Abstract
Background and Purpose
Because of its association with atrial fibrillation and heart failure, we hypothesized that amino terminal pro–B-type natriuretic peptide (NT-proBNP) would identify a subgroup of patients from the Warfarin–Aspirin Recurrent Stroke Study (WARSS), diagnosed with inferred non-cardioembolic ischemic strokes, where anticoagulation would be more effective than antiplatelet agents in reducing risk of subsequent events.
Methods
NT-proBNP was measured in stored serum collected at baseline from participants enrolled in WARSS, a previously reported randomized trial. Relative effectiveness of warfarin and aspirin in preventing recurrent ischemic stroke or death over two years was compared based on NT-proBNP concentrations.
Results
About 95% of 1028 patients with assays had NT-proBNP below 750 pg/mL, and among them, no evidence for treatment effect modification was evident. For 49 patients with NT-proBNP >750 pg/mL, the two-year rate of events per 100 person-years was 45.9 for the aspirin group and 16.6 for the warfarin group, while for 979 patients with NT-proBNP ≤750 pg/mL, rates were similar for both treatments. For those with NT-proBNP >750 pg/mL, the hazard ratio was 0.30 (95% confidence interval 0.12 to 0.84, p-value=0.021) significantly favoring warfarin over aspirin. A formal test for interaction of NT-proBNP with treatment was significant (p-value=0.01).
Conclusion
For secondary stroke prevention, elevated NT-proBNP concentrations may identify a subgroup of ischemic stroke patients without known atrial fibrillation, about 5% based on the current study, who may benefit more from anticoagulants than antiplatelet agents.
Clinical Trial Registration
This trial was not registered because enrollment began prior to 2005.
Keywords: aspirin, warfarin, NT-proBNP, secondary stroke prevention
Introduction
For secondary prevention after ischemic stroke, only a few subgroups of patients have been identified, most notably those with atrial fibrillation, among whom anticoagulants are more effective than antiplatelet agents at reducing subsequent ischemic events. Increased levels of the hormone brain (or B-type) natriuretic peptide (BNP), or its inert co-metabolite amino terminal pro–B-type natriuretic peptide (NT-proBNP),1 are associated with prevalent and incident atrial fibrillation and heart failure.2,3 Patients with elevated BNP or NT-proBNP levels around the time of their incident stroke are more likely to have a cardio-embolic mechanism identified,4–12 to have a worse outcome from their stroke,12–16 and to have an increased risk of future events such as recurrent ischemic stroke17 and death.12–14,18–22 We hypothesized that a cutoff of BNP or NT-proBNP levels would exist above which anticoagulation would be superior to antiplatelet agents–namely that the relative efficacy of these treatments would be modified by BNP or NT-proBNP levels. Seeking to test this hypothesis, we analyzed NT-proBNP in serum collected at baseline as part of the Antiphospholipid Antibodies and Stroke Study (APASS),23 a prospective cohort study within the Warfarin–Aspirin Recurrent Stroke Study (WARSS).24 In this double-blind trial, the relative effectiveness of warfarin and aspirin in preventing recurrent ischemic stroke or death in patients with a prior inferred non-cardioembolic ischemic stroke was compared.
Methods
The Warfarin–Aspirin Recurrent Stroke Study (WARSS) was a randomized double-blind trial (N=2206) conducted at multiple US clinical sites from June 1993 through June 2000. It compared adjusted dose warfarin (target international normalized ratio, 1.4–2.8) to aspirin (325 mg/d) for prevention of the primary outcome, first to occur of recurrent ischemic stroke or death from any cause, within two years.24 In WARSS, eligible patients were 30 to 85 years old, were considered acceptable candidates for warfarin therapy, had had an ischemic stroke within the previous 30 days, and had scores of 3 or more on the Glasgow Outcome Scale. On this scale a score of 3 indicates severe disability; a score of 4, moderate disability; and a score of 5, minimal or no disability. Patients were ineligible if they had: a base-line INR above the normal range of 1.4; a stroke that was due to a procedure or that was attributed to high-grade carotid stenosis for which surgery was planned; or a stroke associated with an inferred cardio-embolic source. Most of the last group had atrial fibrillation at the time of stroke.
In APASS, bloods were obtained from consenting WARSS participants at baseline before randomization and treatment.23 We learned from APASS investigators that blood specimens remained from approximately 1000 participants. Serum was assayed for NT-proBNP at the University of Maryland using an approach identical to that described previously.2 Briefly, NT-proBNP was measured on the Elecsys 2010 system (Roche Diagnostics, Indianapolis, Indiana). The coefficient of variation for the NT-proBNP assay was 2% to 5% during the testing period, and its analytical measurement range was 5 to 35,000 pg/mL where 1 pg/mL equals 0.118 pmol/L.1 All samples were stored at −70°C to −80°C and were thawed before testing (maximum of 3 freeze-thaw cycles). This assay has been shown to be reliable on stored specimens, and measurements of NT-proBNP using this assay do not change after 5 freeze-thaw cycles.25 No information about the patient from whom the blood came was available to those performing the assays. On those patients with NT-proBNP levels assayed, the baseline clinical and follow-up data, including treatment assignment, were obtained from the WARSS coordinating center at Columbia University. All of the participants in the original studies provided written informed consent, including for their blood to be studied for future markers of increased risk of stroke, and none of the data mentioned above included personal identifiers.
Analyses compared the relative effectiveness of warfarin and aspirin in preventing the primary outcome of first to occur of recurrent ischemic stroke or death within two years of randomization. Among the participants with NT-proBNP assayed, characteristics were compared between the two treatment groups, using the chi-squared test for categorical variables and the t-test for continuous variables and excluding those with unknown values. In order to show that the patients with NT-proBNP assayed were representative of all patients in WARSS, these same characteristics were similarly compared between those with and without NT-proBNP assayed. A p-value less that 0.05 was considered significant.
Because the distribution of NT-proBNP was highly skewed to the right, we began by stratifying NT-proBNP at 750 pg/mL, representing about the 95th percentile. We chose this arbitrary value because the literature does not suggested a specific cutoff for such analyses. We made this decision without any analyses that included outcome data. To evaluate treatment effect modification, we used a Cox model including age, sex, the treatment variable, the strata variable, and interaction between treatment and strata. Because the interaction test was statistically significant (p=0.01) for the 750 pg/mL cutoff, the treatments were compared separately for each stratum using the Cox proportional hazard model controlling for age, sex, and the natural logarithm of NT-proBNP as a continuous variable. The latter variable was included in these models because it was a predictor of outcome within each stratum and could increase the power of the test for treatment effect. For descriptive purposes, Kaplan-Meier cumulative event rate curves were displayed for each stratum comparing the two treatments. Also, for descriptive purposes, curves were generated with a cubic spline smoother for each treatment group relating NT-proBNP levels to the probability of the primary outcome. Analyses were performed with Stata 11 (StataCorp LP, College Station, Texas).
Results
Overall, the mean level for NT-proBNP was 220 pg/mL (standard deviation = 663), and the median was 75 pg/mL (interquartile range = 32 to 185). The median time between stroke onset and blood draw was 13 days (interquartile range = 8 to 23). The two treatment groups were similar on the characteristics listed in Table 1, except for the warfarin group being significantly older than the aspirin group. In addition, patients enrolled in WARSS with and without NT-proBNP assayed were similar, without any significant differences in the characteristics listed in Table 1.
Table 1.
Baseline characteristics of the patients with NT-proBNP levels according to treatment group in the Warfarin–Aspirin Recurrent Stroke Study (WARSS) and comparison of WARSS patients with and without NT-proBNP assayed.
| Characteristics* | Patients with NT-proBNP
|
Patients without NT-proBNP (N=1177) | ||
|---|---|---|---|---|
| Aspirin (N=505) | Warfarin (N=524) | Total (N=1029) | ||
| Randomized Treatment Group no. (%) | ||||
| Aspirin | 505 (100) | 0 | 505 (50.9) | 598 (50.8) |
| Warfarin | 0 | 524 (100) | 524 (49.1) | 579 (49.2) |
| Age – years (standard deviation) | 61.9 (11.5) | 63.5 (10.9) | 62.7 (11.2) | 62.2 (11.4) |
| Female sex – no. (%) | 206 (40.8) | 230 (43.9) | 436 (42.4) | 461 (39.2) |
| Race or ethnic group – no. (%) | ||||
| White | 284 (56.2) | 287 (54.8) | 571 (55.5) | 682 (57.9) |
| Black | 155 (30.7) | 159 (30.3) | 314 (30.5) | 349 (29.7) |
| Hispanic | 55 (10.9) | 56 (10.7) | 111 (10.8) | 112 (9.5) |
| Other | 11 (2.2) | 22 (4.2) | 33 (3.2) | 34 (2.9) |
| Education – no. (%) | ||||
| High school or less | 373 (73.9) | 386 (73.6) | 759 (73.8) | 842 (71.5) |
| After high school | 126 (25.0) | 133 (25.4) | 259 (25.2) | 323 (27.4) |
| Unknown | 6 (1.1) | 5 (1.0) | 11 (1.1) | 12 (1.0) |
| Hypertension – no. (%) | ||||
| Yes | 347 (68.7) | 357 (68.1) | 704 (68.4) | 795 (67.5) |
| No | 154 (30.5) | 160 (30.5) | 314 (30.5) | 367 (31.2) |
| Unknown | 4 (0.8) | 7 (1.3) | 11 (1.1) | 12 (1.0) |
| Diabetes – no. (%) | ||||
| Yes | 177 (35.0) | 171 (32.6) | 348 (33.8) | 358 (30.4) |
| No | 328 (65.0) | 351 (67.0) | 679 (66.0) | 817 (69.4) |
| Unknown | 0 (0.0) | 2 (0.4) | 2 (0.2) | 2 (0.2) |
| Any cardiac disease – no. (%) | ||||
| Yes | 113 (22.4) | 117 (22.3) | 230 (22.4) | 274 (23.3) |
| No | 392 (77.6) | 407 (77.7) | 799 (77.7) | 903 (76.7) |
| Unknown | 0 | 0 | 0 | 0 |
| History of transient ischemic attack, amaurosis fugax, or stroke – no. (%) | ||||
| Yes | 151 (29.9) | 146 (27.9) | 297 (28.9) | 332 (28.2) |
| No or unknown | 354 (70.1) | 378 (72.1) | 732 (71.1) | 845 (71.8) |
| Current smoking – no. (%) | ||||
| Yes | 154 (30.5) | 139 (26.5) | 293 (28.5) | 350 (29.7) |
| No | 349 (69.1) | 382 (72.9) | 731 (71.0) | 822 (69.8) |
| Unknown | 2 (0.4) | 3 (0.6) | 5 (0.5) | 5 (0.4) |
| Heavy alcohol intake (≥4 drinks/day) | ||||
| Yes | 12 (2.4) | 17 (3.2) | 29 (2.8) | 45 (3.8) |
| No | 488 (96.6) | 505 (96.4) | 993 (96.5) | 1127 (95.8) |
| Unknown | 5 (1.0) | 2 (0.4) | 7 (0.7) | 5 (0.4) |
| Presumed cause of qualifying stroke | ||||
| Cryptogenic | 127 (25.2) | 133 (25.4) | 260 (25.3) | 316 (26.9) |
| Small-vessel or lacunar | 294 (58.2) | 280 (53.4) | 574 (55.8) | 663 (56.3) |
| Large-artery, severe stenosis, or occlusion | 53 (10.5) | 73 (13.9) | 126 (12.2) | 133 (11.3) |
| Other | 31 (6.1) | 38 (7.3) | 69 (6.7) | 65 (5.5) |
For patients with NT-proBNP assayed, none of the characteristics differed significantly by treatment groups, except for age (p-value < 0.05). Considering all patients in WARSS, none of the characteristics differed significantly between those with and without NT-proBNP assayed. In one patient, the quantity of the sample was insufficient to allow the assay to be performed successfully; therefore, subsequent analyses were based on 1028 patients.
For NT-proBNP less than or equal to 750 pg/mL, the two-year rate of events per 100 person-years was 6.8 for the aspirin group and 8.5 for the warfarin group, while for NT-proBNP greater than 750 pg/mL, the rate was 45.9 for the aspirin group and 16.6 for the warfarin group (Table 2). In adjusted Cox proportional hazard models, for those with NT-proBNP less than or equal to 750 pg/mL, the hazard ratio was 1.21 (95% confidence interval 0.87 to 1.69, p-value = 0.243), favoring aspirin over warfarin but not significantly. For those with NT-proBNP greater than 750 pg/mL, the hazard ratio was 0.30 (95% confidence interval 0.12 to 0.84, p-value = 0.021), significantly favoring warfarin over aspirin (Table 2). Statistically controlling individually for the time between the stroke onset and blood draw and the other factors listed in Table 1 did not alter these conclusions.
Table 2.
Comparison of aspirin and warfarin in preventing the primary outcome of recurrent ischemic stroke and death over two years among patients whose NT-proBNP was less than or equal to 750 pg/mL or greater than 750 pg/mL.
| Treatment by NT-proBNP | Number at Risk (Number of Events†) | Rate per 100 person-years | Hazard Ratio* | 95% Confidence Interval | p-value |
|---|---|---|---|---|---|
| ≤750 pg/mL | |||||
| Aspirin | 477 (49, 13) | 6.8 | 1.0 | ||
| Warfarin | 502 (63, 17) | 8.5 | 1.21 | 0.87, 1.69 | 0.24 |
| >750 pg/mL | |||||
| Aspirin | 28 (7, 9) | 45.9 | 1.0 | ||
| Warfarin | 21 (4, 2) | 16.6 | 0.30 | 0.12, 0.84 | 0.02 |
Adjusted for age, sex, and natural logarithm of NT-proBNP level as a continuous variable.
Stroke, death.
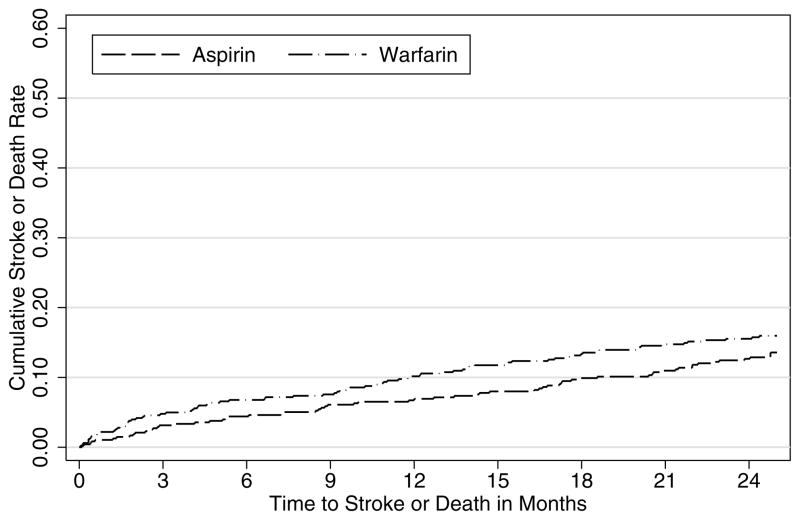
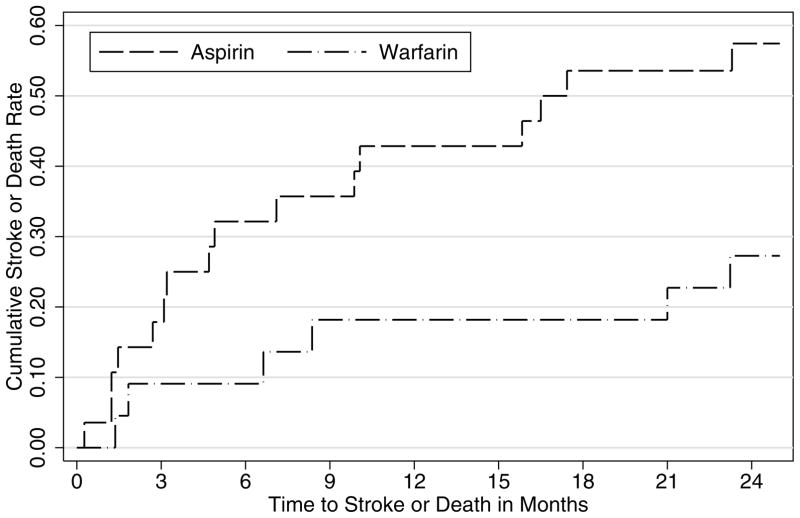
For descriptive purposes, the Kaplan-Meier cumulative event rate curves for the two treatment groups are shown in Figure 1 based on the cutoff of 750 pg/mL for NT-proBNP. Although warfarin and aspirin were comparable in preventing the primary outcome of recurrent ischemic stroke or death among those with NT-proBNP less than or equal to 750 pg/mL (Figure 1A), warfarin was more effective than aspirin among those with NT-proBNP greater than 750 pg/mL (Figure 1B).
Figure 1.
Cumulative event rates of recurrent ischemic stroke or death from any cause for those treated by aspirin or warfarin in the Warfarin–Aspirin Recurrent Stroke Study (WARSS) by NT-proBNP levels: A for less than or equal to 750 pg/mL (N=979) and B for greater than 750 pg/mL (N=49).
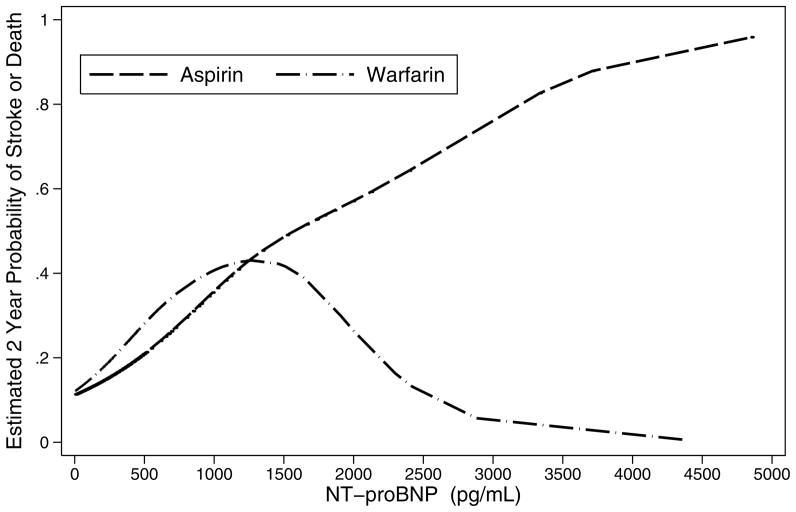
Finally, smoothed curves were generated for both treatments to estimate the probability of recurrent ischemic stroke or death over two years as a function of NT-proBNP levels. Figure 2 shows that, at lower levels of NT-proBNP, the probability of the primary outcome increased with increasing levels of NT-proBNP for those on either aspirin or warfarin. At higher levels of NT-proBNP, the probability in those on warfarin declined markedly, while the probability in those on aspirin continued to rise. The small number of patients with high levels of NT-proBNP prevented precise identification of the best cutoff and also imposes a need for caution in interpreting the substantial differences between treatments at the higher levels of NT-proBNP.
Figure 2.
Estimated probability of recurrent ischemic stroke or death from any cause over two years separately for aspirin and warfarin users as a function of NT-proBNP levels using a cubic spline smoother. For readability the graph is truncated at NT-proBNP levels of less than 5000 pg/mL. Three patients had values greater than 5000 pg/mL: one warfarin patient and two aspirin patients. Both aspirin patients had events, while the one warfarin patient did not.
Discussion
We hypothesized that NT-proBNP concentrations could be used to define a subgroup of inferred non-cardioembolic ischemic stroke patients who would benefit more from anticoagulants than from antiplatelet agents in preventing recurrent ischemic stroke or death over two years following their stroke. These data from the WARSS and its collaborative study, APASS, suggest that such a subgroup may exist with NT-proBNP levels above 750 pg/mL, representing about 5% of the patients in whom assays were performed. Patients in this subgroup had a significantly reduced risk of recurrent ischemic stroke or death when treated with warfarin compared to aspirin, with a point estimate of the relative risk reduction of 70%.
The benefit of warfarin over aspirin in these patients may arise because the elevated NT-proBNP is a marker for a subgroup of patients with heart failure and sinus rhythm who may benefit more than others from anticoagulation. Additional analyses from a recent large trial26 may help to identify such a subgroup. In the meantime, BNP and NT-proBNP levels are being evaluated to optimize therapy in outpatients with chronic heart failure.27 Elevated NT-proBNP is also a marker for patients at high risk of atrial fibrillation. In the Cardiovascular Health Study, a large cohort study of elderly people followed for cardiovascular outcomes, the risk of developing incident atrial fibrillation was increased in those with elevated NT-proBNP but not during the initial two years.3 Follow-up in WARSS was a maximum of two years. Had it been longer, perhaps a level of NT-proBNP lower than 750 pg/mL would have been evident where warfarin would have been more effective than aspirin in preventing recurrent ischemic stroke or death. Finally, prolonged cardiac rhythm monitoring was not performed in WARSS but may have identified episodic atrial fibrillation, as has been shown by others,28 especially in those with elevated NT-proBNP. Unfortunately, which patient in WARSS may have developed atrial fibrillation during the two-year follow up is unknown.
Our analyses were driven by our pre-specified hypothesis that warfarin would be superior to aspirin in those with elevated NT-proBNP. The results are subject to the criticism that other cutoffs might have been both more or less statistically significant, but we stuck with the original choice so as not to bias our results. The scarcity of events in the upper tail of the distribution of NT-proBNP precludes: defining the optimal cutoff; examining the outcomes of stroke and death separately; and evaluating the effect of ischemic stroke subtypes. Furthermore, these results come from analyses in a subset of patients from the WARSS trial who may differ in some unknown way from others entered into that trial. Also those who were entered into the WARSS trial may not be representative of all such ischemic stroke patients. Many patients in whom a cardioembolic source was suspected but not confirmed may not have been entered into the trial because of a clinician’s conviction that anticoagulation was indicated, especially over the years that the trial was conducted between 1993 and 2000. Finally the findings may be confounded by some other factor or factors that were not balanced between the two treatment groups despite randomization.
Although the results of these secondary analyses raise a strong possibility that warfarin may be superior to aspirin in those with high NT-proBNP and be equally or less efficacious in those with lower values, these findings require replication before recommendations can be made to measure BNP or NT-proBNP after ischemic stroke and to base treatment decisions on the results. A randomized trial to confirm these findings would face a major challenge in identifying eligible patients. Although BNP and NT-proBNP levels are known to fall after an acute ischemic stroke,14,15,19,29–31 the optimal timing for levels to be assayed in such a trial is unknown, as is the value of repeated measures. In WARSS and APASS, bloods were collected at baseline, and patients needed to be randomized within 30 days of the ischemic stroke onset. In these analyses, the time from stroke onset to blood draw did not affect these results.
Levels of NT-proBNP may have implications for primary as well as secondary stroke prevention. Bloods were assayed for NT-proBNP in the recently reported trial of warfarin versus dabigatran for primary stroke prevention in patients with atrial fibrillation.32 Investigators found that, even among those participants all of whom had atrial fibrillation, NT-proBNP was associated with an increase risk of stroke, especially among those in the highest quartile defined as NT-proBNP greater than 1402 pg/mL. The corollary to our question of secondary stroke prevention is whether low NT-proBNP would identify a subgroup of patients with atrial fibrillation but without stroke where antiplatelet agents would not be inferior to anticoagulants.
For secondary stroke prevention, NT-proBNP measurements may identify a subgroup of ischemic stroke patients without known atrial fibrillation, about 5% of those with assays in the current study, who may benefit more from anticoagulants than from antiplatelet agents. Replication of these findings supporting such a role for NT-proBNP is needed. The utility and timing of serial measurements of NT-proBNP in influencing treatment decisions aimed at primary or secondary stroke prevention is unknown and also worthy of investigation.
Acknowledgments
Sources of Funding
Roche Diagnostics provided funding and laboratory reagents for the NT-proBNP assay. The WARSS was supported by a grant from the National Institute of Neurological Disorders and Stroke (NS 28371). In the original trial, Dupont Pharmaceuticals and Bayer supplied medications and placebos. The APASS was supported by the National Institute of Neurological Disorders and Stroke (NS052417, NS43992, NS28371, and NS30896) and the National Heart, Lung, and Blood Institute (HL096944).
Footnotes
Disclosures
RHC receives research funding from Siemens Medical Diagnostics and Response Biomedical. SLS receives grant support from Roche Diagnostics. CRdF receives honorarium, consulting, and grant support from Roche Diagnostics and Siemens. The remaining authors declare that they have no conflicts of interest relevant to this report.
References
- 1.Weber M, Hamm C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart. 2006;92:843–849. doi: 10.1136/hrt.2005.071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ, Seliger SL. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol. 2010;55:441–450. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, et al. N-terminal pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation. 2009;120:1768–1774. doi: 10.1161/CIRCULATIONAHA.109.873265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Angelantonio E, De Castro S, Toni D, Sacchetti ML, Biraschi F, Prencipe M, et al. Determinants of plasma levels of brain natriuretic peptide after acute ischemic stroke or TIA. J Neurol Sci. 2007;260:139–142. doi: 10.1016/j.jns.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Montaner J, Perea-Gainza M, Delgado P, Ribo M, Chacon P, Rosell A, et al. Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke. 2008;39:2280–2287. doi: 10.1161/STROKEAHA.107.505354. [DOI] [PubMed] [Google Scholar]
- 6.Naya T, Yukiiri K, Hosomi N, Takahashi T, Ohkita H, Mukai M, et al. Brain natriuretic peptide as a surrogate marker for cardioembolic stroke with paroxysmal atrial fibrillation. Cerebrovasc Dis. 2008;26:434–440. doi: 10.1159/000155640. [DOI] [PubMed] [Google Scholar]
- 7.Yukiiri K, Hosomi N, Naya T, Takahashi T, Ohkita H, Mukai M, et al. Plasma brain natriuretic peptide as a surrogate marker for cardioembolic stroke. BMC Neurol. 2008;8:45. doi: 10.1186/1471-2377-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibazaki K, Kimura K, Iguchi Y, Okada Y, Inoue T. Plasma brain natriuretic peptide can be a biological marker to distinguish cardioembolic stroke from other stroke types in acute ischemic stroke. Intern Med. 2009;48:259–264. doi: 10.2169/internalmedicine.48.1475. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Yanez M, Sobrino T, Blanco M, de la Ossa NP, Brea D, Rodriguez-Gonzalez R, et al. High serum levels of pro-brain natriuretic peptide (pro BNP) identify cardioembolic origin in undetermined stroke. Dis Markers. 2009;26:189–195. doi: 10.3233/DMA-2009-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonseca AC, Matias JS, Pinho e Melo T, Falcao F, Canhao P, Ferro JM. N-terminal probrain natriuretic peptide as a biomarker of cardioembolic stroke. Int J Stroke. 2011;6:398–403. doi: 10.1111/j.1747-4949.2011.00606.x. [DOI] [PubMed] [Google Scholar]
- 11.Shibazaki K, Kimura K, Fujii S, Sakai K, Iguchi Y. Brain natriuretic peptide levels as a predictor for new atrial fibrillation during hospitalization in patients with acute ischemic stroke. Am J Cardiol. 2012;109:1303–1307. doi: 10.1016/j.amjcard.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Rost NS, Biffi A, Cloonan L, Chorba J, Kelly P, Greer D, et al. Brain natriuretic peptide predicts functional outcome in ischemic stroke. Stroke. 2012;43:441–445. doi: 10.1161/STROKEAHA.111.629212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma JC, Ananda K, Ross I, Hill R, Vassallo M. N-terminal proBrain natriuretic peptide levels predict short-term poststroke survival. J Stroke Cerebrovasc Dis. 2006;15:121–127. doi: 10.1016/j.jstrokecerebrovasdis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Yip HK, Sun CK, Chang LT, Chen MC, Liou CW. Time course and prognostic value of plasma levels of N-terminal pro-brain natriuretic peptide in patients after ischemic stroke. Circ J. 2006;70:447–452. doi: 10.1253/circj.70.447. [DOI] [PubMed] [Google Scholar]
- 15.Tomita H, Metoki N, Saitoh G, Ashitate T, Echizen T, Katoh C, et al. Elevated plasma brain natriuretic peptide levels independent of heart disease in acute ischemic stroke: correlation with stroke severity. Hypertens Res. 2008;31:1695–1702. doi: 10.1291/hypres.31.1695. [DOI] [PubMed] [Google Scholar]
- 16.Whiteley W, Wardlaw J, Dennis M, Lowe G, Rumley A, Sattar N, et al. The use of blood biomarkers to predict poor outcome after acute transient ischemic attack or ischemic stroke. Stroke. 2012;43:86–91. doi: 10.1161/STROKEAHA.111.634089. [DOI] [PubMed] [Google Scholar]
- 17.Campbell DJ, Woodward M, Chalmers JP, Colman SA, Jenkins AJ, Kemp BE, et al. Soluble vascular cell adhesion molecule 1 and N-terminal pro-B-type natriuretic peptide in predicting ischemic stroke in patients with cerebrovascular disease. Arch Neurol. 2006;63:60–65. doi: 10.1001/archneur.63.1.noc50221. [DOI] [PubMed] [Google Scholar]
- 18.Makikallio AM, Makikallio TH, Korpelainen JT, Vuolteenaho O, Tapanainen JM, Ylitalo K, et al. Natriuretic peptides and mortality after stroke. Stroke. 2005;36:1016–1020. doi: 10.1161/01.STR.0000162751.54349.ae. [DOI] [PubMed] [Google Scholar]
- 19.Jensen JK, Mickley H, Bak S, Korsholm L, Kristensen SR. Serial measurements of N-terminal pro-brain natriuretic peptide after acute ischemic stroke. Cerebrovasc Dis. 2006;22:439–444. doi: 10.1159/000094997. [DOI] [PubMed] [Google Scholar]
- 20.Shibazaki K, Kimura K, Okada Y, Iguchi Y, Uemura J, Terasawa Y, et al. Plasma brain natriuretic peptide as an independent predictor of in-hospital mortality after acute ischemic stroke. Intern Med. 2009;48:1601–1606. doi: 10.2169/internalmedicine.48.2166. [DOI] [PubMed] [Google Scholar]
- 21.Jensen JK, Atar D, Kristensen SR, Mickley H, Januzzi JL., Jr Usefulness of natriuretic peptide testing for long-term risk assessment following acute ischemic stroke. Am J Cardiol. 2009;104:287–291. doi: 10.1016/j.amjcard.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 22.Shibazaki K, Kimura K, Iguchi Y, Aoki J, Sakai K, Kobayashi K. Plasma brain natriuretic peptide predicts death during hospitalization in acute ischaemic stroke and transient ischaemic attack patients with atrial fibrillation. Eur J Neurol. 2011;18:165–169. doi: 10.1111/j.1468-1331.2010.03101.x. [DOI] [PubMed] [Google Scholar]
- 23.Levine SR, Brey RL, Tilley BC, Thompson JL, Sacco RL, Sciacca RR, et al. Antiphospholipid antibodies and subsequent thrombo-occlusive events in patients with ischemic stroke. JAMA. 2004;291:576–584. doi: 10.1001/jama.291.5.576. [DOI] [PubMed] [Google Scholar]
- 24.Mohr JP, Thompson JLP, Lazar RM, Levin B, Sacco RL, Furie KL, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345:1444–1451. doi: 10.1056/NEJMoa011258. [DOI] [PubMed] [Google Scholar]
- 25.Ordonez-Llanos J, Collinson PO, Christenson RH. Amino-terminal pro-B-type natriuretic peptide: analytic considerations. Am J Cardiol. 2008;101:9–15. doi: 10.1016/j.amjcard.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Homma S, Thompson JLP, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–1869. doi: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Januzzi JL., Jr The role of natriuretic peptide testing in guiding chronic heart failure management: review of available data and recommendations for use. Arch Cardiovasc Dis. 2012;105:40–50. doi: 10.1016/j.acvd.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Seet RC, Friedman PA, Rabinstein AA. Prolonged rhythm monitoring for the detection of occult paroxysmal atrial fibrillation in ischemic stroke of unknown cause. Circulation. 2011;124:477–486. doi: 10.1161/CIRCULATIONAHA.111.029801. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa K, Yamaguchi T, Seida M, Yamada S, Imae S, Tanaka Y, et al. Plasma concentrations of brain natriuretic peptide in patients with acute ischemic stroke. Cerebrovasc Dis. 2005;19:157–164. doi: 10.1159/000083249. [DOI] [PubMed] [Google Scholar]
- 30.Giannakoulas G, Hatzitolios A, Karvounis H, Koliakos G, Charitandi A, Dimitroulas T, et al. N-terminal pro-brain natriuretic peptide levels are elevated in patients with acute ischemic stroke. Angiology. 2005;56:723–730. doi: 10.1177/000331970505600610. [DOI] [PubMed] [Google Scholar]
- 31.Shibazaki K, Kimura K, Okada Y, Iguchi Y, Terasawa Y, Aoki J. Heart failure may be associated with the onset of ischemic stroke with atrial fibrillation: a brain natriuretic peptide study. J Neurol Sci. 2009;281:55–57. doi: 10.1016/j.jns.2009.02.374. [DOI] [PubMed] [Google Scholar]
- 32.Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH, et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation. 2012;125:1605–1616. doi: 10.1161/CIRCULATIONAHA.111.038729. [DOI] [PubMed] [Google Scholar]