Abstract
Excess nicotinamide, a form of vitamin B3, is metabolized through two enzymatic systems and eventually excreted from the body. The first system starts with the methylation of nicotinamide by nicotinamide N-methyltransferase, which can subsequently be oxidized by aldehyde oxidase. The second enzymatic system oxidizes nicotinamide to nicotinamide N-oxide. It is located in the endoplasmic reticulum of hepatocytes but the precise enzyme is unknown. We have used human liver microsomes in combination with selective cytochrome P450 inhibitors, specific substrates, and antibodies to identify CYP2E1 as the main activity producing nicotinamide N-oxide. Our results suggest the potential use of nicotinamide N-oxide as a biomarker of CYP2E1 activity from urine or blood samples.
Introduction
Nicotinamide (NAM) is one of the forms of vitamin B3. It is a precursor for nicotinamide adenine dinucleotide, which is best known as an electron carrier in oxidative phosphorylation and as a cofactor for many dehydrogenases (Bogan and Brenner, 2008; Houtkooper et al., 2010). NAM cannot be broken down and excess is excreted. Two enzymatic systems that remove NAM from the body have been described. The first is cytoplasmic and starts with the methylation of NAM to N1-methylnicotinamide (MNAM) by the enzyme nicotinamide N-methyltransferase (Cantoni, 1951). MNAM can also be further oxidized by aldehyde oxidase to two related compounds, N1-methyl-2-pyridone-5-carboxamide (2-pyr) and N1-methyl-4-pyridone-3-carboxamide (4-pyr) (Fig. 1A) (Leifer et al., 1951; Felsted and Chaykin, 1967). The second clearance pathway consists of an unknown microsomal enzyme, most likely a cytochrome P450 (P450) system, which oxidizes NAM to nicotinamide N-oxide (NAM N-oxide) (Fig. 1A) (Bonavita et al., 1961; Nomura et al., 1983). Both the methylated and the oxidized forms of NAM can be detected in the blood and urine of humans and rodents. Under normal conditions, the cytoplasmic clearance pathway predominates; however, pharmacologic doses of vitamin B3 increase NAM N-oxide, which can become the most abundant NAM metabolite in mouse blood (Chaykin et al., 1965; Shibata et al., 1990; Stratford and Dennis, 1992). In humans, NAM N-oxide is detected after therapeutic doses of niacin for the treatment of hyperlipidemia (Menon et al., 2007). Traditionally, the NAM clearance metabolites were not considered to have biologic activity. However, recent studies suggest that MNAM possesses antithrombotic and anti-inflammatory properties in vivo with the precise mechanism of action not well understood (Chlopicki et al., 2007; Bartuś et al., 2008). NAM N-oxide currently has no assigned biologic function although high doses have been reported to affect the differentiation of leukemia cells (Iwata et al., 2003).
Fig. 1.
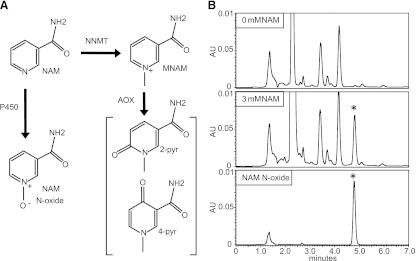
(A) Schematic representation of the nicotinamide clearance pathways. NAM is methylated by nicotinamide N-methyltransferase (NNMT) to MNAM and subsequently oxidized by aldehyde oxidase (AOX) to pyridone carboxamides (2-pyr and 4-pyr). Nicotinamide is oxidized to NAM N-oxide by an unknown microsomal activity (most likely P450). (B) Human microsomes exhibit NAM N-oxidizing activity. HPLC chromatograms of microsomal extracts in the absence of NAM (top panel) and presence of NAM (middle panel). HPLC chromatogram of NAM N-oxide standard (bottom panel). NAM N-oxide peak is indicated by an asterisk (*).
In this study, we have used pooled microsomes from human donors to identify CYP2E1 as the main microsomal enzyme oxidizing nicotinamide and we discuss its potential as a natural biomarker of CYP2E1 activity.
Materials and Methods
Nicotinamide, NAM N-oxide, NADPH, chlorzoxazone, diethyldithiocarbamate, tranylcypromine, fluconazole, ketoconazole, α-naphthoflavone, quinidine, and orphenadrine were from Sigma-Aldrich (St. Louis, MO). Human liver microsomes (HLMs), control, CYP2A6, CYP2B6, CYP2E1, and flavin-containing monooxygenase (FMO)3 overexpressing insect cell microsomes (Supersomes) were from BD Biosciences (San Jose, CA). Control anti-mouse IgG (cat#12-371) was from Millipore (Billerica, MA) and anti-CYP2E1 inhibitory antibody (MAB-2E1, cat#458321) was from BD Biosciences.
Microsomal assay.
HLMs were diluted to 1 mg/ml in 0.1 M NaH2PO4, pH 7.4 supplemented with 1 mM NADPH and preincubated at 37°C for 2 minutes. Total volume was 50 μl. Reaction was started by the addition of substrate NAM, continued for 30 minutes, and stopped by the addition of equal volume of ice-cold methanol. Precipitate was removed by centrifugation. The supernatant was dried under vacuum and resuspended in 50 μl of high-performance liquid chromatography (HPLC) mobile phase. Preliminary experiments showed that the formation of NAM N-oxide was linear with time up to 60 minutes. P450 inhibitors were dissolved in methanol and chlorzoxazone in acetonitrile. The final concentration of the solvent was 0.1% methanol and 1% acetonitrile. At these concentrations, CYP2E1 activity is not significantly inhibited (Easterbrook et al., 2001). Microsomes were preincubated for 30 minutes with the inhibitors before the addition of substrate, 2 mM NAM. Concentrations of the inhibitors were at least 10 times the reported Ki for standard substrates [Kobayashi et al., 1999; Zhang et al., 2001; US Food and Drug Administration, 2011 (http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm#inVitro)]. Final concentrations of inhibitors were 100 μM diethyldithiocarbamate, 1 μM tranylcypromine, 100 μM fluconazole, 10 μM ketoconazole, 100 μM α-naphthoflavone, 1 μM quinidine, and 300 μM orphenadrine. For CYP2E1 antibody inhibition, 15 μg of control mouse antibody or anti-CYP2E1 antibodies were mixed with microsomes, preincubated for 5 minutes at 37°C before the addition of the 2 mM NAM and processed as described above. Control antibody was dialyzed overnight against excess 25 mM Tris-Cl pH 7.5 in a Slide-A-Lyzer Dialysis Cassette (cat#66333) from Pierce (Rockford, IL) to remove sodium azide, which inhibits NAM N-oxide activity.
CYP2E1-overexpressing insect microsomes were used essentially as the human microsomes but were diluted 4-fold with control microsomes because of high CYP2E1 activity.
HPLC assay for NAM N-oxide.
The samples were analyzed on the Breeze system (Waters, Millford, MA) consisting of an in-line degasser, the 1525 binary pump, the UV/Vis detector 2487 and the 717plus autosampler controlled by Breeze software (version 3.2). Thirty microliters of the microsomal extract were injected on a HILIC Atlantis T3 column Waters 100 mm, 4.6 mm i.d. that was protected by a 20 mm guard column of the same material. The compounds were eluted isocratically in 90% acetonitrile, 0.125% acetic acid, and 10 mM ammonium acetate and detected by UV absorbance at 254 nm. NAM N-oxide was identified by coelution with standards and quantified based on a standard curve of known quantities of NAM N-oxide.
Statistical analysis.
All results are from triplicate determination and presented as mean ± S.D. Significance was evaluated by the two-tailed Student’s t test (P < 0.05) in JMP Pro software (version 10; SAS Institute, Cary, NC). The Michaelis constant (Km) and Vmax were calculated based on the Michaelis-Menten equation using the nonlinear fit function of JMP Pro software (version 10; SAS Institute).
Results
We developed the HPLC assay to monitor the NAM N-oxide forming activity of HLMs. Robust NAM N-oxide peaks were detected upon incubation with low millimolar concentrations of NAM (Fig. 1B). Next, we carried dose-response experiments in HLMs to characterize the enzymatic reaction. The apparent Km for NAM N-oxidation under our conditions was 2.98 mM and maximal activity (Vmax) was 60.14 pmol/mg per minute (Fig. 2A). In addition to the P450 enzymes, HLMs also include FMOs, which are similarly capable of broad range oxidations. The two systems have overlapping substrates and both require NADPH (Krueger and Williams, 2005). To gauge the relative contributions of each, we incubated liver microsomes for 5 minutes at 45°C with and without NADPH. Heat treatment without NADPH is known to abolish FMO activity (Tugnait et al., 1997). However, the NAM N-oxide activity was not significantly affected (Fig. 2B). Experiments using insect microsomes overexpressing FMO3 did not show any detectable NAM N-oxidizing activity (not shown) reflecting a likely involvement of P450s. Next, we preincubated the HLMs with selective inhibitors for major hepatic P450 enzymes, ketoconazole (CYP3A4), fluconazole (CYP2C9), α-naphthoflavone (CYP1A2), tranylcypromine (CYP2A6), quinidine (CYP2D6), orphenadrine (CYP2B6), and diethyldithiocarbamate (CYP2E1). Only the CYP2E1 inhibitor significantly reduced (8-fold) the formation of NAM N-oxide (Fig. 2C). Additional experiments using microsomes overexpressing CYP2A6 and CYP2B6 did not show any detectable NAM N-oxidizing activity (not shown).
Fig. 2.
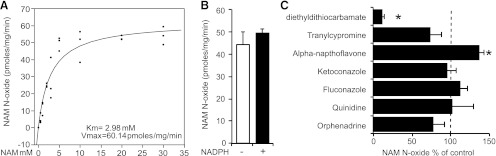
(A) Dose response of NAM versus NAM N-oxide production by HLMs (Km = 2.98 mM, Vmax = 60.14 pmol/mg per minute). (B) NAM N-oxide production by HLMs is not significantly inhibited by heat in the absence of NADPH (substrate NAM at 2 mM). (C) Effect of selective P450 inhibitors against major hepatic P450 enzymes on NAM N-oxide production (100 μM diethyldithiocarbamate, CYP2E1; 1 μM tranylcypromine, CYP2A6; 100 μM fluconazole, CYP2C9; 10 μM ketoconazole, CYP3A4; 100 μM α-naphthoflavone, CYP1A2; 1 μM quinidine, CYP2D6; and 300 μM orphenadrine, CYP2B6). Substrate NAM was at 2 mM. Mean ± S.D. of triplicate determinations. *P < 0.05 by Student’s t test.
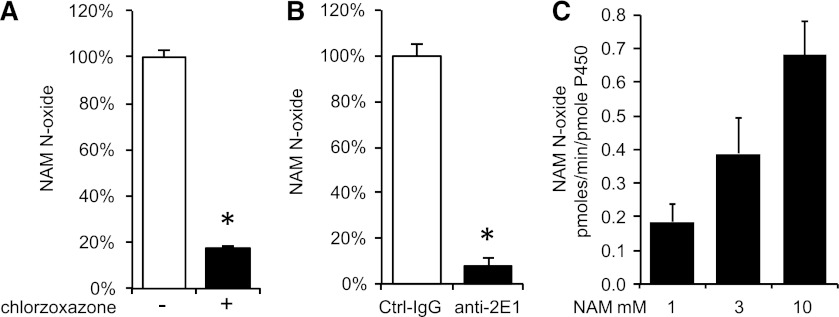
The large decrease in NAM N-oxide formation in the presence of the CYP2E1 inhibitor indicated that CYP2E1 might be the major enzyme oxidizing NAM in HLMs. To further support this idea, we tested the sensitivity of NAM N-oxidizing activity to CYP2E1 substrate and antibody inhibition. We incubated HLMs with 2 mM NAM in the presence of excess chlorzoxazone (100 μM), a standard substrate for CYP2E1 (Peter et al., 1990). Chlorzoxazone addition decreased NAM N-oxide formation more than 5-fold (Fig. 3A). Similarly, preincubation of liver microsomes with specific inhibitory antibody against CYP2E1 decreased NAM N-oxide to less than 8% of control antibody (Fig. 3B). Finally, insect cell microsomes overexpressing CYP2E1 showed dose-dependent NAM N-oxidizing activity corroborating the inhibitor results (Fig. 3C).
Fig. 3.
(A) Inhibition of NAM N-oxide production by 100 μM chlorzoxazone (substrate NAM at 2 mM). (B) Inhibition of NAM N-oxide production by specific anti-CYP2E1 antibody (substrate NAM at 2 mM). (C) Dose-dependent increase in NAM N-oxide production by CYP2E1 overexpressing insect cell microsomes. Mean ± S.D. of triplicate determinations. *P < 0.05 by Student’s t test.
Discussion
Clearance of excess NAM is mediated by two enzymatic systems in the liver, cytoplasmic and microsomal (Fig. 1A). The cytoplasmic system consists of the enzymes nicotinamide N-methyltransferase and aldehyde oxidase. The precise identity of the microsomal system was previously not known. In this report, we identify CYP2E1 as the main microsomal enzyme capable of NAM N-oxidation. Using a combination of inhibitors, substrates, and antibodies, we show that CYP2E1 is the major and possibly only enzyme producing NAM N-oxide in HLMs, although absolute proof of this statement, at least in mice, would require the use of the CYP2E1 knockout animals.
CYP2E1 metabolizes a broad spectrum of small molecular weight compounds. Among them, pyridine-3-carboxamide, a compound structurally similar to NAM, is also converted by CYP2E1 to pyridine N-oxide (Kim et al., 1988). Metabolism of carcinogens, such as azoxymethane, and industrial chemicals, such as carbon tetrachloride, benzene, and acrylamide, by CYP2E1 increases their toxicity (Sohn et al., 1991; Ghanayem et al., 2000). Thus, variation in CYP2E1 activity might predispose individuals to toxicity due to environmental pollutants, and numerous reports have attempted to correlate genotypic variations in CYP2E1 with various cancers (Neafsey et al., 2009). These studies suffer from limitations because the expression and the activity of CYP2E1 are also influenced by a disease state and are under nutritional control (Peng et al., 1983; Gonzalez et al., 1991; Chalasani et al., 2003). A more direct approach would involve the use of a biomarker to estimate CYP2E1 activity. Published attempts have used the hydroxylation of the standard substrate chlorzoxazone after ingestion by volunteers; however, this approach is impractical for large populations (Marchand et al., 1999; Piccoli et al., 2010). NAM N-oxide is a natural metabolite of NAM and can be assayed in the blood or urine and in historical samples, and it could therefore be amenable to large-scale screening. NAM clearance metabolites have recently been proposed as potential biomarkers of peroxisome proliferator-activated receptor-α activation by fibrates (Delaney et al., 2005; Zhen et al., 2007). The large increase in NAM metabolites has been attributed to the suppression of the enzyme α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase by peroxisome proliferator-activated receptor-α, which increases the tryptophan flux through the quinolinic-nicotinamide adenine dinucleotide pathway, giving rise eventually to NAM clearance products (Sanada, 1985; Fukuoka et al., 2002; Shin et al., 2006). Our results suggest that NAM N-oxide might actually be a more direct biomarker of CYP2E1 provided that it shows the required specificity and correlation with CYP2E1 activity. In conclusion, we identified CYP2E1 as the main microsomal enzyme producing the endogenous metabolite nicotinamide N-oxide and raise the potential of this pathway for biomarker development.
Abbreviations
- 2-pyr
N1-methyl-2-pyridone-5-carboxamide
- 4-pyr
N1-methyl-4-pyridone-3-carboxamide
- FMO
flavin-containing monooxygenase
- HLM
human liver microsome
- HPLC
high-performance liquid chromatography
- MNAM
N1-methylnicotinamide
- NAM
nicotinamide
- NAM N-oxide
nicotinamide N-oxide
- NNMT
nicotinamide N-methyltransferase
- P450
cytochrome P450
Authorship Contributions
Participated in research design: Pissios.
Conducted experiments: Real, Hong.
Performed data analysis: Real, Hong, Pissios.
Wrote or contributed to the writing of the manuscript: Real, Hong, Pissios.
Footnotes
This research was supported by the National Institutes of Health [Grant 5R01DK083694].
References
- Bartuś M, Łomnicka M, Kostogrys RB, Kaźmierczak P, Watała C, Słominska EM, Smoleński RT, Pisulewski PM, Adamus J, Gebicki J, et al. (2008) 1-Methylnicotinamide (MNA) prevents endothelial dysfunction in hypertriglyceridemic and diabetic rats. Pharmacol Rep 60:127–138 [PubMed] [Google Scholar]
- Bogan KL, Brenner C. (2008) Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr 28:115–130 [DOI] [PubMed] [Google Scholar]
- Bonavita V, Narrod SA, Kaplan NO. (1961) Metabolites of nicotinamide in mouse urine: effects of azaserine. J Biol Chem 236:936–939 [PubMed] [Google Scholar]
- Cantoni GL. (1951) Methylation of nicotinamide with soluble enzyme system from rat liver. J Biol Chem 189:203–216 [PubMed] [Google Scholar]
- Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, Crabb DW. (2003) Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology 37:544–550 [DOI] [PubMed] [Google Scholar]
- Chaykin S, Dagani M, Johnson L, Samli M. (1965) The fate of nicotinamide in the mouse. Urinary metabolites. J Biol Chem 240:932–938 [PubMed] [Google Scholar]
- Chlopicki S, Swies J, Mogielnicki A, Buczko W, Bartus M, Lomnicka M, Adamus J, Gebicki J. (2007) 1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway. Br J Pharmacol 152:230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney J, Hodson MP, Thakkar H, Connor SC, Sweatman BC, Kenny SP, McGill PJ, Holder JC, Hutton KA, Haselden JN, et al. (2005) Tryptophan-NAD+ pathway metabolites as putative biomarkers and predictors of peroxisome proliferation. Arch Toxicol 79:208–223 [DOI] [PubMed] [Google Scholar]
- Easterbrook J, Lu C, Sakai Y, Li AP. (2001) Effects of organic solvents on the activities of cytochrome P450 isoforms, UDP-dependent glucuronyl transferase, and phenol sulfotransferase in human hepatocytes. Drug Metab Dispos 29:141–144 [PubMed] [Google Scholar]
- Felsted RL, Chaykin S. (1967) N1-methylnicotinamide oxidation in a number of mammals. J Biol Chem 242:1274–1279 [PubMed] [Google Scholar]
- Fukuoka S-I, Ishiguro K, Yanagihara K, Tanabe A, Egashira Y, Sanada H, Shibata K. (2002) Identification and expression of a cDNA encoding human alpha-amino-beta-carboxymuconate-epsilon-semialdehyde decarboxylase (ACMSD). A key enzyme for the tryptophan-niacine pathway and “quinolinate hypothesis”. J Biol Chem 277:35162–35167 [DOI] [PubMed] [Google Scholar]
- Ghanayem BI, Wang H, Sumner S. (2000) Using cytochrome P-450 gene knock-out mice to study chemical metabolism, toxicity, and carcinogenicity. Toxicol Pathol 28:839–850 [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Ueno T, Umeno M, Song BJ, Veech RL, Gelboin HV. (1991) Microsomal ethanol oxidizing system: transcriptional and posttranscriptional regulation of cytochrome P450, CYP2E1. Alcohol Alcohol Suppl 1:97–101 [PubMed] [Google Scholar]
- Houtkooper RH, Cantó C, Wanders RJ, Auwerx J. (2010) The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev 31:194–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata K, Ogata S, Okumura K, Taguchi H. (2003) Induction of differentiation in human promyelocytic leukemia HL-60 cell line by niacin-related compounds. Biosci Biotechnol Biochem 67:1132–1135 [DOI] [PubMed] [Google Scholar]
- Kim SG, Williams DE, Schuetz EG, Guzelian PS, Novak RF. (1988) Pyridine induction of cytochrome P-450 in the rat: role of P-450j (alcohol-inducible form) in pyridine N-oxidation. J Pharmacol Exp Ther 246:1175–1182 [PubMed] [Google Scholar]
- Kobayashi K, Abe S, Nakajima M, Shimada N, Tani M, Chiba K, Yamamoto T. (1999) Role of human CYP2B6 in S-mephobarbital N-demethylation. Drug Metab Dispos 27:1429–1433 [PubMed] [Google Scholar]
- Krueger SK, Williams DE. (2005) Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol Ther 106:357–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifer E, Roth LJ, Hogness DS, Corson MH. (1951) The metabolism of radioactive nicotinic acid and nicotinamide. J Biol Chem 190:595–602 [PubMed] [Google Scholar]
- Marchand LL, Wilkinson GR, Wilkens LR. (1999) Genetic and dietary predictors of CYP2E1 activity: a phenotyping study in Hawaii Japanese using chlorzoxazone. Cancer Epidemiol Biomarkers Prev 8:495–500 [PubMed] [Google Scholar]
- Menon RM, Adams MH, González MA, Tolbert DS, Leu JH, Cefali EA. (2007) Plasma and urine pharmacokinetics of niacin and its metabolites from an extended-release niacin formulation. Int J Clin Pharmacol Ther 45:448–454 [DOI] [PubMed] [Google Scholar]
- Neafsey P, Ginsberg G, Hattis D, Johns DO, Guyton KZ, Sonawane B. (2009) Genetic polymorphism in CYP2E1: Population distribution of CYP2E1 activity. J Toxicol Environ Health B Crit Rev 12:362–388 [DOI] [PubMed] [Google Scholar]
- Nomura K, Shin M, Sano K, Umezawa C, Shimada T. (1983) Nicotinamide N-oxide formation by rat liver microsomes. Biochem Pharmacol 32:934–936 [DOI] [PubMed] [Google Scholar]
- Peng R, Tennant P, Lorr NA, Yang CS. (1983) Alterations of microsomal monooxygenase system and carcinogen metabolism by streptozotocin-induced diabetes in rats. Carcinogenesis 4:703–708 [DOI] [PubMed] [Google Scholar]
- Peter R, Böcker R, Beaune PH, Iwasaki M, Guengerich FP, Yang CS. (1990) Hydroxylation of chlorzoxazone as a specific probe for human liver cytochrome P-450IIE1. Chem Res Toxicol 3:566–573 [DOI] [PubMed] [Google Scholar]
- Piccoli P, Carrieri M, Padovano L, Di Mare M, Bartolucci GB, Fracasso ME, Lepera JS, Manno M. (2010) In vivo CYP2E1 phenotyping as a new potential biomarker of occupational and experimental exposure to benzene. Toxicol Lett 192:29–33 [DOI] [PubMed] [Google Scholar]
- Sanada H. (1985) Suppressive effect of dietary unsaturated fatty acids on alpha-amino-beta-carboxymuconate-epsilon-semialdehyde decarboxylase, a key enzyme of tryptophan-niacin metabolism in rat liver. J Nutr Sci Vitaminol (Tokyo) 31:327–337 [DOI] [PubMed] [Google Scholar]
- Shibata K, Kakehi H, Matsuo H. (1990) Niacin catabolism in rodents. J Nutr Sci Vitaminol (Tokyo) 36:87–98 [DOI] [PubMed] [Google Scholar]
- Shin M, Kim I, Inoue Y, Kimura S, Gonzalez FJ. (2006) Regulation of mouse hepatic alpha-amino-beta-carboxymuconate-epsilon-semialdehyde decarboxylase, a key enzyme in the tryptophan-nicotinamide adenine dinucleotide pathway, by hepatocyte nuclear factor 4alpha and peroxisome proliferator-activated receptor alpha. Mol Pharmacol 70:1281–1290 [DOI] [PubMed] [Google Scholar]
- Sohn OS, Ishizaki H, Yang CS, Fiala ES. (1991) Metabolism of azoxymethane, methylazoxymethanol and N-nitrosodimethylamine by cytochrome P450IIE1. Carcinogenesis 12:127–131 [DOI] [PubMed] [Google Scholar]
- Stratford MR, Dennis MF. (1992) High-performance liquid chromatographic determination of nicotinamide and its metabolites in human and murine plasma and urine. J Chromatogr A 582:145–151 [DOI] [PubMed] [Google Scholar]
- Tugnait M, Hawes EM, McKay G, Rettie AE, Haining RL, Midha KK. (1997) N-oxygenation of clozapine by flavin-containing monooxygenase. Drug Metab Dispos 25:524–527 [PubMed] [Google Scholar]
- Zhang W, Kilicarslan T, Tyndale RF, Sellers EM. (2001) Evaluation of methoxsalen, tranylcypromine, and tryptamine as specific and selective CYP2A6 inhibitors in vitro. Drug Metab Dispos 29:897–902 [PubMed] [Google Scholar]
- Zhen Y, Krausz KW, Chen C, Idle JR, Gonzalez FJ. (2007) Metabolomic and genetic analysis of biomarkers for peroxisome proliferator-activated receptor alpha expression and activation. Mol Endocrinol 21:2136–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]