Abstract
The clinical significance of circulating tumor cells (CTCs) including cancer stem cells (CSCs) (CTC/CSC) in the tumor drainage vein blood of patients with colorectal cancer (CRC) is unclear. In this study, we investigated the prognostic value of CTC/CSC that express carcinoembryonic antigen (CEA) cytokeratin 19 (CK19), CK20 and/or CD133 (CEA/CK/CD133) mRNA in the tumor drainage blood of CRC patients with Dukes' stage B and C. We examined tumor drainage blood from 197 patients with Dukes' stage B and C CRC. CTCs that expressed CEA, CK19, CK20 and CD133 mRNA were detected using the quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) assay. Each mRNA level was normalized with GAPDH mRNA levels. In the relationship between the expression of CEA/CK/CD133 in the tumor drainage blood and clinicopathological factors, a significant correlation was observed between CEA/CK/CD133 expression and Dukes' stage (p<0.041). In CRC patients with Dukes' stage B and C, disease-free (DFS) and overall survival (OS) of patients with CEA/CK/CD133 positive in the tumor drainage blood were significantly worse than that of marker gene negative patients. In contrast, in patients with Dukes' stage A, no significant differences were shown between these groups. By Cox progression analysis, it was shown that CEA/CK/CD133 mRNA in tumor drainage blood was an independent prognostic factor for DFS and OS in patients with Dukes' stage B and C. These results suggest that detecting CEA/CK/CD133 mRNA in tumor drainage blood by the real-time RT-PCR method would have a prognostic value in CRC patients with Dukes' stage B and C.
Keywords: colorectal cancer, circulating tumor cells, cancer stem cell, CD133, CEA, CK20, CK19, tumor drainage vein blood
Introduction
Colorectal cancer (CRC) is one of the leading causes of worldwide cancer-associated morbidity and mortality (1). CRC is staged according to the extent of primary organ involvement and metastatic spread to lymph nodes or distant organs. The 5-year survival rates of CRC patients with Dukes' stage B and C who underwent surgery were 75–80% and 65–70%, respectively (2). Despite surgical resection being highly effective for localized disease, a significant proportion of these patients develop recurrence. Of particular concern is the fact that it is not possible to accurately differentiate between good and poor prognosis of Dukes' stage B. Effective biomarkers for the detection of Dukes' stage B patients who are at high risk are needed since the role of adjuvant chemotherapy in these patients remains controversial (3–7).
The utility of circulating tumor cells (CTCs) as biomarkers for predicting the clinical outcome of patients with various cancers has been shown by many studies (8–11). The detection of CTC in blood may not only provide the mechanism for the early metastatic spreading of isolated cancer cells, but it can also potentially indicate substantial predictive and prognostic information on CRC patients (9,12–15). In the detection of aggressive CTCs in blood, the possibility of a cancer stem-like cell (CSCs) marker is currently attracting attention (16). CSCs have been defined as a unique subpopulation in tumors that possess the ability to initiate tumor growth and sustain tumor self-renewal (17,18). Accumulating evidence shows that CSCs are associated with metastasis, resistance to chemotherapy and radiotherapy, and recurrence. It is known that CSC markers are frequently over-expressed in the CTC of patients with metastatic breast cancer, and most CTCs have CSC phenotypes that are not proliferating and resistant to chemotherapy (11,19). These properties suggest that the founder cells of metastases may arise from the CTC population. Recently, we demonstrated that detection of CTC, including CSC (CTC/CSC) in PB, is a useful tool for determining high risk for recurrence and poor prognosis in patients with Dukes' stage B and C CRC (16).
Peripheral blood (PB) and tumor drainage vein blood were used to obtain a CTC sample (9). As compared to the utility of CTCs in PB, the property of CTCs in tumor drainage vein blood is still unclear. It is known that the detection rate of CTCs in tumor drainage vein blood is higher than that of PB (9). Therefore, the detection of CTC/CSC in tumor drainage vein blood may show a high sensitivity for selection of the high risk patients for recurrence in Dukes' B and C patients. However, little is known about the clinical significance of CTC/CSC in the tumor drainage vein blood of these patients.
In this study, we aimed to clarify the usefulness of CTC/CSC in the tumor drainage vein blood as a prognostic biomarker in CRC patients with Dukes' stage B and C. To detect the CTC/CSC, we utilized the real-time RT-PCR method using multiple marker genes consisting of CEA, CK19 and CK20 mRNA for the general CRC-associated marker, and CD133 mRNA for the CSC marker.
Patients and methods
Patients
A total of 197 CRC patients (107 male and 90 female) with Dukes' stage B and C who underwent surgery at Teikyo University Hospital between 2000 and 2004 were enrolled in this study. The observation period ranged from 22 to 60 months, and the median follow-up period was 37 months. Study protocol conformed to the guidelines of the ethics committee of each university. Written informed consent was obtained from all patients. Their ages ranged from 27–82 years, with a mean age of 68 years. The stages of the tumors were determined according to the Dukes classification system. As a follow-up, all patients were re-evaluated at 3-month intervals during the first year, and then at 18 months, 24 months and yearly thereafter. Each evaluation consisted of a pertinent medical history, physical examination and repetition of imaging studies, including CT scans of the abdomen.
Blood sampling and cDNA preparation
As controls, portal system blood samples collected from 20 patients with benign diseases were prepared. In CRC patients, blood samples were obtained from the mesenteric vein draining the tumor immediately after laparotomy. These samples were collected in PAXgene tubes, stored at −80˚C, and transferred to Teikyo University for real-time RT-PCR assay. Total RNA of the blood samples in PAXgene tubes was extracted using a PAXgene blood RNA Kit (Qiagen K.K. GmbH, Germany). Extracted total RNA was reverse transcribed into cDNA using oligo-p(dT)12–18 primers according to the manufacturer's protocol (Invitrogen Corp., CA, USA).
Quantitative real-time RT-PCR
The primer and probe sequences of CEA, CK19, CK20, CD133 and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) have been previously described (16). GAPDH was utilized as an internal control. Real-time quantitative RT-PCR of these transcripts was performed with the LightCycler instrument (Roche Diagnostics, Mannheim, Germany) as previously described. The expression levels of CEA CK19, CK20 and CD133 were normalized by GAPDH, and the ratio of CEA, CK19, CK20 or CD133 copies to the GAPDH copies was calculated.
Statistical analysis
The correlation between the presence of CEA, CK19, CK20 and CD133 mRNA in the blood and the various clinical parameters was evaluated using the chi-squared test. Disease-free survival (DFS) and overall survival (OS) were analyzed using the Kaplan-Meier method, and the differences were examined using the log-rank test. Univariate and multivariate analysis were performed using Cox regression analysis. P-values <0.05 were considered statistically significant.
Results
Cut-offs of markers and expression of CEA/CK/CD133 mRNA
The CEA, CK19, CK20 and CD133 mRNA levels were normalized with GAPDH mRNA levels in the portal system blood samples from 20 patients with benign diseases prepared for the control of tumor drainage blood to determine the cut-off levels. Based on the range of CEA/GAPDH, CK19/GAPDH, CK20/GAPDH and CD133/GAPDH, we determined the cut-off value by the 95% confidence intervals (mean plus 1.96 standard deviation) of the control groups. In the portal system blood samples, cut-off ratios were 5.7×10−6 in CEA/GAPDH and 9.5×10−5 in CK19/GAPDH, 2.9×10−5 in CK20/GAPDH and 4.7×10−4 in CD133/GAPDH. The positive rates, sensitivity and specificity of various combinations of genetic markers were examined (data not shown). As previous reported, CEA+, CK19+, CK20+, and/or CD133+ (CEA/CK/CD133) showed the highest positivity, sensitivity and specificity in the multimarker groups (16). Therefore, we selected the CEA/CK/CD133 group as representative of PCR positivity and used it for the following prognostic analysis.
Relationship between CEA/CK/CD133 mRNA and clinicopathological factors
The relationship between the expression of CEA/CK/CD133 in the tumor drainage blood, and the clinicopathological factors was examined. A significant correlation was observed between CEA/CK/CD133 expression and the Dukes' stage (Table I). These results suggest that the presence of CTC in the tumor drainage blood correlated to the tumor progression.
Table I.
Relationship between clinicopathological factors and PCR positivity rates.
| Variables | No. of patients (n=197) | PCR positive cases (n=124) | Positive rate (%) | p-value |
|---|---|---|---|---|
| Age (years) | 68±11 | |||
| Sex | ||||
| Male | 107 | 63 | 58.88 | 0.198 |
| Female | 90 | 61 | 67.78 | |
| Tumor size (cm) | ||||
| <5 | 109 | 72 | 66.06 | 0.314 |
| ≥5 | 88 | 52 | 59.09 | |
| Histological type | ||||
| Well differentiated | 138 | 92 | 66.67 | 0.098 |
| Poorly differentiated | 59 | 32 | 54.24 | |
| Lymphatic invasion | ||||
| Negative | 130 | 83 | 63.85 | 0.715 |
| Positive | 67 | 41 | 61.19 | |
| Venous invasion | ||||
| Negative | 84 | 50 | 59.52 | 0.391 |
| Positive | 113 | 74 | 65.49 | |
| Dukes' stage | ||||
| B | 111 | 63 | 56.76 | 0.041a |
| C | 86 | 61 | 70.93 | |
P<0.05.
Kaplan-Meier OS and DFS curve analysis
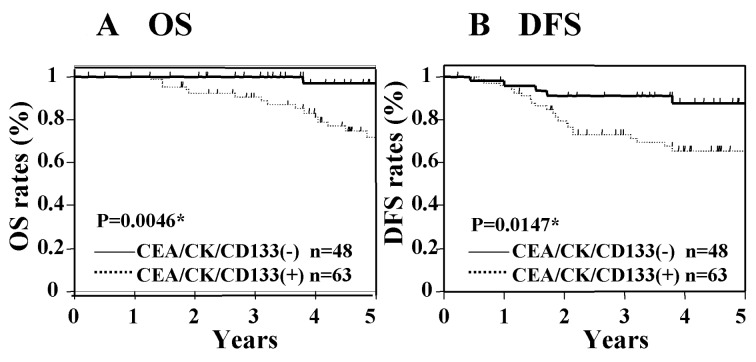
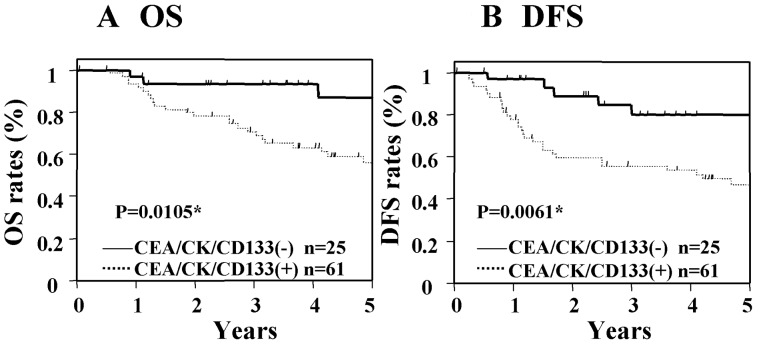
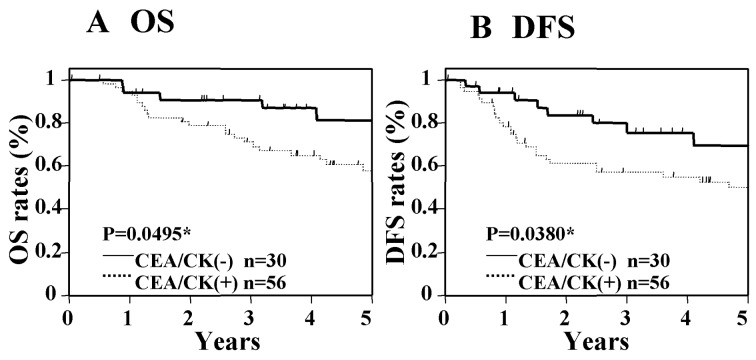
The Kaplan-Meier survival curves show the OS and DFS rates according to the CEA/CK/CD133 gene expression status in CRC patients with Dukes' stage B and C. In this analysis, the average follow-up time for OS was 36.1±20.7 months and that of DFS was 38.8±16.2 months. Fig. 1 shows the OS and DFS in Dukes' stage B cancer according to the PCR status. The OS and DFS of those patients with CEA/CK/CD133 positivity were significantly worse than those who were negative for these markers (Fig. 1). In patients with Dukes' stage C cancer, the OS and DFS of the group with CEA/CK/CD133 positivity were significantly worse than those patients who were negative for these markers (Fig. 2). These results suggest that the expression of CEA/CK/CD133 in tumor drainage blood is associated with poor prognosis in patients with Dukes' stage B and C cancer.
Figure 1.
Kaplan-Meier survival curves for OS and DFS and status of CEA/CK/CD133 positivity in patients with Dukes' stage B cancer. Significant differences were shown between the CEA/CK/CD133 positive (+) and negative group (−). *P-value<0.05.
Figure 2.
Kaplan-Meier survival curves for OS and DFS and status of CEA/CK/CD133 positivity in patients with Dukes' stage C cancer. Significant differences were shown between the CEA/CK/CD133 positive (+) and negative group (−). *P-value<0.05.
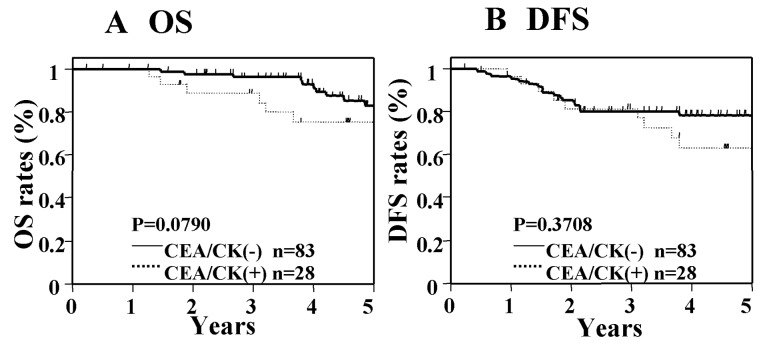
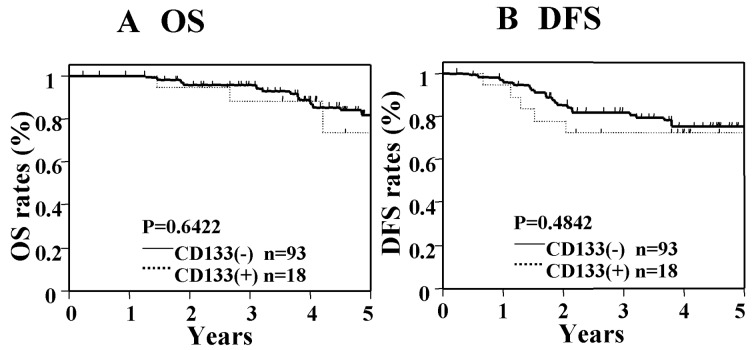
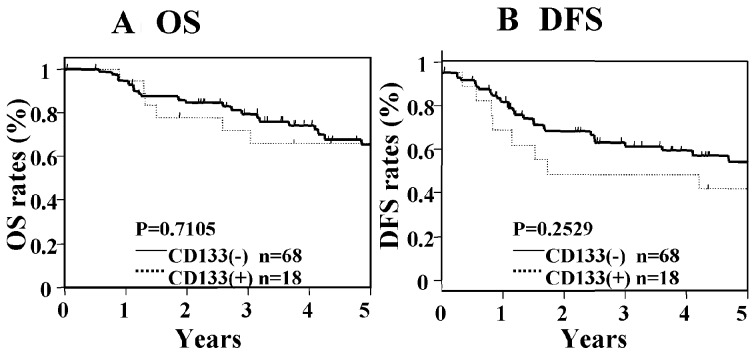
Next, we analyzed the OS and DFS in the general CTCs markers (CEA, CK19, and/or CK20: CEA/CK), and in the CD133 single marker. In patients with Dukes' stage B cancer, significant differences in OS and DFS were not seen between those who were positive for CEA/CK and those who were negative for CEA/CK (Fig. 3), nor were significant differences found between patients who were positive for CD133 as compared with those who were negative for CD133 (Fig. 4). In contrast, in patients with Dukes' stage C cancer, significant differences were seen in OS and DFS between those who were positive for CEA/CK and those who were negative for CEA/CK (Fig. 5). However, in the CD133 single-marker analysis, significant differences in OS and DFS were not found between patients who were positive for CD133 and those who were negative for CD133 (Fig. 6). These results suggest that the addition of CD133 to general CTCs markers is important for the determination of patients at high risk of recurrence and poor prognosis in Dukes' stage B cancer.
Figure 3.
Kaplan-Meier survival curves for OS and DFS and status of CEA/CK positivity in patients with Dukes' stage B cancer. Significant differences were not seen between the CEA/CK positive (+) and negative group (−).
Figure 4.
Kaplan-Meier survival curves for OS and DFS and status of CD133 positivity in patients with Dukes' stage B cancer. Significant differences were not seen between the CD133 positive (+) and negative group (−).
Figure 5.
Kaplan-Meier survival curves for OS and DFS and status of CEA/CK positivity in patients with Dukes' stage C cancer. Significant differences were shown between the CEA/CK positive (+) and negative group (−). *P-value<0.05.
Figure 6.
Kaplan-Meier survival curves for OS and DFS and status of CD133 positivity in patients with Dukes' stage C cancer. Significant differences were not seen between the CD133 positive (+) and negative group (−).
Cox univariate and multivariate analysis of prognostic factors
Table II shows the results of univariate and multivariate Cox analysis of various factors for OS and DFS in patients with Dukes' stage B cancer. In univariate analysis of these patients, venous invasion and the CEA/CK/CD133 showed significance for OS and DFS. Then multivariate analyses were evaluated in factors which showed significance in univariate analyses. In these analyses, only CEA/CK/CD133 showed significance for OS and DFS. Table III show the results of univariate and multivariate Cox analysis of various factors for OS and DFS in patients with Dukes' stage C cancer. In univariate analyses of patients with Dukes' stage C cancer, lymphatic invasion, venous invasion, serum CEA and CEA/CK/CD133 showed significance for OS, and venous invasion, serum CEA, serum CA19-9 and CEA/CK/CD133 showed significance for DFS. In multivariate analyses, venous invasion and CEA/CK/CD133 showed significances for OS and DFS. These results suggest that CEA/CK/CD133 is an independent significant prognostic factor in patients with Dukes' stage B and C cancer.
Table II.
Univariate and multivariate analysis of prognostic factors for OS and DFS in Dukes' stage B patients.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variables | Regression coefficient | Hazard ratio (95% CI) | P-value | Regression coefficient | Hazard ratio (95% CI) | P-value |
| OS | ||||||
| Tumor size | 0.349 | 1.417 (0.521–3.858) | 0.488 | (−) | ||
| Lymphatic invasion | 0.674 | 1.962 (0.665–5.303) | 0.209 | (−) | ||
| Venous invasion | 1.109 | 3.030 (1.054–10.846) | 0.039a | 1.017 | 2.765 (0.961–9.905) | 0.060 |
| Histological type | −0.235 | 0.791 (0.181–2.455) | 0.708 | (−) | ||
| Serum CEA | 0.744 | 2.105 (0.768–5.777) | 0.145 | (−) | ||
| Serum CA19-9 | 0.588 | 1.800 (0.394–6.235) | 0.409 | (−) | ||
| CEA/CK/CD133 | 2.347 | 10.456 (2.119–189.993) | 0.001a | 2.284 | 9.819 (1.987–177.568) | 0.002a |
| DFS | ||||||
| Tumor size | 0.053 | 1.055 (0.479–2.286) | 0.892 | (−) | ||
| Lymphatic invasion | 0.570 | 1.768 (0.775–3.844) | 0.169 | (−) | ||
| Venous invasion | 0.840 | 2.316 (1.040–5.645) | 0.040a | 0.619 | 1.857 (0.824–4.569) | 0.139 |
| Histological type | 0.376 | 1.456 (0.597–3.242) | 0.389 | (−) | ||
| Serum CEA | 0.798 | 2.222 (0.823–4.901) | 0.064 | (−) | ||
| Serum CA19-9 | 0.240 | 1.271 (0.360–3.546) | 0.680 | (−) | ||
| CEA/CK/CD133 | 1.150 | 3.158 (1.286–9.462) | 0.011a | 1.282 | 3.602 (1.454–10.863) | 0.005a |
P<0.05.
Table III.
Univariate and multivariate analysis of prognostic factors for OS and DFS in Dukes' stage C patients.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variables | Regression coefficient | Hazard ratio (95% CI) | P-value | Regression coefficient | Hazard ratio (95% CI) | P-value |
| OS | ||||||
| Tumor size | −0.273 | 0.761 (0.326–1.654) | 0.498 | (−) | ||
| Lymphatic invasion | 0.810 | 2.248 (1.048–5.004) | 0.038a | 0.647 | 1.910 (0.856–4.373) | 0.114 |
| Venous invasion | 1.113 | 3.043 (1.243–9.110) | 0.013a | 1.156 | 3.178 (1.177–11.084) | 0.021a |
| Histological type | 0.401 | 1.493 (0.655–3.229) | 0.328 | (−) | ||
| Serum CEA | 0.838 | 2.313 (1.006–5.958) | 0.048a | 0.560 | 1.751 (0.755–4.543) | 0.198 |
| Serum CA19-9 | 0.721 | 2.056 (0.900–4.613) | 0.086 | (−) | ||
| CEA/CK/CD133 | 1.439 | 4.215 (1.473–17.737) | 0.005a | 1.497 | 4.468 (1.537–18.948) | 0.004a |
| DFS | ||||||
| Tumor size | 0.316 | 1.372 (0.692–2.696) | 0.360 | (−) | ||
| Lymphatic invasion | 0.271 | 1.311 (0.660–2.579) | 0.434 | (−) | ||
| Venous invasion | 1.263 | 3.535 (1.562–9.476) | 0.002a | 1.172 | 3.228 (1.383–8.834) | 0.006a |
| Histological type | 0.644 | 1.903 (0.934–3.777) | 0.075 | (−) | ||
| Serum CEA | 0.713 | 2.039 (1.008–4.373) | 0.047a | 0.555 | 1.742 (0.762–4.164) | 0.190 |
| Serum CA19-9 | 0.833 | 2.300 (1.133–4.622) | 0.022a | 0.291 | 1.337 (0.611–2.958) | 0.466 |
| CEA/CK/CD133 | 1.246 | 3.475 (1.466–10.222) | 0.003a | 1.253 | 3.502 (1.423–10.542) | 0.005a |
P<0.05.
Discussion
Our study demonstrates that the detection of CEA/CK/CD133 mRNA in tumor drainage vein blood samples has prognostic significance in patients with Dukes' stage B and C CRC.
Evidence is rapidly accumulating that cancers are composed of heterogeneous populations of cancer cells. This suggests that CTCs may contain CSCs which are more aggressive and have invasive and metastatic capacity. We hypothesize that founder cells at the metastatic site may be CSCs disseminated from the primary tumor to a distant metastatic site. This hypothesis is supported by the similarities between the properties of CTCs and CSCs (19,20). It has been reported that CSCs or tumor-initiating cell markers are frequently overexpressd in the CTCs of patients with metastatic breast cancer, and most of them have CSC phenotypes that are do not proliferate and resistant to chemotherapy (19). Based on these reports, we selected multimarkers which included the general CTCs and the CSCs marker of CRC. Regarding the general marker of CRCs, several studies support the usefulness of CEA, CK19 and CK20 (9,10,15,21). In contrast, CSCs markers for CRC patients are still controversial (17). Previously, several interesting studies have demonstrated that CD133-positive cells of CRC have high tumorigenic ability in nude mice (22–24). Thereafter, it was reported that CD133-negative cells are capable of initiating CRC growth. The expression of EpCAM, CD166 and CD44 in CRC is also associated with aggressive tumor phenotypes (25). Thus far, several markers such as CD133, CD44, CD166, CD24, CD29, leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5), and aldehyde dehydrogenase 1 (ALDH1) have been reported (24). Although CD133 may have limitation for CSCs, we elected the CD133, which is a key marker for CSCs. In this study, we used five genetic markers consisting of CEA, CK19, and CK20 for the general CTC markers, and CD133 for the CSC.
Next, we evaluated the prognostic value of CTC/CSC in the tumor drainage vein blood of patients with Dukes' stage B and C. Using general CTCs markers such as CEA and CK, many studies have reported a significant correlation between the presence of CTCs of PB and survival (10,21,26). Previously, we demonstrated that CEA/CK20 in the tumor drainage vein blood was an independent prognostic marker for OS and DFS when using Kaplan-Meier and Cox multivariate analysis (9). In contrast, no studies have examined the prognostic value of CTCs of tumor drainage vein blood in each Dukes' stage. In the present study, we have demonstrated that the detection of CEA/CK/CD133 in tumor drainage vein blood samples has prognostic value in patients with Dukes' stage B and C. To the best of our knowledge, our study is the first to demonstrate the prognostic significance of CTC/CSC in the tumor drainage vein blood of these patients.
Furthermore, to clarify the clinical value of CD133 addition in Dukes' stage B and C, we re-analyzed the prognostic significance of the general CTC marker (CEA/CK) group and the CD133 group separately using the Kaplan-Meier survival curve analysis. Our results demonstrated that CEA/CK/CD133, but not CEA/CK, is prognostic factor in patients with Dukes' stage B. In contrast, not only CEA/CK/CD133 but also CEA/CK showed significance in patients with Dukes' stage C. These results were similar to ones we obtained earlier when we examined PB samples (16). To date, the determination of patients with Dukes' stage B who are at high risk for recurrence is difficult, and efforts to find a tool to select them are ongoing. In this study, we used the real-time RT-PCR method, and it was difficult to examine whether individual cells express all markers or not. We speculate that CTC/CSC, which includes the CD133-positive cells, may enable identification of a certain subgroup that has aggressive cancer stem-like cell properties and that this may increase their prognostic value in Dukes' stage B and C cancer. As for the reason why the CD133 single marker did not show any prognostic value in the Dukes' B and C patients, we speculate that it may be due to the potential limitation of CD133 as CSC marker.
In this study, we established that CEA/CK/CD133 mRNA detection of tumor drainage vein blood is a useful tool for the determination of high risk patients with Dukes' stage B and C who are in need of postoperative adjuvant therapy.
Acknowledgements
We thank Ms. J. Tamura for her excellent technical support and members of colorectal group for the sampling of clinical specimens. This study was supported in part by the Grant-in-Aid for Scientific Research, no. C21591734, from the Ministry of Health, Labor and Welfare of Japan.
Abbreviations
- CRC
colorectal cancer
- CTC
circulating tumor cells
- CSC
cancer stem cells
- RT-PCR
reverse transcription-polymerase chain reaction
- CEA
carcinoembryonic antigen
- CK20
cytokeratin 20
- CK19
cytokeratin 19
- GAPDH
glyceraldehyde-3-phosphate-dehydrogenase
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 3.Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979–994. doi: 10.5858/2000-124-0979-PFICC. [DOI] [PubMed] [Google Scholar]
- 4.Guerra A, Borda F, Javier Jimenez F, Martinez-Penuela JM, Larrinaga B. Multivariate analysis of prognostic factors in resected colorectal cancer: a new prognostic index. Eur J Gastroenterol Hepatol. 1998;10:51–58. doi: 10.1097/00042737-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Hundt S, Haug U, Brenner H. Blood markers for early detection of colorectal cancer: a systematic review. Cancer Epidemiol Biomarkers Prev. 2007;16:1935–1953. doi: 10.1158/1055-9965.EPI-06-0994. [DOI] [PubMed] [Google Scholar]
- 6.Chung KY, Kelsen D. Adjuvant therapy for stage II colorectal cancer; who and with what? Cut Treat Options Gastroenterol. 2006;9:272–280. doi: 10.1007/s11938-006-0046-z. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Jatokoe T, Zhang Y, et al. Gene expression profiles and molecular markers to predict recurrence of Dukes' B colon cancer. J Clin Oncol. 2004;22:1564–1571. doi: 10.1200/JCO.2004.08.186. [DOI] [PubMed] [Google Scholar]
- 8.Engell HC. Cancer cells in the circulating blood; a clinical study on the occurrence of cancer cells in the peripheral blood and in venous blood draining the tumor area at operation. Ugeskr Laeger. 1995;117:822–823. [PubMed] [Google Scholar]
- 9.Iinuma H, Okinaga K, Egami H, et al. Usefulness and clinical significance of quantitative real-time RT-PCR to detect isolated tumor cells in the peripheral blood and tumor drainage blood of patients with colorectal cancer. Int J Oncol. 2006;28:297–306. [PubMed] [Google Scholar]
- 10.Peach G, Kim C, Zacharakis E, Purkayastha S, Ziprin P. Prognostic significance of circulating tumour cells following surgical resection of colorectal cancers: a systematic review. Br J Cancer. 2010;102:1327–1334. doi: 10.1038/sj.bjc.6605651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riethdorf S, Wikman H, Pantel K. Biological relevance of disseminated tumor cells in cancer patients. Int J Cancer. 2008;123:1991–2006. doi: 10.1002/ijc.23825. [DOI] [PubMed] [Google Scholar]
- 12.Tol J, Koopman M, Miller MC, et al. Circulating tumour cells early predict progression-free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Ann Oncol. 2010;21:1006–1012. doi: 10.1093/annonc/mdp463. [DOI] [PubMed] [Google Scholar]
- 13.Yen LC, Yeh YS, Chen CW, et al. Detection of KRAS oncogene in peripheral blood as a predictor of the response to cetuximab plus chemotherapy in patients with metastatic colorectal cancer. Clin Cancer. 2009;15:4508–4513. doi: 10.1158/1078-0432.CCR-08-3179. [DOI] [PubMed] [Google Scholar]
- 14.De Bono JS, Attard G, Adjei A, et al. Potential applications for circulating tumor cells expressing the insulin-like growth factor-I receptor. Clin Cancer. 2007;13:3611–3616. doi: 10.1158/1078-0432.CCR-07-0268. [DOI] [PubMed] [Google Scholar]
- 15.Thorsteinsson M, Jess P. The clinical significance of circulating tumor cells in non-metastatic colorectal cancer: a review. Eur J Surg Oncol. 2011;37:459–465. doi: 10.1016/j.ejso.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Iinuma H, Watanabe T, Mimori K, et al. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes' stage B and C colorectal cancer. J Clin Oncol. 2011;29:1547–1555. doi: 10.1200/JCO.2010.30.5151. [DOI] [PubMed] [Google Scholar]
- 17.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumors:accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–758. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 18.Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology. 2010;138:2151–2162. doi: 10.1053/j.gastro.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 19.Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11:R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balic M, Lin H, Young L, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer. 2006;12:5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 21.Sergeant G, Penninckx F, Topal B. Quantitative RT-PCR detection of colorectal tumor cells in peripheral blood: a systematic review. J Surg Res. 2008;150:144–152. doi: 10.1016/j.jss.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumor growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 23.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 24.Richardson GD, Robson CN, Lang SH, et al. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 25.Lugli A, Iezzi G, Hostettler I, et al. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br J Cancer. 2010;103:382–390. doi: 10.1038/sj.bjc.6605762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahbari NN, Aigner M, Thorlund K, et al. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010;138:1714–1726. doi: 10.1053/j.gastro.2010.01.008. [DOI] [PubMed] [Google Scholar]