Abstract
Enterococci are commensal organisms well suited to survival in intestinal and vaginal tracts and the oral cavity. However, as for most bacteria described as causing human disease, enterococci also possess properties that can be ascribed roles in pathogenesis. The natural ability of enterococci to readily acquire, accumulate, and share extrachromosomal elements encoding virulence traits or antibiotic resistance genes lends advantages to their survival under unusual environmental stresses and in part explains their increasing importance as nosocomial pathogens. This review discusses the current understanding of enterococcal virulence relating to (i) adherence to host tissues, (ii) invasion and abscess formation, (iii) factors potentially relevant to modulation of host inflammatory responses, and (iv) potentially toxic secreted products. Aggregation substance, surface carbohydrates, or fibronectin-binding moieties may facilitate adherence to host tissues. Enterococcus faecalis appears to have the capacity to translocate across intact intestinal mucosa in models of antibiotic-induced superinfection. Extracellular toxins such as cytolysin can induce tissue damage as shown in an endophthalmitis model, increase mortality in combination with aggregation substance in an endocarditis model, and cause systemic toxicity in a murine peritonitis model. Finally, lipoteichoic acid, superoxide production, or pheromones and corresponding peptide inhibitors each may modulate local inflammatory reactions.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Boyce S. T., Babcock G. F., Gianotti L., Peck M. D., Dunn D. L., Pyles T., Childress C. P., Ash S. K. The process of microbial translocation. Ann Surg. 1990 Oct;212(4):496–512. doi: 10.1097/00000658-199010000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkan M. L., Beachey E. H. Excretion of lipoteichoic acid by group A streptococci. Influence of penicillin on excretion and loss of ability to adhere to human oral mucosal cells. J Clin Invest. 1978 Mar;61(3):671–677. doi: 10.1172/JCI108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arduino R. C., Murray B. E., Rakita R. M. Roles of antibodies and complement in phagocytic killing of enterococci. Infect Immun. 1994 Mar;62(3):987–993. doi: 10.1128/iai.62.3.987-993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur M., Courvalin P. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob Agents Chemother. 1993 Aug;37(8):1563–1571. doi: 10.1128/aac.37.8.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod P., Talbot G. H. Risk factors for acquisition of gentamicin-resistant enterococci. A multivariate analysis. Arch Intern Med. 1989 Jun;149(6):1397–1401. [PubMed] [Google Scholar]
- BLEIWEIS A. S., ZIMMERMAN L. N. PROPERTIES OF PROTEINASE FROM STREPTOCOCCUS FAECALIS VAR. LIQUEFACIENS. J Bacteriol. 1964 Sep;88:653–659. doi: 10.1128/jb.88.3.653-659.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK T. D., DAVIE J. M. PROBABLE IDENTITY OF A GROUP D HEMOLYSIN WITH A BACTERIOCINE. J Bacteriol. 1963 Oct;86:708–712. doi: 10.1128/jb.86.4.708-712.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK T. D., PEACHER B., PIERSON D. SURVEY OF THE BACTERIOCINES OF ENTEROCOCCI. J Bacteriol. 1963 Oct;86:702–707. doi: 10.1128/jb.86.4.702-707.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddour L. M., Christensen G. D., Lowrance J. H., Simpson W. A. Pathogenesis of experimental endocarditis. Rev Infect Dis. 1989 May-Jun;11(3):452–463. doi: 10.1093/clinids/11.3.452. [DOI] [PubMed] [Google Scholar]
- Barrall D. T., Kenney P. R., Slotman G. J., Burchard K. W. Enterococcal bacteremia in surgical patients. Arch Surg. 1985 Jan;120(1):57–63. doi: 10.1001/archsurg.1985.01390250049008. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Onderdonk A. B., Louie T., Kasper D. L., Gorbach S. L. A review. Lessons from an animal model of intra-abdominal sepsis. Arch Surg. 1978 Jul;113(7):853–857. doi: 10.1001/archsurg.1978.01370190075013. [DOI] [PubMed] [Google Scholar]
- Basinger S. F., Jackson R. W. Bacteriocin (hemolysin) of Streptococcus zymogenes. J Bacteriol. 1968 Dec;96(6):1895–1902. doi: 10.1128/jb.96.6.1895-1902.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A. S., Seidel J. S., Yoshikawa T. T., Anthony B. F., Guze L. B. Group D enterococcal meningitis. Clinical and therapeutic considerations with report of three cases and review of the literature. Arch Intern Med. 1976 Aug;136(8):883–886. doi: 10.1001/archinte.136.8.883. [DOI] [PubMed] [Google Scholar]
- Beachey E. H., Dale J. B., Simpson W. A., Evans J. D., Knox K. W., Ofek I., Wicken A. J. Erythrocyte binding properties of streptococcal lipoteichoic acids. Infect Immun. 1979 Mar;23(3):618–625. doi: 10.1128/iai.23.3.618-625.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev. 1994 Apr;7(2):213–264. doi: 10.1128/cmr.7.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Black C. M., Doughty F., Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun. 1985 Jan;47(1):135–141. doi: 10.1128/iai.47.1.135-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensing B. A., Dunny G. M. Cloning and molecular analysis of genes affecting expression of binding substance, the recipient-encoded receptor(s) mediating mating aggregate formation in Enterococcus faecalis. J Bacteriol. 1993 Nov;175(22):7421–7429. doi: 10.1128/jb.175.22.7421-7429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk S. L., Verghese A., Holtsclaw S. A., Smith J. K. Enterococcal pneumonia. Occurrence in patients receiving broad-spectrum antibiotic regimens and enteral feeding. Am J Med. 1983 Jan;74(1):153–154. doi: 10.1016/0002-9343(83)91132-4. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Klonisch T., Nuber P., Fischer W. Stimulation of monokine production by lipoteichoic acids. Infect Immun. 1991 Dec;59(12):4614–4620. doi: 10.1128/iai.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Copass M. C., Falkow S. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect Immun. 1993 Sep;61(9):3914–3921. doi: 10.1128/iai.61.9.3914-3921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonten M. J., van Tiel F. H., van der Geest S., Stobberingh E. E., Gaillard C. A. Enterococcus faecalis pneumonia complicating topical antimicrobial prophylaxis. N Engl J Med. 1993 Jan 21;328(3):209–210. doi: 10.1056/NEJM199301213280311. [DOI] [PubMed] [Google Scholar]
- Boulanger J. M., Ford-Jones E. L., Matlow A. G. Enterococcal bacteremia in a pediatric institution: a four-year review. Rev Infect Dis. 1991 Sep-Oct;13(5):847–856. doi: 10.1093/clinids/13.5.847. [DOI] [PubMed] [Google Scholar]
- Boyle J. F., Soumakis S. A., Rendo A., Herrington J. A., Gianarkis D. G., Thurberg B. E., Painter B. G. Epidemiologic analysis and genotypic characterization of a nosocomial outbreak of vancomycin-resistant enterococci. J Clin Microbiol. 1993 May;31(5):1280–1285. doi: 10.1128/jcm.31.5.1280-1285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge P. D., Sneath P. H. Numerical taxonomy of Streptococcus. J Gen Microbiol. 1983 Mar;129(3):565–597. doi: 10.1099/00221287-129-3-565. [DOI] [PubMed] [Google Scholar]
- Britton L., Malinowski D. P., Fridovich I. Superoxide dismutase and oxygen metabolism in Streptococcus faecalis and comparisons with other organisms. J Bacteriol. 1978 Apr;134(1):229–236. doi: 10.1128/jb.134.1.229-236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I. Effect of Streptococcus faecalis on the growth of Bacteroides species and anaerobic cocci in mixed infection. Surgery. 1988 Jan;103(1):107–110. [PubMed] [Google Scholar]
- Brook I., Walker R. I. Significance of encapsulated Bacteroides melaninogenicus and Bacteroides fragilis groups in mixed infections. Infect Immun. 1984 Apr;44(1):12–15. doi: 10.1128/iai.44.1.12-15.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan C. S., Reynolds K. L., Brown J. J. Mortality associated with enterococcal bacteremia. Surg Gynecol Obstet. 1985 Jun;160(6):557–561. [PubMed] [Google Scholar]
- Burroughs M., Rozdzinski E., Geelen S., Tuomanen E. A structure-activity relationship for induction of meningeal inflammation by muramyl peptides. J Clin Invest. 1993 Jul;92(1):297–302. doi: 10.1172/JCI116565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush L. M., Calmon J., Cherney C. L., Wendeler M., Pitsakis P., Poupard J., Levison M. E., Johnson C. C. High-level penicillin resistance among isolates of enterococci. Implications for treatment of enterococcal infections. Ann Intern Med. 1989 Apr 1;110(7):515–520. doi: 10.7326/0003-4819-110-7-515. [DOI] [PubMed] [Google Scholar]
- Caprioli T., Zaccour F., Kasatiya S. S. Phage typing scheme for group D streptococci isolated from human urogenital tract. J Clin Microbiol. 1975 Oct;2(4):311–317. doi: 10.1128/jcm.2.4.311-317.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizosa J., Kaye D. Antibiotic synergism in enterococcal endocarditis. J Lab Clin Med. 1976 Jul;88(1):132–141. [PubMed] [Google Scholar]
- Chen H. Y., Williams J. D. Transferable resistance and aminoglycoside-modifying enzymes in enterococci. J Med Microbiol. 1985 Oct;20(2):187–196. doi: 10.1099/00222615-20-2-187. [DOI] [PubMed] [Google Scholar]
- Chirurgi V. A., Oster S. E., Goldberg A. A., Zervos M. J., McCabe R. E. Ampicillin-resistant Enterococcus raffinosus in an acute-care hospital: case-control study and antimicrobial susceptibilities. J Clin Microbiol. 1991 Nov;29(11):2663–2665. doi: 10.1128/jcm.29.11.2663-2665.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J. W., Kuritza A., Shlaes D. M., Green M., Sahm D. F., Zervos M. J. Clonal spread of vancomycin-resistant Enterococcus faecium between patients in three hospitals in two states. J Clin Microbiol. 1993 Jun;31(6):1609–1611. doi: 10.1128/jcm.31.6.1609-1611.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J. W., Thal L. A., Perri M. B., Vazquez J. A., Donabedian S. M., Clewell D. B., Zervos M. J. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1993 Nov;37(11):2474–2477. doi: 10.1128/aac.37.11.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y. J., Hansen J. N. Determination of the sequence of spaE and identification of a promoter in the subtilin (spa) operon in Bacillus subtilis. J Bacteriol. 1992 Oct;174(20):6699–6702. doi: 10.1128/jb.174.20.6699-6702.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Bacterial sex pheromone-induced plasmid transfer. Cell. 1993 Apr 9;73(1):9–12. doi: 10.1016/0092-8674(93)90153-h. [DOI] [PubMed] [Google Scholar]
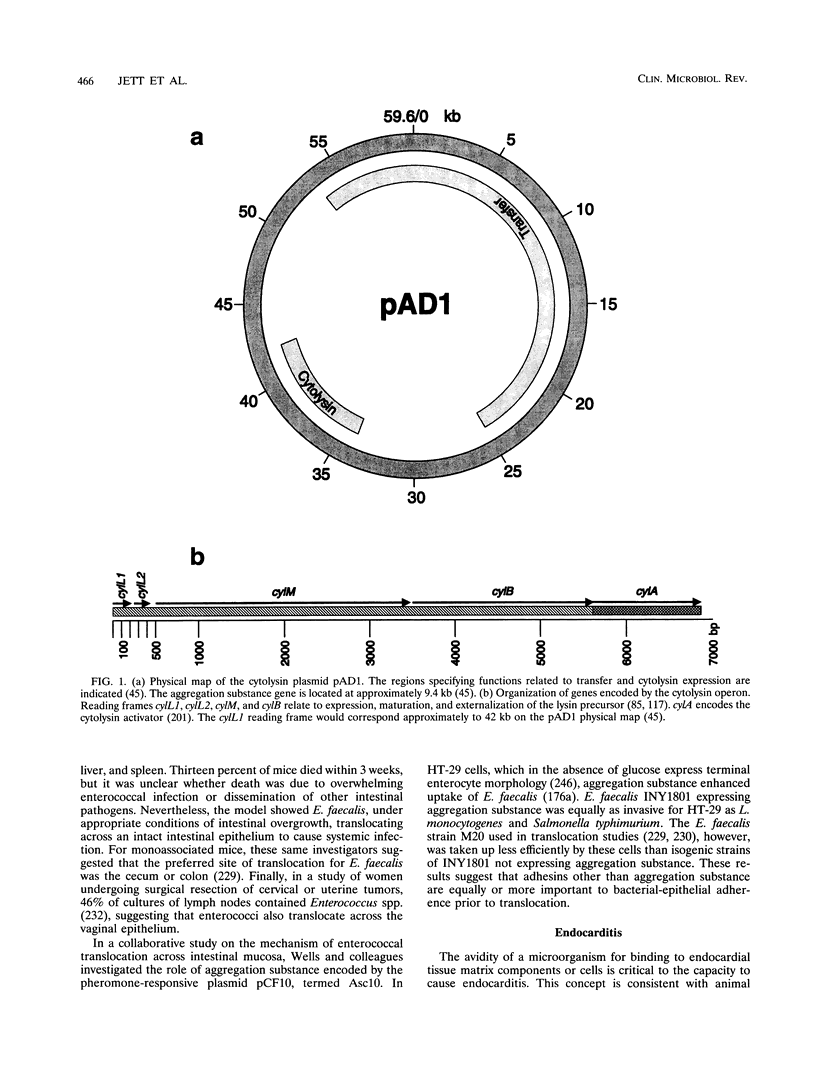
- Clewell D. B. Movable genetic elements and antibiotic resistance in enterococci. Eur J Clin Microbiol Infect Dis. 1990 Feb;9(2):90–102. doi: 10.1007/BF01963632. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Tomich P. K., Gawron-Burke M. C., Franke A. E., Yagi Y., An F. Y. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J Bacteriol. 1982 Dec;152(3):1220–1230. doi: 10.1128/jb.152.3.1220-1230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Weaver K. E. Sex pheromones and plasmid transfer in Enterococcus faecalis. Plasmid. 1989 May;21(3):175–184. doi: 10.1016/0147-619x(89)90041-3. [DOI] [PubMed] [Google Scholar]
- Colmar I., Horaud T. Enterococcus faecalis hemolysin-bacteriocin plasmids belong to the same incompatibility group. Appl Environ Microbiol. 1987 Mar;53(3):567–570. doi: 10.1128/aem.53.3.567-570.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I., Russell C. Comparative adhesion of seven species of streptococci isolated from the blood of patients with sub-acute bacterial endocarditis to fibrin-platelet clots in vitro. J Appl Bacteriol. 1986 Feb;60(2):127–133. doi: 10.1111/j.1365-2672.1986.tb03369.x. [DOI] [PubMed] [Google Scholar]
- Desnottes J. F., Bensman A., Ave-Virat A., Fontaine J. L. Experimental retrograde pyelonephritis and cystitis induced in rabbits by a group D Streptococcus sp.: serum antibody assay by a hemagglutination test. Infect Immun. 1981 Sep;33(3):647–650. doi: 10.1128/iai.33.3.647-650.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet-Populaire F., Trieu-Cuot P., Dosbaa I., Andremont A., Courvalin P. Inducible transfer of conjugative transposon Tn1545 from Enterococcus faecalis to Listeria monocytogenes in the digestive tracts of gnotobiotic mice. Antimicrob Agents Chemother. 1991 Jan;35(1):185–187. doi: 10.1128/aac.35.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty S. H., Flohr A. B., Simmons R. L. 'Breakthrough' enterococcal septicemia in surgical patients. 19 cases and a review of the literature. Arch Surg. 1983 Feb;118(2):232–238. doi: 10.1001/archsurg.1983.01390020076013. [DOI] [PubMed] [Google Scholar]
- Doyle P. W., Woodham J. D. Evaluation of the microbiology of chronic ethmoid sinusitis. J Clin Microbiol. 1991 Nov;29(11):2396–2400. doi: 10.1128/jcm.29.11.2396-2400.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Brown B. L., Clewell D. B. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Clewell D. B. Transmissible toxin (hemolysin) plasmid in Streptococcus faecalis and its mobilization of a noninfectious drug resistance plasmid. J Bacteriol. 1975 Nov;124(2):784–790. doi: 10.1128/jb.124.2.784-790.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M. Genetic functions and cell-cell interactions in the pheromone-inducible plasmid transfer system of Enterococcus faecalis. Mol Microbiol. 1990 May;4(5):689–696. doi: 10.1111/j.1365-2958.1990.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Döring G., Goldstein W., Röll A., Schiøtz P. O., Høiby N., Botzenhart K. Role of Pseudomonas aeruginosa exoenzymes in lung infections of patients with cystic fibrosis. Infect Immun. 1985 Sep;49(3):557–562. doi: 10.1128/iai.49.3.557-562.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard W. G. Why do bacterial plasmids carry some genes and not others? Plasmid. 1989 May;21(3):167–174. doi: 10.1016/0147-619x(89)90040-1. [DOI] [PubMed] [Google Scholar]
- Edelstein H., McCabe R. E. Perinephric abscess. Modern diagnosis and treatment in 47 cases. Medicine (Baltimore) 1988 Mar;67(2):118–131. [PubMed] [Google Scholar]
- Ehrenfeld E. E., Clewell D. B. Transfer functions of the Streptococcus faecalis plasmid pAD1: organization of plasmid DNA encoding response to sex pheromone. J Bacteriol. 1987 Aug;169(8):3473–3481. doi: 10.1128/jb.169.8.3473-3481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E. E., Kessler R. E., Clewell D. B. Identification of pheromone-induced surface proteins in Streptococcus faecalis and evidence of a role for lipoteichoic acid in formation of mating aggregates. J Bacteriol. 1986 Oct;168(1):6–12. doi: 10.1128/jb.168.1.6-12.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Eliopoulos C. T. Therapy of enterococcal infections. Eur J Clin Microbiol Infect Dis. 1990 Feb;9(2):118–126. doi: 10.1007/BF01963636. [DOI] [PubMed] [Google Scholar]
- Ember J. A., Hugli T. E. Characterization of the human neutrophil response to sex pheromones from Streptococcus faecalis. Am J Pathol. 1989 Apr;134(4):797–805. [PMC free article] [PubMed] [Google Scholar]
- Emori T. G., Gaynes R. P. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993 Oct;6(4):428–442. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A. C., Chinn A. L. The Enterococci: With Special Reference to Their Association with Human Disease. J Bacteriol. 1947 Oct;54(4):495–512. doi: 10.1128/jb.54.4.495-512.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R., Collins M. D. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989 Apr;27(4):731–734. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcioni G. C., Coderoni S., Tedeschi G. G., Brunori M., Rotilio G. Red cell lysis induced by microorganisms as a case of superoxide- and hydrogen peroxide-dependent hemolysis mediated by oxyhemoglobin. Biochim Biophys Acta. 1981 Dec 18;678(3):437–441. doi: 10.1016/0304-4165(81)90125-2. [DOI] [PubMed] [Google Scholar]
- Fath M. J., Kolter R. ABC transporters: bacterial exporters. Microbiol Rev. 1993 Dec;57(4):995–1017. doi: 10.1128/mr.57.4.995-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Wong W. W. Complement ligand-receptor interactions that mediate biological responses. Annu Rev Immunol. 1983;1:243–271. doi: 10.1146/annurev.iy.01.040183.001331. [DOI] [PubMed] [Google Scholar]
- Felmingham D., Wilson A. P., Quintana A. I., Grüneberg R. N. Enterococcus species in urinary tract infection. Clin Infect Dis. 1992 Aug;15(2):295–301. doi: 10.1093/clinids/15.2.295. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989 Jul;2(3):285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Repesh L. A. The hyaluronidase associated with Treponema pallidum facilitates treponemal dissemination. Infect Immun. 1987 May;55(5):1023–1028. doi: 10.1128/iai.55.5.1023-1028.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J., Isenberg H. D. Antibiotic resistance of microorganisms isolated from root canals. Oral Surg Oral Med Oral Pathol. 1967 Feb;23(2):230–235. doi: 10.1016/0030-4220(67)90101-6. [DOI] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima J., Yamamoto S., Morihara K., Atsumi Y., Takeuchi H., Kawamoto S., Okuda K. Structural gene and complete amino acid sequence of Pseudomonas aeruginosa IFO 3455 elastase. J Bacteriol. 1989 Mar;171(3):1698–1704. doi: 10.1128/jb.171.3.1698-1704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUZE L. B., GOLDNER B. H., KALMANSON G. M. Pyelonephritis. I. Observations on the course of chronic non-obstructed enterococcal infection in the kidnev of the rat. Yale J Biol Med. 1961 Apr;33:372–385. [PMC free article] [PubMed] [Google Scholar]
- Galli D., Lottspeich F., Wirth R. Sequence analysis of Enterococcus faecalis aggregation substance encoded by the sex pheromone plasmid pAD1. Mol Microbiol. 1990 Jun;4(6):895–904. doi: 10.1111/j.1365-2958.1990.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Galli D., Wirth R. Comparative analysis of Enterococcus faecalis sex pheromone plasmids identifies a single homologous DNA region which codes for aggregation substance. J Bacteriol. 1991 May;173(9):3029–3033. doi: 10.1128/jb.173.9.3029-3033.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison R. N., Fry D. E., Berberich S., Polk H. C., Jr Enterococcal bacteremia: clinical implications and determinants of death. Ann Surg. 1982 Jul;196(1):43–47. doi: 10.1097/00000658-198207000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore M. S., Segarra R. A., Booth M. C. An HlyB-type function is required for expression of the Enterococcus faecalis hemolysin/bacteriocin. Infect Immun. 1990 Dec;58(12):3914–3923. doi: 10.1128/iai.58.12.3914-3923.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold O. G., Jordan H. V., van Houte J. The prevalence of enterococci in the human mouth and their pathogenicity in animal models. Arch Oral Biol. 1975 Jul;20(7):473–477. doi: 10.1016/0003-9969(75)90236-8. [DOI] [PubMed] [Google Scholar]
- Granato P. A., Jackson R. W. Bicomponent nature of lysin from Streptococcus zymogenes. J Bacteriol. 1969 Nov;100(2):865–868. doi: 10.1128/jb.100.2.865-868.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato P. A., Jackson R. W. Characterization of the A component of Streptococcus zymogenes lysin. J Bacteriol. 1971 Aug;107(2):551–556. doi: 10.1128/jb.107.2.551-556.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
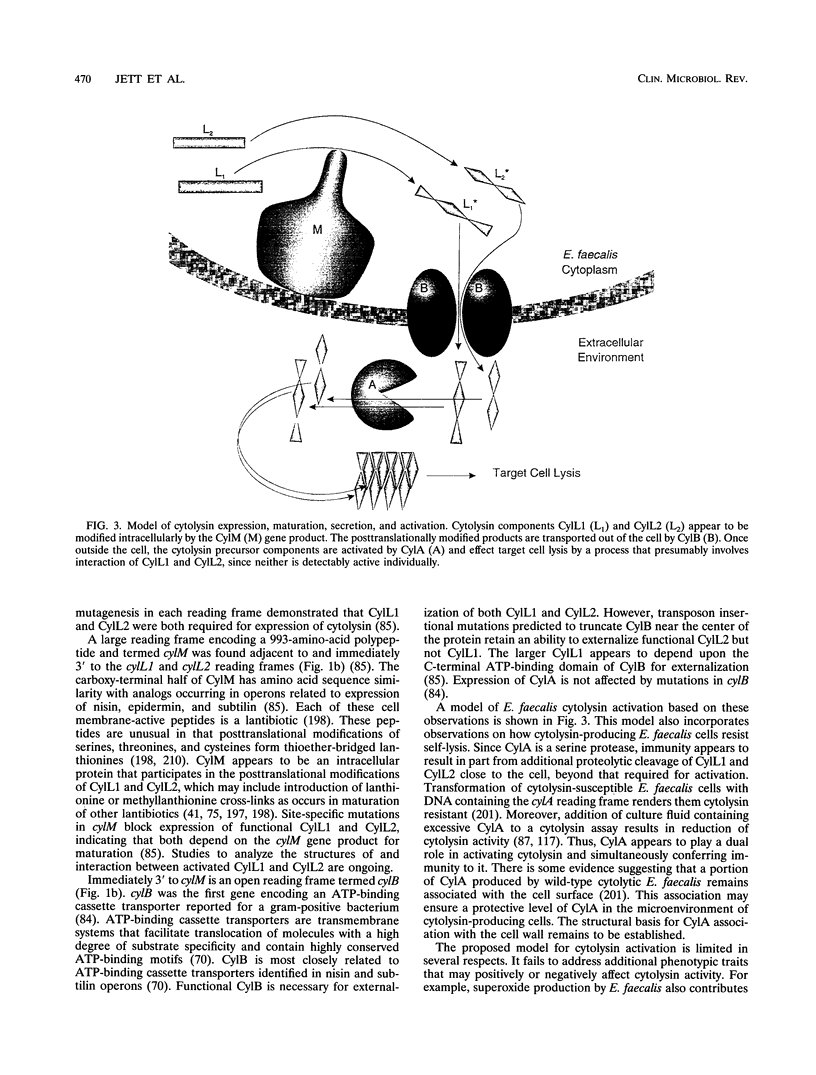
- Granato P. A., Jackson R. W. Purification and characterization of the L component of Streptococcus zymogenes lysin. J Bacteriol. 1971 Nov;108(2):804–808. doi: 10.1128/jb.108.2.804-808.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graninger W., Ragette R. Nosocomial bacteremia due to Enterococcus faecalis without endocarditis. Clin Infect Dis. 1992 Jul;15(1):49–57. doi: 10.1093/clinids/15.1.49. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973 May;114(2):543–548. doi: 10.1128/jb.114.2.543-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross P. A., Harkavy L. M., Barden G. E., Flower M. F. The epidemiology of nosocomial enterococcal urinary tract infection. Am J Med Sci. 1976 Jul-Aug;272(1):75–81. doi: 10.1097/00000441-197607000-00009. [DOI] [PubMed] [Google Scholar]
- Gullberg R. M., Homann S. R., Phair J. P. Enterococcal bacteremia: analysis of 75 episodes. Rev Infect Dis. 1989 Jan-Feb;11(1):74–85. doi: 10.1093/clinids/11.1.74. [DOI] [PubMed] [Google Scholar]
- Guzmàn C. A., Pruzzo C., LiPira G., Calegari L. Role of adherence in pathogenesis of Enterococcus faecalis urinary tract infection and endocarditis. Infect Immun. 1989 Jun;57(6):1834–1838. doi: 10.1128/iai.57.6.1834-1838.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmàn C. A., Pruzzo C., Platè M., Guardati M. C., Calegari L. Serum dependent expression of Enterococcus faecalis adhesins involved in the colonization of heart cells. Microb Pathog. 1991 Dec;11(6):399–409. doi: 10.1016/0882-4010(91)90036-a. [DOI] [PubMed] [Google Scholar]
- Gálvez A., Maqueda M., Martínez-Bueno M., Valdivia E. Bactericidal and bacteriolytic action of peptide antibiotic AS-48 against gram-positive and gram-negative bacteria and other organisms. Res Microbiol. 1989 Jan;140(1):57–68. doi: 10.1016/0923-2508(89)90060-0. [DOI] [PubMed] [Google Scholar]
- Gálvez A., Maqueda M., Martínez-Bueno M., Valdivia E. Permeation of bacterial cells, permeation of cytoplasmic and artificial membrane vesicles, and channel formation on lipid bilayers by peptide antibiotic AS-48. J Bacteriol. 1991 Jan;173(2):886–892. doi: 10.1128/jb.173.2.886-892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez A., Valdivia E., Martínez-Bueno M., Maqueda M. Induction of autolysis in Enterococcus faecalis S-47 by peptide AS-48. J Appl Bacteriol. 1990 Sep;69(3):406–413. doi: 10.1111/j.1365-2672.1990.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Haglund L. A., Flournoy D. J., Gilmore M. S., Huycke M. M. Enterococcus: an old pathogen with new tricks. J Okla State Med Assoc. 1991 Jul;84(7):305–309. [PubMed] [Google Scholar]
- Hall L. M., Duke B., Guiney M., Williams R. Typing of Enterococcus species by DNA restriction fragment analysis. J Clin Microbiol. 1992 Apr;30(4):915–919. doi: 10.1128/jcm.30.4.915-919.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L. M., Duke B., Urwin G., Guiney M. Epidemiology of Enterococcus faecalis urinary tract infection in a teaching hospital in London, United Kingdom. J Clin Microbiol. 1992 Aug;30(8):1953–1957. doi: 10.1128/jcm.30.8.1953-1957.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey B. S., Baker C. J., Edwards M. S. Contributions of complement and immunoglobulin to neutrophil-mediated killing of enterococci. Infect Immun. 1992 Sep;60(9):3635–3640. doi: 10.1128/iai.60.9.3635-3640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty D. L., Ofek I., Courtney H. S., Doyle R. J. Multiple adhesins of streptococci. Infect Immun. 1992 Jun;60(6):2147–2152. doi: 10.1128/iai.60.6.2147-2152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman D. J., Gerding D. N. Antimicrobial resistance among enterococci. Antimicrob Agents Chemother. 1991 Jan;35(1):1–4. doi: 10.1128/aac.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt H., Wanner G., Galli D., Wirth R. Biochemical, immunological and ultrastructural characterization of aggregation substances encoded by Enterococcus faecalis sex-pheromone plasmids. Eur J Biochem. 1993 Feb 1;211(3):711–716. doi: 10.1111/j.1432-1033.1993.tb17600.x. [DOI] [PubMed] [Google Scholar]
- Hoepelman A. I., Tuomanen E. I. Consequences of microbial attachment: directing host cell functions with adhesins. Infect Immun. 1992 May;60(5):1729–1733. doi: 10.1128/iai.60.5.1729-1733.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook E. W., 3rd, Roberts R. B., Sande M. A. Antimicrobial therapy of experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1975 Nov;8(5):564–570. doi: 10.1128/aac.8.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz R. A., Von Graevenitz A. A clinical study of the role of enterococci as sole agents of wound and tissue infection. Yale J Biol Med. 1977 Jul-Aug;50(4):391–395. [PMC free article] [PubMed] [Google Scholar]
- Hotez P. J., Narasimhan S., Haggerty J., Milstone L., Bhopale V., Schad G. A., Richards F. F. Hyaluronidase from infective Ancylostoma hookworm larvae and its possible function as a virulence factor in tissue invasion and in cutaneous larva migrans. Infect Immun. 1992 Mar;60(3):1018–1023. doi: 10.1128/iai.60.3.1018-1023.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummell D. S., Winkelstein J. A. Bacterial lipoteichoic acid sensitizes host cells for destruction by autologous complement. J Clin Invest. 1986 May;77(5):1533–1538. doi: 10.1172/JCI112468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Z., Kuhn M., Lannigan R., Austin T. W. Microbiological investigation of an outbreak of bacteraemia due to Streptococcus faecalis in an intensive care unit. J Hosp Infect. 1988 Nov;12(4):263–271. doi: 10.1016/0195-6701(88)90068-0. [DOI] [PubMed] [Google Scholar]
- Huycke M. M., Gilmore M. S., Jett B. D., Booth J. L. Transfer of pheromone-inducible plasmids between Enterococcus faecalis in the Syrian hamster gastrointestinal tract. J Infect Dis. 1992 Nov;166(5):1188–1191. doi: 10.1093/infdis/166.5.1188. [DOI] [PubMed] [Google Scholar]
- Huycke M. M., Spiegel C. A., Gilmore M. S. Bacteremia caused by hemolytic, high-level gentamicin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1991 Aug;35(8):1626–1634. doi: 10.1128/aac.35.8.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes W. L., Ferretti J. J. Sequence analysis and expression in Escherichia coli of the hyaluronidase gene of Streptococcus pyogenes bacteriophage H4489A. Infect Immun. 1989 Feb;57(2):533–539. doi: 10.1128/iai.57.2.533-539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häse C. C., Finkelstein R. A. Bacterial extracellular zinc-containing metalloproteases. Microbiol Rev. 1993 Dec;57(4):823–837. doi: 10.1128/mr.57.4.823-837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Clewell D. B. Evidence that the hemolysin/bacteriocin phenotype of Enterococcus faecalis subsp. zymogenes can be determined by plasmids in different incompatibility groups as well as by the chromosome. J Bacteriol. 1992 Dec;174(24):8172–8177. doi: 10.1128/jb.174.24.8172-8177.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Clewell D. B. Genetic analysis of the pAD1 pheromone response in Streptococcus faecalis, using transposon Tn917 as an insertional mutagen. J Bacteriol. 1984 Jun;158(3):777–783. doi: 10.1128/jb.158.3.777-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Clewell D. B., Segarra R. A., Gilmore M. S. Genetic analysis of the pAD1 hemolysin/bacteriocin determinant in Enterococcus faecalis: Tn917 insertional mutagenesis and cloning. J Bacteriol. 1990 Jan;172(1):155–163. doi: 10.1128/jb.172.1.155-163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Flannagan S. E., Clewell D. B. Hyperhemolytic phenomena associated with insertions of Tn916 into the hemolysin determinant of Enterococcus faecalis plasmid pAD1. J Bacteriol. 1992 Mar;174(6):1801–1809. doi: 10.1128/jb.174.6.1801-1809.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Hashimoto H., Clewell D. B. Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect Immun. 1984 Aug;45(2):528–530. doi: 10.1128/iai.45.2.528-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Hashimoto H., Clewell D. B. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J Clin Microbiol. 1987 Aug;25(8):1524–1528. doi: 10.1128/jcm.25.8.1524-1528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerman M., Pitsakis P. G., Rosenberg A., Hessen M. T., Abrutyn E., Murray B. E., Levison M. E. beta-Lactamase production in experimental endocarditis due to aminoglycoside-resistant Streptococcus faecalis. J Infect Dis. 1987 Jun;155(6):1226–1232. doi: 10.1093/infdis/155.6.1226. [DOI] [PubMed] [Google Scholar]
- Jackson R. W. Bacteriolysis and inhibition of gram-positive bacteria by components of Streptococcus zymogenes lysin. J Bacteriol. 1971 Jan;105(1):156–159. doi: 10.1128/jb.105.1.156-159.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Douglas G. J., Hobbs S. J. Self-transferable plasmids determining the hemolysin and bacteriocin of Streptococcus faecalis var. zymogenes. J Bacteriol. 1975 Mar;121(3):863–872. doi: 10.1128/jb.121.3.863-872.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett B. D., Gilmore M. S. The growth-inhibitory effect of the Enterococcus faecalis bacteriocin encoded by pAD1 extends to the oral streptococci. J Dent Res. 1990 Oct;69(10):1640–1645. doi: 10.1177/00220345900690100301. [DOI] [PubMed] [Google Scholar]
- Jett B. D., Jensen H. G., Nordquist R. E., Gilmore M. S. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun. 1992 Jun;60(6):2445–2452. doi: 10.1128/iai.60.6.2445-2452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. H., Jacob-Dubuisson F., Dodson K., Kuehn M., Slonim L., Striker R., Hultgren S. J. Adhesin presentation in bacteria requires molecular chaperones and ushers. Infect Immun. 1992 Nov;60(11):4445–4451. doi: 10.1128/iai.60.11.4445-4451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D., Sackin M. J., Sneath P. H. A numerical taxonomic study of streptococci of serological group D. J Gen Microbiol. 1972 Oct;72(3):439–450. doi: 10.1099/00221287-72-3-439. [DOI] [PubMed] [Google Scholar]
- Jones R. N. Gram-positive superinfections following beta-lactam chemotherapy: the significance of the enterococcus. Infection. 1985;13 (Suppl 1):S81–S88. doi: 10.1007/BF01644225. [DOI] [PubMed] [Google Scholar]
- Jones W. G., Barie P. S., Yurt R. W., Goodwin C. W. Enterococcal burn sepsis. A highly lethal complication in severely burned patients. Arch Surg. 1986 Jun;121(6):649–653. doi: 10.1001/archsurg.1986.01400060043004. [DOI] [PubMed] [Google Scholar]
- Kaufhold A., Ferrieri P. Isolation of Enterococcus mundtii from normally sterile body sites in two patients. J Clin Microbiol. 1991 May;29(5):1075–1077. doi: 10.1128/jcm.29.5.1075-1077.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A. Y., Henig E. F. The microbiologic approach in endodontics. Oral Surg Oral Med Oral Pathol. 1976 Dec;42(6):810–816. doi: 10.1016/0030-4220(76)90104-3. [DOI] [PubMed] [Google Scholar]
- Kaye D. Enterococci. Biologic and epidemiologic characteristics and in vitro susceptibility. Arch Intern Med. 1982 Oct 25;142(11):2006–2009. doi: 10.1001/archinte.142.11.2006. [DOI] [PubMed] [Google Scholar]
- Khardori N., Wong E., Carrasco C. H., Wallace S., Patt Y., Bodey G. P. Infections associated with biliary drainage procedures in patients with cancer. Rev Infect Dis. 1991 Jul-Aug;13(4):587–591. doi: 10.1093/clinids/13.4.587. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Bayer A. S. Significance of in-vitro penicillin tolerance in experimental enterococcal endocarditis. J Antimicrob Chemother. 1987 Apr;19(4):475–485. doi: 10.1093/jac/19.4.475. [DOI] [PubMed] [Google Scholar]
- Kreft B., Marre R., Schramm U., Wirth R. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun. 1992 Jan;60(1):25–30. doi: 10.1128/iai.60.1.25-30.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers J. M., Proctor R. A. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect Immun. 1989 Aug;57(8):2306–2312. doi: 10.1128/iai.57.8.2306-2312.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühnen E., Richter F., Richter K., Andries L. Establishment of a typing system for group D streptococci. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Jan;267(3):322–330. doi: 10.1016/s0176-6724(88)80048-8. [DOI] [PubMed] [Google Scholar]
- Landry S. L., Kaiser D. L., Wenzel R. P. Hospital stay and mortality attributed to nosocomial enterococcal bacteremia: a controlled study. Am J Infect Control. 1989 Dec;17(6):323–329. doi: 10.1016/0196-6553(89)90001-1. [DOI] [PubMed] [Google Scholar]
- Law D. Adhesion and its role in the virulence of enteropathogenic Escherichia coli. Clin Microbiol Rev. 1994 Apr;7(2):152–173. doi: 10.1128/cmr.7.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Lee L. N., Clewell D. B., Behnke D. Broad geographical distribution of a cytotoxin gene mediating beta-hemolysis and bacteriocin activity among Streptococcus faecalis strains. Infect Immun. 1983 Jun;40(3):1015–1022. doi: 10.1128/iai.40.3.1015-1022.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Selsted M. E., Babior B. M., Curnutte J. T. Neutrophils and host defense. Ann Intern Med. 1988 Jul 15;109(2):127–142. doi: 10.7326/0003-4819-109-2-127. [DOI] [PubMed] [Google Scholar]
- Lemoine L., Hunter P. R. Enterococcal urinary tract infections in a teaching hospital. Eur J Clin Microbiol. 1987 Oct;6(5):574–575. doi: 10.1007/BF02014250. [DOI] [PubMed] [Google Scholar]
- Leopold K., Fischer W. Separation of the poly(glycerophosphate) lipoteichoic acids of Enterococcus faecalis Kiel 27738, Enterococcus hirae ATCC 9790 and Leuconostoc mesenteroides DSM 20343 into molecular species by affinity chromatography on concanavalin A. Eur J Biochem. 1991 Mar 14;196(2):475–482. doi: 10.1111/j.1432-1033.1991.tb15839.x. [DOI] [PubMed] [Google Scholar]
- Lewis C. M., Zervos M. J. Clinical manifestations of enterococcal infection. Eur J Clin Microbiol Infect Dis. 1990 Feb;9(2):111–117. doi: 10.1007/BF01963635. [DOI] [PubMed] [Google Scholar]
- Libertin C. R., Dumitru R., Stein D. S. The hemolysin/bacteriocin produced by enterococci is a marker of pathogenicity. Diagn Microbiol Infect Dis. 1992 Feb;15(2):115–120. doi: 10.1016/0732-8893(92)90033-p. [DOI] [PubMed] [Google Scholar]
- Libit S. A., Michael A. F., Vernier R. L., Fish A. J. Hematogenous Streptococcus faecalis pyelonephritis in the rat. A histologic, immunopathologic and bacteriologic study. Am J Pathol. 1974 Sep;76(3):419–432. [PMC free article] [PubMed] [Google Scholar]
- Livornese L. L., Jr, Dias S., Samel C., Romanowski B., Taylor S., May P., Pitsakis P., Woods G., Kaye D., Levison M. E. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med. 1992 Jul 15;117(2):112–116. doi: 10.7326/0003-4819-117-2-112. [DOI] [PubMed] [Google Scholar]
- Lowell S. H., Juhn S. K. The role of bacterial enzymes in inducing inflammation in the middle ear cavity. Otolaryngol Head Neck Surg (1979) 1979 Nov-Dec;87(6):859–870. doi: 10.1177/019459987908700621. [DOI] [PubMed] [Google Scholar]
- Maki D. G., Agger W. A. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine (Baltimore) 1988 Jul;67(4):248–269. [PubMed] [Google Scholar]
- Malone D. A., Wagner R. A., Myers J. P., Watanakunakorn C. Enterococcal bacteremia in two large community teaching hospitals. Am J Med. 1986 Oct;81(4):601–606. doi: 10.1016/0002-9343(86)90544-9. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J Clin Invest. 1975 Mar;55(3):561–566. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco W. A., Phan S. H., Krutzsch H., Showell H. J., Feltner D. E., Nairn R., Becker E. L., Ward P. A. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J Biol Chem. 1984 May 10;259(9):5430–5439. [PubMed] [Google Scholar]
- Martens M. G., Faro S., Riddle G. Female genital tract abscess formation in the rat. Use of pathogens including enterococci. J Reprod Med. 1993 Sep;38(9):719–724. [PubMed] [Google Scholar]
- Matlow A. G., Bohnen J. M., Nohr C., Christou N., Meakins J. Pathogenicity of enterococci in a rat model of fecal peritonitis. J Infect Dis. 1989 Jul;160(1):142–145. doi: 10.1093/infdis/160.1.142. [DOI] [PubMed] [Google Scholar]
- Miyake Y., Yasuhara T., Fukui K., Suginaka H., Nakajima T., Moriyama T. Purification and characterization of neutrophil chemotactic factors of Streptococcus sanguis. Biochim Biophys Acta. 1983 Jul 29;758(2):181–186. doi: 10.1016/0304-4165(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr Emergence of Enterococcus as a significant pathogen. Clin Infect Dis. 1992 Jun;14(6):1173–1176. doi: 10.1093/clinids/14.6.1173. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr Enterococcal infections in patients treated with moxalactam. Rev Infect Dis. 1982 Nov-Dec;4 (Suppl):S708–S711. doi: 10.1093/clinids/4.supplement_3.s708. [DOI] [PubMed] [Google Scholar]
- Montgomerie J. Z., Kalmanson G. M., Guze L. B. Virulence of enterococci in experimental pyelonephritis. Urol Res. 1977;5(3):99–102. doi: 10.1007/BF00256860. [DOI] [PubMed] [Google Scholar]
- Morelli L., Sarra P. G., Bottazzi V. In vivo transfer of pAM beta 1 from Lactobacillus reuteri to Enterococcus faecalis. J Appl Bacteriol. 1988 Nov;65(5):371–375. doi: 10.1111/j.1365-2672.1988.tb01905.x. [DOI] [PubMed] [Google Scholar]
- Morrison A. J., Jr, Wenzel R. P. Nosocomial urinary tract infections due to enterococcus. Ten years' experience at a university hospital. Arch Intern Med. 1986 Aug;146(8):1549–1551. [PubMed] [Google Scholar]
- Murray B. E., Lopardo H. A., Rubeglio E. A., Frosolono M., Singh K. V. Intrahospital spread of a single gentamicin-resistant, beta-lactamase-producing strain of Enterococcus faecalis in Argentina. Antimicrob Agents Chemother. 1992 Jan;36(1):230–232. doi: 10.1128/aac.36.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Heath J. D., Sharma B. R., Weinstock G. M. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990 Sep;28(9):2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Markowitz S. M., Lopardo H. A., Patterson J. E., Zervos M. J., Rubeglio E., Eliopoulos G. M., Rice L. B., Goldstein F. W. Evidence for clonal spread of a single strain of beta-lactamase-producing Enterococcus (Streptococcus) faecalis to six hospitals in five states. J Infect Dis. 1991 Apr;163(4):780–785. doi: 10.1093/infdis/163.4.780. [DOI] [PubMed] [Google Scholar]
- Murray B. E. The life and times of the Enterococcus. Clin Microbiol Rev. 1990 Jan;3(1):46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscholl A., Galli D., Wanner G., Wirth R. Sex pheromone plasmid pAD1-encoded aggregation substance of Enterococcus faecalis is positively regulated in trans by traE1. Eur J Biochem. 1993 May 15;214(1):333–338. doi: 10.1111/j.1432-1033.1993.tb17928.x. [DOI] [PubMed] [Google Scholar]
- Mäkinen P. L., Clewell D. B., An F., Mäkinen K. K. Purification and substrate specificity of a strongly hydrophobic extracellular metalloendopeptidase ("gelatinase") from Streptococcus faecalis (strain 0G1-10). J Biol Chem. 1989 Feb 25;264(6):3325–3334. [PubMed] [Google Scholar]
- Nagao S., Tanaka A., Yamamoto Y., Koga T., Onoue K., Shiba T., Kusumoto K., Kotani S. Inhibition of macrophage migration by muramyl peptides. Infect Immun. 1979 May;24(2):308–312. doi: 10.1128/iai.24.2.308-312.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natkin E. Isolation and host range of bacteriophages active against human oral enterococci. Arch Oral Biol. 1967 May;12(5):669–680. doi: 10.1016/0003-9969(67)90085-4. [DOI] [PubMed] [Google Scholar]
- Nord C. E., Wadström T. Characterization of haemolytic enterococci isolated from oral infections. Acta Odontol Scand. 1973 Dec;31(6):387–393. doi: 10.3109/00016357309002526. [DOI] [PubMed] [Google Scholar]
- Noskin G. A., Till M., Patterson B. K., Clarke J. T., Warren J. R. High-level gentamicin resistance in Enterococcus faecalis bacteremia. J Infect Dis. 1991 Dec;164(6):1212–1215. doi: 10.1093/infdis/164.6.1212. [DOI] [PubMed] [Google Scholar]
- Novak R. M., Holzer T. J., Libertin C. R. Human neutrophil oxidative response and phagocytic killing of clinical and laboratory strains of Enterococcus faecalis. Diagn Microbiol Infect Dis. 1993 Jul;17(1):1–6. doi: 10.1016/0732-8893(93)90061-b. [DOI] [PubMed] [Google Scholar]
- ORLAND F. J., BLAYNEY J. R., HARRISON R. W., REYNIERS J. A., TREXLER P. C., ERVIN R. F., GORDON H. A., WAGNER M. Experimental caries in germfree rats inoculated with enterococci. J Am Dent Assoc. 1955 Mar;50(3):259–272. doi: 10.14219/jada.archive.1955.0061. [DOI] [PubMed] [Google Scholar]
- Olmsted S. B., Erlandsen S. L., Dunny G. M., Wells C. L. High-resolution visualization by field emission scanning electron microscopy of Enterococcus faecalis surface proteins encoded by the pheromone-inducible conjugative plasmid pCF10. J Bacteriol. 1993 Oct;175(19):6229–6237. doi: 10.1128/jb.175.19.6229-6237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Bartlett J. G., Louie T., Sullivan-Seigler N., Gorbach S. L. Microbial synergy in experimental intra-abdominal abscess. Infect Immun. 1976 Jan;13(1):22–26. doi: 10.1128/iai.13.1.22-26.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Kasper D. L., Cisneros R. L., Bartlett J. G. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977 Jul;136(1):82–89. doi: 10.1093/infdis/136.1.82. [DOI] [PubMed] [Google Scholar]
- Patel R., Keating M. R., Cockerill F. R., 3rd, Steckelberg J. M. Bacteremia due to Enterococcus avium. Clin Infect Dis. 1993 Dec;17(6):1006–1011. doi: 10.1093/clinids/17.6.1006. [DOI] [PubMed] [Google Scholar]
- Patterson J. E., Zervos M. J. High-level gentamicin resistance in Enterococcus: microbiology, genetic basis, and epidemiology. Rev Infect Dis. 1990 Jul-Aug;12(4):644–652. doi: 10.1093/clinids/12.4.644. [DOI] [PubMed] [Google Scholar]
- Pompei R., Lampis G., Berlutti F., Thaller M. C. Characterization of yellow-pigmented enterococci from severe human infections. J Clin Microbiol. 1991 Dec;29(12):2884–2886. doi: 10.1128/jcm.29.12.2884-2886.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole L. B., Claiborne A. Interactions of pyridine nucleotides with redox forms of the flavin-containing NADH peroxidase from Streptococcus faecalis. J Biol Chem. 1986 Nov 5;261(31):14525–14533. [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS C. G., SARLES W. B. CHARACTERIZATION OF ENTEROCOCCUS BACTERIOPHAGES FROM THE SMALL INTESTINE OF THE RAT. J Bacteriol. 1963 Jun;85:1378–1385. doi: 10.1128/jb.85.6.1378-1385.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSAN B., WILLIAMS N. B. HYALURONIDASE PRODUCTION BY ORAL ENTEROCOCCI. Arch Oral Biol. 1964 May-Jun;9:291–298. doi: 10.1016/0003-9969(64)90061-5. [DOI] [PubMed] [Google Scholar]
- Rhinehart E., Smith N. E., Wennersten C., Gorss E., Freeman J., Eliopoulos G. M., Moellering R. C., Jr, Goldmann D. A. Rapid dissemination of beta-lactamase-producing, aminoglycoside-resistant Enterococcus faecalis among patients and staff on an infant-toddler surgical ward. N Engl J Med. 1990 Dec 27;323(26):1814–1818. doi: 10.1056/NEJM199012273232606. [DOI] [PubMed] [Google Scholar]
- Richet H., Hubert B., Nitemberg G., Andremont A., Buu-Hoi A., Ourbak P., Galicier C., Veron M., Boisivon A., Bouvier A. M. Prospective multicenter study of vascular-catheter-related complications and risk factors for positive central-catheter cultures in intensive care unit patients. J Clin Microbiol. 1990 Nov;28(11):2520–2525. doi: 10.1128/jcm.28.11.2520-2525.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimailho A., Lampl E., Riou B., Richard C., Rottman E., Auzepy P. Enterococcal bacteremia in a medical intensive care unit. Crit Care Med. 1988 Feb;16(2):126–129. doi: 10.1097/00003246-198802000-00006. [DOI] [PubMed] [Google Scholar]
- Ruoff K. L., de la Maza L., Murtagh M. J., Spargo J. D., Ferraro M. J. Species identities of enterococci isolated from clinical specimens. J Clin Microbiol. 1990 Mar;28(3):435–437. doi: 10.1128/jcm.28.3.435-437.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ-HAUDT S. D., SCHERP H. W. Production of hyaluronidase and beta-glucuronidase by Viridans streptococci isolated from gingival crevices. J Dent Res. 1955 Dec;34(6):924–929. doi: 10.1177/00220345550340061901. [DOI] [PubMed] [Google Scholar]
- STARK J. M. Antibiotic activity of haemolytic enterococci. Lancet. 1960 Apr 2;1(7127):733–734. doi: 10.1016/s0140-6736(60)90620-6. [DOI] [PubMed] [Google Scholar]
- Sannomiya P., Craig R. A., Clewell D. B., Suzuki A., Fujino M., Till G. O., Marasco W. A. Characterization of a class of nonformylated Enterococcus faecalis-derived neutrophil chemotactic peptides: the sex pheromones. Proc Natl Acad Sci U S A. 1990 Jan;87(1):66–70. doi: 10.1073/pnas.87.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg D. R., Culver D. H., Gaynes R. P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991 Sep 16;91(3B):72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- Schaberg D. R., Zervos M. J. Intergeneric and interspecies gene exchange in gram-positive cocci. Antimicrob Agents Chemother. 1986 Dec;30(6):817–822. doi: 10.1128/aac.30.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Strunk R. W., Balian G., Calderone R. A. Microbial adhesion to fibronectin in vitro correlates with production of endocarditis in rabbits. Proc Soc Exp Biol Med. 1985 Dec;180(3):474–482. doi: 10.3181/00379727-180-42205. [DOI] [PubMed] [Google Scholar]
- Schnell N., Engelke G., Augustin J., Rosenstein R., Ungermann V., Götz F., Entian K. D. Analysis of genes involved in the biosynthesis of lantibiotic epidermin. Eur J Biochem. 1992 Feb 15;204(1):57–68. doi: 10.1111/j.1432-1033.1992.tb16605.x. [DOI] [PubMed] [Google Scholar]
- Scott J. R. Sex and the single circle: conjugative transposition. J Bacteriol. 1992 Oct;174(19):6005–6010. doi: 10.1128/jb.174.19.6005-6010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra R. A., Booth M. C., Morales D. A., Huycke M. M., Gilmore M. S. Molecular characterization of the Enterococcus faecalis cytolysin activator. Infect Immun. 1991 Apr;59(4):1239–1246. doi: 10.1128/iai.59.4.1239-1246.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman J. M. THE STREPTOCOCCI. Bacteriol Rev. 1937 Dec;1(1):3–97. doi: 10.1128/br.1.1.3-97.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Levy J., Wolinsky E. Enterococcal bacteremia without endocarditis. Arch Intern Med. 1981 Apr;141(5):578–581. [PubMed] [Google Scholar]
- Shorrock P. J., Lambert P. A. Binding of fibronectin and albumin to Enterococcus (Streptococcus) faecalis. Microb Pathog. 1989 Jan;6(1):61–67. doi: 10.1016/0882-4010(89)90008-9. [DOI] [PubMed] [Google Scholar]
- Smith R. F., Willett N. P. Rapid plate method for screening hyaluronidase and chondroitin sulfatase-producing microorganisms. Appl Microbiol. 1968 Sep;16(9):1434–1436. doi: 10.1128/am.16.9.1434-1436.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S. Relationship of bacteria to the etiology of periodontal disease. J Dent Res. 1970 Mar-Apr;49(2):203–222. doi: 10.1177/00220345700490020401. [DOI] [PubMed] [Google Scholar]
- Stevens S. X., Jensen H. G., Jett B. D., Gilmore M. S. A hemolysin-encoding plasmid contributes to bacterial virulence in experimental Enterococcus faecalis endophthalmitis. Invest Ophthalmol Vis Sci. 1992 Apr;33(5):1650–1656. [PubMed] [Google Scholar]
- Su Y. A., Sulavik M. C., He P., Makinen K. K., Makinen P. L., Fiedler S., Wirth R., Clewell D. B. Nucleotide sequence of the gelatinase gene (gelE) from Enterococcus faecalis subsp. liquefaciens. Infect Immun. 1991 Jan;59(1):415–420. doi: 10.1128/iai.59.1.415-420.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullam P. M., Täuber M. G., Hackbarth C. J., Sande M. A. Antimicrobial activity of gentamicin in experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1985 Feb;27(2):224–226. doi: 10.1128/aac.27.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supersac G., Prevost G., Piemont Y. Sequencing of leucocidin R from Staphylococcus aureus P83 suggests that staphylococcal leucocidins and gamma-hemolysin are members of a single, two-component family of toxins. Infect Immun. 1993 Feb;61(2):580–587. doi: 10.1128/iai.61.2.580-587.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauvin C., Eliopoulos G. M., Willey S., Wennersten C., Moellering R. C., Jr Continuous-infusion ampicillin therapy of enterococcal endocarditis in rats. Antimicrob Agents Chemother. 1987 Feb;31(2):139–143. doi: 10.1128/aac.31.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tight R. R. Ampicillin therapy of experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1980 Aug;18(2):307–310. doi: 10.1128/aac.18.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toon P., Brown P. E., Baddiley J. The lipid-teichoic acid complex in the cytoplasmic membrane of Streptococcus faecalis N.C.I.B. 8191. Biochem J. 1972 Apr;127(2):399–409. doi: 10.1042/bj1270399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter K. M., Dunny G. M. Mutants of Enterococcus faecalis deficient as recipients in mating with donors carrying pheromone-inducible plasmids. Plasmid. 1990 Jul;24(1):57–67. doi: 10.1016/0147-619x(90)90025-8. [DOI] [PubMed] [Google Scholar]
- Tsutsui O., Kokeguchi S., Matsumura T., Kato K. Relationship of the chemical structure and immunobiological activities of lipoteichoic acid from Streptococcus faecalis (Enterococcus hirae) ATCC 9790. FEMS Microbiol Immunol. 1991 Aug;3(4):211–218. doi: 10.1111/j.1574-6968.1991.tb04217.x. [DOI] [PubMed] [Google Scholar]
- Unsworth P. F. Hyaluronidase production in Streptococcus milleri in relation to infection. J Clin Pathol. 1989 May;42(5):506–510. doi: 10.1136/jcp.42.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L. Pseudomonas aeruginosa: biology, mechanisms of virulence, epidemiology. J Pediatr. 1986 May;108(5 Pt 2):800–805. doi: 10.1016/s0022-3476(86)80748-x. [DOI] [PubMed] [Google Scholar]
- Vollaard E. J., Clasener H. A. Colonization resistance. Antimicrob Agents Chemother. 1994 Mar;38(3):409–414. doi: 10.1128/aac.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICKEN A. J., ELLIOTT S. D., BADDILEY J. The identity of streptococcal group D antigen with teichoic acid. J Gen Microbiol. 1963 May;31:231–239. doi: 10.1099/00221287-31-2-231. [DOI] [PubMed] [Google Scholar]
- WILLIAMS N. B., FORBES M. A., BLAU E., EICKENBERG C. F. A study of the simultaneous occurrence of Enterococci, Lactobacilli, and yeasts in saliva from human beings. J Dent Res. 1950 Oct;29(5):563–570. doi: 10.1177/00220345500290050201. [DOI] [PubMed] [Google Scholar]
- Wanner G., Formanek H., Galli D., Wirth R. Localization of aggregation substances of Enterococcus faecalis after induction by sex pheromones. An ultrastructural comparison using immuno labelling, transmission and high resolution scanning electron microscopic techniques. Arch Microbiol. 1989;151(6):491–497. doi: 10.1007/BF00454864. [DOI] [PubMed] [Google Scholar]
- Weinstein A. J., Lentnek A. L. Cephalosporin-aminoglycoside synergism in experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1976 Jun;9(6):983–987. doi: 10.1128/aac.9.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M. P., Murphy J. R., Reller L. B., Lichtenstein K. A. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. II. Clinical observations, with special reference to factors influencing prognosis. Rev Infect Dis. 1983 Jan-Feb;5(1):54–70. doi: 10.1093/clinids/5.1.54. [DOI] [PubMed] [Google Scholar]
- Weinstein M. P., Reller L. B., Murphy J. R., Lichtenstein K. A. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observations. Rev Infect Dis. 1983 Jan-Feb;5(1):35–53. doi: 10.1093/clinids/5.1.35. [DOI] [PubMed] [Google Scholar]
- Wells C. L., Erlandsen S. L. Localization of translocating Escherichia coli, Proteus mirabilis, and Enterococcus faecalis within cecal and colonic tissues of monoassociated mice. Infect Immun. 1991 Dec;59(12):4693–4697. doi: 10.1128/iai.59.12.4693-4697.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells C. L., Jechorek R. P., Erlandsen S. L. Evidence for the translocation of Enterococcus faecalis across the mouse intestinal tract. J Infect Dis. 1990 Jul;162(1):82–90. doi: 10.1093/infdis/162.1.82. [DOI] [PubMed] [Google Scholar]
- Wells C. L., Jechorek R. P., Maddaus M. A., Simmons R. L. Effects of clindamycin and metronidazole on the intestinal colonization and translocation of enterococci in mice. Antimicrob Agents Chemother. 1988 Dec;32(12):1769–1775. doi: 10.1128/aac.32.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells C. L., Jechorek R. P., Twiggs L. B., Brooker D. C. Recovery of viable bacteria from pelvic lymph nodes of patients with gynecologic tumors. J Infect Dis. 1990 Nov;162(5):1216–1218. doi: 10.1093/infdis/162.5.1216-a. [DOI] [PubMed] [Google Scholar]
- Wells C. L., Maddaus M. A., Reynolds C. M., Jechorek R. P., Simmons R. L. Role of anaerobic flora in the translocation of aerobic and facultatively anaerobic intestinal bacteria. Infect Immun. 1987 Nov;55(11):2689–2694. doi: 10.1128/iai.55.11.2689-2694.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells C. L., Maddaus M. A., Simmons R. L. Proposed mechanisms for the translocation of intestinal bacteria. Rev Infect Dis. 1988 Sep-Oct;10(5):958–979. doi: 10.1093/clinids/10.5.958. [DOI] [PubMed] [Google Scholar]
- Wells V. D., Wong E. S., Murray B. E., Coudron P. E., Williams D. S., Markowitz S. M. Infections due to beta-lactamase-producing, high-level gentamicin-resistant Enterococcus faecalis. Ann Intern Med. 1992 Feb 15;116(4):285–292. doi: 10.7326/0003-4819-116-4-285. [DOI] [PubMed] [Google Scholar]
- Willey S. H., Hindes R. G., Eliopoulos G. M., Moellering R. C., Jr Effects of clindamycin and gentamicin and other antimicrobial combinations against enterococci in an experimental model of intra-abdominal abscess. Surg Gynecol Obstet. 1989 Sep;169(3):199–202. [PubMed] [Google Scholar]
- Woodford N., Morrison D., Johnson A. P., Briant V., George R. C., Cookson B. Application of DNA probes for rRNA and vanA genes to investigation of a nosocomial cluster of vancomycin-resistant enterococci. J Clin Microbiol. 1993 Mar;31(3):653–658. doi: 10.1128/jcm.31.3.653-658.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A. J., Wilson W. R., Matsumoto J. Y., Washington J. A., 2nd, Geraci J. E. Influence of gentamicin dose size on the efficacies of combinations of gentamicin and penicillin in experimental streptomycin-resistant enterococcal endocarditis. Antimicrob Agents Chemother. 1982 Dec;22(6):972–975. doi: 10.1128/aac.22.6.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervos M. J., Bacon A. E., 3rd, Patterson J. E., Schaberg D. R., Kauffman C. A. Enterococcal superinfection in patients treated with ciprofloxacin. J Antimicrob Chemother. 1988 Jan;21(1):113–115. doi: 10.1093/jac/21.1.113. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Dembinski S., Mikesell T., Schaberg D. R. High-level resistance to gentamicin in Streptococcus faecalis: risk factors and evidence for exogenous acquisition of infection. J Infect Dis. 1986 Jun;153(6):1075–1083. doi: 10.1093/infdis/153.6.1075. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Kauffman C. A., Therasse P. M., Bergman A. G., Mikesell T. S., Schaberg D. R. Nosocomial infection by gentamicin-resistant Streptococcus faecalis. An epidemiologic study. Ann Intern Med. 1987 May;106(5):687–691. doi: 10.7326/0003-4819-106-5-687. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Mikesell T. S., Schaberg D. R. Heterogeneity of plasmids determining high-level resistance to gentamicin in clinical isolates of Streptococcus faecalis. Antimicrob Agents Chemother. 1986 Jul;30(1):78–81. doi: 10.1128/aac.30.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweibaum A., Pinto M., Chevalier G., Dussaulx E., Triadou N., Lacroix B., Haffen K., Brun J. L., Rousset M. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugar-free medium and its inhibition by glucose. J Cell Physiol. 1985 Jan;122(1):21–29. doi: 10.1002/jcp.1041220105. [DOI] [PubMed] [Google Scholar]