Abstract
Degenerin/epithelial sodium channels (DEG/ENaC) represent a large family of animal-specific membrane proteins. Although the physiological functions of most family members are not known, some have been shown to act as nonvoltage gated, amiloride-sensitive sodium channels. The DEG/ENaC family is exceptionally large in genomes of Drosophila species relative to vertebrates and other insects. To elucidate the evolutionary history of the DEG/ENaC family in Drosophila, we took advantage of the genomic and genetic information available for 12 Drosophila species that represent all the major species groups in the Drosophila clade. We have identified 31 family members (termed pickpocket genes) in Drosophila melanogaster, which can be divided into six subfamilies, which are represented in all 12 species. Structure prediction analyses suggested that some subunits evolved unique structural features in the large extracellular domain, possibly supporting mechanosensory functions. This finding is further supported by experimental data that show that both ppk1 and ppk26 are expressed in multidendritic neurons, which can sense mechanical nociceptive stimuli in larvae. We also identified representative genes from five of the six DEG/ENaC subfamilies in a mosquito genome, suggesting that the core DEG/ENaC subfamilies were already present early in the dipteran radiation. Spatial and temporal analyses of expression patterns of the various pickpocket genes indicated that paralogous genes often show very different expression patterns, possibly indicating that gene duplication events have led to new physiological or cellular functions rather than redundancy. In summary, our analyses support a rapid early diversification of the DEG/ENaC family in Diptera followed by physiological and/or cellular specialization. Some members of the family may have diversified to support the physiological functions of a yet unknown class of ligands.
Keywords: Degenerin/epithelial sodium channel, chemosensation, mechanosensation, phylogeny, fruit fly
All cells use a complex array of ion channels to maintain the appropriate ionic gradients across membrane barriers, including the plasma membrane and intracellular compartments and organelles. One enigmatic group of ion channels is the Degenerin/epithelial Na+ channel (DEG/ENaC) family. Although the physiological functions of most family members are not well understood, at least some members seem to act as nonvoltage-gated, amiloride-sensitive sodium channels (Bianchi and Driscoll 2002; Garty and Palmer 1997). Various natural ligands and mechanical stimuli can activate or modulate channel functions. These include the neuropeptides FMRFamide (Askwith et al. 2000; Durrnagel et al. 2010; Golubovic et al. 2007; Green et al. 1994; Kellenberger and Schild 2002; Lingueglia et al. 1995; Xie et al. 2003), FFamide, SFamide (Deval et al. 2003; Sherwood and Askwith, 2008, 2009), and dynorphin-related opioid peptides (Sherwood and Askwith 2009). In addition, some mammalian family members are gated by extracellular protons (Benson et al. 2002; Price et al. 2001; Waldmann et al. 1997; Xie et al. 2003; Xiong et al. 2004). Recently, several sulfhydryl compounds (Cho and Askwith 2007) and small polyamines such as agmatine (Yu et al. 2010) also were shown to modulate the channel functions of specific mammalian family members. Finally, data also support a role for specific DEG/ENaC subunits in pheromone-dependent behaviors as well as in chemosensory functions underlying male courtship behaviors in Drosophila (Ben-Shahar 2011; Ben-Shahar et al. 2007; 2010; Lin et al. 2005; Lu et al. 2012; Starostina et al. 2012; Thistle et al. 2012; Toda et al. 2012).
DEG/ENaC family members also have been implicated in mechanosensation in Caenorhabditis elegans, mammals, and Drosophila (Arnadottir et al. 2011; Bazopoulou et al. 2007; Geffeney et al. 2011; Lu et al. 2009; O’Hagan et al. 2005; Price et al. 2001; Simon et al. 2010; Tsubouchi et al. 2012; Zhang et al. 2004; Zhong et al. 2010). Together, these data indicate that DEG/ENaC channels have evolved to serve many different physiological functions, acting as ionotropic receptors to diverse extracellular stimuli.
Functional and structural studies of DEG/ENaC channels demonstrated that channels are likely hetero or homotrimeric (Benson et al. 2002; Canessa et al. 1994; Eskandari et al. 1999; Jasti et al. 2007; Kellenberger and Schild 2002; Zha et al. 2009b). Electrophysiological studies indicated that subunit composition has a significant effect on the pharmacological and kinetic properties of assembled channels, suggesting that channel subunit composition plays a critical regulatory mechanism (Askwith et al. 2004; Benson et al. 2002; Chu et al. 2004; Xie et al. 2003; Zha et al. 2009a; Zhang et al. 2008). Hence, channel subunit diversity in a single animal is likely to represent diversity in activating stimuli and/or complex channel regulation.
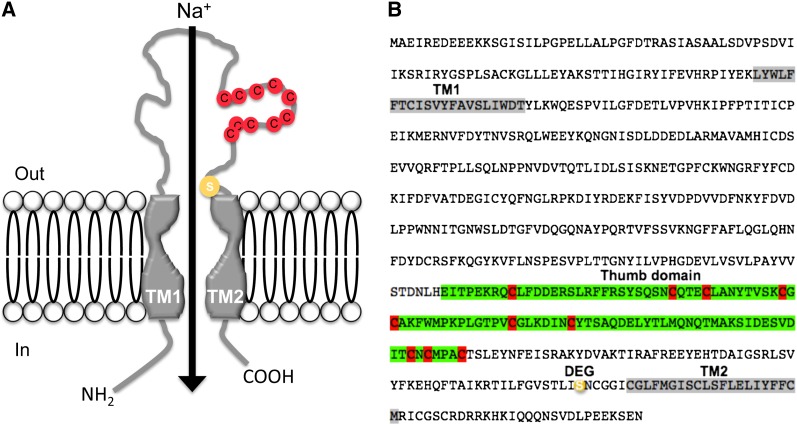
Although the DEG/ENaC family is highly diverse across animalia, all family members share several highly conserved structural and topological features (Bianchi 2007; Bianchi and Driscoll 2002; Corey and Garcia-Anoveros 1996; Tavernarakis and Driscoll, 2000, 2001). Conserved topologies include two transmembrane helixes, two short intracellular domains, and a large cysteine-rich extracellular loop (Figure 1) (Ben-Shahar 2011).
Figure 1 .
(A) Illustration depicting a typical DEG/ENaC subunit. TM, transmembrane domain; Red circles represent conserved cysteines; yellow circle represents the “DEG” residue, which in some subunits results in a constitutively open channel state when mutated (Adams et al. 1998; Kellenberger et al. 2002; Snyder et al. 1998, 2000). (B) The protein sequence of PPK, one of the first DEG/ENaC subunits that was identified in the Drosophila genome (Adams et al. 1998). Alignment of all the Drosophila subunits described in Table 1 and Table S1 indicate the presence of a highly conserved cysteine-enriched domain (also see Figure 7A, thumb domain), highlighted in green. Conserved cysteines are highlighted in red; DEG, a predicted “deg” residue, is highlighted in yellow. TM1 and TM2 represent the predicted transmembrane domains 1 and 2, respectively.
Surprisingly, mammalian genomes encode only eight to nine independent DEG/ENaC subunits, whereas the genomes of the worm C. elegans and various Drosophila species harbor a significantly larger number of DEG/ENaC-like genes [31 in Drosophila melanogaster and 30 in C. elegans (Bazopoulou et al. 2007; Ben-Shahar 2011; Liu et al. 2003a; Liu et al. 2003b; Studer et al. 2011)]. Consequently, DEG/ENaC genes represent one of the largest ion channel families in the Drosophila genome. The high diversification of DEG/ENaC protein sequences across distant animal species makes it difficult to evaluate whether the family expanded in some invertebrate species or whether it contracted in vertebrates. Nevertheless, the remarkable diversity of ppk genes in Drosophila suggests two alternative hypotheses. The first would suggest DEG/ENaC ion channels serve a wider range of physiological functions relative to their roles in mammals. An alternative hypothesis would be that DEG/ENaC channels in Drosophila evolved to serve highly specialized functions, predicting that each specific DEG/ENaC channel type in flies is responsible for a narrow slice of the physiological functions performed by a mammalian family member. However, identifying physiological and functional homology between family members across distant species is often impossible due to the poor overall protein sequence conservation of the extracellular loop domains. Thus, protein alignment analyses alone are typically not sufficient to draw physiological homology conclusions. Consequently, newly identified family members typically require physiological analyses de novo.
The increasing interest in DEG/ENaC-dependent signaling, their emerging importance in diverse physiological functions, and their high variability across different animal genomes suggests these ion channels may have played an important role in animal evolution. Here we reason that the dramatic diversity of the DEG/ENaC family in the Drosophila lineage represents an excellent opportunity to use evolutionary and molecular studies to gain new insights into the possible unique role of these channels in diverse physiological systems in general and insect biology in particular.
Materials and Methods
Phylogenetic analyses
Drosophila melanogaster ppk family member protein sequences were mined in FlyBase and multiply aligned using Clustal Omega (Sievers et al. 2011). To determine the best model of protein evolution for our data, we entered the alignment into ProtTest v 2.4. The appropriate substitution matrix was selected from the Akaike information criterion and Bayesian information criterion scores (Abascal et al. 2005; Darriba et al. 2011; Drummond and Strimmer 2001; Guindon and Gascuel 2003). Phylogenetic analysis was then completed using a maximum likelihood approach and rapid bootstrapping algorithm within RAxML v 7.2.8 Black Box (Stamatakis 2006; Stamatakis et al. 2008), on the Cipres web portal (Miller et al. 2010). Visualizations of the bipartition files were made using FigTree v 1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/).
Expression of ppk genes
Expression patterns of each member of the ppk gene family across different fly tissues were mined from FlyAtlas (Chintapalli et al. 2007). Microarray expression data from four independent microarrays were normalized and then graphed according to the expression level in different tissues. Temporal expression patterns of the ppk gene family were extracted from the modENCODE RNA-sequencing database (Celniker et al. 2009; Graveley et al. 2011). Normalized maximum expression was represented at different developmental stages, from the embryo to the adult fly in both males and females. To observe the spatial expression patterns of ppk and ppk26 at a single cell resolution, we used the UAS-GAL4 binary expression system (Brand and Perrimon 1993) to express a membrane tethered version of EGFP (UAS-mCD8::GFP) using a previously published ppk-GAL4 line and a new ppk26-GAL4 line we have generated. ppk-GAL4 line was obtained from the Bloomington Drosophila Stock Center (stock no. 32078). The ppk26-GAL4 line was produced by amplifying a 2.2-kb fragment that included the first intron as well as sequences upstream of ppk26 transcriptional start site (coordinates were 3L: 7447230-7449432 in release 5.47 of the Drosophila genome)
PPK protein structure modeling
There are currently seven different accession numbers for structural models of DEG/ENaC channels in the PDB database, all which are based on the chicken acid-sensing ion channel (ASIC)1a protein. We chose to base our structural analyses of the Drosophila ppk gene family on the original 2QTS model (Jasti et al. 2007) because of the following reasons: (1) The 2QTS model has the best resolution (1.9 Å), which serves better as a template of homology modeling; and (2) 2QTS is a ligand-free model, which we predicted would work better as a modeling template since ASIC1a is a proton receptor, which is not necessarily a general property of DEG/ENaC channels. To generate structural predictions in silico, all PPK reference sequences and the template sequence (PDB ID: 2QTS) were aligned onto Hidden Markov model of amiloride-sensitive sodium channel family from PFAM [PFAM ID: PF00858(Punta et al. 2012)] by the program hmmalign in HMMER3 (Finn et al. 2011) and visualized by CLC Sequence Viewer. From the pair-wise sequence alignment of each PPK protein and the template, multiple structural models were generated by MODELER with default homology modeling protocol (Sali and Blundell 1993). The model with the best score was selected for further analysis. The molecular graphics software UCSF Chimera was used for structural visualization and analysis (Pettersen et al. 2004).
Results and Discussion
The ppk family in Drosophila melanogaster
The authors of previous studies have identified several DEG/ENaC family members, which were termed pickpocket (ppk) genes (Darboux et al. 1998; Liu et al. 2003a,b). However, a comprehensive scan of the fly genome for all family members has not been performed to date. We used a combination of current genome annotations as well as various homology search engines to identify 31 independent genes encoding for family members, which we named ppk-ppk31 in complete agreement with prior annotations (Table 1).
Table 1. ppk genes identified in the Drosophila melanogaster genome.
| Name | Symbol | Alternative Name | CG No. | FB ID | Location |
|---|---|---|---|---|---|
| pickpocket 1 | ppk | ppk1 | CG3478 | FBgn0020258 | 2L: 35B1-35B1 |
| ripped pocket | rpk | ppk2 | CG1058 | FBgn0022981 | 3R: 82C5-82C5 |
| pickpocket 3 | ppk3 | CG30181 | FBgn0050181 | 2R: 59E3-59E3 | |
| Nach | Nach | ppk4 | CG8178 | FBgn0024319 | 2R: 53C14-53C14 |
| pickpocket 5 | ppk5 | CG33289 | FBgn0053289 | 3L: 78D5-78D5 | |
| pickpocket 6 | ppk6 | CG11209 | FBgn0034489 | 2R: 56F11-56F11 | |
| pickpocket 7 | ppk7 | CG9499 | FBgn0031802 | 2L: 26C3-26C3 | |
| pickpocket 8 | ppk8 | CG32792 | FBgn0052792 | X: 3D6-3D6 | |
| pickpocket 9 | ppk9 | CG34369 | FBgn0085398 | 2R: 58A4-58A4 | |
| pickpocket 10 | ppk10 | CG34042 | FBgn0065110 | 2L: 31E3-31E4 | |
| pickpocket 11 | ppk11 | CG34058 | FBgn0065109 | 2L: 30C8-30C9 | |
| pickpocket 12 | ppk12 | CG10972 | FBgn0034730 | 2R: 58E1-58E1 | |
| pickpocket 13 | ppk13 | CG33508 | FBgn0053508 | 2L: 39A1-39A1 | |
| pickpocket 14 | ppk14 | CG9501 | FBgn0031803 | 2L: 26C3-26C3 | |
| pickpocket 15 | ppk15 | CG14239 | FBgn0039424 | 3R: 97B1-97B1 | |
| pickpocket 16 | ppk16 | CG34059 | FBgn0065108 | 2L: 30C8-30C8 | |
| pickpocket 17 | ppk17 | CG13278 | FBgn0032602 | 2L: 36A14-36A14 | |
| pickpocket 18 | ppk18 | CG13120 | FBgn0032142 | 2L: 30C7-30C8 | |
| pickpocket 19 | ppk19 | CG18287 | FBgn0039679 | 3R: 99B7-99B7 | |
| pickpocket 20 | ppk20 | CG7577 | FBgn0039676 | 3R: 99B7-99B7 | |
| pickpocket 21 | ppk21 | CG12048 | FBgn0039675 | 3R: 99B6-99B6 | |
| pickpocket 22 | ppk22 | CG31105 | FBgn0051105 | 3R: 96B1-96B1 | |
| pickpocket 23 | ppk23 | CG8527 | FBgn0030844 | X: 16B4-16B4 | |
| pickpocket 24 | ppk24 | CG15555 | FBgn0039839 | 3R: 100B9-100B9 | |
| pickpocket 25 | ppk25 | lounge lizard (llz) | CG33349 | FBgn0053349 | 2R: 42E1-42E1 |
| pickpocket 26 | ppk26 | CG8546 | FBgn0035785 | 3L: 66A1-66A1 | |
| pickpocket 27 | ppk27 | CG10858 | FBgn0035458 | 3L: 63E9-63E9 | |
| pickpocket 28 | ppk28 | CG4805 | FBgn0030795 | X: 15A9-15A10 | |
| pickpocket 29 | ppk29 | CG13568 | FBgn0034965 | 2R: 60B6-60B6 | |
| pickpocket 30 | ppk30 | CG18110 | FBgn0039677 | 3R: 99B7-99B7 | |
| pickpocket 31 | ppk31 | CG31065 | FBgn0051065 | 3R: 97E5-97E6 |
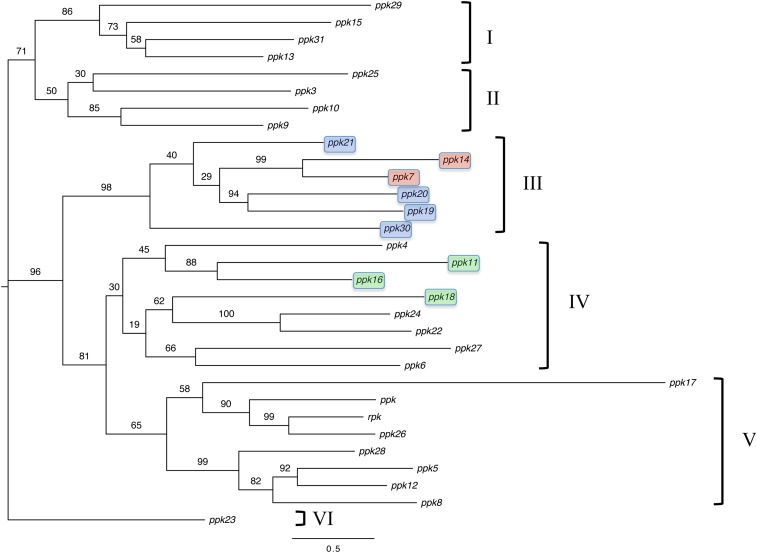
Alignment of all identified PPK sequences revealed a highly conserved cysteine-enriched domain, which contains five disulfide bonds by 10 highly conserved cysteines in the thumb domain (Figure 1, A and B). Unrooted protein phylogenetic analysis of all identified ppk genes in the D. melanogaster genome indicated that this protein family is composed of at least six distinct subfamilies (labeled as I-VI; Figure 2). Overall, the relationship between ppk genes in subfamilies III, IV, and V are well resolved and supported by high bootstrap values. However, few genes such as ppk17 and ppk23 are not well resolved in our phylogeny, despite multiple (N = 4) runs of the alignment and phylogenetic tree programs, which produced the same results for each run. The inability to resolve certain ppk relationships is likely due to the high amount of divergence in amino acid sequence between ppk family members (Supporting Information, Table S1).
Figure 2 .
Maximum-likelihood unrooted phylogenetic tree inferred from multiply aligned amino acid sequences for D. melanogaster DEG/ENaC ppk genes. A total of 31 DEG/ENaC amino acid sequences are divided into six clusters and labeled as groups I-VI. Bootstrap values are given on branches and amino acid substitution rate is given at the bottom of the figure. Colors represent chromosomally clustered subunits (see Figure 5 for details).
ppk genes are highly conserved in the Drosophila lineage
We subsequently extended our gene search analyses to the sequenced genomes of additional 11 Drosophila species as well as to the genome of Anopheles gambiae (African malaria mosquito), which served as a dipteran outgroup (Table S2) (Holt et al. 2002). These analyses revealed that the majority of the D. melanogaster ppk radiation is preserved in all 12 sequenced Drosophila genomes (Bhutkar et al. 2008; Singh et al. 2009), indicating ppk diversification occurred early in the evolution of the Drosophila lineage.
Expression patterns, structural variations, and predictions of function
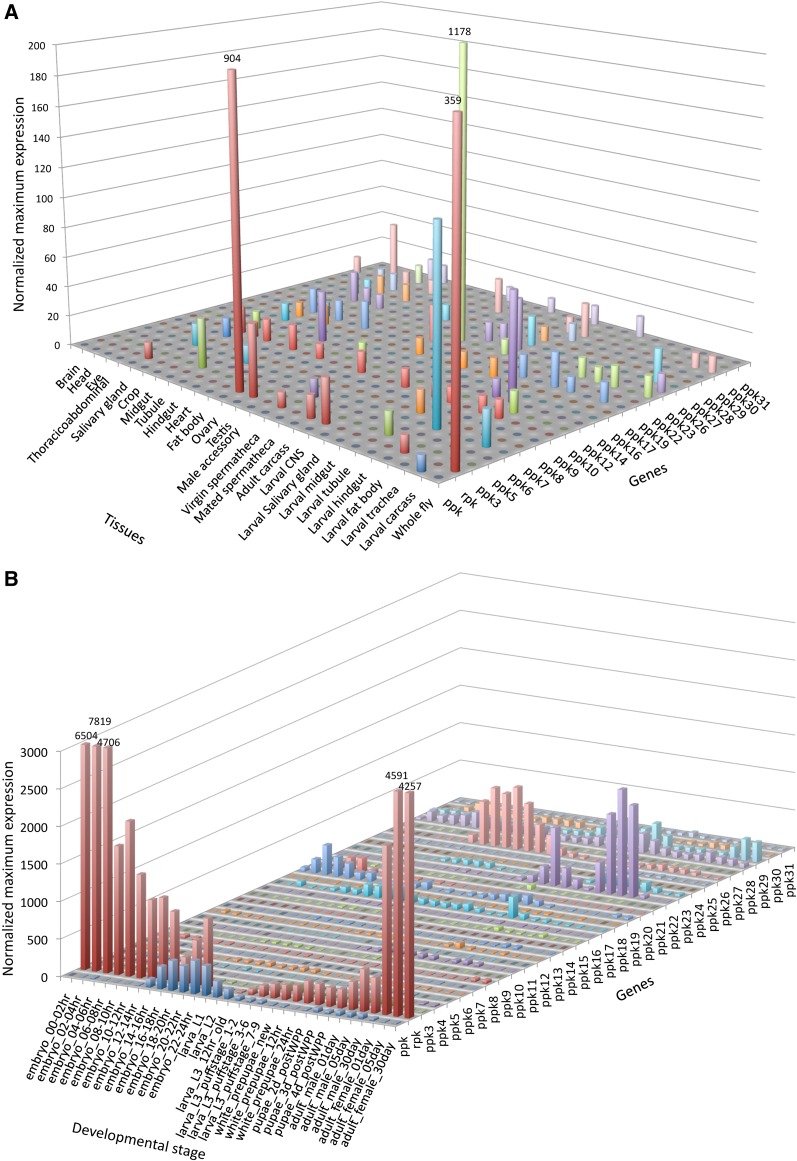
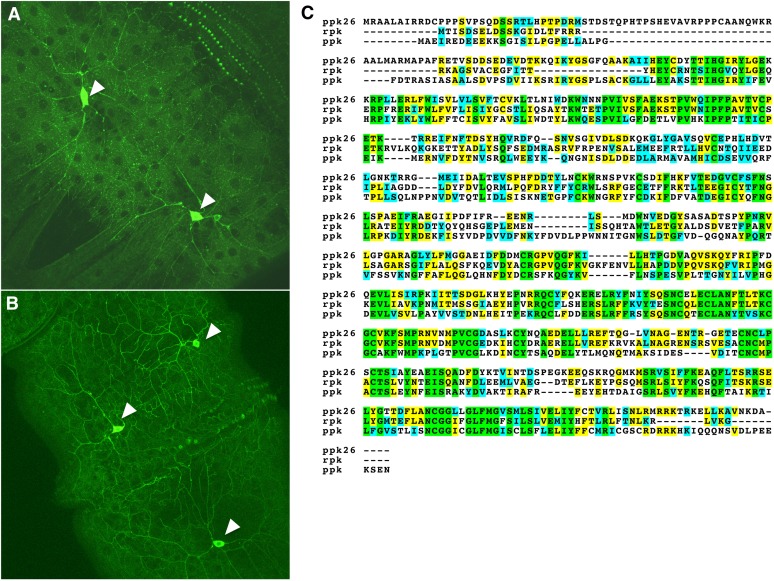
Analyses of mRNA expression levels across various D. melanogaster tissues (Figure 3A) and developmental stages (Figure 3B) indicated that individual ppk family members show different expression profiles in both mRNA expression level and temporal and spatial expression patterns. These data suggest that this family has evolved to serve a wide variety of physiological functions. Although a handful of subunits have been implicated in mechanosensation and chemosensory perception, the contribution of sequence variation to physiological function remains unclear. Of particular interest is subfamily V, which includes the ppk, rpk, and ppk26 cluster (Figures 2 and 4). Both rpk and ppk have been implicated in mechanosensation in larvae, although in different types of multidendritic neurons, and are likely to have similar but independent functions in neurons (Adams et al. 1998; Kim et al. 2012; Tsubouchi et al. 2012; Zhong et al. 2010). The spatial expression pattern of ppk26, which is a close paralogue of the ppk and rpk subunits is very similar to ppk suggesting the two subunits might be co-expressed (Figure 3A). To further explore this, we generated a transgenic Drosophila line that can report the expression patterns of the gene using the UAS-GAL4 system (Brand and Perrimon 1993). As predicted by the mRNA expression data, the expression of the ppk26 gene is enriched in class IV multidendritic sensory neurons, which also express ppk (Figure 4). These data suggest that ppk26 and ppk are either redundant or are corequired for some aspect of mechanosensation in these nociceptive neurons. In sum, though the functions of all DEG/ENaC subunits are not yet known, we hypothesize that ppk, rpk, and ppk26, which show sequence and structural similarities and are expressed in multidendritic neurons, may have similar functions in nociceptive mechanosensation.
Figure 3 .
(A) Spatial expression patterns of ppk genes. Microarray expression data were extracted from FlyAtlas (Chintapalli et al. 2007). Expression represents the average signal from four independent microarrays. (B) Temporal expression patterns of ppk genes. Data were extracted from the modENCODE RNA-seq database (Celniker et al. 2009). Expression levels are represented as log2 values of the original coverage. Numbers at the tops of truncated bars show actual expression values.
Figure 4 .
ppk and ppk26 expression in larval multidendritic neurons. (A) ppk-GAL4 x UAS-mCD8::GFP. (B) ppk26-GAL4 x UAS-mCD8::GFP. White arrows indicate cell body. (C) Alignment of ppk, rpk, and ppk26 amino acid sequence. Green, residues are conserved across all proteins examined; yellow, residues are conserved in some species; blue, conserved substitutions.
Subfamily III is not present in mosquitoes
As expected, ppk family gene conservation between the D. melanogaster and the mosquito genomes was lower than across the Drosophila lineage (Table S2). We identified only 18 family members in the genome of A. gambiae, of which 17 had homologs in the Drosophila genome and one that seemed to be a mosquito-specific subunit (AGAP006704; Table S2). These data suggest that the extreme diversity we observed in the Drosophila lineage is not shared by all dipteran species.
Closer examination of the conservation of Drosophila ppk subfamilies in A. gambiae revealed that none of the genes represented in subfamily III was present in the mosquito genome, suggesting this subfamily is not common in all dipteran species. (Figure 2 and Table S2). In contrast, we have indentified at least one homologous gene from each of the remaining ppk subfamilies in the mosquito genome (Table S2). These data may suggest that each ppk subfamily (with the exception subfamily III) represents a core DEG/ENaC physiological function in Diptera.
Diversity, duplications, gene syntenies, and sequence homologies
Examination of overall gene conservation across all sequenced Drosophila species indicated that protein phylogeny followed closely the predicted species phylogeny (Clark et al. 2007). We examined in more detail several subfamilies of conserved ppk genes across the 12 sequenced Drosophila genomes as well as the malaria mosquito A. gambiae. We first examined the highly conserved subgroup that included ppk, rpk, and ppk26. All three genes are highly conserved across all 12 genomes (Table S2).
Although each Drosophila genome includes one subunit that corresponds most closely to ppk, rpk, or ppk26, the mosquito genome encodes four related subunits, all of which are clustered with the Drosophila ppk26 (Table S2). These data suggest that ppk26 represents an early dipteran subunit, which may have independently diversified in the Drosophila and mosquito lineages.
Nine of the 31 ppk genes we have identified in the D. melanogaster genome are chromosomally clustered (Figure 5). Protein phylogeny indicated that the majority of genomic clusters were likely the result of gene duplications since the clustered genes showed high sequence similarities and belonged to the same ppk subfamilies (Boxed genes names in Figure 2). An exception is ppk18, which is clustered with ppk11 and ppk16 (Figure 5B), two less related subunits (Figure 2). These data suggest that the clustering of these three subunits might have been the result of selection underlying shared physiological and/or cellular functions. ppk11 has been implicated in salt taste (Liu et al. 2003b). We speculate that these three subunits might contribute to salt taste in Drosophila by forming the sodium sensitive ion channel. (Adams et al. 1997; Chandrashekar et al. 2006; Chandrashekar et al. 2010; McDonald et al. 1995; Snyder et al. 1995). We found that all identified D. melanogaster ppk genomic clusters are conserved across all 12 Drosophila species genomes (not shown), indicating that the molecular events that led to clusters formation happened early in the species radiation of the Drosophila genus.
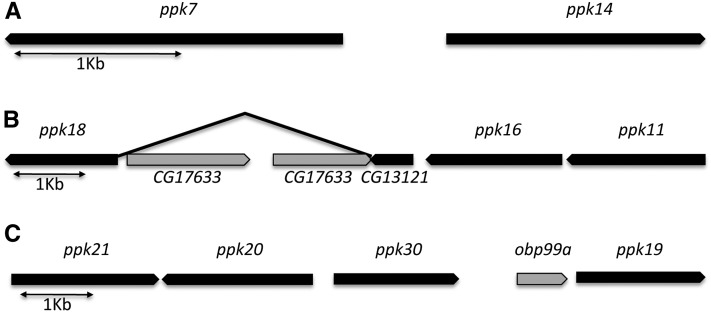
Figure 5 .
Chromosomal clusters of ppk genes. (A) Cluster of ppk7 and ppk14 located at 2L: 26C3-26C3. (B) Cluster of ppk18, ppk16, and ppk11 located at 2L: 30C8-30C9. Note that although CG13121 is currently annotated as a separate gene, molecular analyses of mRNA clones indicate that it is part of the ppk18 locus (not shown). (C) Cluster of ppk21, ppk20, ppk30, and ppk19 located at 3R: 99B6-99B7. Black boxes, ppk genes; gray boxes, none-ppk genes.
In addition to linear protein sequence analyses, we also built structural models of all PPK proteins by using the published crystal structure of the chicken ASIC (Jasti et al. 2007) as a guide. According to the protein conservation information from multiple alignment of the ppk family, we rendered a general Drosophila PPK model (Figure 6A). Furthermore, we used the resolved ASIC structure to predict structural models for all individual Drosophila ppk subunits (Figure 6B). Close inspection of the structure and the overall protein alignment revealed 10 highly conserved cysteines (>90% conservation), which are likely to form up to five disulfide bonds.
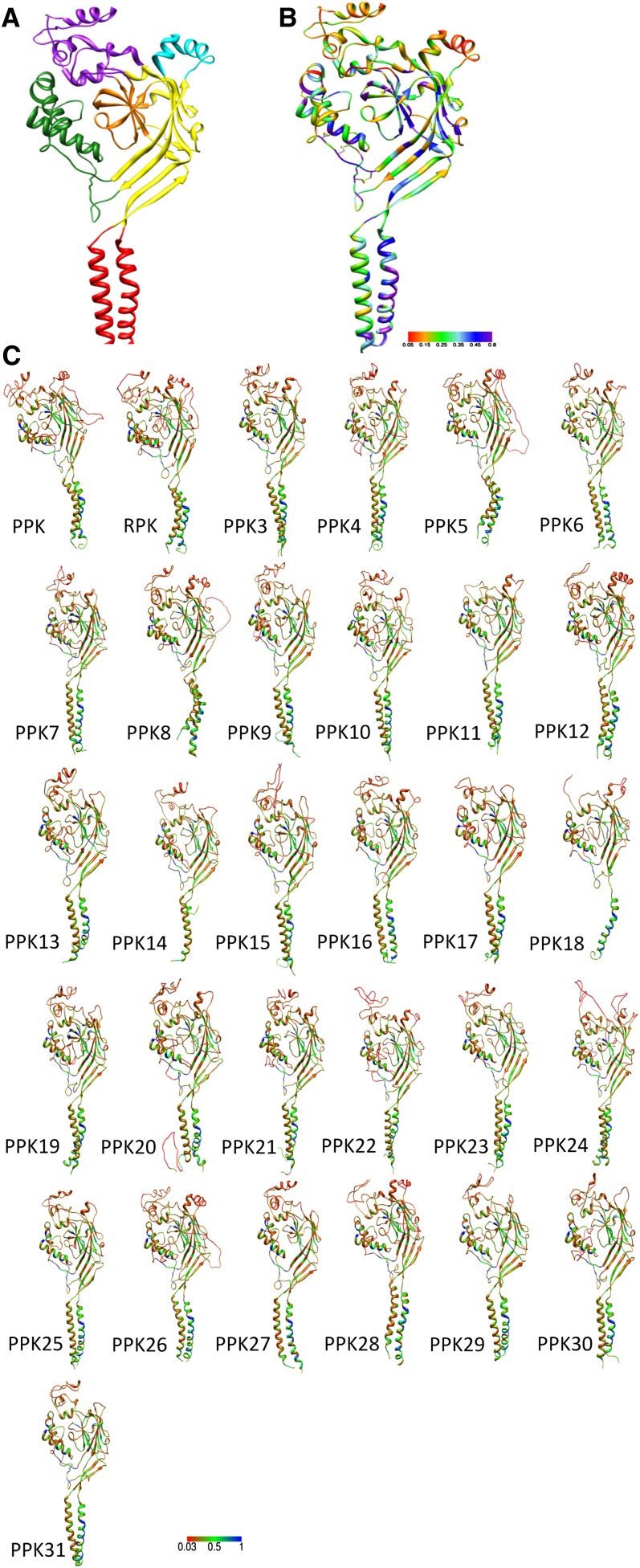
Figure 6 .
Structural modeling of the ppk family in Drosophila. (A) Domain organization of the chicken ASIC1a subunit (Jasti et al. 2007) Red: TM1 (left helix), TM2 (right helix); yellow: Palm; cyan: Knuckle; orange: beta-ball; purple: Finger; green: Thumb. (B) ASIC1a subunit rendered by conservation information from its alignment with the ppk family. The regions colored in purple are highly conserved residues, whereas those colored in red are most variable in the alignment. (C) Predicted structure for all Drosophila PPK subunits. The rainbow scale represents the residue conservation scores. The regions colored in red are most variable whereas regions in blue are highly conserved.
We also found that most family members from group V (Figure 2) have a long unstructured loop without a matched structural template in the resolved vertebrate model (Figure 7, with the exception of PPK17). Whether this unstructured loop plays a functional role is unknown. However, ppk is expressed in type IV multidendritic neurons, which play a role in thermal and mechanical nociception in fly larvae (Adams et al. 1998; Ainsley et al. 2003; Hwang et al. 2007; Kim et al. 2012; Zhong et al. 2010). The recent publication, which implicates rpk in mechanosensotive functions in Class III multidendritic neurons, and our finding that ppk26 is expressed in Class IV multidendritic neurons in a similar pattern to ppk suggest that other members of this cluster might be playing similar roles in mechanotransduction pathways. Further, our data raise the intriguing hypothesis that the large unstructured side loop that is a signature of cluster V may be playing a role in mechanosensory functions, possibly by interacting with extracellular matrix proteins (Arnadottir and Chalfie 2010; Arnadottir et al. 2011; Brown et al. 2008; Chalfie 2009; Geffeney et al. 2011; Huber et al. 2006; Zhang et al. 2004).
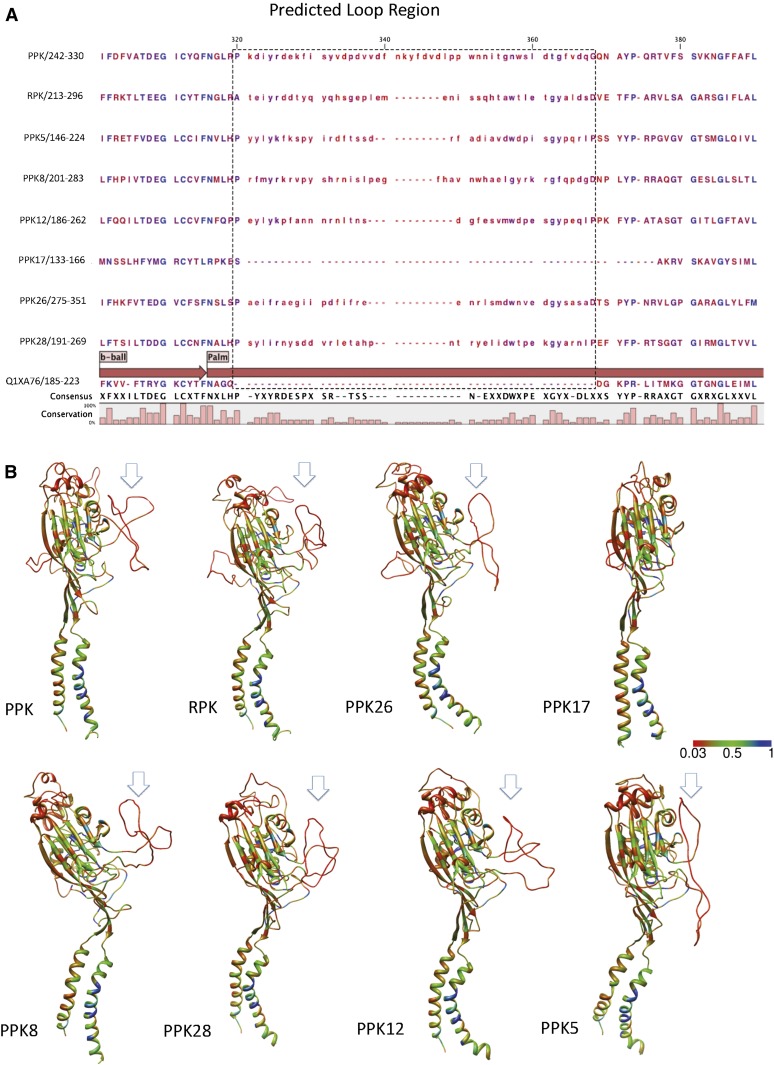
Figure 7 .
(A) The alignment of individual subunits from ppk subfamily Group V (for full Group V alignment, see Figure S1). The dashed frame marks the unstructured loop region. Note that PPK17 does not have the unstructured loop region. Q1XA76 is the chicken ASIC Uniprot Accession ID. Consensus sequence was built from the majority of the aligned residues. The bars in the bottom represent conservation percentage after alignment. (B) Unstructured loop region in the subfamily Group V. Predicted structures for all D. melanogaster PPK subunits are shown in Figure 6. The rainbow scale represents residue conservation as in Figure 6.
Here we show a comprehensive analysis of an emerging and important family of ion channels in the genetically tractable fruit fly model. As the importance of the DEG/ENaC family continues to increase, studies in Drosophila could reveal novel insights into the physiological functions of this enigmatic group of ion channels. Taking advantage of the wealth of genetic and evolutionary data in the Drosophila group as well as other insect species, we intend to generate novel testable structure-function hypotheses that would likely shed additional light on the physiological functions of these proteins in species ranging from the worm to humans.
Supplementary Material
Acknowledgments
We gratefully acknowledge members of the Ben-Shahar laboratory for useful comments on the manuscript. We also thank Yun He for providing technical assistance with protein structure modeling. This work was supported by the National Institutes of Health (R03 DC010244) and an award from the Klingenstein Fund to Y.B.-S.
Footnotes
Communicating editor: S. Celniker
Literature Cited
- Abascal F., Zardoya R., Posada D., 2005. ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21: 2104–2105 [DOI] [PubMed] [Google Scholar]
- Adams C. M., Snyder P. M., Welsh M. J., 1997. Interactions between subunits of the human epithelial sodium channel. J. Biol. Chem. 272: 27295–27300 [DOI] [PubMed] [Google Scholar]
- Adams C. M., Anderson M. G., Motto D. G., Price M. P., Johnson W. A., et al. , 1998. Ripped pocket and pickpocket, novel Drosophila DEG/ENaC subunits expressed in early development and in mechanosensory neurons. J. Cell Biol. 140: 143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsley J. A., Pettus J. M., Bosenko D., Gerstein C. E., Zinkevich N., et al. , 2003. Enhanced locomotion caused by loss of the Drosophila DEG/ENaC protein Pickpocket1. Curr. Biol. 13: 1557–1563 [DOI] [PubMed] [Google Scholar]
- Arnadottir J., Chalfie M., 2010. Eukaryotic mechanosensitive channels. Annu Rev Biophys 39: 111–137 [DOI] [PubMed] [Google Scholar]
- Arnadottir J., O’Hagan R., Chen Y. S., Goodman M. B., Chalfie M., 2011. The DEG/ENaC protein MEC-10 regulates the transduction channel complex in Caenorhabditis elegans touch receptor neurons. J. Neurosci. 31: 12695–12704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askwith C. C., Cheng C., Ikuma M., Benson C., Price M. P., et al. , 2000. Neuropeptide FF and FMRFamide potentiate acid-evoked currents from sensory neurons and proton-gated DEG/ENaC channels. Neuron 26: 133–141 [DOI] [PubMed] [Google Scholar]
- Askwith C. C., Wemmie J. A., Price M. P., Rokhlina T., Welsh M. J., 2004. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J. Biol. Chem. 279: 18296–18305 [DOI] [PubMed] [Google Scholar]
- Bazopoulou D., Voglis G., Tavernarakis N., 2007. The role of DEG/ENaC ion channels in sensory mechanotransduction, pp. 3–31 in Molecular Sensors for Cardiovascular Homeostasis, edited by Wang D. H. Springer, New York [Google Scholar]
- Ben-Shahar Y., 2011. Sensory functions for degenerin/epithelial sodium channels (DEG/ENaC). Adv. Genet. 76: 1–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar Y., Nannapaneni K., Casavant T. L., Scheetz T. E., Welsh M. J., 2007. Eukaryotic operon-like transcription of functionally related genes in Drosophila. Proc. Natl. Acad. Sci. USA 104: 222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar Y., Lu B., Collier D. M., Snyder P. M., Schnizler M., et al. , 2010. The Drosophila gene CheB42a is a novel modifier of Deg/ENaC channel function. PLoS ONE 5: e9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson C. J., Xie J., Wemmie J. A., Price M. P., Henss J. M., et al. , 2002. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc. Natl. Acad. Sci. USA 99: 2338–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutkar A., Schaeffer S. W., Russo S. M., Xu M., Smith T. F., et al. , 2008. Chromosomal rearrangement inferred from comparisons of 12 Drosophila genomes. Genetics 179: 1657–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi L., 2007. Mechanotransduction: touch and feel at the molecular level as modeled in Caenorhabditis elegans. Mol. Neurobiol. 36: 254–271 [DOI] [PubMed] [Google Scholar]
- Bianchi L., Driscoll M., 2002. Protons at the gate: DEG/ENaC ion channels help us feel and remember. Neuron 34: 337–340 [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415 [DOI] [PubMed] [Google Scholar]
- Brown A. L., Liao Z., Goodman M. B., 2008. MEC-2 and MEC-6 in the Caenorhabditis elegans sensory mechanotransduction complex: auxiliary subunits that enable channel activity. J. Gen. Physiol. 131: 605–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa C. M., Schild L., Buell G., Thorens B., Gautschi I., et al. , 1994. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467 [DOI] [PubMed] [Google Scholar]
- Celniker S. E., Dillon L. A., Gerstein M. B., Gunsalus K. C., Henikoff S., et al. , 2009. Unlocking the secrets of the genome. Nature 459: 927–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., 2009. Neurosensory mechanotransduction. Nat. Rev. Mol. Cell Biol. 10: 44–52 [DOI] [PubMed] [Google Scholar]
- Chandrashekar J., Hoon M. A., Ryba N. J., Zuker C. S., 2006. The receptors and cells for mammalian taste. Nature 444: 288–294 [DOI] [PubMed] [Google Scholar]
- Chandrashekar J., Kuhn C., Oka Y., Yarmolinsky D. A., Hummler E., et al. , 2010. The cells and peripheral representation of sodium taste in mice. Nature 464: 297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli V. R., Wang J., Dow J. A., 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39: 715–720 [DOI] [PubMed] [Google Scholar]
- Cho J. H., Askwith C. C., 2007. Potentiation of acid-sensing ion channels by sulfhydryl compounds. Am. J. Physiol. Cell Physiol. 292: C2161–C2174 [DOI] [PubMed] [Google Scholar]
- Chu X. P., Wemmie J. A., Wang W. Z., Zhu X. M., Saugstad J. A., et al. , 2004. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J. Neurosci. 24: 8678–8689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. G., Eisen M. B., Smith D. R., Bergman C. M., Oliver B., et al. , 2007. Evolution of genes and genomes on the Drosophila phylogeny. Nature 450: 203–218 [DOI] [PubMed] [Google Scholar]
- Corey D. P., Garcia-Anoveros J., 1996. Mechanosensation and the DEG/ENaC ion channels. Science 273: 323–324 [DOI] [PubMed] [Google Scholar]
- Darboux I., Lingueglia E., Champigny G., Coscoy S., Barbry P., et al. , 1998. dGNaC1, a gonad-specific amiloride-sensitive Na+ channel. J. Biol. Chem. 273: 9424–9429 [DOI] [PubMed] [Google Scholar]
- Darriba D., Taboada G. L., Doallo R., Posada D., 2011. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27: 1164–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval E., Baron A., Lingueglia E., Mazarguil H., Zajac J. M., et al. , 2003. Effects of neuropeptide SF and related peptides on acid sensing ion channel 3 and sensory neuron excitability. Neuropharmacology 44: 662–671 [DOI] [PubMed] [Google Scholar]
- Drummond A., Strimmer K., 2001. PAL: an object-oriented programming library for molecular evolution and phylogenetics. Bioinformatics 17: 662–663 [DOI] [PubMed] [Google Scholar]
- Durrnagel S., Kuhn A., Tsiairis C. D., Williamson M., Kalbacher H., et al. , 2010. Three homologous subunits form a high affinity peptide-gated ion channel in Hydra. J. Biol. Chem. 285: 11958–11965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari S., Snyder P. M., Kreman M., Zampighi G. A., Welsh M. J., et al. , 1999. Number of subunits comprising the epithelial sodium channel. J. Biol. Chem. 274: 27281–27286 [DOI] [PubMed] [Google Scholar]
- Finn R. D., Clements J., Eddy S. R., 2011. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 39: W29–W37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H., Palmer L. G., 1997. Epithelial sodium channels: function, structure, and regulation. Physiol. Rev. 77: 359–396 [DOI] [PubMed] [Google Scholar]
- Geffeney S. L., Cueva J. G., Glauser D. A., Doll J. C., Lee T. H. C., et al. , 2011. DEG/ENaC but not TRP channels are the major mechanoelectrical transduction channels in a C. elegans nociceptor. Neuron 71: 845–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovic A., Kuhn A., Williamson M., Kalbacher H., Holstein T. W., et al. , 2007. A peptide-gated ion channel from the freshwater polyp Hydra. J. Biol. Chem. 282: 35098–35103 [DOI] [PubMed] [Google Scholar]
- Graveley B. R., Brooks A. N., Carlson J. W., Duff M. O., Landolin J. M., et al. , 2011. The developmental transcriptome of Drosophila melanogaster. Nature 471: 473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. A., Falconer S. W., Cottrell G. A., 1994. The neuropeptide Phe-Met-Arg-Phe-NH2 (FMRFamide) directly gates two ion channels in an identified Helix neurone. Pflugers Arch. 428: 232–240 [DOI] [PubMed] [Google Scholar]
- Guindon S., Gascuel O., 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52: 696–704 [DOI] [PubMed] [Google Scholar]
- Holt R. A., Subramanian G. M., Halpern A., Sutton G. G., Charlab R., et al. , 2002. The genome sequence of the malaria mosquito Anopheles gambiae. Science 298: 129–149 [DOI] [PubMed] [Google Scholar]
- Huber T. B., Schermer B., Muller R. U., Hohne M., Bartram M., et al. , 2006. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc. Natl. Acad. Sci. USA 103: 17079–17086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang R. Y., Zhong L., Xu Y., Johnson T., Zhang F., et al. , 2007. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr. Biol. 17: 2105–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasti J., Furukawa H., Gonzales E. B., Gouaux E., 2007. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449: 316–323 [DOI] [PubMed] [Google Scholar]
- Kellenberger S., Schild L., 2002. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 82: 735–767 [DOI] [PubMed] [Google Scholar]
- Kellenberger S., Gautschi I., Schild L., 2002. An external site controls closing of the epithelial Na+ channel ENaC. J. Physiol. 543: 413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. E., Coste B., Chadha A., Cook B., Patapoutian A., 2012. The role of Drosophila Piezo in mechanical nociception. Nature. 483: 209–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Mann K. J., Starostina E., Kinser R. D., Pikielny C. W., 2005. A Drosophila DEG/ENaC channel subunit is required for male response to female pheromones. Proc. Natl. Acad. Sci. USA 102: 12831–12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingueglia E., Champigny G., Lazdunski M., Barbry P., 1995. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel. Nature 378: 730–733 [DOI] [PubMed] [Google Scholar]
- Liu L., Johnson W. A., Welsh M. J., 2003a Drosophila DEG/ENaC pickpocket genes are expressed in the tracheal system, where they may be involved in liquid clearance. Proc. Natl. Acad. Sci. USA 100: 2128–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Leonard A. S., Motto D. G., Feller M. A., Price M. P., et al. , 2003b Contribution of Drosophila DEG/ENaC genes to salt taste. Neuron 39: 133–146 [DOI] [PubMed] [Google Scholar]
- Lu B., LaMora A., Sun Y., Welsh M. J., Ben-Shahar Y., 2012. ppk23-Dependent chemosensory functions contribute to courtship behavior in Drosophila melanogaster. PLoS Genet. 8: e1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Ma X., Sabharwal R., Snitsarev V., Morgan D., et al. , 2009. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron 64: 885–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald F. J., Price M. P., Snyder P. M., Welsh M. J., 1995. Cloning and expression of the beta- and gamma-subunits of the human epithelial sodium channel. Am. J. Physiol. 268: C1157–C1163 [DOI] [PubMed] [Google Scholar]
- Miller M. A., Pfeiffer W., Schwartz T., 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Gateway Computing Environments Workshop (GCE), November 14, 2010, pp. 1–8.
- O’Hagan R., Chalfie M., Goodman M. B., 2005. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 8: 43–50 [DOI] [PubMed] [Google Scholar]
- Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., et al. , 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25: 1605–1612 [DOI] [PubMed] [Google Scholar]
- Price M. P., McIlwrath S. L., Xie J., Cheng C., Qiao J., et al. , 2001. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 32: 1071–1083 [DOI] [PubMed] [Google Scholar]
- Punta M., Coggill P. C., Eberhardt R. Y., Mistry J., Tate J., et al. , 2012. The Pfam protein families database. Nucleic Acids Res. 40: D290–D301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali A., Blundell T. L., 1993. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234: 779–815 [DOI] [PubMed] [Google Scholar]
- Sherwood T. W., Askwith C. C., 2008. Endogenous arginine-phenylalanine-amide-related peptides alter steady-state desensitization of ASIC1a. J. Biol. Chem. 283: 1818–1830 [DOI] [PubMed] [Google Scholar]
- Sherwood T. W., Askwith C. C., 2009. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J. Neurosci. 29: 14371–14380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F., Wilm A., Dineen D., Gibson T. J., Karplus K., et al. , 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A., Shenton F., Hunter I., Banks R. W., Bewick G. S., 2010. Amiloride-sensitive channels are a major contributor to mechanotransduction in mammalian muscle spindles. J. Physiol. 588: 171–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. D., Larracuente A. M., Sackton T. B., Clark A. G., 2009. Comparative Genomics on the Drosophila Phylogenetic Tree. Annu. Rev. Ecol. Evol. Syst. 40: 459–480 [Google Scholar]
- Snyder P. M., Price M. P., McDonald F. J., Adams C. M., Volk K. A., et al. , 1995. Mechanism by which Liddle’s syndrome mutations increase activity of a human epithelial Na+ channel. Cell 83: 969–978 [DOI] [PubMed] [Google Scholar]
- Snyder P. M., Cheng C., Prince L. S., Rogers J. C., Welsh M. J., 1998. Electrophysiological and biochemical evidence that DEG/ENaC cation channels are composed of nine subunits. J. Biol. Chem. 273: 681–684 [DOI] [PubMed] [Google Scholar]
- Snyder P. M., Bucher D. B., Olson D. R., 2000. Gating induces a conformational change in the outer vestibule of ENaC. J. Gen. Physiol. 116: 781–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A., 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690 [DOI] [PubMed] [Google Scholar]
- Stamatakis A., Hoover P., Rougemont J., 2008. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 57: 758–771 [DOI] [PubMed] [Google Scholar]
- Starostina E., Liu T., Vijayan V., Zheng Z., Siwicki K. K., et al. , 2012. A Drosophila DEG/ENaC subunit functions specifically in gustatory neurons required for male courtship behavior. J. Neurosci. 32: 4665–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer R. A., Person E., Robinson-Rechavi M., Rossier B. C., 2011. Evolution of the epithelial sodium channel and the sodium pump as limiting factors of aldosterone action on sodium transport. Physiol. Genomics 43: 844–854 [DOI] [PubMed] [Google Scholar]
- Tavernarakis N., Driscoll M., 2000. Caenorhabditis elegans degenerins and vertebrate ENaC ion channels contain an extracellular domain related to venom neurotoxins. J. Neurogenet. 13: 257–264 [DOI] [PubMed] [Google Scholar]
- Tavernarakis N., Driscoll M., 2001. Degenerins. At the core of the metazoan mechanotransducer? Ann. N. Y. Acad. Sci. 940: 28–41 [PubMed] [Google Scholar]
- Thistle R., Cameron P., Ghorayshi A., Dennison L., Scott K., 2012. Contact chemoreceptors mediate male-male repulsion and male-female attraction during drosophila courtship. Cell 149: 1140–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda H., Zhao X., Dickson B. J., 2012. The Drosophila female aphrodisiac pheromone activates ppk23+ sensory neurons to elicit male courtship behavior. Cell Rep. 1: 599–607 [DOI] [PubMed] [Google Scholar]
- Tsubouchi A., Caldwell J. C., Tracey W. D., 2012. Dendritic Filopodia, Ripped Pocket, NOMPC, and NMDARs contribute to the sense of touch in Drosophila larvae. Curr. Biol. 22: 2121–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R., Champigny G., Bassilana F., Heurteaux C., Lazdunski M., 1997. A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177 [DOI] [PubMed] [Google Scholar]
- Xie J., Price M. P., Wemmie J. A., Askwith C. C., Welsh M. J., 2003. ASIC3 and ASIC1 mediate FMRFamide-related peptide enhancement of H+-gated currents in cultured dorsal root ganglion neurons. J. Neurophysiol. 89: 2459–2465 [DOI] [PubMed] [Google Scholar]
- Xiong Z. G., Zhu X. M., Chu X. P., Minami M., Hey J., et al. , 2004. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118: 687–698 [DOI] [PubMed] [Google Scholar]
- Yu Y., Chen Z., Li W. G., Cao H., Feng E. G., et al. , 2010. A nonproton ligand sensor in the acid-sensing ion channel. Neuron 68: 61–72 [DOI] [PubMed] [Google Scholar]
- Zha X. M., Costa V., Harding A. M., Reznikov L., Benson C. J., et al. , 2009a ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J. Neurosci. 29: 8438–8446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha X. M., Wang R., Collier D. M., Snyder P. M., Wemmie J. A., et al. , 2009b Oxidant regulated inter-subunit disulfide bond formation between ASIC1a subunits. Proc. Natl. Acad. Sci. USA 106: 3573–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Arnadottir J., Keller C., Caldwell G. A., Yao C. A., et al. , 2004. MEC-2 is recruited to the putative mechanosensory complex in C. elegans touch receptor neurons through its stomatin-like domain. Curr. Biol. 14: 1888–1896 [DOI] [PubMed] [Google Scholar]
- Zhang W., Bianchi L., Lee W. H., Wang Y., Israel S., et al. , 2008. Intersubunit interactions between mutant DEG/ENaCs induce synthetic neurotoxicity. Cell Death Differ. 15: 1794–1803 [DOI] [PubMed] [Google Scholar]
- Zhong L., Hwang R. Y., Tracey W. D., 2010. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr. Biol. 20: 429–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.