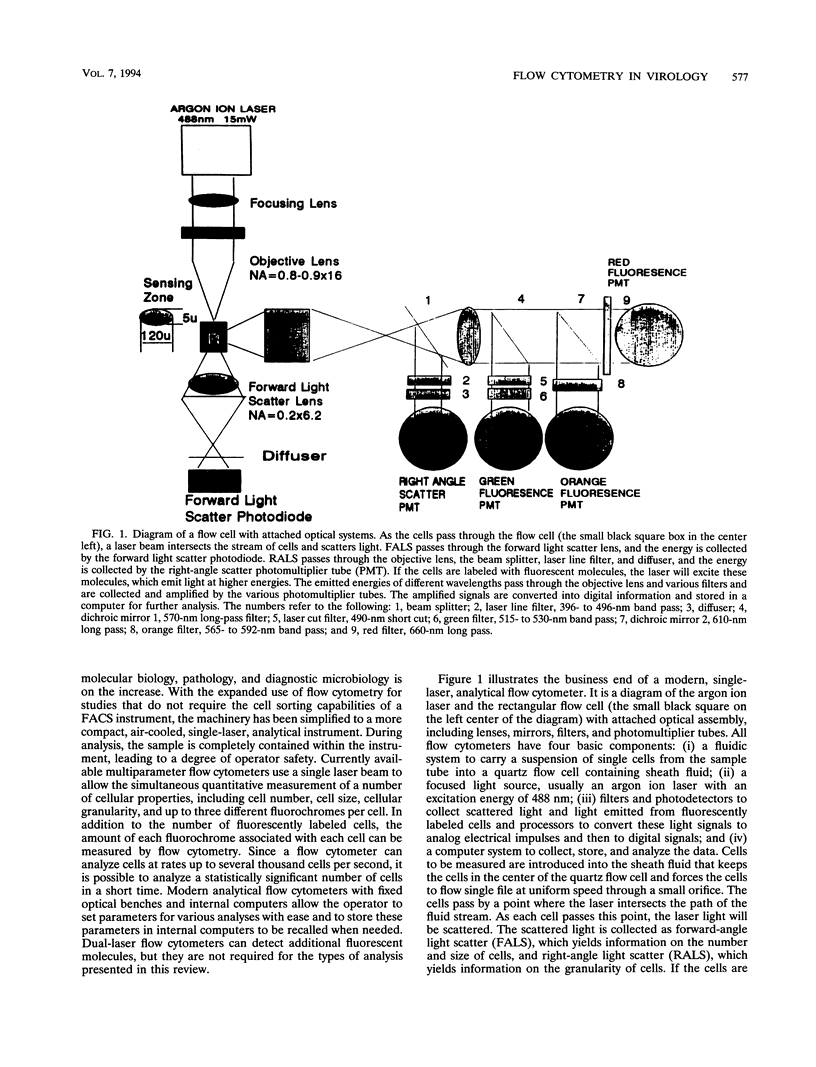
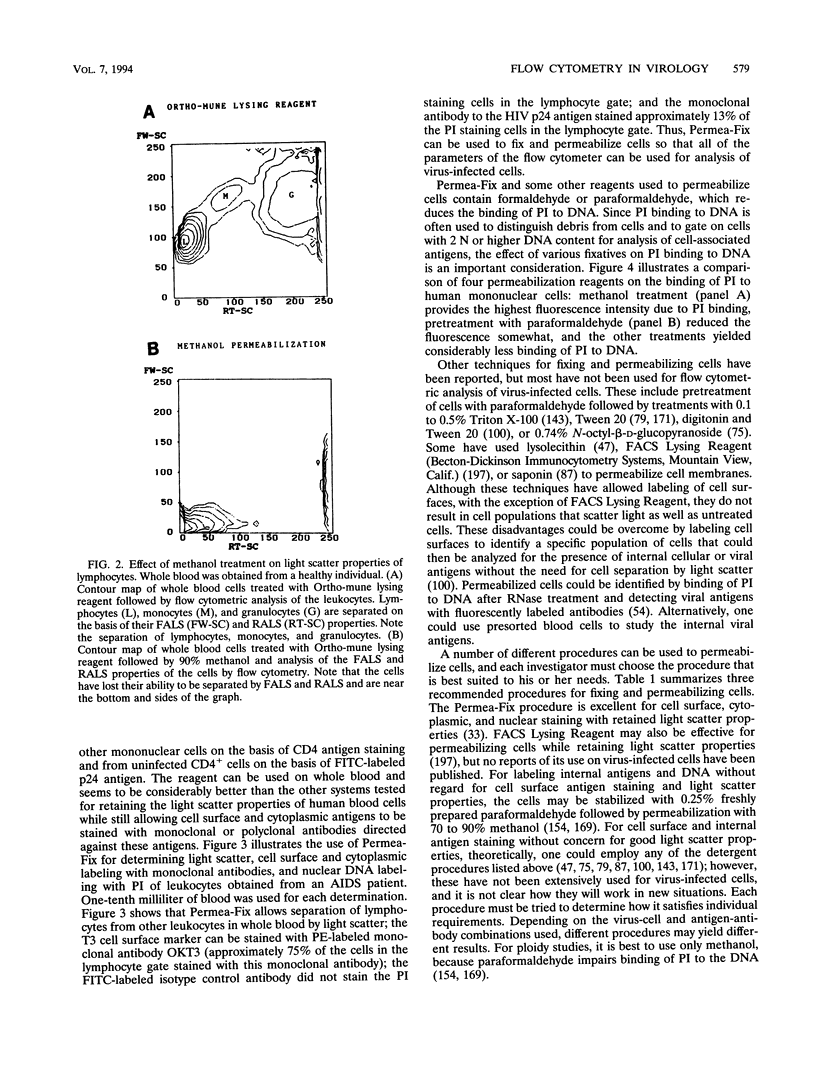
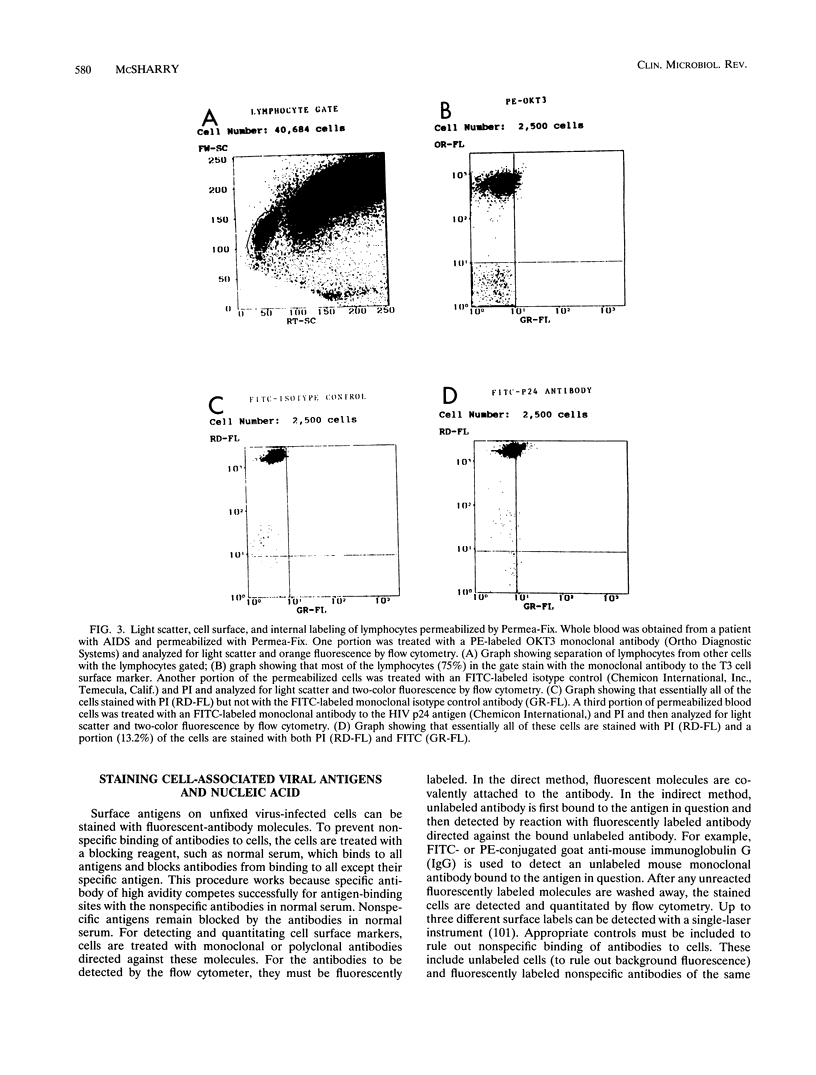
Abstract
This article reviews some of the published applications of flow cytometry for in vitro and in vivo detection and enumeration of virus-infected cells. Sample preparation, fixation, and permeabilization techniques for a number of virus-cell systems are evaluated. The use of flow cytometry for multiparameter analysis of virus-cell interactions for simian virus 40, herpes simplex viruses, human cytomegalovirus, and human immunodeficiency virus and its use for determining the effect of antiviral compounds on these virus-infected cells are reviewed. This is followed by a brief description of the use of flow cytometry for the analysis of several virus-infected cell systems, including blue tongue virus, hepatitis C virus, avian reticuloendotheliosis virus, African swine fever virus, woodchuck hepatitis virus, bovine viral diarrhea virus, feline leukemia virus, Epstein-Barr virus, Autographa californica nuclear polyhedrosis virus, and Friend murine leukemia virus. Finally, the use of flow cytometry for the rapid diagnosis of human cytomegalovirus and human immunodeficiency virus in peripheral blood cells of acutely infected patients and the use of this technology to monitor patients on antiviral therapy are reviewed. Future prospects for the rapid diagnosis of in vivo viral and bacterial infections by flow cytometry are discussed.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afanasyev V. N., Korol B. A., Matylevich N. P., Pechatnikov V. A., Umansky S. R. The use of flow cytometry for the investigation of cell death. Cytometry. 1993;14(6):603–609. doi: 10.1002/cyto.990140604. [DOI] [PubMed] [Google Scholar]
- Akbar A. N., Borthwick N., Salmon M., Gombert W., Bofill M., Shamsadeen N., Pilling D., Pett S., Grundy J. E., Janossy G. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. J Exp Med. 1993 Aug 1;178(2):427–438. doi: 10.1084/jem.178.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz C., Alvarez A., Escribano J. M. Flow cytometric analysis of African swine fever virus-induced plasma membrane proteins and their humoral immune response in infected pigs. Virology. 1992 Jul;189(1):266–273. doi: 10.1016/0042-6822(92)90702-q. [DOI] [PubMed] [Google Scholar]
- Alimenti A., O'Neill M., Sullivan J. L., Luzuriaga K. Diagnosis of vertical human immunodeficiency virus type 1 infection by whole blood culture. J Infect Dis. 1992 Nov;166(5):1146–1148. doi: 10.1093/infdis/166.5.1146. [DOI] [PubMed] [Google Scholar]
- Andrei G., Snoeck R., Schols D., Goubau P., Desmyter J., De Clercq E. Comparative activity of selected antiviral compounds against clinical isolates of human cytomegalovirus. Eur J Clin Microbiol Infect Dis. 1991 Dec;10(12):1026–1033. doi: 10.1007/BF01984924. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Arends M. J., Wyllie A. H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Argall K. G., Armati P. J., King N. J., Douglas M. W. The effects of West Nile virus on major histocompatibility complex class I and II molecule expression by Lewis rat Schwann cells in vitro. J Neuroimmunol. 1991 Dec;35(1-3):273–284. doi: 10.1016/0165-5728(91)90181-6. [DOI] [PubMed] [Google Scholar]
- Ascher D. P., Roberts C., Fowler A. Acidification modified p24 antigen capture assay in HIV seropositives. J Acquir Immune Defic Syndr. 1992;5(11):1080–1083. [PubMed] [Google Scholar]
- Baba M., Schols D., Pauwels R., Nakashima H., De Clercq E. Sulfated polysaccharides as potent inhibitors of HIV-induced syncytium formation: a new strategy towards AIDS chemotherapy. J Acquir Immune Defic Syndr. 1990;3(5):493–499. [PubMed] [Google Scholar]
- Bagasra O., Seshamma T., Oakes J. W., Pomerantz R. J. Frequency of cells positive for HIV-1 sequences assessed by in situ polymerase chain reaction. AIDS. 1993 Nov;7 (Suppl 2):S7–10. doi: 10.1097/00002030-199311002-00003. [DOI] [PubMed] [Google Scholar]
- Balzarini J., Pérez-Pérez M. J., San-Félix A., Schols D., Perno C. F., Vandamme A. M., Camarasa M. J., De Clercq E. 2',5'-Bis-O-(tert-butyldimethylsilyl)-3'-spiro-5''-(4''-amino-1'',2''- oxathiole-2'',2'-dioxide)pyrimidine (TSAO) nucleoside analogues: highlyselective inhibitors of human immunodeficiency virus type 1 that are targeted at the viral reverse transcriptase. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4392–4396. doi: 10.1073/pnas.89.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt-Boyes S. M., Rossitto P. V., Stott J. L., MacLachlan N. J. Flow cytometric analysis of in vitro bluetongue virus infection of bovine blood mononuclear cells. J Gen Virol. 1992 Aug;73(Pt 8):1953–1960. doi: 10.1099/0022-1317-73-8-1953. [DOI] [PubMed] [Google Scholar]
- Basak S., Turner H., Compans R. W. Expression of SV40 receptors on apical surfaces of polarized epithelial cells. Virology. 1992 Sep;190(1):393–402. doi: 10.1016/0042-6822(92)91225-j. [DOI] [PubMed] [Google Scholar]
- Basgoz N., Qadri I., Navarro D., Sears A., Lennette E., Youngblom J., Pereira L. The amino terminus of human cytomegalovirus glycoprotein B contains epitopes that vary among strains. J Gen Virol. 1992 Apr;73(Pt 4):983–988. doi: 10.1099/0022-1317-73-4-983. [DOI] [PubMed] [Google Scholar]
- Beilke M. A. Detection of HTLV-I in clinical specimens. J Virol Methods. 1992 Nov;40(2):133–144. doi: 10.1016/0166-0934(92)90062-i. [DOI] [PubMed] [Google Scholar]
- Best L. M., Veldhuyzen van Zanten S. J., Bezanson G. S., Haldane D. J., Malatjalian D. A. Serological detection of Helicobacter pylori by a flow microsphere immunofluorescence assay. J Clin Microbiol. 1992 Sep;30(9):2311–2317. doi: 10.1128/jcm.30.9.2311-2317.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner B. C., Poulton T. A. Cytofluorometric analysis of anti-lymphocyte antibodies in AIDS. FEMS Microbiol Immunol. 1991 Dec;4(1):33–40. doi: 10.1111/j.1574-6968.1991.tb04967.x. [DOI] [PubMed] [Google Scholar]
- Borrow P., Oldstone M. B. Characterization of lymphocytic choriomeningitis virus-binding protein(s): a candidate cellular receptor for the virus. J Virol. 1992 Dec;66(12):7270–7281. doi: 10.1128/jvi.66.12.7270-7281.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher C. A., Lange J. M., Miedema F. F., Weverling G. J., Koot M., Mulder J. W., Goudsmit J., Kellam P., Larder B. A., Tersmette M. HIV-1 biological phenotype and the development of zidovudine resistance in relation to disease progression in asymptomatic individuals during treatment. AIDS. 1992 Nov;6(11):1259–1264. doi: 10.1097/00002030-199211000-00003. [DOI] [PubMed] [Google Scholar]
- Bouffard P., Hayashi P. H., Acevedo R., Levy N., Zeldis J. B. Hepatitis C virus is detected in a monocyte/macrophage subpopulation of peripheral blood mononuclear cells of infected patients. J Infect Dis. 1992 Dec;166(6):1276–1280. doi: 10.1093/infdis/166.6.1276. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Rakusan T. A., Sison A. V., Josephs S. H., Saxena E. S., Herzog K. D., Parrott R. H., Sever J. L. Detection of human immunodeficiency virus type 1 infection in young pediatric patients by using polymerase chain reaction and biotinylated probes. J Clin Microbiol. 1992 Jan;30(1):36–40. doi: 10.1128/jcm.30.1.36-40.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breau W. C., Atwood W. J., Norkin L. C. Class I major histocompatibility proteins are an essential component of the simian virus 40 receptor. J Virol. 1992 Apr;66(4):2037–2045. doi: 10.1128/jvi.66.4.2037-2045.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgard M., Mayaux M. J., Blanche S., Ferroni A., Guihard-Moscato M. L., Allemon M. C., Ciraru-Vigneron N., Firtion G., Floch C., Guillot F. The use of viral culture and p24 antigen testing to diagnose human immunodeficiency virus infection in neonates. The HIV Infection in Newborns French Collaborative Study Group. N Engl J Med. 1992 Oct 22;327(17):1192–1197. doi: 10.1056/NEJM199210223271702. [DOI] [PubMed] [Google Scholar]
- Böhm D., Nick S., Voss G., Hunsmann G. Detection of viral surface antigens on HIV-2ben infected human tumor cell lines by flow cytometry. Cytometry. 1992;13(3):259–266. doi: 10.1002/cyto.990130307. [DOI] [PubMed] [Google Scholar]
- Cameron P. U., Pope M., Gezelter S., Steinman R. M. Infection and apoptotic cell death of CD4+ T cells during an immune response to HIV-1-pulsed dendritic cells. AIDS Res Hum Retroviruses. 1994 Jan;10(1):61–71. doi: 10.1089/aid.1994.10.61. [DOI] [PubMed] [Google Scholar]
- Carbonari M., Cibati M., Cherchi M., Sbarigia D., Pesce A. M., Dell'Anna L., Modica A., Fiorilli M. Detection and characterization of apoptotic peripheral blood lymphocytes in human immunodeficiency virus infection and cancer chemotherapy by a novel flow immunocytometric method. Blood. 1994 Mar 1;83(5):1268–1277. [PubMed] [Google Scholar]
- Chemin I., Baginski I., Vermot-Desroches C., Hantz O., Jacquet C., Rigal D., Trepo C. Demonstration of woodchuck hepatitis virus infection of peripheral blood mononuclear cells by flow cytometry and polymerase chain reaction. J Gen Virol. 1992 Jan;73(Pt 1):123–129. doi: 10.1099/0022-1317-73-1-123. [DOI] [PubMed] [Google Scholar]
- Cohen C. Y., Sahar E. Rapid flow cytometric bacterial detection and determination of susceptibility to amikacin in body fluids and exudates. J Clin Microbiol. 1989 Jun;27(6):1250–1256. doi: 10.1128/jcm.27.6.1250-1256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J. Apoptosis. Immunol Today. 1993 Mar;14(3):126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- Cone R. W., Huang M. L., Ashley R., Corey L. Human herpesvirus 6 DNA in peripheral blood cells and saliva from immunocompetent individuals. J Clin Microbiol. 1993 May;31(5):1262–1267. doi: 10.1128/jcm.31.5.1262-1267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor R. I., Mohri H., Cao Y., Ho D. D. Increased viral burden and cytopathicity correlate temporally with CD4+ T-lymphocyte decline and clinical progression in human immunodeficiency virus type 1-infected individuals. J Virol. 1993 Apr;67(4):1772–1777. doi: 10.1128/jvi.67.4.1772-1777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory J. M., Ohlsson-Wilhelm B. M., Brock E. J., Sheaffer N. A., Steck M. E., Eyster M. E., Rapp F. Detection of human immunodeficiency virus-infected lymphoid cells at low frequency by flow cytometry. J Immunol Methods. 1987 Dec 4;105(1):71–78. doi: 10.1016/0022-1759(87)90415-7. [DOI] [PubMed] [Google Scholar]
- Cory J. M., Ohlsson-Wilhelm B. M., Steck M. E., Smithgall M. D., Rozday V., Eyster M. E., Rapp F. Kinetics of infected cell appearance as a determinant of number of human immunodeficiency virus-1 infectious units. AIDS Res Hum Retroviruses. 1989 Feb;5(1):97–106. doi: 10.1089/aid.1989.5.97. [DOI] [PubMed] [Google Scholar]
- Cory J. M., Rapp F., Ohlsson-Wilhelm B. M. Effects of cellular fixatives on human immunodeficiency virus production. Cytometry. 1990;11(5):647–651. doi: 10.1002/cyto.990110514. [DOI] [PubMed] [Google Scholar]
- Costigliola P., Tumietto F., Ricchi E., Chiodo F. Detection of circulating p24 antigen-positive CD4+ cells during HIV infection by flow cytometry. AIDS. 1992 Oct;6(10):1121–1125. doi: 10.1097/00002030-199210000-00009. [DOI] [PubMed] [Google Scholar]
- Cozon G., Roure C., Lizard G., Greenland T., Larget-Piet D., Gandilhon F., Peyron F. An improved assay for the detection of Toxoplasma gondii antibodies in human serum by flow cytometry. Cytometry. 1993;14(5):569–575. doi: 10.1002/cyto.990140518. [DOI] [PubMed] [Google Scholar]
- Crawford S. W., Bowden R. A., Hackman R. C., Gleaves C. A., Meyers J. D., Clark J. G. Rapid detection of cytomegalovirus pulmonary infection by bronchoalveolar lavage and centrifugation culture. Ann Intern Med. 1988 Feb;108(2):180–185. doi: 10.7326/0003-4819-108-2-180. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Regulation of HIV-1 gene expression. FASEB J. 1991 Jul;5(10):2361–2368. doi: 10.1096/fasebj.5.10.1712325. [DOI] [PubMed] [Google Scholar]
- Daar E. S., Moudgil T., Meyer R. D., Ho D. D. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med. 1991 Apr 4;324(14):961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Bruno S., Del Bino G., Gorczyca W., Hotz M. A., Lassota P., Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13(8):795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- De Rossi A., Ometto L., Roncella S., D'Andrea E., Menin C., Calderazzo F., Rowe M., Ferrarini M., Chieco-Bianchi L. HIV-1 induces down-regulation of bcl-2 expression and death by apoptosis of EBV-immortalized B cells: a model for a persistent "self-limiting" HIV-1 infection. Virology. 1994 Jan;198(1):234–244. doi: 10.1006/viro.1994.1026. [DOI] [PubMed] [Google Scholar]
- Dean G. A., Groshek P. M., Mullins J. I., Hoover E. A. Hematopoietic target cells of anemogenic subgroup C versus nonanemogenic subgroup A feline leukemia virus. J Virol. 1992 Sep;66(9):5561–5568. doi: 10.1128/jvi.66.9.5561-5568.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent G. A., Leglise M. C., Pryzwansky K. B., Ross D. W. Simultaneous paired analysis by flow cytometry of surface markers, cytoplasmic antigens, or oncogene expression with DNA content. Cytometry. 1989 Mar;10(2):192–198. doi: 10.1002/cyto.990100210. [DOI] [PubMed] [Google Scholar]
- Dhawan S., Streicher H. Z., Wahl L. M., Miller N., Louie A. T., Goldfarb I. S., Jackson W. L., Casali P., Notkins A. L. Model for studying virus attachment. II. Binding of biotinylated human T cell leukemia virus type I to human blood mononuclear cells potential targets for human T cell leukemia virus type I infection. J Immunol. 1991 Jul 1;147(1):102–108. [PubMed] [Google Scholar]
- Dolter K. E., Goins W. F., Levine M., Glorioso J. C. Genetic analysis of type-specific antigenic determinants of herpes simplex virus glycoprotein C. J Virol. 1992 Aug;66(8):4864–4873. doi: 10.1128/jvi.66.8.4864-4873.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörig R. E., Marcil A., Chopra A., Richardson C. D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell. 1993 Oct 22;75(2):295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Eizuru Y., Minamishima Y. Evidence for putative immediate early antigens in human herpesvirus 6-infected cells. J Gen Virol. 1992 Aug;73(Pt 8):2161–2165. doi: 10.1099/0022-1317-73-8-2161. [DOI] [PubMed] [Google Scholar]
- Elmendorf S., McSharry J., Laffin J., Fogleman D., Lehman J. M. Detection of an early cytomegalovirus antigen with two-color quantitative flow cytometry. Cytometry. 1988 May;9(3):254–260. doi: 10.1002/cyto.990090311. [DOI] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Etingin O. R., Silverstein R. L., Hajjar D. P. Identification of a monocyte receptor on herpesvirus-infected endothelial cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7200–7203. doi: 10.1073/pnas.88.16.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. L., Hampton R. G., Boots E. Flow cytometric assays for monitoring production of recombinant HIV-1 gp160 in insect cells infected with a baculovirus expression vector. J Virol Methods. 1989 Dec;26(3):279–290. doi: 10.1016/0166-0934(89)90110-9. [DOI] [PubMed] [Google Scholar]
- Flamand L., Stefanescu I., Ablashi D. V., Menezes J. Activation of the Epstein-Barr virus replicative cycle by human herpesvirus 6. J Virol. 1993 Nov;67(11):6768–6777. doi: 10.1128/jvi.67.11.6768-6777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forthal D. N., Aarnaes S., Blanding J., de la Maza L., Tilles J. G. Degree and length of viremia in adults with measles. J Infect Dis. 1992 Aug;166(2):421–424. doi: 10.1093/infdis/166.2.421. [DOI] [PubMed] [Google Scholar]
- Freistadt M. S., Fleit H. B., Wimmer E. Poliovirus receptor on human blood cells: a possible extraneural site of poliovirus replication. Virology. 1993 Aug;195(2):798–803. doi: 10.1006/viro.1993.1433. [DOI] [PubMed] [Google Scholar]
- Friedrich T. D., Laffin J., Lehman J. M. Simian virus 40 large T-antigen function is required for induction of tetraploid DNA content during lytic infection. J Virol. 1992 Jul;66(7):4576–4579. doi: 10.1128/jvi.66.7.4576-4579.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadol N., Crutcher G. J., Busch M. P. Detection of intracellular HIV in lymphocytes by flow cytometry. Cytometry. 1994 Apr 1;15(4):359–370. doi: 10.1002/cyto.990150412. [DOI] [PubMed] [Google Scholar]
- Gelman R., Cheng S. C., Kidd P., Waxdal M., Kagan J. Assessment of the effects of instrumentation, monoclonal antibody, and fluorochrome on flow cytometric immunophenotyping: a report based on 2 years of the NIAID DAIDS flow cytometry quality assessment program. Clin Immunol Immunopathol. 1993 Feb;66(2):150–162. doi: 10.1006/clin.1993.1019. [DOI] [PubMed] [Google Scholar]
- Gerna G., Percivalle E., Grazia Revello M., Morini F. Correlation of quantitative human cytomegalovirus pp65-, p72- and p150-antigenemia, viremia and circulating endothelial giant cells with clinical symptoms and antiviral treatment in immunocompromised patients. Clin Diagn Virol. 1993 Mar;1(1):47–59. doi: 10.1016/0928-0197(93)90033-2. [DOI] [PubMed] [Google Scholar]
- Gerna G., Revello M. G., Percivalle E., Morini F. Comparison of different immunostaining techniques and monoclonal antibodies to the lower matrix phosphoprotein (pp65) for optimal quantitation of human cytomegalovirus antigenemia. J Clin Microbiol. 1992 May;30(5):1232–1237. doi: 10.1128/jcm.30.5.1232-1237.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Zipeto D., Percivalle E., Parea M., Revello M. G., Maccario R., Peri G., Milanesi G. Human cytomegalovirus infection of the major leukocyte subpopulations and evidence for initial viral replication in polymorphonuclear leukocytes from viremic patients. J Infect Dis. 1992 Dec;166(6):1236–1244. doi: 10.1093/infdis/166.6.1236. [DOI] [PubMed] [Google Scholar]
- Gilbert M. J., Riddell S. R., Li C. R., Greenberg P. D. Selective interference with class I major histocompatibility complex presentation of the major immediate-early protein following infection with human cytomegalovirus. J Virol. 1993 Jun;67(6):3461–3469. doi: 10.1128/jvi.67.6.3461-3469.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi J. V., Cheng H. L., Margolick J. B., Bauer K. D., Ferbas J., Waxdal M., Schmid I., Hultin L. E., Jackson A. L., Park L. Quality control in the flow cytometric measurement of T-lymphocyte subsets: the multicenter AIDS cohort study experience. The Multicenter AIDS Cohort Study Group. Clin Immunol Immunopathol. 1990 May;55(2):173–186. doi: 10.1016/0090-1229(90)90096-9. [DOI] [PubMed] [Google Scholar]
- Gleaves C. A., Smith T. F., Shuster E. A., Pearson G. R. Rapid detection of cytomegalovirus in MRC-5 cells inoculated with urine specimens by using low-speed centrifugation and monoclonal antibody to an early antigen. J Clin Microbiol. 1984 Jun;19(6):917–919. doi: 10.1128/jcm.19.6.917-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goolsby C. L., Steiner M., Nemeth J. Viral and cellular oncogene expression during progressive malignant transformation of SV40 transformed human fibroblasts. Cytometry. 1991;12(8):748–756. doi: 10.1002/cyto.990120809. [DOI] [PubMed] [Google Scholar]
- Goolsby C., Gay H., Docherty J. J., Todd P. Flow cytometric detection of herpes simplex virus type 2 infected and transformed cells by immunoenzymatic and by indirect immunofluorescence staining. Cytometry. 1988 Mar;9(2):126–130. doi: 10.1002/cyto.990090205. [DOI] [PubMed] [Google Scholar]
- Gorse G. J., Frey S. E., Newman F. K., Belshe R. B. Detection of binding antibodies to native and recombinant human immunodeficiency virus type 1 envelope glycoproteins following recombinant gp160 immunization measured by flow cytometry and enzyme immunoassays. The AIDS Vaccine Clinical Trials Network. J Clin Microbiol. 1992 Oct;30(10):2606–2612. doi: 10.1128/jcm.30.10.2606-2612.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L., Petersen B., Steimel L., Haeber P., Current W. Rapid determination of antifungal activity by flow cytometry. J Clin Microbiol. 1994 Apr;32(4):1088–1091. doi: 10.1128/jcm.32.4.1088-1091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldén G., Andersson U., Hed J., Johansson S. G. A new membrane permeabilization method for the detection of intracellular antigens by flow cytometry. J Immunol Methods. 1989 Nov 13;124(1):103–109. doi: 10.1016/0022-1759(89)90191-9. [DOI] [PubMed] [Google Scholar]
- Harabuchi Y., Koizumi S., Osato T., Yamanaka N., Kataura A. Flow cytometric analysis of Epstein-Barr virus receptor among the different B-cell subpopulations using simultaneous two-color immunofluorescence. Virology. 1988 Jul;165(1):278–281. doi: 10.1016/0042-6822(88)90683-6. [DOI] [PubMed] [Google Scholar]
- Hart L., Donovan R. M., Goldstein E., Brady F. P. Detection of human immunodeficiency virus in infected CEM cells using fluorescent DNA probes and a laser-based computerized image cytofluorometry system. Anal Quant Cytol Histol. 1990 Apr;12(2):127–134. [PubMed] [Google Scholar]
- Heynen C. A., Holzer T. J. Evaluation of a flow cytometric model for monitoring HIV antigen expression in vitro. J Immunol Methods. 1992 Jul 31;152(1):25–33. doi: 10.1016/0022-1759(92)90085-8. [DOI] [PubMed] [Google Scholar]
- Hollander Z., Loken M. R. Simultaneous analysis of DNA content and surface antigens in human bone marrow. Cytometry. 1988 Sep;9(5):485–490. doi: 10.1002/cyto.990090513. [DOI] [PubMed] [Google Scholar]
- Holzer T. J., Heynen C. A., Novak R. M., Pitrak D. L., Dawson G. J. Frequency of cells positive for HIV-1 p24 antigen assessed by flow cytometry. AIDS. 1993 Nov;7 (Suppl 2):S3–S5. doi: 10.1097/00002030-199311002-00002. [DOI] [PubMed] [Google Scholar]
- Horan M., Horan P. K., Williams C. A. Quantitative measurement of SB 40 T-antigen production. Exp Cell Res. 1975 Mar 15;91(2):247–252. doi: 10.1016/0014-4827(75)90101-9. [DOI] [PubMed] [Google Scholar]
- Horan P. K., Jett J. H., Romero A., Lehman J. M. Flow microfluorometry analysis of DNA content in Chinese hamster cells following infection with simian virus 40. Int J Cancer. 1974 Oct 15;14(4):514–521. doi: 10.1002/ijc.2910140411. [DOI] [PubMed] [Google Scholar]
- Hotz M. A., Gong J., Traganos F., Darzynkiewicz Z. Flow cytometric detection of apoptosis: comparison of the assays of in situ DNA degradation and chromatin changes. Cytometry. 1994 Mar 1;15(3):237–244. doi: 10.1002/cyto.990150309. [DOI] [PubMed] [Google Scholar]
- Ikeda M. K., Andiman W. A., Mezger J. L., Shapiro E. D., Miller G. Quantitative leukoviremia and immune complex-dissociated antigenemia as predictors of infection status in children born to mothers infected with human immunodeficiency virus type 1. J Pediatr. 1993 Apr;122(4):524–531. doi: 10.1016/s0022-3476(05)83530-9. [DOI] [PubMed] [Google Scholar]
- Inghirami G., Nakamura M., Balow J. E., Notkins A. L., Casali P. Model for studying virus attachment: identification and quantitation of Epstein-Barr virus-binding cells by using biotinylated virus in flow cytometry. J Virol. 1988 Jul;62(7):2453–2463. doi: 10.1128/jvi.62.7.2453-2463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti P., Ottmann M., Morand P., Leclercq P., Seigneurin J. M. HIV-1 in blood monocytes: frequency of detection of proviral DNA using PCR and comparison with the total CD4 count. AIDS Res Hum Retroviruses. 1992 Feb;8(2):261–268. doi: 10.1089/aid.1992.8.261. [DOI] [PubMed] [Google Scholar]
- Jacob M. C., Favre M., Bensa J. C. Membrane cell permeabilization with saponin and multiparametric analysis by flow cytometry. Cytometry. 1991;12(6):550–558. doi: 10.1002/cyto.990120612. [DOI] [PubMed] [Google Scholar]
- Jacobberger J. W., Fogleman D., Lehman J. M. Analysis of intracellular antigens by flow cytometry. Cytometry. 1986 Jul;7(4):356–364. doi: 10.1002/cyto.990070410. [DOI] [PubMed] [Google Scholar]
- Jennings S. R., Lippe P. A., Pauza K. J., Spear P. G., Pereira L., Tevethia S. S. Kinetics of expression of herpes simplex virus type 1-specific glycoprotein species on the surfaces of infected murine, simian, and human cells: flow cytometric analysis. J Virol. 1987 Jan;61(1):104–112. doi: 10.1128/jvi.61.1.104-112.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings S. R., Rice P. L., Kloszewski E. D., Anderson R. W., Thompson D. L., Tevethia S. S. Effect of herpes simplex virus types 1 and 2 on surface expression of class I major histocompatibility complex antigens on infected cells. J Virol. 1985 Dec;56(3):757–766. doi: 10.1128/jvi.56.3.757-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J., Knobler R. L., Lublin F. D., Hart M. N. Mouse hepatitis virus (MHV-4, JHM) blocks gamma-interferon-induced major histocompatibility complex class II antigen expression on murine cerebral endothelial cells. J Neuroimmunol. 1991 Sep;33(3):181–190. doi: 10.1016/0165-5728(91)90105-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurriaans S., Dekker J. T., de Ronde A. HIV-1 viral DNA load in peripheral blood mononuclear cells from seroconverters and long-term infected individuals. AIDS. 1992 Jul;6(7):635–641. doi: 10.1097/00002030-199207000-00004. [DOI] [PubMed] [Google Scholar]
- Kadan M. J., Sturm S., Anderson W. F., Eglitis M. A. Detection of receptor-specific murine leukemia virus binding to cells by immunofluorescence analysis. J Virol. 1992 Apr;66(4):2281–2287. doi: 10.1128/jvi.66.4.2281-2287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koot M., Keet I. P., Vos A. H., de Goede R. E., Roos M. T., Coutinho R. A., Miedema F., Schellekens P. T., Tersmette M. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993 May 1;118(9):681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- Kuhar S. G., Lehman J. M. T antigen and p53 in pre- and post-crisis simian virus 40-transformed human cell lines. Oncogene. 1991 Sep;6(9):1499–1506. [PubMed] [Google Scholar]
- Laffin J., Fogleman D., Lehman J. M. Correlation of DNA content, p53, T antigen, and V antigen in simian virus 40-infected human diploid cells. Cytometry. 1989 Mar;10(2):205–213. doi: 10.1002/cyto.990100212. [DOI] [PubMed] [Google Scholar]
- Laffin J., Lehman J. M. Detection of intracellular virus and viral products. Methods Cell Biol. 1990;33:271–284. doi: 10.1016/s0091-679x(08)60531-2. [DOI] [PubMed] [Google Scholar]
- Lagakos S. W. Surrogate markers in AIDS clinical trials: conceptual basis, validation, and uncertainties. Clin Infect Dis. 1993 Feb;16 (Suppl 1):S22–S25. doi: 10.1093/clinids/16.supplement_1.s22. [DOI] [PubMed] [Google Scholar]
- Landay A., Jennings C., Forman M., Raynor R. Whole blood method for simultaneous detection of surface and cytoplasmic antigens by flow cytometry. Cytometry. 1993;14(4):433–440. doi: 10.1002/cyto.990140413. [DOI] [PubMed] [Google Scholar]
- Landay A., Ohlsson-Wilhelm B., Giorgi J. V. Application of flow cytometry to the study of HIV infection. AIDS. 1990 Jun;4(6):479–497. doi: 10.1097/00002030-199006000-00001. [DOI] [PubMed] [Google Scholar]
- Lapinsky S. E., Glencross D., Car N. G., Kallenbach J. M., Zwi S. Quantification and assessment of viability of Pneumocystis carinii organisms by flow cytometry. J Clin Microbiol. 1991 May;29(5):911–915. doi: 10.1128/jcm.29.5.911-915.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman J. M., Friedrich T. D., Laffin J. Quantitation of simian virus 40 T-antigen correlated with the cell cycle of permissive and non-permissive cells. Cytometry. 1993;14(4):401–410. doi: 10.1002/cyto.990140409. [DOI] [PubMed] [Google Scholar]
- Lehman J. M., Laffin J., Friedrich T. D. Flow cytometry of DNA increase after simian virus 40 infection of CV-1 cells. In Vitro Cell Dev Biol. 1992 May;28A(5):306–308. [PubMed] [Google Scholar]
- Lehman J. M., Laffin J., Jacobberger J. W., Fogleman D. Analysis of simian virus 40 infection of CV-1 cells by quantitative two-color fluorescence with flow cytometry. Cytometry. 1988 Jan;9(1):52–59. doi: 10.1002/cyto.990090109. [DOI] [PubMed] [Google Scholar]
- Li Q. X., Young L. S., Niedobitek G., Dawson C. W., Birkenbach M., Wang F., Rickinson A. B. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature. 1992 Mar 26;356(6367):347–350. doi: 10.1038/356347a0. [DOI] [PubMed] [Google Scholar]
- Liang X., Tang M., Zamb T. J., Babiuk L. A., Kowalski J., Tykocinski M. L. Expression of glycoprotein gIII-human decay-accelerating factor chimera on the bovine herpesvirus 1 virion via a glycosyl phosphatidylinositol-based membrane anchor. J Virol. 1993 Aug;67(8):4896–4904. doi: 10.1128/jvi.67.8.4896-4904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. M., Muirhead K. A., George S. P., Landay A. L. Flow cytometric monitoring of human immunodeficiency virus-infected patients. Simultaneous enumeration of five lymphocyte subsets. Am J Clin Pathol. 1989 Dec;92(6):721–728. doi: 10.1093/ajcp/92.6.721. [DOI] [PubMed] [Google Scholar]
- Lizard G., Chignol M. C., Chardonnet Y., Souchier C., Bordes M., Schmitt D., Revillard J. P. Detection of human papillomavirus DNA in CaSki and HeLa cells by fluorescent in situ hybridization. Analysis by flow cytometry and confocal laser scanning microscopy. J Immunol Methods. 1993 Jan 4;157(1-2):31–38. doi: 10.1016/0022-1759(93)90067-h. [DOI] [PubMed] [Google Scholar]
- Lombardi S., Garzelli C., La Rosa C., Zaccaro L., Specter S., Malvaldi G., Tozzini F., Esposito F., Bendinelli M. Identification of a linear neutralization site within the third variable region of the feline immunodeficiency virus envelope. J Virol. 1993 Aug;67(8):4742–4749. doi: 10.1128/jvi.67.8.4742-4749.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. Y., Koga Y., Tanaka K., Sasaki M., Kimura G., Nomoto K. Apoptosis induced in CD4+ cells expressing gp160 of human immunodeficiency virus type 1. J Virol. 1994 Jan;68(1):390–399. doi: 10.1128/jvi.68.1.390-399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzuriaga K., McQuilken P., Alimenti A., Somasundaran M., Hesselton R., Sullivan J. L. Early viremia and immune responses in vertical human immunodeficiency virus type 1 infection. J Infect Dis. 1993 May;167(5):1008–1013. doi: 10.1093/infdis/167.5.1008. [DOI] [PubMed] [Google Scholar]
- Lydy S. L., Compans R. W. Role of the cytoplasmic domains of viral glycoproteins in antibody-induced cell surface mobility. J Virol. 1993 Oct;67(10):6289–6294. doi: 10.1128/jvi.67.10.6289-6294.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A. B., Samuel K., Sanderson A., Maddy A. H. Simultaneous analysis of immunophenotype and apoptosis of murine thymocytes by single laser flow cytometry. Cytometry. 1992;13(8):809–821. doi: 10.1002/cyto.990130803. [DOI] [PubMed] [Google Scholar]
- Maciejewski J. P., Bruening E. E., Donahue R. E., Sellers S. E., Carter C., Young N. S., St Jeor S. Infection of mononucleated phagocytes with human cytomegalovirus. Virology. 1993 Aug;195(2):327–336. doi: 10.1006/viro.1993.1383. [DOI] [PubMed] [Google Scholar]
- Martin E., Bhakdi S. Flow cytometric assay for quantifying opsonophagocytosis and killing of Staphylococcus aureus by peripheral blood leukocytes. J Clin Microbiol. 1992 Sep;30(9):2246–2255. doi: 10.1128/jcm.30.9.2246-2255.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E., Bhakdi S. Quantitative analysis of opsonophagocytosis and of killing of Candida albicans by human peripheral blood leukocytes by using flow cytometry. J Clin Microbiol. 1991 Sep;29(9):2013–2023. doi: 10.1128/jcm.29.9.2013-2023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E., Stüben A., Görz A., Weller U., Bhakdi S. Novel aspect of amphotericin B action: accumulation in human monocytes potentiates killing of phagocytosed Candida albicans. Antimicrob Agents Chemother. 1994 Jan;38(1):13–22. doi: 10.1128/aac.38.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. M., Veis D., Korsmeyer S. J., Sugden B. Latent membrane protein of Epstein-Barr virus induces cellular phenotypes independently of expression of Bcl-2. J Virol. 1993 Sep;67(9):5269–5278. doi: 10.1128/jvi.67.9.5269-5278.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbida A. D., Pozzetto B., Sabido O., Akono Y., Grattard F., Habib M., Gaudin O. G. Competition binding studies with biotinylated echovirus 11 in cytofluorimetry analysis. J Virol Methods. 1991 Nov-Dec;35(2):169–176. doi: 10.1016/0166-0934(91)90132-j. [DOI] [PubMed] [Google Scholar]
- McHugh T. M., Miner R. C., Logan L. H., Stites D. P. Simultaneous detection of antibodies to cytomegalovirus and herpes simplex virus by using flow cytometry and a microsphere-based fluorescence immunoassay. J Clin Microbiol. 1988 Oct;26(10):1957–1961. doi: 10.1128/jcm.26.10.1957-1961.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh T. M., Stites D. P., Casavant C. H., Fulwyler M. J. Flow cytometric detection and quantitation of immune complexes using human C1q-coated microspheres. J Immunol Methods. 1986 Dec 4;95(1):57–61. doi: 10.1016/0022-1759(86)90317-0. [DOI] [PubMed] [Google Scholar]
- McSharry J. J., Costantino R., McSharry M. B., Venezia R. A., Lehman J. M. Rapid detection of herpes simplex virus in clinical samples by flow cytometry after amplification in tissue culture. J Clin Microbiol. 1990 Aug;28(8):1864–1866. doi: 10.1128/jcm.28.8.1864-1866.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Costantino R., Robbiano E., Echols R., Stevens R., Lehman J. M. Detection and quantitation of human immunodeficiency virus-infected peripheral blood mononuclear cells by flow cytometry. J Clin Microbiol. 1990 Apr;28(4):724–733. doi: 10.1128/jcm.28.4.724-733.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellencamp M. W., O'Brien P. C., Stevenson J. R. Pseudorabies virus-induced suppression of major histocompatibility complex class I antigen expression. J Virol. 1991 Jun;65(6):3365–3368. doi: 10.1128/jvi.65.6.3365-3368.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercure L., Brenner B. J., Phaneuf D., Tsoukas C., Wainberg M. A. Effect of 3'-azido-3'-deoxythymidine and 2',3'-dideoxyinosine on establishment of human immunodeficiency virus type 1 infection in cultured CD8+ lymphocytes. Antimicrob Agents Chemother. 1994 May;38(5):986–990. doi: 10.1128/aac.38.5.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael N. L., Vahey M., Burke D. S., Redfield R. R. Viral DNA and mRNA expression correlate with the stage of human immunodeficiency virus (HIV) type 1 infection in humans: evidence for viral replication in all stages of HIV disease. J Virol. 1992 Jan;66(1):310–316. doi: 10.1128/jvi.66.1.310-316.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles S. A., Balden E., Magpantay L., Wei L., Leiblein A., Hofheinz D., Toedter G., Stiehm E. R., Bryson Y. Rapid serologic testing with immune-complex-dissociated HIV p24 antigen for early detection of HIV infection in neonates. Southern California Pediatric AIDS Consortium. N Engl J Med. 1993 Feb 4;328(5):297–302. doi: 10.1056/NEJM199302043280501. [DOI] [PubMed] [Google Scholar]
- Miller C. L., Longnecker R., Kieff E. Epstein-Barr virus latent membrane protein 2A blocks calcium mobilization in B lymphocytes. J Virol. 1993 Jun;67(6):3087–3094. doi: 10.1128/jvi.67.6.3087-3094.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T., Risser R. Interference established in mice by infection with Friend murine leukemia virus. J Virol. 1992 Sep;66(9):5696–5702. doi: 10.1128/jvi.66.9.5696-5702.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori D. C., Robinson W. E., Jr, Modliszewski A., Mitchell W. M. Effective inactivation of human immunodeficiency virus with chlorhexidine antiseptics containing detergents and alcohol. J Hosp Infect. 1990 Apr;15(3):279–282. doi: 10.1016/0195-6701(90)90036-n. [DOI] [PubMed] [Google Scholar]
- Moody D. J., Casavant C. H., Fulwyler M. J., McHugh T. M., Stites D. P. Multiparameter flow cytometric analysis of mononuclear cells from HIV-infected individuals. Cytometry Suppl. 1988;3:44–47. doi: 10.1002/cyto.990090810. [DOI] [PubMed] [Google Scholar]
- Morris T. D., Miller L. K. Characterization of productive and non-productive AcMNPV infection in selected insect cell lines. Virology. 1993 Nov;197(1):339–348. doi: 10.1006/viro.1993.1595. [DOI] [PubMed] [Google Scholar]
- Naniche D., Varior-Krishnan G., Cervoni F., Wild T. F., Rossi B., Rabourdin-Combe C., Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993 Oct;67(10):6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyts J., Snoeck R., Schols D., Himpens B., De Clercq E. Sensitive, reproducible and convenient fluorometric assay for the in vitro evaluation of anti-cytomegalovirus agents. J Virol Methods. 1991 Nov;35(1):27–38. doi: 10.1016/0166-0934(91)90082-b. [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., Browning S. W., Orloff S. L., McDougal J. S. Inactivation of HIV-infected H9 cells in whole blood preparations by lysing/fixing reagents used in flow cytometry. J Immunol Methods. 1993 Apr 2;160(2):215–218. doi: 10.1016/0022-1759(93)90180-f. [DOI] [PubMed] [Google Scholar]
- Nishino Y., Ohki K., Kimura T., Morikawa S., Mikami T., Ikuta K. Major core proteins, p24s, of human, simian, and feline immunodeficiency viruses are partly expressed on the surface of the virus-infected cells. Vaccine. 1992;10(10):677–683. doi: 10.1016/0264-410x(92)90089-3. [DOI] [PubMed] [Google Scholar]
- Ohlsson-Wilhelm B. M., Cory J. M., Kessler H. A., Eyster M. E., Rapp F., Landay A. Circulating human immunodeficiency virus (HIV) p24 antigen-positive lymphocytes: a flow cytometric measure of HIV infection. J Infect Dis. 1990 Nov;162(5):1018–1024. doi: 10.1093/infdis/162.5.1018. [DOI] [PubMed] [Google Scholar]
- Ordóez J. V., Wehman N. M. Rapid flow cytometric antibiotic susceptibility assay for Staphylococcus aureus. Cytometry. 1993 Oct;14(7):811–818. doi: 10.1002/cyto.990140714. [DOI] [PubMed] [Google Scholar]
- Oyaizu N., McCloskey T. W., Coronesi M., Chirmule N., Kalyanaraman V. S., Pahwa S. Accelerated apoptosis in peripheral blood mononuclear cells (PBMCs) from human immunodeficiency virus type-1 infected patients and in CD4 cross-linked PBMCs from normal individuals. Blood. 1993 Dec 1;82(11):3392–3400. [PubMed] [Google Scholar]
- Pan L. Z., Werner A., Levy J. A. Detection of plasma viremia in human immunodeficiency virus-infected individuals at all clinical stages. J Clin Microbiol. 1993 Feb;31(2):283–288. doi: 10.1128/jcm.31.2.283-288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Parker J. W., Adelsberg B., Azen S. P., Boone D., Fletcher M. A., Gjerset G. F., Hassett J., Kaplan J., Niland J. C., Odom-Maryon T. Leukocyte immunophenotyping by flow cytometry in a multisite study: standardization, quality control, and normal values in the Transfusion Safety Study. The Transfusion Safety Study Group. Clin Immunol Immunopathol. 1990 May;55(2):187–220. doi: 10.1016/0090-1229(90)90097-a. [DOI] [PubMed] [Google Scholar]
- Pattanapanyasat K., Udomsangpetch R., Webster H. K. Two-color flow cytometric analysis of intraerythrocytic malaria parasite DNA and surface membrane-associated antigen in erythrocytes infected with Plasmodium falciparum. Cytometry. 1993;14(4):449–454. doi: 10.1002/cyto.990140415. [DOI] [PubMed] [Google Scholar]
- Patterson B. K., Till M., Otto P., Goolsby C., Furtado M. R., McBride L. J., Wolinsky S. M. Detection of HIV-1 DNA and messenger RNA in individual cells by PCR-driven in situ hybridization and flow cytometry. Science. 1993 May 14;260(5110):976–979. doi: 10.1126/science.8493534. [DOI] [PubMed] [Google Scholar]
- Pauwels R., De Clercq E., Desmyter J., Balzarini J., Goubau P., Herdewijn P., Vanderhaeghe H., Vandeputte M. Sensitive and rapid assay on MT-4 cells for detection of antiviral compounds against the AIDS virus. J Virol Methods. 1987 Jun;16(3):171–185. doi: 10.1016/0166-0934(87)90002-4. [DOI] [PubMed] [Google Scholar]
- Piatak M., Jr, Saag M. S., Yang L. C., Clark S. J., Kappes J. C., Luk K. C., Hahn B. H., Shaw G. M., Lifson J. D. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993 Mar 19;259(5102):1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- Pollice A. A., McCoy J. P., Jr, Shackney S. E., Smith C. A., Agarwal J., Burholt D. R., Janocko L. E., Hornicek F. J., Singh S. G., Hartsock R. J. Sequential paraformaldehyde and methanol fixation for simultaneous flow cytometric analysis of DNA, cell surface proteins, and intracellular proteins. Cytometry. 1992;13(4):432–444. doi: 10.1002/cyto.990130414. [DOI] [PubMed] [Google Scholar]
- Posner M. R., Elboim H. S., Cannon T., Cavacini L., Hideshima T. Functional activity of an HIV-1 neutralizing IgG human monoclonal antibody: ADCC and complement-mediated lysis. AIDS Res Hum Retroviruses. 1992 May;8(5):553–558. doi: 10.1089/aid.1992.8.553. [DOI] [PubMed] [Google Scholar]
- Posner M. R., Hideshima T., Cannon T., Mukherjee M., Mayer K. H., Byrn R. A. An IgG human monoclonal antibody that reacts with HIV-1/GP120, inhibits virus binding to cells, and neutralizes infection. J Immunol. 1991 Jun 15;146(12):4325–4332. [PubMed] [Google Scholar]
- Potts B. J., Field K. G., Wu Y., Posner M., Cavacini L., White-Scharf M. Synergistic inhibition of HIV-1 by CD4 binding domain reagents and V3-directed monoclonal antibodies. Virology. 1993 Nov;197(1):415–419. doi: 10.1006/viro.1993.1604. [DOI] [PubMed] [Google Scholar]
- Quinn T. C., Kline R., Moss M. W., Livingston R. A., Hutton N. Acid dissociation of immune complexes improves diagnostic utility of p24 antigen detection in perinatally acquired human immunodeficiency virus infection. J Infect Dis. 1993 May;167(5):1193–1196. doi: 10.1093/infdis/167.5.1193. [DOI] [PubMed] [Google Scholar]
- Qvist P., Houe H., Aasted B., Meyling A. Comparison of flow cytometry and virus isolation in cell culture for identification of cattle persistently infected with bovine viral diarrhea virus. J Clin Microbiol. 1991 Mar;29(3):660–661. doi: 10.1128/jcm.29.3.660-661.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razvi E. S., Welsh R. M. Programmed cell death of T lymphocytes during acute viral infection: a mechanism for virus-induced immune deficiency. J Virol. 1993 Oct;67(10):5754–5765. doi: 10.1128/jvi.67.10.5754-5765.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re M. C., Zauli G., Gibellini D., Furlini G., Ramazzotti E., Monari P., Ranieri S., Capitani S., La Placa M. Uninfected haematopoietic progenitor (CD34+) cells purified from the bone marrow of AIDS patients are committed to apoptotic cell death in culture. AIDS. 1993 Aug;7(8):1049–1055. doi: 10.1097/00002030-199308000-00004. [DOI] [PubMed] [Google Scholar]
- Reinhart T. A., Ghosh A. K., Hoover E. A., Mullins J. I. Distinct superinfection interference properties yet similar receptor utilization by cytopathic and noncytopathic feline leukemia viruses. J Virol. 1993 Sep;67(9):5153–5162. doi: 10.1128/jvi.67.9.5153-5162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remick S. C., McSharry J. J., Wolf B. C., Blanchard C. G., Eastman A. Y., Wagner H., Portuese E., Wighton T., Powell D., Pearce T. Novel oral combination chemotherapy in the treatment of intermediate-grade and high-grade AIDS-related non-Hodgkin's lymphoma. J Clin Oncol. 1993 Sep;11(9):1691–1702. doi: 10.1200/JCO.1993.11.9.1691. [DOI] [PubMed] [Google Scholar]
- Ritzi E. M. Quantitative flow cytometry of mouse mammary tumor virus envelope glycoprotein (gp52): alternative measures of hormone-mediated change in a viral cell surface antigen. J Virol Methods. 1992 Oct;40(1):11–30. doi: 10.1016/0166-0934(92)90003-v. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. S., Hodnichak C. M., Summers J. L. Flow cytometric evaluation of anti-herpes drugs. Cytometry. 1987 Jul;8(4):392–395. doi: 10.1002/cyto.990080408. [DOI] [PubMed] [Google Scholar]
- Sachsenmeier K. F., Schell K., Morrissey L. W., Pennell D. R., West R. M., Callister S. M., Schell R. F. Detection of borreliacidal antibodies in hamsters by using flow cytometry. J Clin Microbiol. 1992 Jun;30(6):1457–1461. doi: 10.1128/jcm.30.6.1457-1461.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Takamura C., Yasuda A., Miyamoto M., Kamogawa K., Yasui K. High-level expression of the Japanese encephalitis virus E protein by recombinant vaccinia virus and enhancement of its extracellular release by the NS3 gene product. Virology. 1993 Feb;192(2):483–490. doi: 10.1006/viro.1993.1064. [DOI] [PubMed] [Google Scholar]
- Sattar S. A., Springthorpe V. S. Survival and disinfectant inactivation of the human immunodeficiency virus: a critical review. Rev Infect Dis. 1991 May-Jun;13(3):430–447. doi: 10.1093/clinids/13.3.430. [DOI] [PubMed] [Google Scholar]
- Schimenti K. J., Jacobberger J. W. Fixation of mammalian cells for flow cytometric evaluation of DNA content and nuclear immunofluorescence. Cytometry. 1992;13(1):48–59. doi: 10.1002/cyto.990130109. [DOI] [PubMed] [Google Scholar]
- Schlicht H. J. Biosynthesis of the secretory core protein of duck hepatitis B virus: intracellular transport, proteolytic processing, and membrane expression of the precore protein. J Virol. 1991 Jul;65(7):3489–3495. doi: 10.1128/jvi.65.7.3489-3495.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid I., Uittenbogaart C. H., Giorgi J. V. A gentle fixation and permeabilization method for combined cell surface and intracellular staining with improved precision in DNA quantification. Cytometry. 1991;12(3):279–285. doi: 10.1002/cyto.990120312. [DOI] [PubMed] [Google Scholar]
- Schmid I., Uittenbogaart C. H., Giorgi J. V. Sensitive method for measuring apoptosis and cell surface phenotype in human thymocytes by flow cytometry. Cytometry. 1994 Jan 1;15(1):12–20. doi: 10.1002/cyto.990150104. [DOI] [PubMed] [Google Scholar]
- Schmid I., Uittenbogaart C. H., Keld B., Giorgi J. V. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J Immunol Methods. 1994 Apr 15;170(2):145–157. doi: 10.1016/0022-1759(94)90390-5. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Psallidopoulos M. C., Lane H. C., Thompson L., Baseler M., Massari F., Fox C. H., Salzman N. P., Fauci A. S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989 Jul 21;245(4915):305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- Schnorr J. J., Schneider-Schaulies S., Simon-Jödicke A., Pavlovic J., Horisberger M. A., ter Meulen V. MxA-dependent inhibition of measles virus glycoprotein synthesis in a stably transfected human monocytic cell line. J Virol. 1993 Aug;67(8):4760–4768. doi: 10.1128/jvi.67.8.4760-4768.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schols D., Baba M., Pauwels R., De Clercq E. Flow cytometric method to demonstrate whether anti-HIV-1 agents inhibit virion binding to T4+ cells. J Acquir Immune Defic Syndr. 1989;2(1):10–15. [PubMed] [Google Scholar]
- Schols D., Pauwels R., Baba M., Desmyter J., De Clercq E. Syncytium formation and destruction of bystander CD4+ cells cocultured with T cells persistently infected with human immunodeficiency virus as demonstrated by flow cytometry. J Gen Virol. 1989 Sep;70(Pt 9):2397–2408. doi: 10.1099/0022-1317-70-9-2397. [DOI] [PubMed] [Google Scholar]
- Schols D., Pauwels R., Desmyter J., De Clercq E. Dextran sulfate and other polyanionic anti-HIV compounds specifically interact with the viral gp120 glycoprotein expressed by T-cells persistently infected with HIV-1. Virology. 1990 Apr;175(2):556–561. doi: 10.1016/0042-6822(90)90440-3. [DOI] [PubMed] [Google Scholar]
- Schols D., Pauwels R., Desmyter J., De Clercq E. Flow cytometric method to monitor the destruction of CD4+ cells following their fusion with HIV-infected cells. Cytometry. 1990;11(6):736–743. doi: 10.1002/cyto.990110611. [DOI] [PubMed] [Google Scholar]
- Schols D., Pauwels R., Vanlangendonck F., Balzarini J., De Clercq E. A highly reliable, sensitive, flow cytometric/fluorometric assay for the evaluation of the anti-HIV activity of antiviral compounds in MT-4 cells. J Immunol Methods. 1988 Nov 10;114(1-2):27–32. doi: 10.1016/0022-1759(88)90149-4. [DOI] [PubMed] [Google Scholar]
- Schwartz O., Rivière Y., Heard J. M., Danos O. Reduced cell surface expression of processed human immunodeficiency virus type 1 envelope glycoprotein in the presence of Nef. J Virol. 1993 Jun;67(6):3274–3280. doi: 10.1128/jvi.67.6.3274-3280.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scillian J. J., McHugh T. M., Busch M. P., Tam M., Fulwyler M. J., Chien D. Y., Vyas G. N. Early detection of antibodies against rDNA-produced HIV proteins with a flow cytometric assay. Blood. 1989 May 15;73(7):2041–2048. [PubMed] [Google Scholar]
- Shang F., Huang H., Revesz K., Chen H. C., Herz R., Pinter A. Characterization of monoclonal antibodies against the human immunodeficiency virus matrix protein, p17gag: identification of epitopes exposed at the surfaces of infected cells. J Virol. 1991 Sep;65(9):4798–4804. doi: 10.1128/jvi.65.9.4798-4804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoff A. M., Holland T. C. The effect of cytoplasmic domain mutations on membrane anchoring and glycoprotein processing of herpes simplex virus type 1 glycoprotein C. Virology. 1993 Oct;196(2):804–816. doi: 10.1006/viro.1993.1538. [DOI] [PubMed] [Google Scholar]
- Sladek T. L., Jacobberger J. W. Dependence of SV40 large T-antigen cell cycle regulation on T-antigen expression levels. Oncogene. 1992 Jul;7(7):1305–1313. [PubMed] [Google Scholar]
- Sladek T. L., Jacobberger J. W. Simian virus 40 large T-antigen expression decreases the G1 and increases the G2 + M cell cycle phase durations in exponentially growing cells. J Virol. 1992 Feb;66(2):1059–1065. doi: 10.1128/jvi.66.2.1059-1065.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sligh J. M., Roodman S. T., Tsai C. C. Flow cytometric indirect immunofluorescence assay with high sensitivity and specificity for detection of antibodies to human immunodeficiency virus (HIV). Am J Clin Pathol. 1989 Feb;91(2):210–214. doi: 10.1093/ajcp/91.2.210. [DOI] [PubMed] [Google Scholar]
- Snoeck R., Schols D., Andrei G., Neyts J., De Clercq E. Antiviral activity of anti-cytomegalovirus agents (HPMPC, HPMPA) assessed by a flow cytometric method and DNA hybridization technique. Antiviral Res. 1991 Jul;16(1):1–9. doi: 10.1016/0166-3542(91)90053-t. [DOI] [PubMed] [Google Scholar]
- Snoeck R., Schols D., Sadzot-Delvaux C., Cloes J. M., Andrei G., De Clercq E., Piette J., Rentier B. Flow cytometric method for the detection of gpI antigens of varicella zoster virus and evaluation of anti-VZV agents. J Virol Methods. 1992 Aug;38(2):243–254. doi: 10.1016/0166-0934(92)90114-s. [DOI] [PubMed] [Google Scholar]
- Sodora D. L., Eisenberg R. J., Cohen G. H. Characterization of a recombinant herpes simplex virus which expresses a glycoprotein D lacking asparagine-linked oligosaccharides. J Virol. 1991 Aug;65(8):4432–4441. doi: 10.1128/jvi.65.8.4432-4441.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear G. T., Ou C. Y., Kessler H. A., Moore J. L., Schochetman G., Landay A. L. Analysis of lymphocytes, monocytes, and neutrophils from human immunodeficiency virus (HIV)-infected persons for HIV DNA. J Infect Dis. 1990 Dec;162(6):1239–1244. doi: 10.1093/infdis/162.6.1239. [DOI] [PubMed] [Google Scholar]
- Steele-Mortimer O. A., Meier-Ewert H., Löser R., Hasmann M. J. Flow cytometric analysis of virus-infected cells and its potential use for screening antiviral agents. J Virol Methods. 1990 Mar;27(3):241–252. doi: 10.1016/0166-0934(90)90092-t. [DOI] [PubMed] [Google Scholar]
- Syrjälä M. T., Tiirikainen M., Jansson S. E., Krusius T. Flow cytometric analysis of terminal deoxynucleotidyl transferase. A simplified method. Am J Clin Pathol. 1993 Mar;99(3):298–303. doi: 10.1093/ajcp/99.3.298. [DOI] [PubMed] [Google Scholar]
- Söderberg C., Larsson S., Bergstedt-Lindqvist S., Möller E. Definition of a subset of human peripheral blood mononuclear cells that are permissive to human cytomegalovirus infection. J Virol. 1993 Jun;67(6):3166–3175. doi: 10.1128/jvi.67.6.3166-3175.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddeo B., Federico M., Titti F., Rossi G. B., Verani P. Homologous superinfection of both producer and nonproducer HIV-infected cells is blocked at a late retrotranscription step. Virology. 1993 Jun;194(2):441–452. doi: 10.1006/viro.1993.1283. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Okamoto H., Kishimoto S., Munekata E., Tachibana K., Akahane Y., Yoshizawa H., Mishiro S. Demonstration of a hepatitis C virus-specific antigen predicted from the putative core gene in the circulation of infected hosts. J Gen Virol. 1992 Mar;73(Pt 3):667–672. doi: 10.1099/0022-1317-73-3-667. [DOI] [PubMed] [Google Scholar]
- Takizawa T., Matsukawa S., Higuchi Y., Nakamura S., Nakanishi Y., Fukuda R. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J Gen Virol. 1993 Nov;74(Pt 11):2347–2355. doi: 10.1099/0022-1317-74-11-2347. [DOI] [PubMed] [Google Scholar]
- Thomas M. S., Gao M., Knipe D. M., Powell K. L. Association between the herpes simplex virus major DNA-binding protein and alkaline nuclease. J Virol. 1992 Feb;66(2):1152–1161. doi: 10.1128/jvi.66.2.1152-1161.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm E. A., Jr, Stewart C. C. Fluorescent in situ hybridization en suspension (FISHES) using digoxigenin-labeled probes and flow cytometry. Biotechniques. 1992 Mar;12(3):362–367. [PubMed] [Google Scholar]
- Tsai W. P., Oroszlan S. Site-directed cytotoxic antibody against the C-terminal segment of the surface glycoprotein gp90 of avian reticuloendotheliosis virus. Virology. 1988 Oct;166(2):608–611. doi: 10.1016/0042-6822(88)90535-1. [DOI] [PubMed] [Google Scholar]
- Wallner G., Amann R., Beisker W. Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry. 1993;14(2):136–143. doi: 10.1002/cyto.990140205. [DOI] [PubMed] [Google Scholar]
- Whitt M. A., Buonocore L., Prehaud C., Rose J. K. Membrane fusion activity, oligomerization, and assembly of the rabies virus glycoprotein. Virology. 1991 Dec;185(2):681–688. doi: 10.1016/0042-6822(91)90539-n. [DOI] [PubMed] [Google Scholar]
- Whitt M. A., Rose J. K. Fatty acid acylation is not required for membrane fusion activity or glycoprotein assembly into VSV virions. Virology. 1991 Dec;185(2):875–878. doi: 10.1016/0042-6822(91)90563-q. [DOI] [PubMed] [Google Scholar]
- Wood R., Dong H., Katzenstein D. A., Merigan T. C. Quantification and comparison of HIV-1 proviral load in peripheral blood mononuclear cells and isolated CD4+ T cells. J Acquir Immune Defic Syndr. 1993 Mar;6(3):237–240. [PubMed] [Google Scholar]
- Zauli G., Vitale M., Re M. C., Furlini G., Zamai L., Falcieri E., Gibellini D., Visani G., Davis B. R., Capitani S. In vitro exposure to human immunodeficiency virus type 1 induces apoptotic cell death of the factor-dependent TF-1 hematopoietic cell line. Blood. 1994 Jan 1;83(1):167–175. [PubMed] [Google Scholar]
- del Llano A. M., Amieiro-Puig J. P., Kraiselburd E. N., Kessler M. J., Málaga C. A., Lavergne J. A. The combined assessment of cellular apoptosis, mitochondrial function and proliferative response to pokeweed mitogen has prognostic value in SIV infection. J Med Primatol. 1993 Feb-May;22(2-3):147–153. [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Parker M. D., Fitzpatrick D. R., Zamb T. J., van den Hurk J. V., Campos M., Harland R., Babiuk L. A. Expression of bovine herpesvirus 1 glycoprotein gIV by recombinant baculovirus and analysis of its immunogenic properties. J Virol. 1991 Jan;65(1):263–271. doi: 10.1128/jvi.65.1.263-271.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Parker M. D., Fitzpatrick D. R., Zamb T. J., van den Hurk J. V., Campos M., Harland R., Babiuk L. A. Expression of bovine herpesvirus 1 glycoprotein gIV by recombinant baculovirus and analysis of its immunogenic properties. J Virol. 1991 Jan;65(1):263–271. doi: 10.1128/jvi.65.1.263-271.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vianen P. H., van Engen A., Thaithong S., van der Keur M., Tanke H. J., van der Kaay H. J., Mons B., Janse C. J. Flow cytometric screening of blood samples for malaria parasites. Cytometry. 1993;14(3):276–280. doi: 10.1002/cyto.990140307. [DOI] [PubMed] [Google Scholar]