Abstract
Preterm birth (PTB, <37 weeks) may be a marker of endothelial dysfunction and a pro-inflammatory phenotype; both are risk factors for cardiovascular disease. We studied 916 women (46% Black) with 1,181 live births between enrollment in the Coronary Artery Risk Development in Young Adults (CARDIA) study (age 18-30 years) and 20 years later. C-reactive protein (CRP) was measured at years 7, 15 and 20. Interleukin-6 (IL-6) and carotid intima-media thickness (IMT) which incorporated the common carotid arteries, bifurcations, and internal carotid arteries were measured at year 20. Blood pressure, lipids, anthropometrics, and pregnancy events were assessed at all visits. Change in risk factors and differences in inflammatory markers and IMT according to PTB were evaluated. Women with PTBs (n=226) had higher mean systolic blood pressures (SBP) before pregnancy (106 vs. 105 mmHg, respectively; p=0.03). Systolic and diastolic blood pressure increased more rapidly over 20 years compared to women with term births (p<0.01 time interaction) even after removing women with self-reported hypertension in pregnancy. Women with PTB vs. term births had similar mean IMT adjusted for age, BMI, race, lifestyle and cardiovascular risk factors. CRP and IL-6 did not differ according to PTB. Women with PTB, regardless of hypertension during pregnancy, had higher blood pressure after pregnancy compared to women with term births. In the U.S. where rates of PTB are high and race disparities persist, PTB may identify women with higher blood pressure the years after pregnancy.
Keywords: hypertension, inflammation, intima-media thickness, pregnancy
Preterm birth (PTB, delivery < 37 weeks) complicates more than 12% of births in the U.S. and is responsible for most perinatal mortality and morbidity. Until recently PTB was thought to have no long term sequelae for mothers; however emerging evidence indicates that women with PTB have a two to three-fold excess risk for cardiovascular disease.[1-3] Mechanisms that could link these conditions are not understood, and whether this association is completely due to hypertension during pregnancy is equivocal.[4, 5]
Medically indicated preterm births (30% of cases) are dominantly related to hypertensive disorders of pregnancy and are mediated by placental under perfusion.[6] Causes of spontaneous preterm births (70% of cases) are largely unknown, but associations with inflammation and infection, decidual hemorrhage, or stress are well documented. Analysis of the serum of women early in gestation as well as those presenting in preterm labor reveals an elevation in C-reactive protein (CRP) and Interleukin-6 (IL-6).[7, 8] These proinflammatory markers have also been associated with atherosclerosis and coronary artery disease.[9, 10] Although the etiology of coronary disease, like PTB, is multifactorial, one paradigm for its development suggests an important role for chronic systemic inflammation in plaque formation and subsequent destabilization.[11, 12]
It is possible that common predisposing factors such as endothelial dysfunction or a pro-inflammatory phenotype are associated with PTB during the reproductive years and coronary disease later in adulthood. We hypothesized that women with PTB would have elevated blood pressure as a marker of endothelial dysfunction, higher markers of inflammation (C-reactive protein [CRP] and Interleukin-6 [IL-6]) as well as higher common carotid intima medial thickness (IMT) in the years following pregnancy compared to women with term births. We also explored how blood pressure, weight gain, and lipid markers measured longitudinally may change in women with and without preterm births. Further, we considered that vascular and inflammatory differences in women with preterm vs. term births would persist after removing PTB cases due to self-reported hypertensive disorders of pregnancy, the primary reason for medically indicated PTB.
METHODS
Study participants
The CARDIA Study is a multi-center, longitudinal, observational study designed to describe the development of risk factors for coronary heart disease in young black and white men and women.[13, 14] Participants were recruited from four geographic areas: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. From 1985-1986, 5,115 subjects (2,787 women; 52% black) aged 18 to 30 years were enrolled and provided written informed consent. By design, the cohort was recruited from the general population at each site to have adequate representation of subgroups according to sex, race, age and education.[15] Retention rates were 92, 86, 81, 79, 74, and 72 percent of the surviving cohort at years 2, 5, 7, 10, 15, and 20 after baseline.
Of the 2,787 women enrolled in CARDIA we excluded women with diabetes mellitus at baseline based on self-report or medication use (n=41), those with hysterectomy at baseline (n=24), or those with no incident births between baseline and year 20 of follow up (n=1435). We further excluded births with no gestational age (n=8), and 46 twin births. Of the remaining 1,261 women, those with IMT measurements at year 20 comprised the final study population (n=916, 72.6%). Maternal characteristics of those included did not differ from those who did not attend the year 20 visit.
Pregnancies and Preterm birth status
CARDIA represents a childbearing cohort in which reproductive events were assessed at baseline and at each follow up exam. Women were categorized into those who were nulliparous (no births prior to baseline) vs. parous at baseline. All births that occurred after enrollment in CARDIA are included in this analysis. In contrast to a pregnancy cohort which studies a single pregnancy, this approach evaluates the pre-pregnancy profile and all births during 20 years of follow-up. This approach provides a more complete summary of pregnancy characteristics that may be importantly related to later maternal health. For each post-baseline birth women reported the gestational age at delivery (weeks) and birth weight. Preterm births were those delivered <37 completed weeks. For women with more than one birth, we characterized each birth as preterm or term. For women with all term births, we selected the first birth for analysis. For women with at least one preterm birth we analyzed the first preterm birth, as it is unknown if first or subsequent preterm births are related to excess maternal CVD risk.
We conducted a validation study of maternal report of gestational age at delivery among a subset of 211 CARDIA women enrolled at all 4 sites using medical record abstractions. Sensitivity for preterm birth <34 weeks was 100%; specificity was 99%. Sensitivity was 67% and specificity was 89% for preterm births delivered at 34-36 weeks. The overall sensitivity for maternal report of ever delivering preterm (<37 weeks) was 84% (16/19), and the specificity was 89% (170/192).
At CARDIA exams women were asked for each pregnancy whether they had developed high blood pressure with or without protein in the urine. Hypertensive disorders of pregnancy were classified as either gestational hypertension (hypertension without protein) or preeclampsia (with protein) based on self-report for each pregnancy. When compared to the medical record, self-reported hypertensive disorders of pregnancy were over-reported (sensitivity was 40%) but the negative predictive value of no self-report of preeclampsia or gestational hypertension was 90%. [16] Thus, women with no reported hypertension during pregnancy were largely normotensive during pregnancy.
Measurement of inflammatory markers and IMT
IMT measures of the common carotid artery (CCA), the carotid bulb, and the internal carotid artery (ICA) were obtained using B-mode ultrasound (GE-Logiq-700, Issaquoah IL) at each study site by certified sonographers utilizing a standardized protocol.[17] Images of the far and near wall of the distal CCA, the bulb, and the proximal ICA were obtained on the right and left sides. Images were read at the ultrasound reading center (Tufts Medical Center, Boston MA). The mean of the maximum wall thickness of the respective carotid artery segment was defined as the mean of the near and far wall thickness for each of the images taken on the left and right sides, 4 for the common carotid and 8 segments for the bulb and ICAs. The composite IMT measure averaged the maximal result from the CCA, the bulb and ICA.
Serum hs-CRP was measured in blood samples by the University of Vermont using an enzyme-linked immunosorbent assay method improved with a nephelometry-based high-throughput assay that offers greater sensitivity and reproducibility.[18] IL-6 was measured with a high-sensitivity enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minnesota).[19]
Blood Pressure, Anthropometry, and Blood Lipid Measurements
Anthropometric measurements, blood pressure, and blood specimens were collected at baseline and each follow-up exam by trained personnel using standardized research methods.[13, 14] Study participants were asked to fast for 12 hours before their clinic examination. Fasting blood samples were sent to the Northwest Lipid Research Laboratories, University of Washington (Seattle, WA, USA) for lipid determination. The laboratory participates in the Center for Disease Control and Prevention (CDC) lipids standardization program, and the samples were analyzed continuously.[20] Total cholesterol, triglycerides and HDL-cholesterol were measured enzymatically within 6 weeks of collection. LDL-cholesterol was calculated using the Friedewald equation.[21] LDL was not calculated for participants with triglyceride levels ≥400 mg/dl (n=25). Three resting seated blood pressure measurements were obtained with a random-zero sphygmomanometer through year 15 and with the Omron (Omron Corp., Schaumburg, IL) HEM907XL oscillometer at year 20; the mean of the second and third readings was used for this report. Omron results were calibrated to be consistent with the random-zero results.[16] Hypertension at each visit was defined as measured blood pressure >140/90 mmHg or self-report of anti-hypertension medication.
Information on demographic characteristics (age, sex, and race) was obtained at baseline; educational achievement was self-reported on standardized questionnaires during each examination. Height and weight were measured during each examination. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Waist circumference was measured as the abdominal girth midway between the iliac crest and the bottom of the ribcage. Smoking was measured as self-reported smoking status (nonsmoker, ex-smoker, and current smoker). Habitual physical activity was measured by use of the CARDIA Physical Activity History, a simplified version of the Minnesota Leisure Time Physical Activity Questionnaire.[22] CARDIA did not query duration of bouts of physical activity; therefore, physical activity is characterized as exercise units, which increase with frequency and intensity of performance.
Statistical methods
Maternal characteristics at baseline (before reported pregnancies) and at year 20 were compared in women with and without a history of preterm birth using Wilcoxon rank sum tests. We modeled change in blood pressure, BMI, and lipid markers from 7 examinations starting at baseline (before pregnancies evaluated in this study) to year 20 using generalized estimating equations (GEE). Differences in inflammatory markers and IMT at year 20 were estimated in linear regression models sequentially adjusted for age and BMI at year 20. Other covariates with established associations with both preterm birth and hypertension were then added (smoking, education, physical activity, parity, and change in blood pressure). Race was added to the final model, and GEE and linear models were also stratified by race given the well established disparities in both preterm birth and hypertension. Change in log-transformed CRP concentrations over three measurements was also evaluated using GEE, and risk of elevated CRP (>3 mg/L) [23] according to preterm birth status was evaluated using logistic regression. All analyses were then replicated after stratifying by those with any pregnancies reported to have been complicated by hypertension. Effect measure modification (p<0.1) by race and obesity at year 20 was evaluated given the relevance of each of these factors to preterm birth risk and inflammation. SAS 9.2 was utilized for all analyses.
RESULTS
Births evaluated in this study occurred on average 6 years after baseline enrollment. A total of 226 women (24.7%) reported at least one pregnancy resulting in a preterm birth. Of these, 182 were first births to women in the cohort, and 44 were subsequent births. Women with preterm births were more likely to be of Black race, to have less than a high school education, and to be parous at baseline compared to women with term births (Supplemental Table 1). They were also more likely to report pregnancies complicated by hypertension and to develop hypertension in the years after pregnancy.
Longitudinal changes in blood pressure and BMI
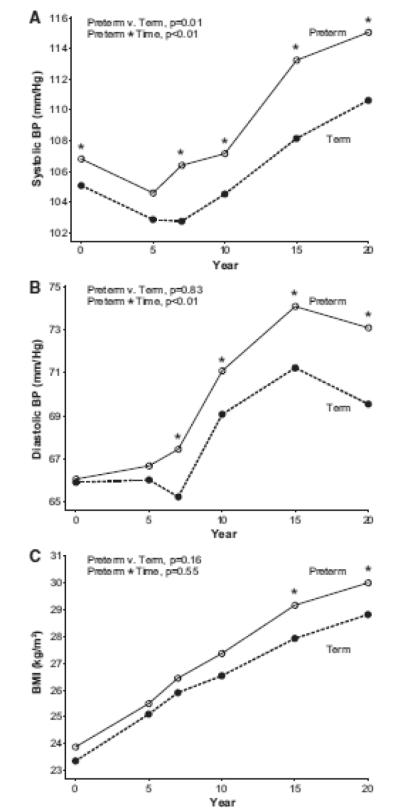
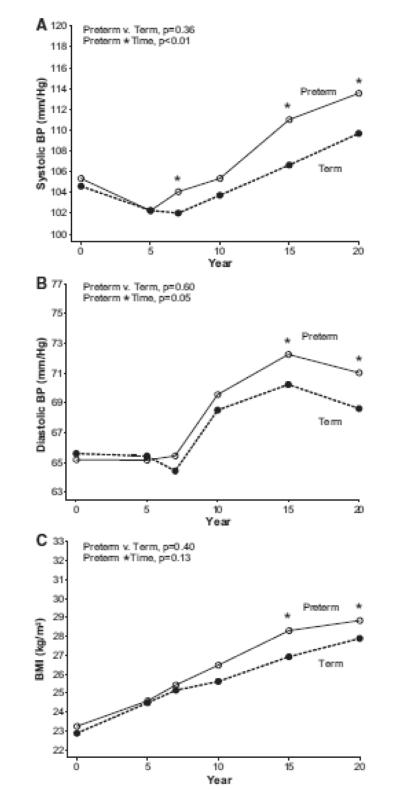
Women with preterm births had higher systolic blood pressure, on average, at baseline which was before pregnancies reported for this study (106.3 vs. 104.9 mmHg, p<0.01) and at year 20 (115.05 vs. 110.63,<0.01; Table 1) compared to women who delivered term births. Repeated measures analysis indicated that systolic blood pressure was higher across follow-up in women with preterm birth (p=0.03 for group differences) and increased more rapidly compared to women with term births (p<0.01 for group*time interaction, Figure 1 Panel A). Diastolic blood pressure was not different according to preterm birth status before pregnancy, but by year 7 of follow up those with preterm births had higher mean diastolic blood pressure (group*time interaction, p<0.01 Figure 1, Panel B). These changes in blood pressure (group*time interactions) remained significant after removal of women who reported no pregnancies complicated by hypertension (Figure 2, Panels A and B). In addition, systolic and diastolic blood pressures increased over time more rapidly, on average, when models were adjusted for age, race and BMI (group*time interaction, p <0.01). When stratified by race, blood pressure results were similar. For example, systolic blood pressure increased more rapidly in White and Black women (group*time interaction p=0.03 for White women; p=0.05 for Black women; Supplemental Table 2).
Table 1.
Cardiovascular risk factors at bseline and at year 20 study visit according to preterm birth status
| Baseline | Year 20 | |||||
|---|---|---|---|---|---|---|
| Characteristic | Term | Preterm | P value |
Term | Preterm | P value |
| BMI, kg/m2 | 23.36(4.55) | 23.88(4.56) | 0.08 | 28.81(7.50) | 29.99(7.15) | 0.01 |
| Waist circumference, cm | 71.88(9.09) | 72.23(9.51) | 0.67 | 86.43(14.87) | 88.25(14.32) | 0.06 |
| Smoking (n, %) | 143(20.82) | 56(24.89) | 0.22 | 84(12.26) | 36(16.07) | 0.17 |
| Systolic blood pressure, mmHg | 105.08(9.07) | 106.81(9.14) | <0.01 | 110.63(13.48) | 115.05(15.99) | <0.001 |
| Diastolic blood pressure, mmHg | 65.91(8.73) | 66.06(9.18) | 1.00 | 69.54(10.69) | 73.09(11.91) | <0.001 |
| Fasting plasma , mg/dl | ||||||
| Total cholesterol | 177.34(30.72) | 175.69(31.14) | 0.42 | 184.20(31.40) | 183.40(33.28) | 0.55 |
| HDL-cholesterol | 56.06(12.44) | 56.61(12.73) | 0.90 | 59.84(16.61) | 58.66(17.28) | 0.13 |
| Triglycerides | 65.28(32.29) | 62.50(33.26) | 0.16 | 92.69(62.68) | 90.43(48.71) | 0.86 |
| LDL-cholesterol | 108.23(29.20) | 106.57(26.81) | 0.49 | 105.92(28.65) | 106.65(28.45) | 0.88 |
| CRP, mg/L (median, IQR) | -- | -- | -- | 1.17(0.44,3.69) | 1.25(0.50,4.67) | 0.22 |
| IL-6, pg/ml (median, IQR) | -- | -- | -- | 1.71(0.98,3.16) | 1.88(1.10,3.37) | 0.26 |
| IMT, cm | -- | -- | -- | 0.67(0.11) | 0.68(0.13) | 0.07 |
Figure 1.
Maternal systolic blood pressure (Panel A), diastolic blood pressure (Panel B), and BMI (Panel C) from baseline to year 20, according to preterm birth history (n=916). Asterisks indicate cross sectional differences according to preterm status with p<0.05 using Wilcoxon test.
Figure 2.
Maternal systolic blood pressure (Panel A), diastolic blood pressure (Panel B), and BMI (Panel C) from baseline to year 20, according to preterm birth history in women with no pregnancies complicated by hypertension (n=680). Asterisks indicate cross sectional differences according to preterm status with p<0.05 using Wilcoxon test.
Women with preterm births had modestly higher mean BMI’s at baseline compared to women with term births. While BMI was not different according to preterm birth status when averaged across all 20 years of follow-up (p=0.34), women with preterm births had higher mean BMI beginning in year 15 of follow up compared to women with term births (Figure 1, Panel C). This was also true when limited to women who reported that no pregnancies were complicated by hypertension (Figure 2, Panel C). Although not different before pregnancy, women with PTB had higher waist circumferences at year 20 compared to women with term births (Table 1). Triglycerides measured prior to pregnancies reported during CARDIA follow-up were lower in women with subsequent preterm births, but by year 20 concentrations were no longer different according to preterm birth status. Other lipid concentrations increased over time as expected, however, there were no differences according to preterm birth history in longitudinal analyses.
IMT and inflammatory markers
Women with PTB had borderline higher IMT at year 20 of follow-up, on average, adjusted for age and body mass index compared to women with term births (difference 0.016 mm, p=0.06; Table 2). Additional adjustment for lifestyle and cardiovascular risk factors (smoking, education, physical activity, and difference in blood pressure from baseline to year 20) appeared to account for this difference, and the addition of race to the model made all differences according to preterm birth history null (difference 0.008, p=0.33). Results were similar when limited to women with no hypertension reported during pregnancy. For these women preterm birth was associated with higher IMT adjusted for age and BMI (difference 0.019, p=0.07) that was attenuated to no difference when adjusted for race, lifestyle and cardiovascular risk factors (difference 0.11, p=0.28). There was no evidence that the association between preterm birth and later life IMT varied according to maternal race (p for interaction =0.69) or obesity (p for interaction 0.31), and estimates were similar when stratified by these characteristics.
Table 2.
Inflammatory markers and Intima Medial Thickness markers on Preterm Birth
|
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Model 1 | Model 2 | Model 3 | Model 4 | |||||||||||
|
|
|||||||||||||||
| Markers | Estimate | SE | P | Estimate | SE | P | Estimate | SE | P | Estimate | SE | P | Estimate | SE | P |
| log(CRP) | 0.135 | 0.106 | 0.20 | 0.129 | 0.106 | 0.22 | −0.001 | 0.083 | 0.99 | −0.032 | 0.084 | 0.70 | −0.025 | 0.084 | 0.76 |
| log(IL-6) | 0.049 | 0.067 | 0.46 | 0.046 | 0.066 | 0.49 | −0.015 | 0.059 | 0.80 | −0.057 | 0.060 | 0.34 | −0.065 | 0.059 | 0.27 |
| IMT | 0.019 | 0.009 | 0.03 | 0.020 | 0.009 | 0.02 | 0.016 | 0.009 | 0.06 | 0.011 | 0.009 | 0.19 | 0.008 | 0.009 | 0.34 |
Model 1, adjusted for age at year 20
Model 2, adjusted for age and BMI at year 20
Model 3, adjusted for factors in model 2 and education, physical activity, smoking, and difference in systolic blood pressure from baseline to year 20
Model 4, adjusted for factors in model 3 and maternal race
Women with a history of PTB did not have higher concentrations of CRP or IL-6 measured at year 20 (Table 1). CRP was also measured at years 7 and 15, and it did not change according to preterm birth status (data not shown). Nor was there evidence that women with a prior PTB were more likely to have CRP >3.0 mg/dl at year 20 than their counterparts with term births (OR 1.17, 95% CI 0.86,1.59; adjusted for age).
DISCUSSION
In a longitudinal child-bearing cohort of Black and White women, systolic blood pressure was moderately higher before preterm birth, and these women appeared to differentially accumulate risk factors (increasing systolic and diastolic blood pressure and higher BMI) across 20 years of follow up that was independent of hypertension status during pregnancy. Women with preterm births did not have higher mean IMT two decades after delivery compared to women with term births after accounting for race, lifestyle and cardiovascular risk factors. There was no evidence that pro-inflammatory markers were elevated in the years following preterm v. term births.
Record linkage studies have consistently indicated that women with preterm births, regardless of hypertension status during pregnancy, have excess CVD morbidity and mortality.[1, 4, 24, 25] Non-preeclamptic preterm births have also been associated with higher maternal CVD mortality risk than term preeclampsia.[2] There are limited data, however, on mechanisms that may link these reproductive and later life conditions. In contrast to our results, Fraser, et al recently reported that higher blood pressure assessed 18 years after preterm vs. term births in the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort in England was explained by hypertension in pregnancy.[5] In our current CARDIA findings, blood pressure increased more rapidly over 20 years of follow-up in women with preterm compared to term births regardless of self-reported hypertension status during pregnancy. Perhaps different study populations with different predisposing risk for both preterm birth and CVD may explain our discordant findings. For example, the preterm birth rate in the Fraser study was 4.3% compared to 24.7% in CARDIA where half of the women were Black. It is well established that Black women in the U.S. have preterm birth rates twice as high as their White counterparts. In addition, White women in the U.S. have preterm birth rates that are twice as high as their European counterparts. In addition, mean systolic blood pressure in CARDIA assessed on average 14 years after the index pregnancy was higher in women with preterm and term births compared to the mean values assessed in the Fraser study 18 years after pregnancy (115.05 and 110.63 mmHg, respectively in CARDIA; 105.02 and 103.06 in ALSPAC).
An advantage of the CARDIA study is its longitudinal assessment of classic atherogenic factors, including measurements before pregnancy. Our findings raise the possibility that preclinical endothelial dysfunction may be related to a portion of preterm births, and that the accumulation of vascular injury due to aging and weight gain may accelerate atherogenesis in women with preterm births. Evidence that normotensive preterm births have excess placental vascular lesions[26-28] and that larger changes in blood pressure within the normal range across gestation are associated with preterm birth risk support this possibility.[29] Thus, similar to other pregnancy complications, the preterm birth may unmask endothelial dysfunction during the stressed state of state of pregnancy. The inclusion of both White and Black women in the CARDIA cohort is also a strength given the profound and persistent race disparities in PTB and CVD.
Contrary to our hypothesis, IMT was not higher in women with prior preterm births, nor were pro-inflammatory markers. Longer follow up and additional evaluation of subclinical organ damage such as flow mediated dilation or pulse wave velocity may be warranted. Our data suggests that increasing blood pressure and perhaps weight gain may be more important markers of elevated CVD risk in women with a prior preterm birth, including those not complicated by hypertension during pregnancy. Interestingly, risk factor trajectories that may relate pregnancy complications such as preterm birth to later life maternal cardiovascular disease have been hypothesized.[30, 31] To our knowledge our results are the first to utilize longitudinal data collected at multiple time points across the reproductive years to characterize a trajectory of blood pressure and body mass index in women with preterm and term deliveries. These results raise the possibility that subtle risk differences may be detected before pregnancy for some factors, and that risk may increase more rapidly across the lifecourse in women with preterm births. This intriguing possibility, and mechanisms that might explain it, warrants additional study.
Our findings should be considered in light of important limitations. Pregnancy data were self-reported every 2 to 5 years in CARDIA. Although recall was good for preterm birth classification, our findings were likely diluted by misclassification that occurred in late preterm births. Women in our study over-reported hypertension during pregnancy. The high specificity of this reported complication (90%), however, ensures that analysis of normotensive preterm births was likely unbiased. This limitation also sheds light on the practical consequences for inclusion of pregnancy history in assessing women’s CVD risk as discussed in the most recent American Heart Association screening guidelines for women.[32] Preterm birth is common, is reported more accurately than hypertension during pregnancy, and is associated with excess CVD morbidity making it a potentially powerful tool for identifying subclinical risk early in the life course when lifestyle changes may delay or prevent disease progression.
Perspectives
Women with PTBs have higher blood pressure before pregnancy that increased more rapidly in the two decades after pregnancy compared to women with term births. This post-pregnancy increase in maternal blood pressure is independent of hypertension status during pregnancy, but may not be related to higher intimal medial thickness or systemic inflammation. In the U.S. where rates of preterm birth are much higher than other developed countries and race disparities persist, PTB may mark women at excess risk of higher blood pressure in the years after pregnancy.
Supplementary Material
S 1. Maternal characteristics at baseline according to preterm birth status (n=916)
S2. Blood pressure and BMI at baseline and year 20, stratified by race
Novelty and Significance.
What is new?
The present study is the first to assess blood pressure before and after pregnancies delivered preterm in a racially diverse cohort.
Independent of hypertension status during pregnancy, blood pressure increases more rapidly in the two decades after pregnancy among women who delivered preterm compared to term births.
What is relevant?
Preterm birth is common in the U.S., affecting 12% of deliveries. The present study demonstrates that the trajectory of increasing blood pressure across 20 years is worse in women who deliver preterm, even among those without hypertension during pregnancy.
Summary
In the U.S. where rates of PTB are high and race disparities persist, PTB may identify women with higher blood pressure and excess cardiovascular risk in the years after pregnancy.
Acknowledgments
Funding Sources: This work is supported by National Institute and Child Health Development K12 HD43441 Preterm birth and maternal cardiovascular disease and National Lung, Heart and Blood Institute R01 HL076532 Fetal growth restriction and maternal cardiovascular risk.
Footnotes
Disclosures: None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Janet M Catov, Department of Obstetrics, Gynecology & Reproductive Sciences University of Pittsburgh School of Medicine 300 Halket Street Pittsburgh, PA 15213.
Cora E Lewis, University of Alabama at Birmingham Preventive Medicine.
Minjae Lee, University of Texas Health Science Center at Houston.
Melissa F Wellons, University of Alabama School of Medicine.
Erica P Gunderson, Kaiser Permanente Division of Research.
References
- 1.Davey Smith G, Whit ley E, Gissler M, Hemminki E. Birth dimensions of offspring, premature birth, and the mortality of mothers. Lancet. 2000;356:2066–2067. doi: 10.1016/S0140-6736(00)03406-1. [DOI] [PubMed] [Google Scholar]
- 2.Irgens H, Reisaeter L, Irgens L, Lie R. Long term mortality of mothers and fathers after pre-eclampsia. BMJ. 2001;323:1213–1217. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catov JM, Wu CS, Olsen J, Sutton-Tyrrell K, Li J, Nohr EA. Early or Recurrent Preterm Birth and Maternal Cardiovascular Disease Risk. Annals of Epidemiology. 2010;20:604–609. doi: 10.1016/j.annepidem.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonamy A-KE, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth Characteristics and Subsequent Risks of Maternal Cardiovascular Disease / Clinical Perspective. Circulation. 2011;124:2839–2846. doi: 10.1161/CIRCULATIONAHA.111.034884. [DOI] [PubMed] [Google Scholar]
- 5.Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, Lawlor DA. Associations of Pregnancy Complications With Calculated Cardiovascular Disease Risk and Cardiovascular Risk Factors in Middle Age / Clinical Perspective. Circulation. 2012;125:1367–1380. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khong T, Wolf F, Robertson W, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Brit J Obstet Gynaec. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 7.Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and Dyslipidemia Related to Risk of Spontaneous Preterm Birth. American Journal of Epidemiology. 2007;166:1312–1319. doi: 10.1093/aje/kwm273. [DOI] [PubMed] [Google Scholar]
- 8.Pitiphat W, Gillman MW, Joshipura KJ, Williams PL, Douglass CW, Rich-Edwards JW. Plasma C-Reactive Protein in Early Pregnancy and Preterm Delivery. Am J Epidemiol. 2005;162:1108–1113. doi: 10.1093/aje/kwi323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luc G, Bard J-M, Juhan-Vague I, Ferrieres J, Evans A, Amouyel P, Arveiler D, Fruchart J-C, Ducimetiere P, on behalf of the PSG C-Reactive Protein, Interleukin-6, and Fibrinogen as Predictors of Coronary Heart Disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:1255–1261. doi: 10.1161/01.ATV.0000079512.66448.1D. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM. Evaluating Novel Cardiovascular Risk Factors: Can We Better Predict Heart Attacks? Annals of Internal Medicine. 1999;130:933–937. doi: 10.7326/0003-4819-130-11-199906010-00018. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM. Clinical Application of C-Reactive Protein for Cardiovascular Disease Detection and Prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 12.Ross R. Atherosclerosis — An Inflammatory Disease. New England Journal of Medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 13.Cutter G, Burke G, Dyer A, Friedman G, Hilner J, Huges G, Hulley S, Jacobs D, Lie K, Manolio T. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials. 1991;12:1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 14.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Liu K, Savage PJ. Cardia: study design, recruitment, and some characteristics of the examined subjects. Journal of Clinical Epidemiology. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 15.Hughes G, Cutter G, Donahue R, Friedman G, Hulley SB, Hunkeler E, Jacobs D, Liu K, Orden S, Pirie P, Tucker B, Wagenknecht L. Recruitment in the Coronary Artery Disease Risk in Young Adults (CARDIA) Study. Controlled Clinical Trials. 1987;8:68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 16.Gunderson EP, Chiang V, Lewis CE, Catov J, Quesenberry CP, Jr., Sidney S, Wei GS, Ness R. Long-Term Blood Pressure Changes Measured From Before to After Pregnancy Relative to Nonparous Women. Obstet Gynecol. 2008;112:1294–1302. doi: 10.1097/AOG.0b013e31818da09b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polak JF, Person SD, Wei GS, Godreau A, Jacobs DR, Harrington A, Sidney S, O’Leary DH. Segment-Specific Associations of Carotid Intima-Media Thickness With Cardiovascular Risk Factors. Stroke. 2010;41:9–15. doi: 10.1161/STROKEAHA.109.566596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rifai N, Tracy RP, Ridker PM. Clinical Efficacy of an Automated High-Sensitivity C-Reactive Protein Assay. Clinical Chemistry. 1999;45:2136–2141. [PubMed] [Google Scholar]
- 19.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated Interleukin-6 and C-Reactive protein levels with mortality in the elderly. The American Journal of Medicine. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 20.Bild DE, Jacobs DR, Jr, Liu K, Williams OD, Hilner JE, Perkins LL, Marcovina SM, Hulley SB. Seven-year trends in pasma low-density-lipoprotein-cholesterol in young Adults: the CARDIA Study. Annals of Epidemiology. 1996;6:235–245. doi: 10.1016/1047-2797(96)00005-1. [DOI] [PubMed] [Google Scholar]
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 22.Jacobs D, Hahn L, Haskell W, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil. 1989;9:448–459. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Taubert K, Tracy RP, Vinicor F. Markers of Inflammation and Cardiovascular Disease. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 24.Lykke JA, Paidas MJ, Damm P, Triche EW, Kuczynski E, Langhoff-Roos J. Preterm delivery and risk of subsequent cardiovascular morbidity and type-II diabetes in the mother. BJOG. 2010;117:274–281. doi: 10.1111/j.1471-0528.2009.02448.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith G, Pell J, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357:2002–2006. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 26.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–591. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 27.Germain AM, Carvajal J, Sanchez M, Valenzuela GJ, Tsunekawa H, Chuaqui B. Preterm labor: placental pathology and clinical correlation. Obstet Gynecol. 1999;94:284–289. doi: 10.1016/s0029-7844(99)00324-5. [DOI] [PubMed] [Google Scholar]
- 28.Kelly R, Holzman C, Senagore P, Wang J, Tian Y, Rahbar MH, Chung H. Placental Vascular Pathology Findings and Pathways to Preterm Delivery. American Journal of Epidemiology. 2009;170:148–158. doi: 10.1093/aje/kwp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Villar J, Sun W, Merialdi M, Abdel-Aleem H, Mathai M, Ali M, Yu KF, Zavaleta N, Purwar M, Nhu Ngoc NT, Campodonico L, Landoulsi S, Lindheimer M, Carroli G. Blood pressure dynamics during pregnancy and spontaneous preterm birth. American Journal of Obstetrics and Gynecology. 2007;197:162.e1–e6. doi: 10.1016/j.ajog.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rich-Edwards JW, McElrath TF, Karumanchi SA, Seely EW. Breathing Life Into the Lifecourse Approach. Hypertension. 2010;56:331–334. doi: 10.1161/HYPERTENSIONAHA.110.156810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sattar I, Greer I. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325:157–160. doi: 10.1136/bmj.325.7356.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC, Jr., Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. Journal of the American College of Cardiology. 2011;57:1404–1423. doi: 10.1016/j.jacc.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S 1. Maternal characteristics at baseline according to preterm birth status (n=916)
S2. Blood pressure and BMI at baseline and year 20, stratified by race