics | ANV1* | ANV2* | Delivery |
|---|---|---|---|
| Body mass index (kg/m2) | |||
| Mean | 22.6 (22.4–22.8) | 23.6 (23.3–23.8) | 24.7 (24.4–24.9) |
| Middle upper arm circumference (cm) | |||
| Mean | 25.6 (25.4–25.7) | 26.0 (25.8–26.2) | 26.6 (26.4–26.8) |
| Gestational age (weeks) | |||
| Mean | 22.1 (21.8–22.3) | 28.9 (28.6–29.1) | 39.3 (39.1–39.5) |
| Duration between Hb assessments (days) | |||
| Mean | Not applicable | 44.8 (44.0–45.6) | 84.5 (82.4–86.6) |
| Gravidity (%) | |||
| 1 | 18.9 | 19.0 | 18.8 |
| ≥ 2 | 81.1 | 81.0 | 81.2 |
| Hb (g/L) | |||
| Mean | 103.2 (102.4–103.9) | 105.1 (104.4–105.8) | 111.4 (110.5–112.4) |
| < 80 | 3.4 | 1.7 | 2.3 |
| 80–99 | 32.3 | 26.1 | 14.5 |
| 100–109 | 32.6 | 36.9 | 23.8 |
| ≥ 110 | 31.7 | 35.3 | 59.4 |
| Serum ferritin† (μg/L) | |||
| Mean | 24.9 (23.7–26.3) | 18.2 (17.4–19.1) | 36.8 (34.5–39.2) |
| ID (%) | |||
| No | 66.7 | 63.7 | 69.3 |
| Yes | 33.3 | 36.3 | 30.7 |
| IDA (%) | |||
| No | 75.7 | 73.5 | 86.5 |
| Yes | 24.3 | 26.5 | 13.5 |
| Serum folic acid† (ng/L) | |||
| Mean | 8.2 (7.9–8.4) | 9.4 (9.1–9.7) | 7.7 (7.3–8.1) |
| Folic acid deficiency (%) | |||
| No | 68.7 | 83.1 | 61.3 |
| Yes | 31.3 | 16.9 | 38.7 |
| Serum vitamin B12† (pg/L) | |||
| Mean | 359.3 (348.9–369.9) | 335.1 (325.5–344.9) | 295.9 (285.9–306.3) |
| Vitamin B12 deficiency (%) | |||
| No | 96.4 | 96.5 | 92.8 |
| Yes | 3.6 | 3.5 | 7.2 |
| Malaria (%) | |||
| No | 84.9 | 96 | 90.4 |
| Yes | 15.1 | 4.0 | 9.6 |
| Placental malaria (%) | |||
| No | – | – | 90.8 |
| Yes | – | – | 9.2 |
| Helminths (%) | |||
| No | 88.8 | 92.8 | 97.6 |
| Yes | 11.1 | 7.2 | 2.4 |
| Inflammation (%) | |||
| No | 79.5 | 87.7 | 65.7 |
| Yes | 20.5 | 12.3 | 34.3 |
First and second doses of IPTp administrations.
Geometric means; 95% CI values are in parentheses.
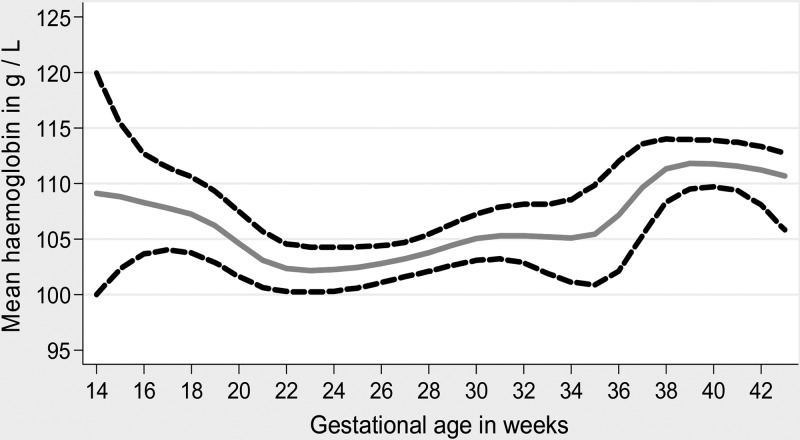
Figure 3 shows the mean Hb variations during pregnancy according to gestational age; they decreased progressively at the end of the first trimester and then increased in the second one-half of the third trimester after 36 weeks of gestation.
Figure 3.
Mean Hb variation according to gestational age.
Factors influencing Hb concentration during pregnancy.
In univariate analysis, malaria, helminth infestations, iron, folic acid, and vitamin B12 deficiencies, inflammation, low body mass index (BMI), rainy season, age below 20 years, gestational age more than 16 weeks, primigravidity, and absence of latrines and electricity in the houses were associated with maternal Hb concentration at ANV1 with P < 0.2. On ANV2, malaria, iron and folic acid deficiencies, inflammation, low BMI, duration between ANV1 and ANV2, and rainy season remained associated to maternal Hb concentration with P < 0.2. At delivery, in addition to malaria, iron and folic acid deficiencies, inflammation, and primigravidity, duration between IPTp2 and delivery, number of ANVs, malaria episodes, and placental malaria were also associated with Hb concentration with P < 0.2. The proportion of ID did not change between ANV1 and ANV2 (McNemar test; P = 0.1) or ANV1 and delivery (McNemar test; P = 0.4). All these variables are included in the multivariate linear models.
In multivariate analyses, the association of mean Hb at ANV1, ANV2, and delivery with identified risk factors is shown in Table 2. The presence of malaria infection was associated with a lower mean Hb at ANV1 and delivery. However, the prevalence of malaria differed between ANV1 and ANV2 (McNemar test; P < 0.0001) and between ANV1 and delivery (McNemar test; P = 0.0001). It decreased from 15.1% (at ANV1) to 4.0% (at ANV2), and it increased again at delivery to 9.6%. Intestinal helminths were not associated with Hb concentrations at ANV1, ANV2, or delivery. The proportions of women infested by these parasites decreased at each blood assessment: 11.1% at IPTp1, 7.2% at IPTp2, and 2.4% at delivery.
Table 2.
Factors associated with maternal Hb concentrations at different times of gestation (multivariate linear regression)
| Factors | ANV1 (N = 1,005) | ANV2 (N = 944) | Delivery (N = 837) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P | Coefficient | 95% CI | P | Coefficient | 95% CI | P | |
| Malaria | −4.8 | (−6.8, −2.7) | < 0.001 | NS | NS | NS | −4.4 | (−7.7, −1.1) | 0.008 |
| Iron deficiency | −2.6 | (−4.2, −1.1) | 0.001 | −3.1 | (−4.5, −1.6) | < 0.001 | NS | NS | NS |
| Folate deficiency | −2.6 | (−4.2, −1.0) | 0.001 | −3.6 | (−5.4, −1.8) | < 0.001 | −2.9 | (−4.9, −0.9) | 0.004 |
| Low BMI at inclusion | −2.8 | (−4.3, −1.4) | < 0.001 | −1.7 | (−3.1, −0.4) | 0.012 | NA | NA | NA |
| Rainy season | −2.5 | (−3.9, −1.0) | 0.001 | NS | NS | NS | NA | NA | NA |
| Gestational age (weeks) | −0.5 | (−0.7, −0.4) | < 0.001 | NA | NA | NA | NA | NA | NA |
| Primigravidity | −4.3 | (−6.2, −2.4) | < 0.001 | NA | NA | NA | NS | NS | NS |
| Constant | 120.3 | (116.2, 124.3) | < 0.001 | 107.6 | (106.5, 108.7) | < 0.001 | 113.3 | (112.0, 114.5) | < 0.001 |
NA = not applicable; NS = no significant association.
On each visit (ANV1, ANV2, and delivery), mean Hb was lower in women with folic acid deficiencies compared with non-deficient women. ID and a low BMI before the beginning of pregnancy were associated with a lower mean Hb at ANV1 and ANV2 but not at delivery. Rainy season, primigravidity, and gestational age over 16 weeks remained associated to a lower level of Hb at ANV1, even after adjustment.
The multilevel linear regression (Table 3) confirmed that malaria, malnutrition, and iron and folic acid deficiencies were significantly associated with a lower Hb level from inclusion until delivery (P < 0.001, P = 0.002, P < 0.001, and P < 0.007, respectively).
Table 3.
Factors influencing maternal Hb level (g/L) during pregnancy (multilevel linear regression)
| Variables | Estimates | 95% CI | P value |
|---|---|---|---|
| Malaria (reference = no) | −5.3 | (−6.6, −3.9) | < 10−3 |
| Malnutrition (reference = no) | −2.0 | (−3.2, −0.7) | 0.002 |
| Iron deficiency (reference = no) | −1.9 | (−2.8, −0.9) | < 10−3 |
| Folic acid deficiency (reference = no) | −1.2 | (−2.2, −0.3) | 0.007 |
| Gestational age (weeks) | 0.4 | (0.3, 0.4) | < 10−3 |
Estimated by maximum likelihood.
The intraclass coefficient of variation of Hb was estimated at 0.42. Thus, 58% of the total variance could be explained by the model.
Risk factors for maternal anemia.
When maternal Hb status was considered as a categorical variable (anemia or no anemia), a multivariate logistic regression showed that most of the factors kept in the multilinear regression (malaria and helminth infestations, iron, folic acid, and vitamin B12 deficiencies, low BMI, rainy season, gestational age over 16 weeks, and primigravidity) were associated with anemia at ANV1. Iron and folic acid deficiencies were associated with maternal anemia at ANV2, whereas only malaria and folic acid deficiency increased the risk for maternal anemia at delivery (Table 4).
Table 4.
Risk factors for maternal anemia at different times of gestation (multivariate logistic regression)
| Factors | ANV1 (N = 989) | ANV2 (N = 944) | Delivery (N = 865) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Malaria | |||||||||
| No | 1 | NS | 1 | ||||||
| Yes | 2.2 | (1.4–3.5) | < 0.001 | NS | NS | NS | 1.7 | (1.1–2.8) | 0.017 |
| Gestational age (weeks) | |||||||||
| < 16 | 1 | NS | NA | ||||||
| ≥ 16 | 1.7 | (1.1–2.8) | 0.025 | NS | NS | NS | NA | NA | NA |
| Season of visit | |||||||||
| Dry | 1 | NS | NS | ||||||
| Rainy | 1.6 | (1.2–2.1) | 0.002 | NS | NS | NS | NS | NS | NS |
| BMI (kg/m2) | |||||||||
| ≥ 20 | 1 | NS | NA | ||||||
| < 20 | 1.6 | (1.2–2.2) | 0.001 | NS | NS | NS | NA | NA | NA |
| Iron deficiency | |||||||||
| No | 1 | 1 | 1 | ||||||
| Yes | 1.4 | (1.1–1.9) | 0.029 | 1.7 | (1.3–2.3) | < 0.001 | 1.3 | (1.0–1.8) | 0.09 |
| Folic acid deficiency | |||||||||
| No | 1 | 1 | 1 | ||||||
| Yes | 1.4 | (1.0–1.9) | 0.045 | 1.5 | (1.0–2.2) | 0.046 | 1.3 | (1.0–1.8) | 0.045 |
| Vitamin B12 deficiency | |||||||||
| No | 1 | NA | NA | ||||||
| Yes | 2.4 | (1.0–6.2) | 0.049 | NA | NA | NA | NA | NA | NA |
| Helminth infestations | |||||||||
| No | 1 | NA | NA | ||||||
| Yes | 1.7 | (1.0–2.7) | 0.037 | NA | NA | NA | NA | NA | NA |
NA = not applicable, because the variable was not included in the multivariate analysis; NS = no significant association.
We did not find any association between the number of ANVs, malaria episodes, Hb genotypes, and maternal Hb status in this population.
Discussion
In Benin, pregnant women are given IPTp for the prevention of malaria under the supervision of health staff as well as antihelminthics and nutritional supplements to be taken home. We showed a significant reduction in the parasitic causes of anemia from the ANV1 until delivery, whereas the effect of preventive measures was less pronounced (when not unchanged) on micronutrient deficiencies.
According to previous findings in industrialized9,16,17 and developing countries,4,10 we observed that the mean Hb decreased from late second trimester to early third trimester, whereas it increased at the end of the third trimester of pregnancy. These variations are partly because of the profound hemodynamic changes associated with pregnancy per se.18,19 It is known that, during the second and early third trimesters, there is an expansion of plasma volume.18 Conversely, at the end of pregnancy, an increase of red blood cells mass is observed.19 All these changes aim to compensate for the increased demand in nutrients and oxygen for both the mother and the fetus. In developing countries, additional causes, such as parasitic infestations and nutrient deficiencies, act as aggravating factors to maternal anemia.7
Because malaria and intestinal helminths are known to be associated with anemia,20,21 efforts to prevent anemia in pregnancy in sub-Saharan Africa include the administration of IPTp and antihelminthic treatments on ANVs.12,22
In this study, we observed a decrease in the prevalence of malaria after IPTp, particularly after the first dose of SP or MQ. In a previous study, we had shown that 15% of anemia was attributable to malaria in this population.8 At the time of the administration of the second dose of IPTp, malaria was not associated with anemia. Our findings confirm the beneficial effects of IPTp in pregnant women in terms of reduction of the prevalence of malaria as well as maternal anemia, which have been shown in other studies.23–26 At delivery, however, there was a slight increase in malaria prevalence, although not reaching the level observed in first ANVs. The shorter duration of time intervals between ANV1 and ANV2, compared with ANV2 and delivery, suggests reinfection of the women after the levels of antimalarials have dropped below subtherapeutic concentrations. Similar findings had been made in a cohort study of pregnant women receiving SP-IPTp in a neighboring area in Benin, which established an increased risk for maternal anemia at delivery in women infected by malaria parasites after 6 months of gestation.27 An alternative explanation to the increase of malaria infection at delivery might be the resistance of malaria parasites to SP (but not MQ), because high SP resistance has been shown in children of the study area (more than 80%).28 However, this hypothesis was not supported by the results of the investigations made on pregnant women in a nearby area, showing that there was no variation in the prevalence of triple and quadruple mutants of P. falciparum strains implicated in SP resistance from the beginning to the end of pregnancy.29
The prevalence of intestinal helminth infestations dropped at ANV2 and delivery after the antihelminthic administrations. Assuming that the women adhered to the treatments (or at least the first dose), the long lifecycle of intestinal worms explains that, contrary to malaria, no reinfestation could result before the end of pregnancy. Accordingly, the association between helminths and anemia disappeared after the first treatments (ANV2 and delivery visits).
In this study, iron and folic acid deficiencies remained high throughout pregnancy, although women were provided with iron and folate supplements at ANV1 to cover the entire duration of pregnancy. However, IDA was less prevalent at delivery than at ANV1 and ANV2, dropping from 24% at ANV1 to 13% at the end of pregnancy. Because ferritin is an acute-phase protein, its value has been interpreted according to the concentration of CRP to avoid misclassification of ID in the presence of inflammation, which occurred in more than one-third of the women at delivery. Therefore, we cannot preclude that the concurrent measurement of the two indicators has led to an underestimation of the actual prevalence of ID at delivery. However, restricted analyses to women with low ferritin concentrations and normal CRP levels show a high prevalence of IDA at ANV1 and ANV2 (76%) followed by a decrease at delivery (61%; data not shown), which seems to contradict this hypothesis. Overall, the high prevalence of inflammations at the end of pregnancy, despite the reduction of malaria and helminth infections, deserves additional investigation.
In developing countries, where diets are poor in iron but often rich in inhibitors of iron absorption,7 the issue of iron supplementation in pregnant women arises differently compared with in industrialized countries. In tropical areas, many women start pregnancy with a lower store of iron30 and undergo higher risks of anemia because of the increase in iron needs during gestation. As pregnancy progresses, it is known that iron requirements for fetal growth rise in proportion to the weight of the fetus, with most of the iron accumulating in the fetus during the third trimester.31 A possible explanation for the persistence of iron and folic acid deficiencies despite supplementation is the inability to compensate the iron mobilization for the development of the fetus and restore the mother's stores because of an insufficient intake of supplements. However, it has been shown that iron side effects are dose-dependant,32 and an increase in the administered dose could result in a lower compliance to supplementation. Additionally, several placebo-controlled trials in pregnant women in industrialized33,34 and developing countries35,36 using 200 mg oral ferrous sulfate daily (60 mg elemental iron; corresponding to WHO recommendations) or less have shown that iron supplementations could reduce maternal anemia markedly and improve pregnancy outcomes, indicating that the lack of efficiency of micronutrient supplementation in our study is not a matter of dosage.
The question of the start date of supplementation may also be considered. In our study, the first iron and folic acid supplements were provided relatively late to the women, because it was given, on average, at 22.1 weeks gestation. This delay probably hampers the correction of an iron deficiency that exists in most cases before the beginning of pregnancy.
Unlike in randomized placebo-controlled trials, the current iron and folic acid supplementation is not supervised. Women are provided with the amount of iron to cover the entire duration of the gestation and are encouraged to take the treatment daily at home. The work by Bonnar and others37 in 1969 showed, in a trial assessing the compliance of pregnant women with iron supplementation, that more than 30% of participants were not taking their treatment adequately, and consequently, the proportion of anemia at term was higher in women who were not compliant with iron supplementation than their counterparts. Similarly, the work by Habib and others38 in 2009 showed that almost 50% of pregnant women discontinued use of iron supplements in Saudi Arabia. Moreover, Hb concentration increased significantly among compliant women (by 3 g/L), whereas it decreased in non-compliant women (by 1.4 g/L).38 Similar proportions of non-compliance were reported in a trial studying the factors influencing compliance among Senegalese pregnant women. The main reasons for low compliance were the experience of side effects, the misunderstanding of the treatment duration, and oversight.39 Contrary to IPTp, in which the directly observed therapy (DOT) scheme is implemented, there is no supervision of the intake of supplements by the health staff, and we think that poor compliance, combined with the late administration of micronutrients, is the main reason for the lack of effectiveness of these preventive measures in our study. As we previously mentioned in this article, our study was nested into a multicenter clinical trial (MiPPAD). It did not allow us to monitor the compliance of the women to routine oral iron and folic acid supplementation or antihelminthic treatment to avoid biases in the follow-up of our population compared with the other MiPPAD sites.
An efficient strategy to increase the beneficial effects of iron supplementation should have the advantage of improving women's compliance to iron prophylaxis by decreasing the incidence of its side effects.32 A Cochrane review on the efficacy of a weekly intermittent preventive treatment with iron compared with daily iron administration concluded that weekly iron supplementation was as effective as daily supplementation in preventing low Hb concentrations, which are associated with negative consequences on maternal health and pregnancy outcomes.40
Absorption of iron has been described as a function of dose and stage of pregnancy. As suggested in the work by Beaton,16 at any stage of pregnancy, low supplement doses correspond to a better efficiency of iron absorption compared with higher doses, even if the absolute amount of iron absorbed is more important. Therefore, early administrations of lower doses of iron could be an optimal solution to increase the beneficial effects of iron supplementation. This strategy has the advantage to improve the compliance of women with iron supplementation by decreasing the incidence of iron side effects.33 Its implementation in tropical Africa, where pregnancies are not scheduled in most cases, requires a huge effort of sensitization for earlier ANVs.
Conclusion
Anemia is a great public health issue in Beninese pregnant women, where almost 50% of women are anemic throughout gestation. For the first time, we were able to follow a cohort of pregnant women from the first ANV to delivery and study the evolution of their Hb status throughout pregnancy before and after the implementation of preventive measures. P. falciparum, helminth infestations, and nutrient deficiencies seemed to be the main preventable causes of anemia. IPTp and antihelminthic treatment efficaciously protected the women from parasitic infections. However, the effectiveness of daily iron and folic acid supplements to prevent iron and folic acid deficiencies as well as maternal anemia was less perceptible in this population, and a lower compliance to this strategy seems to be the most likely explanation. Therefore, additional works comparing weekly intermittent oral iron supplements with daily supplementation starting early in pregnancy or pre-gestationally and containing lower doses than the recommended 60 mg in sub-Saharan Africa should be considered.
ACKNOWLEDGMENTS
The authors thank the women who participated in this study. We also thank the study nurses, laboratory technicians, field worker, driver, Dr. Didier Tonakpon Agbozognibè, the midwives of the district of Allada, and their assistants for their help in conducting this study.
Footnotes
Financial support: The study was supported by the Malaria in Pregnancy (MiP) Consortium, which is funded through a grant from the Bill and Melinda Gates Foundation to the Liverpool School of Tropical Medicine. The Malaria in Pregnancy Preventive Alternative Drugs (MiPPAD) trial is cofunded by the European and Developing Countries Clinical Trials Partnership (EDCTP-IP.07.31080.002). We also thank the MiPPAD executive committee and MiPc reviewers for valuable input in this work. S.O. was supported by an Institut de Recherche pour le Développement (IRD) grant while writing this paper.
Authors' addresses: Smaïla Ouédraogo, Unité Mixte de Recherche, Mère et Enfant Face aux Infections Tropicales/Faculté des Sciences de la Santé, Paris, France, E-mail: smaila11@yahoo.fr. Ghislain K. Koura and Michel Cot, Unité Mixte de Recherche, Mère et Enfant Face aux Infections Tropicales, Paris, France, and Faculté de Pharmacie, Université Paris Descartes, Paris, France, E-mails: kourakobtoghislain@yahoo.fr and michel.cot@ird.fr. Florence Bodeau-Livinec, Ecole des Hautes Etudes en Santé Publique, Rennes, France, E-mail: Florence.Bodeau-Livinec@ehesp.fr. Manfred M. K. Accrombessi and Achille Massougbodji, Faculté des Sciences de la Santé, Cotonou, Benin, E-mails: accrombessimanfred@yahoo.fr and massougbodjiachille@yahoo.fr.
References
- 1.WHO . Iron Deficiency Anaemia Assessment, Prevention, and Control. A Guide for Programme Managers. Geneva, Switzerland: WHO; 2001. [Google Scholar]
- 2.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12:444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 3.Stoltzfus RJ. Iron deficiency: global prevalence and consequences. Food Nutr Bull. 2003;24:S99–S103. doi: 10.1177/15648265030244S206. [DOI] [PubMed] [Google Scholar]
- 4.Bodeau-Livinec F, Briand V, Berger J, Xiong X, Massougbodji A, Day KP, Cot M. Maternal anemia in Benin: prevalence, risk factors, and association with low birth weight. Am J Trop Med Hyg. 2011;85:414–420. doi: 10.4269/ajtmh.2011.10-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Broek N. Anaemia in pregnancy in developing countries. Br J Obstet Gynaecol. 1998;105:385–390. doi: 10.1111/j.1471-0528.1998.tb10120.x. [DOI] [PubMed] [Google Scholar]
- 6.Tolentino K, Friedman JF. An update on anemia in less developed countries. Am J Trop Med Hyg. 2007;77:44–51. [PubMed] [Google Scholar]
- 7.Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV. Anaemia in low-income and middle-income countries. Lancet. 2011;378:2123–2135. doi: 10.1016/S0140-6736(10)62304-5. [DOI] [PubMed] [Google Scholar]
- 8.Ouédraogo S, Koura GK, Accrombessi MMK, Bodeau-Livinec F, Massougbodji A, Cot M. Maternal anaemia at first antenatal visit: prevalence and risk factors in a malaria endemic area in Benin. Am J Trop Med Hyg. 2012;87:418–424. doi: 10.4269/ajtmh.2012.11-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephansson O, Dickman PW, Johansson A, Cnattingius S. Maternal hemoglobin concentration during pregnancy and risk of stillbirth. JAMA. 2000;284:2611–2617. doi: 10.1001/jama.284.20.2611. [DOI] [PubMed] [Google Scholar]
- 10.Xiong X, Buekens P, Fraser WD, Guo Z. Anemia during pregnancy in a Chinese population. Int J Gynaecol Obstet. 2003;83:159–164. doi: 10.1016/s0020-7292(03)00214-5. [DOI] [PubMed] [Google Scholar]
- 11.De Maeyer EM. Preventing and Controlling Iron Deficiency Through Primary Care. Geneva, Switzerland: WHO; 1989. [Google Scholar]
- 12.WHO . WHO Expert Committee on Malaria. Twentieth Report. Geneva, Switzerland: WHO; 2000. [Google Scholar]
- 13.WHO . Weekly Iron-Folic Acid Supplementations (WIFS) in Women of Reproductive Age: Its Role in Promoting Optimal Maternal and Child Health Position Statement. Geneva, Switzerland: WHO; 2009. [PubMed] [Google Scholar]
- 14.Ndibazza J, Muhangi L, Akishule D, Kiggundu M, Ameke C, Oweka J, Kizindo R, Duong T, Kleinschmidt I, Muwanga M, Elliott AM. Effects of deworming during pregnancy on maternal and perinatal outcomes in Entebbe, Uganda: a randomized controlled trial. Clin Infect Dis. 2010;50:531–540. doi: 10.1086/649924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabyemela ER, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. Decreased susceptibility to Plasmodium falciparum infection in pregnant women with iron deficiency. J Infect Dis. 2008;198:163–166. doi: 10.1086/589512. [DOI] [PubMed] [Google Scholar]
- 16.Beaton GH. Iron needs during pregnancy: do we need to rethink our targets? Am J Clin Nutr. 2000;72:265S–271S. doi: 10.1093/ajcn/72.1.265S. [DOI] [PubMed] [Google Scholar]
- 17.Milman N. Prepartum anaemia: prevention and treatment. Ann Hematol. 2008;949:959. doi: 10.1007/s00277-008-0518-4. [DOI] [PubMed] [Google Scholar]
- 18.Chesley LC. Plasma and red cell volumes during pregnancy. Am J Obstet Gynecol. 1972;112:440–450. doi: 10.1016/0002-9378(72)90493-0. [DOI] [PubMed] [Google Scholar]
- 19.Hytten F. Blood volume changes in normal pregnancy. Clin Haematol. 1985;14:601–612. [PubMed] [Google Scholar]
- 20.Menendez C, Fleming AF, Alonso PL. Malaria-related anaemia. Parasitol Today. 2000;16:469–476. doi: 10.1016/s0169-4758(00)01774-9. [DOI] [PubMed] [Google Scholar]
- 21.Jennifer LS, Simon B. Impact of hookworm infection and deworming on anaemia in non-pregnant populations: a systematic review. Trop Med Int Health. 2010;15:776–795. doi: 10.1111/j.1365-3156.2010.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoltzfus RJ, Dreyfuss ML. Guidelines for the Use of Iron Supplements to Prevent and Treat Iron Deficiency Anemia. Washington, DC: International Life Sciences Institute Press; 1998. [Google Scholar]
- 23.Menendez C, Bardaji A, Sigauque B, Romagosa C, Sanz S, Serra-Casas E, Macete E, Berenguera A, David C, Dobano C, Naniche D, Mayor A, Ordi J, Mandomando I, Aponte JJ, Mabunda S, Alonso PL. A randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinic. PLoS One. 2008;3:e1934. doi: 10.1371/journal.pone.0001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayentao K, Kodio M, Newman RD, Maiga H, Doumtabe D, Ongoiba A, Coulibaly D, Keita AS, Maiga B, Mungai M, Parise ME, Doumbo O. Comparison of intermittent preventive treatment with chemoprophylaxis for the prevention of malaria during pregnancy in Mali. J Infect Dis. 2005;191:109–116. doi: 10.1086/426400. [DOI] [PubMed] [Google Scholar]
- 25.Tukur IU, Thacher TD, Sagay AS, Madaki JK. A comparison of sulfadoxine-pyrimethamine with chloroquine and pyrimethamine for prevention of malaria in pregnant Nigerian women. Am J Trop Med Hyg. 2007;76:1019–1023. [PubMed] [Google Scholar]
- 26.ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA. 2007;297:2603–2616. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- 27.Huynh BT, Fievet N, Gbaguidi G, Dechavanne S, Borgella S, Guezo-Mevo B, Massougbodji A, Ndam NT, Deloron P, Cot M. Influence of the timing of malaria infection during pregnancy on birth weight and on maternal anemia in Benin. Am J Trop Med Hyg. 2011;85:214–220. doi: 10.4269/ajtmh.2011.11-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faucher JF, Aubouy A, Adeothy A, Cottrell G, Doritchamou J, Gourmel B, Houze P, Kossou H, Amedome H, Massougbodji A, Cot M, Deloron P. Comparison of sulfadoxine-pyrimethamine, unsupervised artemether-lumefantrine, and unsupervised artesunate-amodiaquine fixed-dose formulation for uncomplicated Plasmodium falciparum malaria in Benin: a randomized effectiveness noninferiority trial. J Infect Dis. 2009;200:57–65. doi: 10.1086/599378. [DOI] [PubMed] [Google Scholar]
- 29.Bertin G, Briand V, Bonaventure D, Carrieu A, Massougbodji A, Cot M, Deloron P. Molecular markers of resistance to sulphadoxine-pyrimethamine during intermittent preventive treatment of pregnant women in Benin. Malar J. 2011;10:196. doi: 10.1186/1475-2875-10-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO . Preventing and Controlling Iron Deficiency Anaemia Through Primary Health Care. A Guide for Health Administrators and Programme Managers. Geneva, Switzerland: WHO; 1989. [Google Scholar]
- 31.Widdowson EM, Spray CM. Chemical development in utero. Arch Dis Child. 1951;26:205–214. doi: 10.1136/adc.26.127.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuizon MD, Desnacido JA, Placon CP, Ancheta LD, Macapinlac MP. Iron supplementation using different dose levels in prenant Phillipinois. Nutr Res. 1983;3:257–264. [Google Scholar]
- 33.Makrides M, Crowther CA, Gibson RA, Gibson RS, Skeaff CM. Efficacy and tolerability of low-dose iron supplements during pregnancy: a randomized controlled trial. Am J Clin Nutr. 2003;78:145–153. doi: 10.1093/ajcn/78.1.145. [DOI] [PubMed] [Google Scholar]
- 34.Milman N, Bergholt T, Eriksen L, Byg K-E, Graudal N, Pedersen P, Hertz J. Iron prophylaxis during pregnancy—how much iron is needed? A randomised, controlled study of 20 to 80 mg ferrous iron daily to pregnant women. Acta Obstet Gynecol Scand. 2005;84:238–247. doi: 10.1111/j.0001-6349.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- 35.Menendez C, Todd J, Alonso P, Francis N, Lulat S, Ceesay S, M'boge B, Greenwood B. The effects of iron supplementation during pregnancy, given by traditional birth attendants, on the prevalence of anaemia and malaria. Trans R Soc Trop Med Hyg. 1994;88:590–593. doi: 10.1016/0035-9203(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 36.Zavaleta N, Caulfield LE, Garcia T. Changes in iron status during pregnancy in Peruvian women receiving prenatal iron and folic acid supplements with or without zinc. Am J Clin Nutr. 2000;71:956–961. doi: 10.1093/ajcn/71.4.956. [DOI] [PubMed] [Google Scholar]
- 37.Bonnar J, Goldberg A, Smith JA. Do pregnant women take their iron? Lancet. 1969;1:457–458. doi: 10.1016/s0140-6736(69)91492-5. [DOI] [PubMed] [Google Scholar]
- 38.Habib F, Alabdin EH, Alenazy M, Nooh R. Compliance to iron supplementation during pregnancy. J Obstet Gynaecol. 2009;29:487–492. doi: 10.1080/01443610902984961. [DOI] [PubMed] [Google Scholar]
- 39.Seck BC, Jackson RT. Determinants of compliance with iron supplementation among pregnant women in Senegal. Public Health Nutr. 2008;11:596–605. doi: 10.1017/S1368980007000924. [DOI] [PubMed] [Google Scholar]
- 40.Pena-Rosas JP, Viteri FE. Effects and safety of preventive oral iron or iron+folic acid supplementation for women during pregnancy. Cochrane Database Syst Rev. 2009;4:CD004736. doi: 10.1002/14651858.CD004736.pub3. [DOI] [PubMed] [Google Scholar]