Abstract
The human landing catch (HLC) has long been the gold standard for estimating malaria transmission by mosquitoes, but has come under scrutiny because of ethical concerns of exposing collectors to infectious bites. We estimated the incidence of Plasmodium falciparum malaria infection in a cohort of 152 persons conducting HLCs and compared it with that of 147 non-collectors in western Kenya. Participants were presumptively cleared of malaria with Coartem™ (artemether-lumefantrine) and tested for malaria every 2 weeks for 12 weeks. The HLC collections were conducted four nights per week for six weeks. Collectors were provided chemoprophylaxis with Malarone™ (atovaquone-proguanil) during the six weeks of HLC activities and one week after HLC activities were completed. The incidence of malaria was 96.6% lower in collectors than in non-collectors (hazard ratio = 0.034, P < 0.0001). Therefore, with proper prophylaxis, concern about increased risk of malaria among collectors should not be an impediment to conducting HLC studies.
Introduction
Malaria control in Africa and other malaria-endemic regions relies heavily on vector control measures such as insecticide-treated nets (ITNs) and indoor residual spraying.1 Given the reliance on these vector control measures, many malaria control programs also implement some form of entomologic monitoring to assess their impact on vector populations and vector behavior. The traditional gold standard method to monitor vector populations has been the human landing catch (HLC) which involves persons sitting with their lower legs exposed and collecting mosquitoes that come to feed on them during the night.2 The HLC is a simple, elegant, and powerful tool. It is the most direct measure of mosquito biting, it can be implemented indoors or outdoors, and it provides information on the time of biting. Data on temporal and spatial distribution of bites is important in many areas where mosquitoes bite outside or early in the evening and may be even more important in areas such as Africa where malaria vectors that traditionally feed indoors late at night may be shifting their behaviors in the face of intense pressure from vector control interventions, specifically ITNs and indoor residual spraying.3 Furthermore, the mosquitoes collected are generally held alive until processing. This feature enables numerous manipulations in the laboratory that cannot easily be done in specimens that are killed or that have abdomens that are full of blood or eggs. These mosquitoes can be dissected for parity determination as an indicator of mosquito age4,5 or counts of oocysts as an indicator of mosquito infection rates.6 The live mosquitoes can also be used in insecticide resistance assays as the most direct measure of the efficacy of insecticides used for vector control.7
However, the HLC has at least two disadvantages. One is that to obtain reliable data, the HLC requires intensive supervision of personnel who must remain awake through most of the night performing a task that can be very tedious. The other disadvantage is an ethical and safety concern. The World Health Organization now recommends universal ITN coverage for all persons living in malaria-endemic areas.8 Therefore, employing persons to stay up all night for the express purpose of collecting host-seeking mosquitoes exposes them to malaria that they might have otherwise avoided if they were protected under an ITN. In the past, implementation of vector control interventions was limited, if not completely absent, and most adults in malaria-endemic areas were considered to have sufficient acquired immunity against malaria such that exposure to malaria-infected mosquitoes while conducting HLCs was believed to pose minimal to no additional risk. With decreasing malaria transmission in many areas, malaria infection may be detrimental to adults who may have lower acquired immunity because of reduced malaria exposure than they have had in the past. Furthermore, in the setting of universal ITN policy, purposeful exposure of adults to malaria infection is no longer acceptable. One way to continue to obtain the important information available only through HLCs, while protecting adults from malaria infection is to provide collectors with malaria chemoprophylaxis to prevent the acquisition of malaria infection even if exposure to an infected mosquito occurs. Chemoprophylaxis has been shown to be very effective in preventing malaria in non-immune travelers as well as in semi-immune populations living in malaria endemic areas.9–11
Despite many attempts to find a suitable replacement for the HLC,12–14 none have consistently proven to be as sensitive and as versatile as the HLC, and given the risks of malaria infection and illness, the use of the HLC remains controversial. Increasingly, ethical review committees are reluctant to approve studies that include the use of HLCs given the concern about increased risk of disease among collectors. The result is that entomologic monitoring increasingly will rely on indirect approaches to estimate malaria transmission indoors and outdoors, such as the recent study inferring a highly efficient, exophilic vector in Burkina Faso based on genetic comparisons between mosquitoes resting indoors and those collected from larval habitats,15 a conclusion that was disputed by some investigators.16 As vector control is scaled up in malaria-endemic areas, the need for high-quality entomologic monitoring using methods that are broadly comparable across many sites and under conditions of varying vector behavior and ecology is more urgent than ever. The HLC provides just such a method.
The controversy over the use of the HLC is fueled by a lack of data on the actual risks to collectors. We were unable to find a single study that scientifically evaluated risk to collectors in the published literature. Therefore, to provide concrete data to guide researchers, national malaria control programs, and ethical review committees, we conducted a prospective trial to estimate the incidence of Plasmodium falciparum malaria infection in a cohort of persons conducting HLCs four nights per week during a six-week period while taking effective malaria chemoprophylaxis compared with a matched cohort of persons residing in the same villages who were not conducting HLCs and who were not taking chemoprophylaxis. The study was designed to provide concrete data to guide researchers, national malaria control programs, and ethical review committees on the potential risks of conducting HLCs.
Materials and Methods
Study site and population.
The study was conducted in the area of Asembo in Rarieda District in western Kenya. Asembo includes 75 villages covering approximately 200 km2 of gently rolling hills bisected by small streams that discharge into Lake Victoria, which forms the southern boundary of Asembo. The area is characterized by high, year-round transmission of malaria. In the early 1990s, the annual entomologic inoculation rate (EIR) was estimated to be > 300 infectious bites per person per year.17 In the late 1990s, a large scale ITN trial was conducted in Asembo and entomologic measures of transmission were estimated to have been reduced by > 90%.18 Insecticide-treated nets were scaled up throughout western Kenya and in May 2011, a mass campaign was conducted targeting one net for every two persons. A survey conducted in Asembo during the current study found that 82% of households had at least one ITN (Desai M, unpublished data). Experiments with light traps or pyrethrum spray catches have shown that annual estimated EIRs since 2003 were < 15 infectious bites per person per year (Bayoh MN, unpublished data). Historically, the primary vectors in this area were Anopheles gambiae s.s. and An. funestus; An. arabiensis was a secondary vector. Numbers of An. funestus decreased precipitously during and after the ITN trial in the late 1990s, and the proportion of An. gambiae s.s. relative to An. arabiensis shifted in 2007, and An. arabiensis has been the predominant vector captured in indoor collections since that time.19 There are two main peaks of malaria transmission after the long rains (March–June) and the short rains (October–November), but rainfall and malaria transmission occur year round.
The population of Asembo is predominantly of the Luo ethnic group. Residents live in compounds, scattered clusters of houses surrounded by agricultural fields. Houses vary in size and style from traditional huts made of mud walls and thatched roofs to permanent structures with cement walls and iron sheet or tiled roofs. Most of the houses have open eaves, which enable unimpeded entry and exit of mosquitoes. Most residents are subsistence farmers; maize, millet, cassava, and ground nuts are the primary crops. The population has been monitored under a Health and Demographic Surveillance System (HDSS) since 2003.20
Enrollment.
Of the 75 villages in Asembo, one was administratively split because of its large size and one village was not included in the study. Thus, 75 villages were included in the study. In each village, two men ≥ 18 years of age were recruited to participate in the study as collectors. Given the nature of the HLC work, persons with experience in entomologic projects were recruited along with other members of the community who were known to study staff as reliable. A non-collector was matched to each collector based on village of residence and age (within 10 years). Initially, non-collectors were matched to collectors by using the HDSS database. However, this matching proved unreliable given the mobility of the target population and therefore most of the matches were conducted manually by village reporters used as part of the HDSS project. Enrollment of all the collectors was completed on June 6, 2011. Enrollment of the non-collectors began on June 13, 2011. However, because of difficulties in matching collectors to non-collectors, enrollment of the non-collectors was not completed until June 29, 2011. Before enrollment, the study was explained to all participants in their local language and written consent was obtained from all study participants.
At enrollment, a short questionnaire was administered on a personal digital assistant to all participants and a blood smear was taken to test for malaria. However, all participants (collectors and non-collectors) were provided with a treatment dose of Coartem™ (20 mg of artemether and 120 mg of lumefantrine, 4 tablets taken twice a day for 3 days) to clear any parasites, regardless of symptoms or the results of their blood smear.
Human landing catches.
Collectors were provided a directly observed dose of Malarone™ (atovaquone-proguanil) on June 12, 2011. Collectors were then provided 2 weeks of Malarone™ and directed to take one tablet per day at the same time with food. Malarone™ was provided again at each follow-up visit, conducted every two weeks, until the end of the HLCs. To monitor adherence to chemoprophylaxis, each collector recorded a daily log of when they took Malarone™, which was self-reported during each follow-up visit.
Human landing catches began on June 13, 2011. Collectors were trained to sit in a chair with their pants pulled up to their knees and to collect mosquitoes that landed on their lower legs by using a mouth aspirator. Collectors were instructed to wear long-sleeve shirts during the collections to prevent mosquitoes landing and biting on the arms. However, the collectors did not use face or head protection. Mosquitoes were transferred to a paper cup and provided with cotton soaked in sugar water. New cups were used for each hour and each location.
Collectors were organized into 38 teams of 4 persons from two neighboring villages with the exception of 1 team that consisted of only 2 collectors. The collection sites were the compounds where each collector lived and each night, and the teams worked in one team member's compound. The teams rotated among the four compounds so that every week there were two nights of collections per village in Asembo. Collections began at 5:00 pm with one person collecting inside and one person collecting outside. The other two collectors rested. At midnight, the collectors switched, enabling the two collectors who collected from 5:00 pm to rest while the other two collected mosquitoes until 7:00 am. Collectors were instructed to collect for 45 minutes during each hour and to take a 15-minute break before resuming collections for the next hour. The person collecting outdoors was given discretion to stop collecting in the event of rain and to indicate that collections had stopped because of rain at a given hour. Collectors working indoors were instructed to continue regardless of rainfall.
A team leader was appointed for each collection team to ensure that collections were made properly and on time and to assist with monitoring of adherence to chemoprophylaxis. Each night, all teams were contacted by supervisors by mobile phone to determine who was working and if there were any problems. Each team was contacted 4–5 times throughout the night. In addition, a team of supervisors performed random spot checks on 3–4 teams per night to ensure that the collectors were collecting mosquitoes as directed.
Collections were made four nights per week over the course of six weeks at the end of the peak transmission season. The last collections were conducted on the night of July 22, 2011. Collectors were instructed to continue taking Malarone™ for one week after the final night of collection consistent with the recommendations for Malarone™ during a period of potential malaria exposure. All collectors were provided compensation for each night worked.
Follow-ups.
All participants were asked to come to the nearest clinic for follow-up with a study clinician at two-week intervals or sooner if the participant became ill. During these follow-up visits, a short questionnaire was administered and a blood sample was taken for malaria microscopy regardless of symptoms. The blood smears were stained with Giemsa and examined independently for the presence of P. falciparum by two expert microscopists. In the event of discordant readings, a third expert microscopist re-examined the slide to determine whether P. falciparum parasites were present. However, any participant found to have malaria by blood smear upon the first reading was treated immediately. If at any time, a participant was ill, he was instructed to come to the clinic to see the study clinician. As with the routine follow-ups, a short questionnaire was administered and a blood smear was prepared. Study clinics were also provided with rapid diagnostic tests to assist in diagnosis and treatment during these sick visits without waiting for the results of the blood smear.
If a participant missed a follow-up, he was visited by a community interviewer who encouraged the person to come to the clinic for follow-up. If a participant was late for a follow-up visit, the next visit was scheduled for two weeks after the date of the actual visit. The final follow-ups for collectors were completed on September 20, 2011, and the final follow-ups for non-collectors were completed on September 28, 2011.
If a non-collector became blood smear positive for malaria at any time during the study, he was provided malaria treatment and dropped from the study. If collectors became malaria positive during the study, they were provided treatment and asked to continue with all study activities, including HLCs and clinic visits, until the end of the study. The HLCs continued as a peripheral research study on mosquito behavior, and collectors were asked to continue with collections as well as to return for their regularly scheduled clinic visits as a safeguard against malaria infection. However, because incidence of first or only malaria episode was the primary end point of this study, data collected after a collector became malaria positive were not used in the analysis.
Statistical analysis.
Baseline differences between the two study groups were compared by using bivariate analyses. Ordinal logistic regression adjusting for correlation within matched pairs of collectors and non-collectors using generalized estimating equations was conducted for house type. For age, the difference between paired collectors and non-collectors was calculated and then tested by using a paired t-test. For all other outcome variables, McNemar's test was used to compare paired collectors and non-collectors.
The primary endpoint of the study was the time to first infection. Person time was estimated, reduced by 10.5 days for treatment with Coartem™ at baseline and whenever a participant received Coartem™ during the study, and the incidence was compared between collectors and non-collectors. Matched survival analysis was conducted by using a stratified Cox proportional hazards model. Matching was accounted for in the models by including a strata statement with the matching variable (robust sandwich estimator of the standard errors). The assumption of proportionality was tested and met for the Cox proportional hazards analysis. Regression models were adjusted for P. falciparum infection, and use of artemisinin combination therapies (ACTs) and mosquito prevention methods such as mosquito coils and sprays at baseline. However, models did not converge with the inclusion of any of these variables and all final analyses were unadjusted. Assuming the period at risk for malaria infection as part of the HLCs would be effective for approximately 65 days, additional models were constructed before and after that cut-off value to compare the incidence during the risk period and after when the effects of prophylaxis were likely to have waned.
Kaplan-Meier survival analysis was used to estimate median times to first infection. However, the Kaplan-Meier analysis could not be adjusted for the matched design. Using only the matched data with persons who either completed at least five scheduled follow-up visits or were positive for malaria during the study, we used McNemar's test to compare the frequency of discordant pairs in which collectors became malaria positive during the study and non-collectors remained malaria negative throughout the study versus those discordant pairs in which non-collectors became malaria positive during the study while collectors remained malaria negative. For the McNemar's test, only persons who either became malaria positive or completed at least five of the six scheduled follow-up visits were included in the analysis.
The EIR for the six weeks of HLCs was estimated by summing the numbers collected over each night and each site of collection and calculating an average number of bites per person per night for each location of collection (indoors or outdoors). This was divided by 0.75 because collectors worked for 45 minutes each hour and then multiplied by the sporozoite infection rate estimated by using enzyme-linked immunosorbent assays.21
Ethical approval.
This study was approved by the Institutional Review Boards of the U.S. Centers for Disease Control and Prevention and Michigan State University and by the Ethical Review Committee of the Kenya Medical Research Institute.
Results
A total of 152 persons were enrolled as collectors. Two persons withdrew from the study soon after enrollment and did not participate in any collections. Of the remaining 150 collectors, 147 were matched to a non-collector based on village of residence and age (within 10 years). Matching proved difficult because the target population (men ≥ 18 years of age) was mobile and less willing to participate in studies that required multiple repeated follow-ups.
Characteristics of the collectors and non-collectors as determined at enrollment are shown in Table 1. At baseline, there were no differences between the collectors and non-collectors in house type, use of a bed net the previous night, or parasite prevalence. There were also no differences in history of fever, antimalarial drug use, or Coartem™ use in the previous two weeks. A paired t-test showed that collectors were significantly older than non-collectors. However, the mean difference in age was only 1.0 years, and the median difference in age was only 1.7 years (range −9.0 to 9.4 years).
Table 1.
Characteristics of collectors and non-collectors at enrollment, western Kenya*
| Characteristic | Collectors | Non-collectors | P |
|---|---|---|---|
| No. enrolled | 152 | 147 | |
| Median age, years (range) | 29 (18.6–51.5) | 28 (18.1–52.2) | 0.003 |
| House type | |||
| Traditional mud hut (%) | 44 | 46 | |
| Semi-permanent (iron sheet roof) (%) | 39 | 41 | |
| Permanent (concrete or stone walls) (%) | 17 | 13 | 0.327 |
| Mosquito coils, insecticide sprays, or repellents used in previous 2 weeks (%) | 3 | 9 | 0.059 |
| Used bed net previous night (%) | 90 | 90 | 0.835 |
| Fever in previous 2 weeks (%) | 22 | 22 | 0.763 |
| Took antimalarial drug in previous 2 weeks (%) | 5 | 8 | 0.197 |
| Took ACT in previous 2 weeks (%) | 4 | 7 | 0.083 |
| Plasmodium falciparum parasitemia prevalence (%) | 17 | 17 | 1.000 |
Differences in age were compared by paired t-test, and differences in house type were compared by ordinal logistic regression controlling for correlation within the matched pairs. Differences in all other variables were compared by McNemar's test. Descriptive statistics include all 152 collectors, and comparisons included only those matched to a non-collector (n = 147). ACT = artemisinin combination therapy.
Of the 299 persons originally enrolled, 253 (84.6%) either completed the 6 scheduled follow-up visits or had a positive blood smear during one of the follow-up visits. Allowing for one missed visit, 266 (88.9%) of the study participants completed the study and 26 (17.7%) of the non-collectors were lost to follow-up compared with 7 (4.6%) of the collectors (χ2 = 13.0, P < 0.001). For eight participants (one collector and seven non-collectors), follow-ups were considered not completed because the participants' blood smears were initially read as positive for P. falciparum malaria but upon re-examination, the blood smears were later determined to be negative. Follow-up visits for these eight participants ended after the initial malaria-positive blood smear reading. One mosquito collector who had a chronic illness withdrew from the study at the end of the HLCs and died of that chronic illness approximately seven weeks later. He did not test positive for malaria and his death was unrelated to the study.
During the follow-up period, 37 participants tested positive for malaria. Of those who tested positive, 5 were collectors (3.5% of collectors who completed at least 5 follow-ups or became positive) and 32 were non-collectors (26.5% of non-collectors who completed at least 5 follow-ups or became positive). Based on an estimated person-time at risk of 35.0 years for the collectors and 28.3 years for the non-collectors, the rates of malaria were 0.14 infections per person-year for collectors and 1.13 infections per person-year for non-collectors. Persons performing HLCs while taking Malarone™ prophylaxis were 96.6% less likely to become infected with P. falciparum malaria than other persons living in their community (P < 0.0001) (Table 2). Models constructed by using follow-up visits that occurred within 65 days after enrollment failed to converge because no mosquito collectors became infected during this time. Using only follow-up data collected more than 65 days after enrollment, we found that collectors were still less likely to become infected although the difference was not statistically significant (P = 0.069).
Table 2.
Malaria parasitemia incidence among collectors and non-collectors and the hazard ratio estimated from a Cox proportional hazards model, western Kenya
| Group | Events | Person-years at risk | Rate per person-year | Hazard ratio | P |
|---|---|---|---|---|---|
| Collectors | 5 | 35.0 | 0.14 | 0.034 | < 0.0001 |
| Non-collectors | 32 | 28.3 | 1.13 | Referent | Referent |
A total of 117 of 147 matched pairs of collectors and non-collectors completed at least 5 follow-up visits or were positive for malaria during the follow-up period. Among these matched pairs, there were 32 discordant pairs in which one participant was malaria positive and the other was malaria negative. Within the 32 discordant pairs, non-collectors became positive in 30 (93.7% of discordant pairs) pairs, and collectors became positive in 2 (6.3% of discordant pairs) pairs. Non-collectors became positive in a significantly higher proportion of the discordant pairs compared with collectors (McNemar's statistic = 24.5, P < 0.0001) (Table 3).
Table 3.
Summary of paired analysis among collectors and non-collectors, western Kenya*
| Collectors | Non-collectors | ||
|---|---|---|---|
| Negative | Positive | ||
| Negative | 84 (71.8) | 30 (25.6) | |
| Positive | 2 (1.7) | 1 (0.9) | |
| McNemar's statistic | 24.5 | – | – |
| Degrees of freedom | 1 | – | – |
| P value | < 0.0001 | – | – |
Numbers in each cell indicate the number of matched pairs with respective outcomes. The numbers in parentheses indicate the proportion of matched pairs in each cell (n = 117 matched pairs).
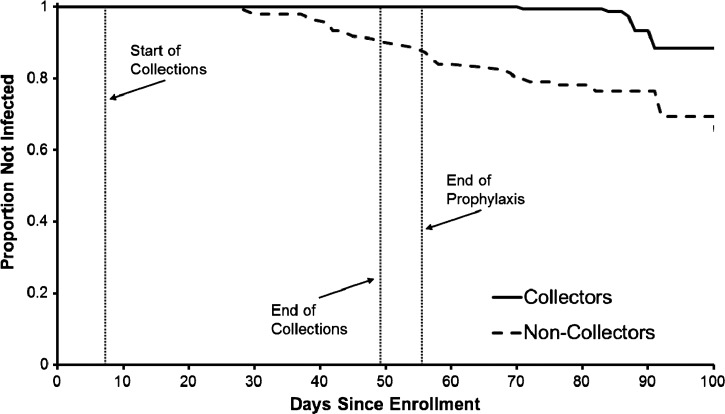
A Kaplan-Meier survival analysis is shown in Figure 1 along with the days on which HLC began and ended as well as the day that prophylaxis ended. Overall, the survival curves for the collectors and non-collectors were significantly different (Wilcoxon χ2 = 33.3, P < 0.0001). The timing of events related to the study shows that all infections among the collectors occurred after the completion of prophylaxis. One collector became positive approximately 3.5 weeks after the completion of the HLCs, and the remaining four collectors became positive more than one month after the completion of the HLCs.
Figure 1.
Kaplan-Meier survival analysis showing time to first infection for collectors and non-collectors, western Kenya. The start of human landing catches, the end of human landing catches, and the end of prophylaxis for the collectors are indicated by vertical lines. The collectors began prophylaxis one day before the start of catches.
Reported rates of Malarone™ use among the collectors were high. Among follow-up visits that occurred before August 1, 2011, nearly all collectors reported taking Malarone™ every day as originally instructed (496 of 523 = 94.8% of all follow-up visits). Similarly, reported net use among the non-collectors was high. Nearly all non-collectors reported using a net the night before their follow-up visit (98.8%) and 93.6% (685 of 732 follow-up visits) reported using a net every night.
Indoor biting rates by anopheline mosquitoes were estimated to be 1.8 per person per night, and outdoor biting rates were estimated to be 1.0 per person per night. Sporozoite rates were 8.5% in mosquitoes collected indoors and 7.9% in mosquitoes collected outdoors. The resulting EIRs were estimated to be 0.15 infectious bites per person per night indoors and 0.08 infectious bites per person per night outdoors. Therefore, exposure during the 6 weeks of the HLCs was estimated to be 6.4 and 3.3 infectious bites per person, indoors and outdoors, respectively.
Discussion
Persons conducting HLCs while receiving malaria chemoprophylaxis had a 96.6% lower incidence of malaria infection compared with other members of their communities who were not performing mosquito collections late at night. The actual incidence during the HLCs was likely even lower because we continued follow-up visits for five weeks after completion of Malarone™ prophylaxis to assess whether there was an increase in malaria incidence and because all five infections in the collectors were detected over three weeks after the completion of the HLC collection period. Of these, one case of malaria occurred 3.5 weeks after the completion of the HLC collection and the remaining four occurred over a month after the completion of HLC collection. The pre-patent period for P. falciparum is estimated to be 9–10 days.22 It is therefore unlikely that any of the five collectors became infected until after the end of mosquito collections.
We attempted to construct Cox proportional hazards models by using only data collected during the 65 days after enrollment because this was the period in which collectors were mostly likely to have become infected during the HLCs. However, these models failed to converge because no collectors became positive during this period. This finding suggests that prophylaxis mitigated the risk of HLCs, and the collectors who eventually were positive likely were infected after the HLCs were completed. Models constructed using only follow-ups > 65 days after enrollment indicated that collectors were less likely to become malaria positive. These latter models were likely biased because of loss to follow-up that occurred < 65 days after enrollment. However, these models suggest that there is no immediate increase in infections among collectors or that the effects of prophylaxis may extend longer than expected, at least in this population of semi-immune adults.
The incidence of malaria among the non-collectors was estimated to be 1.13 infections per person per year. Because non-collectors reported frequent use of ITNs, this figure represents the estimated minimum rate of infection in the collector cohort, had the collectors not been provided prophylaxis. Furthermore, entomologic data indicated an EIR of approximately 6.4 infectious bites per person indoors during the course of the six-week HLC study. The efficacy of Malarone™ in an area with a high EIR and a high incidence of infections in humans indicates that prophylaxis in a semi-immune population is effective even in areas of high transmission.23
One question that we could not address given our study design was whether HLCs in the absence of prophylaxis place collectors at increased risk for malaria. Given the high incidence of malaria in the non-collectors, the incidence of malaria in a cohort of collectors not receiving prophylaxis might not have been significantly higher than that of the non-collectors. Nevertheless, with changing ethical standards, it was not possible to conduct HLCs without providing prophylaxis.
Although this study showed a reduced incidence of malaria among collectors on prophylaxis in an area of high transmission compared with persons not engaged in HLCs, additional studies are required to assess the risk of malaria in areas with lower transmission such as South America or Southeast Asia. In many of these settings, the HLC may be even more important as a vector monitoring tool than in settings in Africa because vectors of malaria may be less endophagic and/or endophilic and alternatives to the HLC may not be reliable. However, many vectors in these settings feed primarily outdoors or earlier in the evening, and collectors may be at increased risk for infectious bites compared with those sleeping under ITNs. Furthermore, because of lower transmission, collectors may have lower acquired immunity and therefore may be at even greater risk for severe disease because of malaria. Given the reported efficacy of Malarone™ in non-immune persons,24–27 it is likely that prophylaxis with this drug would also be effective in areas with lower transmission. However, with a lower incidence of malaria among the non-collectors, the effects may not be as dramatic as observed in this study.
Human landing catches have been used for decades, often without the benefit of prophylaxis among collectors. In several studies in which the HLC was used in malaria-endemic areas of Africa, none indicated that persons making the collections were protected by prophylaxis from malaria infection.28–32 In a review of field sampling methods for mosquitoes, provision of prophylaxis for mosquito collectors conducting HLCs was reported in only two studies.33 However, despite numerous examples in which HLC was conducted without malaria prophylaxis, there are, to our knowledge, no reports of serious adverse events as a result of HLCs. For malaria, clinical illness among adults is much less frequent than in children because of acquired immunity in high transmission areas,34 and it has been assumed that semi-immune adults are unlikely to become ill because of infections acquired during HLCs. Furthermore, until recently, because there were rarely any vector control measures in place, collectors were typically not at higher risk for malaria infection than they would have been if they were non-collectors. Most of these studies were conducted before institutional review boards and systems to reduce risks of human subjects in research were in place or before the details of the HLC came under scrutiny of institutional review boards. Ethical standards evolve as new risks are identified or new tools become available for risk mitigation. The development and deployment of ITNs has altered the landscape because all persons are now expected to be protected by ITNs when mosquitoes are active. Therefore, asking persons to perform HLCs potentially places them at higher risk. However, giving collectors prophylaxis not only mitigates the risk, it provides additional protection to the collectors and places them at a much lower risk for malaria than other persons in their community.
We provided daily prophylaxis with Malarone™ for the mosquito collectors. Malarone™ is one of the more expensive options for prophylaxis but has been shown to be highly effective in travelers24–27 and semi-immune persons23,35–37 living in malaria-endemic regions. Furthermore, Malarone™ is well tolerated24–26,38 and needs to be taken for only one week after potential exposure to malaria. However, other less expensive but effective options for malaria prophylaxis are available for use during HLCs. In addition to the lower cost, there may be other factors to consider when selecting a drug for prophylaxis, such as the convenience of supervising weekly prophylaxis for mefloquine rather than daily prophylaxis for Malarone™ or doxycycline, and the time after HLC completion that the antimalarial drug will be continued (one week for Malarone™ and four weeks for doxycycline or mefloquine). The selection of chemoprophylaxis for mosquito collectors should be determined by cost, ease of administration, and concerns about potential side effects.
This study had several limitations. The main limitation was the lack of randomization among the collectors and non-collectors. The Kenya Medical Research Institute and the U.S. Centers for Disease Control and Prevention have had a long history of working on malaria in Asembo, and most of the collectors were recruited from a pool of persons who had previously worked on various projects. This lack of randomization was used primarily to ensure the quality of the HLCs. We originally planned to match non-collectors to collectors by using the HDSS database. However, the target population proved to be highly mobile and was not well captured in the HDSS database. Therefore, we relied on village reporters to non-randomly identify and recruit eligible persons as non-collectors. In addition, there were several differences in the two groups at baseline, which suggest a lack of comparability between them. First, there were differences in age of the two study populations at baseline. However, this difference was small and is unlikely to have affected our results. Second, there was a higher rate of loss to follow-up in the non-collectors. This finding is not surprising given the high mobility of the population and the fact that the collectors were provided compensation for the nights they worked. However, the likely effect of the differential loss to follow-up would be to bias our results to the null because additional non-collectors might have been screened as being malaria positive had they attended all their follow- up visits. Third, although not statistically significant, there was some evidence that the non-collectors were more likely to have taken an ACT or used mosquito prevention methods such as mosquito coils or insecticide sprays at the baseline. Although baseline malaria prevalence was similar in both groups, the differences in ACT use and mosquito prevention methods suggest that there may have been a higher risk of malaria in non-collectors. We attempted to control for use of ACTs and mosquito prevention methods at baseline in the Cox proportional hazards model but the models failed to converge when these variables were included. Given the low frequency of use of ACTs, mosquito coils, and insecticide sprays among both groups, it is likely that any bias was minimal.
We relied on reported rates of use of bed nets by the non-collectors and Malarone™ by the collectors, and the high rates of use among both groups might have been overestimates. However, the area of Asembo has a long history of net use,39,40 and the Kenya Division of Malaria Control had recently completed as mass campaign targeting universal coverage throughout western Kenya. In a survey conducted in Asembo in July 2011, 90% of persons ≥ 15 years of age reported using a net the night before and 78% reported sleeping under an ITN. The fact that no mosquito collectors became malaria positive while receiving prophylaxis suggests that compliance was high. In addition, supervision of HLCs is critical to ensure high-quality data. With a large cohort of collectors, we relied on mobile phones to check in with all teams several times through the night. Although it was rare for teams to fail to check in, we cannot guarantee that they were actually engaged in HLCs throughout each night. However, during numerous unannounced spot checks, on only one occasion was a team found not performing HLCs. Despite these reservations, the study provided a robust and conclusive result.
Our study demonstrated that the incidence of malaria among persons conducting HLCs is low relative to the general population, provided they are given appropriate chemoprophylaxis. Before undertaking studies or surveillance including HLCs, it is important to assess other risks including other vector-borne diseases such as lymphatic filariasis, yellow fever, and dengue, which were not present in our study area and for which there is no prophylaxis or treatment. However, the risk of malaria among collectors taking chemoprophylaxis is low and concerns about malaria risk alone should not be an impediment to conducting HLCs.
ACKNOWLEDGMENTS
We thank the clinical officers for conducting the enrollment survey and follow-up visits, Kephas Otieno for assisting with reading of blood smears, Jodi Vanden Eng for helping with statistical analysis, and study participants for their cooperation. This paper was published with the permission of the director of the Kenya Medical Research Institute.
Disclaimer: The opinions or assertions contained in this manuscript are the private ones of the authors and are not to be construed as official or reflecting the views of the U.S. Public Health Service or Department of Health and Human Services. Use of trade names is for identification only and does not imply endorsement by U.S. Public Health Service or Department of Health and Human Services.
Footnotes
Financial support: This study was supported by the Bill and Melinda Gates Foundation through the Malaria Transmission Consortium (grant no. 45114) and by a National Science Foundation Ecology of Infectious Diseases grant (grant no. EF-072377).
Authors' addresses: John E. Gimnig and Mary J. Hamel, Division of Parasitic Diseases and Malaria, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: jgimnig@cdc.gov and mhamel@cdc.gov. Edward D. Walker, Department of Microbiology and Molecular Genetics, Michigan State University, East Lansing, MI, E-mail: walker@msu.edu. Peter Otieno, Jackline Kosgei, George Olang, Maurice Ombok, John Williamson, Doris Marwanga, Daisy Abong'o, Meghna Desai, Simon Kariuki, and M. Nabie Bayoh, Kenya Medical Research Institute/Centers for Disease Control and Prevention Research and Public Health Collaboration, Kisumu, Kenya, E-mails: potieno@kemricdc.org, jkosgei@kemricdc.org, golang@kemricdc.org, mombok@kemricdc.org, dmarwanga@kemricdc.org, dabongo@kemricdc.org, mdesai@kemricdc.org, skariuki@kemricdc.org, and nbayoh@kemricdc.org. Neil F. Lobo, Eck Institute for Global Health, Department of Biological Sciences, University of Notre Dame, Notre Dame, IN, E-mail: nlobo@nd.edu. John Vulule, Centre for Global Health Research, Kenya Medical Research Institute, Kisumu, Kenya, E-mail: jvulule@kemricdc.org.
References
- 1.World Health Organization . World Malaria Report 2011. Geneva: World Health Organization; 2011. [Google Scholar]
- 2.Service MW. Mosquito Ecology. Field Sampling Methods. Essex, UK: Elsevier Science Publishers; 1993. [Google Scholar]
- 3.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Detinova TS. Age-Grouping Methods in Diptera of Medical Importance. Geneva: World Health Organization; 1962. Monograph Series. [PubMed] [Google Scholar]
- 5.Beklemishev WN, Detinova TS, Polovodova VP. Determination of physiological age in anophelines and of age distribution in anopheline populations in the USSR. Bull World Health Organ. 1959;21:223–232. [PMC free article] [PubMed] [Google Scholar]
- 6.Killeen GF, Ross A, Smith T. Infectiousness of malaria-endemic human populations to vectors. Am J Trop Med Hyg. 2006;75:38–45. doi: 10.4269/ajtmh.2006.75.2_suppl.0750038. [DOI] [PubMed] [Google Scholar]
- 7.Brogdon WG, McAllister JC. Simplification of adult mosquito bioassays through use of time-mortality determinations in glass bottles. J Am Mosq Control Assoc. 1998;14:159–164. [PubMed] [Google Scholar]
- 8.RBM-Partnership Global Malaria Action Plan. 2008. http://www.rbm.who.int/gmap/index.html Available at.
- 9.Boggild AK, Parise ME, Lewis LS, Kain KC. Atovaquone-proguanil: report from the CDC expert meeting on malaria chemoprophylaxis (II) Am J Trop Med Hyg. 2007;76:208–223. [PubMed] [Google Scholar]
- 10.Tan KR, Magill AJ, Parise ME, Arguin PM. Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am J Trop Med Hyg. 2011;84:517–531. doi: 10.4269/ajtmh.2011.10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacquerioz FA, Croft AM. Drugs for preventing malaria in travellers. Cochrane Database Syst Rev. 2009;4:CD006491. doi: 10.1002/14651858.CD006491.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Govella NJ, Chaki PP, Mpangile JM, Killeen GF. Monitoring mosquitoes in urban Dar es Salaam: evaluation of resting boxes, window exit traps, CDC light traps, Ifakara tent traps and human landing catches. Parasites and Vectors. 2011;4:40. doi: 10.1186/1756-3305-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kweka EJ, Mahande AM. Comparative evaluation of four mosquito sampling methods in rice irrigation schemes of lower Moshi, northern Tanzania. Malar J. 2009;8:149. doi: 10.1186/1475-2875-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnard DR, Knue GJ, Dickerson CZ, Bernier UR, Kline DL. Relationship between mosquito (Diptera: Culicidae) landing rates on a human subject and numbers captured using CO2-baited light traps. Bull Entomol Res. 2011;101:277–285. doi: 10.1017/S0007485310000453. [DOI] [PubMed] [Google Scholar]
- 15.Riehle MM, Guelbeogo WM, Gneme A, Eiglmeier K, Holm I, Bischoff E, Garnier T, Snyder GM, Li X, Markianos K, Sagnon N, Vernick KD. A cryptic subgroup of Anopheles gambiae is highly susceptible to human malaria parasites. Science. 2011;331:596–598. doi: 10.1126/science.1196759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Della Torre A, Pombi M, Petrarca V, Coluzzi M. New mosquito subgroup breeds questions. Science. 2011;332:419–420. doi: 10.1126/science.332.6028.419-b. [DOI] [PubMed] [Google Scholar]
- 17.Beier JC, Oster CN, Onyango FK, Bales JD, Sherwood JA, Perkins PV, Chumo DK, Koech DV, Whitmire RE, Roberts CR, Diggs CL, Hoffman SL. Plasmodium falciparum incidence relative to entomologic inoculation rates at a site proposed for testing malaria vaccines in western Kenya. Am J Trop Med Hyg. 1994;50:529–536. doi: 10.4269/ajtmh.1994.50.529. [DOI] [PubMed] [Google Scholar]
- 18.Gimnig JE, Vulule JM, Lo TQ, Kamau L, Kolczak MS, Phillips-Howard PA, Mathenge EM, ter Kuile FO, Nahlen BL, Hightower AW, Hawley WA. Impact of permethrin-treated bednets on entomological indices in an area of intense year-round malaria transmission. Am J Trop Med Hyg. 2003;68((Suppl)):16–22. [PubMed] [Google Scholar]
- 19.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, Vulule JM, Hawley WA, Hamel MJ, Walker ED. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adazu K, Lindblade KA, Rosen DH, Odhiambo F, Ofware P, Kwach J, Van Eijk AM, Decock KM, Amornkul P, Karanja D, Vulule JM, Slutsker L. Health and demographic surveillance in rural western Kenya: a platform for evaluating interventions to reduce morbidity and mortality from infectious diseases. Am J Trop Med Hyg. 2005;73:1151–1158. [PubMed] [Google Scholar]
- 21.Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Burkot TR, Schneider I, Esser KM, Beaudoin RL, Andre RG. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65:39–45. [PMC free article] [PubMed] [Google Scholar]
- 22.Warrell DA. Clinical features of malaria. In: Gilles HM, Warrell DA, editors. Bruce-Chwatt's Essential Malariology. London: Edward Arnold; 1993. pp. 12–34. [Google Scholar]
- 23.Shanks GD, Gordon DM, Klotz FW, Aleman GM, Oloo AJ, Sadie D, Scott TR. Efficacy and safety of atovaquone/proguanil as suppressive prophylaxis for Plasmodium falciparum malaria. Clin Infect Dis. 1998;27:494–499. doi: 10.1086/514710. [DOI] [PubMed] [Google Scholar]
- 24.Camus D, Djossou F, Schilthuis HJ, Høgh B, Dutoit E, Malvy D, Roskell NS, Hedgley C, De Boever EH, Miller GB, Team IMS. Atovaquone-proguanil versus chloroquine-proguanil for malaria prophylaxis in nonimmune pediatric travelers: results of an international, randomized, open-label study. Clin Infect Dis. 2004;38:1716–1723. doi: 10.1086/421086. [DOI] [PubMed] [Google Scholar]
- 25.Høgh B, Clarke PD, Camus D, Nothdurft H, Overbosch D, Gunther M, Joubert I, Kain K, Shaw D, Roskell N, Chulay J, Team IMS. Atovaquone-proguanil versus chloroquine-proguanil for malaria prophylaxis in non-immune travelers: a randomized, double-blind study. Lancet. 2000;356:1888–1894. doi: 10.1016/s0140-6736(00)03260-8. [DOI] [PubMed] [Google Scholar]
- 26.Overbosch D. Post-marketing surveillance: adverse events during long-term use of atovaquone/proguanil for travelers to malaria-endemic countries. J Travel Med. 2003;10:S16–S20. doi: 10.2310/7060.2003.35079. [DOI] [PubMed] [Google Scholar]
- 27.Soto J, Toledo J, Luzz M, Gutierrez P, Berman J, Duparc S. Randomized, double-blind, placebo-controlled study of Malarone for malaria prophylaxis in nonimmune Colombian soldiers. Am J Trop Med Hyg. 2006;75:430–433. [PubMed] [Google Scholar]
- 28.Service MW. The ecology of the mosquitos of the northern Guinea savannah of Nigeria. Bull Entomol Res. 1963;54:601–632. [Google Scholar]
- 29.Beier JC, Perkins PV, Onyango FK, Gargan TP, Oster CN, Whitmore RE, Koech DK, Roberts CR. Characterization of malaria transmission by Anopheles (Diptera: Culicidae) in preparation for malaria vaccine trials. J Med Entomol. 1990;27:570–577. doi: 10.1093/jmedent/27.4.570. [DOI] [PubMed] [Google Scholar]
- 30.Githeko AK, Service MW, Mbogo CM, Atieli FK, Juma FO. Plasmodium falciparum sporozoite and entomological inoculation rates at the Ahero rice irrigation scheme and the Miwani sugar-belt in western Kenya. Ann Trop Med Parasitol. 1993;87:379–391. doi: 10.1080/00034983.1993.11812782. [DOI] [PubMed] [Google Scholar]
- 31.Beier JC, Oster CN, Onyango FK, Bales JD, Sherwood JA, Perkins PV, Chumo DK, Koech DV, Whitmire RE, Roberts CR, Diggs CL, Hoffman SL. Plasmodium falciparum incidence relative to entomologic inoculation rates at a site proposed for testing malaria vaccines in western Kenya. Am J Trop Med Hyg. 1994;50:529–536. doi: 10.4269/ajtmh.1994.50.529. [DOI] [PubMed] [Google Scholar]
- 32.Githeko AK, Adungo NI, Karanja DM, Hawley WA, Vulule JM, Seroney IK, Ofulla AV, Atieli FK, Ondijo SO, Genga IO, Odada PK, Situbi PA, Oloo JA. Some observations on the biting behavior of Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus and their implications for malaria control. Exp Parasitol. 1996;82:306–315. doi: 10.1006/expr.1996.0038. [DOI] [PubMed] [Google Scholar]
- 33.Silver JB. Mosquito Ecology: Field Sampling Methods. New York: Springer; 2007. [Google Scholar]
- 34.Petersen E, Høgh B, Marbiah NT, David K, Hanson AP. Development of immunity against Plasmodium falciparum malaria: clinical and parasitologic immunity cannot be separated. J Infect Dis. 1991;164:949–953. doi: 10.1093/infdis/164.5.949. [DOI] [PubMed] [Google Scholar]
- 35.Sukwa TY, Mulenga M, Chisdaka N, Roskell NS, Scott TR. A randomized, doubleblind, placebo-controlled field trial to determine the efficacy and safety of Malarone (atovaquone/proguanil) for the prophylaxis of malaria in Zambia. Am J Trop Med Hyg. 1999;60:521–525. doi: 10.4269/ajtmh.1999.60.521. [DOI] [PubMed] [Google Scholar]
- 36.Lell B, Luckner D, Ndjavé M, Scott T, Kremsner PG. Randomised placebo-controlled study of atovaquone plus proguanil for malaria prophylaxis in children. Lancet. 1998;351:709–713. doi: 10.1016/S0140-6736(97)09222-2. [DOI] [PubMed] [Google Scholar]
- 37.Faucher JF, Binder R, Missinou MA, Matsiegui PB, Gruss H, Neubauer R, Lell B, Que JU, Miller GB, Kremsner PG. Efficacy of atovaquone/proguanil for malaria prophylaxis in children and its effect on the immunogenicity of live oral typhoid and cholera vaccines. Clin Infect Dis. 2002;35:1147–1154. doi: 10.1086/342908. [DOI] [PubMed] [Google Scholar]
- 38.Schlagenhauf P, Tschopp A, Johnson R, Nothdurft HD, Beck B, Schwartz E, Herold M, Krebs B, Veit O, Allwinn R, Steffen R. Tolerability of malaria chemoprophylaxis in non-immune travellers to sub-Saharan Africa: multicentre, randomised, double blind, four arm study. BMJ. 2003;327:1078. doi: 10.1136/bmj.327.7423.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindblade KA, Eisele TP, Gimnig JE, Alaii JA, Odhiambo F, ter Kuile FO, Hawley WA, Wannemuehler KA, Phillips-Howard PA, Rosen DH, Nahlen BL, Terlouw DJ, Adazu K, Vulule JM, Slutsker L. Sustainability of reductions in malaria transmission and infant mortality in western Kenya with use of insecticide-treated bednets: 4 to 6 years of follow-up. JAMA. 2004;291:2571–2580. doi: 10.1001/jama.291.21.2571. [DOI] [PubMed] [Google Scholar]
- 40.Alaii JA, van den Borne HW, Kachur SP, Mwenesi H, Vulule JM, Hawley WA, Meltzer MI, Nahlen BL, Phillips-Howard PA. Perceptions of bednets and malaria prevention before and after a randomized controlled trial of bednets in western Kenya. Am J Trop Med Hyg. 2003;68((Suppl)):142–148. [PubMed] [Google Scholar]