Abstract
In this review, we discuss the prospective medical application of magnetic carriers microfabricated by top-down techniques. Physical methods allow the fabrication of a variety of magnetic structures with tightly controlled magnetic properties and geometry, which makes them very attractive for a cost-efficient mass-production in the fast growing field of nanomedicine. Stand-alone fabricated particles along with integrated devices combining lithographically defined magnetic structures and synthesized magnetic tags will be considered. Applications of microfabricated multifunctional magnetic structures for future medicinal purposes range from ultrasensitive in vitro diagnostic bioassays, DNA sequencing and microfluidic cell sorting to magnetomechanical actuation, cargo delivery, contrast enhancement and heating therapy.
Keywords: cells, ferromagnetic nanoparticles, lithography, microdisks, microfabrication, sensors
Nanotechnology has rapidly placed itself at the forefront of virtually all areas of science from solar cells to medicine. The advancement of nanotechnology-based innovations is supported by efforts of government-supported agencies all over the world. The National Nanotechnology Initiative (NNI) was established by the United States government in 2000 and since then has continued to provide resources and strategic planning for developing the applications of nanotechnology, with healthcare being among the top priority areas of research, as indicated by significant budget allocations for the NIH from the NNI [201]. NANOfutures is a part of European Technology Integrating and Innovation Platform bringing together industry and academic partners to promote growth of the field and facilitate commercialization of nanotechnological tools [202]. Nanotechnology has been shown to be particularly promising for advancing medical technologies, biology and biotechnology due to the unique electronic, optical, magnetic and biological properties of nanoscale materials [1–4]. Magnetic particles are very attractive for medical applications since they can be detected and manipulated remotely using magnetic fields, which opens the opportunity to utilize them in vivo.
The importance of magnetic particles as active agents for studying biological systems has been recognized long before the advent of sophisticated fabrication techniques (BOX 1). Magnetic powders and particles synthesized by wet chemistry methods were historically the first magnetic carriers employed for biological studies. The ability to control the magnetization, localization and to change temperature or magnetic particles remotely by the external magnetic field gave rise to a number of their biological applications including molecular sensing, drug delivery, cell sorting, magnetic field-mediated transfection (magnetofection) of living cells, hyperthermia and MRI, where the particles are serving as contrast enhancement agents. Detailed discussions about different functionalities of such particles can be found elsewhere [5–12]. It is important to note that toxicity of nanoparticles employed in biomedicine should always be taken into account. Readers are directed to [13] for the recent updates on this topic. The key routes for synthesis and functionalization of superparamagnetic particles have also been reviewed extensively [6,7,14,15]. Depending on a specific application, the particles can be synthesized using either aqueous [11,12] or organic-based routes [14,15]. The first method involves co-precipitation of Fe3+ ions using an inorganic base (NaOH or NH·H2O) and the latter employs high-temperature decomposition of an iron precursor, for example, iron pentacarbonyl Fe(CO)5 or ferric acetylacetonate Fe(acac)3 in an organic solvent in the presence of stabilizing and capping agents, such as oleic acid. The organic synthesis yields nanoparticles with diameters below 20 nm with tightly controlled size distribution, which is required for maintaining their superparamagnetic properties. Also, nanoparticles in the 11–13 nm diameter range are very efficient for hyperthermia since their size allows for achieving large specific heat absorption rates [16]. On the other hand, nanoparticles dispersed in organic solutions are not compatible with biological systems, so the additional functionalization steps, such as ligand exchange or coating with a polymer, should be made in order to eliminate the problem of cytotoxicity.
Box 1. Key terms.
Bottom-up fabrication: a method for building a large structure from smaller subunits, atoms and molecules, which by themselves cannot perform a function or possesses the properties of the final complex structure.
Nanoparticle: a particle with a characteristic size below 1000 nm, which is functionally independent and may exhibit unique properties compared to a bulk material.
Top-down fabrication: a method for shaping a bulk material in order to create a structure with desired dimensions and properties. This paper focuses on magnetic structures fabricated using top-down methods as opposed to chemically synthesized magnetic nanoparticles that are typically realized using bottom-up techniques.
Microfabrication: a top-down approach for making microstructures with characteristic sizes in the order of 1–1000 μm. This method allows for creating multilayered structures made of different materials that can be shaped down either sequentially or simultaneously resulting in highly complex functional units. The term nanofabrication applies to the methods for creating nanostructures typically no larger than 1000 nm.
Magnetization vortex state: a kind of magnetic domain structure, a curling spin configuration. This state is characteristic for magnetically soft materials with lateral dimensions that are much larger than the thickness. The vortex is characterized by an in-plane continuous swirling closure spin structure. The vortex state is stable because it generates minimal stray magnetic fields. In other words, ferromagnetic particles with spin vortices are not ‘magnetized’ in the absence of an external magnetic field.
While chemical synthesis methods are instrumental for producing small spherical particles, they are difficult to use for creating multilayer structures with anisotropic shapes, with the exception of nanowires, which have been synthesized using template electroporation [17–19]. Physical methods (BOX 1), such as photolithography or electron-beam lithography, combined with physical vapor deposition, allow a variety of magnetic structures with tightly controlled magnetic properties and geometry to be fabricated, which makes them very attractive for the fast growing field of magnetism-based nanomedicine. Moreover, it should be noted that the wet chemistry synthesis of superparamagnetic particles remains to be very difficult to adapt for mass production owing to a relatively low yield. Novel approaches for automatic combinatorial syntheses and post-processing of colloid magnetic nanoparticles are required for advancing existing methods. Automatic systems with precise reaction condition control are under development to secure reproducibility of synthetic methods [20,101]. At the same time, microfabrication (BOX 1) is compliant with the demands of mass-production requirements for material yield and automation requirements. This is especially important in view of the medical applications of magnetic carriers, which will require large amounts of particles and structures with reproducible sizes and material properties.
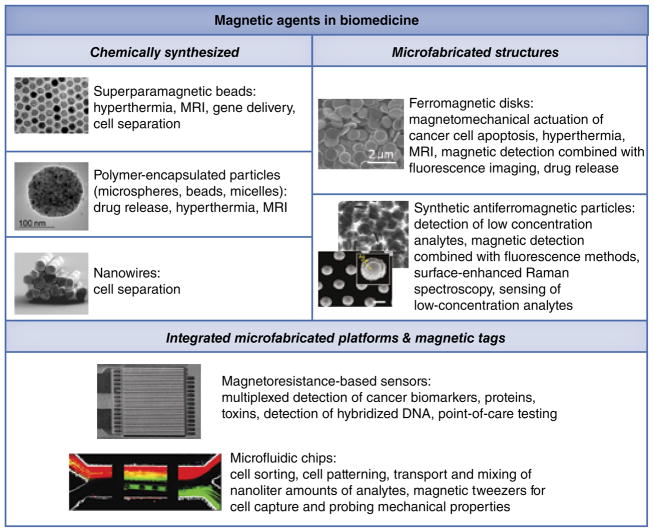
Microfabrication of magnetic structures has experienced a tremendous growth since the 1980s when the thin film deposition methods, such as molecular beam epitaxy, progressed rapidly. The technology, enabling growth of films with precisely controlled geometry and crystalline structure, has led to the discovery of a giant magneto-resistance effect, perpendicular surface anisotropy [21] and oscillatory exchange coupling [22]. Although originally these phenomena were viewed primarily in the context of microelectronics application such as magnetic recording, it was later realized that microfabricated structures open a new realm of opportunities for manipulating cells and biological molecules. To better define the importance that microfabricated magnetic structures have already gained in this area, FIGURE 1 compares the chemically synthesized magnetic particles with microfabricated ones, essentially presenting an overview of this paper where we aim to summarize the latest applications of microfabricated magnetic structures in biomedicine. The manuscript is divided into sections according to the applications. We will first discuss the historically original application of magnetic particles for studying the mechanical properties of cells. Second, the multifunctional ferromagnetic disks developed by our group will be considered. Third, we discuss a novel type of fabricated synthetic antiferromagnetic particles whose structure originated from the field of magnetic random access memory. Importantly, microfabricated magnetic structures should not be viewed as a pure alternative to chemically synthesized particles. On the contrary, both types of particles/structures can be combined to complement each other in the integrated platforms employing microfabricated magnetic structures together with nanoparticle-based magnetic tags. Thus, in the last section we will discuss magnetoresistive sensors and microfluidic chips, which have found a vast number of applications for high sensitivity molecular recognition and cell sorting. Before moving on to the discussion, it is important to note that the studies employing microfabricated magnetic particles are still in their early stages and this review is one of the first attempts to provide a systematic outlook at this emerging field.
Figure 1. Applications of magnetic agents in biomedicine.
The images of chemically synthesized superparamagnetic beads [14], nanowires [19], SAF particles [50,53] and a microfluidic magnetic chip for cell sorting [76] are reproduced with permission from the corresponding references.
Images from [14,19] are copyright of the American Chemical Society.
Probing mechanical properties of living cells
Mechanical and mechanoresponsive properties play critical roles on virtually every stage of the cell lifecycle. In living organisms, cell mechanosensitivity regulates the process of organism homeostasis and interaction with the ambient environment through self-propulsion, hearing and touch response. It has been established that stem cell differentiation can be directed by applying shear stress [23,24] or by growing cells on substrates with different magnitudes of stiffness [25]. One of the earliest applications of magnetic particles (16 μm nickel powder) proposed by Heilbronn and Seifriz in the 1920s was investigating the mechanical properties of cell cytoplasm [26,27]. The displacement of particles incorporated in the cytoplasm was tracked as a function of the applied magnetic field in order to measure the viscosity and elasticity of the cytoplasm. Later, a number of similar approaches for studying cell rheology using smaller superparamagnetic particles have been developed [28–30]. De Vries et al. utilized microfabricated magnetic tweezers for studying the rheological properties of cell chromatin [31]. A human cervical adenocarcinoma HeLa cell was microinjected with a single 0.5-μm Dynal bead and its movement was tracked using an optical microscope. The results show that the chromatin network is a relatively stiff structure with the Young’s modulus of 2.5*102 Pa.
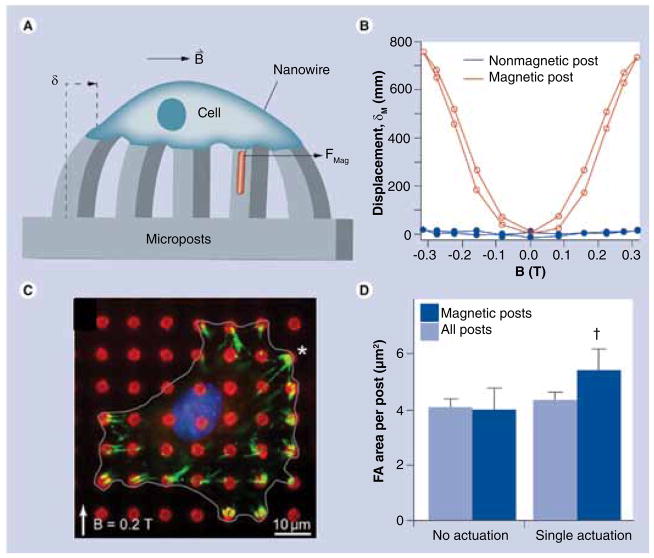
Sniadecki et al. have shown that microfabricated cobalt posts can be used for applying controlled forces to cells and studying their response to mechanical forces [32]. FIGURES 2A & B show the principle of this approach where the microposts impregnated with magnetic nanowires deflect in response to an external magnetic field, thus causing the tensile stress on the cells placed atop the microposts. The cells are known to attach to the substrate through focal adhesions, which emerge from clustering of integrins expressed upon cell adhesion. The increase in the focal adhesion area was observed as a result of mechanical stimulation, which indicates that the cells respond to the traction forces (FIGURE 2C & D). Using a similar approach, Lin et al. have applied magnetic nanowires for measuring the cellular contractile forces in response to magnetic stimuli [33].
Figure 2. Modulation of cell adhesion by mechanical stimulation.
(A) Schematic of applying forces to a single cell through a micropost with embedded cobalt nanowires. (B) Displacement of magnetic and nonmagnetic posts in response to external field. (C) Fluorescent imaging of FAs (green) forming upon magnetic actuation. The asterisk denotes the location of a magnetic post. (D) FA area before and after magnetic post actuation. The results demonstrate that focal adhesions are growing as a result of applying an external force.
FA: Focal adhesion.
Reproduced with permission from [32].
Inducing apoptosis in cancer cells with ferromagnetic disks
Lithographically defined disk-shaped particles have been successfully employed for modulating cell function [34,35] Composed of Fe20Ni80 permalloy, these particles possess zero net magnetization due to their anisotropic (flat) shape, which leads to the formation of spin-vortices (BOX 1). In other words, in the absence of a magnetic field these particles are not magnetized. This eliminates the problem of particle aggregation, which is known to limit the applications of superparamagnetic particles synthesized by wet chemistry. Moreover, the intrinsic properties of 3d metals of the permalloy allow for weak external magnetic fields for achieving high magnetization of the microdisks to be used, which makes them highly responsive to magnetic stimuli.
The authors’ group has previously shown that microdisks are instrumental in both low- and high-frequency magnetic field regimes, while they are also promising as contrast agents for MRI high frequency field applications (100–300 kHz), including magnetic hyperthermia. In the low-frequency regime, the microdisks can serve as mediators of mechanical stimuli applied directly to living cells. This aspect of microdisk applications is considered in more detail in the following sections.
Apoptosis is a natural process of cell death, which, unlike necrosis, occurs as a result of a specific biochemical signaling cascade. Apoptosis controls fundamental processes, such as the embryonic development in higher vertebrates and deletion of lymphocytes for supporting the homeostasis of the immune system [36]. Disruption of apoptotic pathways may result in uncontrollable cell proliferation, which occurs in cancer tumors. The methods for triggering apoptosis in cancer cells are therefore being thoroughly investigated. Ferromagnetic microdisks have been proven to be a promising tool for inducing apoptosis of human glioblastoma cells.
The disk fabrication process has been reported previously [35,37]. Briefly, the shape of the disks is defined using contact photolithographic patterning of a negative photoresist. Photoresist development yields a structure of 0.5-μm tall, 1-μm diameter pillars, which are then coated with three layers of metal by means of electron beam deposition, as shown in FIGURE 3A. The central 60-nm thick layer is permalloy, which defines the magnetic properties of the disk. The outer layers consist of 5-nm gold, which allows for biocompatibility and chemical functionalization of the disks to enable targeting to specific cell receptors and labeling the disks with, for example, fluorescent tags [34,35,38].
Figure 3. Fabrication of ferromagnetic disks.
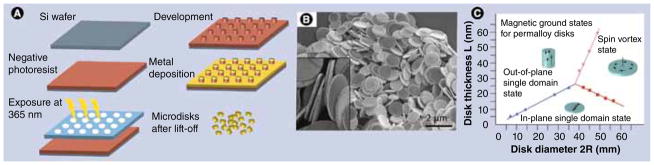
(A) Schematic of fabricating ferromagnetic microdisks using lithography and thin film deposition techniques. (B) Scanning electron micrograph of microdisks after photoresist lift-off. The inset shows a zoomed cross-section of individual disks. (C) Theoretical phase diagram of permalloy disk-shaped particles showing their magnetization state as a function of aspect ratio. Existence of magnetic vortices in submicron disks has been experimentally confirmed by many research groups. From the energy minimization viewpoint, the magnetic vortex state remains a stable (‘ground’) spin state for magnetically soft disks, down to sub-100 nm lateral dimensions.
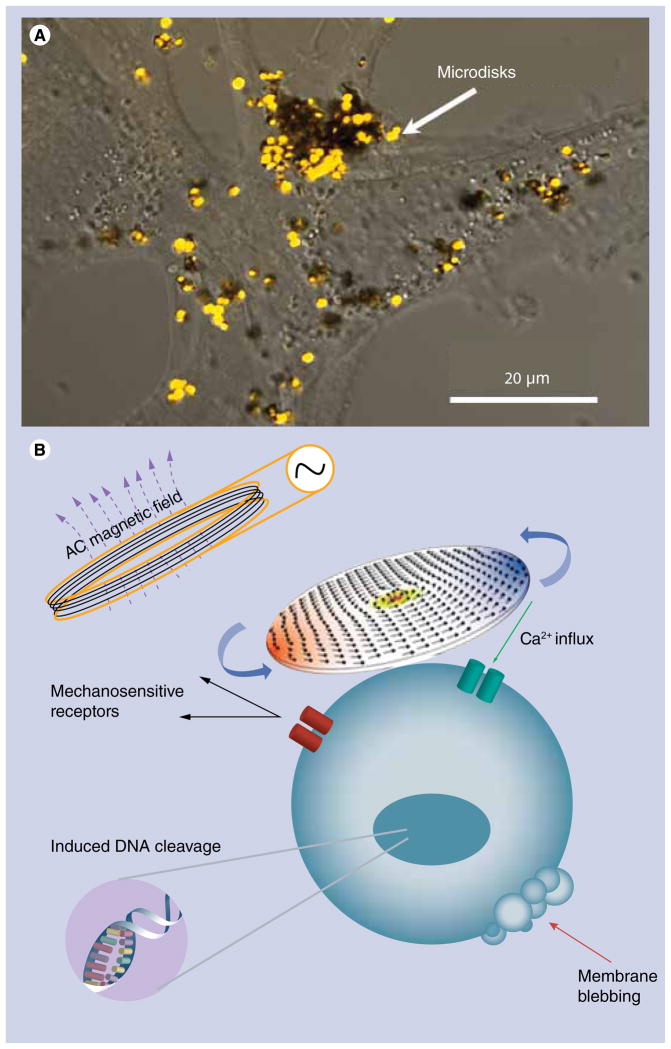
Due to their large scattering cross-section, the disks can be easily visualized under an optical microscope. FIGURE 4A shows a pseudo-color image of the disks on the cell surface acquired in the confocal regime. To enable specific attachment of the disks to cancer cells, the disks were functionalized with IL-13α2R antibody, which targets the IL-13α2 receptor expressed on human glioma cells. FIGURE 4B illustrates the principle of magnetomechnical actuations of cancer cells using ferromagnetic disks. Low frequency AC magnetic field induces high magnetomotive force causing the disks to oscillate and stimulate the cell membrane.
Figure 4. Ferromagnetic microdisks targeted to a cancer cell for magnetomechanical cell stimulation.
(A) Confocal optical image of microdisks on the A172 glioblastoma cell surface. (B) The principle of magnetomechanical actuation of cell membrane triggering apoptosis.
While the exact mechanism of magnetomechanical cell stimulation is yet to be fully understood, the experimental data clearly supports the hypothesis about mechanical origin of the apoptotic response. The stimulation elevates the intracellular calcium level suggesting the recruitment of mechanosensitive receptors as a result of cell membrane stretching by oscillating disks [34,39,40]. The natural lack of calcium metabolism in a cell makes it highly susceptible to elevated intracellular calcium concentrations, which, on prolonged exposure, may trigger the apoptosis [41]. At the same time, acridine orange and ethidium bromide staining of magnetomechnically treated cells reveals the signs of apoptotic DNA damage. FIGURE 5 shows the confocal fluorescence images of the A172 human glioblastoma cells overlaid with their optical transmission images before and after the treatment with ferromagnetic microdisks in a weak low frequency AC magnetic field. The enhanced green nuclear staining by acridine orange indicates that the cells are undergoing apoptosis. At the same time, the absence of orange nuclear staining suggests that for the given cell line, the magnetomechanically induced apoptosis does not necessarily involve the rupture of the cell membrane since ethidium bromide does not enter the nucleus, at least in the early stages of apoptosis. Importantly, magnetic disks are not cytotoxic in the absence of the external field [42].
Figure 5. Acridine orange/ethidium bromide (14 μg/ml) staining of A172 human glioblastoma cells for assessing magnetomechanically induced DNA damage.
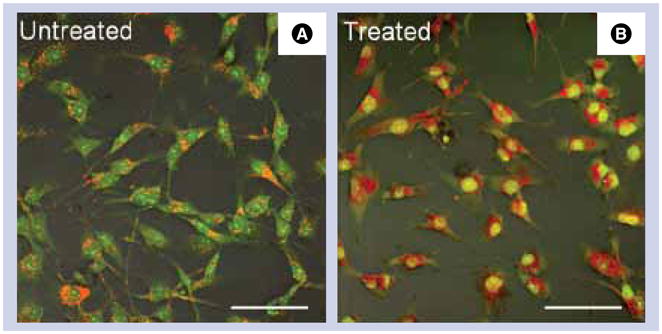
Overlay of differential interference contrast and confocal fluorescence images of (A) control untreated cells and (B) magnetomechanically stimulated cells, which were incubated with microdisks and then exposed to AC magnetic field (10 Hz, 100 Oe) for 15 min. The treated cells exhibit the signs of early apoptosis as indicated by bright nuclear staining with acridine orange. Scale bar: 100 μm; 20× magnification objective.
From an alternative point of view, it is interesting to note that while a number of theories about the underlying principles of cell architecture exist, it is generally agreed that the cytoskeleton is the key organizational unit responsible for cell mechanomechnical signal transduction, cell shape regulation and migration. The tensegrity model considers the cell as a structure with tensional integrity as opposed to compressional continuity [43]. For example, Buckminster fullerenes represent one of the most well-known tensegrity-based structures. The fundamental idea of tensegrity is that every element of the system would sense and respond to either an external or internal physical stimulus [43]. In the context of this work, this translates to reasoning behind the principle of magnetomechnically induced cell activation leading to apoptosis.
Ferromagnetic disks appear to be one of the promising currently available microfabricated magnetic structures. Importantly, due to their simple shape and the ease of fabrication, involving only a one-step metal deposition on the patterned photoresist layer, the disks can be scaled down to less than a 100-nm diameter while still preserving the vortex state [44,45]. From a fabrication point of view, this can be achieved by employing the latest advances in the nanoimprint as well as deep-UV lithographies [46]. An interesting approach for fabricating sub-100 nm disks was demonstrated by Liu et al. where a porous aluminum membrane was used as a mask for depositing 60 nm Fe dots on a substrate [47]. Submicron disks are likely to have a large number of applications for in vivo therapy. Moreover, compared to superparamagnetic particles, ferromagnetic materials offer the advantages of:
High saturation magnetization
Large magnetic susceptibility (in soft ferromagnets)
Zero remanence achievable in particles with anisotropic shapes due to spin–vortex state or in synthetic antiferromagnetic (SAF) particles.
Taken together, this opens the opportunity to manipulate the behavior of such particles with very weak magnetic fields, thus making them additionally attractive and practical for in vivo applications.
Multiplexed molecular sensing
Detection with microfabricated magnetic particles
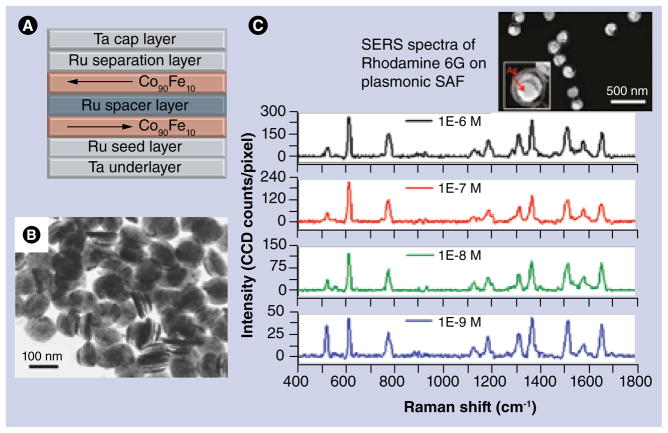
Microfabricated magnetic structures are actively exploited in microelectronics, with magnetoresistive random access memory (MRAM) being one of its most well-known applications. It is therefore not surprising that some of the fabrication principles for creating biologically relevant magnetic particles have been borrowed from this field. In 2005, Engel et al. demonstrated a novel type of MRAM based on a SAF structure that comprises two ferromagnetic layers separated by a thin nonmagnetic spacer [48]. This structure exhibits unique magnetic properties compared to a single ferromagnetic layer. The thickness and material of the nonmagnetic spacer controls the exchange coupling between ferromagnetic layers, which results in magnetization of these layers in the opposite directions thus yielding a structure with zero net magnetization. At the same time, the magnetization can be easily toggled in the external field. Employing this principle, Hu et al. recently reported on the SAF nanoparticles that were fabricated by means of nanoimprint lithography and vapor deposition [49,50]. Quartz stamps were used for creating the pattern of 100 nm dots on a silicon substrate coated with copper sacrificial layer as shown schematically in FIGURE 6A. FIGURE 6B shows the transmission electron micrograph of the SAF particles. Subsequent deposition of two Co90Fe10 layers with Ru spacers and capping layers of Ta on both sides results in the formation of particles that are strongly magnetic but have zero remanence. Moreover, magnetic susceptibility of these particles can be tuned by controlling the interlayer interactions in the SAF structure while preserving the particle size and magnetic saturation moment. It has been shown that by varying the thickness of the SAF-constituting layers and thus the resulting magnetic susceptibility, it is possible to control the dispersion of the particles in solution [51,52] Moreover, there is large potential in tailoring the properties of SAF particles for multiplex molecular sensing where the particles with different susceptibilities may be used for detecting different types of molecules. Recently, the same research group led by Wang demonstrated another type of SAF particles that were specially designed for plasmonic applications. The so-called ‘magnetic sombreros’ were successfully employed for high sensitivity surface-enhanced Raman spectroscopy detection of Rhodamine 6G [53], as shown in FIGURE 6C.
Figure 6. Synthetic antiferromagnetic particles: fabrication and applications.
(A) Layer structure of synthetic antiferromagnetic particles. (B) TEM image of released SAF particles with 100-nm diameter. (C) Surface-enhanced Raman spectra of Rhodamine 6G at different concentrations measured using a different type of plasmonic SAF particle with a ‘sombrero’-like shape, coated with silver.
(B) Reproduced with permission from [50].
(C) Reproduced with permission from [53].
Chemically resistant magnetic digital planar tags fabricated by deep-UV photolithography were introduced for high-throughput biological sensing applications [54,55]. Thus, each magnetic tag contains five elements (bits) with different coercive fields as controlled by the aspect ratio of magnetic structure corresponding to each bit. The bit magnetization switching can be imaged using a Kerr magneto-optical microscope. The ability to address each bit individually opens the opportunity for high resolution molecular sensing and DNA sequencing.
Magnetoresistive sensors
The discovery of spin-dependent transport phenomena such as in the giant magneto-resistance (GMR) effect is a remarkable achievement in modern magnetism. The resistance of certain magnetic heterostructures drops dramatically as a magnetic field is applied. It is termed giant since it is a much larger effect than had previously been seen in conventional magnetic alloys, and its mechanistic origins also differ from conventional materials. Therefore, it has generated tremendous interest from both physicists and device engineers, as there are both new physics to be investigated and major technological applications for magnetic field sensors. Magnetoresistive sensors are based on the change of electrical resistance in response to applied magnetic fields [56,57]. GMR was first reported on Fe/Cr super-lattices prepared by molecular beam epitaxy [58]. GMR has since become one of the key principles of operation for hard drives and magnetic memory chips. More recently, GMR has been successfully incorporated into the methods for high sensitivity magnetic label detection for biological applications [59–61]. A combination of magnetic tags with GMR sensors allows for simultaneous detection of multiple analytes, making this approach very attractive for high sensitivity detection of proteins, low concentration analytes, various types of biomarkers and toxins [60,62,63]. It has been shown that magnetoresistance-based sensors offer the advantage of higher sensitivities compared with fluorescence-based assays [64]. Fabrication methods are still being actively developed. Recently, Kennedy et al. demonstrated a method for depositing magnetic nanoclusters surface using ion implantation on dielectric SiO2 followed by electron beam annealing at 1000°C [65]. The nanoclusters with particles ranging from 5 to 40 nm were obtained. This method holds potential for the development of high sensitivity magnetoresistive sensors.
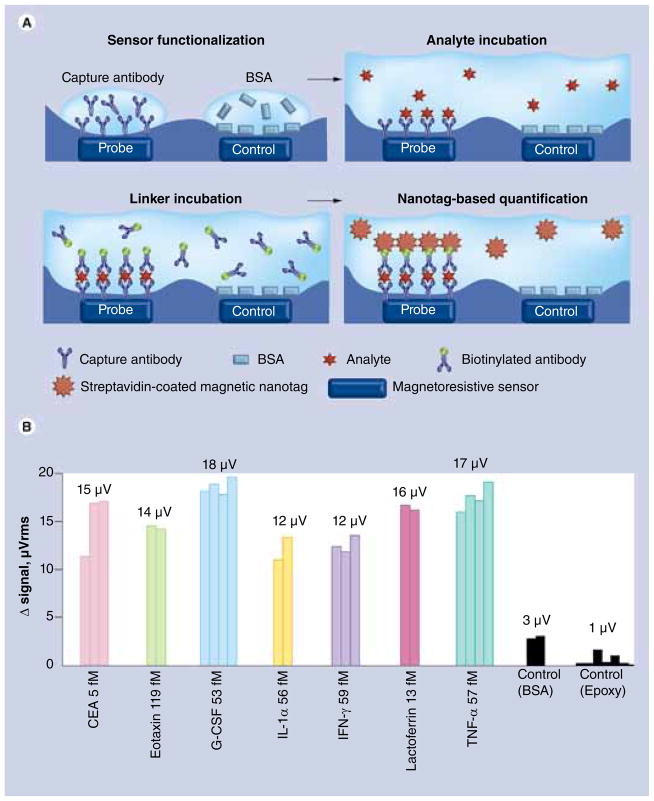
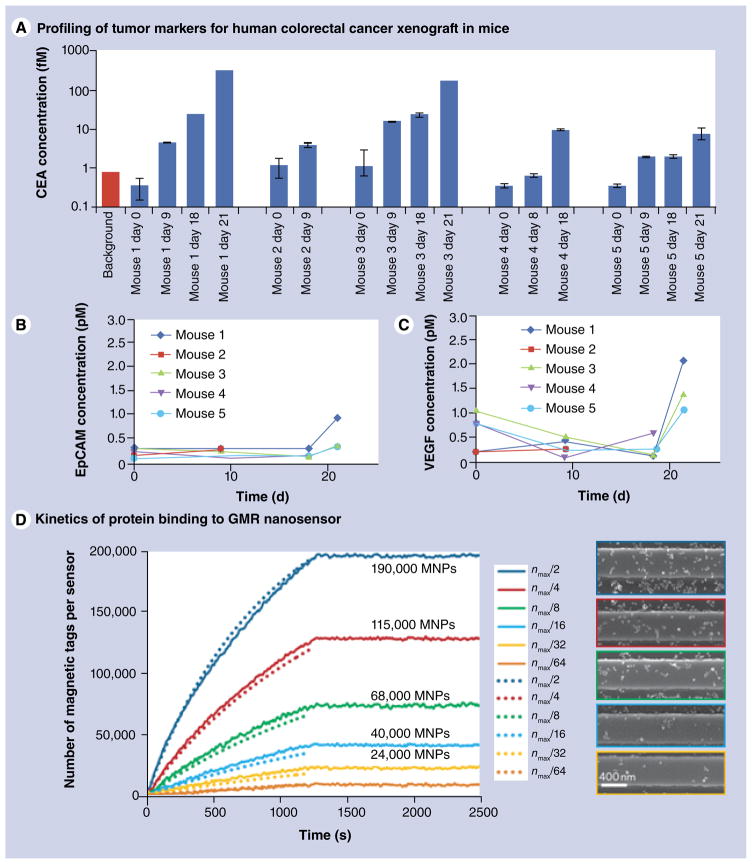
Osterfeld et al. utilized a GMR sensor for detecting magnetically labeled cancer markers. Magnetic particles with a 50-nm diameter were used as magnetic tags [60]. A spin-valve type of a GMR sensor was functionalized with antibodies enabling specific binding of the analyte molecules. The magnetic tags were attached to the analyte through biotin–streptavidin interaction. The results shown in FIGURE 7 demonstrate that this method allows for multiplexed detection of different types of proteins. The same GMR nanosensor has also been employed for correlating the amount of expressed tumor markers with the tumor growth [66]. Human colorectal adenocarcinoma was xenografted in atymic nude mice and the concentration of blood tumor markers, namely carcinoembryonic antigen (CEA), VEGF and epithelial cell adhesion molecule (EpCAM), was measured using the GMR assay. The results shown in FIGURE 8A–C demonstrate that the amount of CEA expressed correlates with tumor progression, whereas the concentration of EpCAM and VEGF does not depend on the tumor growth stage. Moreover, the variations of femtomolar level concentrations of CEA detected with the GMR sensor are well below the picomolar limit of detection of ELISA, which is currently a standard technique for protein analysis. This technique has been further improved by eliminating the need for washing the non-specifically attached proteins by employing the autoassembly of macromolecular complexes [67]. With this modification, the feasibility of using the GMR nanosensor for monitoring protein binding kinetics was demonstrated [68]. Adapting the two-compartment model of a ligand surface binding in solution to the GMR nanosensors, the authors showed that the number of magnetic tags attached to the sensor surface and, therefore, the number of tag-associated protein molecules, can be found by fitting the experimental results.
Figure 7. GMR-based sensing of cancer biomarkers.
(A) Principle of giant magneto-resistance-based sensing with streptavidin-coated magnetic nanotags. (B) Multianalyte protein assay using the GMR sensor functionalized with antibodies specific to the measured proteins. The sample volume was 20 μl at 1 pg/ml concentration.
BSA: Bovine serum albumin; CEA: Carcinoembryonic antigen.
Reproduced with permission from [60].
Figure 8. Applications of GMR-magnetic nanotag sensors.
(A–C) Applications of GMR-magnetic nanotag sensor for profiling of the human colorectral adenocarcinoma xenografted in mouse model biomarkers – CEA, VEGF and EpCAM. Time-dependent biomarker concentration in animal blood was measured daily. The results show that CEA expression level correlates with tumor progression. (D) Application of GMR sensor for studying the kinetics of protein–magnetic tag complexes binding to the antigen immobilized on the sensor surface. The information about the absolute number of magnetic tags bound to the surface is extracted from experimental data (solid lines) using the theoretical model (dotted lines). Scanning electron micrographs of the sensor loaded with different amounts of magnetic tags, corresponding to the experimental data are shown. EpCAM: Epithelial cell adhesion molecule; CEA: Carcinoembryonic antigen; GMR: Giant magneto-resistance; MNP: Magnetic nanoparticle.
Cell sorting in microfluidic channels
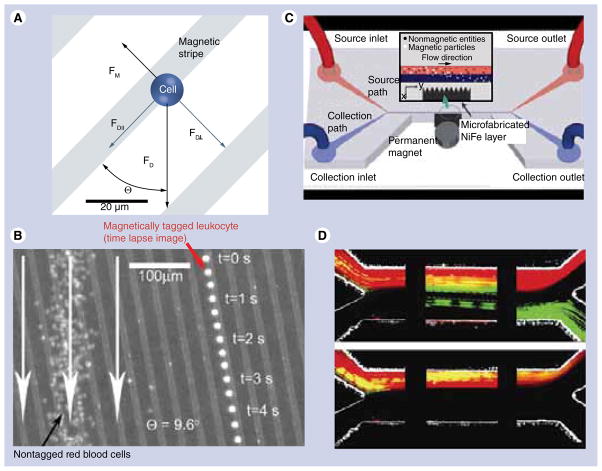
Microfluidics mainly relies on the devices produced by microfabrication techniques. For biological applications, microfluidics is largely used for counting, sorting, separating and mixing various materials [69–74]. While magnetically assisted cell sorting can be achieved simply by attaching magnetic particles to the cells and applying a permanent magnet, this technique is greatly enhanced in combination with microfluidics. In 1997, Duke and Austin proposed a method for 2D ‘sieving’ of macromolecules, which was later employed for cell sorting [70,72,75]. Analyte cell fractionation was achieved by combining the hydrodynamic forces with the magnetic field gradients created by lithographically deposited Co-Cr-Ta wires. FIGURE 9A–B illustrates the principle of this method and demonstrates the application of the magnetic sieve for trapping of magnetically tagged leukocytes [72]. Xia et al. later showed a modified version of this device, also known as high-gradient magnetic field concentrator, where the ferromagnetic Ni80Fe20 comb was placed outside the microfluidic channel [76].
Figure 9. Cell sorting in magnetic microfluidic chips.
(A) Principle of cell separation by combining the hydrodynamic force with magnetic field gradient provided by ferromagnetic nickel stripes. The cells are coupled with superparamagnetic iron oxide beads to enable magnetically assisted sorting. (B) Overlay of optical reflection image of the microfluidic magnetic chip and the fluorescent images of a single magnetically labeled leukocyte (right) and a large number of free-flowing red blood cells (left). The image demonstrates that the magnetically tagged cell is trapped between magnetic stripes and it follows the defined path in contrast with untagged cells. (C) Schematic diagram of the cell sorting device with microfluidic channel and microfabricated permalloy comb separated spatially for improved biocompatibility. (D) Separation of green fluorescent magnetic beads and red blood cells labeled with a red fluorescent dye SYTO 64. The snapshots represent the distribution of cells/beads mixture as it enters the channel (left), proceeds to the middle (middle) and separates at the end of the channel (right). The images were acquired with (top) and without (bottom) the permanent magnet, which is used for magnetizing the permalloy comb. In the absence of the external magnetizing field no separation is observed (bottom).
(A & B) Reproduced with permission from [72].
(C & D) Reproduced with permission from [76].
Another type of magnetic particle, which is likely to find novel applications in combination with microfluidics, is nanowires. The method for templated growth of metal nanowires has been known for over four decades. In 1970, Possin employed etched mica as a template for electro-deposition of 40 nm, 15-μm long wires of zinc, indium and tin [74–77]. Using anodized aluminum membranes, Kawai and Ueda prepared Co and Co–Ni nanowires [78]. In their paper, they pointed out that these structures are very promising for vertical magnetic recording media. More recently, a method for fabricating a 20-nm diameter permalloy nanowire was proposed [79,80]. A 4-nm wide InAs modulation doped quantum well grown by molecular beam epitaxy, was used as a template for electrodepositing a single permalloy wire. An interesting approach combining carbon nanotubes, which could also be viewed as wires in the context of our discussion, with superparamagnetic particles was shown by Korneva et al. [81]. The nanotubes, prepared by chemical vapor deposition in anodized aluminum membranes, were filled with magnetite particles by capillary action and the resulting magnetic nanotubes were successfully manipulated by an external magnetic field in solution. A detailed study about the mechanisms of filling carbon nanotubes with metal particles can be found in [82].
Overall, magnetic nanowires synthesized using aluminum templates have so far been more actively used compared with the substrate-bound structures, which are more suitable for sensors.
In addition to ‘monomaterial’ anisotropic particles, multisegmented magnetic nanowires have also been a subject of active research. Kline et al. proposed a method for controlling the movement of catalytic nanomotors based on striped Pt–Ni–Au–Ni–Au nanorods [83]. In a static magnetic field, the rods orient orthogonally to the field due to the presence of Ni segments, which are shorter than the diameter of the rod. Transverse rod magnetization results in its preferential movement with the platinum end forward. This approach potentially has a large number of applications for targeted bio-sensing and magnetic separation, especially in conjunction with microfluidics. Furthermore, significant work on multisegemented nanowires as nanomotors for biological cargo delivery has been performed by the group of Wang at the University of California San Diego (CA, USA) [84].
Apart from applications involving manipulation of magnetic wires by external field only, there are reports on using microfabricated permalloy (Ni79Fe21) and Ni structures, which were utilized for trapping Ni, Pt–Ni–Pt and Au–Ni nanowires, and controlled assembly of suspended fibroblast cells bound to these wires [85–87]. Nanowires with an average diameter of 175 nm were synthesized using anodized aluminum membranes as templates for electrodeposition. Magnetic ellipses were fabricated on glass substrates by first depositing a 400-nm thick layer of permalloy, which was then photolithographically patterned and etched with 10% wt. nitric acid [86]. Using different masks, the distance between the permalloy magnets was varied and it was demonstrated that a higher cell trapping efficiency can be achieved with smaller inter-magnet pitch.
Future perspective
Magnetic nanostructures have proven to be highly promising for future biomedical applications. As seen from the example of synthetic ferromagnetic particles, the progress in fabrication of novel biologically relevant magnetic particles can benefit from the advances in the seemingly distant field of microelectronics. Giant magnetoresistance biological sensors represent another equally important example that emerged due to interdisciplinary connections. It is therefore reasonable to expect that the new advancements in magnetism-based nanomedicine are likely to owe their development to the latest technological advances in the fields of micro- and nano-fabrication. The nanoimprint and photolithography discussed in this paper are only a part of fabrication techniques which are available. We believe that hybrid lithographic structures might move to the forefront for magnetic nanomedicine in the near future. The highly promising methods, which are yet to be applied for creating magnetic structures, include molecular-ruler technique, nanotransfer printing and nanoskiving [88–90]. Ferromagnetic lithographically defined disks discussed in this work represent a very potent type of magnetic carriers for biological applications such as hyperthermia, MRI, drug release and even the induction of cancer cell apoptosis. Scaling down the dimensions of the disks to sub-100 nm diameter will open new opportunities for in vivo applications.
Executive summary.
-
The studies employing microfabricated magnetic particles in biology and medicine are still in their early stages and this review is one of the first attempts to provide a systematic outlook at this emerging field. The key applications demonstrated to date include:
Probing mechanical properties of living cells using magnetic tweezers and microfabricated structures enabling application of controlled forces to the cells.
Inducing apoptosis in brain cancer cells with lithographically defined ferromagnetic disks. This type of particle has also shown the promising properties for hyperthermia, controlled drug delivery and release and MRI.
High sensitivity molecular detection enabled by synthetic antiferromagnetic particles. Giant magnetoresistance-based sensors are being actively explored for simultaneous detection of multiple low concentration analytes such as toxins and cancer biomarkers.
Cell sorting in microfluidic channels enabled by conjugating cells with superparamagnetic particles, which can be manipulated by a magnetic field. The techniques employing lithographically fabricated magnetic structures to direct the flow of magnetically tagged cells have been devised.
Acknowledgments
The authors thank their collaborators SD Bader, T Rajh, VG Yefremenko, R Divan and Mr J Pearson from Argonne National Laboratory, as well as members of MS Lesniak’s and E Cohen’s groups from the University of Chicago.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Disclaimer
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Financial & competing interests disclosure
The work at Argonne, including use of the Center for Nanoscale Materials, was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357, and in part by Grant Number R01NS077388 from the National Institute of Neurological Disorders And Stroke. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
of considerable interest
- 1.Caruthers SD, Wickline SA, Lanza GM. Nanotechnological applications in medicine. Current Opin Biotechnol. 2007;18(1):26–30. doi: 10.1016/j.copbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Kim J, Park S, Lee JE, et al. Designed fabrication of multifunctional magnetic gold nanoshells and their application to magnetic resonance imaging and photothermal therapy. Angew Chem Int Ed Engl. 2006;45(46):7754–7758. doi: 10.1002/anie.200602471. [DOI] [PubMed] [Google Scholar]
- 3▪ ▪.Rozhkova EA. Nanoscale materials for tackling brain cancer: recent progress and outlook. Adv Mater. 2011;23(24):H136–H150. doi: 10.1002/adma.201004714. Reviews different types of nanoscale materials, including magnetic particles, used in medical diagnostics and therapy. [DOI] [PubMed] [Google Scholar]
- 4.Hook AL, Voelcker NH, Thissen H. Patterned and switchable surfaces for biomolecular manipulation. Acta Biomaterialia. 2009;5(7):2350–2370. doi: 10.1016/j.actbio.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 5.Pankhurst QA, Thanh NTK, Jones SK, Dobson J. Progress in applications of magnetic nanoparticles in biomedicine. J Phys D: Appl Phys. 2009;42:224001. [Google Scholar]
- 6.Tartaj P, Morales MD, Veintemillas-Verdaguer S, Gonzalez-Carreno T, Serna CJ. The preparation of magnetic nanoparticles for applications in biomedicine. J Phys D: Appl Phys. 2003;36(13):R182–R197. [Google Scholar]
- 7.Berry CC, Curtis ASG. Functionalisation of magnetic nanoparticles for applications in biomedicine. J Phys D: Appl Phys. 2003;36(13):R198–R206. [Google Scholar]
- 8.Mornet S, Vasseur S, Grasset F, Duguet E. Magnetic nanoparticle design for medical diagnosis and therapy. J Mater Chem. 2004;14(14):2161–2175. [Google Scholar]
- 9.Dobson J. Magnetic nanoparticles for drug delivery. Drug Dev Res. 2006;67(1):55–60. [Google Scholar]
- 10▪ ▪.Dobson J. Remote control of cellular behaviour with magnetic nanoparticles. Nat Nanotechnol. 2008;3(3):139–143. doi: 10.1038/nnano.2008.39. Summarizes the methods for modulating cell function using magnetic nanoparticles. [DOI] [PubMed] [Google Scholar]
- 11.Cheng FY, Su CH, Yang YS, et al. Characterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applications. Biomaterials. 2005;26(7):729–738. doi: 10.1016/j.biomaterials.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Babes L, Denizot B, Tanguy G, Le Jeune JJ, Jallet P. Synthesis of iron oxide nanoparticles used as MRI contrast agents: a parametric study. J Colloid Interface Sci. 1999;212(2):474–482. doi: 10.1006/jcis.1998.6053. [DOI] [PubMed] [Google Scholar]
- 13.Kim JE, Shin JY, Cho MH. Magnetic nanoparticles: an update of application for drug delivery and possible toxic effects. Archives Toxicol. 2012;86(5):685–700. doi: 10.1007/s00204-011-0773-3. [DOI] [PubMed] [Google Scholar]
- 14▪ ▪.Hyeon T, Lee SS, Park J, Chung Y, Bin Na H. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J Am Chem Soc. 2001;123(51):12798–12801. doi: 10.1021/ja016812s. This is a seminal work on one-step wet chemistry synthesis of superparamagnetic iron oxide nanoparticles with tightly controlled size distribution. [DOI] [PubMed] [Google Scholar]
- 15.Sun SH, Zeng H, Robinson DB, et al. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J Am Chem Soc. 2004;126(1):273–279. doi: 10.1021/ja0380852. [DOI] [PubMed] [Google Scholar]
- 16.Hergt R, Andra W, d’Ambly CG, et al. Physical limits of hyperthermia using magnetite fine particles. IEEE Trans Magn. 1998;34(5):3745–3754. [Google Scholar]
- 17.Bangar MA, Hangarter CM, Yoo B, et al. Magnetically assembled multisegmented nanowires and their applications. Electroanalysis. 2009;21(1):61–67. [Google Scholar]
- 18.Fert A, Piraux L. Magnetic nanowires. J Mag Mag Mater. 1999;200(1–3):338–358. [Google Scholar]
- 19.Love JC, Urbach AR, Prentiss MG, Whitesides GM. Three-dimensional self-assembly of metallic rods with submicron diameters using magnetic interactions. J Am Chem Soc. 2003;125(42):12696–12697. doi: 10.1021/ja037642h. [DOI] [PubMed] [Google Scholar]
- 20.Rades S, Kornowski A, Weller H, Albert B. Wet-chemical synthesis of nanoscale iron boride, XAFS analysis and crystallisation to α-FeB. Chemphyschem. 2011;12:1756–1760. doi: 10.1002/cphc.201001072. [DOI] [PubMed] [Google Scholar]
- 21.Gay JG, Richter R. Spin anisotropy of ferromagnetic films. Phys Rev Letters. 1986;56(25):2728–2731. doi: 10.1103/PhysRevLett.56.2728. [DOI] [PubMed] [Google Scholar]
- 22.Parkin SSP, More N, Roche KP. Oscillations in exchange coupling and magnetoresistance in metallic superlattive structures – Co/Ru, Co/Cr, and Fe/Cr. Phys Rev Letters. 1990;64(19):2304–2307. doi: 10.1103/PhysRevLett.64.2304. [DOI] [PubMed] [Google Scholar]
- 23.Illi B, Scopece A, Nanni S, et al. Epigenetic histone modification and cardiovascular lineage programming in mouse embryonic stem cells exposed to laminar shear stress. Circulation Res. 2005;96(5):501–508. doi: 10.1161/01.RES.0000159181.06379.63. [DOI] [PubMed] [Google Scholar]
- 24.Stolberg S, McCloskey KE. Can shear stress direct stem cell fate? Biotechnol Prog. 2009;25(1):10–19. doi: 10.1002/btpr.124. [DOI] [PubMed] [Google Scholar]
- 25.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 26.Heilbronn A. A new method for the estimation of viscosity in living protoplasts. Jahrb Wiss Bot. 1922;61:284–338. [Google Scholar]
- 27.Seifriz W. An elastic value of protoplasm, with further observations on the viscosity of protoplasm. J Exper Biol. 1924;2:1–11. [Google Scholar]
- 28.Crick FHC, Hughes AFW. The physical properties of cytoplasm – a study by means of the magnetic particle method. Exp Cell Res. 1950;1:37–80. [Google Scholar]
- 29.Hiramoto Y. Mechanical properties of the protoplasm of the sea urchin egg. I Unfertilized egg. Exp Cell Res. 1969;56(2):201–208. doi: 10.1016/0014-4827(69)90003-2. [DOI] [PubMed] [Google Scholar]
- 30.Gehr P, Brain JD, Bloom SB, Valberg PA. Magnetic particles in the liver – a probe for intracellular movement. Nature. 1983;302(5906):336–338. doi: 10.1038/302336a0. [DOI] [PubMed] [Google Scholar]
- 31.de Vries AHB, Krenn BE, van Driel R, Subramaniam V, Kanger JS. Direct observation of nanomechanical properties of chromatin in living cells. Nano Letters. 2007;7(5):1424–1427. doi: 10.1021/nl070603+. [DOI] [PubMed] [Google Scholar]
- 32.Sniadecki NJ, Anguelouch A, Yang MT, et al. Magnetic microposts as an approach to apply forces to living cells. Proc Natl Acad Sci USA. 2007;104(37):14553–14558. doi: 10.1073/pnas.0611613104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin YC, Kramer CM, Chen CS, Reich DH. Probing cellular traction forces with magnetic nanowires and microfabricated force sensor arrays. Nanotechnology. 2012;23(7):8. doi: 10.1088/0957-4484/23/7/075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34▪ ▪.Kim D-H, Rozhkova EA, Ulasov IV, et al. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nat Mater. 2009;9(2):165–171. doi: 10.1038/nmat2591. Reports on the unique application of microfabricated ferromagnetic particles for inducing apoptosis in cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rozhkova EA, Novosad V, Kim DH, et al. Ferromagnetic microdisks as carriers for biomedical applications. J Appl Physics. 2009;105:07B306. [Google Scholar]
- 36.Kerr JF. In: Apoptosis: The Molecular Basis of Cell Death. Tomei LD, Cope FO, editors. Cold Spring Harbor Laboratory Press; NY, USA: 1991. [Google Scholar]
- 37.Vitol EA, Yefremenko VG, Jain S, et al. Optical transmission modulation by disk-shaped ferromagnetic particles. J Appl Physics. 2012;111:07A945. [Google Scholar]
- 38.Kim D-H, Karavayev P, Rozhkova EA, et al. Mechanoresponsive system based on sub-micron chitosan-functionalized ferromagnetic disks. J Mater Chem. 2011;21(23):8422–8426. [Google Scholar]
- 39.Sigurdson W, Ruknudin A, Sachs F. Calcium imaging of mechanically induced fluxes in tissue-cultured chick heart – role of stretch-activated ion channels. Am J Physiol. 1992;262(4):H1110–H1115. doi: 10.1152/ajpheart.1992.262.4.H1110. [DOI] [PubMed] [Google Scholar]
- 40.Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci. 2004;117(12):2449–2460. doi: 10.1242/jcs.01232. [DOI] [PubMed] [Google Scholar]
- 41.Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 42.Vitol EA, Novosad V, Rozhkova EA. Multifunctional ferromagnetic disks for modulating cell function. IEEE Trans Magn. 2012 doi: 10.1109/tmag.2012.2198209. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ingber DE. Cellular tensegrity – defining new rules of biological design that govern the cytoskeleton. J Cell Sci. 1993;104:613–627. doi: 10.1242/jcs.104.3.613. [DOI] [PubMed] [Google Scholar]
- 44.Scholz W, Guslienko KY, Novosad V, et al. Transition from single-domain to vortex state in soft magnetic cylindrical nanodots. J Mag Mag Mater. 2003;266(1–2):155–163. [Google Scholar]
- 45.Koh AL, Hu W, Wilson RJ, Earhart CM, Wang SX, Sinclair R. Structural and magnetic characterizations of high moment synthetic antiferromagnetic nanoparticles fabricated using self-assembled stamps. J Appl Physics. 2010;107(9):9B522. doi: 10.1063/1.3358067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moritz J, Dieny B, Nozieres JP, Landis S, Lebib A, Chen Y. Domain structure in magnetic dots prepared by nanoimprint and e-beam lithography. J Appl Physics. 2002;91(10):7314–7316. [Google Scholar]
- 47.Liu K, Nogues J, Leighton C, et al. Fabrication and thermal stability of arrays of Fe nanodots. Appl Physics Letters. 2002;81(23):4434–4436. [Google Scholar]
- 48.Engel BN, Akerman J, Butcher B, et al. A 4-mb toggle MRAM based on a novel bit and switching method. IEEE Trans Mag. 2005;41(1):132–136. [Google Scholar]
- 49▪ ▪.Hu W, Wilson CRJ, Koh A, et al. High-moment antiferromagnetic nanoparticles with tunable magnetic properties. Adv Mater. 2008;20(8):1479–1483. Presents the first synthetic antiferromagnetic nanoparticles fabricated by the top-down approach that have shown a large potential for biological applications. [Google Scholar]
- 50.Hu W, Wilson RJ, Earhart CM, Koh AL, Sinclair R, Wang SX. Synthetic antiferromagnetic nanoparticles with tunable susceptibilities. J Appl Physics. 2009;105(7):7B508. doi: 10.1063/1.3072028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joisten H, Courcier T, Balint P, et al. Self-polarization phenomenon and control of dispersion of synthetic antiferromagnetic nanoparticles for biological applications. Appl Physics Letters. 2010;97(25):253112. [Google Scholar]
- 52.Courcier T, Joisten H, Sabon P, et al. Tumbling motion yielding fast displacements of synthetic antiferromagnetic nanoparticles for biological applications. Appl Physics Letters. 2011;99(9):093107. [Google Scholar]
- 53.Wi J-S, Barnard ES, Wilson RJ, et al. Sombrero-shaped plasmonic nanoparticles with molecular-level sensitivity and multifunctionality. ACS Nano. 2011;5(8):6449–6457. doi: 10.1021/nn201649n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong B, Hayward TJ, Jeong JR, et al. Design and fabrication of SU8 encapsulated digital magnetic carriers for high throughput biological assays. J Appl Physics. 2009;105(3):6. [Google Scholar]
- 55.Hong B, Jeong JR, Llandro J, et al. High throughput biological analysis using multi-bit magnetic digital planar tags. AIP Conf Proc. 2008;1025:74–81. [Google Scholar]
- 56.Tsymbal EY, Pettifor DG. Perspectives of giant magnetoresistance. Solid State Physics. 2001;56:113–237. [Google Scholar]
- 57.Binasch G, Grunberg P, Saurenbach F, Zinn W. Enhanced magnetoresistance in layered magnetic-structures with antiferromagnetic interlayer exchange. Phys Rev B. 1989;39(7):4828–4830. doi: 10.1103/physrevb.39.4828. [DOI] [PubMed] [Google Scholar]
- 58.Baibich MN, Broto JM, Fert A, et al. Giant magnetoresistance of (001)Fe/(001) Cr magnetic superlattices. Phys Rev Letters. 1988;61(21):2472–2475. doi: 10.1103/PhysRevLett.61.2472. [DOI] [PubMed] [Google Scholar]
- 59.Baselt DR, Lee GU, Natesan M, Metzger SW, Sheehan PE, Colton RJ. A biosensor based on magnetoresistance technology. Biosensors Bioelectronics. 1998;13(7–8):731–739. doi: 10.1016/s0956-5663(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 60▪ ▪.Osterfeld SJ, Yu H, Gaster RS, et al. Multiplex protein assays based on real-time magnetic nanotag sensing. Proc Natl Acad Sci USA. 2008;105(52):20637–20640. doi: 10.1073/pnas.0810822105. A key work describing the approach for multiplex protein detection using a giant magnetoresistance-based sensor. References [58–60] report on more recent advances of this method by the same group of scientists. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li GX, Joshi V, White RL, et al. Detection of single micron-sized magnetic bead and magnetic nanoparticles using spin valve sensors for biological applications. J Appl Physics. 2003;93(10):7557–7559. [Google Scholar]
- 62.Graham DL, Ferreira H, Bernardo J, Freitas PP, Cabral JMS. Single magnetic microsphere placement and detection on-chip using current line designs with integrated spin valve sensors: biotechnological applications. J Appl Physics. 2002;91(10):7786–7788. [Google Scholar]
- 63.Mak AC, Osterfeld SJ, Yu H, et al. Sensitive giant magnetoresistive-based immunoassay for multiplex mycotoxin detection. Biosensors Bioelectronics. 2009;25(7):1635–1639. doi: 10.1016/j.bios.2009.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schotter J, Kamp PB, Becker A, Puhler A, Reiss G, Bruckl H. Comparison of a prototype magnetoresistive biosensor to standard fluorescent DNA detection. Biosensors Bioelectronics. 2004;19(10):1149–1156. doi: 10.1016/j.bios.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Kennedy J, Leveneur J, Williams GVM, Mitchell DRG, Markwitz A. Fabrication of surface magnetic nanoclusters using low energy ion implantation and electron beam annealing. Nanotechnology. 2011;22(11):6. doi: 10.1088/0957-4484/22/11/115602. [DOI] [PubMed] [Google Scholar]
- 66.Gaster RS, Hall DA, Nielsen CH, et al. Matrix-insensitive protein assays push the limits of biosensors in medicine. Nat Med. 2009;15(11):1327–1332. doi: 10.1038/nm.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaster RS, Hall DA, Wang SX. Autoassembly protein arrays for analyzing antibody cross-reactivity. Nano Letters. 2011;11(7):2579–2583. doi: 10.1021/nl1026056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gaster RS, Xu L, Han S-J, et al. Quantification of protein interactions and solution transport using high-density GMR sensor arrays. Nat Nanotechnol. 2011;6(5):314–320. doi: 10.1038/nnano.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yi CQ, Li CW, Ji SL, Yang MS. Microfluidics technology for manipulation and analysis of biological cells. Analytica Chimica Acta. 2006;560(1–2):1–23. doi: 10.1016/j.aca.2018.06.054. [DOI] [PubMed] [Google Scholar]
- 70.Berger M, Castelino J, Huang R, Shah M, Austin RH. Design of a microfabricated magnetic cell separator. Electrophoresis. 2001;22(18):3883–3892. doi: 10.1002/1522-2683(200110)22:18<3883::AID-ELPS3883>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 71.Burdick J, Laocharoensuk R, Wheat PM, Posner JD, Wang J. Synthetic nanomotors in microchannel networks: Directional microchip motion and controlled manipulation of cargo. J Am Chem Soc. 2008;130(26):8164–8165. doi: 10.1021/ja803529u. [DOI] [PubMed] [Google Scholar]
- 72.Inglis DW, Riehn R, Austin RH, Sturm JC. Continuous microfluidic immunomagnetic cell separation. Appl Physics Letters. 2004;85(21):5093–5095. [Google Scholar]
- 73.Johansson L, Gunnarsson K, Bijelovic S, et al. A magnetic microchip for controlled transport of attomole levels of proteins. Lab Chip. 2009;10(5):654–661. doi: 10.1039/b919893h. [DOI] [PubMed] [Google Scholar]
- 74.Liu RH, Yang JN, Lenigk R, Bonanno J, Grodzinski P. Self-contained, fully integrated biochip for sample preparation, polymerase chain reaction amplification, and DNA microarray detection. Anal Chem. 2004;76(7):1824–1831. doi: 10.1021/ac0353029. [DOI] [PubMed] [Google Scholar]
- 75.Duke TAJ, Austin RH. Microfabricated sieve for the continuous sorting of macromolecules. Phys Rev Letters. 1998;80(7):1552–1555. [Google Scholar]
- 76.Xia N, Hunt TP, Mayers BT, et al. Combined microfluidic-micromagnetic separation of living cells in continuous flow. Biomedical Microdevices. 2006;8(4):299–308. doi: 10.1007/s10544-006-0033-0. [DOI] [PubMed] [Google Scholar]
- 77.Possin GE. A method for forming very small diameter wires. Rev Sci Instrum. 1970;41:772. [Google Scholar]
- 78.Kawai S, Ueda R. Magnetic properties of anodic oxide coatings on aluminum containing electrodeposited Co and Co-Ni. J Electrochem Soc. 1975;122(1):32–36. [Google Scholar]
- 79.Fasol G. Applied physics – nanowires: small is beautiful. Science. 1998;80(5363):545–546. [Google Scholar]
- 80.Fasol G. Runge Selective electrodeposition of nanometer scale magnetic wires. Appl Physics Letters. 1997;70(18):2467–2468. [Google Scholar]
- 81.Korneva G, Ye HH, Gogotsi Y, et al. Carbon nanotubes loaded with magnetic particles. Nano Letters. 2005;5(5):879–884. doi: 10.1021/nl0502928. [DOI] [PubMed] [Google Scholar]
- 82.Guerret-Piecourt C, Lebouar Y, Loiseau A, Pascard H. Relation between metal electronic structure and morphology of metal compounds inside carbon nanotubes. Nature. 1994;372(6508):761–765. [Google Scholar]
- 83.Kline TR, Paxton WF, Mallouk TE, Sen A. Catalytic nanomotors: remote-controlled autonomous movement of striped metallic nanorods. Angew Chem Int Ed Engl. 2005;44(5):744–746. doi: 10.1002/anie.200461890. [DOI] [PubMed] [Google Scholar]
- 84.Wang J. Biomolecule-functionalized nanowires: from nanosensors to nanocarriers. Chemphyschem. 2009;10(11):1748–1755. doi: 10.1002/cphc.200900377. [DOI] [PubMed] [Google Scholar]
- 85.Reich DH, Tanase M, Hultgren A, Bauer LA, Chen CS, Meyer GJ. Biological applications of multifunctional magnetic nanowires (invited) J Appl Physics. 2003;93(10):7275–7280. [Google Scholar]
- 86.Tanase M, Felton EJ, Gray DS, Hultgren A, Chen CS, Reich DH. Assembly of multicellular constructs and microarrays of cells using magnetic nanowires. Lab on a Chip. 2005;5(6):598–605. doi: 10.1039/b500243e. [DOI] [PubMed] [Google Scholar]
- 87.Tanase M, Silevitch DM, Hultgren A, et al. Magnetic trapping and self-assembly of multicomponent nanowires. J Appl Physics. 2002;91(10):8549–8551. [Google Scholar]
- 88▪ ▪.Saavedra HM, Mullen TJ, Zhang P, Dewey DC, Claridge SA, Weiss PS. Hybrid strategies in nanolithography. Rep Progress Physics. 2010;73:036501. his work would be of significant interest for readers looking to learn advanced fabrication methods for both magnetic and non-magnetic structures. [Google Scholar]
- 89.Luttge R. Massively parallel fabrication of repetitive nanostructures: nanolithography for nanoarrays. J Phys D: Appl Phys. 2009;42(12):18. [Google Scholar]
- 90.Xu Q, Rioux RM, Whitesides GM. Fabrication of complex metallic nanostructures by nanoskiving. ACS Nano. 2007;1(3):215–227. doi: 10.1021/nn700172c. [DOI] [PubMed] [Google Scholar]
Patent
- 101.Weller H, Niehaus J. Reactor for the manufacture of nanoparticles. 2011/0042611. US. 2011:A1.
Websites
- 201.National Nanotechnology Initiative Strategic Plan. www.whitehouse.gov/sites/default/files/microsites/ostp/nni_strategic_plan_2011.pdf.
- 202.European initiative for sustainable development by nanotechnologies. www.nanofutures.eu.