Abstract
NO MAS (NM) mosquito repellent was evaluated in two farming villages (4 km apart) in the Kassena Nankana district of northern Ghana. We determined its efficacy against local malaria vectors, degree of user acceptance, and its effect on malaria prevalence in households using insecticide-treated bed nets. The average protective efficacy of NM against Anopheles mosquitoes over 9 hours was 89.6%. Controls averaged 86 bites/person/night versus 9 bites/person/night with the use of NM. Use of repellent was associated with a decrease of absolute malaria prevalence by 19.2% in the repellent village and by 6.5% in the control village (45.5 to 26.3, and 29.5 to 23.0, respectively). The user-acceptance rate of NM repellent was 96.1%. Ten percent (10%) of repellent users reported irritation as the main adverse effect during the period. Eighty-five percent (85%) of the users found the odor of NM appealing and 87% reported no inconvenience in applying the repellent daily.
Introduction
Repellents have long been known to offer protection against mosquito-borne diseases by reducing the contact between man and mosquitoes.1–3 Repellents have been available in developed countries for decades, but their application to infectious disease problems in less developed countries has been frustrated by doubts about their efficacy, affordability, and user-acceptance. For example, N,N-Diethyl-meta-toluamide (DEET), the “gold standard” of repellents, provides good protection against numerous biting insects and produces a chemical odor and skin sensation that is offensive to many users and has potentially toxic effects.4,5 Thus, a search continues for safer, more effective compounds in the formulation of repellents.6,7
A few other plant-based formulations (e.g., celery seed and American beautyberry) have tested well against some mosquitoes. For example, Eucalyptus oil, the principal ingredient of which is para-menthane-diol (PMD), provided protection comparable to DEET in repelling Anopheles mosquitoes in Tanzania.8 However, lack of convincing user-acceptance data, and the high cost of producing active ingredients from the plant sources have contributed to the exclusion of repellents from global disease prevention programs. The NO MAS (NM) repellent is a low-cost water-based lotion whose active ingredients are PMD and lemongrass oil. The PMD is found in numerous plant oils and provides a very high degree of extended protection from a broad range of insect vectors and has been advocated for use in disease-endemic areas because of its proven clinical efficacy to prevent malaria and its lower risk to human health.9–13
In a Guatemalan field trial in 2005,13 an early iteration of NM with 15% PMD provided 98% protection for 5 hours against 13 species of mosquitoes, including Anopheles albimanus, Culex quinquefasciatus, and Aedes aegypti. This exceeded the 92% protection provided by 15% DEET.
In a Peruvian field trial conducted over 9 days in 2006,13 the same formulation provided 95% protection after 6 hours against 17 species of mosquitoes, including Anopheles darlingi (86% of all landings) and Anopheles nuneztovari. This exceeded the 64% protection provided by 20% DEET. In a laboratory study employing multiple test subjects (Carroll S, personal communication), NM provided 9 hours of complete protection time against Anopheles gambiae sensu stricto (s.s.).
Additional cage studies conducted at Bentley University and the Harvard School of Public Health, Laboratory of Public Health Entomology (Kiszewski A, personal communication), confirmed the superior repellency of this formulation against An. albimanus and Anopheles stephensi in head-to-head comparisons with DEET.
This study sought to measure the efficacy, adverse effects, and user acceptance of NM in Ghana by estimating the biting pressure and sporozoite rate of local mosquitoes along with user acceptance rates and the epidemiological efficacy of NM on local inhabitants using the repellent.
Methods
Study sites.
The field test was carried out in Korania, a community within the Kassena Nankana District (KND) of Northern Ghana. The efficacy, adverse effects, and user acceptance study was carried out from September to November 2010 to coincide with the rainy season. The baseline malaria prevalence survey was carried out at the beginning of September 2010 and the post-intervention was done in May 2011. The district is located in the Upper East region of Ghana and covers about 1,674 sq km of Sahelian savannah with a population of ∼140,000. It lies between latitude 10°30′ and 11°00′ N and longitude 1°00′ and 1°30′ W. The area is characterized by Guinea savannah vegetation and receives an annual rainfall of about 800 mm. Malaria transmission is highly seasonal with transmission coinciding with the rainy season from May to September/October. Anopheles gambiae and Anopheles funestus are the main vectors and the average biting pressure in the area is 36.7 bites/man/night.14 The entomological inoculation rate has been estimated at 418 infective bites/person/year, with ∼60% of malaria transmission in KND occurring indoors during the second half of the night, peaking again at daybreak between 04:00 am and 06:00 am.14 Malaria and anemia were responsible for 41% and 18%, respectively, of hospital deaths in 1996 and parasitemia was 71% and 54.3% at high and low transmission seasons, respectively.15
Efficacy trial.
Using a Latin Square design, every night for 16 days, four (4) trained technicians (two controls and two treatments) performed mosquito-landing collections while seated at four fixed positions, one inside and one outside of each of the two households selected for the trials. Collectors rotated sequentially to new positions each night until the study was completed. On any given night, two of the collectors used the NM repellent (Treatment), whereas the other two used 20% mineral oil in ethanol (Control). Except for one 10-minute break every hour, collectors remained at their assigned positions from 21:00 pm to 06:00 am. To minimize the “relativity effect,” wherein mosquitoes are forced to choose between two hosts simultaneously, collectors were situated at least 10 m apart.16
All solutions were placed in unmarked containers labeled by code. The length and circumference of the legs were measured to calculate the surface area, and the correct dose of treatment measured with a micropipette. Each night of the study, mosquito collectors using a latex glove to minimize absorption of material by the hand, treated both lower legs with either the NM repellent (Treatment) or 20% mineral oil in ethanol (Control) at a rate of 5 mL/1,000 cm2 between the ankle and the knee.
Mosquito collectors were made to wear long sleeve shirts to ensure that blood-seeking mosquitoes will only have access to their lower legs. To minimize variation in the mosquito attraction, collectors were not allowed to smoke, consume alcohol, or use soap when washing. Using a mouth aspirator, flashlight and collection vessel, mosquitoes were collected once they landed on the exposed lower legs of the collectors, but before biting commenced. Collection vessels were changed each hour to provide hourly measures of repellence. Umbrellas were also provided to protect the collectors from any rain showers that might wash away their repellent. Data on relative humidity in the study communities during the period was obtained from the Meteorological Station in the area.
User acceptance study.
At the beginning of the study, 1-L repellent containers were given to each participating household after the landlord was made aware of the study's goals and risks and agreed to participate by signing an informed consent document. All household members were shown how to use the repellent on themselves or on their children. Care was taken to select houses at least 30 meters between the houses of repellent users and non-users.
A total of 77 landlords received 1 L each of NM. A total of 419 participants from these households used the repellent. Repellents were first weighed before being given to the landlords and after the end of the study. Basic demographic information on each landlord was also obtained for future follow-up on compliance or non-compliance, as the case may be.
Identification and sporozoite infectivity of mosquitoes.
Mosquitoes collected were sorted and identified into species by their morphological characteristics using taxonomic keys.17 Heads and thoraces of a sample of An. gambiae s.l. and An. funestus were removed and tested for the presence of circumsporozoite antigens of Plasmodium falciparum using the enzyme-linked immunosorbent assay (ELISA).18
Malaria parasite prevalence study.
Two closely-situated millet farming villages of comparable size, demographics, insecticide-treated net (ITN) coverage and other characteristics were selected to compare the impact of repellents on malaria prevalence. Korania (Cohort1) served as the intervention village, whereas Bonia (Cohort 2) served as the control.
Baseline P. falciparum malaria prevalence was measured in both communities before repellent was distributed in Korania in September 2010 and repeated in May 2011 after the conclusion of the study. Consenting members of the households (200–350) in each community were recruited for the malaria parasite prevalence survey. This sample size provided a power of 74–90% to detect a 20–25% reduction in the prevalence rate, respectively, assuming the prevalence in the community not using NM was 50%. Participants were diagnosed for malaria using the rapid diagnostic test (Hexagon Malaria Immunochromatographic), which gave on the spot malaria diagnosis. This test detected only P. falciparum, the most common malaria parasite in Ghana. Participants with a positive rapid test and fever (axillary temp > 37.5°C) were offered treatment according to national guidelines.
Data analysis.
The biting pressure and sporozoite rates were analyzed based on standard World Health Organization (WHO) methods.19 The percentage protection time or percentage repellency was calculated based on the formulae, % R = ([C − T]/C) × 100, where C is the total number of mosquitoes biting on the legs of the control subject and T is the total number of mosquitoes biting on the legs of repellent-treated subjects.20
Comparisons of malaria incidence between the control and treatment villages were performed using Fisher's exact test (Minitab 15.1, Minitab, Inc., State College, PA). Parameters derived from this study were also used in a model of malaria infection21 to derive predictions of repellent efficacy under varying degrees of biting pressure from malaria vectors.
Ethical consideration.
The study was reviewed by the scientific and ethical review committees of the Noguchi Memorial Institute for Medical Research and safety issues for this repellent were fully considered. We also explained all risks and benefits involved to potential study participants and they voluntarily agreed to take part. The individual decision was only after seeking acceptance for the study at the community level with the authorities including the local chiefs, etc. Individuals consented to take part of their own free will and were at liberty to withdraw from the study at any time point without penalty. However, arrangements were made to report any adverse events to the District hospital, which was about 1 km from the study community. Free treatment was also offered to participants as a requirement for ethical handling of human subjects.
Results
Biting pressure of Anopheles mosquitoes.
A total of 64 man-nights captured a total of 6,097 mosquitoes. Anopheles mosquitoes constituted 99.4% (6,062), whereas Culex and Aedes mosquitoes formed only 1.6% (35) of the entire collection. The 9.51% (576) of the total Anopheles were collected in the treatment arm (NM users) and 90.49% (5,486) in the control arm (non-NM users). The biting pressure of Anopheles on unprotected individuals averaged 86 bites/man/night, which was significantly (P < 0.001) reduced to 9 bites/person/night among collectors using NM repellent.
The average maximum and minimum temperature in the area was 32.3 and 23.1°C. The humidity in the area during the study was about 80% (Ghana Meteorological Agency).
Level of protection of NM in the community.
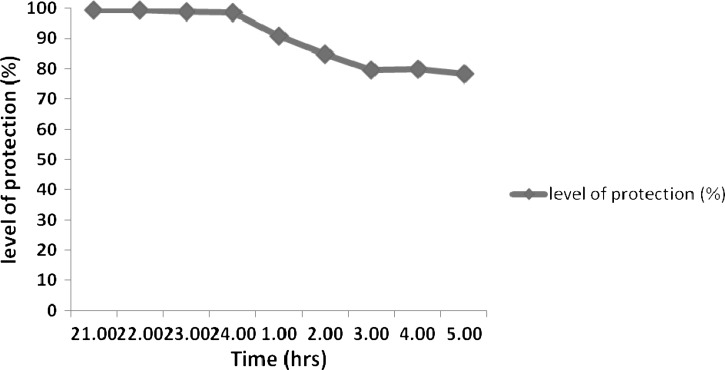
The total number of Anopheles collected from NM users and non-NM users, respectively, was used to estimate repellent efficacy. During 9 hours of capture, the NM repellent provided an average of 89.6% protection against malaria vectors (Figure 1) compared with non-users. During the peak time of biting (i.e., 24:00–02:00), NM provided about 92% of protection against Anopheles mosquitoes.
Figure 1.
Percentage level of protection (efficacy) of NO MAS (NM) repellent when used in the night.
Sporozoite rates of Anopheles mosquitoes.
A total of 501 Anopheles gambiae sensu lacto and Anopheles funestus were assayed by ELISA for the presence of Plasmodium falciparum circumsporozoite proteins. In all, only five mosquitoes tested positive for the antigen, giving a sporozoite rate of 1.0% (501).
Malaria prevalence in the communities.
Baseline parasite prevalence in the study villages were 45.5% in Korania versus 29.5% in Bonia (Z = 3.35, P = 0.01). Prevalence dropped in both villages between surveys (Table 1). There was a 42.2% drop in prevalence in Korania (study village) compared with a 22.03% drop in the Bonia (control village), a relative drop in prevalence of about 3 times in the intervention village compared with the control village. Thus, the repellent intervention significantly reduced malaria prevalence in the intervention community.
Table 1.
Malaria prevalence during baseline and NO MAS (NM) post-intervention surveys based on rapid diagnostic tests (RDTs)
| Study site | Baseline | Post-intervention | P value | ||||
|---|---|---|---|---|---|---|---|
| No. examined | No. positive | Prevalence (%) | No. examined | No. positive | Prevalence (%) | ||
| Korania (study village) | 200 | 91 | 45.5 | 205 | 54 | 26.3 | P = 0.000 |
| Bonia (control village) | 203 | 60 | 29.5 | 204 | 47 | 23.0 | P = 0.144 |
Community acceptance of NM repellent.
Three months after the repellent was distributed, a survey on user acceptance of NM was conducted in all households receiving repellent. A total of 77 household heads agreed to participate and 419 members of these households used the repellent. When asked whether they would like to continue using the repellent after the study (37,710 user-days), 96.7% of repellent users said YES.
Adverse effects of NM repellent.
After 3 months of NM use, when the inhabitants were asked how frequently they used the repellent, 96.1% said they used NM every day.
Commonly reported symptoms after use of the repellent, as reported by study participants were irritation (10.0%), headache (3.9%), nausea (3.9%), and rashes (2.0%).
Community perception of NM repellent.
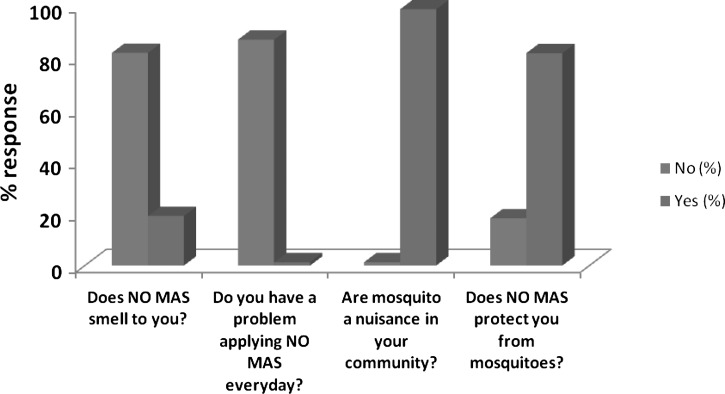
A questionnaire-based survey was also used to determine the perceptions of the community on their use of NM repellent. In the community, many people (98.7%) admitted that mosquitoes pose a problem to them and 81.8% said that NM protected them from mosquito bites. Over 85% said the smell of NM was appealing and not offensive. Many (87.0%) users of NM did not have any problem applying the repellent every day (Figure 2).
Figure 2.
Perceptions of the community on NO MAS (NM) repellent after 3 months usage.
Epidemiological efficacy of NM repellent.
A model was used to estimate the probability of avoiding malaria infections in a population protected by a repellent20; this model assumed that each mosquito-biting attempt in a transmission season is an independent event. Using values of 86 bites/man/night for biting pressure (b), 1.0% sporozoite rates, 90% efficacy and 97% acceptance obtained from this study, the daily probability that members of the community at risk of vector borne infections can escape infection by using repellent-based intervention was calculated as Fe = 0.997549 translating to a 0.25% daily infection probability. In the absence of the repellent, the probability of escaping malaria infection was Fe = 0.981256 translating to a 1.87% daily infection probability.
The implication is that by using the NM repellent in the community, malaria cases per day will be reduced by 86.9%, and for the 3 months that members of the community used the repellent, malaria infections will be reduced by 75.8%. We used a presumptive value (0.022) for human infection rate (h) for moderate-intense malaria transmission in semi-immune populations from a data set published by Nedelman22 and evaluated by Pull.23
Discussion
The study reported here aimed to evaluate the efficacy of NM against local mosquitoes, especially anopheline vectors of malaria in Ghana, while simultaneously investigating how likely it is that rural Ghanaians would accept the use of NM as a routine malaria preventive measure. Anopheles mosquitoes were the predominant mosquito species in the study communities. Culex and Aedes species of mosquitoes were also collected but in small numbers and therefore were not included in the analysis. The study proved that NM repellent was both persistent and efficacious against the major malaria vectors present in the region, and users predominantly indicated their willingness to continue using it. The NM repellent had an average efficacy of 90% for the 9 hours of capture against the two major vectors of An. gambiae and An. funestus, even in an area where the relative humidity and ambient temperature were high. Under equally challenging conditions in a Guatemala study, the PMD/LG repellent provided 98% protection for 5 hours, whereas in Peru, it provided 95% protection for 6 hours.13
By reducing man-vector contact,3 repellents offer an important means of personal protection against insect vectors. Indeed, the most striking result of this Ghana trial is that the absolute malaria prevalence significantly decreased by 19.2% (95% confidence interval [CI], 10.0 to 61.9, P < 0.0001) in the repellent village but by only 6.5% (95% CI, −2.0 to 18.1, P > 0.05) in the control village.
Seasonal differences are known to affect the transmission and malaria prevalence. This was controlled through the assessment of the control village and therefore any seasonal changes in the study community also occurred in the control village, which is very close geographically and subject to the same seasonal dynamics. Participants were also offered free treatments as a policy in both the study and control villages and therefore the study was able to assess the effect of the repellent as a separate factor. These findings indicate that NM can be a useful addition to a strategy of malaria reduction, perhaps by protecting bed net users who are exposed to infective bites before they retire to their beds and people who might not use bed nets. Repellents may also offer a second line of defense against mosquitoes that are refractory to the insecticides used to treat ITNs, and are thus able to enter bed nets through holes that inevitably develop in them after long-term use.
Additionally, the repellent had a high user acceptance rate of 97%. Minimal adverse effects were observed and it proved to be well tolerated by users at least during the entire 9 months duration of the study. This was not surprising because safety issues and data on the repellent were fully considered before the start of this pilot study. However, long-term follow-up on adverse events may be useful in subsequent trials. This result compares favorably with a repellent/malaria study conducted near Iquitos, Peru in 2007. In that study, more than 98% of the nearly 2,200 people who used NM daily for 4 months said they would continue using it voluntarily when the study ended13; after applying NM to their arms, legs, and necks for 120 days, study subjects reported only two cases of mild dermatitis. This outcome produced from 264,000 user-days of data, added to the high level of user-acceptance measured in the study suggests that NM is particularly well suited for use at the community level in public health interventions against vector-borne diseases.
Repellents have been in use for many years in developing countries and quite recently, their use has been encouraged in Africa.8,24 In the past few years, a plant-derived repellent, PMD has been proven to be suitably efficacious and safe to compete with DEET in the field of disease prevention, and repellents have been recognized by WHO as a useful disease prevention tool to complement insecticide-based means of vector control.24 Insect repellents can provide protection against malaria. The PMD is the only plant-based repellent that has been advocated for use in disease-endemic areas by the Centers for Disease Control (CDC),10 because of its proven clinical efficacy to prevent malaria25 and is considered to pose no risk to human health.10 A study in Bolivia found a highly significant 80% reduction in episodes of Plasmodium vivax in the group that used treated nets and repellent (incidence rate ratio 0.20, 95% CI, 0.11 to 0.38, P < 0.001).26 In areas where vectors feed in the early evening, effectiveness of treated nets can be significantly increased by using repellent between dusk and bedtime.26
In Ghana, An. gambiae s.s., An. funestus, and An. melas are the major vectors of malaria and lymphatic filariasis in the country.14,27 The primary interventions used against these vectors are ITNs and indoor residual spraying (IRS). The use of ITNs has helped to reduce malaria morbidity in some parts of the country28 and IRS is currently being implemented in some areas of the country. These interventions target mostly indoor resting mosquitoes, and in some areas where people stay outdoors for part of the night and in areas where mosquitoes bite mostly outdoors, the efficacy of these interventions may be compromised29,30; there is, therefore, the need to incorporate other interventions, such as repellents, that are capable of preventing both indoor and outdoor mosquito bites.
With funding from two rounds of grants from the Global Fund Against Aids, Tuberculosis and Malaria (GFATM), Ghana has begun to implement a comprehensive array of anti-malarial interventions, including artemisinin-based combination therapy, intermittent preventive treatment, and ITNs.
Ghana's effort to achieve greater reductions in new infections from malaria might be significantly improved by incorporating an effective personal repellent in its integrated vector management strategies. An effective repellent will offer additional protection to those who lack access to a bed net.
In conclusion, this study showed that NM repellent is efficacious in preventing bites, reducing malaria prevalence, and is attractive to most users in the Korania community. With a protection level of about 90% and the relatively high epidemiological efficacy, the repellent when used in combination with ITNs and IRS, could offer a useful integrated strategy that might lead to significant reduction of not only malaria but other diseases such as lymphatic filariasis because the same vectors14,27 transmit both diseases in the country.
ACKNOWLEDGMENTS
We thank the chief and people of Korania in Northern Ghana for participating in the study. Our gratitude also goes to the fieldworkers for their hard work.
Disclaimer: Permission to publish from the Director of NMIMR is greatly appreciated.
Footnotes
Financial support: This study received financial support from the Del Cielo Project, British Columbia, Canada.
Disclosure: A U.S. patent for the repellent mentioned in this manuscript was awarded to S.T. Darling in 2010. The research that appears here was partly funded by him. All co-authors of this paper gave their services freely to this effort and, therefore, retained their independence from the funder.
Authors' addresses: Samuel Dadzie, Noguchi Memorial Institute for Medical Research, Accra, Ghana, E-mail: sdadzie@noguchi.mimcom.org. Daniel Boakye and Maxwell Appawu, Noguchi Memorial Institute for Medical Research, Parasitology, Accra, Ghana, E-mails: dboakye@noguchi.mimcom.org and mappawu@noguchi.mimcom.org. Victor Asoala, Navrongo Health Research Centre, Health, Navrongo, Ghana, E-mail: vasoala@navrongo.mimcom.org. Kwadwo Koram, Noguchi Memorial Institute of Medical Research, Epidemiology, University of Ghana, Accra, Ghana, E-mail: kkoram@noguchi.mimcom.org. Anthony Kiszewski, Bentley College, Natural and Applied Sciences, Waltham, MA, E-mail: akiszewski@bentley.edu.
References
- 1.Anonymous Are insect repellents safe? Lancet. 1988;332:610–611. [PubMed] [Google Scholar]
- 2.Gupta RK, Rutledge LC. Role of repellents in vector control and disease prevention. Am J Trop Med Hyg. 1994;50:82–86. doi: 10.4269/ajtmh.1994.50.82. [DOI] [PubMed] [Google Scholar]
- 3.Curtis CF. Personal protection methods against vectors of disease. Rev Med Vet Entomol. 1992;80:543–551. [Google Scholar]
- 4.Rutlege LC, Sofield RK, Moussa MA. A bibliography of diethyl toulamide. Bull Entomol Soc Am. 1978;24:431–439. [Google Scholar]
- 5.Corbel V, Stankiewicz M, Pennetier C, Fournier D, Stojan J, Girard E, Dimitrov M, Molgó J, Hougard J-M, Lapied B. Evidence for inhibition of cholinesterases in insect and mammalian nervous systems by the insect repellent DEET. BMC Biol. 2009;7:1741–1747. doi: 10.1186/1741-7007-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Govindarajan M, Mathivanan T, Elumalai K, Krishnappa K, Anandan A. Mosquito larvicidal, ovicidal, and repellent properties of botanical extracts against Anopheles stephensi, Aedes aegypti, and Culex quinquefasciatus (Diptera: Culicidae) Parasitol Res. 2011;109:353–367. doi: 10.1007/s00436-011-2263-1. [DOI] [PubMed] [Google Scholar]
- 7.Walker TW, Robert LL, Copeland RA, Githeko AK, Wirtz RA, Githure JI, Klein TA. Field evaluation of arthropod repellents, DEET and a piperidine compound, A13-37220, against Anopheles funestus and Anopheles arabiensis in western Kenya. J Am Mosq Control Assoc. 1996;12:172–176. [PubMed] [Google Scholar]
- 8.Trigg JK. Evaluation of a eucalyptus-based repellent against Anopheles spp. in Tanzania. J Am Mosq Control Assoc. 1996;12:243–246. [PubMed] [Google Scholar]
- 9.Carroll SP, Loye J. PMD, a registered botanical mosquito repellent with deet-like efficacy. J Am Mosq Control Assoc. 2006;22:507–514. doi: 10.2987/8756-971X(2006)22[507:PARBMR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Environmental Protection Agency p-Menthane-3, 8-diol (011550) Fact Sheet. 2000. htttp://www.epa.gov/oppbppd1/biopesticides/ingredients/factsheets/factsheet_011550.htm Available at. Accessed May 28, 2012.
- 11.Zielinski-Gutierrez E, Wirtz RA, Nasci RS, Brogdon WG. CDC Health Information for International Travel (“The Yellow Book”) Atlanta, GA: Centers for Disease Control and Prevention; 2012. Protection against mosquitoes, ticks and other insects and arthropods. [Google Scholar]
- 12.Hill N, Lenglet A, Arnez AM, Cainero I. Randomized, double-blind control trial of p-menthane diol repellent against malaria in Bolivia. BMJ. 2007;335:1023. doi: 10.1136/bmj.39356.574641.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore SJ, Darling ST, Sihuincha M, Padilla N, Devine GJ. A low-cost repellent for malaria vectors in the Americas: results of two field trials in Guatemala and Peru. Malar J. 2007;6:101. doi: 10.1186/1475-2875-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appawu M, Owusu-Agyei S, Dadzie S, Asoala V, Anto F, Koram K, Rogers W, Nkrumah F, Hoffman SL, Fryauff DJ. Malaria transmission dynamics at a site in northern Ghana proposed for testing malaria vaccines. Trop Med Int Health. 2004;9:164–170. doi: 10.1046/j.1365-3156.2003.01162.x. [DOI] [PubMed] [Google Scholar]
- 15.Koram KA, Owusu-Agyei S, Greg UTZ, Binka FN, Kevin BJ, Hoffman SL, Nkrumah FK. Severe anemia in young children after high and low malaria transmission seasons in the Kassena-Nankana district of Northern Ghana. Am J Trop Med Hyg. 2000;62:670–674. doi: 10.4269/ajtmh.2000.62.670. [DOI] [PubMed] [Google Scholar]
- 16.Gillies MT, Wilkes TJ. Range of attraction of single baits for some West-African mosquitoes. Bull Entomol Res. 1970;60:225–235. doi: 10.1017/S000748530004075X. [DOI] [PubMed] [Google Scholar]
- 17.Gilles MT, De Meillon B. The anophelinae of Africa south of Sahara (Ethiopian Zoogeographical Region) Publ S African Instit Med Res. 1968;54:343. [Google Scholar]
- 18.Wirtz RD, Burkot TR, Graves PM. Field evaluation of enzyme-linked immunosorbent assays for Plasmodium falciparum and Plasmodium vivax sporozoites in mosquitoes (Diptera: Culicidae) from Papua New Guinea. J Med Entomol. 1987;24:433–437. doi: 10.1093/jmedent/24.4.433. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization . Manual on Practical Entomology in Malaria. Vol. 13. WHO offset publication; Geneva: 1975. Part II. Methods and techniques; p. 191. [Google Scholar]
- 20.Barnard DR, Bernier UR, Posey KH, Xue R. Repellency of IR3535, KBR3023, para-menthane-3,8-diol, and Deet to black salt marsh mosquitoes (Diptera: Culicidae) in the Everglades National Park. J Med Entomol. 2002;39:895–899. doi: 10.1603/0022-2585-39.6.895. [DOI] [PubMed] [Google Scholar]
- 21.Kiszewski AE, Darling ST. Estimating a mosquito repellent's potential to reduce malaria in communities. J Vector Borne Dis. 2010;47:217–221. [PubMed] [Google Scholar]
- 22.Nedelman J. Introductory review: some new thoughts about some old malaria models. Math Biosci. 1985;73:159–182. [Google Scholar]
- 23.Pull JH, Grab B. A simple epidemiological model for evaluating the malaria inoculation rate and the risk of infection in infants. Bull World Health Organ. 1974;51:507–516. [PMC free article] [PubMed] [Google Scholar]
- 24.Maia MF, Moore SJ. Plant-based insect repellents: a review of their efficacy, development and testing. Malar J. 2011;10((Suppl 1)):S11. doi: 10.1186/1475-2875-10-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill N, Lenglet A, Arnéz AM, Cainero I. Randomized, double-blind control trial of p-menthane diol repellent against malaria in Bolivia. BMJ. 2007;335:1023. doi: 10.1136/bmj.39356.574641.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill N, Lenglet A, Arnéz AM, Carneiro I. Plant-based insect repellent and insecticide treated bed nets to protect against malaria in areas of early evening biting vectors: double blind randomized placebo controlled clinical trial in the Bolivian Amazon. BMJ. 2007;335:1023. doi: 10.1136/bmj.39356.574641.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Appawu MA, Dadzie SK, Baffoe-Wilmot A, Wilson MD. Lymphatic filariasis in Ghana: entomological investigation of transmission dynamics and intensity in communities served by irrigation systems in the Upper East Region of Ghana. Trop Med Int Health. 2001;6:511–516. doi: 10.1046/j.1365-3156.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 28.Binka FN, Kubaje A, Adjuik M, Williams LA, Lengeler C, Maude GH, Armah GE, Kajihara B, Adiamah JH, Smith PG. Impact of permethrin impregnated bednets on child mortality in Kassena-Nankana district, Ghana: a randomized controlled trial. Trop Med Int Health. 1996;1:147–154. doi: 10.1111/j.1365-3156.1996.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 29.Oyewole IO, Awolola TS. Impact of urbanization on bionomics and distribution of malaria vectors in Lagos, southwestern Nigeria. J Vector Borne Dis. 2006;43:173–178. [PubMed] [Google Scholar]
- 30.Pates H, Curtis C. Mosquito behavior and vector control. Annu Rev Entomol. 2005;50:53–70. doi: 10.1146/annurev.ento.50.071803.130439. [DOI] [PubMed] [Google Scholar]