Abstract
Adolescence is an ontogenetic period characterized by numerous hormonal, neural, and behavioral changes. In animal models, adolescents exhibit greater levels of novelty-seeking behavior and risk-taking relative to adults, behaviors associated in humans with increases in impulsivity and elevated propensities to engage in drug and alcohol seeking behaviors. The current series of experiments sought to explore possible age-related differences in impulsivity when indexed using delay discounting in adolescent (postnatal day [P] 25-27) and adult (P68-71) female (Experiment 1) and male (Experiment 2) Sprague-Dawley rats. In both experiments, adolescents exhibited significantly greater levels of impulsive-like behavior in this test relative to adults—even when data were adjusted to account for baseline differences in activity levels (i.e. general nose-poking behavior) across age. Taken together, these results extend to both sexes previous findings of adolescent-associated elevations in impulsivity observed among male mice using delay discounting, as well as among male rats using other procedures to index impulsivity. That these age differences were observed among both male and female rats suggests that impulsivity may be a pervasive feature of adolescence, and contributes to the expression of risky behaviors during this ontogenetic period.
Keywords: Adolescence, Rat, Impulsivity, Delay Discounting, Sex differences
Adolescents are often risk-takers, with over 50% of adolescents in the United States engaging in risky behaviors that include drunk driving, unprotected sex, drug use, and minor criminal activities (e.g., Arnett, 1992). For most, risk-taking is largely adolescent-limited, and declines in frequency as individuals reach adulthood. Though multiple factors likely influence the expression of risk-taking behaviors during adolescence, impulsive behavior may be one major contributor, with high levels of impulsivity in humans reported to be associated with greater engagement in risk-taking behaviors during adolescence (Pfefferbaum & Wood, 1994). Such associations are not always evident, however (Galvan et al., 2006), perhaps in part because the construct of impulsivity encompasses a number of seemingly independent factors—e.g., impulsive choice, impulsive action, inattention (de Wit, 2009)—each assessed differently and with its own neurobiology and ontogeny (e.g., see Evenden, 1999; Steinberg et al., 2009; Winstanley, Dalley, Theobald, & Robbins, 2004; Winstanley, Theobald, Dalley, Glennon, & Robbins, 2004).
Delay discounting is one of a number of paradigms through which impulsive-like behavior has been examined experimentally, with greater impulsivity in this test indexed as a more rapid shift in preference for a smaller immediate reward as delays are increased to obtain a larger reward. Using this type of procedure, several groups have examined changes in delay discounting as a function of age in humans. Indeed, it has generally been shown that human adolescents behave more impulsively (i.e., display more delay discounting) than their adult counterparts. (e.g., Green, Fry, & Myerson, 1994; Olson, Cooper, Collins, and Luciana, 2007; Prencipe et al., 2011; Steinberg et al., 2009)
To the extent that developmental differences in the proclivity for making impulsive choices are biologically driven, adolescent-typical increases in impulsivity using a delay discounting task should also be apparent using simple models of adolescence in laboratory animals. Such studies can be challenging, however, given that operant delay discounting procedures designed for adult rodents typically subsume time intervals substantially longer than the approximate 2 week period during which adolescent-typical neural, physiological, and behavioral changes are seen in rats and mice (for review see Spear, 2000). Pioneering work conducted by Adriani and Laviola showed considerable success in adapting the operant delay discounting task to the adolescent time window of 30–45 days postnatally [P30-45] using both mice (e.g. Adriani & Laviola, 2003) and rats (e.g. Adriani et al., 2004; Adriani & Laviola 2006; Adriani, Zoratto, Romano, & Laviola, 2010). More specifically, in a direct comparison of adolescent and adult male mice (Adriani & Laviola, 2003), adolescents were reported to show greater impulsive responding relative to adults, as indexed by more rapid declines in responding for a larger reward with increasing reward delays. More recently, Pinkston & Lamb (2011), again found that male C57BL/6J adolescent mice exhibited more impulsive behavior than adults in a delay discounting procedure, although this age difference was not apparent among male DBA/2J mice. Age differences have not, however, been explored in male rats nor in either female rats or mice. Consequently, the goal of these studies was to determine whether age differences in impulsivity sometimes observed between adolescent and adult male mice would also be evident in rats using a modified delay discounting procedure, and whether such age differences would also be apparent among female subjects.
General Methods
Subjects
Adolescent (P25-27) and adult (P68-71) male and female Sprague-Dawley rats used in these experiments were derived from our breeding colony and reared in our vivarium (14:10 hr light:dark cycle; lights on at 0700). Litters were culled to 8–10 pups on P1, weaned at P21 into same-sex littermate pairs and given ad libitum access to food (Purina Rat Chow, Lowell, MA) and water unless otherwise noted. Animals used in these experiments were maintained and treated in accordance with the National Institutes of Health Guide for Animal Care (NIH Publication No.: 80–23, revised 1996), using protocols approved by the Binghamton University Institutional Animal Care and Use Committee (IACUC).
Food Restriction
Each adolescent and adult housing pair received a set amount (20 g) of food prior to the first training day. Thereafter, animals were weighed daily and the amount of food provided following each operant session was adjusted slightly upward or downward from the previous day’s allocation in order to allow maintenance of approximately 85% of free-feeding weight. Free-feeding weights were determined from age/weight charts previously developed in our laboratory (Vetter & Spear, 2007). By the last day of delay testing, female adolescent housing pairs received approximately 25 g (± 2g) of food and adult females about 20 g (± 2 g) of food per pair. In contrast, males from Exp. 2 were given more food per day, with both adolescent and adult male housing pairs receiving approximately 30 g (± 2 g) of food prior to the final day of the delay phase.
Apparatus
Standard rat-sized operant chambers (30.5 × 24.1 × 21 cm) housed inside sound-attenuating boxes (55.9 × 38 × 35.6 cm) (Med Associates, Inc., St. Albans, VT) were used. A nose-poking device (2.54 cm diameter) was located on both the left and right panels of one wall. A trough-style pellet receptacle (5.1 × 5.1 cm) and stimulus light (directly above the trough; 2.5 cm, 18 lux) were located between the nose-poking devices. The opposite wall of each chamber contained a house light (3 lux).
Delay Discounting Procedure
To avoid the stress of isolate housing (Hall, 1998), and to permit testing of both animals in each pair without assigning more than one animal per litter to an experimental group (Holson & Pearce, 1992), animals were re-housed with a same age and sex partner from a different litter on the day prior to training. At this time, and throughout the remainder of the experiment, animals were weighed daily and food-restricted, with daily food rations distributed approximately 30 to 60 minutes after each operant session. The procedures for this modified delay discounting paradigm (see below) were based on those used by Adriani, Laviola and colleagues (e.g. Adriani et al., 2004; Adriani & Laviola, 2003; 2006; Adriani, Zoratto, Romano, & Laviola, 2010).
Training
Sessions were conducted between 1300 and 1600 hr. Following an initial night of food restriction, training began the next day (P25-26 for adolescents; P70-72 for adults). During training (5 days), a nose poke into the short-delay hole (SDH) resulted in immediate delivery of one pellet (45 mg chocolate pellets, Bio-Serv, Frenchtown, NJ), whereas a poke into the long-delay hole (LDH) resulted in immediate illumination of the house light followed 1-sec later by delivery of a larger reward (5 or 4 pellets for adults and adolescents, respectively). After pellet delivery, the stimulus light was illuminated for 25 sec. During this 25-sec “timeout,” pokes into either hole or the food trough were without consequence. Once the timeout was complete, the stimulus light was turned off and the nose poking devices reactivated. All training and testing sessions lasted for 30 minutes.
Delay Testing
During the 7-day test (delay) phase, increasing delays were interpolated over days between nose pokes into the LDH and reward delivery, whereas entry into the SDH resulted in the same consequence as in the training phase. Delays of 5, 10, 20, 30, 40, 60, and 70 sec corresponded to testing days 7 through 12, respectively. The house light remained illuminated and nose pokes into either hole or the trough were without effect throughout the delay period. As was the case for training, the stimulus light above the magazine was turned on for a 25-sec timeout period after each reward delivery. For both training and testing, number of rewarded entries into each nose poke device was recorded daily for later statistical analyses.
Data Analysis
Before analysis, data from all experiments were checked for outliers, with a score greater than two standard deviations from the mean of an age group indicating a significant outlier. One adult animal in each experiment met this criterion and, thus, these animals were removed from all analyses. Fisher’s Least Significant Difference (LSD) post hoc tests were used to explore the locus of significant main effects and interactions.
Potential age differences in overall responding were first assessed by examining number of rewarded pokes. Behavior directed at both the SDH and LDH were also each separately assessed as potential indices of impulsive-like behavior, with data from the training and testing phases examined independently. Given that significant baseline differences in behavior would complicate interpretation of potential age differences in levels of impulsivity, number of SDH pokes and LDH pokes were subsequently analyzed on the fifth day of training in order to determine whether age differences in responding were present before the onset of the delay phase. In the majority of these instances, age differences were in fact present when examining these measures. Therefore, percentage of LDH pokes, defined as: [number of rewarded pokes at the LDH/(total rewarded pokes)] * 100, was also examined as an index of conditioning during training (and on the last training day), and was used to index impulsivity during the delay phase, with greater declines in %LDH with increasing delays used to reflect increased impulsivity.
The indifference point (the delay interval at which each animal exhibited approximately equal preference for both the smaller and larger reward) was also calculated as an additional index of impulsivity (e.g., see Harty, Whaley, Halperin, Ranaldi, 2011). In these experiments, the indifference point was obtained by selecting the delay length at which %LDH first reached 50% or below, with shorter indifference points indicating increased impulsivity. In a few instances (2 adult females and 2 adult males), the preference for the LDH never declined to 50% (or lower), and these animals were conservatively assigned an indifference point of 70 sec (the longest delay interval tested).
Experiment 1
Methods
To assess possible age differences in impulsivity among female rodents, adolescent and adult Sprague-Dawley rats (n=10/group, N=20) were exposed to the modified delay discounting procedure detailed above.
Results
Overall Responding
When total number of rewarded pokes (i.e. sum of rewarded LDH and SDH pokes) was examined during acquisition, significant main effects of Age [F(1,17) = 7.94, p ≤ 0.05] and Day [F(4, 68) = 4.16, p ≤ 0.001] emerged. Adults overall responded more than adolescents, with total number of rewarded pokes significantly increasing across days at both ages (Table 1). This age difference was no longer significant during the delay phase, however. An overall main effect of day [main effect of Day: F(6, 102) = 4.80, p ≤ 0.001] indicated that total responding slightly but significantly increased during throughout the delay phase (Table 1).
Table 1.
Number of Rewarded Hole Pokes, Short-Delay Hole (SDH) Pokes, and Long-Delay Hole (LDH) pokes Exhibited by both Female (Experiment 1) and Male (Experiment 2) Adolescent and Adult Rats.
| Experiment 1 | Experimental Day
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Training Phase | Delay Phase | ||||||||||||
|
| |||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
|
|
|||||||||||||
| Total rewarded pokes | |||||||||||||
| Adolescents | 12.3 (1.2) | 8.7 (2.5) | 10.5 (3.0) | 11.1 (2.5) | 15.6 (2.5) | 19.9 (2.4) | 22.4 (3.0) | 23.1 (3.3) | 25.3 (3.0) | 29.4 (2.9) | 24.0 (3.2) | 33.7 (3.7) | |
| Adults | 11.0 (1.3) | 17.7 (2.7) | 19.1 (3.1) | 18.3 (2.7) | 24.1 (2.6) | 26.8 (2.5) | 29.0 (3.2) | 30.0 (3.5) | 27.1 (3.2) | 30.2 (3.0) | 21.1 (3.6) | 31.3 (3.9) | |
| SDH pokes | |||||||||||||
| Adolescents | 5.0 (0.9) | 4.6 (1.5) | 5.2 (1.6) | 4.3 (1.3) | 6.3 (1.2) | 10.0 (1.7) | 9.8 (2.5) | 11.5 (3.3) | 15.2 (3.0) | 19.2 (3.0) | 14.8 (3.3) | 27.8 (4.6) | |
| Adults | 5.6 (0.9) | 9.1 (1.6) | 6.7 (1.6) | 4.7 (1.3) | 6.6 (1.2) | 7.6 (1.8) | 8.9 (2.6) | 11.1 (3.4) | 10.6 (3.2) | 14.9 (3.2) | 9.7 (3.5) | 21.2 (4.8) | |
| LDH pokes | |||||||||||||
| Adolescents | 7.3 (1.0) | 4.1 (1.2) | 5.3 (2.3) | 6.8 (1.9) | 9.3 (1.8) | 9.9 (1.9) | 12.6 (1.9) | 11.6 (1.2) | 10.1 (1.3) | 10.2 (0.8) | 9.2 (1.1) | 5.9 (1.1) | |
| Adults | 5.4 (1.0) | 8.6 (1.3) | 12.2 (2.5) | 13.7 (2.0) | 17.6 (1.9) | 19.2 (2.0) | 20.1 (2.0) | 18.9 (1.3) | 16.6 (1.4) | 15.3 (0.8) | 11.4 (1.2) | 10.1 (1.2) | |
|
| |||||||||||||
| Experiment 2 | |||||||||||||
| Total rewarded pokes | |||||||||||||
| Adolescents | 8.3 (1.1) | 12.0 (2.7) | 12.4 (2.4) | 12.4 (2.1) | 21.9 (3.4) | 18.8 (3.9) | 24.1 (3.0) | 32.1 (2.5) | 32.6 (3.7) | 35.6 (3.8) | 41.5 (4.1) | 43.3 (4.0) | |
| Adults | 6.7 (1.7) | 8.4 (3.0) | 13.1 (3.9) | 14.1 (4.3) | 22.7 (5.2) | 23.9 (5.7) | 20.7 (3.5) | 18.7 (4.5) | 21.7 (2.8) | 20.1 (3.1) | 22.4 (4.9) | 24.9 (4.5) | |
| SDH pokes | |||||||||||||
| Adolescents | 5.4 (0.8) | 7.8 (2.0) | 6.3 (1.3) | 5.3 (1.0) | 8.6 (1.2) | 6.8 (1.6) | 10.9 (3.3) | 19.8 (3.1) | 22.6 (4.6) | 27.1 (4.9) | 34.6 (5.2) | 37.8 (5.2) | |
| Adults | 3.4 (1.1) | 3.6 (1.2) | 5.6 (1.9) | 4.0 (1.1) | 5.6 (1.1) | 4.6 (1.5) | 4.4 (1.0) | 4.4 (1.4) | 7.4 (2.0) | 8.6 (2.2) | 13.4 (5.5) | 15.6 (5.8) | |
| LDH pokes | |||||||||||||
| Adolescents | 2.9 (0.8) | 4.3 (0.8) | 6.1 (2.0) | 7.1 (1.7) | 13.3 (2.8) | 12.0 (3.2) | 13.3 (3.5) | 12.4 (0.9) | 10.0 (1.9) | 8.5 (1.3) | 6.9 (1.2) | 5.5 (1.4) | |
| Adults | 3.3 (1.2) | 4.9 (2.2) | 7.6 (2.3) | 10.1 (3.7) | 17.1 (4.4) | 19.3 (4.7) | 16.3 (3.8) | 14.3 (3.6) | 14.3 (3.0) | 11.6 (2.3) | 9.0 (2.0) | 9.3 (2.2) | |
Note. Data represent means for each day of the experiment, with standard error of the mean following in parentheses; Bold values indicate a significant age difference from adults.
Training Phase
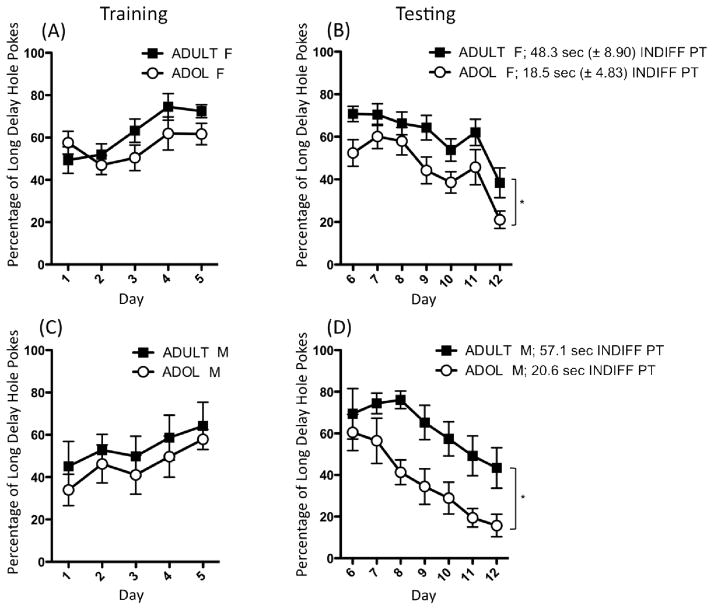
When %LDH pokes were analyzed, no significant age differences were observed during training (Figure 1a), with rats of both ages showing similar increases in the percentage of rewarded pokes obtained from the hole delivering the larger reward during training [main effect of Day: F(4,68) = 4.39, p ≤ 0.01]. When age differences in LDH and SDH responding were separately examined on the last day of training (day 5) using t-tests, adults were found to exhibit significantly more LDH pokes [t(17) = 3.23, p ≤ 0.01] than adolescents, although no significant age differences in SDH pokes were observed on this day (see Table 1).
Fig. 1.
Percentage of long-delay hole pokes (%LDH) [number of long-delay pokes/(total rewarded pokes) * 100] are shown for female (F) adolescent (ADOL) and adult rats from Experiment 1 during (a) training and (b) testing. Bottom panels depict %LDH for adolescent and adult males (M) from Experiment 2, also during (c) training and (d) testing. Asterisks (*) denote a significant main effect of age, with adolescents overall exhibiting a lower ratio of LDH pokes relative to adults. Indifference point (INDIFF PT) values (in seconds, ± standard error of the mean) for animals from both experiments are also included in the figure legend.
Testing Phase
When rewarded pokes into the SDH were examined during the delay phase, a main effect of Day [F(6,102) = 12.19, p ≤ 0.000001] (Table 1) revealed that SDH pokes increased significantly across days. No significant effects of age were observed for this measure. Post hoc analyses of a significant interaction of Day with Age [F(6,102) = 2.72, p ≤ 0.05] for the number of rewarded LDH pokes revealed that adults exhibited significantly more LDH pokes than adolescents on all days of the delay phase except day 11. Additionally, the number of LDH pokes significantly decreased across the testing period (Table 1). In the analysis of %LDH pokes (Figure 1b), adolescent females were found to exhibit an overall lower percentage of rewarded LDH pokes (i.e. greater impulsivity) than adults [main effect of Age: F(1,17) = 5.86, p ≤ 0.05], as well as a significantly lower indifference point [t(17) = 3.03, p ≤ 0.01] (Figure 1b). A main effect of Day [F(6,102) = 15.27, p ≤ 0.000001] in this analysis reflected a significant decline in %LDH pokes with increasing delays regardless of age.
Percent body weight gain
Adolescents were shown to gain an average of 4.1 g/day—a rate of gain that cumulatively resulted in body weights at the end of the experiment that were approximately 83.5% of what same aged, free-feeding Sprague-Dawley female rats would have weighed at that age. Adult females exhibited an average daily loss of about -0.8 g/day, which by the experiment’s conclusion, lead to body weights that were approximately 86% of projected free-feeding weights of female Sprague-Dawley rats of the same age.
Experiment 2
The purpose of Experiment 2 was to determine whether similar age differences would emerge in impulsivity in adolescent and adult male rats (n=8/age group), as in the female rats in Experiment 1 using the delay discounting paradigm described in the General Methods.
Results
Overall Responding
Total number of rewarded pokes analyzed during acquisition significantly increased across days [main effect of Day: F(4,52) = 17.20, p ≤ 0.00001], with no main effect or interaction involving Age. During testing, however, Day interacted with Age [F(6,78) = 3.59, p < 0.01]. Adolescents exhibited progressively more responses across days and made more responses than adults on days 8, and 10–12, whereas total responses made by adult animals remained generally similar across the testing period (Table 1).
Training
The analysis of percentage of LDH pokes failed to reveal any significant main effects of Age or Day, nor was there a significant interaction of these two variables (Figure 1c). Likewise, t tests conducted on the last day of training for this measure, as well as for number of SDH and LDH pokes (Table 1), revealed no significant age differences at the end of acquisition.
Testing
During the delay phase, analysis of SDH pokes (Table 1) showed main effects of Age [F(1,13) = 13.92, p ≤ 0.01] and Day [F(6,78) = 14.36, p ≤ .000001], as well as their interaction [F(6,78) = 3.25, p ≤ 0.01]. Specifically, although adolescents and adults both exhibited an increasing number of SDH pokes across the testing, this rise was more marked among adolescents, with adolescents having significantly more SDH pokes than adults on days 8–12. Conversely, while rewarded LDH pokes decreased throughout the delay phase [main effect of Day F(6,78) = 7.45, p ≤ 0.00001], no significant main effects or interactions involving Age were observed (Table 1). When %LDH pokes were examined (Figure 1d), adolescents exhibited a significantly lower percentage of LDH pokes compared to adults [main effect of Age: F(1,13) = 8.01, p ≤ 0.05] and a significantly shorter indifference point [t(13) = 4.14, p ≤ 0.01]. For both ages, %LDH pokes decreased during the delay phase [main effect of Day: F(6,78) = 14.43, p ≤ 0.000001].
Percent body weight gain
In this experiment, adolescent males exhibited a daily average weight gain of 5.2 g/day, which resulted in body weights that were approximately 82% of what a free-feeding adolescent male would have weighed by the end of the experiment. Adult males lost an average of about −0.9 g/day. By the end of the experiment, therefore, adult body weights were roughly 85% of their projected free-feeding weight.
Discussion
Using a modified delay-discounting procedure (e.g. Adriani et al., 2004; Adriani & Laviola, 2006; Adriani, Zoratto, Romano, & Laviola, 2010), both male (Exp. 2) and female (Exp. 1) adolescent rats were demonstrated to be significantly more impulsive relative to adults. This impulsive behavior was evidenced by significantly lower percentages of LDH pokes during the delay phase and significantly shorter indifference points among adolescents compared to adults. These results extend prior work showing increased delay discounting in adolescent male mice relative to their adult counterparts in some (Adriani & Laviola, 2003; Pinkston & Lamb, 2011), although not all (Pinkston & Lamb, 2011), studies. To our knowledge, the present findings are the first to demonstrate increased impulsivity in both male and female adolescent rats compared to adults using a delay discounting paradigm. A recent study (Andrzejewski et al., 2011) reporting impaired behavioral inhibition and self-control in male adolescent rats compared to adults using responding during extinction, differential reinforcement of low-rate (DRO) and differential reinforcement of other behavior (DRO) procedures further corroborates this age difference among male rats. These findings are reminiscent of human studies where adolescents have also been found to exhibit an intolerance to delay, more quickly increasing their preference for a smaller more immediate reward when a delay for a larger reward is instituted (Green, Myerson, & Ostaszewski, 1999; Olson, Hooper, Collins, & Luciana, 2007; Steinberg et al., 2009).
Increased levels of impulsivity have been linked to alterations in several brain neurotransmitter systems. Specifically, previous studies reported evidence that dopamine (e.g. Adriani et al., 2009; Dalley, Theobald, Pereira, Li, & Robbins, 2002; Kobayashi & Schultz, 2008; Koffarnus, Newman, Grundt, Rice, & Woods, 2011) and serotonin (Bizot, Le Bihan, Puech, Hamon, & Thiebot, 1999; Dalley, Theobald, Pereira, Li, & Robbins, 2002; Poulos, Parker, & Le, 1996) activity within the corticostriatal circuit may account for trait or state differences in impulsivity, though the relationship between neurotransmitter level (i.e. reduced versus elevated state) is not clear and may depend on changes relative to basal neurotransmitter levels and also brain region of interest. Ontogenetic differences in brain dopaminergic and serotonergic systems have also been reported during adolescence (for review see Crews, He, & Hodge, 2007; Doremus-Fitzwater, Varlinskaya, & Spear, 2009; Spear, 2000) and may contribute to increased expression of impulsive-like behavior in this developmental stage. Clearly though, other brain systems also influence impulsive behaviors (e.g. endogenous cannabinoids; Pattij et al., 2007) and more research is needed in order to further elucidate the role of normal maturational changes in adolescent brain in the elevated impulsivity seen during this ontogenetic period.
The cognitive processes underlying age-related differences in impulsive behavior are also largely unknown. One possible contributor to this age effect could be ontogenetic alterations in internal timing processes, as perception of the length of the delay interval would likely be influenced by any possible developmental differences in an “internal clock.” Some previous studies have reported ontogenetic differences in the ability to estimate time duration (e.g., Coelho et al., 2004; Gunstad, Cohen, Paul, Luyster & Gordon, 2006), however, these studies have focused on differences late in life among aged individuals. More recently, evidence has emerged which indicates that the ability to judge the duration of a short stimulus may differ early in life, specifically between children, young adults and older adults (Lustig and Meck, 2011). Frontostriatal circuitry has been implicated in the ability to estimate timing events (e.g., Hinton and Meck, 2004), and adolescence is known to be a developmental period characterized by dramatic brain transformations, including alterations in frontocortical regions (for review see Casey and Jones, 2010). Examination of age differences in internal timing abilities as they relate to the expression of impulsivity across age therefore warrants future investigation.
Taken together, these results demonstrate that both adolescent male and female rats exhibit increased levels of impulsivity in a modified delayed discounting paradigm (Adriani et al., 2004; Adriani & Laviola, 2006; Adriani, Zoratto, Romano, & Laviola, 2010). These findings corroborate and extend prior data from both mouse and human studies showing that elevations in impulsive behavior may be a pervasive behavioral feature of adolescence and may contribute to the poor behavioral control seen under some circumstances at this age. Though relatively limited thus far, results from animal studies have indicated that impulsive behavior may be positively associated with propensity for drug/alcohol intake (e.g., Perry, Nelson, and Carroll, 2008; Poulos, Le, and Parker, 1995). Furthermore, studies with humans have shown a similar relationship between adolescent impulsivity and proclivity for drug and alcohol abuse (e.g., Wulfert, Block, Santa Ana, Rodriguez, and Colsman, 2002), with recent clinical evidence also showing that levels of impulsivity may predict substance abuse treatment outcomes among adolescents (Stanger, et al., 2011). Thus, future studies using this simple rat model of impulsivity may prove useful in detailing the links between impulsivity and drug seeking/taking during ontogeny, their underlying neural mechanisms, genetic contributors, and potential sex differences in these effects.
Acknowledgments
This research was supported by grants AA018026, AA017355 and DA019071 to LPS. Animals used in these experiments were maintained and treated in accordance with the National Institutes of Health Guide for Animal Care (NIH Publication No.: 80-23, revised 1996), using protocols approved by the Binghamton University Institutional Animal Care and Use Committee (IACUC).
Footnotes
The authors have no conflicts of interest to disclose.
References
- Adriani W, Boyer F, Gioiosa L, Macri S, Dreyer JL, Laviola G. Increased impulsive behavior and risk proneness following lentivirus-mediated dopamine transporter over-expression in rats’ nucleus accumbens. Neuroscience. 2009;159(1):47–58. doi: 10.1016/j.neuroscience.2008.11.042. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behav Neurosci. 2003;117(4):695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Delay aversion but preference for large and rare rewards in two choice tasks: implications for the measurement of self-control parameters. BMC Neurosci. 2006;7:52. doi: 10.1186/1471-2202-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriani W, Rea M, Baviera M, Invernizzi W, Carli M, Ghirardi O. Acetyl-L-carnitine reduces impulsive behaviour in adolescent rats. Psychopharmacology (Berl) 2004;176(3–4):296–304. doi: 10.1007/s00213-004-1892-9. [DOI] [PubMed] [Google Scholar]
- Adriani W, Zoratto F, Romano E, Laviola G. Cognitive impulsivity in animal models: role of response time and reinforcing rate in delay intolerance with two-choice operant tasks. Neuropharmacology. 2010;58(4–5):694–701. doi: 10.1016/j.neuropharm.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Andrzejewski ME, Schochet TL, Feit EC, Harris R, McKee BL, Kelley AE. A comparison of adult and adolescent rat behavior in operant learning, extinction, and behavioral inhibition paradigms. Behav Neurosci. 2011;125(1):93–105. doi: 10.1037/a0022038. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: a developmental perspective. Developmental Review. 1992;12:339–373. [Google Scholar]
- Bizot J, Le Bihan C, Puech AJ, Hamon M, Thiebot M. Serotonin and tolerance to delay of reward in rats. Psychopharmacology (Berl) 1999;146(4):400–412. doi: 10.1007/PL00005485. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1189–1201. doi: 10.1016/j.jaac.2010.08.017. quiz 1285 S0890-8567(10)00670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho M, Ferreira JJ, Dias B, Sampaio C, Pavao Martins I, Castro-Caldas A. Assessment of time perception: the effect of aging. J Int Neuropsychol Soc. 2004;10(3):332–341. doi: 10.1017/S1355617704103019. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86(2):189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Pereira EA, Li PM, Robbins TW. Specific abnormalities in serotonin release in the prefrontal cortex of isolation-reared rats measured during behavioural performance of a task assessing visuospatial attention and impulsivity. Psychopharmacology (Berl) 2002;164(3):329–340. doi: 10.1007/s00213-002-1215-y. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14(1):22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2009;72(1):114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146(4):348–361. doi: 10.1007/PL00005487. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Fry AF, Myerson J. Discounting of delayed rewards: a life-span comparison. Psychological Science. 1994;5(1):33–36. [Google Scholar]
- Green L, Myerson J, Ostaszewski P. Discounting of delayed rewards across the life span: Age differences in individual discounting functions. Behavioural Processes. 1999;46:89–96. doi: 10.1016/S0376-6357(99)00021-2. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Cohen RA, Paul RH, Luyster FS, Gordon E. Age effects in time estimation: relationship to frontal brain morphometry. J Integr Neurosci. 2006;5(1):75–87. doi: 10.1142/s0219635206001045. S0219635206001045. [DOI] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998;12(1–2):129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Harty SC, Whaley JE, Halperin JM, Ranaldi R. Impulsive choice, as measured in a delay discounting paradigm, remains stable after chronic heroin administration. Pharmacol Biochem Behav. 98(3):337–340. doi: 10.1016/j.pbb.2011.02.004. S0091-30571100043-8. [DOI] [PubMed] [Google Scholar]
- Hinton SC, Meck WH. Frontal-striatal circuitry activated by human peak-interval timing in the supra-seconds range. Brain Res Cogn Brain Res. 2004;21(2):171–182. doi: 10.1016/j.cogbrainres.2004.08.005. S0926-6410(04)00219-8. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14(3):221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Research, C. o. L. S., National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, D.C: 1996. [Google Scholar]
- Kobayashi S, Schultz W. Influence of reward delays on responses of dopamine neurons. J Neurosci. 2008;28(31):7837–7846. doi: 10.1523/JNEUROSCI.1600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Newman AH, Grundt P, Rice KC, Woods JH. Effects of selective dopaminergic compounds on a delay-discounting task. Behav Pharmacol. 2011 doi: 10.1097/FBP.0b013e3283473bcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Meck WH. Modality differences in timing and temporal memory throughout the lifespan. Brain Cogn. 2011;77(2):298–303. doi: 10.1016/j.bandc.2011.07.007. S0278-2626(11)00124-2. [DOI] [PubMed] [Google Scholar]
- Olson EA, Hooper CJ, Collins P, Luciana M. Adolescents’ performance on delay and probability discounting tasks: contributions of age, intelligence, executive functioning, and self-reported externalizing behavior. Pers Individ Dif. 2007;43(7):1886–1897. doi: 10.1016/j.paid.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Schepers I, Gonzalez-Cuevas G, de Vries TJ, Schoffelmeer AN. Effects of the cannabinoid CB1 receptor antagonist rimonabant on distinct measures of impulsive behavior in rats. Psychopharmacology (Berl) 2007;193(1):85–96. doi: 10.1007/s00213-007-0773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of IV cocaine self- administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol. 2008;16(2):165–177. doi: 10.1037/1064-1297.16.2.165. doi: 2008-03846-007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum B, Wood PB. Self-report study of impulsive and delinquent behavior in college students. J Adolesc Health. 1994;15(4):295–302. doi: 10.1016/1054-139x(94)90602-5. [DOI] [PubMed] [Google Scholar]
- Pinkston JW, Lamb RJ. Delay discounting in C57BL/6J and DBA/2J mice: adolescent-limited and life-persistent patterns of impulsivity. Behav Neurosci. 2011;125(2):194–201. doi: 10.1037/a0022919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6(8):810–814. [PubMed] [Google Scholar]
- Poulos CX, Parker JL, Le AD. Dexfenfluramine and 8-OH-DPAT modulate impulsivity in a delay-of-reward paradigm: implications for a correspondence with alcohol consumption. Behav Pharmacol. 1996;7(4):395–399. doi: 10.1097/00008877-199608000-00011. [DOI] [PubMed] [Google Scholar]
- Prencipe A, Kesek A, Cohen J, Lamm C, Lewis MD, Zelazo PD. Development of hot and cool executive function during the transition to adolescence. J Exp Child Psychol. 2011;108(3):621–637. doi: 10.1016/j.jecp.2010.09.008. S0022-0965(10)00182-7. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Fu H, Landes RD, Jones BA, Bickel WK. Delay discounting predicts adolescent substance abuse treatment outcome. Exp Clin Psychopharmacol. 2011 doi: 10.1037/a0026543. 2011-29376-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Dev. 2009;80(1):28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Spear LP. Age-associated trajectories of consumption and body weight gain in pair- and isolate-housed adolescent and adult Sprague-Dawley rats. Paper presented at the International Society for Developmental Psychobiology; San Diego, CA.. Nov, 2007. [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2004;29(7):1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 2004;176(3–4):376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- Wulfert E, Block JA, Santa Ana E, Rodriguez ML, Colsman M. Delay of gratification: impulsive choices and problem behaviors in early and late adolescence. J Pers. 2002;70(4):533–552. doi: 10.1111/1467-6494.05013. [DOI] [PubMed] [Google Scholar]