Abstract
Differences in the ability of opioid drugs to promote regulated endocytosis of μ-opioid receptors are related to their tendency to produce drug tolerance and dependence. Here we show that drug-specific differences in receptor internalization are determined by a conserved, 10-residue sequence in the receptor’s carboxyl-terminal cytoplasmic tail. Diverse opioids induce receptor phosphorylation at serine (S)375, present in the middle of this sequence, but opioids differ markedly in their ability to drive higher-order phosphorylation on flanking residues [threonine (T)370, T376, and T379]. Multi-phosphorylation is required for the endocytosis-promoting activity of this sequence and occurs both sequentially and hierarchically, with S375 representing the initiating site. Higher-order phosphorylation involving T370, T376, and T379 specifically requires GRK2/3 isoforms, and the same sequence controls opioid receptor internalization in neurons. These results reveal a biochemical mechanism differentiating the endocytic activity of opioid drugs.
Introduction
Opioids are an extremely important class of drugs used in medicine, and they remain among the most effective drugs for the treatment of severe pain. Morphine, an alkaloid derived from the opium poppy, is the prototypical opioid. A vast number of additional drugs have been developed and are presently used in the clinic, some related in structure to morphine and others chemically diverse. The primary mechanism by which most of these drugs work is by binding to the μ-type opioid neuropeptide receptor (MOR), and this receptor mediates essentially all of the acute pharmacological actions of morphine (Handa et al., 1981; Matthes et al., 1996). However, the clinical benefits of opioid drugs are counteracted by longer-term regulatory processes that result in the development of tolerance and addiction (Aghajanian, 1978; Bozarth and Wise, 1981; Trujillo and Akil, 1991). Chemically diverse opioid drugs, despite similarity in their acute actions, differ remarkably in longer-term regulatory effects, and such differences significantly impact the clinical utility of particular drugs. Drug-induced internalization of opioid receptors has attracted considerable interest because several studies have reported some relationship between the ability of opioids to induce MOR internalization and their tendency to produce tolerance (Grecksch et al., 2006; Martini and Whistler, 2007; Koch and Hollt, 2008; Grecksch et al., 2011; Williams et al., 2013).
MOR internalization is stimulated by phosphorylation of the receptor’s carboxyl-terminal cytoplasmic domain (Zhang et al., 1998; Deng et al., 2000). MORs are basally phosphorylated and this is increased by opioid neuropeptides. Various opioid drugs differ in their effects on overall MOR phosphorylation, with increases in phosphorylation generally correlating with their ability to induce internalization (Zhang et al., 1998; McPherson et al., 2010). Analysis of serial truncation and site-directed mutants suggested that phosphorylation of μ receptors occurs primarily at a cluster of three serine and threonine residues, namely serine (S)363, threonine (T)370, and S375, within the cytoplasmatic tail of the receptor (El Kouhen et al., 2001). On a functional level mutation of S375 was found to inhibit receptor internalization, whereas mutation of S363 or T370 had either no or the opposite effect (El Kouhen et al., 2001). More recent evidence suggests that mutation of T376 or T379 leads to a similar internalization defect as mutation of S375, and that an opioid peptide agonist can promote phosphorylation of receptors at several residues in this region of the cytoplasmic tail (Lau et al., 2011). Indeed, considerable progress has been made recently in defining specific residues and regions of the cytoplasmic tail that determine phosphorylation-dependent control of MOR internalization induced by opioid peptide relative to morphine (Schulz et al., 2004; McPherson et al., 2010; Doll et al., 2011; Lau et al., 2011; Doll et al., 2012). However, the biochemical mechanism of this phosphorylation remains poorly understood. Further, most of what is known about agonist-selective differences is based on comparison of the effects of opioid peptide with morphine. Very little is known about how differences in the endocytic effects of nonpeptide opioid drugs are specified, despite the considerable potential importance of this question based on the wide variety of structurally distinct opioid drugs deployed in the clinic. Here we address these two questions and propose a simple principle, based on multi-site phosphorylation involving the coordinated activity of more than one GRK family member, that may underlie how drug-specific regulatory differences are encoded at the level of discrete opioid receptors.
Materials and Methods
Plasmids.
DNA for mouse MOR and MOR mutants were generated via artificial gene synthesis and cloned into pcDNA3.1 by imaGenes (Berlin, Germany). In addition, the coding sequence for an amino-terminal HA- or FLAG-tag was added.
Antibodies.
Phosphosite-specific antibodies for the S375/T376-phosphorylated form of the μ-opioid receptor were generated against the following sequence that contained a phosphorylated serine and a phosphorylated threonine residue: REHP(pSpT)ANTV. This sequence corresponds to amino acids 371–380 of the mouse μ-opioid receptor. Phosphosite-specific antibodies for the T379-phosphorylated form of the μ receptor were generated against the following sequence that contained a phosphorylated threonine residue: STAN(pT)VDRT. This sequence corresponds to amino acids 375–383 of the mouse μ-opioid receptor. The peptides were purified by HPLC and coupled to keyhole limpet hemocyanin. The conjugates were mixed 1:1 with Freund’s adjuvant and injected into groups of four rabbits each {3721–3723, 3725} for anti-pS375/pS376 antibody production and {3684–3687} for anti-pT379 antibody production. Animals were injected at 4-week intervals, and serum was obtained 2 weeks after immunizations beginning with the second injection. The specificity of the antisera was initially tested using dot blot analysis. For subsequent analysis, antibodies were affinity purified against their immunizing peptide using the SulfoLink kit (Thermo Scientific, Rockford, IL). The rabbit polyclonal phosphosite-specific antibodies anti-pS363 {3199}, anti-pT370 {3196}, and anti-pS375 {2493} were generated and extensively characterized previously (Doll et al., 2011). The phosphorylation-independent rabbit monoclonal anti-MOR antibody {UMB-3} was obtained from Epitomics (Burlingame, CA) (Lupp et al., 2011).
Agonists.
The following μ-opioid receptor agonists were obtained from commercial suppliers: morphine (Merck Pharma, Darmstadt, Germany), fentanyl (Rotexmedica, Trittau, Germany), etonitazene (Novartis, Basel, Switzerland), [d-Ala2-MePhe4-Gly-ol]enkephalin (DAMGO), pethidine (Sanofi-Aventis, Frankfurt, Germany), nortilidine (Pfizer, Karlsruhe, Germany), buprenorphine (Grünenthal, Aachen, Germany), and oxycodone (Mundipharma, Limburg, Germany).
Cell Culture and Transfection.
Human embryonic kidney 293 (HEK 293) cells obtained from DSMZ (Braunschweig, Germany) were cultured at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum. HEK 293 cells were stably transfected with Lipofectamine2000 (Invitrogen, Carlsbad, CA). Stable transfected cells were grown in medium supplemented with 500 μg/ml G418. HEK 293 cells stably expressing μ-opioid receptors were characterized using radioligand-binding assays, Western blot analysis, immunocytochemistry, and cAMP assays as described previously (Koch et al., 2001). Clones expressing similar amounts of receptors (∼800 fmol/mg membrane protein) were selected and used for further studies.
Analysis of Opioid Receptor Internalization in HEK 293 Cells.
Stably transfected cells were grown on poly-l-lysine-coated cover slips overnight. Cells were then incubated with primary antibody rabbit anti-HA antibody in serum-free medium for 2 hours at 4°C. After agonist exposure, cells were fixed with 4% paraformaldehyde and 0.2% picric acid in phosphate buffer (pH 6.9) for 30 minutes at room temperature and washed several times with phosphate-buffered saline (PBS). Specimens were permeabilized and then incubated with an Alexa488-conjugated goat antirabbit antibody (Amersham, Braunschweig, Germany). Specimens were mounted and examined using a Zeiss LSM510 META laser scanning confocal microscope (Zeiss, Jena, Germany). For quantitative internalization assays, cells were seeded onto 24-well plates. On the next day, cells were preincubated with anti-HA antibody for 2 hours at 4°C. Cells were then exposed to agonist at 37°C, fixed, and developed with peroxidase-conjugated secondary antibody as described (Lesche et al., 2009; Poll et al., 2010).
Analysis of Opioid Receptor Internalization in Neurons.
Striatal neurons were prepared from embryonic day 17–18 Sprague Dawley rats, transfected upon plating (Amaxa nucleofector) and studied 10–14 days in vitro. All animal methods were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted by the National Institutes of Health and the UCSF Institutional Animal Care and Use Committee. Surface-accessible receptors were labeled in intact cells by addition of Alexa 488–conjugated M1 anti-FLAG antibody (2 μg/ml) to the culture medium for 30 minutes at 37°C, then cells were incubated for an additional 20 minutes in the presence or absence of 10 μM morphine or 10 μM DAMGO as indicated. After a quick wash with PBS, cells were fixed in 4% formaldehyde in PBS for 15 minutes, then stained with Alexa 555-conjugated antimouse secondary (1:1000; Invitrogen), washed four times with PBS and mounted onto glass slides with Vectashield (Vector Laboratories, Burlingame, CA). Epifluorescence microscopy was performed with an inverted Nikon Diaphot microscope equipped with a 60×/numerical aperture (NA) 1.4 objective, mercury arc lamp illumination, and standard dichroic filter sets (Omega Optical, Brattleboro, VT). Images were collected with a CCD camera (Princeton Instruments, Trenton, NJ) interfaced to a PC running Micromanager software (www.micro-manager.org). Ratiometric determination of agonist-induced changes in surface-receptor fluorescence was carried out as described previously (Kotowski et al., 2011).
Western Blot Analysis.
Cells were seeded onto poly-l-lysine-coated 60-mm dishes and grown to 80% confluence. After treatment with agonist, cells were lysed in detergent buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 10 mM NaF, 10 mM disodium pyrophosphate, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) in the presence of protease and phosphatase inhibitors Complete Mini and PhosSTOP (Roche Diagnostics, Mannheim, Germany). Glycosylated proteins were partially enriched using wheat germ lectin-agarose beads as described (Koch et al., 2001; Schulz et al., 2004). Proteins were eluted from the beads using SDS-sample buffer for 20 minutes at 45°C. Samples were split, resolved on 7.5% SDS–polyacrylamide gels, and after electroblotting, membranes were incubated with either anti-pT370 {3196}, anti-pS375 {2493}, anti-pT376 {3722}, or anti-pT379 {3686} antibodies followed by detection using an enhanced chemiluminescence detection system (Amersham, Braunschweig, Germany). Blots were stripped and incubated again using the phosphorylation-independent anti-MOR antibody {UMB-3} (Lupp et al., 2011), anti-HA antibody, or antitransferrin receptor antibody (Invitrogen) to ensure equal loading of the gels.
Stable Isotope Labeling by Amino Acids in Cell Culture Labeling and Mass Spectrometry Analysis of Opioid Receptor Phosphorylation.
Stably transfected HEK 293 cells expressing Flag-tagged μ-opioid receptors were grown in arginine-depleted Dulbecco’s modified Eagle’s medium that was supplemented with either regular arginine, 13C-arginine, or 13C15N-arginine. To allow for efficient incorporation of isotopic arginine, cells were allowed to double five to six times prior to agonist application. Subsequent analysis of arginine levels showed that approximately 99% of arginines within a peptide contained the heavy isotope. Regular arginine supplemented cells acted as the control. 13C-arginine–supplemented cells were treated with 10 μM morphine. 13C15N-arginine–supplemented cells were treated with 10 μM DAMGO. Dishes were incubated with the indicated agonist for 2 minutes at 37°C, then rapidly harvested on ice, and flash frozen in liquid nitrogen. Anti-Flag immunoaffinity purification of receptors and LC–MS methods were carried out as described previously (Lau et al., 2011).
Small Interfering RNA Silencing of Gene Expression.
Double--stranded small-interfering RNA (siRNA) duplexes with 3′-dTdT overhangs were synthesized by Qiagen (Hilden, Germany) for following targets: GRK2 (5′-CCGGGAGATCTTCGACTCATA-3′ and 5′-AAGAAGTACGAGAAGCTGGAG-3′), GRK3 (5′-AAGCAAGCTGTAGAACACGTA-3′ and 5′-GCAGAAGTCGACAAATTTA-3′), GRK5 (5′-AGCGTCATAACTAGAACTGAA-3′ and 5′-AAGCCGTGCAAAGAACTCTTT-3′) and nonsilencing RNA duplex (5′-GCTTAGGAGCATTAGTAAA-3′ or 5′-AAACTCTATCTGCACGCTGAC-3′). HEK 293 cells were transfected using HiPerFect transfection reagent (Quiagen) with 200 nM for single or 150-nM siRNA for double transfection for 3 days. Silencing was quantified by immunoblotting as described (Poll et al., 2010; Poll et al., 2011; Doll et al., 2012). All experiments showed protein levels reduced by ≥80%.
Data Analysis.
Protein bands detected on Western blots were quantified using ImageJ 1.40g or BIO-1D analysis software. Data were analyzed using GraphPad Prism 4.0 software (La Jolla, CA). Statistical analysis was calculated with two-way ANOVA or Student’s t test. P values <0.05 were considered statistically significant.
Results
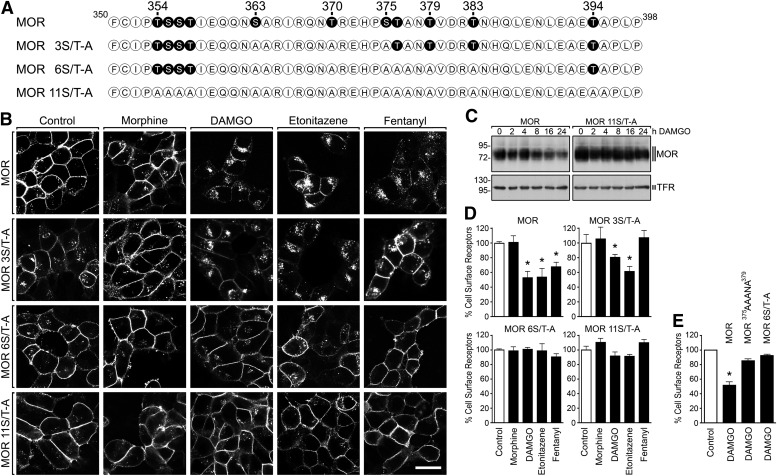
MORs internalized rapidly in transfected human embryonic kidney cells following application of the opioid peptide agonist DAMGO. The nonpeptide agonist drugs etonitazine and fentanyl also induced robust internalization, whereas morphine induced very little internalization (Fig. 1). We next mapped putative phosphorylation sites controlling MOR internalization. Mutation of all Ser/Thr residues present in the carboxyl-terminal cytoplasmic tail (Fig. 1A) inhibited the rapid internalization of receptors as visualized by fluorescence microscopy (Fig. 1B) and, consistent with this, also inhibited proteolytic down-regulation of receptors observed after more prolonged agonist exposure (Fig. 1C). Comparison of a series of mutant-receptor constructs (Fig. 1A) identified a middle portion the cytoplasmic tail in which Ser/Thr mutation (MOR 6S/T-A) inhibited receptor internalization to a similar degree as mutating all Ser/Thr residues in the cytoplasmic tail. Endocytic inhibition was verified using a cell-surface enzyme-linked immunosorbent assay (ELISA) assay to quantify opioid-induced reduction of surface-receptor number (Fig. 1D), as well as using fluorescence flow cytometry that established a reduced rate of MOR 6S/T-A mutant–receptor internalization. In the MOR 3S/T-A mutant, DAMGO, and etonitazene were able to stimulate a measurable internalization, whereas fentanyl-induced internalization was strongly compromised (Fig. 1D). Mutating only S375, T376, and T379 within this region produced a similar degree of endocytic inhibition (Fig. 1E), focusing our attention on these particular residues for further analysis.
Fig. 1.
Carboxyl-terminal phosphorylation is required for μ-opioid receptor internalization. (A) Sequence of the carboxyl-terminal tail of the μ-opioid receptor showing all potential phospho-acceptor sites. Serine (S) and threonine (T) residues depicted in black were substituted by alanines (A). (B) HEK 293 cells stably expressing the HA-tagged MOR, MOR 3S/T-A, MOR 6S/T-A, or MOR 11S/T-A were preincubated with anti-HA antibody and stimulated with 10 μM morphine, 10 μM DAMGO, 25 nM etonitazene, or 1 μM fentanyl for 30 minutes. Cells were then fixed, immunofluorescently stained, and examined using confocal microscopy. Shown are representative results from one of four independent experiments per condition. Scale bar, 20 μM. (C) HEK 293 cells stably expressing the HA-tagged MOR or MOR 11S/T-A were treated with 10 μM DAMGO for 0, 2, 4, 8, 16, or 24 hours. Cells were lysed and immunoblotted with anti-HA antibody. To ensure equal loading of the gels, blots were stripped and incubated with antitransferrin receptor antibody (TFR). The position of molecular mass marker is indicated on the left (in kilodaltons). (D) HEK 293 cells stably expressing the HA-tagged MOR, MOR 3S/T-A, MOR 6S/T-A, or MOR 11S/T-A were preincubated with anti-HA antibody and stimulated with 10 μM morphine, 10 μM DAMGO, 25 nM etonitazene, or 1 μM fentanyl for 30 minutes. Cells were then fixed and labeled with a peroxidase-conjugated secondary antibody. Receptor sequestration, quantified as the percentage of residual cell-surface receptors in agonist-treated cells, was measured by enzyme-linked immunosorbent assay. (E) HEK 293 cells stably expressing the FLAG-tagged MOR, MOR375AAANA379, or MOR 6S/T-A were preincubated with anti-FLAG antibody and stimulated with 10 μM DAMGO for 30 minutes. Cells were then fixed and labeled with a fluorescently-labeled secondary antibody. Receptor sequestration, quantified as the percentage of residual cell-surface receptors in agonist-treated cells, was measured by flow cytometry. The data are presented as the means ± S.E.M. of four independent experiments performed in triplicate. Results were analyzed by two-way ANOVA followed by the Bonferroni posttest (*P < 0.05).
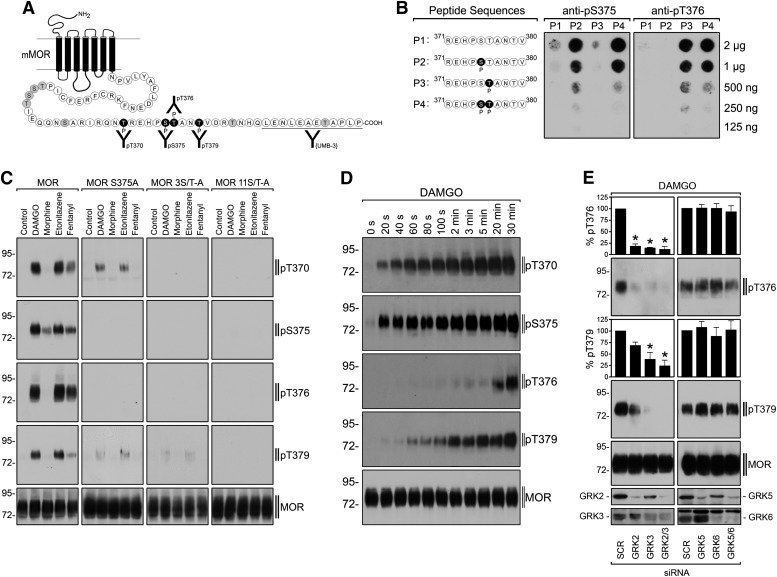
To directly assess receptor phosphorylation at candidate sites, antipeptide antibodies specifically recognizing the phosphorylated form of each were generated (Fig. 2A), and their specificity verified using synthetic phosphopeptides (Fig. 2B). Immunoblot analysis using these antibodies revealed that DAMGO strongly stimulated phosphorylation at all of these residues in the wild type MOR, as did the nonpeptide agonists etonitazine and fentanyl that also robustly promote receptor internalization (Fig. 2C, left column of immunoblots). Morphine stimulated phosphorylation at S375 but, in contrast to the efficiently internalizing agonists, failed to stimulate detectable phosphorylation of the other residues, with equivalent receptor loading verified by detection of a distinct (nonphosphorylated) epitope in the cytoplasmic tail (UMB-3 antibody, Fig. 2C, bottom immunoblot).
Fig. 2.
Hierarchical and sequential multi-site phosphorylation of μ-opioid receptor. (A) Schematic representation of the mouse μ-opioid receptor. T370, S375, T376, and T379 were targeted for the generation of phosphosite-specific antibodies. (B) Characterization of anti-pS375 and anti-pT376 antibodies using dot-blot analysis. Serial dilutions of peptides 1–4 were blotted, and membranes were incubated with the anti-pS375 antibody {2493} or anti-pT376 antibody {3722}. Note that the anti-pS375 antibody {2493} detects the peptide phosphorylated at S375 as well as the peptide phosphorylated at S375 and T376 but not the peptide phosphorylated at T376. Conversely, the anti-pT376 antibody {3722} detects the peptide phosphorylated at T376 as well as the peptide phosphorylated at S375 and T376 but not the peptide phosphorylated at S375. (C) Characterization of phosphosite-specific antibodies using Western blot analysis. HEK 293 cells stably expressing the HA-tagged MOR, MOR 11S/T-A, MOR 3S/T-A, or MOR S375A were stimulated with 10 μM morphine, 10 μM DAMGO, 25 nM etonitazene, or 1 μM fentanyl for 10 minutes. Cells were lysed and immunoblotted with anti-pT370, anti-pS375, anti-pT376, or anti-pT379 antibodies. Blots were stripped and reprobed with the phosphorylation-independent anti-MOR antibody UMB-3 or with anti-HA antibody to confirm equal loading of the gels. (D) Time-course of agonist-induced μ-opioid receptor phosphorylation. HEK 293 cells stably expressing the μ-opioid receptor were exposed to 10 μM DAMGO for the indicated time periods. Cells were lysed and immunoblotted with anti-pT370, anti-pS375, anti-pT376, or anti-pT379 antibodies. Blots were stripped and reprobed with the phosphorylation-independent anti-MOR antibody UMB-3 to confirm equal loading of the gels. (E) GRK2 and GRK3 are responsible for DAMGO-induced T376 and T379 phosphorylation. HEK 293 cells stably expressing the μ-opioid receptor were transfected with siRNA targeted to GRK2, GRK3, GRK2, and GRK3 (GRK2/3), or GRK5, GRK6, GRK5, and GRK6 (GRK5/6), or nonsilencing siRNA control (SCR) for 72 hours and then exposed to 10 μM DAMGO for 30 minutes. Cells were lysed and immunoblotted with anti-pT376 or anti-pT379 antibodies. Blots were stripped and reprobed with the phosphorylation-independent anti-MOR antibody UMB-3 to confirm equal loading of the gels. Blots were quantified and expressed as percentage of maximal phosphorylation in SCR-transfected cells. Data correspond to means ± S.E.M. from four independent experiments. Results were analyzed by two-way ANOVA followed by the Bonferroni posttest (*P < 0.05). Shown are representative results from one of four independent experiments per condition. The positions of molecular mass markers are indicated on the left (in kilodaltons).
As expected, receptor detection by all of the phospho-specific antibodies was blocked by global mutation of Ser/Thr residues present in the cytoplasmic tail (MOR 11S/T-A mutant receptor, Fig. 2C, right column of immunoblots). Further, no signal for pT370 or pS375 was detected in the MOR 3S/T-A mutant receptor that also lacks these residues (Fig. 2C, third column of immunoblots). Remarkably, phosphorylation of T376, and T379 was also greatly reduced in the MOR 3S/T-A mutant construct, even though these residues were not mutated in this receptor (Fig. 2C, third column of immunoblots). Moreover, point mutation of only S375 (MOR S375A), corresponding to one of the substituted residues in the MOR 3S/T-A mutant receptor, strongly reduced DAMGO- and etonitazene-induced phosphorylation of T370, T376, and T379 (Fig. 2C, second column of immunoblots). These results indicate that phosphorylation in this cytoplasmic region is hierarchical, with S375 representing an initiating site required for subsequent phosphorylation at T370, T376, and T379. Interestingly, mutation of S375 prevented fentanyl-induced phosphorylation of T370, T376, and T379, suggesting that these residues are less accessible for GRK-mediated phosphorylation of fentanyl-activated MORs (Fig. 2C, second column of immunoblots).
Based on this hypothesis, one would predict phosphorylation at S375 in the wild type receptor to precede that occurring at either T376 or T379. This was indeed the case, as indicated by careful time-course analysis, which was carried out at reduced temperature (22°C) to slow down the cellular processes (Fig. 2D). Phosphorylation at S375 occurred within 20 seconds after agonist application while phosphorylation at T376 and T379 followed by several minutes. Another prediction is that multiple phosphorylations occur in precisely the same receptor molecule, rather than being distributed over a mixture of singly phosphorylated receptor species. Liquid chromatography–mass spectrometry (LC–MS) analysis verified this, resolving both doubly and triply phosphorylated receptor species in this region of the cytoplasmic tail that were produced within 2 minutes after agonist application at physiologic temperature (Supplemental Fig S1). Whereas phosphosite-specific antibodies detected a selective phosphorylation of S375 in the presence of morphine, LC–MS analysis resolved doubly and triply phosphorylated receptor species after morphine although to a much lesser extent as after DAMGO exposure, most likely reflecting different sensitivities of the techniques.
Ligand-induced endocytosis of MORs is stimulated by G protein-coupled receptor kinase (GRK)-mediated phosphorylation, and GRK2/3 as well as GRK5 isoforms can mediate ligand-induced phosphorylation of S375 (Doll et al., 2012). We next asked about the kinase specificity for phosphorylating T376 and T379. Depleting GRK2/3 isoforms using siRNA markedly inhibited MOR phosphorylation at both of these residues, whereas depleting GRK5/6 isoforms had no effect (Fig. 2E). These results suggest that GRK2/3 or 5/6 isoforms can mediate the initiating phosphorylation of S375, but higher-order phosphorylation involving T376 and T379 specifically requires GRK2/3 isoforms.
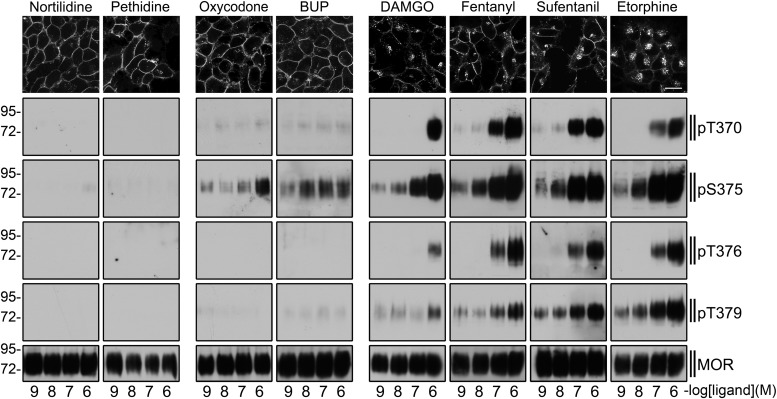
We then surveyed a larger range of opioids to determine if the observed relationship applies more broadly across structurally diverse agonists. This indeed appeared to be the case. In surveying a selection of opioids, differing both in structure and ability to promote regulated internalization of receptors, we consistently observed that endocytic activity of an opioid drug is associated with the ability to stimulate multi-phosphorylation of the residues described. Opioid drugs lacking the ability to induce higher-order phosphorylation involving these residues, even if they are able to stimulate phosphorylation at S375, are inefficient at stimulating regulatory internalization of MORs (Fig. 3).
Fig. 3.
Agonist-selective μ-opioid receptor phosphorylation. (Top) HEK 293 cells stably expressing the HA-tagged μ-opioid receptor were preincubated with anti-HA antibody and then exposed to nortilidine, pethidine, oxycodone, buprenorphine (BUP), DAMGO, fentanyl, sufentanil, or etorphine at a concentration of 10−6 M for 30 minutes. Cells were then fixed, immunofluorescently stained, and examined using confocal microscopy. Shown are representative results from one of three independent experiments per condition. Scale bar, 20 μm. (Bottom) HEK 293 cells stably expressing the HA-tagged μ-opioid receptor were exposed to nortilidine, pethidine, oxycodone, buprenorphine (BUP), DAMGO, fentanyl, sufentanil, or etorphine at concentrations ranging from 10−9 to 10−6 M for 10 minutes. Cells were lysed and immunoblotted with anti-pT370, anti-pS375, anti-pT376, or anti-pT379 antibodies. Blots were stripped and reprobed with the phosphorylation-independent anti-MOR antibody UMB-3 to confirm equal loading of the gels. Shown are representative results from one of three independent experiments per condition. The position of molecular mass markers is indicated on the left (in kilodaltons).
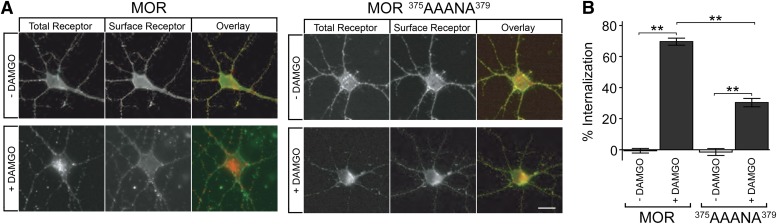
Finally, to investigate the physiologic relevance of the present findings, we asked if the same residues control MOR internalization in CNS-neurons that naturally express MORs. To do so, we prepared primary cultures of medium spiny neurons from rat striatum, and tested the effect of mutating phosphorylation sites that control internalization in the nonneural cell model. The wild type MOR internalized robustly in cultured neurons, whereas mutation of S375, T376, and T379 markedly inhibited this process. This was apparent by simple visual inspection of fluorescence micrographs (Fig. 4A), and was verified quantitatively across multiple experiments by fluorescence-ratio imaging using distinct fluorophores to label surface- and internalized-receptor populations (Fig. 4B). These observations suggest that the same phosphorylation sites control opioid receptor internalization in relevant neurons.
Fig. 4.
Carboxyl-terminal phosphorylation promotes μ-opioid receptor internalization in neurons. (A) Representative confocal fluorescence micrograph of a wild type MOR (left set of panels) or MOR375AAANA379 (right set of panels) localization in medium spiny neurons maintained in the absence of agonist (top row of images) and after exposure to 1 μM DAMGO for 20 minutes (bottom row). The total pool of plasma membrane receptors was initially labeled with Alexa488-conjugated anti-Flag monoclonal antibody (left panels, overlay indicated in red). At the end of the indicated incubation period, cells were fixed without permeabilization and the fraction of receptors remaining in the plasma membrane was selectively labeled using Alexa555-conjugated antimouse secondary antibody (middle panels, overlay indicated in green). Scale bar, 10 μm. (B) Quantification of the receptor internalization results, by fluorescence ratio imaging, across a total of 40 neurons for each condition imaged in three independent experiments. Bars represent the mean percentage internalization of the receptor pool labeled in the plasma membrane at the outset of the experiment, and error bars represent means ± S.E.M. Results were analyzed across the indicated comparisons using unpaired, two-tailed Student’s t test (**P < 0.0001).
Discussion
The present results identify multi-phosphorylation in a specific sequence within the MOR cytoplasmic tail as a discriminator of drug-induced receptor regulation by endocytosis. They define specific phosphorylated residues in this sequence, resolve the biochemical mechanism of this regulation through sequential and hierarchical phosphorylation, and establish that higher-order phosphorylations in this sequence are mediated specifically by GRK2/3 isoforms. Multiple phosphorylations within this sequence are necessary for its endocytosis-promoting activity, and the core phosphorylation sites in this sequence are essential for opioid receptor internalization in neurons. Accordingly, we propose that this multi-phosphorylation mechanism represents a fundamental biochemical basis regulating the different endocytic activity of opioid drugs.
S375, the initiating residue in the hierarchical phosphorylation cascade, can be phosphorylated by GRK2/3 as well as GRK5/6 isoforms, and phosphorylation of this residue is stimulated by a wide variety of opioid drugs (Doll et al., 2012). Phosphorylation at both T376 and T379 requires this priming phosphorylation but specifically requires GRK2/3 isoforms, and phosphorylation at each of these later residues is markedly drug-selective. Multi-phosphorylation of this conserved cytoplasmic sequence thus provides a biochemical code controlling drug-selective MOR internalization, and explains why opioid drugs that differ only moderately in their effects on overall MOR phosphorylation can differ dramatically in their effects on regulated endocytosis. We have recently shown that multi-site phosphorylation including S375, T376, and T379 is required for efficient recruitment of nonvisual arrestin (Lau et al., 2011). Point mutating any of those residues individually significantly reduced arrestin recruitment (Lau et al., 2011), consistent with the inhibition of endocytosis shown here.
Because multi-site phosphorylation can effectively sharpen drug-dependent differences in regulated endocytosis of receptors, we propose that multi-site phosphorylation might represent a relatively widespread principle underlying the generation or expression of functional selectivity among drugs. Functionally selective drug-effects fundamentally involve differentially promoting or stabilizing the interaction of activated receptors with distinct cytoplasmic proteins, but how the receptor’s cytoplasmic surface is altered to do so remains largely unknown (Stallaert et al., 2011). The present results suggest that multi-site phosphorylation, by its inherent ability to generate nonlinear effects in diverse biologic contexts (Deshaies and Ferrell, 2001; Nash et al., 2001), could provide a flexible and quantitative basis for differentiating, and covalently “encoding,” the effects of chemically distinct ligands and drugs. The present results support this concept specifically in the case of endocytic regulation of opioid receptors; further study will be necessary to investigate whether this principle is more widely applicable to other functionally selective drug-effects.
An intriguing possibility is that the mechanism of drug differentiation shown here in cultured cells may contribute to understanding differences among opioids in their tendency to produce deleterious effects such as tolerance and physical dependence in vivo. There is already evidence for such a relationship, particularly for opioid tolerance (Grecksch et al., 2011). However, as most of the evidence supporting this concept remains correlative in nature, caution is warranted. The present identification of specific residues and kinases contributing to multi-phosphorylation regulating different endocytic effects of opioid drugs suggest a useful starting point for definitive experiments testing causation, based on targeted manipulation of defined drug-selective phosphorylation events in vivo. As an immediate but still correlative next step toward this goal, the phospho-specific antibodies described in the present study should provide a means to test whether the key residues whose phosphorylation distinguishes opioid drugs in cultured cells are also differentially phosphorylated in vivo.
In conclusion, the present results elucidate a simple biochemical mechanism, based on the conserved principle of multi-site phosphorylation, by which functional differentiation of the diverse endocytic effects of opioid drugs is achieved. By doing so, the present study reveals an additional level of selectivity in the cell biology of drug action that is potentially widely applicable in pharmacology, and may lead to future development of more precise and effective opioid pharmacotherapy.
Supplementary Material
Acknowledgments
The authors thank Heidrun Guder and Heike Stadler for excellent technical assistance.
Abbreviations
- DAMGO
[d-Ala2-MePhe4-Gly-ol]enkephalin
- GRK
G protein-coupled receptor kinase
- HEK 293
human embryonic kidney 293 cells
- LC–MS
liquid chromatography–mass spectrometry
- MOR
μ-opioid receptor
- S
serine
- siRNA
small-interfering RNA
- T
threonine
Authorship Contributions
Participated in research design: von Zastrow, Schulz.
Conducted experiments: Just, Illing, Trester-Zedlitz, Lau, Kotowski, Miess, Mann, Doll, Trinidad, Burlingame, von Zastrow, Schulz.
Contributed new reagents or analytic tools: Schulz.
Performed data analysis: Just, Illing, Trester-Zedlitz, Lau, Kotowski, Miess, Mann, Doll, Trinidad, Burlingame, von Zastrow, Schulz.
Wrote or contributed to the writing of the manuscript: Just, Schulz, von Zastrow.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft [SCH924/11-2]; the National Institutes of Health National Institute on Drug Abuse [Grants DA010711 and DA012864]; and the National Institutes of Health National Center for Research Resources [Grant P41RR001614].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Aghajanian GK. (1978) Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature 276:186–188 [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. (1981) Heroin reward is dependent on a dopaminergic substrate. Life Sci 29:1881–1886 [DOI] [PubMed] [Google Scholar]
- Deng HB, Yu Y, Pak Y, O’Dowd BF, George SR, Surratt CK, Uhl GR, Wang JB. (2000) Role for the C-terminus in agonist-induced mu opioid receptor phosphorylation and desensitization. Biochemistry 39:5492–5499 [DOI] [PubMed] [Google Scholar]
- Deshaies RJ, Ferrell JE., Jr (2001) Multisite phosphorylation and the countdown to S phase. Cell 107:819–822 [DOI] [PubMed] [Google Scholar]
- Doll C, Konietzko J, Pöll F, Koch T, Höllt V, Schulz S. (2011) Agonist-selective patterns of µ-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Br J Pharmacol 164:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll C, Pöll F, Peuker K, Loktev A, Glück L, Schulz S. (2012) Deciphering µ-opioid receptor phosphorylation and dephosphorylation in HEK293 cells. Br J Pharmacol 167:1259–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kouhen R, Burd AL, Erickson-Herbrandson LJ, Chang CY, Law PY, Loh HH. (2001) Phosphorylation of Ser363, Thr370, and Ser375 residues within the carboxyl tail differentially regulates mu-opioid receptor internalization. J Biol Chem 276:12774–12780 [DOI] [PubMed] [Google Scholar]
- Grecksch G, Bartzsch K, Widera A, Becker A, Höllt V, Koch T. (2006) Development of tolerance and sensitization to different opioid agonists in rats. Psychopharmacology (Berl) 186:177–184 [DOI] [PubMed] [Google Scholar]
- Grecksch G, Just S, Pierstorff C, Imhof AK, Glück L, Doll C, Lupp A, Becker A, Koch T, Stumm R, et al. (2011) Analgesic tolerance to high-efficacy agonists but not to morphine is diminished in phosphorylation-deficient S375A μ-opioid receptor knock-in mice. J Neurosci 31:13890–13896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa BK, Land AC, Lord JA, Morgan BA, Rance MJ, Smith CF. (1981) Analogues of beta-LPH61-64 possessing selective agonist activity at mu-opiate receptors. Eur J Pharmacol 70:531–540 [DOI] [PubMed] [Google Scholar]
- Koch T, Schulz S, Pfeiffer M, Klutzny M, Schröder H, Kahl E, Höllt V. (2001) C-terminal splice variants of the mouse mu-opioid receptor differ in morphine-induced internalization and receptor resensitization. J Biol Chem 276:31408–31414 [DOI] [PubMed] [Google Scholar]
- Koch T, Höllt V. (2008) Role of receptor internalization in opioid tolerance and dependence. Pharmacol Ther 117:199–206 [DOI] [PubMed] [Google Scholar]
- Kotowski SJ, Hopf FW, Seif T, Bonci A, von Zastrow M. (2011) Endocytosis promotes rapid dopaminergic signaling. Neuron 71:278–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau EK, Trester-Zedlitz M, Trinidad JC, Kotowski SJ, Krutchinsky AN, Burlingame AL, von Zastrow M. (2011) Quantitative encoding of the effect of a partial agonist on individual opioid receptors by multisite phosphorylation and threshold detection. Sci Signal 4:ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesche S, Lehmann D, Nagel F, Schmid HA, Schulz S. (2009) Differential effects of octreotide and pasireotide on somatostatin receptor internalization and trafficking in vitro. J Clin Endocrinol Metab 94:654–661 [DOI] [PubMed] [Google Scholar]
- Lupp A, Richter N, Doll C, Nagel F, Schulz S. (2011) UMB-3, a novel rabbit monoclonal antibody, for assessing μ-opioid receptor expression in mouse, rat and human formalin-fixed and paraffin-embedded tissues. Regul Pept 167:9–13 [DOI] [PubMed] [Google Scholar]
- Martini L, Whistler JL. (2007) The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol 17:556–564 [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, et al. (1996) Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383:819–823 [DOI] [PubMed] [Google Scholar]
- McPherson J, Rivero G, Baptist M, Llorente J, Al-Sabah S, Krasel C, Dewey WL, Bailey CP, Rosethorne EM, Charlton SJ, et al. (2010) μ-opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol Pharmacol 78:756–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash P, Tang X, Orlicky S, Chen Q, Gertler FB, Mendenhall MD, Sicheri F, Pawson T, Tyers M. (2001) Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature 414:514–521 [DOI] [PubMed] [Google Scholar]
- Pöll F, Doll C, Schulz S. (2011) Rapid dephosphorylation of G protein-coupled receptors by protein phosphatase 1β is required for termination of β-arrestin-dependent signaling. J Biol Chem 286:32931–32936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöll F, Lehmann D, Illing S, Ginj M, Jacobs S, Lupp A, Stumm R, Schulz S. (2010) Pasireotide and octreotide stimulate distinct patterns of sst2A somatostatin receptor phosphorylation. Mol Endocrinol 24:436–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, Höllt V. (2004) Morphine induces terminal micro-opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J 23:3282–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallaert W, Christopoulos A, Bouvier M. (2011) Ligand functional selectivity and quantitative pharmacology at G protein-coupled receptors. Expert Opin Drug Discov 6:811–825 [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. (1991) Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science 251:85–87 [DOI] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MD. (2013) Regulation of μ-opioid receptors: Desensitization, phosphorylation, internalization and tolerance. Pharmacol Rev 65:223–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. (1998) Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci USA 95:7157–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.