Abstract
Stem cells rely on extracellular signals produced by the niche, which dictate their ability to self-renew, expand and differentiate. It is essential to have sensitive and reproducible methods of either quantifying or isolating these stem cells and progenitors to understand their intrinsic properties and how extrinsic signals regulate their development. However, stem cells are difficult to distinguish from multipotential progenitors, which may look and act like them. Here we define a 4-color flow cytometry panel using CD133, LeX, CD140a, NG2 to define an NSC as well as 4 classes of multipotential progenitors and 3 classes of bipotential progenitors, several of which have not been previously described. We performed gain and loss of function studies for LIF and show a depletion of NSCs, a subset of multipotential neural precursors and immature oligodendrocytes in LIF null mice. Gain of function studies showed that LIF increased the abundance of these precursors. Our studies also show that these NPs have differential requirements for LIF and CNTF and for EGF, FGF-2 and PDGF for their propagation in vitro. Surprisingly, the related cytokine, CNTF was less potent than LIF in increasing the NSCs and more potent than LIF in increasing the PDGF responsive multipotential precursors. Finally, we show that LIF increases the expression of the core transcription factors: Klf4, Fbx15, Nanog, Sox2 and c-Myc. Altogether our FACS analyses reveal that the neonatal SVZ is far more heterogeneous than previously suspected and our studies provide new insights into the signals and mechanisms that regulate their self-renewal and proliferation.
Keywords: Neurogenesis, glial progenitors, gliogenesis, Subventricular zone, oligodendrocyte
INTRODUCTION
Virtually all of the neurons and glia that populate the mature brain arise from neural precursors (NPs) that reside immediately adjacent to the lateral ventricles. By parturition the ventricular zone (VZ) has regressed, but NPs persist within the subventricular zone (SVZ). The SVZ achieves its maximal size and heterogeneity during the neonatal period, when the neonatal brain is in a dynamic state of development. With the recent discovery that adult olfactory bulb neurogenesis in the human brain likely ceases after 18 months of age, there is increased interest in studying the NPs of the neonate [1].
Leukemia inhibitory factor receptor (LIFR) is an important signaling molecule that influences the heterogeneity within the germinal zones. At E11.5 when the VZ and SVZ are both present, the VZ robustly expresses the LIFR while the SVZ is deficient [2]. Within the primitive VZ there is a symmetric expansion of fibroblast growth factor-2 (FGF-2) - responsive neural stem cells (NSCs) [2]. During fetal development the cells of the choroid plexus begin to secrete many small molecules that include leukemia inhibitory factor (LIF) and ciliary neurotrophic factor (CNTF) that signal through the LIFR. LIF/CNTF creates a gradient within the germinal zones that establishe the distinct layers of VZ and SVZ and sustains self-renewal of the NPs [2]. As SVZ cells begin to expand exponentially during the latter third of fetal development epidermal growth factor (EGF)-responsive NPs emerge. Thus, the SVZ becomes a mixture of FGF2- and EGF-responsive precursors [3]. Also coincident with the emergence of the EGF responsive NPs is the shift from neurogenesis to astrogliogenesis and then to oligodendrogenesis.
Many neuroscientists have sought to isolate and characterize the NSC. Studies have identified numerous cell surface antigens that are expressed by subsets of NPs that include CD133, Lewis-X (LeX) and NG2 proteoglycan [4-8]. Unfortunately, these markers are not restricted to NPs. As NSCs fail to express unique lineage markers, investigators have implemented negative selection strategies using neuronal precursor markers like cholera toxin B subunit and tetanus toxin fragment C along with glial progenitor antigens: A2B5, O4 and O1 [9]. However, separations that fail to include CD133 and LeX are of limited utility [10].
With the discovery that adult NSCs express GFAP, recent studies have used hGFAP-GFP mice to study the NSCs [11,12]. Unfortunately, neonatal NSCs do not express GFAP since the transition from GFAP- to GFAP+ NSCs occurs during the second week of life thus these mice have limited utility for studies of embryonic or neonatal NSCs [13]. Here we report a novel 4-color flow cytometry and FACS protocol that enables the identification and characterization of a NSC, 4 classes of multipotential progenitors and 3 classes of bipotential progenitors and have evaluated their requirement for LIF signaling for their growth and maintenance. These studies reveal that the neonatal SVZ is far more heterogeneous than previously suspected and they provide new insights into the signals and mechanisms that regulate their self-renewal and proliferation.
Materials and Methods
C57BL/6 postnatal day 7 (P7) neonatal mice generated from breeders purchased from Jackson Laboratories (Bar Harbor, Maine) were used for neurosphere studies, FACS analysis and studies on the cellular composition of the adult SVZ. CD-1 background LIF P11 heterozygotes and nulls (bred with CD-1 mice purchased from Charles River Laboratories (Wilmington, MA) were genotyped using primers and the polymerase chain reaction-based method, along with age-matched wild type neonates for flow cytometric analysis. All experiments were performed in accordance with research guidelines set forth by the IACUC committee of the New Jersey Medical School.
Neurosphere Experiments
Spheres were prepared from P7 neonates using standard techniques. After 7 days in vitro, spheres were collected, digested with 70% strength Accutase (Millipore, Billerica, MA) and plated at 5×104 cells/cm2 for secondary (2°), tertiary (3°) or quaternary (4°) spheres. Spheres were cultured in chemically defined medium supplemented with 20 ng/ml EGF (PeproTech, Rocky Hill, NJ) and 10 ng/ml FGF2 (PeproTech, Rocky Hill, NJ) with 2ng/ml heparin (referred to as EF). Recombinant mouse LIF (rmLIF; Millipore, Billerica, MA) and recombinant rat CNTF (rrCNTF; R & D Systems, Minneapolis, MN) were used at 5 ng/ml, while soluble receptor alpha for rrCNTF were used at 100 ng/ml. Cells were grown for 6-7 days prior to analysis. A sphere was defined as a free-floating, cohesive cluster that was at least 30 μm in diameter, although the majority of spheres were larger in size. Plates were gently shaken to distribute the spheres before counting. 5 random 10x fields were counted per well and 6 wells were evaluated per group. The frequency of sphere-forming cells was calculated for 3° and 4° (Figure 3) by the average number of spheres per field, the area of the field and the area of the well using a Ziess Observer.Z1. Sphere volume was calculated using Ziess Axiovision software.
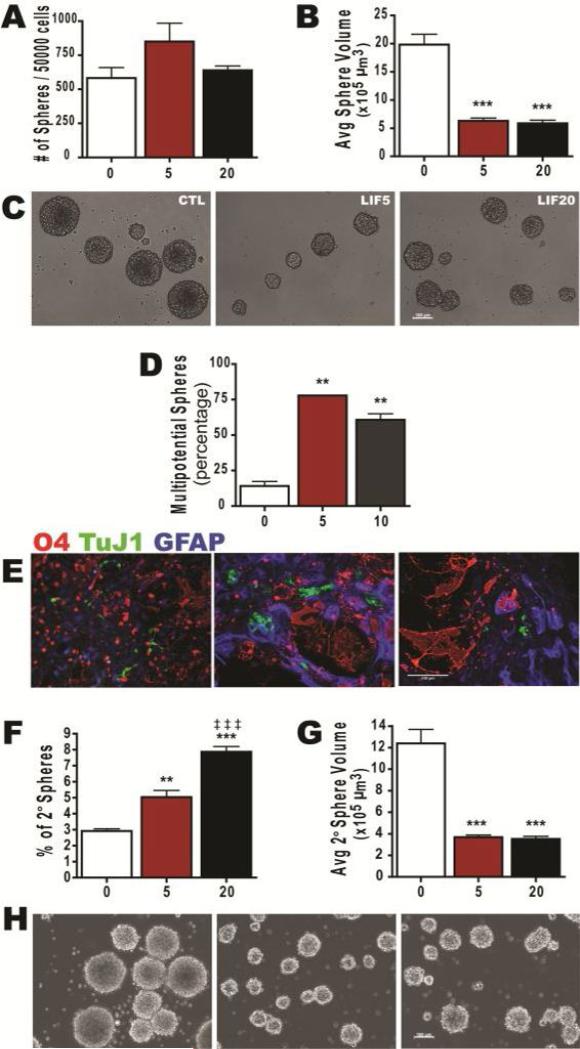
Figure 3. LIF Expands and Sustains Self-Renewing Multipotential Neonatal Neural Precursors.
Neurospheres established from P4-5 C57BL/6 mouse pups were passaged to secondary spheres and amplified in medium containing 20 ng/mL EGF, 20 ng/mL FGF-2 ± LIF. (A,B) Cells were treated with 5 or 20 ng/ml rmLIF for 7 DIV. Then the number (A) and size (B) of generated neurospheres was quantified. (C) Representative phase-contrast images of spheres at 7 DIV. (D) The percentage of multipotential spheres after 6 DIV LIF treatment. (E) Representative images of progeny immunostained for O4 (red), TuJ1 (green) and GFAP (blue). (F,G) Number of secondary spheres formed (F) and volume of spheres (G). (H) Representative phase contrast images of secondary spheres. Data are representative of 6 independent experiments with error bars representing mean ± SEM of n = 4 per experiment. At least 120 spheres were measured per group for volume calculations. **: p<0.01; ***: p<0.001 vs control and ‡ ‡ ‡: p < 0.001 vs 5ng LIF by Tukey's post hoc test. Scale bars represent: 100 μm in panels C and E.
Neurosphere Differentiation
3° spheres were plated onto chamber slides previously coated with 1% poly-d-lysine (w/v) and 10 μg/ml laminin, in ProN with 0.5% FBS without growth factors. After 5-7 days, chamber slides were stained using O4 culture supernatant (1:20) in 10% lamb serum in DMEM/F12 with 15 mM HEPES at room temperature (RT) for 45 min, washed in BCH (10% Newborn bovine serum in DMEM/F12 with HEPES) and then incubated for 30 min in GAM IgM lissamine rhodamine sulfonyl chloride (1:200; Jackson ImmunoResearch, West Grove, PA). Cells were fixed in 3% paraformaldehyde, quenched with 100 mM glycine, rinsed with PBS and then permeabilized with methanol for 20 min at -20°C. Cells were blocked in TGB superblock (0.3% triton-x-100, 10% goat serum, 10% bovine serum albumin in Tris-buffer) for 45 min and immunostained using anti-β-Tubulin III (TUJ1, 1:300; Covance, Princeton, NJ) and anti-GFAP (1:500; Dako, Carpenteria, CA) in TGB diluent O/N at 4°C. After washing thoroughly, cells were incubated for 30 min at RT in GAM dylight 488 IgG2a for TuJ1 and GAR dylight 649 IgG for GFAP (both at 1:300; Jackson ImmunoResearch, West Grove, PA). Cells were coverslipped with ProLong Gold with DAPI (4′,6-diamidino-2-phenylindole; Invitrogen, Carlsbad, CA). Images of stained cells were collected using a SenSys cooled-coupled device camera (CRI) interfaced with IPLab scientific imaging software (Scanalytics) on an Olympus AX-70 microscope.
Flow Cytometric Sample Preparation
2° or 3° spheres were dissociated with 0.2 Wünsch unit (WU)/ml of Liberase DH (Roche) and 250 μg of DNase1 (Sigma) in PGM solution (PBS with 1mM MgCl2 and 0.6% dextrose) and were incubated in a 37°C water bath for 5 min with gentle shaking. Equal volume of PGM was added and spheres were placed on shaker (LabLine) at 220 rpms at 37°C for 10 min. SVZs were dissociated with 0.45 WU/ml of Liberase DH and 250 μg of DNase1 in PGM and were shaken at 220 rpms at 37°C for 30 min. After enzymatic digestion, Liberase DH was quenched with 10 ml of PGB (PBS without Mg2+ and Ca2+ with 0.6% dextrose and 2 mg/ml fraction V of BSA-Fisher) and cells were centrifuged for 5 min at 200 × g. Cells were dissociated by repeated trituration, collected by centrifugation, counted using ViCell (Beckman Coulter, Miami, FL) and diluted to at least 106 cells per 50 μl of PGB. All staining was performed in 96 V-bottom plates using 150 μl volume. For surface marker analysis, cell were incubated in PGB for 25 min with antibodies against CD24-PE/Cy7 (1:300, M1/69; BioLegend), Lewis-X (1:20, LeX/CD15, MMA; BD Bioscience), CD133-APC (1:50,13A4; eBioscience), CD140a (1:400, APA5; BioLegend) and NG2 Chondroitin Sulfate Proteoglycan (1:50, AB5320; Millipore). Cells were washed with PGB by centrifugation at 300 × g. Anti-mouse IgM PerCP-eFluor 710 (1:200; eBioscience) was used for LeX, while goat anti-rabbit IgG Alexa Fluor 700 (1:100; Invitrogen) was used for NG2. Secondary antibodies and DAPI (for spheres – live analysis; Sigma) or LIVE/DEAD fixable Violet (for SVZs – fixed cell analysis; Invitrogen) were incubated in PGB for 20 min. DAPI or LIVE/DEAD was used for dead cell exclusion. To analyze oligodendrocyte lineage, we used a panel comprised of O4 (1:5) detected with anti-mouse IgM PerCP-efluor 710 (1:200). Cells were washed with PGB by centrifugation at 300 × g. Cells from SVZ were fixed with 1% ultra-pure formaldehyde (50000; Polysciences, Inc) for 20 min, collected by centrifugation for 9 min at 609 × g, resuspended in PBS w/o Mg2+ and Ca2+ and stored at 4°C for next day analysis. All sample data were collected on the BD LSR II (BD Biosciences Immunocytometry Systems).
Flow Cytometric Gating
Gating and data were analyzed with FlowJo software (Tree Star, Inc). Matching isotype control for all antibodies were used and gates were set based on these isotype controls [14]. Overall flow cytometric data used a flow cytometric gating profile of debris, dead cell and doublet exclusion. A positive control was used for dead cell exclusion by heating cells to 95°C for 3-5 min, filtering, adding DAPI or LIVE/Dead and then placing on ice prior to analysis. The gating strategy for neurospheres and SVZ were used is as follows: Gate 1) Live cell scatter: SSC-A vs FSC-A; Gate 2) Live cell gating with DAPI or LIVE/DEAD exclusion: FSC-A vs. DAPI or LIVE/DEAD; Gate 3) Single cell gating to exclude doublets: FSC-A vs FSC-W; Gate 4) CD140a-PE vs. NG2-Alexa Fluor 700; and Gate 5) CD133-APC vs. LeX-PerCP eFluor 710.
Cell Sorting
Dissociation and cell staining was performed as described above, with the exception that 15 ml falcon tubes were used instead of a 96-well plates and large volume washes were done. For sorting, cells were resuspended at 4×106/ml in PGB. Cells were isolated by FACSVantage™ SE, using 9 psi pressure and 90 μm nozzle aperture, while its regular sheath fluid was replaced with sterile PGB. Cells were collected with polypropylene tubes in 70% neurosphere conditioned medium (CM) plus EF and kept on ice. Cells were centrifuged at 300 × g and plated into 8-well chamber slides coated with 1% poly-d-lysine and 10 μg/ml laminin. Cells were plated at about 1000 cells (NSC), 8000 (MP4), or about 5000 cell/well and then expanded in either 70% CM plus EF or ProN plus PDGF-AA with FGF2-heparin. Medium was changed every other day. After 4-6 days, growth factors were withdrawn and cells were differentiated in DMEM/F12-HEPES containing B27 supplement (Gibco) with 0.5% FBS for 10 days. Medium changed every 2 days. Immunofluorescence imaging was performed as described above using O4, TuJ1 and GFAP. Chamber slides were also stained using A2B5 supernatant (1:5), GD3 (R24, 1:5; gift from Dr. James E. Goldman), GFAP, O4, GD3 and GFAP.
Flow Cytometry Analysis for adult mouse SVZs
Adult mouse SVZs were dissociated with 0.5 WU/ml of Liberase DH and 250 μg/ml of DNaseI in PGM with agitation at 220 rpms at 37°C for 1 hour. After enzymatic digestion, Liberase DH was quenched with 10 ml of PGB and cells were centrifuged for 5 minutes at 300 × g. Cells were dissociated by repeated trituration, then filtered through a 100 μm cell strainer, washed in 25 ml PGB and collected by centrifugation. To remove dead cells and debris, the cell suspension was layered onto 22% Percoll in PGB and centrifuge for 10 minutes at 500 × g (Lim DA, et al 2000). Cells were resuspended in PGM and processed for flow cytometry as described above.
Quantitative Real-Time PCR Analysis
Tertiary spheres were collected after treatment from EF and EF plus 5 and 20 ng/ml of rmLIF (EFL). Spheres were centrifuged, washed with PBS, snap frozen on dry ice in TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA) and stored at -80°C. Total RNA was extracted by homogenizing ice thawed TRIzol spheres, centrifuging and then following RNeasy Mini protocol (QIAGEN). cDNA was generated using SuperScript III reverse transcriptase (Invitrogen), according to manufacturer's instructions for calibrator (pooled P5 neonatal SVZ cDNA), 18S rRNA (Ambion, Inc.) and sphere sample. cDNA was quantified using Nanodrop (ND-1000; Thermo Scientific) and 100 ng of cDNA was used in each qPCR reaction well. QuantiTect SYBR Green c-Myc, distal-less homeobox 2 (Dlx2), F-box protein 15 (Fbx15), Alpha 1,3-fucosyltransferase IX (Fut9), krueppel-like factor 4 (Klf4), Nanog, sex determining region Y-box 1 (Sox1) and Sox2 primers according to manufacturer's protocol were utilized for quantitative real-time polymerase chain reaction (qPCR), using ABI Prism 7300 Sequence Detection System (Applied Biosystems). 18S RNA was used as the internal, endogenous normalization control. Relative quantification was evaluated by the ΔΔCt method to measure expression of genes reported.
Statistical Analysis
All data were expressed as means ± SEM and were analyzed using one-way ANOVA followed by Tukey's post-hoc analysis (Graphpad Prism 4 software).
RESULTS
Multi-panel isolation of SVZ NSC and seven phenotypically distinct progenitors
Multipotential NSCs that are CD133+LeX+Lin- reside within the SVZ [6,7]. In addition to these NSCs, the SVZ contains a mosaic of different developmentally staged precursors and lineage-restricted cell types [15-17]. Using FACS, investigators in the hematopoietic stem cell field have identified, purified and constructed a complex lineage tree for the formed cells of the blood. Progress has been made in studying “neuropoiesis;” however, there is no consensus for the types of NPs that exist nor is there a widely used panel of markers to classify the different types of precursors that reside within the brain's germinal matrices. Therefore, to enable a more complete characterization of the precursors of the SVZ, we evaluated several panels of cell surface markers.
The panel that proved most informative in distinguishing between NSCs, multi- and bi-potential progenitors and unipotential progenitors was comprised of the following markers: prominin-1: CD133, Lewis-X: LeX, heat stable antigen: CD24, NG2 chondroitin sulphate proteoglycan and platelet-derived growth factor receptor, alpha (PDGFRα): CD140a. To enrich for periventricular precursors dissociated cells from P7 neonatal mouse SVZs were amplified as neurospheres in medium containing EGF and FGF-2 (EF). The neurospheres were dissociated with Liberase-DH, a gentle enzymatic reagent that increases dissociation efficiency, viability, and cell surface antigen retention (particularly CD133 and CD24)[18], and incubated with CD133-APC, LeX-PerCP efluor 710, CD24-PE/Cy7, CD140a-PE, NG2-Alexa 700 and DAPI. We found that all NPs expressed CD24; and thus, CD24 was either ignored or eliminated from the panel. Debris, dead cells and doublets were excluded from analysis using DAPI and forward scatter gates while gates were set using isotype controls. From 9 independent FACS studies, 8 phenotypically different NPs were sorted using a two-gate strategy where the first gate was CD140a vs. NG2 (Figure 1A, quadrants A, B and C) and the second gate was CD133 vs. LeX (Figure 1B-D, boxes 1-8). The first distinct population was NG2+CD140a+ (Figure 1A box A) that were classified separately into three subpopulations: CD133+LeX+ (Box 1), CD133-LeX+ (Box 2) and CD133-LeX- (Box 3). The second population was NG2+CD140a- (Figure 1A box B), that was segmented into three subpopulations: CD133+LeX+ (Box 4), CD133-LeX+ (Box 5) and CD133-LeX- (Box 6), while the third population was double negative for NG2/CD140a (Figure 1A box C) which could be segmented into two subpopulations: CD133+LeX+ (Box 7) and CD133-LeX+ (Box 8).
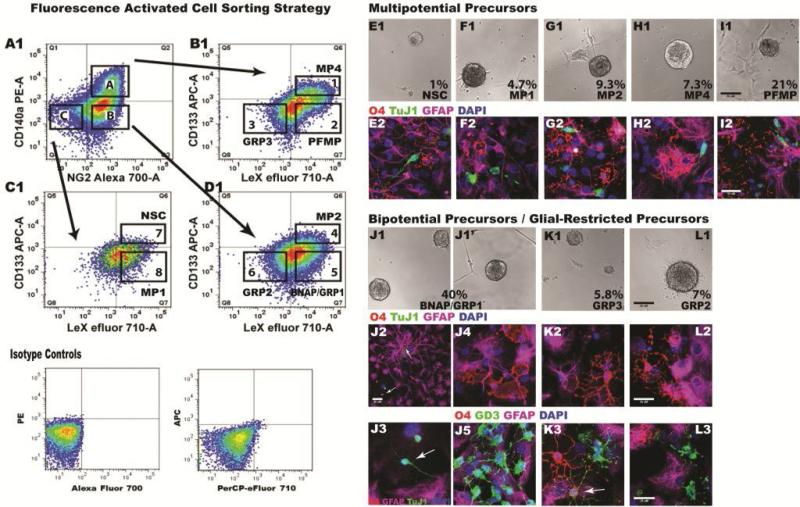
Figure 1. Diversity of Sorted Mouse Neonatal Neural Precursors.
(A1-D1) Secondary neurospheres from P7 C57BL/6 mouse pups were dissociated with Liberase-DH and sorted by FACS using a two-gate strategy. Debris and dead cells were excluded using DAPI and positive staining was established using isotype controls. After gating on CD140/NG2 (A1), cells were separated based on intensity of CD133/LeX (B1-D1). Eight cell types within the boxes in B1-D1 were sorted and analyzed. (E1-L1) Sorted cells were grown on poly-d-lysine and laminin-coated chamber slides in either EGF+FGF2 (NSCs, MP1, MP2 and BNAPs/GRP1) or PDGF-AA+FGF2 (MP4, PFMP, GRP3 and GRP2). Panels depict phase-contrast images of spheres at 4-6 DIV. (E2-L2).
Progeny of multipotential precursors. Growth factors were removed and cells were differentiated for 10 DIV and then fixed and immunostained for oligodendrocytic (O4; red), neuronal (TuJ1; green) and astrocytic (GFAP; purple) markers.
(J2, J3, J4, K2, L2) Progeny of bipotential progenitors stained for O4, GFAP and TuJ1. Arrows in J2 identify TuJ1+ cell and GFAP+ radial glial-like cells. Arrow in J3 identifies a TuJ1+ cell. (J5, K3, L3) Progeny of bipotential progenitors stained for O4 (red), GD3 (green) and GFAP (purple). Arrow in K3 indicates co-expression of GD3 and O4 observed on an immature oligodendrocyte. Scale bars represent: 50 μm in panels E1-l1; 25 μm for panels E2-L3. Data were aggregated from 9 independent FACS studies.
To determine the developmental potential of these eight subpopulations, sorted cells were plated onto poly-d-lysine and laminin coated chamber slides at low density and expanded with growth factors. Initially, all subpopulations were plated with 70% neurosphere-conditioned medium supplemented with EF; however, some precursors could not be expanded in EF. Those sorted precursors that responded to EF included CD133+LeX+NG2–CD140a–, CD133–LeX+NG2–CD140a–, CD133+LeX+NG2+CD140a– and CD133–LeX+NG2+CD140a–. Those precursors that expressed CD140a+ were expanded in PDGF-AA and FGF-2 (with the exception of CD133–LeX-NG2+CD140a– which were also expanded using PDGF-AA and FGF-2). The sorted CD133+LeX+NG2+CD140a+ progenitors required, in addition to PDGF-AA and FGF-2, B27-supplemented medium containing 0.5% FBS. After 4-6 days, all eight sorted NPs formed spheres (Figure 1E-L). The CD133+LeX+NG2–CD140a– precursors formed the smallest spheres (Figure 1E1-1L1).
To determine potentiality, we differentiated the colonies formed from these NPs by withdrawing the growth factors and maintaining them in 0.5% FBS for an additional 10 days. Triple immunostaining was performed for βIII-Tubulin (TUJ1; for immature neurons), GFAP (for astrocytes) and O4 (for oligodendrocytes). Additionally, cells were stained for TUJ1, GFAP and GD3 (b-series ganglioside, for immature cells [19]; or GFAP, GD3 and O4 or GFAP, GD3 and A2B5 (for immature cells and also bi- and uni-potential glial-restricted progenitors [20]). Five of the 8 subpopulations were multipotential precursors (MP), yielding neurons, oligodendrocytes and astrocytes: CD133+LeX+NG2-CD140a– (NSC; Figure 1E2), CD133–LeX+NG2–CD140a– (MP1; Figure 1F2), CD133+LeX+NG2+CD140a– (MP2; Figure 1G2), CD133+LeX+NG2+CD140a+ (MP4; Figure 1H2) and CD133-LeX+NG2+CD140a+ (referred to as PDGFRα-FGF2-responsive MP cell: PFMP; Figure 1I2). Spheres differentiated from CD133–LeX+NG2+CD140a– were bipotential, producing either astrocytes and neurons (referred to as bipotential neuronal-astrocytic precursor, BNAP; Figure 1J2-1J3) or astrocytes and oligodendrocytes (referred to as glial-restricted precursor, GRP1; Figure 1J4-1J5). Two other phenotypically distinct subpopulations were GRPs that produced oligodendrocytes and astrocytes: CD133–LeX–NG2+CD140a+ (GRP3; Figure 1K2-1K3) and CD133-LeX–NG2+CD140a– (GRP2; Figure 1L2-1L3). In separate studies we sorted NPs directly from the neonatal SVZ. The progeny that each SVZ precursor produced was the same, with the exception that cells with the cell surface phenotype of GRP2s when sorted directly from the SVZ differentiated astrocytes and oligodendrocytes; however, some of the colonies produced from these NG2+ precursors also produced neurons (data not shown). Thus, we will refer to this population of cells, which were directly sorted from the SVZ, as MP3/GRP2s (CD133-LeX–NG2+CD140a–).
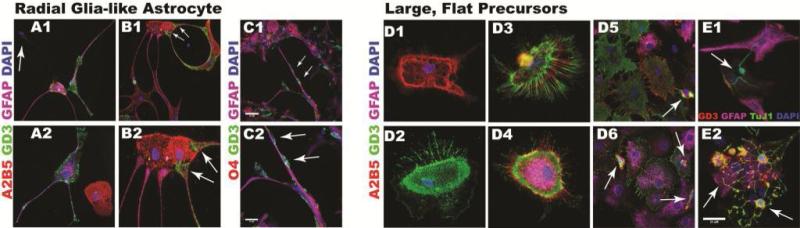
Amongst the progeny from these NPs, a variety of previously described glial subtypes types were seen. For example, a distinct A2B5-GD3+GFAP+ radial glial-like cell (Figure 2A1-2A2) with end-feet (Figure 2B1-2B2) was obtained when MP1 and BNAP/GRP1 colonies were differentiated. This radial glial-like cell was first described by Bonaguidi et. al. (2005) from SVZ NPs maintained in LIF [21]. Adhered to these radial-glial like cells were cells with migratory profiles (Figure 2C1-2C2). A second unique cell was a large flat cell that possessed large nuclei and had multiple, thin-spike-like projections; similar to cells previously described [22]. These flat cells were identified as A2B5+ or GD3+ (Figure 2D1-1D2) or A2B5+GD3+GFAP± (Figure 2D3-D4) and often resided within dense heterogeneous groups (Figure 2D5-2D6). Small A2B5+GD3+GFAP– cells, that were presumably oligodendrocyte precursors, were found on top of these glial cells (Figure 2D6). These large flat cells were observed within colonies containing neurons or oligodendrocytes (Figure 2E1 and 2E2). However, none of the flat cells were O4+. These large flat cells persisted even after 10 days of growth factor withdrawal. The two glial-restricted precursors (GRP2 and GRP3) generated A2B5loGD3-GFAP+ (immature astrocytes) and A2B5+GD3+GFAP- or O4±GD3+GFAP– ((immature oligodendrocytes); Figure 2E2 and 1J5, 1K3, 1L3). To our knowledge this is the first description of the isolation of SVZ precursors capable of producing these radial glia-like and large GD3+ flat cells.
Figure 2. Astroglial Progeny of Sorted Mouse Neural Precursors.
Secondary neurospheres from P7 C57BL/6 mouse pups were dissociated with Liberase-DH and sorted by FACS and differentiated as described in Figure 1. Panels A-E depict representative images of radial glial-like astrocytes from NSC, MP1 and BNAP/GRP1. Cells were immunostained for A2B5 (red), GD3 (green) and GFAP (purple). (A1-A2) depict endfeet of a radial glial-like cell contacting an A2B5+ dividing cell (arrows). (B1-B2) depict radial glial-like cell in an astrocyte (GFAP-purple) and oligodendrocyte (O4 red) rich area. Arrows in C2 indicate two immature GD3+ (green) cells on a radial process. (D-E) illustrate large, flat precursors immunostained with A2B5 (red), GD3 (green) and GFAP (purple). (D1-D6) Arrows indicate small immature cells on top of flat cells. (E1) A small colony of flat cells and a neuron (GD3-red, TUJ1-green, GFAP-purple). Arrow points to the neuron. (E2) A colony of flat cells, immature astrocytes (A2B5+GD3-GFAP+, arrow) and immature oligodendrocytes (A2B5+GD3+GFAP-, arrow). Nuclei in images were stained with DAPI (blue). Scale bars represent: 50 μm for panels C1, C2; and 25 μm for panels A-B, D-E. Data were aggregated from 9 independent FACS studies.
NSA reveals expansion, sustained self-renewal and increased multipotentiality with LIF treatment
LIF is known for its pleiotropic effects on various cell types and at many stages of nervous system development. LIF has been shown to promote the survival, expansion and/or self-renewal of human [23], rodent embryonic [24] and adult mouse putative NSCs [25]. The NSA was used to assess the effects of LIF on expansion, self-renewal and fate of neonatal SVZ precursors and these studies used high doses of LIF (20 ng/ml or greater) to obtain maximum responses [24,25]. However, LIF is known to exert dose-dependent effects; therefore, we evaluated two doses within (5 ng/ml) and above (20 ng/ml) the Kd range of the LIF/LIFR/gp130 complex[26]. Stimulating NPs with 5 or 20 ng/ml LIF for 7 days had no effect on neurosphere number, but decreased sphere size by 75% (Figure 3A-3C). Despite the smaller size of the spheres, LIF increased the proportion of multipotential spheres by 5-fold. Upon passaging neurospheres after LIF treatment, there was a 2-fold increase in sphere number, but again the spheres were significantly smaller (30% control; Figure 3F-3H). Using the NSA, these data suggest that LIF expands and sustains the self-renewal of multipotential NP(s).
Flow cytometry demonstrates that LIF expands neonatal NSCs, LeX+ intermediate progenitors and immature oligodendrocytes
Having established a flow cytometric method that enables the quantification of 8 different types of neural precursors, we used this assay to test the hypothesis that LIF stimulates NSC expansion. The addition of 5ng/mL LIF to defined medium used to grow NPs doubled the percentage of NSCs, increased MP1s (30%), PFMPs (10%) and BNAP/GRP1s (19%; Figure 4). However, LIF also reduced another pool of precursors that we hypothesize are descended from the MP2 progenitor due to their surface marker phenotype: MP2s (33%), MP4s (55%) and MP3/GRP2s (35%). Unexpectedly, LIF reduced the frequency of GRP3s (52%; Figure 4). There was no difference by flow cytometry between 5 ng/ml and 20 ng/ml of LIF (data not shown). These data indicate that LIF specifically promotes the growth and maintenance of three phenotypically distinct multipotent precursors; NSCs, MP1s and PFMPs, and one mixed population of bipotential progenitors (BNAP/GRP1s), while simultaneously repressing the expansion of a separate set of LIF-nonresponsive progenitors.
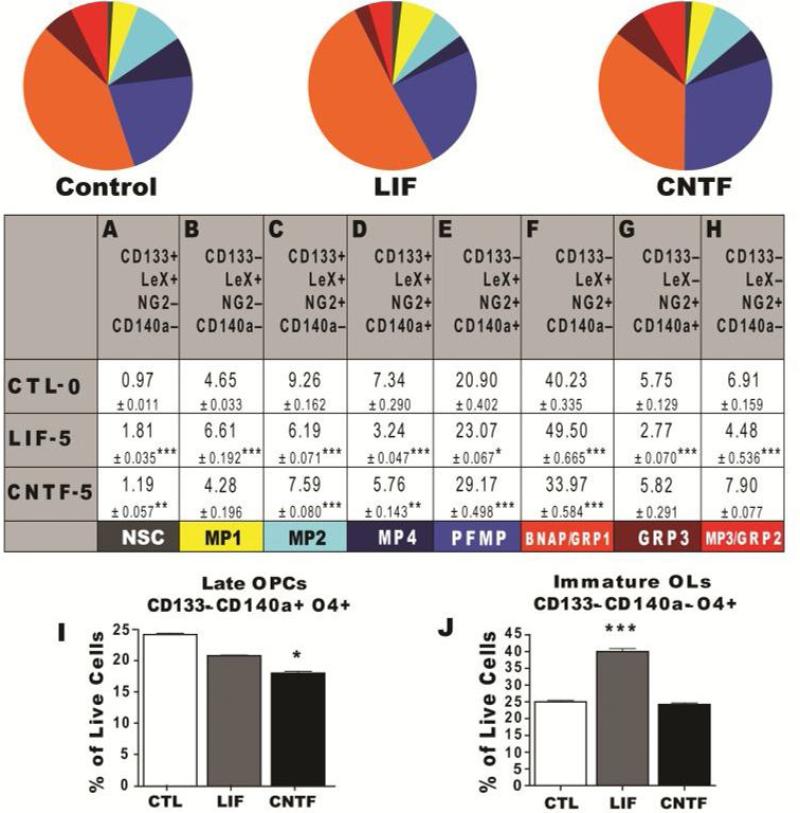
Figure 4. LIF and CNTF Differentially Regulate Neural Precursor Homeostasis.
(A-J) Neurospheres established from P4-5 C57BL/6 mouse pups were passaged to secondary spheres and amplified in medium containing 20 ng/mL EGF, 20 ng/mL FGF-2 with either 5 ng/mL rmLIF or rrCNTF (plus 100ng/mL sCNTF receptor). The proportion of NP subtypes was quantified by flow cytometry, using a NP panel (pie graphs and table): CD133/LeX/NG2/CD140a/CD24 and an oligodendrocyte (OL) panel (bar graphs): CD133/CD140a/O4/CD24. 20000 to 40000 live cell events were analyzed after excluding debris and dead cell using DAPI and gating on positively stained cells based on isotype controls. All cells were CD24+. Data represent averages from 3 independent experiments for CTL, and 5 ng/ml LIF and CNTF. Table and error bars represent mean percentages ± SEM. *: P < 0.05; **: P < 0.01; ***: P < 0.001 versus control by Tukey's post hoc test.
Like LIF, CNTF, which is structurally similar, has been reported to regulate NP self-renewal, multipotency and maturation of the oligodendrocyte lineage[27]. However, it has not been established whether this related, but distinct cytokine exerts similar or different effects on NPs. Therefore, we used flow cytometry with two multimarker panels to determine how LIF and CNTF affect NP population dynamics. These analyses revealed significant differences, but also similarities between LIF and CNTF. As described above, LIF increased the proportion of NSCs, MP1s, PFMPs and BNAP/GRP1s, while it significantly decreased MP2s, MP4s, GRP3s and GRP2s. LIF expanded NSCs and MP1s by 1.5-fold compared to CNTF (ANOVA, Tukey test, P<0.001; Figure 4A and 4B). Both cytokines increased PFMPs; and decreased MP2 and MP4 percentages (Figure 4C and 4D). Using the CD133/CD140a/O4-panel, LIF increased the production of immature oligodendrocytes (CD133-CD140a-O4+) by 43% when compared to control and CNTF (ANOVA, Tukey Test, P<0.001; Figure 4J). Interestingly, CNTF had no effect on the production of pre- or immature oligodendrocytes. Altogether these data demonstrate that LIF is unique in its ability to expand neonatal NSCs, MP1s, BNAP/GRP1s and immature oligodendrocytes.
LIF increases self-renewal genes and regulates LeX expression by FUT9
LIF increased production of secondary neurospheres and the proportion of NPs expressing the LeX marker, suggesting that this cytokine enhances NP self-renewal. To investigate this possibility, the expression of several stem cell self-renewal genes was assessed. Q-PCR revealed that spheres treated with 5 and 20 ng/ml of LIF had increased expression of Klf4, F-box protein 15 (Fbx15), Nanog, sex determining region Y-box 2 (Sox2) and c-Myc (Table 2). At the higher concentration, LIF increased the expression of Sox1 and Dlx2; Table 2). Flow cytometric analysis of the geometric mean fluorescent intensity established that LIF increased LeX expression by 75% (ANOVA, Tukey Test, P<0.001). Fut9 is a synthetic enzyme for LeX expressing proteins and lipids. Fut9 increased in a dose-dependent fashion between 5 ng/ml and 20 ng/ml of LIF (Table 2). Overall, these data are consistent with the interpretation that LIF keeps NPs in a self-renewing state by increasing their expression of Fut9, Klf4, Fbx15, Nanog, Sox2 and c-Myc.
Table 2. LIF induces the expression of specific self-renewal genes.
Neural precursor gene expression was analyzed by qPCR after 7 days in control (EF) and LIF supplemented medium (EFL) at 5 and 20 ng/ml. mRNA levels were measured using QuantiTech primers from Qiagen and SYBR-Green. Levels were normalized to a cDNA pool produced from P5 neonatal SVZ. Values represent average fold increase in LIF treated cultures from 3 independent experiments.
| GENE | EFL5/EF | EFL20/EF |
|---|---|---|
| Klf4 | 6.508 | 7.213 |
| Fbx15 | 3.070 | 2.764 |
| Nanog | 1.876 | 2.306 |
| Sox2 | 1.739 | 2.976 |
| c-Myc | 1.629 | 2.191 |
| Fut9 | 1.294 | 2.311 |
| Dlx2 | 0.813 | 2.180 |
| Sox1 | 0.756 | 1.596 |
LIF deficiency decreases NSCs & immature oligodendrocytes, while intermediate progenitors accumulate
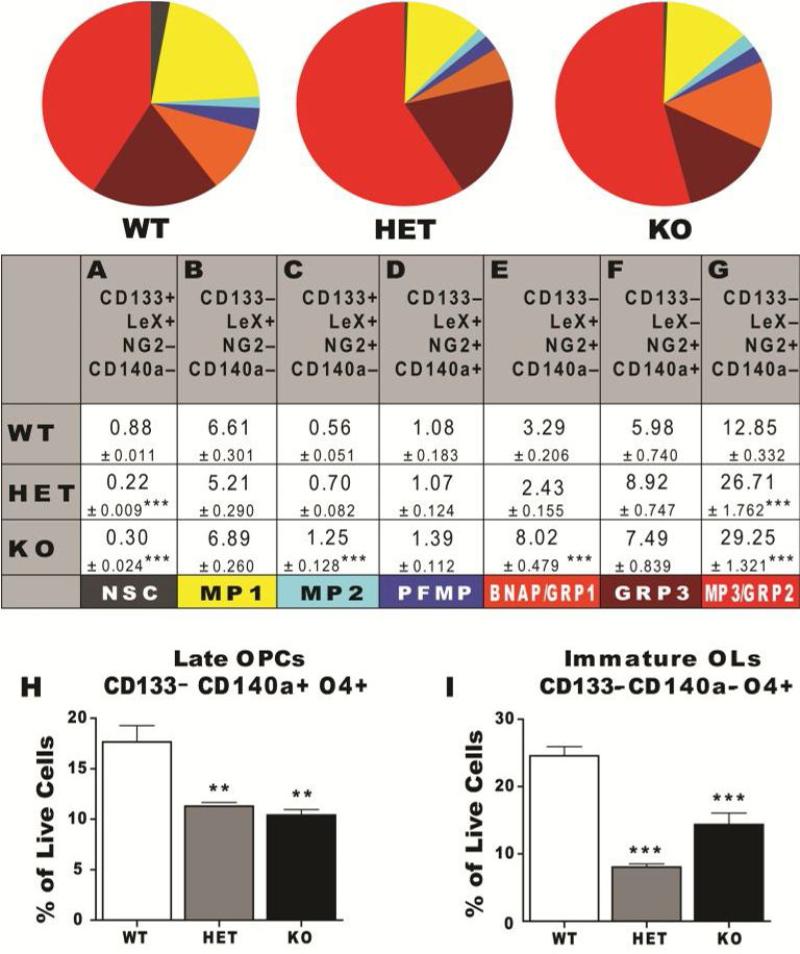
Neural stem and progenitor cells within the SVZ receive signals from their niche to self-renew, expand and differentiate. Prior studies have shown that LIF is secreted by choroid plexus cells [2] and astrocytes [28], two of the main regulators of the periventricular niche. Despite the recognized important role that LIF exerts on stem cell self-renewal, the SVZ of the LIF null mouse has not been analyzed to determine whether there are fewer NPs and/or NSCs. Thus, to test the above in vitro model of LIF function, and also to better understand the role of LIF in neural development, the cellular composition of the SVZ of individual LIF knockout and heterozygous neonates were assessed by flow cytometry. SVZs were micro-dissected from 11-day old wild type (WT), LIF heterozygous (Het) and LIF knockout (KO) neonates. The SVZs were dissociated into single cell suspensions and stained for the two panels of NPs described earlier. Flow cytometry revealed that 7 out of the 8 NPs phenotypically identified NPs present in neurospheres were identified within the SVZ (MP4 cells were undetectable). As mentioned earlier, some of the NG2+ cells directly sorted from the SVZ generated neurons and glia whereas others only produced glia; therefore, the group of cells with phenotype of CD133–LeX–NG2+CD140a– is likely a mixture of two precursors which we will henceforth refer to as MP3/GRP2 cells. By flow cytometry, LIF KO mice displayed a 66% reduction in NSCs and a 42% reduction in immature oligodendrocytes compared to wild-type mice (Figure 5A and 5I), but an increased proportion of MP2s (74%), BNAP/GRP1s (59%), MP3/GRP2s (59%; Figure 5C, 5E and 5G). Surprisingly, LIF Het displayed a similar phenotype as the LIF nulls. These changes in vivo due to the loss of LIF were opposite to those obtained with the gain of LIF studies where there was an expansion in all three bipotential progenitor populations. Similar to the in vitro flow cytometry data, CD24 expression was detected in all SVZ populations [14].
Figure 5. LIF Deficiency Disrupts the Steady State Balance of SVZ Precursors Leading to a Surplus of Intermediate Progenitors.
(A-I) SVZs from P11 WT, LIF Het and LIF KO CD1 mice were analyzed by flow cytometry using the NP panel (pie graph and table): CD133/LeX/NG2/ CD140a/CD24 and OL panel (bar graphs): CD133/CD140a/O4/CD24. Six independent animals per genotype (WT, LIF Het and LIF KO) were analyzed (3 female and 3 male). 50000 live cell events were analyzed per animal after excluding debris and dead cell using LIVE/DEAD violet and gating on positively stained cells based on isotype controls. All cells were CD24+. Table and error bars represent mean percentages ± SEM.
*: P < 0.05; **: P < 0.01; ***: P < 0.001 versus control by Tukey's post hoc test.
Many studies have characterized the adult SVZ as being comprised of 4 classes of precursors: A cells, two types of B cells and C cells. Therefore, it was of interest to compare the composition of the adult mouse SVZ with that of the neonatal SVZ using our multimarker flow cytometry panel. The SVZs from 2 month old mice were microdissected from the periventricular wall, enzymatically and mechanically dissociated and then layered onto 22% Percoll to remove debris prior to stained for the cell surface markers CD133, LeX, NG2 and CD140a. Flow cytometry revealed that the multimarker-panel identified seven distinct progenitors that were present at the following frequencies: NSCs (0.23 ± 0.08%) MP1 (10.99 ± 2.89%), MP2 (0.40 ± 0.20%), PFMP (0.17±0.11%), BNAP/GRP1 (3.48 ± 2.28%), MP3/GRP2 (8.02 ± 0.53) and GRP3 (0.19 ± 0.03%). These results are averages from 3 separate analyses where the SVZs from 8 animals were pooled for each analysis in order to obtain at least 1 × 106 viable cells for flow cytometry.
Altogether, these multimarker flow cytometry studies have defined important new tools to analyze NPs both in vitro and in vivo. These panels have identified new types of neural precursors, demonstrated that both the neonatal and adult SVZ are comprised of complex mosaic of neural precursors and demonstrated the important role that LIF plays in regulating neonatal NPs, especially in NSC maintenance and immature oligodendrocyte production.
DISCUSSION
To evaluate the outcomes of perturbing the signals that regulate neural stem cells and progenitors, sensitive and reproducible methods of quantifying the relative numbers of stem cells and progenitors are needed. Using multimarker flow cytometry we have established the means to isolate NSCs and 7 types of intermediate neural progenitors. Studies of the fetal and neonatal SVZ foreshadowed the diversity that we now document [15,29,30]. By defining precursors as either positive or negative for one of 4 cell surface antigens, 7 classes of cells were partitioned from dissociates of the neonatal SVZ. The descendants that each precursor produced are summarized in Figure 6. NSCs (CD133+LeX+NG2–CD140a–CD24lo) represented a small percentage of the total cells, as expected from estimates of their prevalence of 1% as previously reported within neurospheres [31]. The abundance of NSCs in the adult SVZ agrees with previous estimates of 0.2-0.4% [32]. That the value we report for NSC abundance within the neonatal SVZ is higher than that reported for the adult should not be surprising since the percentage of NSCs decreases with age [33]. The NSCs were extremely slowly dividing cells and they generated smaller spheres than the other NPs. This observed relative quiescence agrees with the estimates of the cell cycle time for a progenitor as 13h, while for a NSC it is 2-3 weeks in vivo [34]. In summing the percentages of identified cells in the P11 SVZ (from both the table as well as from the two graphs in figure 5), our flow cytometric analysis accounts for 73% of the total cells. Missing from our FACs and flow analysis panel are cell surface markers that identify precursors committed to the astrocyte lineage and precursors that are committed neuroblasts. Previous retroviral lineage tracing experiments of SVZ cells have established that approximately 20% of the dividing cells within the P14 SVZ are restricted to the astrocyte lineage and 6% are restricted neuroblasts; thus, these two populations of precursors likely comprise the bulk of the cells that were unclassified using our 4 color flow panel {Young, 1996 #360}. Studies are underway to incorporate additional surface markers for these progenitors into a larger multicolor panel.
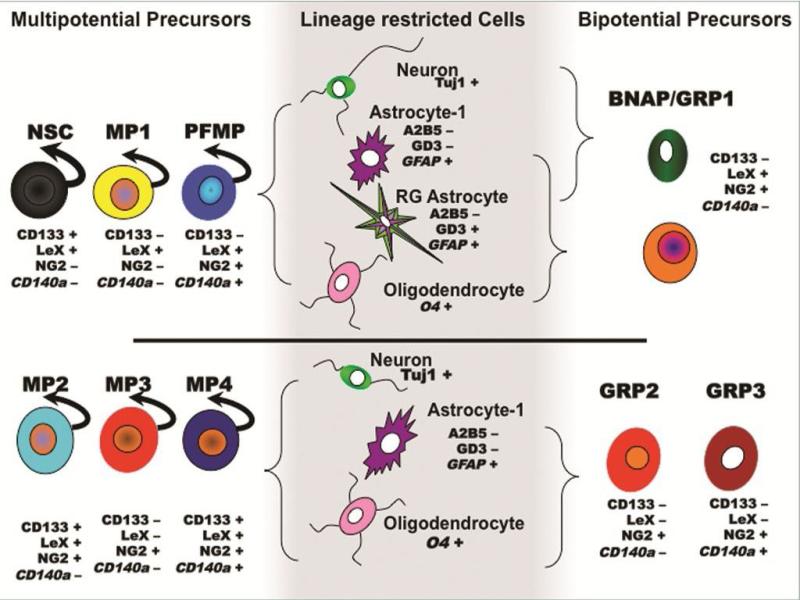
Figure 6. LIF Responsiveness on Neonatal SVZ Neural Precursor Diversity.
This schematic summarizes the diverse set of sorted neonatal NPs, their fate and their responsiveness to LIF. NPs that responded to LIF include NSCs, MP1s and PFMFs which differentiate into neurons, two types of phenotypically distinct astrocytes and oligodendrocytes; BNAP/GRP1 which differentiate into neurons and 2 types of astrocytes (BNAP) or oligodendrocytes and 2 types of astrocytes (GRP1). Sorted NPs unresponsive to LIF include MP2s and MP4s which differentiate into neurons, astrocytes and oligodendrocytes; GRP2s and GRP3s which differentiate into astrocytes and oligodendrocytes. Curved arrows above NPs depict sustained self-renewal in response to LIF.
Our data corroborate previous flow cytometric analyses that demonstrated that CD24 is found on all NPs. Several studies have reported that NSCs and primitive progenitors express low levels of CD24 [4,18], whereas others have reported that NSCs are CD24 negative [7,11]. CD24 is sensitive to enzymatic degradation; therefore, using gentle enzymes like collagenase plus dispase (in Liberase [18]) or collagenase plus hyaluronidase [4], will retain the CD24 antigen.
Of the 4 sorted multipotential precursors (excluding MP4s because they were not found in vivo), one expressed the PDGFRa, which has been predominantly associated with oligodendrocyte progenitors. This CD133-LeX+NG2+CD140a+ cell (PFMP) is likely the NG2+ multipotential precursor that has been isolated from the embryonic forebrain [35] and from neonatal and adult SVZs [36,37]. Recent fate mapping studies have suggested that these multipotential PDGFRa+ precursors produce pyramidal neurons of the piriform cortex [36,38]. Interestingly, we also found that the PFMPs did not self-renew when grown in medium supplemented with EGF and FGF2, but proliferated and self-renewed when placed into medium containing PDGF-AA and FGF2, similar to results obtained by Chojnacki et al., 2004 [35]. In comparing the neonatal vs. the adult SVZ, the PFMPs showed the largest reduction with age.
The existence of bipotential precursors and especially glial-restricted precursors that can generate astrocytes and oligodendrocytes has been a subject of much debate [39,40]. Originally shown to be present within the SVZ of the neonate using retroviral fate mapping analyses in vivo [41], these cells have since been found within the spinal cord [20]. More recently two GRPs were isolated from the embryonic telencephalon, having an A2B5+NG2–CD140a– phenotype [42]. Extending these earlier studies, our data reveal the existence of 3 pools of bipotential progenitors within the neonatal SVZ: CD133–LeX+NG2+CD140a– (BNAP/GRP1), CD133–LeX-NG2+CD140a+ (GRP3) and CD133–LeX–NG2+CD140a–(GRP2). GRPs isolated from spinal cord, optic nerve and from the ventral and dorsal telencephalon depend upon FGFs for survival and proliferation. In agreement with these studies, only one of the three GRPs expressed CD140a (GRP3) which is likely the “Pre-O-2A” cell previously described in cultures of rat telencephon by Ben-Hur et al. (1998) and by Grinspan et al. (1990)[43,44]. Studies using transgenic mice have revealed two NG2+ populations (Plp-NG2+CD140+ and Plp+NG2+CD140a–) that are located within the neonatal SVZ and appear to be closely related, having similar morphology, migratory and proliferative capacity [45,46]. All of the GRPs we isolated were NG2+ immunoreactive. Using NG2 reporter mice, a study found that in vitro NG2 cells produced oligodendrocytes, astrocytes, while in vivo, they produced myelinating oligodendrocytes and protoplasmic astrocytes [47].
LIF is essential for maintaining self-renewal in mESC cultures and, in addition, it sustains cortical, embryonic and adult SVZ NPs in vitro [24,25,48]. These studies have concluded that LIF enhances self-renewal by promoting symmetric expansions at the expense of asymmetric divisions. If LIF only affected NSCs, then one would predict that in the absence of LIF that there would be a reduction in NSCs and an increase in all of the intermediate precursors and that the opposite would be obtained with LIF supplementation; however, this is not what we observed. All previous studies used the NSA, and assumed that an increase in self-renewal and multipotential spheres reflected an increase in NSCs. These earlier studies were indeed partially correct in that LIF maintains NSCs, but our data reveal that LIF expands several LIF-responsive precursors that appear to be lineally related and which consist of two MPs (MP1 and PFMP) and a mixed bipotential neuronal-glial population (BNAP/GRP1; Table 3). Our data also suggest that LIF expands precursors whose progeny include radial glial-like astrocytes, reminiscent of previous observations [21]. Our data further suggest that LIF blocks MP2 expansion; and thus, inhibits production of its progeny (GRP2). With LIF deficiency, NSCs and immature oligodendrocytes are primarily affected. Accompanying the significant reduction in NSCs, there is an expansion of MP2s and their progeny. However, the upsurge of intermediate bipotential progenitors is not completely because the NSCs have lost their ability to self-renew, as concluded from studies of LIFR nulls [2]. The expansion of these restricted progenitors is also due to their inability to further differentiate into immature oligodendrocytes. This accumulation of NG2+ cells was also seen within the optic nerves of LIF nulls at P10 [49]. However, based on their antigen expression and differentiation capacity, the NG2 cells that accumulated within the SVZ are likely less differentiated than those that accumulate in the optic nerve.
Table 3. Summary of In Vitro vs In Vivo Effects of LIF on Neural Precursor Population Dynamics.
Flow cytometry data are summarized from 7 day in vitro treatment with rmLIF at 5 ng/ml and from LIF null mice SVZs. UC indicates no change between control and experimental. Some cell types were identified in one environment, but were not detected (ND) in the other.
| Surface Marker ID | + LIF – | |||||
|---|---|---|---|---|---|---|
| Cell | CD133 LeX NG2 CD140a | In Vitro | In Vivo | |||
| NSC | + | + | – | – | up | down |
| MP1 | – | + | – | – | up | UC |
| MP2 | + | + | + | – | down | up |
| MP4 | + | + | + | – | down | ND |
| PFMP | – | + | + | + | up | UC |
| BNAP/GRP1 | – | + | + | – | up | up |
| GRP3 | – | – | + | + | down | UC |
| MP3/GRP2 | – | – | + | – | down | up |
| Pre-OL | – | – | – | + | UC | down |
| Im-OL | – | – | – | – | up | down |
In conclusion, we have established the means to isolate a NSC and 7 types of intermediate NPs by flow cytometry. Using FACS we have begun to gain insights into the growth factor requirements of each type of intermediate progenitor as well as the progeny that they produce. Our studies suggest that there are at least three multipotential progenitors within the neonatal SVZ that can each produce neurons, astrocytes and oligodendrocytes. We have shown that these precursors are differentially regulated by LIF and that the types of glial cells that they produce are distinct. It is likely that each precursor also produces unique types of neurons, which we did not attempt to evaluate. Clearly many more studies will be required to fully understand the intrinsic properties and lineage relationships of each precursor type and how they respond to environmental cues. Given the interest in cancer stem cells, we anticipate that having the ability to isolate NPs at different stages of developmental restriction will provide new insights into the origins of specific brain tumors, such as glioblastoma. Finally, these studies are not only informative for understanding neural development, but also for promoting nervous system regeneration and repair.
Table 1. Antibody Panels used for Flow Cytometry.
Summary of the 2 antibody panels used to characterize neural precursors by flow cytometry.
| Antibody | Antibody and Source | LSRII Configuration | |
|---|---|---|---|
| Neural Precursor Panel | CD133-APC | 13A4; eBioscience | 660/20: 633-Laser 4 |
| LeX-eFluor 710 | MMA; BD Bioscience | 710/50: 488-Laser 1 | |
| CD140a-PE | APA5; BioLegend | 575/26: 488-Laser 1 | |
| NG2-AF700 | AB5320; Millipore | 730/45: 633-Laser 4 | |
| DAPI | 450/50: UV-Laser 3 | ||
| Oligodendrocyte Precursor Panel | CD133-APC | 13A4; eBioscience | 660/20: 633-Laser 4 |
| CD140a-PE | APA5; BioLegend | 575/26: 488-Laser 1 | |
| O4-eFluor 710 | Anti-mouse IgM PerCP-eFluor 710; eBioscience | 710/50: 488-Laser 1 | |
| CD24-PE/Cy7 | M1/69; BioLegend | 780/60: 488-Laser 1 | |
| DAPI | 450/50: UV-Laser 3 |
ACKNOWLEDGEMENTS
We thank C. Stewart for permission to use his LIF null mice; R.D. Fields for shipping the LIF mice; S. Singh and D. Stein for flow cytometry assistance; T. Galenkamp for cell sorting; J. Goldman for GD3 antibody and A. White and D. Lazzarino for editing the manuscript. This work was presented in part at the American Society for Neurochemistry meeting [50] and was supported by grants from the National Institutes of Health, MH59950 and HD052064, and the Leducq Foundation awarded to S.W.L, along with a NIH NINDS Training Grant fellowship, NS051157 awarded to K.D.B.
REFERENCES CITED
- 1.Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, Rowitch DH, Alvarez-Buylla A. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregg C, Weiss S. CNTF/lif/gp130 receptor complex signaling maintains a vz precursor differentiation gradient in the developing ventral forebrain. Development. 2005;132:565–578. doi: 10.1242/dev.01592. [DOI] [PubMed] [Google Scholar]
- 3.Vescovi AL, Reynolds BA, Fraser DD, Weiss S. Bfgf regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) egf-generated cns progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- 4.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawamoto K, Nakao N, Kakishita K, Ogawa Y, Toyama Y, Yamamoto A, Yamaguchi M, Mori K, Goldman SA, Itakura T, Okano H. Generation of dopaminergic neurons in the adult brain from mesencephalic precursor cells labeled with a nestin-gfp transgene. J Neurosci. 2001;21:3895–3903. doi: 10.1523/JNEUROSCI.21-11-03895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC, Jr., Fan G, de Vellis J, Sun YE. Cd133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci U S A. 2008;105:1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capela A, Temple S. Lex/ssea-1 is expressed by adult mouse cns stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 8.Jablonska B, Aguirre A, Vandenbosch R, Belachew S, Berthet C, Kaldis P, Gallo V. Cdk2 is critical for proliferation and self-renewal of neural progenitor cells in the adult subventricular zone. J Cell Biol. 2007;179:1231–1245. doi: 10.1083/jcb.200702031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maric D, Maric I, Chang YH, Barker JL. Prospective cell sorting of embryonic rat neural stem cells and neuronal and glial progenitors reveals selective effects of basic fibroblast growth factor and epidermal growth factor on self-renewal and differentiation. J Neurosci. 2003;23:240–251. doi: 10.1523/JNEUROSCI.23-01-00240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rietze RL, Valcanis H, Brooker GF, Thomas T, Voss AK, Bartlett PF. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412:736–739. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- 11.Pastrana E, Cheng LC, Doetsch F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc Natl Acad Sci U S A. 2009;106:6387–6392. doi: 10.1073/pnas.0810407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckervordersandforth R, Tripathi P, Ninkovic J, Bayam E, Lepier A, Stempfhuber B, Kirchhoff F, Hirrlinger J, Haslinger A, Lie DC, Beckers J, Yoder B, Irmler M, Gotz M. In vivo fate mapping and expression analysis reveals molecular hallmarks of prospectively isolated adult neural stem cells. Cell Stem Cell. 2010;7:744–758. doi: 10.1016/j.stem.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004 doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buono KD. Ph.D. Dissertation. University of Medicine and Dentistry of New Jersey; Newark: 2011. Analyses of mouse neural precursor responses to leukemia inhibitor factor and hypoxia/ischemia: Graduate School of Biomedical Sciences. pp. 1–200. [Google Scholar]
- 15.Gal JS, Morozov YM, Ayoub AE, Chatterjee M, Rakic P, Haydar TF. Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zones. J Neurosci. 2006;26:1045–1056. doi: 10.1523/JNEUROSCI.4499-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levison SW, Goldman JE. Multipotential and lineage restricted precursors coexist in the mammalian perinatal subventricular zone. J Neurosci Res. 1997;48:83–94. [PubMed] [Google Scholar]
- 18.Panchision DM, Chen HL, Pistollato F, Papini D, Ni HT, Hawley TS. Optimized flow cytometric analysis of central nervous system tissue reveals novel functional relationships among cells expressing cd133, cd15, and cd24. Stem Cells. 2007;25:1560–1570. doi: 10.1634/stemcells.2006-0260. [DOI] [PubMed] [Google Scholar]
- 19.Goldman JE, Hirano M, Yu RK, Seyfried TN. Gd3 ganglioside is a glycolipid characteristic of immature neuroectodermal cells. J Neuroimmunol. 1984;7:179–192. doi: 10.1016/s0165-5728(84)80017-x. [DOI] [PubMed] [Google Scholar]
- 20.Rao MS, Mayer-Proschel M. Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Dev Biol. 1997;188:48–63. doi: 10.1006/dbio.1997.8597. [DOI] [PubMed] [Google Scholar]
- 21.Bonaguidi MA, McGuire T, Hu M, Kan L, Samanta J, Kessler JA. Lif and bmp signaling generate separate and discrete types of gfap-expressing cells. Development. 2005;132:5503–5514. doi: 10.1242/dev.02166. [DOI] [PubMed] [Google Scholar]
- 22.Vaysse PJ, Goldman JE. A distinct type of gd3+, flat astrocyte in rat cns cultures. J Neurosci. 1992;12:330–337. doi: 10.1523/JNEUROSCI.12-01-00330.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright LS, Li J, Caldwell MA, Wallace K, Johnson JA, Svendsen CN. Gene expression in human neural stem cells: Effects of leukemia inhibitory factor. J Neurochem. 2003;86:179–195. doi: 10.1046/j.1471-4159.2003.01826.x. [DOI] [PubMed] [Google Scholar]
- 24.Pitman M, Emery B, Binder M, Wang S, Butzkueven H, Kilpatrick TJ. Lif receptor signaling modulates neural stem cell renewal. Molecular and Cellular Neuroscience. 2004;27:255–266. doi: 10.1016/j.mcn.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Bauer S, Patterson PH. Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J Neurosci. 2006;26:12089–12099. doi: 10.1523/JNEUROSCI.3047-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilton DJ, Nicola NA. Kinetic analyses of the binding of leukemia inhibitory factor to receptor on cells and membranes and in detergent solution. J Biol Chem. 1992;267:10238–10247. [PubMed] [Google Scholar]
- 27.Muller S, Chakrapani BP, Schwegler H, Hofmann HD, Kirsch M. Neurogenesis in the dentate gyrus depends on ciliary neurotrophic factor and signal transducer and activator of transcription 3 signaling. Stem Cells. 2009;27:431–441. doi: 10.1634/stemcells.2008-0234. [DOI] [PubMed] [Google Scholar]
- 28.Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- 30.Young GM, Levison SW. Persistence of multipotential progenitors in the juvenile rat subventricular zone. Dev Neurosci. 1996;18:255–265. doi: 10.1159/000111415. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds BA, Rietze RL. Neural stem cells and neurospheres--re-evaluating the relationship. Nat Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 32.Morshead CM, Craig CG, van der Kooy D. In vivo clonal analyses reveal the properties of endogenous neural stem cell proliferation in the adult mammalian forebrain. Development. 1998;125:2251–2261. doi: 10.1242/dev.125.12.2251. [DOI] [PubMed] [Google Scholar]
- 33.Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16ink4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, Van der Kooy D. Neural stem cells in the adult mammalian forebrain: A relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 35.Chojnacki A, Weiss S. Isolation of a novel platelet-derived growth factor-responsive precursor from the embryonic ventral forebrain. J Neurosci. 2004;24:10888–10899. doi: 10.1523/JNEUROSCI.3302-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. Pdgfra/ng2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chojnacki A, Mak G, Weiss S. Pdgfralpha expression distinguishes gfap-expressing neural stem cells from pdgf-responsive neural precursors in the adult periventricular area. J Neurosci. 2011;31:9503–9512. doi: 10.1523/JNEUROSCI.1531-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo F, Maeda Y, Ma J, Xu J, Horiuchi M, Miers L, Vaccarino F, Pleasure D. Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J Neurosci. 2010;30:12036–12049. doi: 10.1523/JNEUROSCI.1360-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Rao M. Oligodendrocytes, grps and mnops. Trends Neurosci. 2003;26:410–412. doi: 10.1016/S0166-2236(03)00201-7. [DOI] [PubMed] [Google Scholar]
- 40.Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;7:11–18. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levison SW, Chuang C, Abramson BJ, Goldman JE. The migrational patterns and developmental fates of glial precursors in the rat subventricular zone are temporally regulated. Development. 1993;119:611–622. doi: 10.1242/dev.119.3.611. [DOI] [PubMed] [Google Scholar]
- 42.Strathmann FG, Wang X, Mayer-Proschel M. Identification of two novel glial-restricted cell populations in the embryonic telencephalon arising from unique origins. BMC Dev Biol. 2007;7:33. doi: 10.1186/1471-213X-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ben-Hur T, Rogister B, Murray K, Rougon G, Dubois-Dalcq M. Growth and fate of psancam+ precursors of the postnatal brain. J Neurosci. 1998;18:5777–5788. doi: 10.1523/JNEUROSCI.18-15-05777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grinspan JB, Stern JL, Pustilnik SM, Pleasure D. Cerebral white matter contains pdgf-responsive precursors to o2a cells. J Neurosci. 1990;10:1866–1873. doi: 10.1523/JNEUROSCI.10-06-01866.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of ng2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22:876–885. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ivanova A, Nakahira E, Kagawa T, Oba A, Wada T, Takebayashi H, Spassky N, Levine J, Zalc B, Ikenaka K. Evidence for a second wave of oligodendrogenesis in the postnatal cerebral cortex of the mouse. J Neurosci Res. 2003;73:581–592. doi: 10.1002/jnr.10717. [DOI] [PubMed] [Google Scholar]
- 47.Zhu X, Bergles DE, Nishiyama A. Ng2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 48.Hatta T, Moriyama K, Nakashima K, Taga T, Otani H. The role of gp130 in cerebral cortical development: In vivo functional analysis in a mouse exo utero system. J Neurosci. 2002;22:5516–5524. doi: 10.1523/JNEUROSCI.22-13-05516.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishibashi T, Lee PR, Baba H, Fields RD. Leukemia inhibitory factor regulates the timing of oligodendrocyte development and myelination in the postnatal optic nerve. J Neurosci Res. 2009;87:3343–3355. doi: 10.1002/jnr.22173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buono KD, Levison SW. Lif sustains the self-renewal of tripotential svz precursors while inhibiting the production of linage restricted progenitors: Transactions of the 41st American Society for Neurochemistry Meeting; Santa Fe, NM. 2010. [Google Scholar]