Abstract
Progress in the development of asymmetric Heck couplings of arenes and acyclic olefins has been limited by a tenuous understanding of the factors that dictate selectivity in migratory insertion and β-hydride elimination. On the basis of key mechanistic insight recently garnered in the exploration of selective Heck reactions, we report here an enantioselective variant that delivers β-, γ-, or δ-aryl carbonyl products from acyclic alkenol substrates. The catalyst system imparts notable regioselectivity during migratory insertion and promotes the migration of the alkene's unsaturation toward the alcohol to ultimately form the ketone product. The reaction uses aryldiazonium salts as the arene source, yields enantiomeric products from opposite starting alkene configurations, and uses a readily accessible ligand. The racemic nature of the alkenol substrate does not bias the enantioselection.
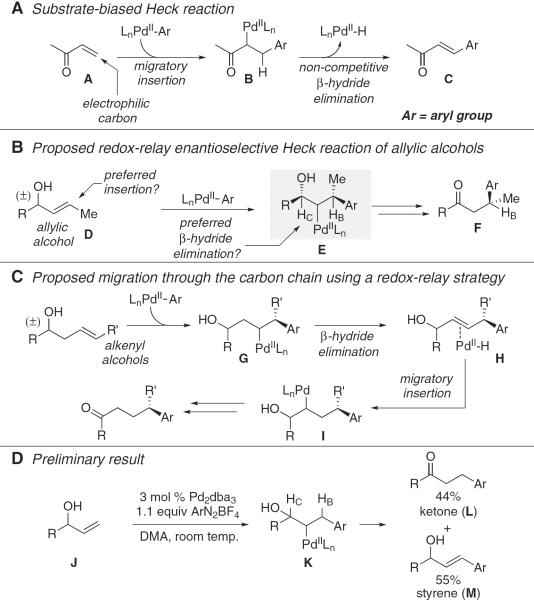
Alkenes that engage in migratory insertion typically require an adjacent functional group to polarize the double bond for site-selective addition and enhanced reactivity (1–3). A classical and long-studied example is the Heck reaction, wherein an organometallic species derived from an aromatic group (Ar) is added to an alkene A followed by β-hydride elimination in B to formally replace a carbon–hydrogen bond with a carbon–carbon bond (Fig. 1A) (4–7). The alkene used in these reactions is generally electron-deficient through conjugation. An electronically biased alkene of this type is necessary for two reasons: First, the charge distribution in A dictates site selectivity of the alkene insertion into the metal–Ar bond, and, second, these simple substrates oblige β-hydride elimination from B of a single C–H bond in the formation of C (8). Unfortunately, the use of biased substrates fundamentally limits the breadth of transformations available (9–11), motivating discovery of methods wherein the outcomes for both the migratory insertion of unactivated alkenes into aryl organometallic reagents, as well as elimination from complexes with different β-C–H bonds are predictable (4, 12, 13). In this context, we report a Pd-catalyzed enantioselective redox-relay Heck reaction of acyclic, alkenyl alcohols where the addition of the organometallic to the alkene is coupled with oxidation of the alcohol by migration of the metal through the alkyl chain (Fig. 1, B and C) (14, 15). This method establishes chiral centers remote from the resultant carbonyl as highlighted by the ability to synthesize β-, γ-, or δ-aryl carbonyl chiral building blocks in high enantioselectivity from the corresponding racemic acyclic alkenol substrates.
Fig. 1.
(A) Classical Heck reaction with electronically biased alkenes. (B) Proposed redox-relay enantioselective Heck reactions and mechanistic analysis. (C) Proposed migration of the metal through the alkyl chain and mechanistic analysis. (D) Preliminary result. DMA indicates dimethylacetamide; dba, dibenzylidene acetone; Me, methyl group.
The inspiration for this approach arose from the observation that an allylic alcohol substrate J, when submitted to a Heck reaction under conditions developed within our laboratory (13), gives rise to about equal amounts of ketone L and styrenyl product M (Fig. 1D). This is an outcome consistent with previous reports describing the use of allylic alcohols, although in some cases improved selectivity for products of type L are observed (10, 14, 15). This product distribution implies that the carbinol (HC) and benzylic (HB) C–H bonds in K are electronically similar and thus competitive in the key C–H bond cleavage event. We hypothesized that an enantio-selective variant might be achieved by submitting 1,2-disubstituted allylic alcohol substrates to a system incorporating a chiral catalyst (Fig. 1B). This strategy would require the discovery of a catalyst sensitive to the subtle electronic differences between the carbons α and β to the alcohol in E and capable of distinguishing between electronically similar C–H bonds on the basis of their relative steric environments. We thought that the judicious choice of a bulky ligand would promote the required selective β-hydride elimination event and that modulating the electronic nature of the metal center would enable discrimination between subtly different alkene carbons in the insertion step.
The process leading to the ketone product requires migration of the alkene's unsaturation toward the tethered alcohol, leading to its oxidation (16, 17). This relay of redox information is attractive for uses in synthesis because it simultaneously promotes functionalization at multiple carbons within the molecule and, more importantly, allows us to envision expansion of the scope to substrates wherein the alcohol and the alkene are separated by additional carbon atoms. Such scope expansion would provide a method for setting a chiral center remotely from the newly formed ketone (Fig. 1C). Mechanistically, the process would require the transposition of the metal, after initial migratory insertion yielded G, whereby β-hydride elimination to form H followed by reinsertion would produce I. Repetition of these steps would ultimately form the ketone product effectively by walking Pd stepwise through the β-and α-carbons relative to the alcohol along the alkyl chain. To favor migration over dissociation of the metal from the alkene, we hypothesized that an electrophilic catalyst produced under mild conditions would be required to promote the binding and insertion of H (4, 18). This suggested the use of aryldiazonium salts as the arene source (13, 19, 20). However, these reagents have not been reported in enantioselective carbon–carbon bond-forming reactions of this sort because of their likely incompatibility with common, chiral phosphine-based ligands (20, 21).
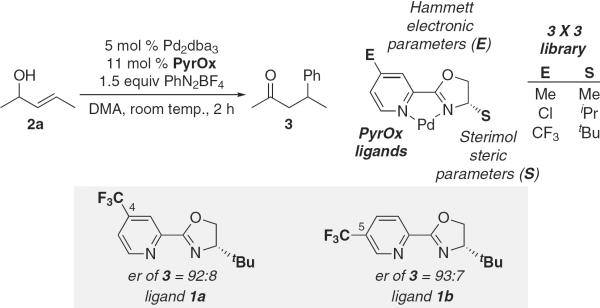
Despite this lack of precedent, we investigated the use of chiral pyridine oxazoline (PyrOx) ligands (22-25), which were hypothesized to be compatible with aryldiazonium salts because of their reduced Lewis basicity relative to phosphine ligands (Fig. 2). These ligands were attractive for two major reasons: First, they are easily synthesized (normally two steps from commercially available materials) in a modular manner, allowing us to take advantage of our recently reported multidimensional parameter-based optimization protocol (26, 27), and, second, the ligands have two coordination sites with distinct electronic properties, which should allow for a well-defined coordination environment envisioned to be required for effective asymmetric catalysis (24, 28, 29). Therefore, a nine-membered ligand library was prepared and evaluated for efficacy in the redox-relay enantioselective Heck reaction of the simple racemic allylic alcohol 2a and aryldiazonium salts. These experiments rapidly revealed that ligand 1a was optimal in terms of rate and enantio-selectivity [reported as enantiomeric ratio (er) in table S1]. However, a 5-CF3 variant called 1b, predicted by the mathematical model to function similarly, was selected for economic reasons. Further modest optimization of the reaction conditions resulted in a transformation that delivers arylated ketone products in generally high enantiomeric ratios (Fig. 3).
Fig. 2.
Ligand selection and optimization protocol of the redox-relay enantioselective Heck reaction of 2a. Pr, propyl; Bu, butyl group.
Fig. 3.
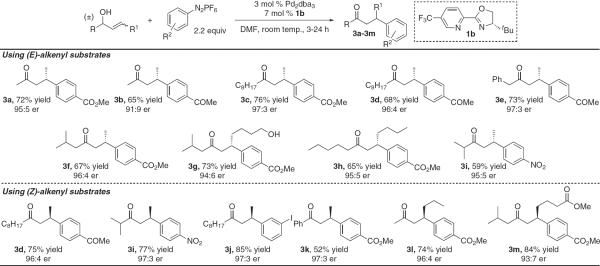
Scope of the enantioselective redox-relay Heck arylations of racemic allylic alcohols. Absolute configuration was determined by the synthesis of ar-(+)-juvabione (3f) and comparison to its reported optical rotation data. The balance of the entries was assigned by analogy (33, 34).
Submission of the simple substrate, 2a, to coupling with electron-poor diazonium salts on a 0.5 mmol scale gave high yields (3a and 3b) with good enantioselectivity (Fig. 3). Improved er (3c, 97:3) was observed when a substrate bearing a bulkier group on the saturated side of the allylic alcohol was submitted. This trend continued because generally higher enantioselectivity was observed with greater substitution on the saturated side of the allylic alcohol (3d to 3f). Alkenes further embedded in the alkyl chain performed well, leading to 3g and 3h in high enantioselectivity. An additional free alcohol did not interfere with catalysis (3g), and it was possible to install a challenging nitro-bearing arene (3i). When (Z)-alkenes were submitted to the same conditions, β-aryl ketone products were isolated in similar yields and enantio-selectivity but for the opposite enantiomer of product (Fig. 3). The yields using (Z)-alkenyl substrates tended to be slightly enhanced relative to products delivered from (E)-olefins (compare 3i for each). Again, generally high enantioselectivity was achieved with a diverse array of substrates, including a carbonyl-bearing alkene (3m). The best observed result in terms of both enantioselectivity and yield entailed the synthesis of 3j, wherein an iodide-bearing arene was installed. This product would be difficult to access if aryldiazonium salts were not used. Conveniently accessed racemic substrates are used in all cases above, strongly suggesting catalyst control overriding the chiral nature of the substrate and highlighting the synthetic utility of this transformation.
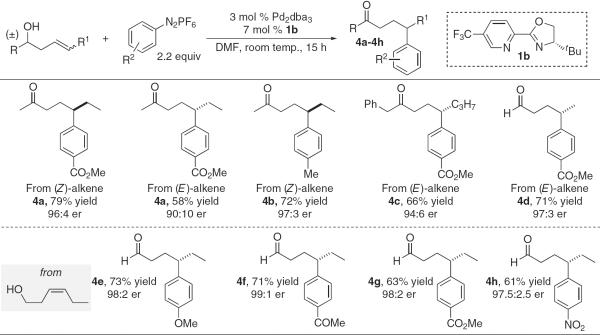
Whereas many of the β-aryl ketones produced above can in principle be accessed by using various alternative strategies (particularly conjugate addition to α,β-unsaturated carbonyls) (25, 30), γ-aryl carbonyl products are much more difficult to access by using reported asymmetric catalytic methods (31, 32). To effectively form this remote chiral center from a homoallylic alcohol would require selective insertion as well as Pd migration along the carbon chain as discussed above. Therefore, it was gratifying that homo-allylic alcohols of various types proved to be excellent substrates for the enantioselective Heck reaction dependent on this redox-relay strategy, leading to generally good yields of γ-aryl ketone products with excellent enantioselectivity (Fig. 4). Minor amounts of the β-aryl carbonyl products, a result of insertion at the alternative alkenyl carbon, were isolated by using these substrates (<5% in most cases). The scope of the process is not limited to homoallylic secondary alcohols, because aldehydes can be accessed from primary alcohols with electronically diverse aryldiazonium salts in good yields and excellent er values (4d to 4h). As previously described, (Z)-alkenes perform modestly better than (E)-alkenes in this reaction and again provide the opposite enantiomer of product.
Fig. 4.
Scope of the enantioselective redox-relay Heck reactions of racemic homoallylic secondary alcohols and primary alcohols.
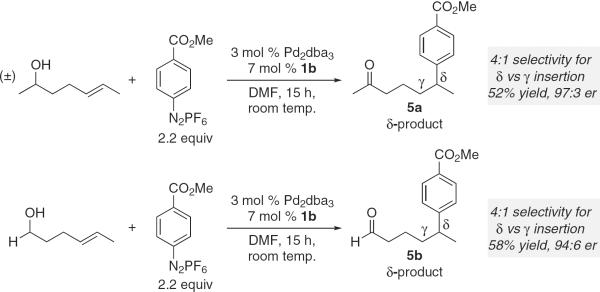
To further evaluate the potential applications of our strategy, we evaluated two substrates wherein the alcohol was an additional carbon away from the alkene (Fig. 5). Under the same reaction conditions, the enantioselective Heck reaction successfully resulted in the formation of δ-substituted carbonyl products 5a and 5b. The selectivity of insertion is diminished somewhat with ~20% of the γ-substituted product observed. However, high enantioselectivity is still achieved, thus showcasing the robustness and potential power of the catalytic system.
Fig. 5.
Evaluation of bis-homoallylic alcohol substrates in the enantioselective Heck reaction.
The data presented suggest that the tactic of asymmetrically functionalizing alkenes and transferring unsaturation to a tethered functional group capable of being oxidized can be applied to the synthesis of remote chiral centers. Further studies will be required to establish what features of the catalyst contribute to high selectivity of the insertion events.
Supplementary Material
Acknowledgments
Supported by NIH grant R01GM063540.
Footnotes
Supplementary Materials www.sciencemag.org/cgi/content/full/338/6113/1455/DC1
References and Notes
- 1.Mc Cartney D, Guiry PJ. Chem. Soc. Rev. 2011;40:5122. doi: 10.1039/c1cs15101k. [DOI] [PubMed] [Google Scholar]
- 2.Shibasaki M, Vogl EM, Ohshima T. Adv. Synth. Catal. 2004;346:1533. [Google Scholar]
- 3.Dounay AB, Overman LE. Chem. Rev. 2003;103:2945. doi: 10.1021/cr020039h. [DOI] [PubMed] [Google Scholar]
- 4.Sigman MS, Werner EW. Acc. Chem. Res. 2012;45:874. doi: 10.1021/ar200236v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yonehara K, et al. J. Organomet. Chem. 2000;603:40. [Google Scholar]
- 6.Yoo KS, et al. Org. Lett. 2007;9:3933. doi: 10.1021/ol701584f. [DOI] [PubMed] [Google Scholar]
- 7.Yoo KS, et al. J. Org. Chem. 2010;75:95. doi: 10.1021/jo901977n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beletskaya IP, Cheprakov AV. Chem. Rev. 2000;100:3009. doi: 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]
- 9.Melpolder JB, Heck RF. J. Org. Chem. 1976;41:265. [Google Scholar]
- 10.Berthiol F, Doucet H, Santelli M. Tetrahedron. 2006;62:4372. [Google Scholar]
- 11.Calò V, Nacci A, Monopoli A, Ferola V. J. Org. Chem. 2007;72:2596. doi: 10.1021/jo070005f. [DOI] [PubMed] [Google Scholar]
- 12.Werner EW, Sigman MS. J. Am. Chem. Soc. 2010;132:13981. doi: 10.1021/ja1060998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werner EW, Sigman MS. J. Am. Chem. Soc. 2011;133:9692. doi: 10.1021/ja203164p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouquillon S, Ganchegui B, Estrine B, Hénin F, Muzart J. J. Organomet. Chem. 2001;634:153. [Google Scholar]
- 15.Larock RC, Leung W-Y, Stolz-Dunn S. Tetrahedron Lett. 1989;30:6629. [Google Scholar]
- 16.Richter JM, et al. J. Am. Chem. Soc. 2008;130:17938. doi: 10.1021/ja806981k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns NZ, Baran PS, Hoffmann RW. Angew. Chem. Int. Ed. 2009;48:2854. doi: 10.1002/anie.200806086. [DOI] [PubMed] [Google Scholar]
- 18.Urkalan KB, Sigman MS. Angew. Chem. Int. Ed. 2009;48:3146. doi: 10.1002/anie.200900218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kikukawa K, Nagira K, Wada F, Matsuda T. Tetrahedron. 1981;37:31. [Google Scholar]
- 20.Taylor JG, Moro AV, Correia CRD. Eur. J. Org. Chem. 2011;2011:1403. [Google Scholar]
- 21.Roglans A, Pla-Quintana A, Moreno-Mañas M. Chem. Rev. 2006;106:4622. doi: 10.1021/cr0509861. [DOI] [PubMed] [Google Scholar]
- 22.Jensen KH, Pathak TP, Zhang Y, Sigman MS. J. Am. Chem. Soc. 2009;131:17074. doi: 10.1021/ja909030c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michel BW, Camelio AM, Cornell CN, Sigman MS. J. Am. Chem. Soc. 2009;131:6076. doi: 10.1021/ja901212h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald RI, White PB, Weinstein AB, Tam CP, Stahl SS. Org. Lett. 2011;13:2830. doi: 10.1021/ol200784y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kikushima K, Holder JC, Gatti M, Stoltz BM. J. Am. Chem. Soc. 2011;133:6902. doi: 10.1021/ja200664x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harper KC, Sigman MS. Science. 2011;333:1875. doi: 10.1126/science.1206997. [DOI] [PubMed] [Google Scholar]
- 27.Harper KC, Bess EN, Sigman MS. Nat. Chem. 2012;4:366. doi: 10.1038/nchem.1297. [DOI] [PubMed] [Google Scholar]
- 28.Michel BW, Steffens LD, Sigman MS. J. Am. Chem. Soc. 2011;133:8317. doi: 10.1021/ja2017043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen KH, Webb JD, Sigman MS. J. Am. Chem. Soc. 2010;132:17471. doi: 10.1021/ja108106h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi T, Yamasaki K. Chem. Rev. 2003;103:2829. doi: 10.1021/cr020022z. [DOI] [PubMed] [Google Scholar]
- 31.Smith SW, Fu GC. J. Am. Chem. Soc. 2009;131:14231. doi: 10.1021/ja9061823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zultanski SL, Fu GC. J. Am. Chem. Soc. 2011;133:15362. doi: 10.1021/ja2079515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayyar KS, Rao GSK. Can. J. Chem. 1968;46:1467. [Google Scholar]
- 34.Tanaka K, et al. Chem. Pharm. Bull. 1999;47:1053. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.