Abstract
Purpose
To compare ultra-widefield fluorescein angiography imaging using the Optos® Optomap® and the Heidelberg Spectralis® noncontact ultra-widefield module.
Methods
Five patients (ten eyes) underwent ultra-widefield fluorescein angiography using the Optos® panoramic P200Tx imaging system and the noncontact ultra-widefield module in the Heidelberg Spectralis® HRA+OCT system. The images were obtained as a single, nonsteered shot centered on the macula. The area of imaged retina was outlined and quantified using Adobe® Photoshop® C5 software. The total area and area within each of four visualized quadrants was calculated and compared between the two imaging modalities. Three masked reviewers also evaluated each quadrant per eye (40 total quadrants) to determine which modality imaged the retinal vasculature most peripherally.
Results
Optos® imaging captured a total retinal area averaging 151,362 pixels, ranging from 116,998 to 205,833 pixels, while the area captured using the Heidelberg Spectralis® was 101,786 pixels, ranging from 73,424 to 116,319 (P = 0.0002). The average area per individual quadrant imaged by Optos® versus the Heidelberg Spectralis® superiorly was 32,373 vs 32,789 pixels, respectively (P = 0.91), inferiorly was 24,665 vs 26,117 pixels, respectively (P = 0.71), temporally was 47,948 vs 20,645 pixels, respectively (P = 0.0001), and nasally was 46,374 vs 22,234 pixels, respectively (P = 0.0001). The Heidelberg Spectralis® was able to image the superior and inferior retinal vasculature to a more distal point than was the Optos®, in nine of ten eyes (18 of 20 quadrants). The Optos® was able to image the nasal and temporal retinal vasculature to a more distal point than was the Heidelberg Spectralis®, in ten of ten eyes (20 of 20 quadrants).
Conclusion
The ultra-widefield fluorescein angiography obtained with the Optos® and Heidelberg Spectralis® ultra-widefield imaging systems are both excellent modalities that provide views of the peripheral retina. On a single nonsteered image, the Optos® Optomap® covered a significantly larger total retinal surface area, with greater image variability, than did the Heidelberg Spectralis® ultra-widefield module. The Optos® captured an appreciably wider view of the retina temporally and nasally, albeit with peripheral distortion, while the ultra-widefield Heidelberg Spectralis® module was able to image the superior and inferior retinal vasculature more peripherally. The clinical significance of these findings as well as the area imaged on steered montaged images remains to be determined.
Keywords: peripheral, retina, wide-angle, widefield, ultra-widefield
Introduction
As the site of considerable pathology, visualization of the peripheral retina has become essential to the screening, diagnosis, monitoring, and treatment of many vision-threatening eye diseases, including diabetic retinopathy.
The first commercially available fundus camera was produced in 1926 by the Carl Zeiss Company and provided a 20-degree field of view of the retina.1 Later, 30-degrees became the “standard” field of view. As fundus cameras with up to 50-degrees field of view emerged, they became widely used in clinical practice. Consequently, imaging angles larger than 30- or 50-degrees have been referred to as “wide-field” or “wide-angle.” More recently, the term “ultra-widefield” fundus imaging has gained popularity, although the exact definition and area of the retina imaged remains ambiguous.
Several imaging platforms have been developed that provide a widefield view of the retina. These include the Pomerantzeff camera,2 the Retcam (Clarity Medical Systems, Inc, Pleasanton, CA, USA), the Panoret-1000™ camera (Medibell Medical Vision Technologies, Haifa, Israel), the Optos® camera (Optos PLC, Dunfermline, UK), the Staurenghi lens (Ocular Staurenghi 230 SLO Retina Lens; Ocular Instruments Inc, Bellevue, WA, USA),3 and other wide-angle contact lenses,4–6 among others. The single-shot, nonsteered widefield imaging capabilities of these modalities range from 20 degrees up to 200 degrees, albeit with variable image quality.
Ultra-widefield imaging has been shown to be valuable for the evaluation of several retinal pathologies,7 including diabetes,8,9 retinal vein occlusions,10 choroidal masses,11,12 uveitis,13 retinal vasculitis,14 retinal detachment,12,15 and retinopathy of prematurity,16 among others.
One of the most widely used commercially available ultra-widefield systems is the Optos® noncontact camera. The Optos® system utilizes an ellipsoid mirror to produce images with approximately 200 internal degrees of view, providing an image of more than 80% of the retina in a single shot.17 This allows for simultaneous evaluation of the peripheral and central retina without the requirement of significant eye steering. Despite its ability to produce dynamic widefield fundus images, the Optos® Optomap® has several limitations. Specifically, its imaging of the far superior and inferior peripheral retina is less complete compared with its imaging of the temporal and nasal retina.18 Moreover, there is noteworthy distortion and decreased resolution of the far temporal and nasal peripheral retina. A method for correcting for this peripheral distortion, when evaluating retinal area and making measurements, has been described.19
More recently, Heidelberg Engineering (Heidelberg, Germany) has developed a noncontact ultra-widefield angiography module for the Spectralis® and Heidelberg Retina Angiograph (HRA 2). Previously, the Heidelberg Spectralis® provided a 25- and 35-degree field of view of the retina, with the possibility of a 55-degree noncontact lens attachment, to produce autofluorescence, fluorescein, and indocyanine green angiographic images. In addition, there was the capability of using the Staurenghi contact lens in conjunction with the Spectralis® to provide up to 150-degrees field of view in a single shot. The newly developed Heidelberg ultra-widefield angiography module consists of an interchangeable noncontact lens that attaches to the camera head to provide high-contrast, undistorted and evenly illuminated images out to the peripheral retina. Both fluorescein and indocyanine green angiography, individually or simultaneously, may be performed with this ultra-widefield Heidelberg Spectralis® module.
With the expanded indications for ultra-widefield imaging and the varying definitions of the exact area of retina imaged, comparison of various imaging platforms is becoming increasingly important. The purpose of this study was to report our initial experience with a Heidelberg Spectralis® noncontact, ultra-widefield module and to evaluate the area of the retina imaged on ultra-widefield fluorescein angiography with the Heidelberg Spectralis® compared with the Optos® Optomap®.
Methods
This retrospective, observational study was performed after obtaining Institutional Review Board approval at Weill Cornell Medical College in New York, NY, USA.
Five patients (ten eyes) underwent nonsteered ultra-widefield fluorescein angiography using the Optos® panoramic P200Tx imaging system as well as the ultra-widefield module for the Heidelberg Spectralis® HRA+OCT module. All photographs were centered on the macula.
All the images were subsequently transferred into Adobe® Photoshop® C5 software (Adobe Systems Inc, San Jose, CA, USA) and independently analyzed by two masked, trained readers (MW and GP). Using the software, each image was split into four quadrants centered on the fovea (Figure 1). The area of visible retina in each image was outlined. A free-style line was made, demarcating the area of visible peripheral retina in each image. This area was then highlighted, and its pixels were quantified using the pixel measurement function of the Adobe® Photoshop® C5 software. An additional grader (SK) verified the outline of the imaged retina for all eyes.
Figure 1.
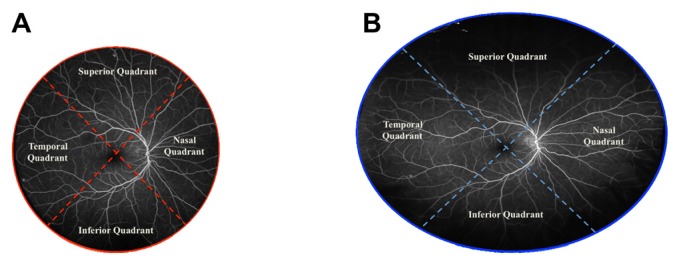
Fluorescein angiogram of the right eye of a patient showing a single-shot, noncontact image centered on the macula. (A) Heidelberg Spectralis® (Heidelberg Engineering, Heidelberg, Germany); (B) Optos® Optomap® (Optos PLC, Dunfermline, UK).
Notes: After outlining the area of the retina imaged (areas included were those with visible retinal and/or choroidal vasculature, while artifacts, including the eyelids and eyelashes, were excluded from the pixel calculation), the photographs were divided into four quadrants, superior, inferior, temporal, and nasal, centered on the macula (dashed lines). The total number of pixels in the image as well as the number of pixels in each quadrant were calculated using Adobe® Photoshop® C5 software (Adobe Systems Inc, San Jose, CA, USA) and were compared between the two modalities.
The total area visualized, as well as the area within each individual quadrant (superior, inferior, nasal, and temporal) (Figure 1), was calculated and compared between the two imaging modalities. Subsequently, the images from each eye in the study were evaluated by three of the authors (MW, GP, SK) to determine which imaging modality was able to image the retinal vasculature at the most distal point within each quadrant.
Statistical analysis
An unpaired t-test was used to compare the means of pixels that were obtained within each quadrant (and in total, per image) using the Heidelberg Spectralis® ultra-widefield lens versus the Optos® Optomap®. A P-value of <0.05 was considered statistically significant.
Results
The Optos® Optomap® imaging captured a total retinal area averaging 151,362 pixels, ranging from 116,998 to 205,833 pixels, while the area captured using the Heidelberg Spectralis® was 101,786 pixels, ranging from 73,424 to 116,319 (P = 0.0002) (Table 1). The average area per individual quadrant imaged by the Optos® Optomap® versus the Heidelberg Spectralis® superiorly was 32,373 versus 32,789, respectively (P = 0.91), inferiorly was 24,665 versus 26,117, respectively (P = 0.71), temporally was 47,948 versus 20,645, respectively (P = 0.0001), and nasally was 46,374 versus 22,234, respectively (P = 0.0001) (Table 2). The Heidelberg Spectralis® was able to image the superior and inferior retinal vasculature to a more distal point than the Optos® Optomap® in nine of ten eyes (18 of 20 quadrants). The Optos® Optomap® was able to image the nasal and temporal retinal vasculature to a more distal point than the Heidelberg Spectralis® in ten of ten eyes (20 of 20 quadrants).
Table 1.
Total area of retina visualized on a single-shot, nonsteered image
| Patient | Eye | Optos® Optomap® | Heidelberg Spectralis® |
|---|---|---|---|
| 1 | OD | 151,611 | 116,319 |
| OS | 164,003 | 101,218 | |
| 2 | OD | 205,833 | 104,443 |
| OS | 194,557 | 102,902 | |
| 3 | OD | 131,730 | 73,424 |
| OS | 123,512 | 103,392 | |
| 4 | OD | 172,662 | 101,995 |
| OS | 133,109 | 105,483 | |
| 5 | OD | 116,998 | 104,485 |
| OS | 119,607 | 104,201 | |
| Average | 151,362* | 101,786* | |
| Standard deviation | 31,850 | 10,820 |
Notes: The total area of the retina visualized was expressed as the number of pixels (and calculated with Adobe® Photoshop® C5 software; Adobe Systems Inc, San Jose, CA, USA) on a single-shot, nonsteered fluorescein angiogram image, using the Optos® Optomap® (Optos PLC, Dunfermline, UK) and Heidelberg Spectralis® (Heidelberg Engineering, Heidelberg, Germany). The Optos® Optomap® captured a significantly greater total retinal area compared with the Heidelberg Spectralis® (151,362 pixels vs 101,786 pixels, respectively;
P < 0.0005).
Abbreviations: OD, right eye; OS, left eye.
Table 2.
Area of visualized retina by quadrant
| Patient | Eye | Superior: Optos | Superior: HB | Inferior: Optos | Inferior: HB | Nasal: Optos | Nasal: HB | Temporal: Optos | Temporal: HB |
|---|---|---|---|---|---|---|---|---|---|
| 1 | OD | 36,210 | 41,781 | 19,603 | 23,815 | 47,594 | 22,856 | 48,204 | 27,867 |
| OS | 37,236 | 33,989 | 26,257 | 23,123 | 43,605 | 13,374 | 56,905 | 30,732 | |
| 2 | OD | 51,452 | 38,582 | 37,957 | 23,812 | 61,566 | 24,474 | 54,858 | 17,575 |
| OS | 44,264 | 37,434 | 35,515 | 23,146 | 54,604 | 24,997 | 60,174 | 17,325 | |
| 3 | OD | 26,314 | 21,765 | 18,075 | 13,637 | 47,508 | 19,621 | 39,833 | 18,401 |
| OS | 27,698 | 37,884 | 18,128 | 23,648 | 40,795 | 22,912 | 36,891 | 18,948 | |
| 4 | OD | 26,428 | 29,349 | 40,420 | 29,997 | 52,429 | 23,445 | 53,385 | 19,204 |
| OS | 24,740 | 31,363 | 25,089 | 31,107 | 36,130 | 22,541 | 47,150 | 20,472 | |
| 5 | OD | 22,899 | 28,244 | 10,309 | 33,820 | 43,790 | 26,458 | 40,000 | 15,963 |
| OS | 26,495 | 27,505 | 15,306 | 35,065 | 35,719 | 21,666 | 42,087 | 19,965 | |
| Average | 32,374 | 32,790 | 24,666 | 26,117 | 46,374* | 22,234* | 47,949* | 20,645* |
Notes: The area of visualized retina was calculated with Adobe® Photoshop® C5 software (Adobe Systems Inc, San Jose, CA, USA) and expressed as the number of pixels, in each superior, inferior, nasal, and temporal quadrant of the retina. The area of temporal and nasal retina visualized by the Optos® Optomap® (Optos PLC, Dunfermline, UK) was significantly greater than seen with the Heidelberg Spectralis® (Heidelberg Engineering, Heidelberg, Germany) (temporal retina: 47,948 vs 20,645, respectively; nasal retina: 46,374 vs 22,234, respectively;
P < 0.0005 for both). The absolute number of pixels noted in the superior and inferior retina did not differ significantly. However, the Heidelberg Spectralis® was able to image the superior and inferior retinal vasculature to a more distal point than the Optos® Optomap®, in nine of ten eyes (18 of 20 quadrants).
Abbreviations: OD, right eye; OS, left eye; Optos, Optos® Optomap®; HB, Heidelberg Spectralis® Ultra-Widefield module.
The three reviewers were in agreement for each comparison 100% of the time (four quadrants per ten eyes for 40 of 40 comparisons).
Discussion
Imaging of the peripheral retina has become essential for the diagnosis, classification, and management of numerous diseases of the retina.7–16,18,19 Noncontact ultra-widefield fluorescein angiography with both the Optos® Optomap® and the Heidelberg Spectralis® imaging systems permit excellent capture of the posterior pole as well as peripheral retinal pathology in a single, nonsteered shot. Herein, we present the first direct comparison of ultra-widefield fluorescein angiography obtained using the Optos® Optomap® and Heidelberg Spectralis® noncontact modules.
Although the Optos® Optomap® and the Heidelberg Spectralis® are both considered ultra-widefield imaging devices, the area of the retina imaged differed considerably between the two instruments. Overall, the Optos® Optomap® imaged a significantly greater total area of the retina compared with the Heidelberg Spectralis® (Table 1). The Optos® Optomap® also showed the temporal and nasal retina to a greater extent compared with the Heidelberg Spectralis® (Table 2; Figures 2 and 3). However, the Heidelberg Spectralis® was able to image the superior and inferior retina to a more distal point compared with the Optos® Optomap® (Figure 3).
Figure 2.
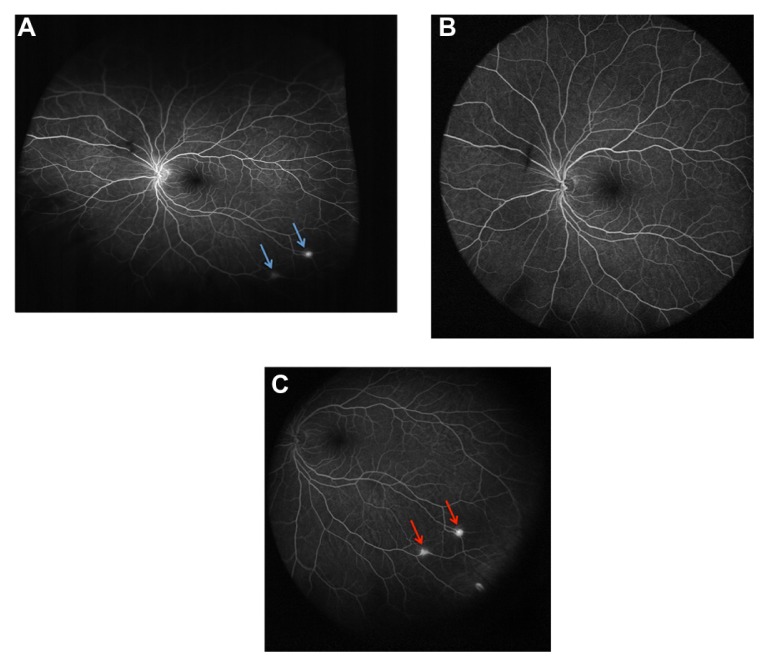
Ultra-widefield fluorescein angiogram of the left eye of a patient with Von Hippel-Lindau syndrome. (A) Optos® Optomap® (Optos PLC, Dunfermline, UK); (B and C) Heidelberg Spectralis® (Heidelberg Engineering, Heidelberg, Germany). The total retinal surface area visualized on a single-shot image was considerably greater with the Optos® Optomap® compared with the Heidelberg Spectralis®. Two retinal hemangioblastomas are noted in the inferotemporal quadrant (arrows in A and C). With the patient in primary gaze (A and B), only the Optos® Optomap®, and not the Heidelberg Spectralis®, clearly shows these two lesions (arrows in A). With the patient looking inferotemporally (C), the Heidelberg Spectralis® ultra-widefield module is able to capture the two retinal hemangioblastomas (arrows in C).
Figure 3.
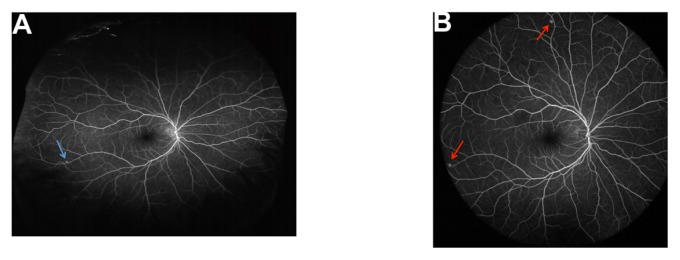
Ultra-widefield fluorescein angiogram of the right eye of the same patient with Von Hippel-Lindau syndrome, shown in Figure 2. (A) Optos® Optomap® (Optos PLC, Dunfermline, UK); (B) Heidelberg Spectralis® (Heidelberg Engineering, Heidelberg, Germany).
Notes: In primary gaze, the Optos® Optomap® shows one retinal hemangioblastoma in the inferotemporal quadrant (arrow). The superior and inferior quadrants are not as distinctly visualized by the Optos® Optomap® compared with the nasal and temporal quadrants. In primary gaze, the Heidelberg Spectralis® ultra-widefield image shows two retinal hemangioblastomas (arrows). The superior retinal hemangioblastoma visualized with the Heidelberg Spectralis® was not seen on single-shot, nonsteered image obtained with the Optos® Optomap® taken in primary position.
The clinical significance of ultra-widefield imaging, as well as the differences between the Heidelberg Spectralis® and the Optos® Optomap® instruments, can be illustrated effectively by a patient with multiple retinal hemangioblastomas, in the setting of Von Hippel-Lindau syndrome (Figures 2 and 3). When taken in primary gaze, a single-shot, nonsteered fluorescein angiogram of the left eye obtained using the Optos® Optomap® reveals two retina hemangioblastomas in the inferotemporal quadrant (Figure 2A). The same image taken in primary gaze with the Heidelberg Spectralis® failed to show either of the retina hemangioblastomas (Figure 2B). However, a steered image (where the patient is asked to look inferotemporally) obtained with the Heidelberg Spectralis® ultra-widefield module showed the two retinal lesions (Figure 2C).
Despite a considerably greater total retinal area imaged, the ultra-widefield fluorescein angiogram of the right eye of the same patient with Von Hippel-Lindau syndrome demonstrated the potential pathology that may still be missed with the Optos® Optomap® device (Figure 3). The single-shot, primary-gaze, nonsteered fluorescein angiogram of the right eye taken with the Optos® Optomap® clearly shows a retinal hemangioblastoma in the inferotemporal quadrant (Figure 3A). The superior and inferior vessels, however, are noticeably less-well visualized compared with the far temporal and nasal vessels in this image. The clinical significance is apparent when the same eye is imaged with Heidelberg Spectralis® ultra-widefield module (Figure 3B). With the Heidelberg Spectralis®, a second retinal hemangioblastoma is noted superiorly (Figure 3B).
Although the Optos® Optomap® consistently imaged a larger total retinal surface area compared with the Heidelberg Spectralis®, the variability among images (and thus the standard deviation of the average number of pixels imaged) was noticeably greater with the Optos®. On thorough inspection of the individual photographs, this variability was attributed to the presence of lid and lash artifacts that were noticed more often in the Optos® Optomap® images compared with the Heidelberg Spectralis® images. This may be related to how the images were acquired (eg, the photographer/patient/device interface) as well as to the fact that the Optos® Optomap® may be more likely to encounter the lids and lashes in its wider field of view.
Yet another distinction between the Optos® Optomap® images and those obtained on the Heidelberg Spectralis® was the peripheral distortion noted in the Optos® images. In order to produce so wide a field of view, the Optos® system utilizes an ellipsoid mirror to image the retina. As such, the resultant image appears distorted, especially in the far temporal and nasal periphery. On the other hand, the Heidelberg Spectralis® noncontact lens produces an undistorted flat image of the retina. Due to the fact that we compared the retinal area of a distorted image (Optos® Optomap®) versus a nondistorted image (Heidelberg Spectralis®) in a quantitative manner in this study, and recognizing that this would bias the outcomes in favor of the distorted image, it was also necessary to evaluate the area of imaged retina in a qualitative manner. In the qualitative evaluation, we found that the Heidelberg ultra-widefield lens outperformed the Optos® Optomap® in the superior and inferior quadrants, despite a nonstatistical quantitative difference.
The limitations of the present study include its retrospective nature as well as the inclusion of only ten eyes of five patients. Larger, prospective studies and inclusion of steered and montaged retinal images may be necessary to more completely evaluate the clinical utility of ultra-widefield retinal imaging and to fully compare various imaging modalities.
In summary, the Optos® Optomap® and the Heidelberg Spectralis® both provide outstanding noncontact ultra-widefield fluorescein angiography, which represents considerable improvement over 30- and 50-degree images. The Optos® Optomap® provides a view of the retina that is substantially greater than that of the Heidelberg Spectralis® noncontact ultra-widefield module temporally and nasally, albeit at the cost of peripheral distortion and with the provision of noticeably less detail. The ultimate clinical significance of these findings remains to be established.
Footnotes
Disclosure
Neither the authors nor the Weill Cornell Medical College have any direct or indirect financial interest in any manufacturer of ultra-widefield imaging devices. No funding of any kind from any company was received to perform the current study. Weill Cornell Medical College has received research funding from Optos PLC that was unrelated to this study, and Heidelberg Engineering Inc loaned the ultra-widefield module, on a trial basis, to the institution. Dr Szilárd Kiss has served as a paid consultant to Optos, PLC. The authors maintained full control of the study design, collection of data, data analysis, and writing of the manuscript. Neither the company nor any of its representatives were involved in any aspect of the presented research. The remaining authors (MW, GP, and SP) have no relevant financial conflicts of interest or financial support to disclose.
References
- 1.Ciardella A, Brown D. Wide-field imaging. In: Agarwal A, editor. Fundus Fluorescein and Indocyanine Green Angiography: A Textbook and Atlas. Thorofare: Slack Inc; 2007. pp. 79–84. [Google Scholar]
- 2.Pomerantzeff O. Equator-plus camera. Invest Ophthalmol. 1975;14(5):401–406. [PubMed] [Google Scholar]
- 3.Staurenghi G, Viola F, Mainster MA, Graham RD, Harrington PG. Scanning laser ophthalmoscopy and angiography with a wide-field contact lens system. Arch Ophthalmol. 2005;123(2):244–252. doi: 10.1001/archopht.123.2.244. [DOI] [PubMed] [Google Scholar]
- 4.Noyori KS, Chino K, Deguchi T. Wide field fluorescein angiography by use of contact lens. Retina. 1983;3(2):131–134. doi: 10.1097/00006982-198300320-00012. [DOI] [PubMed] [Google Scholar]
- 5.Spaide RF, Orlock DA, Herrmann-Delemazure B, et al. Wide-angle indocyanine green angiography. Retina. 1998;18(1):44–49. doi: 10.1097/00006982-199801000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Muraoka K, Kishi S, Shimizu K. Watershed zone in the human peripheral choroid. Ophthalmology. 1996;103(2):336–342. doi: 10.1016/s0161-6420(96)30695-7. [DOI] [PubMed] [Google Scholar]
- 7.Witmer M, Kiss S. Wide-field imaging of the retina. Surv Ophthalmol. doi: 10.1016/j.survophthal.2012.07.003. Epub 2013 Jan 29. [DOI] [PubMed] [Google Scholar]
- 8.Wessel MM, Aaker GD, Parlitsis G, Cho M, D’amico DJ, Kiss S. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32(4):785–791. doi: 10.1097/IAE.0b013e3182278b64. [DOI] [PubMed] [Google Scholar]
- 9.Wessel MM, Nair N, Aaker GD, Ehrlich JR, D’Amico DJ, Kiss S. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96(5):694–698. doi: 10.1136/bjophthalmol-2011-300774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad PS, Oliver SC, Coffee RE, Hubschman JP, Schwartz SD. Ultra wide-field angiographic characteristics of branch retinal and hemicentral retinal vein occlusion. Ophthalmology. 2010;117(4):780–784. doi: 10.1016/j.ophtha.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Pe’er J, Sancho C, Cantu J, et al. Measurement of choroidal melanoma basal diameter by wide-angle digital fundus camera: a comparison with ultrasound measurement. Ophthalmologica. 2006;220(3):194–197. doi: 10.1159/000091765. [DOI] [PubMed] [Google Scholar]
- 12.Shields CL, Materin M, Shields JA. Panoramic imaging of the ocular fundus. Arch Ophthalmol. 2003;121(11):1603–1607. doi: 10.1001/archopht.121.11.1603. [DOI] [PubMed] [Google Scholar]
- 13.Mudvari SS, Virasch VV, Singa RM, MacCumber MW. Ultra-wide-field imaging for cytomegalovirus retinitis. Ophthalmic Surg Lasers Imaging. 2010;41(3):311–315. doi: 10.3928/15428877-20100430-03. [DOI] [PubMed] [Google Scholar]
- 14.Cho M, Kiss S. Detection and monitoring of sickle cell retinopathy using ultra wide-field color photography and fluorescein angiography. Retina. 2011;31(4):738–747. doi: 10.1097/IAE.0b013e3181f049ec. [DOI] [PubMed] [Google Scholar]
- 15.Witmer MT, Cho M, Favarone G, Chan RV, D’Amico DJ, Kiss S. Ultra-wide-field autofluorescence imaging in non-traumatic rhegmatogenous retinal detachment. Eye (Lond) 2012;26(9):1209–1216. doi: 10.1038/eye.2012.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz SD, Harrison SA, Ferrone PJ, Trese MT. Telemedical evaluation and management of retinopathy of prematurity using a fiberoptic digital fundus camera. Ophthalmology. 2000;107(1):25–28. doi: 10.1016/s0161-6420(99)00003-2. [DOI] [PubMed] [Google Scholar]
- 17.Atkinson A, Mazo C. Imaged Area of the Retina. Dunfermline, UK: Optos PLC; 2011. [Accessed January 24, 2013]. Available from: http://www.optos.com/Global/documents/CaseStudies_ImagedAreaOfTheRetina.pdf. [Google Scholar]
- 18.Bonnay G, Nguyen F, Meunier I, Ducasse A, Hamel C, Arndt C. Screening for retinal detachment using wide-field retinal imaging. J Fr Ophtalmol. 2011;34(7):482–485. doi: 10.1016/j.jfo.2011.02.012. French. [DOI] [PubMed] [Google Scholar]
- 19.Spaide RF. Peripheral areas of nonperfusion in treated central retinal vein occlusion as imaged by wide-field fluorescein angiography. Retina. 2011;31(5):829–837. doi: 10.1097/IAE.0b013e31820c841e. [DOI] [PubMed] [Google Scholar]