Abstract
Objective
Morbidly obese patients frequently present with mood and anxiety disorders, which are often treated with serotonin reuptake inhibitors (SRIs). Having observed that patients treated with SRIs frequently relapse after Rouxen-Y gastric bypass surgery, the authors sought to assess whether SRI bioavailability is reduced postoperatively.
Method
Twelve gastric bypass candidates treated with an SRI for primary mood or anxiety disorders were studied prospectively. Timed blood samples for SRI plasma levels were drawn for pharmacokinetic studies before surgery and 1, 6, and 12 months afterward. Maximum concentration, time to maximum concentration, and area under the concentration/time curve (AUC) were determined.
Results
In eight of the 12 patients, AUC values 1 month after surgery dropped to an average of 54% (SD=18) of preoperative levels (range=36%–80%); in six of these patients, AUC values returned to baseline levels (or greater) by 6 months. Four patients had an exacerbation of depressive symptoms, which resolved by 12 months in three of them. Three of the four patients had a reduced AUC level at 1 month and either gained weight or failed to lose weight between 6 and 12 months. Normalization of the AUC was associated with improvement in symptom scores.
Conclusions
Patients taking SRIs in this study were at risk for reduced drug bioavailability 1 month after Rouxen-Y gastric bypass. The authors recommend close psychiatric monitoring after surgery.
Obesity has reached epidemic status in the United States. According to the National Health and Nutrition Examination Survey, the prevalence of obesity among American adults increased from 15% during the period 1976–1980 to 34% in 2007 and 2008 (1). Obesity severity is measured by body mass index (BMI), which is computed by dividing body weight (in kilograms) by the square of height (in meters). A BMI of 25 to 29.9 is considered to indicate overweight; a BMI of 30–34.9 is designated as obese, and a BMI ≥40 is designated as morbidly obese. Obesity is associated with a wide spectrum of medical comorbidities, including sleep apnea, type 2 diabetes mellitus, hypertension, cardiovascular disease, asthma, and cancers of the colon, breast, and endometrium. These conditions contribute to the substantial morbidity and mortality associated with obesity; therefore, in individuals with weight-related comorbidities, a BMI ≥35 is considered morbidly obese.
Morbid obesity has been linked to psychiatric disorders, most commonly major depressive disorder, with a reported odds ratio of 4.98 (95% confidence interval=2.07–11.99) (2). In one study, nearly two-thirds of bariatric surgery candidates received a psychiatric diagnosis, most commonly major depression (3). Bipolar disorder is also prevalent among candidates for bariatric surgery (4). Psychotropic medications (5) and atypical depressive symptoms such as overeating and fatigue contribute to weight gain. Persons with psychiatric disorders die decades earlier than the general population (6, 7), and the majority of premature deaths are due to cardiovascular events (8).
Roux-en-Y Gastric Bypass Surgery
Morbidly obese patients who have failed dietary or medical weight loss methods are potential candidates for bariatric surgery to achieve long-term weight reduction. In 2008, 220,000 bariatric surgeries were performed in the United States and Canada (9). The Roux-en-Y gastric bypass is one of the most common bariatric procedures. It may be performed either through an abdominal midline incision or laparoscopically, using five or six transabdominal access ports. The laparoscopic approach is associated with a more rapid recovery, lower rates of pulmonary complications and wound infections, and less postoperative pain. A gastric pouch 15–30 ml in volume is created to restrict food intake. The jejunum is then divided 40–50 cm distal to the ligament of Treitz, and the distal end is anastomosed to the pouch. The proximal end is anastomosed 75–150 cm downstream to create a bypass, which induces mild malabsorption.
“Ms. Z” is a 48-year-old white married woman who had failed multiple attempts at weight loss and presented for Roux-en-Y gastric bypass surgery. Her height was 1.57 m, and her weight was 114 kg (body mass index=46)
She had a history of obsessive-compulsive disorder and recurrent major depression and had been successfully treated with sertraline, 100 mg/day, with a 2-year period of remission prior to surgery. Ms. Z underwent a laparoscopic retrocolic, retrogastric Roux-en-Y gastric bypass under general anesthesia. She had an uncomplicated postoperative course. Three months after surgery, she reported a relapse during the previous month, with symptoms of marked anxiety. She had a recurrence of obsessions and compulsions involving keeping objects in an orderly and symmetrical fashion.
Ms. Z was the first subject to enter our formal pharmacokinetics study. We confirmed that the bioavailability of sertraline postoperatively was only 36% of the preoperative value. We offered Ms. Z liquid sertraline at the same dosage (100 mg/day) to improve absorption. One month later, she reported a return to baseline, with marked reduction in her anxiety and obsessive-compulsive symptoms.
After Roux-en-Y gastric bypass, patients achieve durable weight loss and experience improvements in weight-related comorbidities, quality of life, and mortality rates (10–12). The mean percent excess weight loss (%EWL) is 75% at 5 years (13). The %EWL, the standard value for expressing weight loss outcomes in bariatric surgery, is defined as the interval weight loss divided by the difference between pre-surgical weight and ideal body weight multiplied by 100. Patients are advised to eat small meals three to five times daily, avoid high-calorie snack foods, drink calorie-free beverages, and exercise at least three times a week.
Pathophysiology
After Roux-en-Y gastric bypass, ingested food no longer passes through the gastric antrum and duodenum; as a result, nutrient absorption is reduced (14–17) and patients are required to take vitamin and mineral supplements. Because food no longer passes through the duodenum, which is the site of calcium and iron absorption, patients must take these supplements for life. Vitamin B12 from ingested food normally binds to intrinsic factor in the stomach for subsequent absorption in the terminal ileum. Because of the separation of the stomach during gastric bypass, the binding of vitamin B12 to intrinsic factor is reduced, and therefore vitamin B12 supplementation is required to prevent anemia.
The drastic alteration in gastrointestinal anatomy resulting from Roux-en-Y gastric bypass induces major changes in drug disposition (18); however, few pharmacokinetic studies have been conducted in gastric bypass patients (17, 19–22). After surgery, the absorption of a number of medications has been found to be reduced, including phenytoin, T4, rifampin, and tamoxifen (23, 24). Antibiotic therapy may require intravenous or intramuscular administration or monitoring of oral antibiotic levels to ensure that dosing is therapeutic (25). In a study comparing Roux-en-Y gastric bypass patients to non-bypass comparison subjects, the pharmacokinetics of tacrolimus, sirolimus, mycophenolic acid, and mycophenolic acid glucuronide were all reduced (20). Roux-en-Y gastric bypass has been shown to have a variable effect on the pharmacokinetics of atorvastatin, ranging from a 2.9-fold decrease to a 2.3-fold increase in AUC0–8 (area under the concentration/time curve, the integral of drug blood level over time from 0 to 8 hours as a measure of quantity of drug absorbed) (21).
Because cytochrome P450 and other metabolizing enzymes are found in the bypassed proximal small intestine, drugs that undergo substantial first-pass metabolism in the intestine may be more bioavailable after Roux-en-Y gastric bypass. Metformin absorption and bioavailability increase after surgery (26); this agent is transported mainly by the organic anion-transporting polypeptides, which may be up-regulated after surgery, leading to an increase in absorption.
Despite the frequency of psychotropic drug use by bar-iatric surgical patients, pharmacokinetic studies are rare. In a study of five Roux-en-Y gastric bypass patients treated with sertraline for major depression, the patients had a lower mean AUC compared with non-surgical comparison subjects after receiving a dose of 100 mg sertraline (19).
Possible Mechanisms of Action
Various factors may affect bioavailability of drugs in Roux-en-Y gastric bypass patients. The mechanism of the postsurgical reduction in serotonin reuptake inhibitor (SRI) levels may be related to the markedly reduced gastric acidity (14); absorption of the tablet form of SRI decreases as a result of reduced solubility. After surgery, the cardia is separated from the distal stomach; therefore, most of the parietal cells are excluded from the pouch and gastric pH is increased. An in vitro drug dissolution model approximating the Roux-en-Y gastric bypass environment demonstrated that 10 of 22 psychiatric medications had significantly less dissolution compared with the control environment (27).
Another contributing factor is the diminished intestinal surface area available for drug absorption as a result of the Roux-en-Y gastric bypass anatomy (17). Gastric emptying may also be altered after gastric transection (18). Furthermore, the rapid weight loss induced by the procedure during the first 18 months dramatically alters the volume of distribution of drugs.
Other explanations for the exacerbation of major depression include reduced absorption of tryptophan, which is a precursor of serotonin (28), and malabsorption of vitamins and minerals that function as enzymatic cofactors in the synthesis of neurotransmitters (29). Because of the risk of iron and folate deficiency (30), gastric bypass patients may be susceptible to both anemia and exacerbation of depression. In young women, iron deficiency is associated with higher depressive symptom scores (31). Folate deficiency has been linked to a reduced response to antidepressants (32).
Method
Based on our observation that Roux-en-Y gastric bypass patients treated with antidepressants frequently experienced exacerbation of psychiatric symptoms postoperatively, we hypothesized that the procedure decreases the absorption of SRIs, which include selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs). We also evaluated whether a reduction in plasma SRI levels and bioavailability was associated with an exacerbation of depressive symptoms.
Morbidly obese adults who were seeking Roux-en-Y gastric bypass and received maintenance treatment with an SRI for a primary mood or anxiety disorder were eligible to participate in the study. A convenience sample of 12 patients met criteria for bariatric surgery (BMI ≥40 or BMI ≥35 with severe obesity-related comorbidities). The patients were studied prospectively over the course of 12 months under a protocol approved by the University of Pittsburgh Institutional Review Board. The SRIs studied included SNRIs (venlafaxine, N=5; duloxetine, N=1) and SSRIs (citalopram, N=2; escitalopram, N=2; sertraline, N=2). Each subject acted as his or her own control throughout the longitudinal study.
Psychiatric Assessment
All patients were assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders (33) to establish the psychiatric diagnoses and psychotropic medication regimens. To measure the severity of depressive symptoms, the Structured Interview Guide for the Hamilton Depression Rating Scale–Atypical Depression Symptom Version (SIGH-ADS) (34) was administered preoperatively within 2 weeks of the procedure and repeated at 1, 6, and 12 months postoperatively. The SIGH-ADS incorporates a set of eight questions designed to assess atypical symptoms of depression, which are associated with weight gain. Adherence to SRI medication regimens was assessed by patient questionnaire at each study visit.
Plasma Drug Analysis
High-performance liquid chromatography with ultraviolet detection methods was used for analysis of venlafaxine, O-des-methylvenlafaxine, sertraline, desmethylsertraline, citalopram, desmethylcitalopram, escitalopram, and desmethylescitalopram. The sertraline/desmethylsertraline analysis has been described previously (35). The method of analysis for citalopram and escitalopram with major metabolites was derived from our sertraline method. Deviations include the use of lipid-stripped normal human serum (Scantibodies Laboratory, Santee, Calif.) as the matrix for standards and controls; 0.1 ml of a 4-mg/ml solution of fluvoxamine as the internal standard; a 26% acetonitrile mobile phase; and ultraviolet detection at 210 nm. The analytical method for the analysis of venlafaxine and O-desmethylvenlafaxine was adapted from our previously published method for chiral fluoxetine (36).
For the analysis of venlafaxine and O-desmethylvenlafaxine, we used a high-performance liquid chromatography (Supelco, Bellefonte, Pa.) Discovery C18, 5-μg, 15-cm, 4.6-mm (catalog no. 504955) column with ultraviolet absorbance detection (225 nm). Risperidone (1.0 μg) was the internal standard, and a 20% ethyl acetate in heptane solution was used for the extraction solvent. The mobile phase was 18/82 vol/vol acetonitrile/0.02 M potassium dihydrogen phosphate with 8.5 ml of triethylamine per liter adjusted to a pH of 6.0 with concentrated phosphoric acid. The retention times were 4.1 minutes for O-desmethylvenlafaxine, 12.0 minutes for venlafaxine, and 26.4 minutes for risperidone. The day-to-day coefficients of variation were 2.7%–8.5% for the medium and high controls and 6.0%–9.0% for the low control. Du-loxetine levels were analyzed by MedTox Scientific, Inc. (St. Paul).
Pharmacokinetic Studies
Pharmacokinetic study sample acquisition was performed at our clinical research center. Patients took their dose of SRI medication at 8:00 a.m., and blood samples were collected through a peripheral intravenous catheter over a 7-hour period for plasma assay of the parent SRI drug and its major metabolite. Data for SRI concentrations over time underwent descriptive model-independent pharmacokinetics using WinNonlin, version 4.1 (Pharsight, Cary, N.C.). Among the pharmacokinetic parameters determined were the maximum concentration (CMAX), time to CMAX (TMAX), and AUC0–7. Like the psychiatric assessments, the pharmacokinetic assays were performed preoperatively within 2 weeks of the procedure and at 1, 6, and 12 months postoperatively.
We evaluated differences in the AUC values under the one-sided hypothesis that the postoperative values would be lower than the baseline values, which reflects lower bioavailability. The mean dose-corrected AUC values at baseline were compared with the values at 1, 6, and 12 months for patients in the overall sample as well as in the SSRI (N=6) and SNRI (N=6) subgroups. The Wilcoxon signed-rank test was used to evaluate significance.
Surgical Procedure
The patients underwent laparoscopic Roux-en-Y gastric bypass surgery under general anesthesia within 2 weeks after their preoperative psychiatric assessment and pharmacokinetic studies. A six-port technique was used, and a 15-ml gastric pouch was created using linear staplers. The biliopancreatic limb was 40 cm long; the Roux limb was 75 cm long for patients with a BMI <50 and 150 cm long for those with a BMI ≥50. A side-to-side jejunoje-junostomy was created using a linear stapler. The Roux limb was anastomosed to the pouch using a combined linear-stapled and hand-sewn technique.
Results
The sample consisted of 11 women and one man. The mean age was 41.9 years (SD=12.5, range=22–56), and the meanpreoperativeBMIwas48.9(SD=5.9, range=37.4–56.4). Psychiatric diagnoses included major depressive disorder (N=6), dysthymic disorder (N=1), bipolar I disorder (N=1), bipolar II disorder (N=2), generalized anxiety disorder (N=1), and anxiety disorder not otherwise specified (N=1). Patients were taking venlafaxine (N=5), citalopram (N=2), escitalopram (N=2), sertraline (N=2), or duloxetine (N=1). Additional demographic data and clinical data, including the values of the dose-corrected pharmacokinetic parameters and SIGH-ADS scores, are presented in Table S1 in the data supplement that accompanies the online edition of this article.
The mean %EWL at 12 months was 48.8% (SD=16.3, range=27.9–80.3). In eight patients (patients 2, 4, 5, 6, 7, 9, 10, and 11), the AUC values for SRIs had decreased 1 month after surgery compared with preoperative levels. Figure 1 presents the pharmacokinetic data for these eight patients, whose AUC values dropped to an average of 54% of the preoperative levels, with a range of 36%–80% of the baseline value. For patient 4, the final preoperative blood sample was drawn at 6 hours, and therefore a preoperative AUC0–7 could not be calculated. However, the AUC0–6 was 0.003 hours/liter, which may be extrapolated to AUC0–7 as a linear estimation. In six of these eight patients (patients 4, 5, 6, 7, 9, and 11), the AUC values had returned to baseline or exceeded preoperative levels at 6 months (Figure 1). For patient 12, the AUC was maintained at 1 month but had decreased at 6 months and again at 12 months. In three patients (patients 1, 3, and 8), the AUC values remained constant or increased postoperatively.
FIGURE 1. Pharmacokinetic Plots for Serotonin Reuptake Inhibitors in Eight Patients Before and After Roux-en-Y Bariatric Bypass Surgerya.
a The log10 plots show dose-corrected plasma concentration over time.
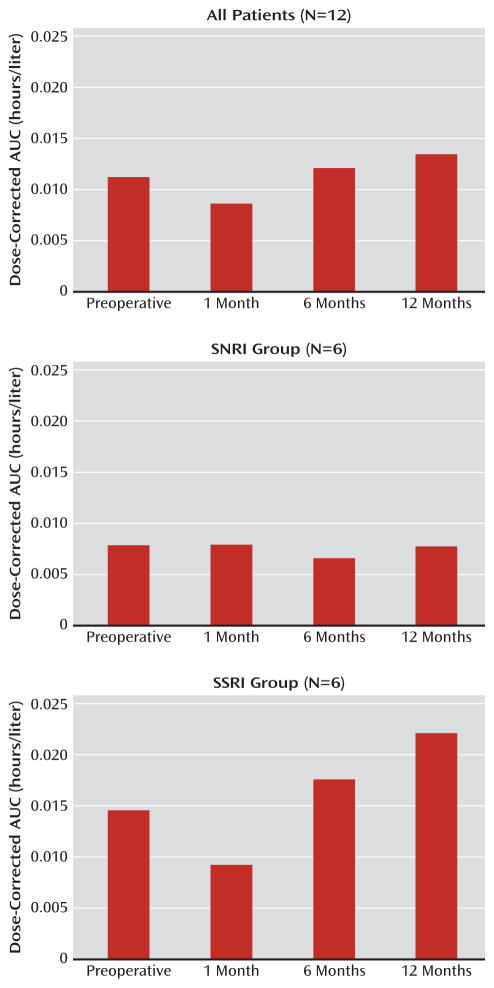
The mean baseline AUC and 1-month AUC values were significantly different for the entire sample (Wilcoxon signed-rank test=−21.0, one-tailed p=0.05) and for the SSRI group (Wilcoxon signed-rank test=−9.5, one-tailed p=0.03), but not for the SNRI group (Wilcoxon signed-rank test=−1.5, one-tailed p=0.42). The comparisons for 6 and 12 months with baseline values were not significant for the entire sample or for either the SSRI or the SNRI group. Figure 2 displays the AUC data for the 12 patients at each assessment.
FIGURE 2. Dose-Corrected AUC Over Time for Serotonin Reuptake Inhibitors Before and After Roux-en-Y Bariatric Bypass Surgerya.
a AUC=area under the concentration/time curve; SNRI=serotonin-norepinephrine reuptake inhibitor; SSRI=selective serotonin reuptake inhibitor.
Normalization of the AUC was associated with an improvement in SIGH-ADS score. Four patients had an exacerbation of depressive symptoms postoperatively (patients 2, 3, 7, and 9), which resolved by 12 months in three patients. Three of the four patients (patients 2, 7, and 9) had a reduced AUC at 1 month. Patient 9 was hospitalized for a suicide attempt by overdose at 3 months, but her symptoms had improved dramatically by 12 months, when her AUC exceeded her baseline value.
Lack of adherence to SRI treatment was examined as a reason for reduction in postoperative SRI levels at 1 month. If the ratios of parent drug to metabolite were variable, then partial adherence, disrupted hepatic metabolism, or erratic absorption might be possible explanations. However, the parent drug-to-metabolite ratios were constant as a function of time, which suggests that the patients were adherent (37).
Clinical Impact
Eight patients had reduced SRI bioavailability 1 month after the procedure, as measured by AUC. The potential impact of lower bioavailability and reduced efficacy of SRI medication is substantial given that mental illness, particularly major depression, plays a major role in weight loss outcomes following surgery (38, 39). Bariatric surgery patients with psychiatric disorders lose significantly less weight compared with those who do not have a preoperative psychiatric diagnosis (38, 39). Patients who experience an exacerbation of symptoms are prone to maladaptive eating behaviors and may struggle to adhere to the required dietary and exercise regimens after surgery (40). These combined factors are likely to promote suboptimal weight loss after surgery (41). Three of our study patients who had an exacerbation of major depression either did not lose weight or gained weight between study visits.
In all but two patients, the AUC values were increased or returned to baseline by 6 months and were sustained through 12 months. One possible explanation for this finding is the adaptive increase in the absorptive surface area of the small intestinal mucosa that occurs after surgery. Elevated levels of glucagon-like peptide-2 result in mucosal crypt cell proliferation, which restores the absorptive surface of the intestine postoperatively, thereby improving drug absorption (39). The substantial weight loss following surgery may also contribute to the improvement in drug availability across time by reducing the volume of distribution (42). Nevertheless, three patients (patients 1, 2, and 12) did not return to their baseline AUC values during the 1-year follow-up period.
To our knowledge, this is the first study in which longitudinal SRI pharmacokinetic studies and psychiatric assessment data have been collected simultaneously for 1 year following bariatric surgery. Determination of the extent to which our findings apply to other orally administered drugs is crucial to the long-term well-being of this highly comorbid population. Limitations of our study include the small sample size and the variability in SRI medications used among the participants. However, each subject acted as his or her own control, which directly addresses the impact of Roux-en-Y gastric bypass on the bioavailability of the SRI for the individual across time and is the primary clinical management concern.
Although a significant reduction in AUC was observed for the entire sample, it was more prominent in the SSRI group than the SNRI group. Differing drug dissolution characteristics may explain these findings. In physiologic solutions created to approximate a presurgical compared with a postsurgical gut environment (27), the SSRIs sertraline, fluoxetine, and paroxetine were significantly less soluble in the postsurgical than in the presurgical solution. No significant difference was observed between solutions for venlafaxine, which supports our observation of less dramatic reductions in the bioavailability for SN-RIs compared with SSRIs. The differences between SNRIs and SSRIs may be related more to solubility characteristics than to drug class, since solubility of the SSRI citalopram did not differ between conditions.
Treatment Considerations
Patients who are taking an SRI and undergoing Roux-en-Y gastric bypass are at substantial risk for reduced drug bioavailability. Control of psychiatric symptoms is critical for maintaining long-term weight loss, safety, and quality of life. An elevated risk for suicide has been reported in postbariatric surgery patients compared with non-surgical controls (43). Based on our clinical experience and research findings, we suggest the following management strategies:
Maintain collaboration between the surgical and psychiatric management teams for monitoring and rapid intervention if psychiatric symptoms recur.
Treat the patient to optimal response of the psychiatric illness to establish the most efficacious drug dosage before surgery.
Educate patients about the potential for worsening psychiatric symptoms after surgery and engage them in self-monitoring.
Use a standard measure of symptom severity to assess systematically the course in the first postoperative year, such as the publicly available Patient Health Questionnaire (44), the Center for Epidemiologic Studies Depression Scale (45), and the Generalized Anxiety Disorder Scale (46).
Because the reduction in SRI bioavailability occurs within the first month after surgery for the majority of patients, symptom evaluation should take place within this time frame. Repeated assessments at 3, 6, and 12 months are recommended.
All patients taking SRIs should have contact information for emergency psychiatric treatment near their area of residence (which may be distant from the surgical facility).
If recurrence of the psychiatric symptoms occurs, the goal is to provide psychosocial support during recovery and to increase the bioavailability of the drug. The latter can be achieved by using a formulation that is absorbed more readily, such as a liquid (as in the case of Ms. Z in the vignette); crushing pills; using immediate- rather than sustained-release preparations; increasing the dosage and/or dividing the dosage into multiple administrations throughout the day; or using transdermal or intravenous agents. Many SRI medications are formulated as liquids, but these may need to be specially ordered at most pharmacies.
Conclusions
Patients taking SRIs are at risk for substantial reduction in drug bioavailability after Roux-en-Y gastric bypass, particularly during the first month after surgery. These patients should be monitored closely for recurrent psychiatric symptoms. In our sample of 12 patients, recovery occurred in the majority by 6 months, but the 12-month course was variable across the sample. Strategies to ensure that therapeutic doses of oral medications are bio-available after surgery and alternative modes of drug delivery warrant further investigation.
In this study, we focused on recurrence of psychiatric symptoms secondary to reduced SRI bioavailability; however, the dramatic improvement in AUC values (and in the majority of cases, in depressive symptoms) at 12 months also warrants exploration. Although we did not observe increased side effects attributable to SRIs, their occurrence is a theoretical possibility. Population pharmacokinetic data and longitudinal modeling would be useful to improve data to support improvement of clinical care for the gastric bypass patient’s dynamic postoperative pharmacotherapy needs.
Acknowledgments
Supported by a research grant award from the American Society for Metabolic and Bariatric Surgery; grant MO1-RR000056 from the NIH National Center for Research Resources, General Clinical Research Centers; and Clinical and Translational Science Award grant 1 UL 1 RR024153-01. Dr. Wisner’s time was supported by NIMH grant R01 075921.
Footnotes
Presented at the 28th annual meeting of the American Society for Metabolic and Bariatric Surgery, Orlando, Fla., June 16, 2011.
Dr. McCloskey has served as a consultant to Allergan. Dr. Perel has served as an expert witness on atomoxetine and other non-psychostimulants in the treatment of ADHD for a consortium of pharmaceutical companies. Dr. Wisner has received advisory board payments from Eli Lilly and has received donations of active and placebo estradiol patches for an NIMH-funded study from Novartis. The other authors report no financial relationships with commercial interests.
References
- 1.Freedman DS. Obesity: United States, 1988–2008. MMWR Surveill Summ. 2011;60(suppl):73–77. [PubMed] [Google Scholar]
- 2.Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158:1139–1147. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- 3.Sarwer DB, Cohn NI, Gibbons LM, Magee L, Crerand CE, Raper SE, Rosato EF, Williams NN, Wadden TA. Psychiatric diagnoses and psychiatric treatment among bariatric surgery candidates. Obes Surg. 2004;14:1148–1156. doi: 10.1381/0960892042386922. [DOI] [PubMed] [Google Scholar]
- 4.Alciati A, D’Ambrosio A, Foschi D, Corsi F, Mellado C, Angst J. Bipolar spectrum disorders in severely obese patients seeking surgical treatment. J Affect Disord. 2007;101:131–138. doi: 10.1016/j.jad.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259–1272. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- 6.Allison DB, Newcomer JW, Dunn AL, Blumenthal JA, Fabricatore AN, Daumit GL, Cope MB, Riley WT, Vreeland B, Hibbeln JR, Alpert JE. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med. 2009;36:341–350. doi: 10.1016/j.amepre.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3:A42. [PMC free article] [PubMed] [Google Scholar]
- 8.Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298:1794–1796. doi: 10.1001/jama.298.15.1794. [DOI] [PubMed] [Google Scholar]
- 9.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide, 2008. Obes Surg. 2009;19:1605–1611. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 10.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 11.Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, MacLean LD. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240:416–423. doi: 10.1097/01.sla.0000137343.63376.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarwer DB, Wadden TA, Moore RH, Eisenberg MH, Raper SE, Williams NN. Changes in quality of life and body image after gastric bypass surgery. Surg Obes Relat Dis. 2010;6:608–614. doi: 10.1016/j.soard.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christou N, Efthimiou E. Five-year outcomes of laparoscopic adjustable gastric banding and laparoscopic Roux-en-Y gastric bypass in a comprehensive bariatric surgery program in Canada. Can J Surg. 2009;52:E249–E258. [PMC free article] [PubMed] [Google Scholar]
- 14.Behrns KE, Smith CD, Sarr MG. Prospective evaluation of gastric acid secretion and cobalamin absorption following gastric bypass for clinically severe obesity. Dig Dis Sci. 1994;39:315–320. doi: 10.1007/BF02090203. [DOI] [PubMed] [Google Scholar]
- 15.Malinowski SS. Nutritional and metabolic complications of bariatric surgery. Am J Med Sci. 2006;331:219–225. doi: 10.1097/00000441-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Pihlajamaki J, Gronlund S, Simonen M, Kakela P, Moilanen L, Paakkonen M, Pirinen E, Kolehmainen M, Karja V, Kainulainen S, Uusitupa M, Alhava E, Miettinen TA, Gylling H. Cholesterol absorption decreases after Roux-en-Y gastric bypass but not after gastric banding. Metabolism. 2010;59:866–872. doi: 10.1016/j.metabol.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Miller AD, Smith KM. Medication and nutrient administration considerations after bariatric surgery. Am J Health Syst Pharm. 2006;63:1852–1857. doi: 10.2146/ajhp060033. [DOI] [PubMed] [Google Scholar]
- 18.Gubbins PO, Bertch KE. Drug absorption in gastrointestinal disease and surgery: clinical pharmacokinetic and therapeutic implications. Clin Pharmacokinet. 1991;21:431–447. doi: 10.2165/00003088-199121060-00004. [DOI] [PubMed] [Google Scholar]
- 19.Roerig JL, Steffen K, Zimmerman C, Mitchell JE, Crosby RD, Cao L. Preliminary comparison of sertraline levels in postbariatric surgery patients versus matched nonsurgical cohort. Surg Obes Relat Dis. doi: 10.1016/j.soard.2010.12.003. (Epub ahead of print, Dec 15, 2010) [DOI] [PubMed] [Google Scholar]
- 20.Rogers CC, Alloway RR, Alexander JW, Cardi M, Trofe J, Vinks AA. Pharmacokinetics of mycophenolic acid, tacrolimus, and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: a pilot study. Clin Transplant. 2008;22:281–291. doi: 10.1111/j.1399-0012.2007.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skottheim IB, Stormark K, Christensen H, Jakobsen GS, Hjelmesaeth J, Jenssen T, Reubsaet JL, Sandbu R, Asberg A. Significantly altered systemic exposure to atorvastatin acid following gastric bypass surgery in morbidly obese patients. Clin Pharmacol Ther. 2009;86:311–318. doi: 10.1038/clpt.2009.82. [DOI] [PubMed] [Google Scholar]
- 22.Tondapu P, Provost D, Adams-Huet B, Sims T, Chang C, Sakhaee K. Comparison of the absorption of calcium carbonate and calcium citrate after Roux-en-Y gastric bypass. Obes Surg. 2009;19:1256–1261. doi: 10.1007/s11695-009-9850-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11:41–50. doi: 10.1111/j.1467-789X.2009.00614.x. [DOI] [PubMed] [Google Scholar]
- 24.Wills SM, Zekman R, Bestul D, Kuwajerwala N, Decker D. Tamoxifen malabsorption after Roux-en-Y gastric bypass surgery: case series and review of the literature. Pharmacotherapy. 2010;30:217. doi: 10.1592/phco.30.2.217. [DOI] [PubMed] [Google Scholar]
- 25.Magee SR, Shih G, Hume A. Malabsorption of oral antibiotics in pregnancy after gastric bypass surgery. J Am Board Fam Med. 2007;20:310–313. doi: 10.3122/jabfm.2007.03.060177. [DOI] [PubMed] [Google Scholar]
- 26.Padwal RS, Gabr RQ, Sharma AM, Langkaas LA, Birch DW, Karmali S, Brocks DR. Effect of gastric bypass surgery on the absorption and bioavailability of metformin. Diabetes Care. 2011;34:1295–1300. doi: 10.2337/dc10-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seaman JS, Bowers SP, Dixon P, Schindler L. Dissolution of common psychiatric medications in a Roux-en-Y gastric bypass model. Psychosomatics. 2005;46:250–253. doi: 10.1176/appi.psy.46.3.250. [DOI] [PubMed] [Google Scholar]
- 28.Brandacher G, Winkler C, Aigner F, Schwelberger H, Schroecksnadel K, Margreiter R, Fuchs D, Weiss HG. Bariatric surgery cannot prevent tryptophan depletion due to chronic immune activation in morbidly obese patients. Obes Surg. 2006;16:541–548. doi: 10.1381/096089206776945066. [DOI] [PubMed] [Google Scholar]
- 29.Bodnar LM, Wisner KL. Nutrition and depression: implications for improving mental health among childbearing-aged women. Biol Psychiatry. 2005;58:679–685. doi: 10.1016/j.biopsych.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brolin RE, Gorman JH, Gorman RC, Petschenik AJ, Bradley LJ, Kenler HA, Cody RP. Are vitamin B12 and folate deficiency clinically important after Roux-en-Y gastric bypass? J Gastrointest Surg. 1998;2:436–442. doi: 10.1016/s1091-255x(98)80034-6. [DOI] [PubMed] [Google Scholar]
- 31.Rangan AM, Blight GD, Binns CW. Iron status and non-specific symptoms of female students. J Am Coll Nutr. 1998;17:351–355. doi: 10.1080/07315724.1998.10718774. [DOI] [PubMed] [Google Scholar]
- 32.Papakostas GI, Petersen T, Mischoulon D, Ryan JL, Nierenberg AA, Bottiglieri T, Rosenbaum JF, Alpert JE, Fava M. Serum folate, vitamin B12, and homocysteine in major depressive disorder, part 1 predictors of clinical response in fluoxetine-resistant depression. J Clin Psychiatry. 2004;65:1090–1095. doi: 10.4088/jcp.v65n0810. [DOI] [PubMed] [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York: New York State Psychiatric Institute, Biometrics Research; 1996. [Google Scholar]
- 34.Williams JBW, Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale With Ayptical Depression Supplement (SIGH-ADS) New York: New York State Psychiatric Institute; 2003. [Google Scholar]
- 35.Wisner KL, Hanusa BH, Perel JM, Peindl KS, Piontek CM, Sit DK, Findling RL, Moses-Kolko EL. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26:353–360. doi: 10.1097/01.jcp.0000227706.56870.dd. [DOI] [PubMed] [Google Scholar]
- 36.Sit D, Perel JM, Luther JF, Wisniewski SR, Helsel JC, Wisner KL. Disposition of chiral and racemic fluoxetine and norfluoxetine across childbearing. J Clin Psychopharmacol. 2010;30:381–386. doi: 10.1097/JCP.0b013e3181e7be23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reis M, Aberg-Wistedt A, Agren H, Akerblad AC, Bengtsson F. Compliance with SSRI medication during 6 months of treatment for major depression: an evaluation by determination of repeated serum drug concentrations. J Affect Disord. 2004;82:443–446. doi: 10.1016/j.jad.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Kinzl JF, Schrattenecker M, Traweger C, Mattesich M, Fiala M, Biebl W. Psychosocial predictors of weight loss after bariatric surgery. Obes Surg. 2006;16:1609–1614. doi: 10.1381/096089206779319301. [DOI] [PubMed] [Google Scholar]
- 39.Legenbauer T, De Zwaan M, Benecke A, Muhlhans B, Petrak F, Herpertz S. Depression and anxiety: their predictive function for weight loss in obese individuals. Obes Facts. 2009;2:227–234. doi: 10.1159/000226278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rusch MD, Andris D. Maladaptive eating patterns after weight-loss surgery. Nutr Clin Pract. 2007;22:41–49. doi: 10.1177/011542650702200141. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Santos R, Del Barrio MJ, Gonzalez C, Madico C, Terrado I, Gordillo ML, Pujol J, Moreno P, Masdevall C. Long-term health-related quality of life following gastric bypass: influence of depression. Obes Surg. 2006;16:580–585. doi: 10.1381/096089206776945084. [DOI] [PubMed] [Google Scholar]
- 42.Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49:71–87. doi: 10.2165/11318100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 43.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 44.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. J Applied Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 46.Kroenke K, Spitzer RL, Williams JB, Monahan PO, Lowe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146:317–325. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]