Abstract
The concept of allostasis suggests that greater cumulative stress burden can influence stress-responsive physiology. Dysregulation of allostatic mediators, including the hypothalamic-pituitary-adrenal (HPA) axis, is thought to precede many other signs of age-related pathology as the persistent burden of stressors accumulates over the individual's lifespan. We predicted that even in young adulthood, HPA regulation would differ between Blacks and Whites reflecting, in part, higher rates of stressor exposure and greater potential for stressors to “get under the skin”. We examined whether stressor exposure, including experiences with racism and discrimination, explained race differences in waking cortisol and the diurnal rhythm. We also examined whether HPA functioning was associated with mental health outcomes previously linked to cortisol. Salivary cortisol was assayed in 275 young adults (127 Blacks, 148 Whites, 19 to 22 years old), four times a day across 3 days. Hierarchical linear models revealed flatter slopes for Blacks, reflecting significantly lower waking and higher bedtime cortisol levels compared to Whites. Associations of HPA functioning with stressors were typically more robust for Whites such that more stress exposure created an HPA profile that resembled that of Black young adults. For Blacks, greater stressor exposure did not further impact HPA functioning, or, when significant, was often associated with higher cortisol levels. Across both races, flatter slopes generally indicated greater HPA dysregulation and were associated with poor mental health outcomes. These differential effects were more robust for Whites. These findings support an allostatic model in which social contextual factors influence normal biorhythms, even as early as young adulthood.
Keywords: allostasis, cortisol, race differences, HPA axis
Introduction
Allostasis and Allostatic Load: Physiological Processes that Instantiate Stressor Exposure
Stress regulatory processes in young adults may provide a window into the hypothesized biobehavioral pathways that link stressful experiences with poor health. Maintaining some degree of physiological stability is a healthy adaptive process that allows an individual to respond to both typical and extraordinary demands (McEwen, 2003). Allostasis presupposes that stress-responsive physiological systems are intended to change over time and adjust to meet the demands of a constantly changing environment. Even when the environment is extremely challenging or stressors are persistent or recurrent, allostatic physiological changes will help the individual to meet the demands of their environment, despite potential costs exerted by such alterations. Stress exposure is not equally distributed across a large population of individuals. Within the US, Blacks reliably experience greater exposure to a variety of stressors, including poverty, poor schools, unsafe neighborhoods, racial discrimination, and concomitant strains on family interactions compared to Whites (Williams & Jackson, 2005). Examining race differences in HPA functioning may capture different parts of the normal distribution of stressor exposure, providing a window into the impact of the cumulative burden of stress.
Persistent stressor exposure and the subsequent allostatic physiological adjustments may, over time, canalize the individual along a deleterious pathway, which may result in physiological hyper- or hypo-responsivity. Chronic activation of stress responsive systems can cause wear-and-tear on regulatory systems (e.g., allostatic load, McEwen, 1998). This concept has helped to explain risk for Metabolic Syndrome (Carroll et al., 2008; Keyes, 2009), and stress-related diseases (Karlamangla, Merkin, Crimmins, & Seeman, 2010; Taylor, Repetti, & Seeman, 1997) including mental health symptoms (Evans, 2003; McEwen, 2000c; Schulkin, McEwen, & Gold, 1994). Not surprisingly, Blacks demonstrate consistent evidence of higher allostatic load by adulthood, and have a high incidence of stress-related morbidity and mortality (Green & Darity, 2010; Szanton, Gill, & Allen, 2005). Evidence for allostatic load may be present in relatively young populations (Evans & English, 2002; Johnston-Brooks, Lewis, Evans, & Whalen, 1998). Geronimus and colleagues (2006) found that Blacks had greater probability of allostatic load as young adults compared to Whites, and that health disparity increased proportionally across adult development (Huffman, 2009). Health disparities in life expectancy and death rates due to all causes and a wide range of specific causes are well-established (Heron, 2007). We need to better understand the biopsychosocial processes underlying the development of stress-related diseases, with a particular focus on the factors underlying individual differences within both races.
Our goal is to use the allostasis model to describe when and how race differences can be ascribed to social and environmental forces. Identifying and understanding early signs of the biological impact of social and environmental forces is imperative for prevention efforts, as it may be possible to diminish the impact of race-related differences through targeted psychosocial prevention efforts. The present study targets young adults aged 19 - 22 years because exposure to social stressors is likely to have accumulated enough to influence stress-related physiology. On the other hand, it is early enough in adulthood that the wear-and-tear on physiology may not yet be permanent, and intervention efforts may still be fruitful before the individual fully attains adult roles and responsibilities. This age range marks an important developmental transition (Schulenberg, Sameroff & Cicchetti, 2004), known as emerging adulthood (Arnett, 2007).
HPA Functioning as a Primary Allostatic Mediator
Many empirical examples of allostatic load are less applicable to young populations because they rely on outcome measures that emerge or become exacerbated much later in development. Lupien and colleagues (2006) describe the first physiological indicators of stressor exposure as primary allostatic mediators. These include physiological systems which are both stress responsive and that have diverse target organs, thereby helping the individual calibrate to their environment (e.g., autonomic, HPA, immune, McEwen, 2000c). Each primary allostatic mediator is theorized to have different thresholds for responding to environmental stimuli (Del Guidice, Ellis, & Shirtcliff, 2011; Hastings et al., 2012). The present investigation targets HPA axis functioning because it (a) is known to be responsive to social contextual factors of both short- and long-term duration (Miller, Chen, & Zhou, 2007; Weems & Carrion, 2009; Yehuda, 2001), (b) has a relatively high threshold for activation, so likely reflects only the most salient stressors (Hastings et al., 2012; Sapolsky, Romero, & Munck, 2000), (c) is capable of changing gene expression and protein transcription for a substantial period of time (De Kloet, 2004), and (d) has been implicated as a risk factor for mental and physical health problems (Lupien, King, Meaney, & McEwen, 2001; Lupien et al., 2006).
Defining risk or pathology for allostatic mediators is challenging because it is difficult to disentangle when physiological adjustments are adaptive or maladaptive (Del Guidice et al., 2011), and both higher or lower activity may be advantageous depending on the context (Cicchetti & Rogosch, 2001; Ruttle et al., 2011; Shirtcliff & Essex, 2008). Stress regulation provides a useful rubric for understanding adaptation (Siever & Davis, 1985). Regulation can be summarized through two large components: flexibility and regularity (or rhythmicity). The purpose of flexibility is to enhance the individual's ability to respond to the environment to maintain stability; because environments frequently change, often the most efficient way of maintaining stability is to promote flexibility within physiological systems. At the same time, regularity encourages these changes to be predictable and consistent. Rhythmic changes can encourage physiological flexibility because these physiological systems fluctuate substantially on a daily basis. By examining cortisol's diurnal (or daytime) rhythm across several days, we were able to examine rhythmicity as well as flexibility.
We consider cortisol's diurnal rhythm to reflect an allostatic process because it can capture consistent (or longitudinal) modulation of the HPA axis often observed as a consequence of chronically stressful experiences (Evans, Lercher, Meis, Ising, & Kofler, 2001; Gunnar & Vazquez, 2001; Ockenfels et al., 1995). The daytime component of the circadian rhythm, diurnal cortisol, is highly stable across days and years (Rosmalen et al., 2005; Ruttle et al., 2011; Shirtcliff et al., 2012; Shirtcliff & Essex, 2008). It is typically characterized by a sharp increase within 30 minutes of waking, followed by a steep decline by mid-morning and then more gradual decline over the course of the day with the lowest values at bedtime (Stone et al., 2001). Morning cortisol levels reflect strong genetic influences and are largely under the control of hypothalamic and pituitary hormone regulation (Van Hulle, Shirtcliff, Lemery-Chalfant, & Goldsmith, under review), but afternoon/evening levels can be more influenced by contextual and environmental events (Bartels, de Geus, Kirschbaum, Sluyter, & Boomsma, 2003; Schreiber et al., 2006). Consequently, the diurnal rhythm may indicate the confluence of intrinsic, biological forces with extrinsic, environmental forces working together within an individual on a daily cycle. The regularity of this rhythm is also important because high morning levels help individuals prepare for the events of the day, whereas the lower evening levels permit critical immune and tissue repair (Dallman et al., 2002). In the present investigation, we captured cortisol levels and diurnal rhythm using saliva samples taken four times per day across 3 separate days.
Two recent cross-sectional studies have linked diurnal cortisol measures with race. DeSantis and colleagues (2007) found that Black adolescents had flatter diurnal cortisol slopes than White adolescents, derived from both lower waking and higher bedtime cortisol in Black youth. The Coronary Artery Risk Development in Young Adults study (Cohen et al., 2006) measured cortisol over the course of one day from 781 adults and found flatter diurnal slopes attributed to both lower morning and higher evening cortisol in Black adults. Additionally, a small convenience sample found Black adults had lower morning cortisol than White adults, but Bennett and colleagues (2004) did not assess the diurnal rhythm or stressor exposure and racial discrimination. For the other two studies, socio-environmental factors did not explain race differences (Cohen et al., 2006; DeSantis et al., 2007), but rather stressor exposure and race had independent and additive effects on the diurnal rhythm. In fact, race had the strongest effect size (Cohen et al., 2006). These additive effects could reflect differences in the underlying distribution of stressor exposure for Blacks than Whites, but that individual differences in stressor exposure are also salient.
Race Differences in Stressor Exposure and Physiological Impact of Stressors
Psychological mechanisms have been posited for how differential exposure to stress may explain race disparities in health and well-being (Szalacha et al., 2003), but the allostasis framework emphasizes that psychological explanations may be psychobiological as well. Higher rates of exposure to a wide range of stressors due to low income, poorer neighborhoods and lower education among Blacks compared to Whites have been born out in a number of studies, particularly on health outcomes where stressor exposure reliably predicts morbidity and mortality (Lantz, House, Mero, & Williams, 2005; Prelow, Danoff-Burg, Swenson, & Pulgiano, 2004; Turner & Avison, 2003). To some extent, race differences have been attributed to differences in poverty and financial stress (Krieger, 2005). Young adults are particularly susceptible to financial strains such as difficulties paying rent, not having money left after paying bills, and unpaid bills (Conger et al., 1992; Duncan & Brooks-Gunn, 1997; Wilkinson & Pickett, 2009). Much of the effects of poverty are transmitted through residential segregation and the increased likelihood that a Black family will live in a distressed neighborhood (Krieger, 2005; Massey, 2004; Williams & Jackson, 2005). A number of studies have linked race, poverty, and distressed neighborhoods (Lantz et al., 2005; Prelow et al., 2004; Turner & Avison, 2003) to a variety of outcomes, including mental health (Caughy, O'Campo, & Muntaner, 2003), violent behavior (Paschall, Flewelling, & Ennett, 1998), and drug problems (Duncan, Duncan, & Strycker, 2002). The association between neighborhood quality and violence provides evidence that living in a poor neighborhood contributes to violence within the family (Cunradi, Caetano, Clark, & Schafer, 2000), and further helps to account for the known relationship between criminal victimization and depression (Simons et al., 2002). Recent stressful life events as well as total lifetime major events are more prevalent among Blacks than Whites even when compared within low SES young adults (Turner & Avison, 2003).
Blacks are also exposed more frequently to race-related stressors, which Peters and Massey (1983) describes as “mundane extreme environmental stress”. This describes a special form of daily hassles in which Blacks are subjected to more scrutiny, treated as suspicious or potentially threatening in everyday situations, often ignored, or assumed to be an employee or server. These stressors are particular to race rather than class or income status (Bulatao & Anderson, 2004). Similarly, racial discrimination is a distinct stressor from other socio-demographic disadvantages (Landrine & Klonoff, 1996; Turner & Avison, 2003) and exerts a unique wear and tear on the individual (Luthar, 2006).
We examined multiple types of stressors (Cicchetti & Curtis, 2007; Cicchetti & Toth, 2009) because types of stressors may exert differential impact on HPA functioning (Boyce & Ellis, 2005; Essex et al., 2012). We do not know whether or how race differences in these domains operate. Concurrent stressors provide information about the co-regulation of stressor exposure and stress responsivity. These particular measures of stressors were selected because they tap into life-history relevant processes (Del Guidice et al., 2011) and have previously been associated with HPA functioning (Evans et al., 2001; Lupien, King, Meaney, & McEwen, 2000; Lupien et al., 2001). Three measures of stressors intentionally captured experiences with racism, discrimination, and daily racial hassles. Race-related stressors have been associated with HPA functioning (DeSantis et al., 2007; Kaholokula et al., 2011; Richman & Jonassaint, 2008).
Association with Mental Health Outcomes
Allostatic load implies that understanding allostatic processes will inform us about stress-related health outcomes. This dovetails nicely with a developmental psychopathology perspective which seeks to identify multiple pathways from risk exposure to mental health disorders (Cicchetti & Cohen, 1995), or with symptomatology within the normal range (Cicchetti & Lynch, 1995; Cicchetti & Toth, 2009). Allostatic load is linked with physical health problems (McEwen, 2000c), yet it is also implicated in the development of mental health problems (Koob & Le Moal, 2001; McEwen, 2000a; Piazza & Le Moal, 1996). We targeted three broad mental health symptom clusters which are prevalent in a young adult population.
First, antisocial behavior has been previously linked with blunted HPA activity (Alink et al., 2008; Hawes, Brennan, & Dadds, 2009; Loney, Butler, Lima, Counts, & Eckel, 2006; McBurnett et al., 2005; O'Leary, Loney, & Eckel, 2007; Popma et al., 2007; Shirtcliff et al., 2009; Susman, 2006; van Goozen et al., 1998). Briefly, the HPA axis has strong reciprocal connections with social- and emotion-related neurocircuitry (Phan, Wager, Taylor, & Liberzon, 2002; Pruessner et al., 2010). High cortisol allows the individual to be open or attentive to relevant emotional and social information in the individual's environment (Shirtcliff et al., 2009), yet may also leave the individual sensitive to experience the pain and distress of another with nearly as much activation in pain neurocircuitry as if they experienced the pain themselves (Decety & Lamm, 2006; Singer et al., 2004). Hypo-arousal of the HPA axis attenuates the openness of the individual to such distress cues, potentially buffering the individual from being open to stress or distress. Antisocial behavior may result, however, as the individual becomes more unemotional and callous to others' distress cues, with hypo-arousal augmenting relative insensitivity to punishment or distress (van Honk, Schutter, Hermans, & Putman, 2003).
Second, Koob and colleagues (2001) posit an allostatic model for substance use risk (see also Piazza & Le Moal, 1996). Similar to the model for antisocial behavior, low cortisol shifts the balance toward insensitivity to punishment cues. It may also enhance reward sensitivity and heightened dependence on activation in reward- neurocircuitry (van Honk et al., 2003). Low cortisol may put individuals at risk for seeking such activation, in part to bring cortisol up to more optimal levels (Raine, 2002). Consistently, studies find that individuals at risk for drug abuse initiation or relapse have lower morning cortisol (Moss, Vanyukov, Yao, & Kirillova, 1999) and potentially blunted diurnal rhythms as well (Adinoff et al., 1991). Thereafter, acute intake of nearly all drugs of abuse stimulates cortisol reactivity (Lovallo, 2006). Initial cortisol reactivity to substance use helps return the HPA axis to basal levels through negative feedback, but also induces an acute allostatic state (Koob & Le Moal, 2008a, 2008b). Persistent use may lead to HPA dysregulation with basal cortisol becoming chronically low, despite continued use.
Third, the present study targets internalizing symptoms of depression, anxiety and somatization which have been theorized to operate in an allostatic framework (Korte, Koolhaas, Wingfield, & McEwen, 2005; McEwen, 2000a, 2000b). Burke and colleagues' (2005) meta-analysis suggests the diurnal rhythm may be especially calibrated to internalizing symptoms. Heightened HPA functioning renders an individual more open to their environment. This allows the individual to benefit from warmth, support, and structure (Ellis & Boyce, 2011; Roy, Steptoe, & Kirschbaum, 1998). In negative social contexts, however, this may leave the individual more vulnerable to a stressor's full impact (Ellis & Boyce, 2011), leading to vigilance, anxiety and depressive symptoms (Del Guidice et al., 2011).
Figure 1 posits a heuristic model based on a developmental allostasis framework that acknowledges the link between race and measures of stressors, including the unique stressors of racism and discrimination. Chronic stressors are anticipated to lead to allostatic changes, as reflected by shifting set-points for the primary allostatic mediators of cortisol level and its diurnal rhythm. In turn, altered HPA functioning is hypothesized to be associated with poor mental health outcomes, an early indicator of an adverse trajectory with more likely adverse outcomes in a still relatively young and healthy age group.
Figure 1.
Heuristic model linking stress and allostatic mediators to outcomes. Moderation by Race was evident at each level.
Methods
Sample
Participants were recruited from a longitudinal study which examined effectiveness of the Parents Who Care substance abuse prevention intervention for families (Haggerty, MacKenzie, Skinner, Harachi, & Catalano, 2006; Haggerty, Skinner, MacKenzie, & Catalano, 2007). Parents of eighth-grade students in the Seattle school district received a letter describing the study, and parents were contacted by phone. Families were included if the teen and one or both parents consented to participate. Eligibility included that the parent identified the teen as Black (or African American) or White (or European American), spoke English as their primary language, and planned to live in the area for at least 6 months. Forty-six percent of families who received letters consented (55% of Blacks and 40% of Whites). Parents who refused were more likely to be White, married, and had a higher education on average than those who consented. Other ethnic groups were not recruited.
The sample was balanced by teen race and gender. Racial differences were evident for several demographic measures. Whites reported higher per capita income and parental education; Blacks reported higher prevalence of single parenthood (Table 1). Most primary caregivers were female (> 80%), with 71.6% being the adolescent's biological mother. Caregiver gender and relationship were similar across race with one exception: more Black youth had another female primary caregiver (e.g., grandmother, aunt) than did White youth (χ2(1) = 13.95, p < .001).
Table 1. Descriptive Data on Demographics, Control, Stressor, and Mental Health Measures by Race.
| Measure | Blacks | Whites | Total Sample |
|---|---|---|---|
| Mean age of child | 13.66 yrs | 13.69 yrs | 13.67 yrs |
| Mean household members | 5.05 | 4.18** | 4.61 |
| % single parent | 57% | 24%** | 40% |
| Parent college graduate | 13% | 61%** | 38% |
| Mean per capita income | $7,807 | $21,970** | $15,042 |
| Sleep duration | 0.94 (1.08) | 0.82 (0.97)** | 0.87 (1.02) |
| Sleep disturbance | 12.51 (3.15) | 11.33 (2.36)** | 11.81 (2.77) |
| Latency to sleep | 0.71 (0.82) | 0.76 (0.81) | 0.74 (0.82) |
| Birth control hormones | 7.5% | 22.7%** | 15.7% |
| Days since start of last period | 16.91 (13.27) | 14.63 (9.66) | 15.51 (11.24) |
| Perceived racism | 2.64 (0.71) | 2.00 (0.52)** | 2.30 (0.70) |
| Perceived discrimination | 3.61 (1.15) | 3.13 (1.03)** | 3.36 (1.09) |
| Racial daily hassles | 2.35 (4.11) | 2.05 (3.02)* | 2.18 (3.54) |
| Financial strain | 46.8% | 27.3% | 36.3% |
| Problems over the past year | 1.60 (0.40) | 1.53 (0.38) | 1.56 (0.38) |
| Stressful life events | 5.39 (2.53) | 4.83 (1.95)* | 5.09 (2.21) |
| Neighborhood quality | 1.98 (0.79) | 1.71 (0.62)** | 1.86 (0.72) |
| Exposure to personal violence | 17.1% | 4.3% | 10.3% |
| Family conflict | 2.45 (0.66) | 2.14 (0.73)*** | 2.29 (0.72) |
| Antisocial frequency | 1.65 (2.71) | 1.75 (2.10) | 1.71 (2.40) |
| Antisocial self-description | 1.40 (0.55) | 1.45 (0.43) | 1.43 (0.50) |
| Smoking | 11.7% | 12.4% | 12.3% |
| Alcohol use | 6.8% | 12.1%** | 9.6% |
| Marijuana use | 14.9% | 10.9%*** | 12.1% |
| BSI | 1.40 (0.53) | 1.40 (0.55) | 1.39 (0.54) |
p<.05,
p<.01
At the follow-up (5 to 6 years after the intervention), we attempted to contact all 331 young adults who now ranged in age from 19 to 22 (mean 19.7 years). Of the original participants, 301 (90.1%) completed the surveys and were asked to participate in the saliva collection phase of the study. Of these, 67 were Black males, 73 Black females, 82 White males and 79 White females. Two participants were not eligible for saliva collection due to incarceration. Most participants were currently enrolled in school (57.8%), while 45.6% were employed and 18% were currently neither employed nor attending school regularly.
Collection Procedure
Participants completed a 50-minute survey on a laptop computer and were given instructions for collecting saliva and a collection kit filled with 12 vials, daily diaries, a card with saliva collection instructions, and freezer packs to ensure samples remained chilled until frozen. At least 1.5 mL of saliva was collected by passive drool. Saliva samples were collected four times each day over 3 days: (1) when the participant woke up, before they were out of bed; (2) 30 minutes after the initial sample before the participants had brushed their teeth or eaten any food; (3) at least 1 hour after lunch; (4) at bedtime, before brushing their teeth. Participants were urged to not eat, drink, exercise, or brush their teeth for 30 minutes before collection to avoid contaminating the sample. Like other steroid hormones (O'Connor et al., 2003), cortisol is stable when stored frozen, and is robust to freeze-thaw cycles (Barrett, Akinsanya, Chang, & Vesterqvis, 2005). Nevertheless, freeze-thaw cycles were strictly avoided. The saliva samples were refrigerated or frozen until picked up by study staff, then frozen at −80 °C until assay. Participants were asked to complete diary entries immediately after each collection in order to record the date and time of each sample and answer questions evaluating demographic, social, emotional and physiological factors. The participant was given $50 for completing the survey and compensated $5 in cash for each filled saliva vial, for a maximum of $60 for 12 vials.
Six data collectors trained participants on the research protocols and facilitated compliance (e.g., coordinating participants' reminder schedules, Fernades, Skinner, Woelfel, Carpenter, & Haggerty, under review). Type and timing of reminders were specific to each participant, depending on work/ school commitments and reminder preferences. Participants were contacted via phone call, direct text (between the data collector and participants), or autotexts (textmelater.com). All participants were contacted on their personal cell phone. If the participant did not have a cell phone, they were provided with a pre-paid cell phone.
Measures
Cortisol
An enzymatic immunoassay was used to measure cortisol using 80μL of saliva. The assay uses a purified polyclonal anti-cortisol antibody, R4866, provided by C. Munro (U.C. Davis) and reference calibrators (Steraloids, cat. no. Q3880). This assay has been validated for use in serum (Munro & Stabenfeldt, 1985), and saliva specimens (Tomblingson, 2005). Intra- and inter-assay coefficients of variation were 2.4% and 13.3%, respectively. Results were further validated by comparison with a commercial kit (Assay Designs, cat. no.900-071), r(55) = 0.71, p<.0001. Cortisol was measured in micrograms per deciliter, with a lower detection limit of .03 μg/dL. Natural log transformation was used to reduce skew to a normal distribution.
Stressor Exposure
Correlations between measures of stressors (see Table 2) are frequently significant, yet the magnitude of the correlations indicates that these stressors capture distinct constructs. Racism and Life Experiences Scales developed by Harrel et al. (2000 Harrel et al. (1997) assessed racism, discrimination across domains, and racial daily hassles. The author has provided extensive documentation of the scale's development, reliability, and psychometric properties. Perceived Racism is the mean of 9 survey items (Cronbach's alpha = .83) from the Harrell Racism Scale. Items include ‘Overall, during your lifetime, how much have you personally experienced racism, racial discrimination or racial prejudice?’ and ‘In general, how much stress has racism caused you during your lifetime?’ Response options ranged from 1 to 5; higher scores indicate more experience with racism.
Table 2. Correlations and P-values for Associations Between Measures of Stressors by Race (Blacks bottom half, Whites top half).
| Whites Blacks |
1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Perceived racism | 1 | .369** | .214* | .166* | .166* | .219** | .095 | .224** | .184* |
| 2. | Perceived discrimination | .445** | 1 | .261** | .277** | .484** | .181* | −.045 | .228** | .265** |
| 3. | Racial daily hassles | .142 | .118 | 1 | −.027 | .248** | .114 | .086 | .137 | .181* |
| 4. | Financial strain | −.056 | .051 | .280** | 1 | .379** | .337** | .076 | .321** | .278** |
| 5. | Problems over the past year | .190* | .184* | .330** | .424** | 1 | .374** | .127 | .285** | .425** |
| 6. | Stressful life events | .273** | .105 | .141 | .350** | .306** | 1 | .148 | .308** | .235** |
| 7. | Neighborhood quality | .223** | .096 | .095 | .232** | .356** | .325** | 1 | .157* | .059 |
| 8. | Exposure to personal violence | .212* | .080 | .226* | .294** | .389** | .290** | .252** | 1 | .187* |
| 9. | Family conflict | −.069 | .031 | .133 | .015 | .113 | .013 | .048 | .194* | 1 |
Perceived discrimination includes 10 survey items from the Harrell Discrimination Scale. The root for all items was ‘During your lifetime, how much have you experienced prejudice or discrimination based on each of these characteristics?’ Characteristics included gender, race, ethnicity, social class, talents (skills, intelligence), religion, physical appearance, sexual orientation, personality, and physical disability. Responses ranged from 1(extremely) to 5 (not at all) so that higher scores indicated greater extent of perceived discrimination. Scores reflected the maximum level reported regardless of the source identified.
The Harrell daily hassles scale was collected at the end of each saliva sampling day to capture mundane extreme environmental stress. The scale consists of 20 items assessing microaggressions, including ‘being ignored, overlooked, or not given service,’ and ‘others reacting to you as if they are afraid or intimidated.’ Participants report how often these occurred in their daily lives because of their race (0 = never, 5 = once a week or more), and how much these events bothered them (0 = never happened, 1 = doesn't bother me at all, 5 = bothers me extremely). The sum of hassles was calculated for each of 3 days then averaged across days.
Financial strain was measured with four survey items ‘Was there a time in the past 12 months when you did any of the following because you did not have enough money?’ Items included ‘Did not pay the full amount of the rent or mortgage on time,’ ‘Needed to see a doctor or go to the hospital but did not go,’ ‘Needed to see a dentist but did not go,’ and ‘Did not pay the full amount of the gas, oil, or electric bills.’ Scores were assigned 0 (did not endorse any of these) and 1 (endorsed one or more items) to form a dichotomous variable.
Problems in the past 12 months was the mean of 11 survey items (Cronbach's alpha = .81) where respondents rated problems in the past 12 months as 1 (not at all), 2(a little), and 3 (a lot). Problems included concerns about health, problems with friends, romantic partners, school, legal, family relationships, work or people at work, teams or organizations, moving house, loss of income, and big disappointments or failure.
Number of stressful events was based on the items endorsed from a list of 14 potentially stressful events (Gray, Litz, Hsu, & Lombardo, 2004), such as death of a parent, a close friend, serious illness, failing a grade, breaking up with a boy/girlfriend, natural disaster, combat, etc.
Neighborhood quality was based on five survey items ‘How much do these things describe the neighborhood you live in now?’ Items included crime or drug selling, fights, undesirable neighbors, people moving in and out, not safe. Responses range from 1 (a lot) to 4 (not at all). Items were reversed then averaged (Cronbach's alpha = .87). High scores indicated more distressed neighborhoods.
Violence victimization was based on six survey items to assess past-year violence exposure. Items included ‘In the past year have you been attacked?’ and ‘In the past year has there been any violence in your home?’ Scores were assigned from 0 (none of the items were endorsed) to 3 (3 or more items endorsed).
Family Conflict was computed as number of positive endorsements of 4 items taken from the Conflict Tactics Scale (Straus, 1990). The family behaviors included fighting a lot, criticizing each other, losing their temper, and being openly angry. Endorsements were made on a 4 point scale: YES, yes, no, NO. Higher scores indicated greater conflict levels.
Mental health outcomes
Frequency of antisocial behavior was computed as the sum of 12 items. Each item was scored for frequency of the behavior in the past year, with scores ranging from 0 (none) through 2(4 or more times). Items included starting fights, using a weapon or force to get something you want, driving recklessly, destroying others' property on purpose, etc. The item ‘Purposely setting fire to a building or car’ was dichotomously scored 0 (none) or 2(at all in the past year). Antisocial self-description was computed as the mean of 5 items. The statements included ‘I ignore rules that get in my way’, and ‘I tell a lot of lies.’ Responses for whether the statement described them were coded on a 4 point scale of YES, yes, no, NO. Higher scores indicated more antisocial attribute endorsement.
Substance use was recorded on all three days for the daily diary at the time of each saliva sample. Scores for smoking, alcohol use, and marijuana use were set to 0 if none reported and 1 if any reported; individual scores across the 12 saliva samples were then averaged.
Measures of mental health included the 18-item Brief Symptom Inventory (BSI-18). The BSI is a global severity measure of depression, anxiety, and somatization on a scale from 1 (not at all distressed) to 5 (extremely distressed) (Derogatis, 1993).
Control variables
Control variables included gender, family income during adolescence, and sleep quality. Income of the family at baseline (eighth grade) was used as an indicator of resources availability while growing up. This prospective measure of income may be a more stable indicator of lifetime SES than concurrent income, which transiently and precipitously declines in emerging adults. Moreover, controlling for income partially rules out influence of stressor exposure stemming from earlier poverty or resource availability. Household per capita income was calculated from parent's endorsement of 1 of 11 categories for annual household income (before taxes). We assigned the midpoint of the range and then divided by the number of people in the household as reported by parents on the survey.
The Pittsburgh Sleep Quality Index (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) was collected on each saliva day and used to create three scales. Sleep duration scores are based on answers to ‘During the past week, how many hours of actual sleep did you get each night?’ Scores range from 0 (7 or more hours) to 3(less than 5 hours). High scores indicate short sleep duration. Disturbance is the sum of nine items, such as ‘Had bad dreams,’ and ‘Coughed or snored loudly,’ with response options 1 (not during the past week), 2 (once or twice a week), and 3 (three or more times a week). The sum ranges from 6 to 22 (mean = 11.8, sd = 2.77). High scores indicate frequently disturbed sleep. Latency is the sum of two items, ‘Couldn’t get to sleep within 30 minutes' and ‘During the past week, how long in minutes has it usually taken you to fall asleep each night?’ The sum was then recoded into three categories. High scores indicate taking longer to get to sleep.
Analyses
Descriptive data are presented on demographics, sleep quality, and exposure to stressors by race in Table 1. Hierarchical linear models (HLM) estimated the trajectory of cortisol across the day using SAS PROC MIXED (Singer, 1998). Repeated cortisol measures (max = 12) are nested within day. The two levels of the model were estimated using a random effect for intercept (to capture cortisol upon awakening) and Time Since Waking (TSW, to capture the linear diurnal slope). The cortisol awakening response (CAR) was modeled using a fixed effect for a dummy variable coded 0/1 for samples collected between 25 and 55 minutes after waking. Cross tabulation with tube number verified that 94.3% of these observations were the designated second tube of the day. Similarly, over 90% of the first tubes of the day were collected prior to 25 min of waking. We also tested for a within person random effect of DAY and DAY*TSW, meaning intercepts and slopes were estimated separately for each day. The DAY variables were dropped after no effects for DAY were found, consistent with Shirtcliff & Essex (2008).
RACE was entered as a main effect on cortisol level, an interaction with CAR, and an interaction with TSW. Post hoc analyses included single degree of freedom significance tests of race differences in point estimates at waking, 30 minutes, 8 hours, and 16 hours after waking. Models were estimated for women separately and indicated neither use of a hormone-based birth control method (pill, nuvo ring, etc.) nor menstrual cycle phase influenced cortisol level or slope. These potential control measures were nonsignificant and dropped from further models.
A base model was estimated including intercept, race, gender, CAR, TSW, income and sleep duration, disturbance and latency, and the 2-way interactions of race with gender, CAR and TSW (see Table 3). This model indicated no significant effects for sleep disturbance or latency so they were dropped from further analyses. Separate HLMs then added a main effect for each of the eight measures of stressors which indicated the impact of that stressor on waking levels (on the intercept). Successive analyses then examined the effect of each stressor on the waking response (stressor × CAR) and on linear decline (stressor × TSW). Next, forward selection procedures tested for the interaction of race with each stressor to determine whether the stressor was associated with waking levels differently for Whites than Blacks. To assess possibly non-linear associations between stressor exposure and waking cortisol, a stressor-squared term was added. Non-linear race moderation was assessed by adding the interaction between race and stressor-squared. Finally, models were estimated including the 3-way interactions (CAR × race × stressor and TSW × race × stressor) to assess whether stressors were associated with the cortisol awakening response or linear decline over the day differently for Whites and Blacks. A parallel strategy was employed for mental health outcomes.
Table 3.
Parameter Estimates and Significance Levels for Models Predicting Cortisol.
| Effect | Base Model, Including Control Variables | Models Including Measures of Stressors |
|---|---|---|
| A. Within-individual difference measures | ||
| 1. Intercept (waking cortisol levels) | −0.89 | −1.03 |
| 2. Time Since Waking (TSW, Diurnal Rhythm) | −0.04*** | −0.05*** |
| 3. Cortisol Awakening Response (CAR) | 0.29*** | 0.3*** |
| B. Between-individual difference measures of prime interest | ||
| 4. Race | 0.12*** | 0.08* |
| 5. Gender | −0.06 | −0.05 |
| 6. Gender*Race | −0.10 | −0.10 |
| C.Cross-level interactions between cortisol measures and between-individual difference measures of prime interest | ||
| 7. Race * CAR | −0.01 | 0.0 |
| 8. Gender *CAR | −0.09 | −0.01 |
| 9. Diurnal Rhythm*Race | −0.03*** | −0.03*** |
| 10. Diurnal Rhythm* Gender | 0.01 | 0.001 |
| D. Inclusion of control variablesa | ||
| 11. Income | −0.01* | −0.01* |
| 12. Income*Race | 0.01 | 0.01 |
| 13. Sleep duration | 0.21** | 0.18** |
| 0.20** | 0.18** | |
| 0.24** | 0.25** | |
| 14. Sleep disturbance | 0.00 | |
| 15. Sleep latency | −0.17 | |
| −0.22 | ||
| −0.18 | ||
NOTE:
p< .05
p< .01
p< .001.
sleep disturbance and latency were entered a priori into the model, but were removed as they were not significant and were not of primary interest.
Results
Were There Race Differences in Stressor Exposure?
Race differences were significant for demographic variables and most control variables and stressors, consistent with typical race differences in the U.S (See Table 1). A higher rate of poverty among Blacks was indicated by large family size, more single-parent households, and lower parent education and per capita income compared to Whites. Blacks reported sleeping longer, but having more disturbed sleep. There wasn't a race difference in sleep latency.
Blacks reported significantly more racism, discrimination, and racial daily hassles. Blacks reported significantly more stressful life events, greater financial strain, higher family conflict and poorer quality neighborhoods. Race differences in exposure to violence were especially notable in the top and bottom category, indicating that Blacks typically report high exposure to violence whereas Whites typically report no exposure. There was no race difference in reports of problems in the past 12 months. Blacks reported lower alcohol use, and higher marijuana use than Whites.
Capturing Cortisol's Diurnal Rhythm with the Hierarchical Model
Time since waking (TSW) represents a significant linear slope from waking until bedtime (β = −.06, t = −16.38, p < .001), indicating that cortisol levels dropped across the day (See Table 3). The dummy variable for CAR was also significant and positive (β = .25, t = 7.09, p < .001), indicating cortisol rose significantly during the period from 25 to 55 minutes after waking. A fixed effect for TSW squared was tested but not significant and was thereafter dropped.
Race and Gender Differences in Cortisol Level and Rhythm
Waking cortisol levels
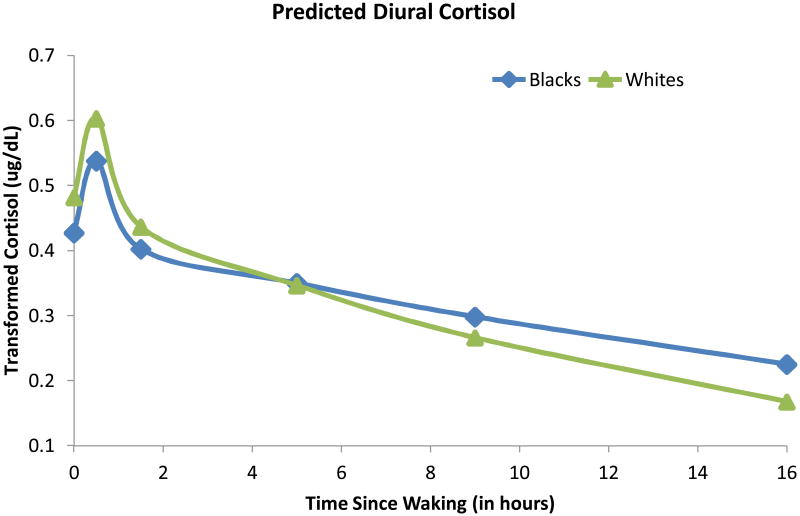
Figure 2 presents the predicted diurnal patterns for Blacks and Whites. There were significant race differences (β = .09, t = 2.55, p = .01), indicating higher cortisol levels at waking for Whites compared to Blacks. There were no gender effects or race by gender interactions predicting waking cortisol levels.
Figure 2.
Race differences in diurnal cortisol rhythm indicate lower waking and higher evening levels for Blacks compared to Whites.
CAR
We then determined if the rise in cortisol after awakening (CAR) showed race or gender differences by examining interactions with the CAR dummy variable. These effects were not significant.
Diurnal rhythm
We examined race and gender differences in cortisol's rate of decline over the day by examining interactions with TSW. Cortisol's diurnal decline was slower (flatter slope) for Blacks than Whites (β = .03, t = 3.97, p < .001). Post hoc tests of race differences at 0, 30 minutes, 8 hours and 16 hours after waking indicated that, compared to Whites, Blacks had lower cortisol at waking (Δ = .12, df = 1, 471, t = 3.09, p = .002), were not different by the mid-afternoon (Δ = −.08, df = 1, 471, t = −1.32, p = .19), but then emerged with significantly higher cortisol at bedtime (Δ = −.29, df = 1, 471, t = −2.56, p = .01).
Income, Gender and Sleep Quality Effects on Cortisol
The predictors included the main effects and interactions in the base model identified above (see also Table 3). There was a significant effect of family income (when participants were 8th graders); individuals with less money as adolescents had higher cortisol by young adulthood. The effect of income on cortisol did not interact with race; nevertheless, both income and income × race were included in subsequent analyses because the interaction controls for possible nonlinear income effects (increases in income are more meaningful when income is low and less meaningful when income is high). The effects of gender or race × gender on the linear slope were not significant; Black males and females both appeared to have flatter diurnal slopes than White males and females. Nevertheless, gender effects were controlled in all analyses. Short sleep duration was associated with higher waking cortisol, so it was controlled in all analyses.
Effects of Stressor Exposure on Cortisol Level and Possible Race Moderation
Of the nine measures of stressor exposure, five were significantly associated with waking cortisol levels directly or moderated by race: daily racial hassles, high family conflict, financial strain, distressed neighborhood, and exposure to personal violence (see Table 4). Main effects were found for racial daily hassles and financial strain, indicating higher waking cortisol associated with both stressors for Blacks and Whites.
Table 4.
Relationships or stressors and mental health outcomes to cortisol waking levels and change over the day (linear slope) by race.
| EffectA | Blacks | Whites | |||
|---|---|---|---|---|---|
| A. Stressors | Waking Level | Linear Slope | Waking Level | Linear Slope | |
| 1. Perceived racism | 0.002 | 0.003 | −0.10 | −0.001 | |
| 2. Perceived discrimination | −0.04 | 0.010* | −0.01 | 0.010* | |
| 3. Racial daily hassles | 0.01* | 0.001 | 0.01* | 0.001 | |
| 4. Financial strain | 0.11** | 0.004 | 0.11** | 0.002 | |
| 5. Problems over the past year | −0.02 | −0.01 | −0.08 | 0.020 | |
| 6. Stressful life eventsB | 0.01 | −0.03 | −0.02 | .005* | |
| 7. Distressed neighborhoodB | 0.01 | −0.01 | −0.14*** | 0.003 | |
| 8. High exposure to person violence | Low | 0.20* | −0.040** | −0.19*** | 0.040 |
| Medium | 0.09 | −0.023 | - | 0.030 | |
| High | - | - | −0.08 | - | |
| 9. High family conflict | 0.02 | −0.008 | −0.14*** | 0.007 | |
| B. Mental Health Outcomes | Waking Level | Linear Slope | Waking Level | Linear Slope | |
| 10. Frequency of Antisocial BehaviorB | .003 | −.002 | −.04*** | .004 | |
| 11. Antisocial self-descriptionB | .020 | .02* | −.30*** | .020* | |
| 12. Cigarette smoking | 0.05 | −.02 | −0.16 | .030** | |
| 13. Alcohol use | −.12* | 0.02 | −.12* | 0.010 | |
| 14. Marijuana use | 0.11 | 0.0001 | −0.10 | 0.020 | |
| 15. Brief Symptom Inventory | 0.03 | 0.005 | 0.06 | 0.002 | |
NOTE:
p< .05
p< .01
p< .001.
each stressor measure and its interactions and mental health outcome were added to the base model in separate analyses. They are shown in single columns for brevity. Parameters for predictors in the base model vary only slightly depending on which stressor is included.
Only linear effects are illustrated. Quadratic effects or quadratic interactions with Race were later found.
The interaction of each stressor with Race then determined if stressors influence waking cortisol more for Blacks or Whites. Three race interactions were significant. The relationship between family conflict and waking cortisol was moderated by race (F = 9.38, df = 1,1621, p = 0.002). In separate follow-up models for Blacks and Whites, we found no significant relationship for Blacks, but lower waking levels were significantly associated with higher conflict for Whites.
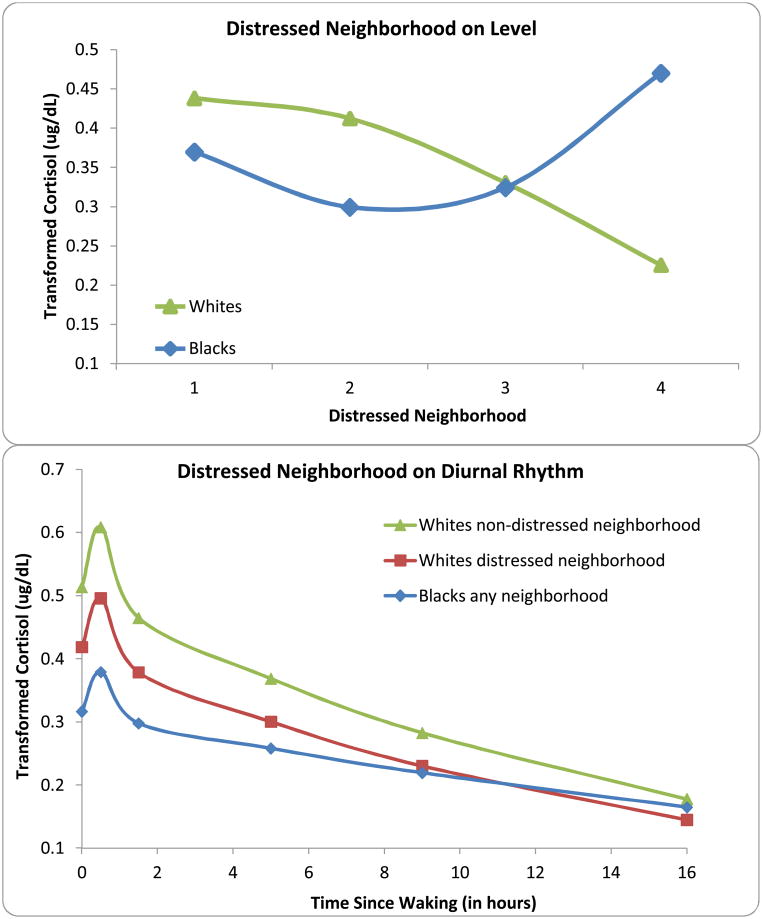
The relationship between distressed neighborhood and waking cortisol was significantly different for Blacks and Whites (F = 7.93, df = 1,1740, p = .005). Distressed neighborhood was associated significantly with lower waking cortisol for Whites (β = − .14, p = .0003), but not associated significantly for Blacks (β = .01, p = .75). Exposure to distressed neighborhoods resulted in diurnal cortisol for Whites that more closely resembled Blacks (Figure 3).
Figure 3.
A. Effect of distressed neighborhood on Cortisol Levels indicated more distressed neighborhood was associated with lower cortisol within Whites, but had a nonlinear curve within Blacks, so that the highest cortisol was observed in Blacks from distressed neighborhoods. B. Distressed Neighborhood further impacted the diurnal rhythm, but only in Whites.
There was also a significant interaction between race and violence victimization on waking cortisol levels (F = 10.89, df = 2,1651, p< .0001). Separate models were run for Blacks and Whites. Compared to Whites exposed to no violence, there was a statistical trend that waking cortisol was lower for Whites exposed to high violence (β = −.19, p = .09). For Blacks, the association was quite different. Compared to Blacks with no exposure, Blacks with high exposure to personal violence had higher waking cortisol levels (β = .20, p = .03).
Given our overarching model and preliminary findings (Del Guidice et al., 2011), we tested for non-linear relationships between stressor exposure and waking cortisol levels by adding an effect for each stressor-squared in addition to linear stressor effect. We added the interaction between stressor-squared and race because non-linear relationships might be moderated by race. These tests are only appropriate for continuously measured variables so no such tests were conducted for financial strain or exposure to personal violence. There was no evidence for curvilinear effects for racism, discrimination, racial hassles, problems in the past year, or family conflict. Significant curvilinear effects were found for stressful life events (F=8.96, df=1, 1809, p=.003) and the interaction between distressed neighborhood and race (F=12.65, df=1, 1599, p=.0004). Compared to individuals with no stressful life events, cortisol levels were lower as individuals experienced greater number of events; as the number of events increased beyond moderate stressor exposure, however, individuals with the greatest number of events had higher cortisol levels. This pattern was comparable for Blacks and Whites. In the case of distressed neighborhood the patterns differed by race, such that the neighborhood-squared effect was significant only for Blacks (F=13.82, df=1, 596, p=.0002). For Whites, the linear effect persisted such that lower waking cortisol was associated with increasingly distressed neighborhoods. For Blacks waking cortisol was somewhat lower in slightly distressed neighborhoods compared to nondistressed neighborhoods; yet in more and more distressed neighborhoods, waking levels were higher.
Effects of Stressor Exposure on Cortisol Awakening Response
No significant effects or interactions with race were found for any of the stressors on CAR.
Effects of Stressor Exposure on Cortisol's Diurnal Rhythm
Models then determined which of the measures of stressors were associated with the rate of decline in cortisol over the day. Each model included the main effect of the stressor on cortisol levels in addition to the interaction between that stressor and TSW; all other control measures identified in Table 3 were likewise included. HLM models estimated the interaction between each stressor and race on TSW to assess race differences in the impact of stressors on the rate of decline in cortisol over the day.
First, regardless of race, higher levels of discrimination were associated with flatter diurnal patterns (β = 0.01, p = .02) (see Table 4). There was also a significant three-way interaction between stressful life events, race and TSW (F = 5.57, df = 1,225, p = 0.02), indicating that the impact of stressful life events on cortisol's diurnal rhythm was different for Whites than Blacks. Subsequent analyses examined the interaction by estimating separate models for Blacks and Whites. Greater lifetime exposure to stressful life events flattened cortisol's diurnal rhythm for Whites (β = .005, p = .047), in addition to the quadratic effects of stressful life events on cortisol's waking level. For Blacks, the impact of stressor exposure on cortisol's diurnal rhythm was not significant (β = − .003, p = .19), though the quadratic-stressor effects on cortisol waking level (described above) persisted.
Finally, a significant three-way interaction was detected for exposure to personal violence (F = 5.45, df = 2, 258, p = 0.005). Follow-up models for each race separately indicated that the effects of personal violence for Whites were specific to waking levels (described above), but for Blacks, the effects of personal violence were associated with the cortisol slope (F = 4.33, df = 2, 176, p = 0.01). While the effects of personal violence on White young adults made them appear indistinguishable from Black young adults in the morning, by the afternoon Blacks nevertheless still had higher cortisol levels as a function of their flatter diurnal rhythms. For Blacks exposed to high levels of personal violence, the impact of race was exacerbated by exposure to personal violence. Not only did they have higher morning cortisol levels than any other group, but their diurnal rhythm was even flatter, so that they ended the day with the highest cortisol levels.
Three-way interactions for the other measures of stressor were not detected.
Relationship between HPA functioning and mental health outcomes
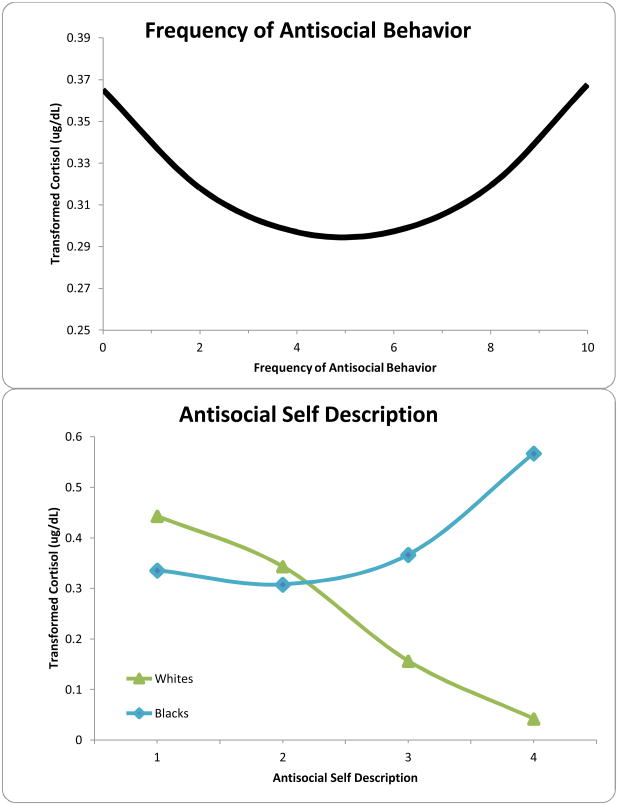
Similar analyses were conducted to assess the relationships between mental health outcomes and cortisol diurnal rhythm (Table 4). We found significant race moderation in the relationship between waking levels and reports of antisocial behavior frequency (F=7.57, df= 1, 1589, p=.006) and self-description (F=18.14, df= 1, 1710, p< .0001). In both cases, the relationship was not significant for Blacks, but greater antisocial behavior was associated with lower waking cortisol for Whites. Additionally, antisocial self-description was significantly associated with blunted cortisol decline over the day for both Blacks and Whites. Similarly, race moderation was indicated for antisocial behavior frequency, but subsequent analyses for each race group detected no significant relationships.
A significant main effect was found for alcohol use such that use was associated with lower waking cortisol for Blacks and Whites. The relationship between cortisol and smoking was moderated by race (F=9.27, df=1, 1696, p=.002) such that smoking was associated with blunted cortisol decline over the day for Whites, but was not significant for Blacks. No relationships were evident between BSI and cortisol waking level, CAR or rate of decline over the day.
Discussion
The present study used the conceptual framework of allostasis to examine whether stressor exposure can “get under the skin” and impact functioning of one of the primary neuroendocrine pathways, the HPA axis. Following the view that allostatic load has behavioral and physiological consequences, we then determined the implications of HPA dysregulation for mental health outcomes evident in young adults. Four findings were notable. First, consistent with previous research (Cohen et al., 2006; DeSantis et al., 2007), we found Blacks had lower morning cortisol and flatter diurnal rhythms, ending the day with higher cortisol than Whites. Second, associations between stressor exposure and HPA functioning were complex, but followed a pattern proposed in recent developmental models (Boyce & Ellis, 2005; Del Guidice et al., 2011). Stressor effects on HPA functioning were typically weaker for Blacks than Whites. Despite strong measures of stressors, the effect of race on HPA functioning persisted. Third, there was some evidence for interactions with race, perhaps suggesting that the underlying allostatic mechanism of how stress can “get under the skin” may operate differently in Blacks than Whites. Fourth, consistent with our prior research (Essex et al., 2012; Ruttle et al., 2011; Shirtcliff & Essex, 2008; Shirtcliff, Granger, Booth, & Johnson, 2005) and our heuristic model, blunted HPA functioning was associated with poorer mental health outcomes relevant to this particular age group (antisocial behavior, substance use) (Schulenberg et al., 2004). These findings could lead us to conclude that Black young adults exhibited less HPA flexibility than their White counterparts which may contribute, over time, to greater risk for allostatic load as the persistent weathering of HPA dysregulation takes its toll (Borrell & Crawford, 2011; Geronimus et al., 2006; Szanton et al., 2005; Taylor et al., 1997). That race differences were already observed within a relatively healthy age range is, unfortunately, consistent with prior literature (Evans & English, 2002; Johnston-Brooks et al., 1998).
Did Stressor Exposure Explain Race-Related Differences in HPA Functioning?
In a simplistic manner, stressor exposure did not account for race differences in waking cortisol or diurnal rhythm. Findings appear consistent with studies where race differences are persistent (Cohen et al., 2006). Within White young adults, greater stressor exposure typically blunted HPA functioning to the extent that Whites with substantial exposure to stressors appeared similar to typical Black young adults. Traditionally, greater stressor exposure has been linked with higher cortisol (Sapolsky, 1998; Selye, 1950), yet several theories have begun to recognize the paradoxical finding that low cortisol (especially low morning cortisol) may be a common outcome of chronic stressor exposure (Cicchetti & Rogosch, 2001; Gunnar & Vazquez, 2001; Yehuda et al., 2000). The acute rise in stress-related HPA activity, over time, diminishes until basal functioning falls below earlier set-points and flexibility is compromised (Miller et al., 2007; Weems & Carrion, 2009). While this may appear as stress dysregulation, an allostatic view emphasizes that such alterations may be adaptations to the individual's environment. Low cortisol may help the individual to be buffered or shielded from the impact of chronic stressors.
Del Giudice and colleagues (2011) build upon the foundation of differential susceptibility (Belsky & Pluess, 2009) and biological sensitivity to context (Boyce & Ellis, 2005; Ellis & Boyce, 2011; Ellis, Essex, & Boyce, 2005). Such adaptive calibration models may be necessary to account for complex and potentially nonlinear stressor-HPA associations (Cicchetti & Rogosch, 2007). There are wide differences in an individual's biological sensitivity to context (BSC) in addition to individual differences in stressor exposure. Biological systems that change across time and development are proposed to filter, encode and amplify salient information about the individual's environment, thereby coordinating or calibrating stress responsive systems to the demands of the environment and moderating the individual's openness to environmental inputs. Notably, Del Guidice and colleagues (2011) point to allostatic mediators as being the primary systems implicated. It may be adaptive for individuals with a high BSC to be open to the benefits of a warm, supportive and protected environment. Likewise, it may be adaptive for individuals with a high BSC to be open to harsh, dangerous or unpredictable environments, allowing them to attend to the environment closely to anticipate and respond early to challenges. Individuals with a low BSC, on the other hand, do not experience extant physiological arousal. Rather, they appear buffered or protected from repeatedly responding to moderate stressors and maintain enough sensitivity to remain responsive to salient social information (Lyons & Parker, 2007; Parker, Buckmaster, Schatzberg, & Lyons, 2004). Some environments are even more toxic (Cicchetti & Lynch, 1995). Such extremely challenging conditions may be powerful enough to shut down physiological responsiveness in order to shield the individual from their stressful lives. In such extreme instances, the individual may become unemotional, callous, or nonresponsive to social cues (Hawes et al., 2009; Shirtcliff et al., 2009).
Applying this perspective, we observed considerable within-race variability in exposure to stressors, yet Blacks on average reported more short-term and chronic exposure to a variety of stressors. This is consistent with prior literature showing Blacks typically experience greater “mundane environmental stress” (Peters & Massey, 1983). Within our study, it appears as though the stress response systems for Whites was typically calibrated by milder environmental stressors so that their cortisol levels largely ranged from “sensitive” to “buffered”, as observed in other largely White samples (Ellis et al., 2005). Conversely, it appears as though the stress response systems for Blacks was typically calibrated to a higher stressor burden such that cortisol levels largely ranged from “buffered” to “vigilant.” Del Guidice and colleagues (2011) postulate that most individuals are “buffered,” or exposed to moderate stressors. The greater frequency of null findings in Blacks may reflect that, typically, Black young adults experience the concomitant calibration of HPA functioning of being buffered.
Our interpretation of race interactions, therefore, is not that underlying psychobiology is disparate in Blacks and Whites. Rather, we contend there is an overlapping yet different range of stressors typically experienced by Blacks and Whites, but underlying psychobiology is similar. Exposure to dangerous, unpredictable environments contributes to allostatic load regardless of race (Geronimus et al., 2006). Understanding the full continuum of environmental experiences, from normal to abnormal, is an important tenet of developmental psychopathology (Cicchetti & Toth, 2009) as well as allostasis which emphasizes how regulatory processes are often nonlinear. Such developmental views may be more fruitful than merely illustrating race differences.
Depending on the range of stressors and the BSC profiles of the population, stress-HPA associations are expected to be three-fold (i.e., linear decline; flat; and linear inclines, Obradovic, Bush, & Boyce, 2011; Obradovic, Bush, Stamperdahl, Adler, & Boyce, 2010). If the measure of stressor is broad, it may be possible to detect more than one section of this curve, thereby strengthening evidence for adaptive calibration (Ellis et al., 2005). Although not apparent for each stressor (stressor-specific effects are predicted, Obradovic & Boyce, 2009), two measures of stressors revealed the anticipated curvilinear association. First, individuals who had low and extremely high numbers of stressful life events by young adulthood had the highest cortisol levels, whereas individuals with a more moderate number of stressful events had lower cortisol. This U-shaped curve was apparent for both Whites and Blacks. Second, cortisol levels were lower as White young adults were exposed to more distressed neighborhoods; for Black young adults, however, there was a J-shaped curve such that individuals with moderately distressed neighborhoods showed lower cortisol but individuals from the most distressed neighborhoods had the highest cortisol levels. These associations were admittedly complex, but followed the predicted pattern of individual differences in both biological sensitivity and stressor exposure.
One concern with this application of the model is that allostasis may be more relevant for stress reactivity as observed in acute laboratory challenges instead of the diurnal rhythm (Dickerson & Kemeny, 2004; Het, Rohleder, Schoofs, Kirschbaum, & Wolf, 2009; Lam, Dickerson, Zoccola, & Zaldivar, 2009). Nevertheless, responsivity is best interpreted with reference to that specific context (Gunnar, Talge, & Herrera, 2009). We do not know how relevant or ecologically-valid such contexts are with reference to more persistent or chronic stressors (van Eck, Nicolson, Berkhof, & Sulon, 1996), nor whether such contexts are similarly salient for Whites and Blacks (Richman & Jonassaint, 2008). Cortisol's diurnal rhythm is not a typical stress reactivity measure. Yet, because momentary stress responsivity quickly habituates and the diurnal rhythm is highly stable, it may shed light on the long-term physiological resources during persistent, ecological stressors. The rhythm partially reflects the accumulation of environmental stressors, with afternoon and evening levels being highly influenced by the social environment (Bartels et al., 2003; Schreiber et al., 2006; Van Hulle et al., under review) including naturally occurring hassles (Adam, Hawkley, Kudielka, & Cacioppo, 2006; Almeida, McGonagle, & King, 2009; Peeters, Nicholson, & Berkhof, 2003). Cortisol's diurnal rhythm may therefore instantiate long-term alterations in stress responsivity. Flatter diurnal rhythms may indicate stress dysregulation, as the individual struggles to match their physiological set-points with the demands of the environment, resulting in a less flexible diurnal rhythm. Yet, flatter diurnal rhythms may also reflect adaptive calibration of the individual's physiology to the level of challenge in their environment, suggestive of a buffering effect in which a flattened rhythm nonetheless maintains some physiological rhythmicity. This buffering may come at a cost, with the trade-off of maintaining rhythmicity being a less flexible or malleable HPA axis.
Does HPA functioning have implications for relevant mental health outcomes in young adults?
We anticipated race-related differences in allostatic load, but were agnostic as to whether Blacks would be more likely to show associations of HPA functioning with mental health outcomes. On the one hand, once stress physiology moves toward allostatic load, vulnerabilities to stress-related pathology, including psychopathology, should be anticipated regardless of race. On the other hand, the extant literature finds greater allostatic load within Black adults (Green & Darity, 2010; Szanton et al., 2005), hinting that these processes may be stronger in Blacks than Whites. For the present study of young adults, we did not observe greater mental health problems in Blacks. Yet, we did replicate that Blacks experienced greater stress exposure, consistent with other investigations in which Blacks report equal or lesser levels of psychological distress or psychiatric disorders than Whites (Kessler, Berglund, Demler, Jin, & Walters, 2005). This may fit with a process called equifinality (Cicchetti & Lynch, 1995; Cicchetti & Rogosch, 1999), in which there are multiple pathways to the same outcomes.
Associations of HPA functioning with mental health outcomes were typically stronger for Whites than Blacks. This may suggest that the process of stressor exposure “getting under the skin” shows race-related differences. Alternatively, it may indicate that the shift of BSC between “sensitive” to “buffered” is highly calibrated to mental health outcomes, whereas that calibration is not tight within the wide range of “buffered” individuals. Individuals with a high BSC with very little stressor exposure are theorized to have better than average mental health (i.e., for better and for worse, Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2011; Knafo, Israel, & Ebstein, 2011). There is some empirical support that high cortisol can culminate in better outcomes than expected (Essex, Armstrong, Burk, Goldsmith, & Boyce, 2011; Cicchetti & Rogosch, 2007; Gunnar, Tout, de Haan, Pierce, & Stansbury, 1997; Obradovic et al., 2011; Sethre-Hofstad, Stansbury, & Rice, 2002). These protective benefits may deteriorate quickly toward more typical levels as individuals become more hardy. Hardiness may be more typical within Blacks (Williams & Lawler, 2001). This emphasizes that higher or lower responsivity of allostatic mediators is not inherently good, but rather indicates a process of adaptive physiological calibration so that the individual can best meet the demands of their environment. A wide variety of physiological profiles can thus be viewed as “best” or “best for the situation”.
As expected, we found that lower cortisol was associated with antisocial behavior, at least within Whites. This fits with a broad literature (Alink et al., 2008; Loney et al., 2006; O'Leary et al., 2007; Oosterlaan, Geurts, Knol, & Sergeant, 2005; Shirtcliff & Essex, 2008; Shirtcliff et al., 2005; van Goozen et al., 1998) and our neurobiological model (Shirtcliff et al., 2009). Moreover, for both White and Black young adults, flatter diurnal rhythms were associated with antisocial behavior, such that low morning cortisol was most clearly linked with antisocial behavior (Popma et al., 2007; Ruttle et al., 2011). Prior trade-offs of flexibility for buffering, as indicated by flattened diurnal rhythms, may render individuals more susceptible to stress-related outcomes. This allostatic process may become deleterious when the physiological adjustments themselves exert problems (Merritt, McCallum, & Fritsch, 2011), or when the environment shifts and the individual's set-points appear mismatched for their present context. Low cortisol, for example, may shift the balance toward punishment insensitivity and reward dependency (van Honk et al., 2003), buffering the individual from learning from correction or discipline.
We found some evidence for effects of HPA functioning on substance use. Within Whites, blunted diurnal rhythms were associated with smoking; for both Blacks and Whites, lower morning cortisol was associated with alcohol use. Lower cortisol and flatter diurnal rhythms were expected to indicate greater allostatic burden and, in turn, be associated with greater substance use (Koob & Le Moal, 2001; Koob & Le Moal, 2008a, 2008b). Koob's allostasis model is not specific to substances (Lovallo, 2006), nor is it clear why diurnal dysregulation would be specific to smoking whereas alcohol would impact morning cortisol. Prior studies have found that higher cortisol is associated with nicotine withdrawal (Granger et al., 2007; Hogle & Curtin, 2006; Ussher et al., 2006); the blunted diurnal rhythm may indicate a growing accumulation of withdrawal or number of use/withdrawal cycles in cigarette smokers by the afternoon/evening hours. Morning cortisol levels, on the other hand, does not likely reflect acute alcohol intake. Instead, low cortisol may indicate a general risk for initiation of alcohol use (Adinoff, Iranmanesh, Veldhuis, & Fisher, 1998; Adinoff, Junghanns, Kiefer, & Krishnan-Sarin, 2005; Croissant & Olbrich, 2004; Moss, Vanyukov, & Martin, 1995). Finally, we found no evidence for HPA functioning in relation to internalizing as measured by the BSI, which is inconsistent with the allostasis theory (McEwen, 2000a, 2000b). We remain cautious about interpretation as a different range of internalizing symptoms may be necessary to observe effects.
Implications for Intervention and Prevention Efforts
Little research has been conducted on the impact of psychosocial intervention on HPA regulation (Dozier et al., 2006; Fisher, Gunnar, Chamberlain, & Reid, 2000; Fisher, Stoolmiller, Gunnar, & Burraston, 2007; Fisher, Van Ryzin, & Gunnar, 2011; Luecken, Hagan, Sandler, Tien, Ayers, & Wolchik, 2009). The extant studies are promising as they make the implications for preventive efforts tangible. Based in part on our own interventions, we know that family-focused preventive interventions that promote the buffering effects of positive parent/child bonding, anger management, predictability and safety within the home reduce future mental health problems (e.g., drug use, antisocial behavior, etc.) (Brody, Kogan, Chen, & Murry, 2008; Haggerty et al., 2007; Hawkins, Kosterman, Catalano, Hill, & Abbott, 2005). The targeted family practices should impact life-history relevant experiences which are salient for establishing set-points for allostatic mediators (Del Guidice et al., 2011). These successful interventions thus may work in part because they facilitate HPA regulation through three underlying mechanisms. First, interventions may reduce stressor exposure within the family, thereby reducing chronic wear-and-tear. Such intervention outcomes are frequently small in magnitude, yet their slow and consistent accumulation over time may result in the largest impact on allostatic load (Fisher et al., 2011; Luecken et al, 2009); large intervention effects may, unfortunately, induce an allostatic state to which the individual's physiology works against to re-establish homeostasis. Small, consistent HPA alterations may be more permanent. Second, prevention and intervention efforts may provide the individual with useful skills for navigating an inherently stressful world (Taylor et al., 2008; Cicchetti & Rogosch, 2009). In turn, these skills could serve to reduce the further impact of stressful experiences. Communication skills and anger management may be particularly useful in reducing the physiological impact of stressor exposure (Antoni et al., 2000; Moons, Eisenberger, & Taylor, 2009). Finally, interventions may change the individual's social support or openness to receiving social support (Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003; Kirschbaum, Klauer, Filipp, & Hellhammer, 1995; Roy et al., 1998).
Regarding the present study, we illustrate that the HPA axis is calibrated by some stressors more than others; and that the direction of calibration may be nonlinear. Rather than attempt to “reduce cortisol”, intervention studies should consider using an allostatic framework to interpret potentially paradoxical findings; high cortisol may indicate that an individual is open to prevention efforts (Cicchetti & Rogosch, 2009; Luecken et al, 2009). The present study does not suggest that White or Black young adults should be more targeted for intervention efforts. Rather, prevention efforts should primarily consider the individual's underlying level of stressor exposure. At one end of the stressor-continuum, encouraging youth to be open, empathic, attentive and emotionally expressive may be beneficial, whereas those same outcomes may not be as beneficial in persistently stressful environments.
Limitations
Several limitations must be acknowledged. First, we did not have earlier measures of HPA functioning or parallel measures of stressors which would permit examination of developmental allostatic processes (Cicchetti & Toth, 2009). Controlling prospectively for income (as a proxy for socioeconomic status) points toward findings being specific to the emergence of allostatic load beyond earlier adversity. Second, this study focused on stressors without examining protective or positive life events which could shield the individual (Heinrichs et al., 2003; Kirschbaum et al., 1995; Roy et al., 1998; Taylor et al., 1997). Preliminary glances showed that Whites exposed to the most positive parenting had the highest cortisol; for Blacks, observed positive parenting was linked with low cortisol levels, whereas the absence of positive parenting was associated with higher cortisol; such nonlinear associations hint toward Whites and Blacks occupying different parts of a continuum of environmental influences. Future investigations should test whether positive environments mitigate the development of allostatic load. Third, this study focused on HPA functioning as a primary allostatic mediator, but allostatic load is defined by alterations across multiple physiological systems which interact with each other (Hastings et al., 2012). Despite these limitations, however, the present investigation sheds light on allostatic processes in young adults, moving beyond simple demonstration of health disparities between Blacks and Whites.
Conclusions
Findings support an allostatic model in which stressors, including experiences with racism and discrimination, influence primary allostatic mediators, even as early as age 20. At first glance, observing significant interactions with race lends to the argument that allostatic processes operate differently in Whites than Blacks. Our view is that race differences in HPA functioning reflect that stressors exposure is not equally distributed across the population, with Blacks and Whites representing different parts of the distribution of stressors. Examining race differences has provided an important glance into the impact of the cumulative burden of stress. Higher stress exposure, nevertheless, did not fully account for observed race differences, suggesting that more work needs to be done to understand the nature of health disparities. Better understanding of which stressors influence HPA regulation most for Blacks requires more attention to the full range of experiences and environments, and how HPA regulation develops over time in response to those experiences. Regardless of race, HPA functioning had implications for mental health outcomes. Lower cortisol and flattened diurnal rhythms were associated with poorer mental health outcomes. While blunted physiological activity may buffer the individual from the stressors in their environment, the trade-off is that some individuals with blunted HPA functioning may be at risk for poor mental health outcomes, especially problems which reflect a propensity toward punishment insensitivity and reward dependency.
Figure 4.
A. Nonlinear association of Frequency of Antisocial Behavior on Cortisol Levels. B. Interaction between Race and Antisocial Self-Description showed lower cortisol was associated with Antisocial Behavior within Whites. The association was nonlinear for Blacks, with the most Antisocial Blacks having the highest cortisol levels.
Acknowledgments
Supported by grant # R01 DA021737-03 from the National Institute on Drug Abuse. Salary support was provided by a Career Development Award for Shirtcliff (K01 MH077687). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
An earlier version of this paper was presented at the annual meeting of the Society for Prevention Research held in Denver, CO in June 2010 and the Society for Research in Child Development, Montreal Canada in April 2011.
Richard F. Catalano is a board member of Channing Bete Company, distributor of the Parents Who Care program which was tested as part of the study described in this paper.
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience--cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Iranmanesh A, Veldhuis J, Fisher L. Disturbances of the stress response: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health and Research World. 1998;22:67–72. [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcoholism: Clinical and Experimental Research. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Risher-Flowers D, De Jong J, Ravitz B, Bone GH, Nutt DJ, et al. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. The American Journal of Psychiatry. 1991;148:1023–1025. doi: 10.1176/ajp.148.8.1023. [DOI] [PubMed] [Google Scholar]
- Alink LR, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM. Cortisol and externalizing behavior in children and adolescents: mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Developmental Psychobiology. 2008;50:427–450. doi: 10.1002/dev.20300. [DOI] [PubMed] [Google Scholar]
- Almeida DM, McGonagle K, King H. Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology. 2009;55:219–237. doi: 10.1080/19485560903382338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Cruess S, Cruess DG, Kumar M, Lutgendorf S, Ironson G, et al. Cognitive-behavioral stress management reduces distress and 24-hour urinary free cortisol output among symptomatic HIV-infected gay men. Annals of Behavioral Medicine. 2000;22:29–37. doi: 10.1007/BF02895165. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Emerging adulthood: What is it, and what is it good for? Child Development Perspectives. 2007;1:68–73. [Google Scholar]
- Barrett YC, Akinsanya B, Chang SY, Vesterqvis O. Automated on-line SPE LC-MS/MS method to quantitate 6beta-hydroxycortisol and cortisol in human urine: use of the 6beta-hydroxycortisol to cortisol ratio as an indicator of CYP3A4 activity. Journal of Chromatography B. 2005;821:159–165. doi: 10.1016/j.jchromb.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Bartels M, de Geus EJ, Kirschbaum C, Sluyter F, Boomsma DI. Heritability of daytime cortisol levels in children. Behavior Genetics. 2003;33:421–433. doi: 10.1023/a:1025321609994. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bennett GG, Merritt MM, Wolin KY. Ethnicity, education, and the cortisol response to awakening: A preliminary investigation. Ethnicity & Health. 2004;9:337–347. doi: 10.1080/1355785042000285366. [DOI] [PubMed] [Google Scholar]
- Borrell LN, Crawford ND. Social disparities in periodontitis among US adults: the effect of allostatic load. Journal of Epidemiology and Community Health. 2011;65:144–149. doi: 10.1136/jech.2009.098269. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brody GH, Kogan SM, Chen Yf, Murry VM. Long-Term Effects of the Strong African American Families Program on Youths' Conduct Problems. Journal of Adolescent Health. 2008;43:474–481. doi: 10.1016/j.jadohealth.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulatao RA. In: Understanding racial and ethnic differences in health in late life: a research agenda. Anderson N, editor. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity. 2008;16:600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- Caughy MOB, O'Campo PJ, Muntaner C. When being alone might be better: neighborhood poverty, social capital, and child mental health. Social Science & Medicine. 2003;57:227–237. doi: 10.1016/s0277-9536(02)00342-8. [DOI] [PubMed] [Google Scholar]
- Cicchetti D. In: Developmental psychopathology, Vol 1: Theory and methods. Cohen DJ, editor. Oxford, England: John Wiley & Sons; 1995. [Google Scholar]
- Cicchetti D, Curtis WJ. Multilevel perspectives on pathways to resilient functioning. Development and Psychopathology. 2007;19:627–629. doi: 10.1017/s0954579407000314. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Lynch M. Developmental psychopathology. Vol. 2. Oxford, England: John Wiley & Sons; 1995. Failures in the expectable environment and their impact on individual development: The case of child maltreatment. [Google Scholar]
- Cicchetti D, Rogosch FA. Psychopathology as risk for adolescent substance use disorders: a developmental psychopathology perspective. Journal of Clinical Child Psychology. 1999;28:355–365. doi: 10.1207/S15374424jccp280308. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Personality, adrenal steroid hormones, and resilience in maltreated children: A multilevel perspective. Development and Psychopathology. 2007;19:787–809. doi: 10.1017/S0954579407000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. The past achievements and future promises of developmental psychopathology: the coming of age of a discipline. Journal of Child Psychology and Psychiatry. 2009;50:16–25. doi: 10.1111/j.1469-7610.2008.01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychosomatic Medicine. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Conger RD, Conger KJ, Elder GH, Lorenz FO, Simons RL, Whitbeck LB. A family process model of economic hardship and adjustment of early adolescent boys. Child Development. 1992;63:526–541. doi: 10.1111/j.1467-8624.1992.tb01644.x. [DOI] [PubMed] [Google Scholar]
- Croissant B, Olbrich R. Stress response dampening indexed by cortisol in subjects at risk for alcoholism. Journal of Studies on Alcohol. 2004;65:701–707. doi: 10.15288/jsa.2004.65.701. [DOI] [PubMed] [Google Scholar]
- Cunradi CB, Caetano R, Clark C, Schafer J. Neighborhood poverty as a predictor of intimate partner violence among White, Black, and Hispanic couples in the United States: a multilevel analysis. Annals of Epidemiology. 2000;10:297–308. doi: 10.1016/s1047-2797(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Viau V, Bhatnagar S, Gomez F, Laugero K, Bell ME. Corticotropin-releasing factor (CRF), corticosteroids, stress and sugar: energy balance the brain and behavior. In: Pfaff D, editor. Hormones brain and behavior. Chap 9. I. San Diego, CA: Academic Press; 2002. pp. 571–631. [Google Scholar]
- De Kloet ER. Hormones and the stressed brain. Annals of the New York Academy of Sciences. 2004;1018:1–15. doi: 10.1196/annals.1296.001. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. Human empathy through the lens of social neuroscience. Scientific World Journal. 2006;6:1146–1163. doi: 10.1100/tsw.2006.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Guidice M, Ellis BJ, Shirtcliff EA. The adaptive calibration of stress responsivity. Neuroscience and Behavioral Reviews. 2011;35(7):1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. BSI, Brief Symptom Inventory : administration scoring & procedures manual. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. Journal of Adolescent Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, et al. Foster children's diurnal production of cortisol: an exploratory study. Child Maltreatment. 2006;11:189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Duncan GJ. In: Consequences of growing up poor. Brooks-Gunn J, editor. New York: Russell Sage Foundation; 1997. [Google Scholar]
- Duncan SC, Duncan TE, Strycker LA. A multilevel analysis of neighborhood context and youth alcohol and drug problems. Prevention Science. 2002;3:125–133. doi: 10.1023/a:1015483317310. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Differential susceptibility to the environment: toward an understanding of sensitivity to developmental experiences and context. Development and Psychopathology. 2011;23:1–5. doi: 10.1017/S095457941000060X. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: an evolutionary--neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Development and Psychopathology. 2005;17:303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Armstrong JM, Burk LR, Goldsmith HH, Boyce WT. Biological sensitivity to context moderates the effects of the early teacher-child relationship on the development of mental health by adolescence. Development and Psychopathology. 2011;23:149–161. doi: 10.1017/S0954579410000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Shirtcliff EA, Burk LR, Armstrong JM, Ruttle PL, Klein MH, et al. Influence of early life stress on later HPA functioning and mental health symptoms: A study of the allostatic process from childhood into adolescence. Development and Psychopathology. 2012 doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002;73:1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Evans GW, Lercher P, Meis M, Ising H, Kofler WW. Community noise exposure and stress in children. The Journal of the Acoustical Society of America. 2001;109:1023–1027. doi: 10.1121/1.1340642. [DOI] [PubMed] [Google Scholar]
- Fernades A, Skinner ML, Woelfel T, Carpenter T, Haggerty KP. Implementing self-collecting of biological specimens with a diverse sample. Manuscript submitted for publication under review. [Google Scholar]
- Fisher PA, Gunnar MR, Chamberlain P, Reid JB. Preventive intervention for maltreated preschool children: impact on children's behavior, neuroendocrine activity, and foster parent functioning. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1356–1364. doi: 10.1097/00004583-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Van Ryzin MJ, Gunnar MR. Mitigating HPA axis dysregulation associated with placement changes in foster care. Psychoneuroendocrinology. 2011;36:531–539. doi: 10.1016/j.psyneuen.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among Blacks and Whites in the United States. American Journal of Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Blair C, Willoughby M, Kivlighan KT, Hibel LC, Fortunato CK, et al. Individual differences in salivary cortisol and alpha-amylase in mothers and their infants: relation to tobacco smoke exposure. Developmental Psychobiology. 2007;49:692–701. doi: 10.1002/dev.20247. [DOI] [PubMed] [Google Scholar]
- Gray MJ, Litz BT, Hsu JL, Lombardo TW. Psychometric properties of the life events checklist. Assessment. 2004;11:330–341. doi: 10.1177/1073191104269954. [DOI] [PubMed] [Google Scholar]
- Green TL, Darity WA., Jr Under the skin: Using theories from biology and the social sciences to explore the mechanisms behind the Black-White health gap. American Journal of Public Health. 2010;100:S36–40. doi: 10.2105/AJPH.2009.171140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: what does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Tout K, de Haan M, Pierce S, Stansbury K. Temperament, social competence, and adrenocortical activity in preschoolers. Developmental Psychobiology. 1997;31:65–85. doi: 10.1002/(sici)1098-2302(199707)31:1<65::aid-dev6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hagan MJ, Luecken LJ, Sandler IN, Tien J. Prospective effects of post-bereavement negative events on cortisol activity in parentally bereaved youth. Developmental Psychobiology. 2010;52:394–400. doi: 10.1002/dev.20433. [DOI] [PubMed] [Google Scholar]
- Haggerty KP, MacKenzie EP, Skinner ML, Harachi TW, Catalano RF. Participation in “Parents Who Care”: Predicting program initiation and exposure in two different program formats. Journal of Primary Prevention. 2006;27:47–65. doi: 10.1007/s10935-005-0019-3. [DOI] [PubMed] [Google Scholar]
- Haggerty KP, Skinner ML, MacKenzie EP, Catalano RF. A randomized trial of Parents Who Care: Effects on key outcomes at 24-month follow-up. Prevention Science. 2007;8:249–260. doi: 10.1007/s11121-007-0077-2. [DOI] [PubMed] [Google Scholar]
- Harrell SP. A multidimensional conceptualization of racism-related stress: Implications for the well-being of people of color. American Journal of Orthopsychiatry. 2000;70:42–57. doi: 10.1037/h0087722. [DOI] [PubMed] [Google Scholar]
- Harrell SP, Merchant M, Young SA. Psychometric properties of the Racism and Life Experience Scales (RaLES) Unpublished manuscript 1997 [Google Scholar]
- Hastings PD, Shirtcliff EA, Klimes-Dougan B, Allison AL, DeRose L, Usher B, et al. Allostasis and the development of internalizing and externalizing problems: Changing relations with physiological systems across adolescence. Development and Psychopathology. 2012 doi: 10.1017/S0954579411000538. [DOI] [PubMed] [Google Scholar]
- Hawes DJ, Brennan J, Dadds MR. Cortisol, callous-unemotional traits, and pathways to antisocial behavior. Current Opinion in Psychiatry. 2009;22:357–362. doi: 10.1097/YCO.0b013e32832bfa6d. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Kosterman R, Catalano RF, Hill KG, Abbott RD. Promoting positive adult functioning through social development intervention in childhood: Long-term effects from the Seattle Social Development Project. Archives of Pediatrics and Adolescent Medicine. 2005;159:25–31. doi: 10.1001/archpedi.159.1.25. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54:1389–1298. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heron M. Deaths: Leading causes for 2004. Hyattsville, MD: U.S. Department of Health and Human Services; 2007. [Google Scholar]
- Het S, Rohleder N, Schoofs D, Kirschbaum C, Wolf OT. Neuroendocrine and psychometric evaluation of a placebo version of the ‘Trier Social Stress Test’. Psychoneuroendocrinology. 2009;34:1075–1086. doi: 10.1016/j.psyneuen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Curtin JJ. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;43:344–356. doi: 10.1111/j.1469-8986.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Huffman R. Metabolic Syndrome racial differences in adolescents. Current Diabetes Reviews. 2009;5:259–265. doi: 10.2174/157339909789804332. [DOI] [PubMed] [Google Scholar]
- Johnston-Brooks CH, Lewis MA, Evans GW, Whalen CK. Chronic stress and illness in children: the role of allostatic load. Psychosomatic Medicine. 1998;60:597–603. doi: 10.1097/00006842-199809000-00015. [DOI] [PubMed] [Google Scholar]
- Kaholokula JK, Grandinetti A, Keller S, Nacapoy AH, Kingi TK, Mau MK. Association between perceived racism and physiological stress indices in Native Hawaiians. Journal of Behavioral Medicine. 2011 doi: 10.1007/s10865-011-9330-z. Online first 2-28-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Merkin SS, Crimmins EM, Seeman TE. Socioeconomic and ethnic disparities in cardiovascular risk in the United States, 2001-2006. Annals of Epidemiology. 2010;20:617–628. doi: 10.1016/j.annepidem.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Keyes CL. The Black-White paradox in health: flourishing in the face of social inequality and discrimination. Journal of Personality. 2009;77:1677–1706. doi: 10.1111/j.1467-6494.2009.00597.x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic Medicine. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Knafo A, Israel S, Ebstein RP. Heritability of children's prosocial behavior and differential susceptibility to parenting by variation in the dopamine receptor D4 gene. Development and Psychopathology. 2011;23:53–67. doi: 10.1017/S0954579410000647. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008a;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. (Series B Biological Sciences).Philosophical Transactions of the Royal Society of London. 2008b;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neuroscience and Biobehavioral Reviews. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Krieger N. Embodiment: a conceptual glossary for epidemiology. Journal of Epidemiology and Community Health. 2005;59:350–355. doi: 10.1136/jech.2004.024562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S, Dickerson SS, Zoccola PM, Zaldivar F. Emotion regulation and cortisol reactivity to a social-evaluative speech task. Psychoneuroendocrinology. 2009;34:1355–1362. doi: 10.1016/j.psyneuen.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Landrine H, Klonoff EA. The schedule of racist events: A measure of racial discrimination and a study of its negative physical and mental health consequences. Journal of Black Psychology. 1996;22:144–168. [Google Scholar]
- Lantz PM, House JS, Mero RP, Williams DR. Stress, life events, and socioeconomic disparities in health: Results from the Americans' Changing Lives Study. Journal of Health and Social Behavior. 2005;46:274–288. doi: 10.1177/002214650504600305. [DOI] [PubMed] [Google Scholar]
- Loney BR, Butler MA, Lima EN, Counts CA, Eckel LA. The relation between salivary cortisol, callous-unemotional traits, and conduct problems in an adolescent non-referred sample. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47:30–36. doi: 10.1111/j.1469-7610.2005.01444.x. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Cortisol secretion patterns in addiction and addiction risk. International Journal of Psychophysiology. 2006;59:195–202. doi: 10.1016/j.ijpsycho.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecken LJ, Hagan MJ, Sandler IN, Tien J, Ayers TS, Wolchik SA. Cortisol levels six-years after participation in the Family Bereavement Program. Psychoneuroendocrinology. 2009;35:785–789. doi: 10.1016/j.psyneuen.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Child's stress hormone levels correlate with mother's socioeconomic status and depressive state. Biological Psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development & Psychopathology. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Ouellet-Morin I, Hupbach A, Tu MT, Buss C, Walker D, et al. Developmental psychopathology: Vol 2 Developmental Neuroscience. 2nd. New York: Wiley; 2006. Beyond the stress concept: Allostatic load--a developmental biological and cognitive perspective; pp. 578–628. [Google Scholar]
- Luthar SS. Resilience in development: A synthesis of research across five decades. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Risk, disorder, and adaptation. 2nd. New York: Wiley; 2006. pp. 739–795. [Google Scholar]
- Lyons DM, Parker KJ. Stress inoculation-induced indications of resilience in monkeys. Journal of Traumatic Stress. 2007;20:423–433. doi: 10.1002/jts.20265. [DOI] [PubMed] [Google Scholar]
- Massey DS. Segregation and stratification: a biosocial perspective. Du Bois Review: Social Science Research on Race. 2004;1:7–25. [Google Scholar]
- McBurnett K, Raine A, Stouthamer-Loeber M, Loeber R, Kumar AM, Kumar M, et al. Mood and hormone responses to psychological challenge in adolescent males with conduct problems. Biological Psychiatry. 2005;57:1109–1116. doi: 10.1016/j.biopsych.2005.01.041. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. In: McCann SM, Lipton JM, editors. Annals of the New York Academy of Sciences Vol 840: Neuroimmunomodulation: Molecular aspects integrative systems and clinical advances. New York: New York Academy of Sciences; 1998. pp. 33–44. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis, allostatic load, and the aging nervous system: role of excitatory amino acids and excitotoxicity. Neurochemical Research. 2000a;25:1219–1231. doi: 10.1023/a:1007687911139. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Research. 2000b;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Progress in Brain Research. 2000c;122:25–34. doi: 10.1016/s0079-6123(08)62128-7. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biological Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- Merritt MM, McCallum TJ, Fritsch T. How much striving is too much? John henryism active coping predicts worse daily cortisol responses for African American but not white female dementia family caregivers. American Journal of Geriatric Psychiatry. 2011;19:451–460. doi: 10.1097/JGP.0b013e3181eaffa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Moons WG, Eisenberger NI, Taylor SE. Anger and fear responses to stress have different biological profiles. Brain Behavior and Immunity. 2009;24:215–219. doi: 10.1016/j.bbi.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov M, Yao JK, Kirillova GP. Salivary cortisol responses in prepubertal boys: the effects of parental substance abuse and association with drug use behavior during adolescence. Biological Psychiatry. 1999;45:1293–1299. doi: 10.1016/s0006-3223(98)00216-9. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov MM, Martin CS. Salivary cortisol responses and the risk for substance abuse in prepubertal boys. Biological Psychiatry. 1995;38:547–555. doi: 10.1016/0006-3223(94)00382-D. [DOI] [PubMed] [Google Scholar]
- Munro C, Stabenfeldt GH. Development of a cortisol enzyme-immunoassay in plasma. Clinical Chemistry. 1985;31:956. [Google Scholar]
- O'Connor KA, Brindle E, Holman DJ, Klein NA, Soules MR, Campbell KL, et al. Urinary estrone conjugate and pregnanediol 3-glucuronide enzyme immunoassays for population research. Clinical Chemistry. 2003;49:1139–1148. doi: 10.1373/49.7.1139. [DOI] [PubMed] [Google Scholar]
- O'Leary MM, Loney BR, Eckel LA. Gender differences in the association between psychopathic personality traits and cortisol response to induced stress. Psychoneuroendocrinology. 2007;32:183–191. doi: 10.1016/j.psyneuen.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Obradovic J, Boyce WT. Individual differences in behavioral, physiological, and genetic sensitivities to contexts: implications for development and adaptation. Developmental Neuroscience. 2009;31:300–308. doi: 10.1159/000216541. [DOI] [PubMed] [Google Scholar]
- Obradovic J, Bush NR, Boyce WT. The interactive effect of marital conflict and stress reactivity on externalizing and internalizing symptoms: the role of laboratory stressors. Development and Psychopathology. 2011;23:101–114. doi: 10.1017/S0954579410000672. [DOI] [PubMed] [Google Scholar]
- Obradovic J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: the interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development. 2010;81:270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenfels MC, Porter L, Smyth J, Kirschbaum C, Hellhammer DH, Stone AA. Effect of chronic stress associated with unemployment on salivary cortisol: overall cortisol levels, diurnal rhythm, and acute stress reactivity. Psychosomatic Medicine. 1995;57:460–467. doi: 10.1097/00006842-199509000-00008. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Geurts HM, Knol DL, Sergeant JA. Low basal salivary cortisol is associated with teacher-reported symptoms of conduct disorder. Psychiatry Research. 2005;134:1–10. doi: 10.1016/j.psychres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress inoculation in young monkeys. Archives of General Psychiatry. 2004;61:933–941. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- Paschall MJ, Flewelling RL, Ennett ST. Racial differences in violent behavior among young adults: Moderating and confounding effects. Journal of Research in Crime and Delinquency. 1998;35:148–165. [Google Scholar]
- Peeters F, Nicholson NA, Berkhof J. Cortisol responses to daily events in major depressive disorder. Psychosomatic Medicine. 2003;65:836–841. doi: 10.1097/01.psy.0000088594.17747.2e. [DOI] [PubMed] [Google Scholar]
- Peters MF, Massey G. Mundane extreme environmental stress in family stress theories: The case of Black families in White America. Marriage & Family Review. 1983;6:193–218. [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. Pathophysiological basis of vulnerability to drug abuse: Role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annual Review of Pharmacology and Toxicology. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Popma A, Doreleijers TA, Jansen LM, Van Goozen SH, Van Engeland H, Vermeiren R. The diurnal cortisol cycle in delinquent male adolescents and normal controls. Neuropsychopharmacology. 2007;32:1622–1628. doi: 10.1038/sj.npp.1301289. [DOI] [PubMed] [Google Scholar]
- Prelow HM, Danoff-Burg S, Swenson RR, Pulgiano D. The impact of ecological risk and perceived discrimination on the psychological adjustment of African American and European American youth. Journal of Community Psychology. 2004;32:375–389. [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, et al. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35:179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of Abnormal Child Psychology. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Richman LS, Jonassaint C. The effects of race-related stress on cortisol reactivity in the laboratory: implications of the Duke lacrosse scandal. Annals of Behavioral Medicine. 2008;35:105–110. doi: 10.1007/s12160-007-9013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmalen JG, Oldehinkel AJ, Ormel J, de Winter AF, Buitelaar JK, Verhulst FC. Determinants of salivary cortisol levels in 10-12 year old children; a population-based study of individual differences. Psychoneuroendocrinology. 2005;30:483–495. doi: 10.1016/j.psyneuen.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Roy MP, Steptoe A, Kirschbaum C. Life events and social support as moderators of individual differences in cardiovascular and cortisol reactivity. Journal of Personality and Social Psychology. 1998;75:1273–1281. doi: 10.1037//0022-3514.75.5.1273. [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Serbin LA, Ben-Dat Fisher D, Stack DM, Schwartzman AE. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: Longitudinal and concurrent associations with cortisol. Hormones and Behavior. 2011;59(1):123–132. doi: 10.1016/j.yhbeh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Why zebras don't get ulcers: an updated guide to stress stress-related diseases, and coping. New York: W.H. Freeman and Co; 1998. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schreiber JE, Shirtcliff E, Van Hulle C, Lemery-Chalfant K, Klein MH, Kalin NH, et al. Environmental influences on family similarity in afternoon cortisol levels: twin and parent-offspring designs. Psychoneuroendocrinology. 2006;31:1131–1137. doi: 10.1016/j.psyneuen.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenberg JE, Sameroff AJ, Cicchetti D. The transition to adulthood as a critical juncture in the course of psychopathology and mental health. Development and Psychopathology. 2004;16:799–806. doi: 10.1017/s0954579404040015. [DOI] [PubMed] [Google Scholar]
- Schulkin J, McEwen BS, Gold PW. Allostasis, amygdala, and anticipatory angst. Neuroscience and Biobehavioral Reviews. 1994;18:385–396. doi: 10.1016/0149-7634(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Selye H. Stress: The physiology and pathology of exposure to stress. Montreal: Acta Medical Publishers; 1950. [Google Scholar]
- Sethre-Hofstad L, Stansbury K, Rice MA. Attunement of maternal and child adrenocortical response to child challenge. Psychoneuroendocrinology. 2002;27:731–747. doi: 10.1016/s0306-4530(01)00077-4. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery M, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and diurnal rhythms from childhood to adolescence. 2012 doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50:690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Vitacco MJ, Graf AR, Gostisha AJ, Merz JL, Zahn-Waxler C. Neurobiology of empathy and callousness: implications for the development of antisocial behavior. Behavioral Sciences & the Law. 2009;27:137–171. doi: 10.1002/bsl.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever LJ, Davis KL. Overview: Toward a dysregulation hypothesis of depression. American Journal of Psychiatry. 1985;142:1017–1031. doi: 10.1176/ajp.142.9.1017. [DOI] [PubMed] [Google Scholar]
- Simons RL, Murry V, McLoyd V, Lin KH, Cutrona C, Conger RD. Discrimination, crime, ethnic identity, and parenting as correlates of depressive symptoms among African American children: A multilevel analysis. Development and Psychopathology. 2002;14:371–393. doi: 10.1017/s0954579402002109. [DOI] [PubMed] [Google Scholar]
- Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;23:323–355. [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Stone AA, Schwartz JE, Smyth J, Kirschbaum C, Cohen S, Hellhammer D, et al. Individual differences in the diurnal cycle of salivary free cortisol: a replication of flattened cycles for some individuals. Psychoneuroendocrinology. 2001;26:295–306. doi: 10.1016/s0306-4530(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Straus MA. The Conflict Tactics Scale and its critics: An evaluation and new data on validity and reliability. In: Straus MA, Gelles RJ, editors. Physical violence in American families: Risk factors and adaptations to violence in 8,145 families. New Brunswick, NJ: Transaction Publishing; 1990. pp. 49–73. [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: stress, early vulnerabilities and the attenuation hypothesis. Neuroscience and Behavioral Reviews. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Szalacha LA, Erkut S, Garcia Coll C, Fields JP, Alarcon O, Ceder I. Perceived discrimination and resilience. In: Luthar SS, editor. Resilience and vulnerability: Adaptation in the context of childhood adversity. New York: Cambridge University Press; 2003. pp. 414–435. [Google Scholar]
- Szanton SL, Gill JM, Allen JK. Allostatic load: A mechanism of socioeconomic health disparities? Biological Research For Nursing. 2005;7:7–15. doi: 10.1177/1099800405278216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, Lieberman MD. Neural bases of moderation of cortisol stress responses by psychosocial resources. Journal of Personality and Social Psychology. 2008;95:197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Repetti RL, Seeman T. Health psychology: what is an unhealthy environment and how does it get under the skin? Annual Review of Psychology. 1997;48:411–447. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- Tomblingson G. Comparison of paired urinary and salivary cortisol measures as biomarkers of stress. Presented at the University of Washington's Eighth Annual Undergraduate Research Symposium; Seattle. 2005. May, [Google Scholar]
- Turner R, Avison WR. Status variations in stress exposure: Implications for the interpretation of research on race, socioeconomic status, and gender. Journal of Health & Social Behavior. 2003;44:488–505. [PubMed] [Google Scholar]
- Ussher M, West R, Evans P, Steptoe A, McEwen A, Clow A, et al. Reduction in cortisol after smoking cessation among users of nicotine patches. Psychosomatic Medicine. 2006;68:299–306. doi: 10.1097/01.psy.0000204926.27215.a1. [DOI] [PubMed] [Google Scholar]
- van Eck MM, Nicolson NA, Berkhof H, Sulon J. Individual differences in cortisol responses to a laboratory speech task and their relationship to responses to stressful daily events. Biological Psychiatry. 1996;43:69–84. doi: 10.1016/0301-0511(95)05159-7. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Matthys W, Cohen-Kettenis PT, Gispen-de Wied C, Wiegant VM, van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biological Psychiatry. 1998;43:531–539. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ, Hermans EJ, Putman P. Low cortisol levels and the balance between punishment sensitivity and reward dependency. Neuroreport. 2003;14:1993–1996. doi: 10.1097/00001756-200310270-00023. [DOI] [PubMed] [Google Scholar]
- Van Hulle CA, Shirtcliff EA, Lemery-Chalfant K, Goldsmith HH. Genetic and Environmental Influences on Individual Differences in Cortisol Level and Circadian Rhythm. doi: 10.1016/j.yhbeh.2012.04.014. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems CF, Carrion VG. Brief report: diurnal salivary cortisol in youth--clarifying the nature of posttraumatic stress dysregulation. Journal of Pediatric Psychology. 2009;34:389–395. doi: 10.1093/jpepsy/jsn087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson RG, Pickett KE. Income inequality and social dysfunction. Annual Review of Sociology. 2009;35:493–511. [Google Scholar]
- Williams D, Lawler KA. Stress and illness in low-income women: the roles of hardiness, John Henryism, and race. Women's Health. 2001;32:61–75. doi: 10.1300/J013v32n04_04. [DOI] [PubMed] [Google Scholar]
- Williams DR, Jackson PB. Social sources of racial disparities in health. Health Affairs. 2005;24:325–334. doi: 10.1377/hlthaff.24.2.325. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. The Journal of Clinical Psychiatry. 2001;62(17):41–46. [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Schmeidler J, Aferiat DH, Breslau I, Dolan S. Low cortisol and risk for PTSD in adult offspring of holocaust survivors. The American Journal of Psychiatry. 2000;157:1252–1259. doi: 10.1176/appi.ajp.157.8.1252. [DOI] [PubMed] [Google Scholar]