Abstract
We have proposed a novel probe design of pyrene-disulfide molecular assembly and demonstrated its application for the fluorescence turn-on detection of mercury (II) ions (Hg2+). By taking advantage of the pyrene-assisted efficient photolysis of disulfide bonds, our proposed sensing system exhibits both high selectivity and sensitivity toward Hg2+ detection with a detection limit of 5 nM (1 ppb).
Keywords: photolysis, pyrene, disulfide bonds, mercury ions
Contamination of the environment with heavy metal ions has created a pressing public health concern in living systems. Mercury is widely considered to be one of the most hazardous pollutants and highly dangerous elements due to its recognized accumulative and toxic characters in the environment and the ecosystem. The sources of mercury pollution mainly include coal-fired power plants, oceanic and volcanic emissions, gold mining and combustion of waste. [1] Bioaccumulation of mercury in vital organs and tissues, such as the liver, brain, and heart muscle, has been reported to cause a number of severe adverse health effects, sometimes even lethal effects, on living systems. [2] Therefore, there is an ever-growing demand to develop some sensitive and selective mercury(II) ions (Hg2+) detection assays, suitable for quick determination in drinking water and food resources. A number of traditional methods have been established to assay trace amounts of Hg2+, such as atomic absorption spectrometry (AAS), cold vapour AAS or flame-AAS-ETA (electrothermal atomisation), anodic stripping voltammetry and inductively coupled plasma-optical emission spectroscopy. However, these methods share the drawbacks of time-consuming, labor-intensive steps, the addition of many exogenous reagents, specialized expertise, or expensive equipment. To overcome the above disadvantages, a great number of diverse Hg2+-sensitive biosensors based on organic chromophores [3] or fluorophores, [4] conjugated polymers, [5] DNAzyme, [6] gold nanoparticles, [7] semiconductor quantum dots, [8] proteins, [9] and genetically engineered bacteria, [10] have been developed. Very recently, a novel approach for the detection of Hg2+ based on DNA–metal coordination chemistry [11] has attracted significant research interest. That is, Hg2+ can interact with thymine bases to form strong and stable thymine-thymine mismatches complexes (T-Hg2+-T), which is more stable than a T-A Watson Crick pair. [12] In addition, Hg2+ shows an excellent selectivity against other heavy and transition metal ions to stabilize the T-T base pair. Thus, diverse Hg2+-selective sensors, based on T-Hg2+-T coordination chemistry, are particularly attractive with the high sensitivity, selectivity and simplicity.
Fluorescence-based probes are widely employed for sensor design due to their easy synthesis, high sensitivity and convenience. Among the probes, attracting most attention are the molecular beacon (MB) probes. Usually, this kind of MB probe is a dual-labeled oligonucleotide probe with a hairpin-shaped structure in which the 5′ and 3′ ends are self-complementary, bringing a fluorophore and a quencher into close proximity and resulting in fluorescence quenching of the fluorophore via fluorescence resonance energy transfer. Ono and Togashi [13] designed that a simple turn-off MB probe, a T-rich single-strand DNA labeled with a fluorophore and a quencher at the 3′ and 5′ end, automatically folded induced by Hg2+ to decrease fluorescence signal (sensitivity up to 40 nM). The fluorescent turn-off sensor may have the problem of “false positive” results because of the external quenchers or other environmental interferents. In contrast, the fluorescent turn-on sensor seems to be more sensitive and less interferential attributed to its intrinsic feature.
Pyrene, a simple hydrocarbon aromatic molecule, has been employed as an alternative to traditional fluorophore labeling in oligonucleotides. It acts as a spatial-sensitive fluorescent dye because of its excellent photochemically property, which gives a large Stokes shift of the fluorescence emission from 375 and 398 nm (pyrene monomer) to ~485 nm (pyrene excimer) [14] upon the formation of ternary complexes between the pyrene-modified probes and target. Therefore, a novel class of molecular beacons using excimer-monomer switching molecular beacons has been developed to detect proteins, [15] nucleic acids, [16] metal ions, [17] and small molecules. [18] Very recently, we have found an interesting phenomenon of pyrene, which can cause efficient disulfide photocleavage at 350 nm light irradiation. [19] To the best of our knowledge, we were the first to report the efficient disulfide photocleavage mediated by pyrene moiety, similar mechanism was found in proteins as well via UV irradiation at 280 nm (λmax of the tryptophan group). [20] As we proven, the radical pathway for the efficient disulfide photocleavage at 350 nm was much enhanced in the presence of pyrene, via producing large amounts of photo-induced radical cations. Based on our findings, the photolysis of disulfide bonds can be facilitated by nearby pyrene moiety in DNA frameworks.
In this study, the property of pyrene-mediated photolysis of disulfide bonds is further employed to develop sensing system, using Hg2+ as a model target. Scheme 1 illustrates the working mechanism of this homogeneous Hg2+ detection assay. We designed two T-rich probes termed as “Hg-Pyr” (5′-CTCTTATCTTGTT/Pyr/TCTCTTTTCTCTT-3′) and “Hg-SS” (5′-TTGTGTTATGTG-/FAM/-/SS/-/Dab/-TCTTGTTATGTG-3′). Hg-Pyr probe contained two T-rich domains spaced by a pyrene moiety. Similar to Hg-Pyr probe, Hg-SS probe also contained two T-rich domains spaced by the moieties of FAM (6-carboxyfluorescein), disulfide, and Dabcyl (quencher of FAM fluorophore), in which a S-S linkage was incorporated into Hg-SS probe to separate 6-FAM and Dabcyl. Initially, the FAM fluorescence was quenched by the nearby quencher Dabcyl moiety. In the presence of Hg2+, Hg-Pyr and Hg-SS probes were associated via the Hg2+-mediated coordination of T-Hg2+-T base pairs. Upon light irradiation at 350 nm (using a UV-B lamp and a 352 nm optical filter), Hg-SS probe was irreversibly cleaved at the disulfide bridge. Following the photolysis, the cleaved pieces in Hg-SS probe were released because of the decreased affinity to Hg-Pyr probe, resulting in the dissociation of Hg-Pyr and Hg-SS complex. Correspondingly, the FAM fluorescence restored, giving out the increased signal (turn-on) for Hg2+ detection. It needs to point out that after cleavage, Hg2+ could be released to react with other Hg-Pyr and Hg-SS probes to initiate a new round of photo-induced disulfide cleavage. Thus via the Hg2+-recycling event, the proposed sensor provided an amplified fluorescent signal. Such fluorescence enhancement phenomenon is sensitive and specific to Hg2+, which can be quantified by spectrophotometer. In the absence of Hg2+, the distance-dependent photolytic cleavage between disulfide and pyrene moiety doesn’t work.
Scheme 1.
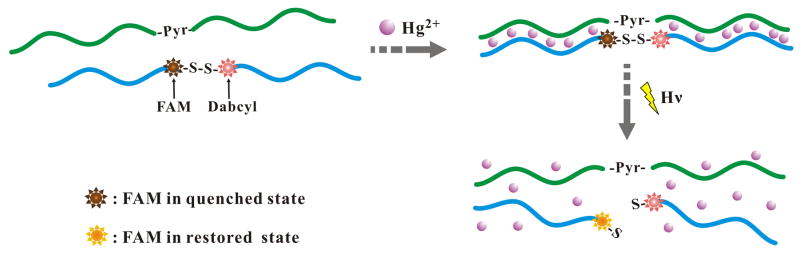
Schematic illustration of Hg2+ detection via pyrene-mediated photolysis of disulfide bonds.
As a proof-of-concept experiment, we first optimized the sensing conditions, including reaction buffer and kinetic behaviors of disulfide photolysis. It is well known that the reaction buffer plays an important role in facilitating the Hg2+-mediated T-T mismatches complexes. Three buffers including NaNO3-MOPS buffer, Tris-acetate buffer and Tris-MOPS buffer were tested at three Hg2+ concentrations. As shown in Figure 1(A), the NaNO3-MOPS buffer exhibited the best performance in the fluorescence restoration for different Hg2+ concentrations. Therefore, the following experiments were all carried out using NaNO3-MOPS buffer. The time-course of photocleavage of disulfide bonds in Hg-SS probe in the presence of Hg2+ was also investigated. The change in fluorescence intensity due to the cleavage of Hg-SS probe was monitored by spectrofluorometer as a function of time. As shown in Figure 1(B), when sensing system was treated with irradiation, the fluorescence intensity increased rapidly within the first 15 min and then achieved equilibrium within ~35 min. A question may arise: if irradiating for longer time, whether the sensing system with different Hg2+ concentrations would reach the same fluorescence intensity finally. Actually, the sensing system would achieve in a photolytic equilibrium between the two disulfide molecular states: the oxidized form (S-S) and the reduced form (-SH), which was confirmed in our previous reported work.19
Figure 1.
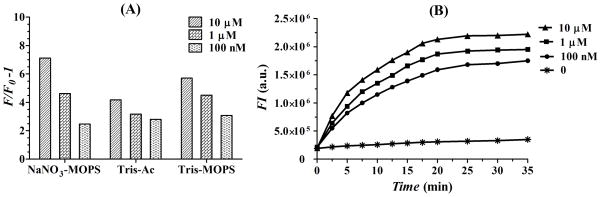
(A) Investigation of reaction buffers for Hg2+ detection when adding Hg2+ with three concentrations (10 μM, 1 μM and 100 nM). Three reaction buffers were investigated, including NaNO3-MOPS buffer (0.1 M NaNO3/10 mM MOPS, pH 7.5), Tris-acetate buffer (25 mM Tris/20 mM KAc/80 mM NaAc, pH 8.0) and Tris-MOPS buffer (10 mM Tris/10 mM MOPS, pH 7.7). (B) Time-course experiment for monitoring the kinetics of pyrene-mediated disulfide bonds cleavage after 350 nm light irradiation in the presence of Hg2+ at four concentrations (10 μM, 1 μM, 100 nM and 0). Fluorescence measurements were performed after 0, 2.5, 5, 7.5, 10, 12.5, 15, 17.5, 20, 25, 30 and 35 min of irradiation reaction, respectively.
The sensitivity of the sensor under the optimized detection condition was investigated in a series of Hg2+ concentrations. Figure 2(A) reveals the fluorescence spectra of the sensing system upon incubation with different Hg2+ concentrations and then irradiated at 350 nm with a UV-B lamp and a 352 nm optical filter for 20 min. It clearly demonstrates that a dramatic increase in the fluorescence emission spectrum was observed with the increasing concentrations of Hg2+ from 0 to 10 μM. Figure 2(B) illustrates the fluorescence intensity changes (F/F0−1) upon addition of different Hg2+ concentrations, where F0 and F are the FAM fluorescence intensity at λ=518 nm in the absence and presence of Hg2+, respectively. The Figure 2(B) inset exhibits a good linear correlation (R2=0.9913) between the (F/F0−1) value and the Hg2+ concentration over the range of 8 nM to 100 nM. An obvious change in the fluorescence emission spectrum was observed when 5 nM Hg2+ was present. Thus the experimentally measured detection limit of this sensing system was as low as 5 nM (>3 SD/m, SD and m represent standard deviation and slope rate, respectively), which was lower than the MAL (maximum allowable level) of 10 nM (2 ppb) Hg2+ in drinking water in the U.S. Environmental Protection Agency (EPA). These experimental data demonstrate that the proposed sensor is sensitively responsive to Hg2+.
Figure 2.
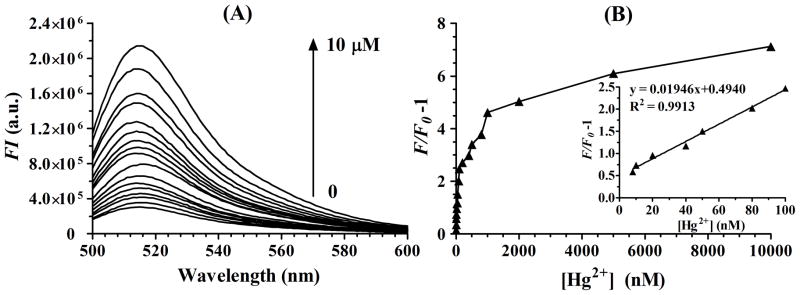
(A) Fluorescence emission spectra (excitation at 480 nm, emission at 518 nm) of probes solution on adding increasing concentrations of Hg2+ (0, 5 nM, 8 nM, 10 nM, 20 nM, 40 nM, 50 nM, 80 nM, 100 nM, 200 nM, 400 nM, 500 nM, 800 nM, 1 μM, 2 μM, 5 μM, 10 μM). (B) Plot of (F/F0−1) as function of the same concentrations of Hg2+ as (A), where F0 and F are the FAM fluorescence intensity in the absence and the presence of Hg2+. Inset: Linear fit plot of (F/F0−1) as function of the increasing concentrations of Hg2+ from 8 nM to 100 nM.
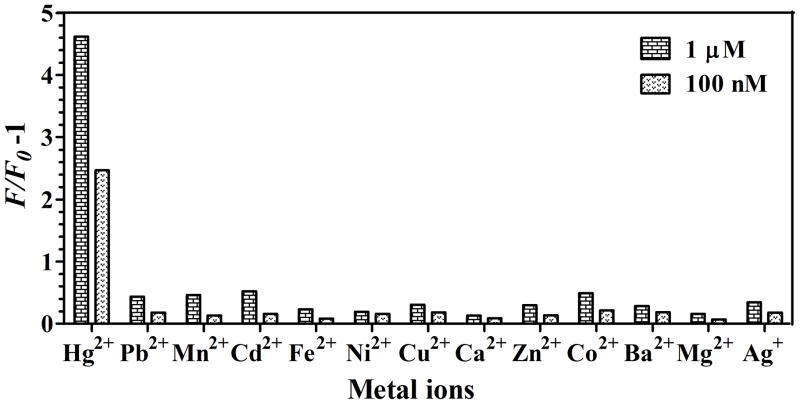
The selectivity of the sensor was also examined by probing the response of the sensing system to twelve competing metal ions including Pb2+, Mn2+, Cd2+, Fe2+, Ni2+, Cu2+, Ca2+, Zn2+, Co2+, Ba2+, Mg2+ and Ag+ ions, under the same conditions as in the case of Hg2+. Each metal ion was tested at two concentrations (1 μM and 100 nM). The emission intensities of the sensing sensors with different metal ions were compared. As shown in Figure 3, the significant fluorescence restoration was only observed in the case of Hg2+. That is none of the competing metal ions gave (F/F0−1) value higher than that of Hg2+. The results demonstrate an excellent selectivity of the proposed sensor over alkali, alkaline earth and other transition heavy metal ions. We further investigated the response of the proposed sensor to Hg2+ in a mixture containing other twelve different interference metal ions. A mixture, containing 1 μM Hg2+and twelve metal ions (each 100 nM), was prepared. The (F/F0−1) value for mixture was 5.01, a littler higher than 4.62 of Hg2+ alone. The result indicates that the competing metal ions had little interference with Hg2+ detection. For a practical application purpose, we studied whether this sensor was applicable to natural samples. The spiked tap water samples with three different concentrations of Hg2+ including 30 nM, 60 nM and 80 nM, were tested. As expected, the sensing system demonstrates the good recoveries of 90.2%, 116.3% and 112.5%, respectively. It suggests that this sensing system can be applied to facile detection of aqueous Hg2+ in real samples.
Figure 3.
Selectivity of photolysis-based Hg2+ sensor over the competing metal ions with two different concentrations of 1 μM and 100 nM.
In summary, we have demonstrated a photolysis-based fluorescence turn-on sensor for the detection of Hg2+ in aqueous solutions. Taking advantages of efficient pyrene-mediate photocleavage of disulfide bonds at 350 nm irradiation, our proposed sensor delivers a detection limit of 5 nM (1 ppb), which is comparable to the most sensitive methods reported without the involvement of complicated procedures or sophisticated instrumentation. Based on the distinct advantages of high sensitivity, good selectivity and practical application, it shows that this sensor would offer an inexpensive and feasible alternative for the quantitative analysis of the Hg2+. Moreover, we believe that this sensing design based on pyrene-disulfide molecular engineering can provide numerous applications in biomedical and proteomics scenarios, with light-induced spatio-temporal control.
Experimental Section
Chemicals and Meterials
The pyrene phosphoramidite was synthesized according to the reported protocol. [21] DNA synthesis reagents including C-6 disulfide phosphoramidite, 6-fluorescein (FAM) phosphoramidite, 5′-4-(4-dimethylaminophenylazo) benzoic acid (Dabcyl) phosphoramidite were purchased from Glen Research (Sterling, VA, USA). The metal salts (Pb(NO3)2, Mn(Ac)2, Cd(NO3)2, Fe(NO3)2, Ni(NO3)2, Cu(NO3)2, Ca(Ac)2, Zn(Ac)2, Co(Ac)2, Ba(NO3)2, Mg(NO3)2, AgNO3 and Hg(Ac)2) were reagent-grade. All solutions were prepared with ultrapure water (18.2 MΩ·cm) from a Millipore Milli-Q system (Bedford, MA).
Instrumentation
The DNA probes (Hg-Pyr: 5′-CTCTTATCTTGTT/Pyr/TCTCTTTTCTCTT -3′, Hg-SS: 5′-TTGTGTTATGTG-/FAM/-/SS/-/Dab/-TCTTGTTATGTG-3′) were synthesized using an ABI 3400 DNA/RNA synthesizer (Applied Bio-systems), purified using a ProStar HPLC (Varian) equipped with a C18 column (Econosil, 5U, 250 × 4.6 mm) from Alltech Associates, and quantified using a Cary Bio 100 UV/vis spectrometer (Varian, Walnut Creek, CA, USA). For the photolysis of the disulfide bonds, the prepared samples were irradiated at 350 nm with a UV-B lamp centered at 302 nm (SANKYO DENKI, Japan) with a 352 nm optical filter (3 nm half bandwidth; Oriel Instruments, Stratford, CT, Newport). Fluorescence emission spectra were recorded by a Fluorolog-Tau-3 spectrofluorometer (Jobin Yvon, Edison, NJ) in quartz fluorescence cell with an optical path length of 1.0 cm, with an excitation wavelength 480 nm and an emission range from 500 nm to 600 nm.
Procedures for Hg2+ Detection
In a typical experiment of Hg2+ detection, the probe solutions containing Hg-Pyr and Hg-SS probes (each 200 nM) were prepared in MOPS buffer (50 mM NaNO3, 10 mM MOPS, pH 7.0). Then a series of Hg(Ac)2 aliquot with different concentrations were added to the probe solutions, respectively. After allowing incubation for 30 min, the probe solutions were irradiated at 350 nm using a UV-B lamp with 352 nm optical filter for 20 min. Following irradiation, fluorescence emission spectra of the probe solutions were recorded with excitation wavelengths of 488 nm. In the selectivity experiment, other metal salts (as control samples) were added to the probe solutions, and the procedures were the same as Hg2+ detection. All the experiments were carried out at room temperature (RT).
Acknowledgments
This study is supported by grants awarded by the China NSF 21075040, the Shanghai Fund (11nm0502500, 11XD1401900, and 09JC1404100), NIH (GM066137, GM079359 and CA133086) and NSF. We thank Dr. Kathryn R. Williams, Da Han, Hui Wang, Zhi Zhu, Yan Chen, Lu Peng, Jian Wang, Tao Chen, Quan Yuan, Xiaohong Tan, Jin Huang, Cuichen Wu for valuable discussions and assistance on the experiment design.
References
- 1.Harris HH, Pickering IJ, George GN. Science. 2003;301:1203. doi: 10.1126/science.1085941. [DOI] [PubMed] [Google Scholar]
- 2.a) Renzoni A, Zino F, Franchi E. Environ Res. 1998;77:68. doi: 10.1006/enrs.1998.3832. [DOI] [PubMed] [Google Scholar]; b) Basu N, Kwan M, Man Chan H. J Toxicol and Env Health A. 2006;69:1133. doi: 10.1080/15287390500362394. [DOI] [PubMed] [Google Scholar]; c) Dorea JG, Donangelo CM. Clinical Nutrition. 2006;25:369. doi: 10.1016/j.clnu.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 3.a) Yang YK, Yook KJ, Tae J. J Am Chem Soc. 2005;127:16760. doi: 10.1021/ja054855t. [DOI] [PubMed] [Google Scholar]; b) Avirah RR, Jyothish K, Ramaiah D. Organic Letters. 2006;9:121. doi: 10.1021/ol062691v. [DOI] [PubMed] [Google Scholar]; c) Nolan EM, Lippard SJ. J Am Chem Soc. 2007;129:5910. doi: 10.1021/ja068879r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Yoon S, Miller EW, He Q, Do PH, Chang CJ. Angew Chem Int Ed. 2007;46:6658. doi: 10.1002/anie.200701785. [DOI] [PubMed] [Google Scholar]; b) Yoon S, Albers AE, Wong AP, Chang CJ. J Am Chem Soc. 2005;127:16030. doi: 10.1021/ja0557987. [DOI] [PubMed] [Google Scholar]; c) Domaille DW, Que EL, Chang CJ. Nat Chem Biol. 2008;4:168. doi: 10.1038/nchembio.69. [DOI] [PubMed] [Google Scholar]
- 5.a) Kim IB, Erdogan B, Wilson JN, Bunz UHF. Chem Eur J. 2004;10:6247. doi: 10.1002/chem.200400788. [DOI] [PubMed] [Google Scholar]; b) Liu X, Tang Y, Wang L, Zhang J, Song S, Fan C, Wang S. Adv Mater. 2007;19:1662. [Google Scholar]; c) Shi HF, Liu SJ, Sun HB, Xu WJ, An ZF, Chen J, Sun S, Lu XM, Zhao Q, Huang W. Chem Eur J. 2010;16:12158. doi: 10.1002/chem.201000748. [DOI] [PubMed] [Google Scholar]
- 6.a) Liu J, Lu Y. Angew Chem Int Ed. 2007;46:7587. doi: 10.1002/anie.200702006. [DOI] [PubMed] [Google Scholar]; b) Zuo P, Yin BC, Ye BC. Biosens Bioelectron. 2009;25:935. doi: 10.1016/j.bios.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 7.a) Ye BC, Yin BC. Angew Chem Int Ed. 2008;47:8386. doi: 10.1002/anie.200803069. [DOI] [PubMed] [Google Scholar]; b) Li D, Wieckowska A, Willner I. Angew Chem Int Ed. 2008;47:3927. doi: 10.1002/anie.200705991. [DOI] [PubMed] [Google Scholar]; c) Kong RM, Zhang XB, Zhang LL, Jin XY, Huan SY, Shen GL, Yu RQ. Chem Commun. 2009:5633. doi: 10.1039/b911163h. [DOI] [PubMed] [Google Scholar]; d) Huy GD, Zhang M, Zuo P, Ye BC. Analyst. 2011;136:3289. doi: 10.1039/c1an15373k. [DOI] [PubMed] [Google Scholar]
- 8.a) Li H, Zhang Y, Wang X, Xiong D, Bai Y. Materials Letters. 2007;61:1474. [Google Scholar]; b) Freeman R, Finder T, Willner I. Angew Chem Int Ed. 2009;48:7818. doi: 10.1002/anie.200902395. [DOI] [PubMed] [Google Scholar]
- 9.Chen P, He C. J Am Chem Soc. 2003;126:728. doi: 10.1021/ja0383975. [DOI] [PubMed] [Google Scholar]
- 10.Hakkila K, Green T, Leskinen P, Ivask A, Marks R, Virta M. J Appl Toxicol. 2004;24:333. doi: 10.1002/jat.1020. [DOI] [PubMed] [Google Scholar]
- 11.Clever GH, Kaul C, Carell T. Angew Chem Int Ed. 2007;46:6226. doi: 10.1002/anie.200701185. [DOI] [PubMed] [Google Scholar]
- 12.Miyake Y, Togashi H, Tashiro M, Yamaguchi H, Oda S, Kudo M, Tanaka Y, Kondo Y, Sawa R, Fujimoto T, Machinami T, Ono A. J Am Chem Soc. 2006;128:2172. doi: 10.1021/ja056354d. [DOI] [PubMed] [Google Scholar]
- 13.Ono A, Togashi H. Angew Chem Int Ed. 2004;43:4300. doi: 10.1002/anie.200454172. [DOI] [PubMed] [Google Scholar]
- 14.Huang J, Wu Y, Chen Y, Zhu Z, Yang X, Yang CJ, Wang K, Tan W. Angew Chem Int Ed. 2011;50:401. doi: 10.1002/anie.201005375. [DOI] [PubMed] [Google Scholar]
- 15.a) Yang CJ, Jockusch S, Vicens M, Turro NJ, Tan W. Proc Natl Acad Sci U S A. 2005;102:17278. doi: 10.1073/pnas.0508821102. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chen Y, Yang CJ, Wu Y, Conlon P, Kim Y, Lin H, Tan W. Chembiochem. 2008;9:355. doi: 10.1002/cbic.200700542. [DOI] [PubMed] [Google Scholar]
- 16.a) Yamana K, Iwai T, Ohtani Y, Sato S, Nakamura M, Nakano H. Bioconjugate Chem. 2002;13:1266. doi: 10.1021/bc025530u. [DOI] [PubMed] [Google Scholar]; b) Marti AA, Li X, Jockusch S, Li Z, Raveendra B, Kalachikov S, Russo JJ, Morozova I, Puthanveettil SV, Ju J, Turro NJ. Nucleic Acids Res. 2006;34:3161. doi: 10.1093/nar/gkl406. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Conlon P, Yang CJ, Wu Y, Chen Y, Martinez K, Kim Y, Stevens N, Marti AA, Jockusch S, Turro NJ, Tan W. J Am Chem Soc. 2008;130:336. doi: 10.1021/ja076411y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagatoishi S, Nojima T, Juskowiak B, Takenaka S. Angew Chem Int Ed. 2005;44:5067. doi: 10.1002/anie.200501506. [DOI] [PubMed] [Google Scholar]
- 18.Freeman R, Li Y, Tel-Vered R, Sharon E, Elbaz J, Willner I. Analyst. 2009;134:653. doi: 10.1039/b822836c. [DOI] [PubMed] [Google Scholar]
- 19.You M, Zhu Z, Liu H, Gulbakan B, Han D, Wang R, Williams KR, Tan W. ACS Appl Mater Interfaces. 2010;2:3601. doi: 10.1021/am1007886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.a) Bent DV, Hayon E. J Am Chem Soc. 1975;97:2612. doi: 10.1021/ja00843a004. [DOI] [PubMed] [Google Scholar]; b) Fung YME, Kjeldsen F, Silivra OA, Chan TWD, Zubarev RA. Angew Chem Int Ed. 2005;44:6399. doi: 10.1002/anie.200501533. [DOI] [PubMed] [Google Scholar]; c) Ly T, Julian RR. Angew Chem Int Ed. 2009;48:7130. doi: 10.1002/anie.200900613. [DOI] [PubMed] [Google Scholar]
- 21.Conlon P, Yang JC, Wu YR, Chen Y, Martinez K, Kim Y, Stevens N, Marti AA, Jockusch S, Turro NJ, Tan W. J Am Chem Soc. 2008;130:336. doi: 10.1021/ja076411y. [DOI] [PMC free article] [PubMed] [Google Scholar]