Abstract
Children with traditionally defined cerebral malaria (CM) can be subcategorized by the presence or absence of malaria retinopathy. We retrospectively reviewed the seasonal pattern of retinopathy status in patients admitted with CM in Blantyre, Malawi from 1997 to 2010. The proportion of children with CM who were retinopathy-positive was significantly greater during the peak seasonal rains when the community incidence of uncomplicated malaria is higher. This finding supports the hypothesis that retinopathy-negative and retinopathy-positive CM categories have different underlying etiologies.
Introduction
Cerebral malaria (CM) is defined by the World Health Organization (WHO) as an otherwise unexplained coma in a person with malarial parasitemia.1 Although five malaria parasite species are infective for humans, Plasmodium falciparum is responsible for the large majority of CM cases. CM is a common disorder in the developing world, and at least 575,000 cases occur worldwide annually.2 Most individuals affected are children less than 5 years old living in sub-Saharan Africa.3 Even with optimal treatment, 15–25% of these children die.4 Of the survivors, 30% are left with neurologic sequelae, including developmental disabilities, behavioral abnormalities, and epilepsy.5,6
In areas of high malaria transmission, asymptomatic parasitemia is common. Up to 50% of the population may harbor circulating parasites but have no symptoms of disease.7 Therefore, febrile children with asymptomatic parasitemia who have lapsed into abnormal consciousness because of an etiology other than malaria and have an underlying etiology for coma that cannot be ascertained with available diagnostic resources will fulfill diagnostic criteria for CM. Sequestration of parasitized erythrocytes in the cerebral vasculature is thought to be a key diagnostic autopsy finding for CM. A clinical autopsy study revealed that 23% of children who fulfilled diagnostic criteria for CM lacked autopsy evidence of P. falciparum sequestration.8 In this study, patients who lacked evidence of typical CM neuropathology had other identifiable causes of death, including Reye syndrome and severe pneumonia.
In 1993, a malaria retinopathy was described,9 and later was well-characterized.10 In an autopsy case series, the value of these clinical findings became clear. In that study, the presence of malaria retinopathy was found to be 95% sensitive and 100% specific for predicting the presence of sequestered parasitized erythrocytes on cerebral pathology.11 Up to one-third of patients who fulfill WHO case criteria for CM are retinopathy-negative, and it seems likely that these patients have a coma etiology other than malaria.12
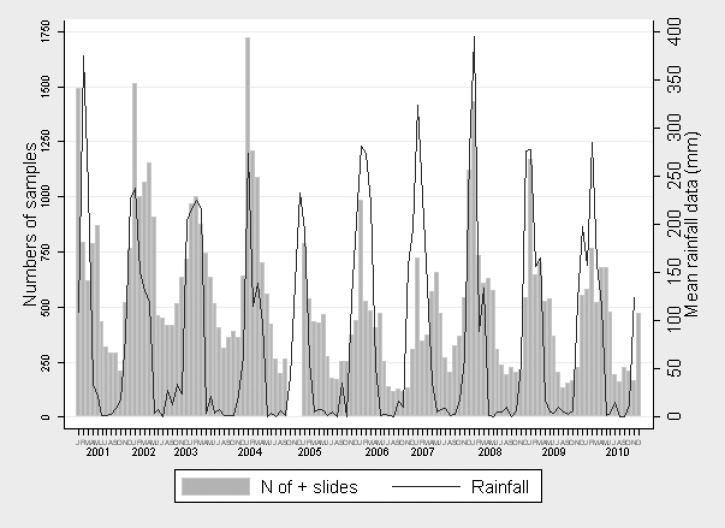
If acute infection with the malaria parasite is not responsible for the coma in retinopathy-negative patients and the underlying cause or causes of these children's comas stays constant in prevalence (is less impacted by rainfall) throughout the calendar year, one would expect the ratio of retinopathy-positive to retinopathy-negative patients to vary seasonally, with retinopathy-positive patients dominating during the high malaria transmission season. In Malawi, uncomplicated malaria usually peaks yearly in January or February, coinciding with the rainy season, and the incidence of malaria infection decreases rapidly as the rains abate (Figure 1).
Figure 1.
Monthly mean rainfall (thin black line; in millimeters) and the number of thick blood film evaluations found positive for malaria parasites (histogram) in patients presenting with clinical malaria at Queen Elizabeth Central Hospital Emergency Room, Blantyre, Malawi, from 2001 to 2010. Used with permission of and adapted by Arantxa Roca-Feltrer.
We conducted a secondary analysis using data collected during an ongoing study of malaria pathogenesis. Patients included in this analysis were enrolled between January and June each year from 1997 and 2010. We compared the seasonal disease proportions between retinopathy-positive and retinopathy-negative CM patients to test the hypothesis that acute P. falciparum infection is not associated with the retinopathy-negative CM syndrome.
Patients
We identified cases of retinopathy-positive and retinopathy-negative CM from children admitted between January and June from 1997 to 2010 to the Pediatric Research Ward at Queen Elizabeth Central Hospital, a tertiary referral center located in Blantyre, Malawi. The Pediatric Research Ward is a section of the pediatric department specially suited for investigations and intensive care required for comatose children. Enrollment in this observational study required explicit written consent from the child's caregiver. The parent study was approved by the University of Malawi College of Medicine Research Ethics Committee and Michigan State University's Biomedical Institutional Review Board.
Children with a clinical diagnosis of CM (Blantyre coma score [BCS] ≤ 2, P. falciparum asexual parasites on thick blood film, and no other clear explanation for their abnormal level of consciousness) were included. On admission, informed consent from a parent or guardian was obtained. Within 6 hours of admission, ophthalmoscopy (direct and indirect) was performed by a trained ophthalmologist to determine malaria retinopathy status. Malarial retinopathy was defined as the presence of retinal whitening, hemorrhages, or orange or white vessels with or without papilledema.
Statistical Methods
To evaluate the seasonal distribution of retinopathy cases, we calculated the proportion of retinopathy-negative CM cases of the total number of CM cases admitted during each calendar month and compared the proportion in January with each individual month from February to June. Statistical significance of the changes in the odds of retinopathy-negative admissions over months and years was assessed by likelihood ratio tests in logistic regression models including indicator variables for months and years.
A P value less than 0.05 was considered evidence of a significant difference between retinopathy groups. Analysis was performed using S-plus 8.0 (Insightful, Seattle, WA) software.
A total of 2,291 children with a clinical diagnosis of CM were admitted during this time period. Of these children, 1,728 children had determination of retinopathy status. Children who were admitted when an ophthalmologist was not available did not have retinopathy status assessed. Of the 1,728 children with known retinopathy status, 1,056 (61.1%) were malaria retinopathy-positive. The proportion of children with CM who were retinopathy-positive varied throughout the malaria season in parallel with fluctuations of the number of patients presenting with uncomplicated malaria at Queen Elizabeth Central Hospital. When data were combined across calendar years, the proportion of total CM cases that were retinopathy-negative varied significantly between the beginning of the rainy season (January) and its end (June) (P = 0.03) (Figure 2).
Figure 2.
Monthly proportion of children who were retinopathy-negative of the total number of patients admitted with CM in all years combined between 1997 and 2010. The proportion of children admitted with retinopathy-negative CM significantly increased in a linear fashion (P value of likelihood ratio test in crude analysis and analysis adjusting by month = 0.03) from January to June. The dashed line represents the proportions estimated in the logistic regression, and the solid line represents the observed proportions.
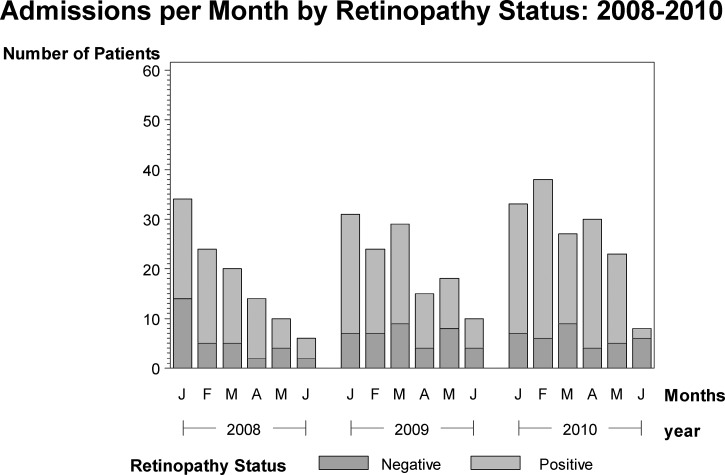
At the time of year when the incidence of malaria is highest in the community, the proportion of total CM cases that are retinopathy-positive is also at its highest. In contrast, in most years, the annual peak in systemic malaria seen in Malawi is not reflected in a peak in retinopathy-negative CM cases (Figure 3).
Figure 3.
Monthly variation in the number of retinopathy-positive and retinopathy-negative CM patients admitted to Queen Elizabeth Central Hospital from January of 2008 to June of 2010. Histograms were constructed using SAS (SAS Corporation, Cary, NC).
Our findings lend support to the hypothesis that the clinical syndrome of retinopathy-negative CM is unlikely to be associated with acute infection with the malaria parasite. These findings are congruent with data from autopsy studies that show that children dying with retinopathy-negative CM lack cerebral malaria parasite sequestration and have other severe non-malarial illnesses at the time of death.8 Alternatively, the retinopathy-negative CM syndrome could be caused by acute malaria infection with a cofactor (perhaps coinfection) varying seasonally. Additional studies to explore this possibility are warranted.
The half-yearly fluctuation in the proportion of retinopathy-positive CM mirrors the fluctuating incidence pattern seen in uncomplicated malarial illness. This finding reflects the clear increase of malaria transmission intensity that occurs in Malawi in the rainy season. At the time of year when the incidence of malaria is highest in the community, the incidence of retinopathy-positive CM is also at its highest. In contrast, in most years, the annual peak in uncomplicated malaria seen in Malawi is not reflected in a peak in retinopathy-negative CM cases. The proportion of CM patients who were retinopathy-negative increased later in the malaria transmission season.
A limitation of this retrospective study is that patients were recruited for the parent study only during the peak malaria transmission season (January to June each year), and therefore, extrapolation to the remainder of the year is not possible.
We have presented temporal disease pattern evidence supporting the hypothesis that the comas associated with retinopathy-positive and retinopathy-negative CM have different etiologies. The seasonal differences in the proportion of total CM cases that are retinopathy-negative versus retinopathy-positive suggest that the etiologies underlying the retinopathy-negative CM syndrome are distinct from acute severe malaria, the etiology of retinopathy-positive CM.
ACKNOWLEDGMENTS
The authors thank the clinical officers, physicians, and nurses who collected the data and provided care for CM patients at Queen Elizabeth Central Hospital. We are grateful to the children and their parents and guardians who took part in this study.
Footnotes
Financial support: This work was supported by National Institutes of Health Grant 5R01AI34969-14 (to T.E.T.) and Wellcome Trust Grant 074125/Z/04/Z.
Authors' addresses: Douglas G. Postels and Gretchen L. Birbeck, International Neurologic and Psychiatric Epidemiology Program, Michigan State University, East Lansing, MI, E-mails: douglas.postels@ht.msu.edu and Gretchen.birbeck@ht.msu.edu. Clarissa Valim, Department of Immunology and Infectious Disease, Harvard School of Public Health, Boston, MA, E-mail: cvalim@hsph.harvard.edu. Kara M. Mannor, Department of Epidemiology, Michigan State University, East Lansing, MI, E-mail: mannorka@msu.edu. Terrie E. Taylor, Departments of Internal Medicine, Emergency Medicine, and International Medicine, Michigan State University, East Lansing, MI, E-mail: ttmalawi@msu.edu.
References
- 1.World Health Organization Communicable Diseases Cluster Severe falciparum malaria. World Health Organization Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94((Suppl 1)):S1–S90. [PubMed] [Google Scholar]
- 2.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C. Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 3.Roca-Feltrer A, Carneiro I, Armstrong Schellenberg JR. Estimates of the burden of malaria morbidity in Africa in children under the age of 5 years. Trop Med Int Health. 2008;13:771–783. doi: 10.1111/j.1365-3156.2008.02076.x. [DOI] [PubMed] [Google Scholar]
- 4.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–459. [PubMed] [Google Scholar]
- 5.Birbeck GL, Molyneux ME, Kaplan PW, Seydel KB, Chimalizeni YF, Kawaza K, Taylor TE. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol. 2010;9:1173–1181. doi: 10.1016/S1474-4422(10)70270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postels DG, Taylor TE, Molyneux M, Mannor K, Kaplan PW, Seydel KB, Chimalizeni YF, Kawaza K, Birbeck GL. Neurologic outcomes in retinopathy-negative cerebral malaria survivors. Neurology. 2012;79:1268–1272. doi: 10.1212/WNL.0b013e31826aacd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Meara WP, Bejon P, Mwangi TW, Okiro EA, Peshu N, Snow RW, Newton CR, Marsh K. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;372:1555–1562. doi: 10.1016/S0140-6736(08)61655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, Lewallen S, Liomba NG, Molyneux ME. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–145. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 9.Lewallen S, Taylor TE, Molyneux ME, Wills BA, Courtright P. Ocular fundus findings in Malawian children with cerebral malaria. Ophthalmology. 1993;100:857–861. doi: 10.1016/s0161-6420(93)31563-0. [DOI] [PubMed] [Google Scholar]
- 10.Beare NA, Glover SJ, Molyneux M. Malarial retinopathy in cerebral malaria. Am J Trop Med Hyg. 2009;80:171. [PubMed] [Google Scholar]
- 11.Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg. 2006;75:790–797. [PMC free article] [PubMed] [Google Scholar]
- 12.Postels DG, Birbeck GL. Children with retinopathy-negative cerebral malaria: a pathophysiological puzzle. Pediatr Infect Dis J. 2011;30:953–956. doi: 10.1097/INF.0b013e3182271c69. [DOI] [PMC free article] [PubMed] [Google Scholar]