Abstract
GABAB receptor-positive modulators are thought to have advantages as potential medications for anxiety, depression, and drug addiction. They may have fewer side effects than GABAB receptor agonists, because selective enhancement of activated receptors could have effects different from nonselective activation of all receptors. To examine this, pigeons were trained to discriminate the GABAB receptor-positive modulator (R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one (rac-BHFF) from its vehicle. The discriminative stimulus effects of rac-BHFF were not mimicked by the GABAB receptor agonists baclofen and γ-hydroxybutyrate (GHB), not by diazepam, and not by alcohol, cocaine, and nicotine, whose self-administration has been reported to be attenuated by GABAB receptor-positive modulators. The discriminative stimulus effects of rac-BHFF were not antagonized by the GABAB receptor antagonist 3-aminopropyl (diethoxymethyl)phosphinic acid (CGP35348) but were attenuated by the less efficacious GABAB receptor-positive modulator 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethylpropyl)phenol (CGP7930), suggesting the possibility that rac-BHFF produces its discriminative stimulus effects by directly activating GABAB2 subunits of GABAB receptors. At a dose 10-fold lower than the training dose, rac-BHFF enhanced the discriminative stimulus effects of baclofen, but not of GHB. This study provides evidence that the effects of GABAB receptor-positive modulators are not identical to those of GABAB receptor agonists. In addition, the results suggest that positive modulation of GABAB receptors does not produce discriminative stimulus effects similar to those of benzodiazepines, alcohol, cocaine, and nicotine. Finally, the finding that rac-BHFF enhanced effects of baclofen but not of GHB is consistent with converging evidence that the populations of GABAB receptors mediating the effects of baclofen and GHB are not identical.
Introduction
GABAB receptors, which are present throughout the central nervous system, are implicated in various central nervous system disorders (Cryan and Kaupmann, 2005; Bowery, 2006), including drug dependence (Maccioni et al., 2008; Addolorato et al., 2009; Vlachou and Markou, 2010). They couple through Gαi/o to inhibit adenylyl cyclase, close voltage-dependent calcium channels, and open inwardly rectifying K+channels (Bowery et al., 2002; Bettler et al., 2004), and function as autoreceptors and heteroreceptors, modulating neurotransmitter release and neuronal firing, and influencing long-term changes in synaptic strength (Pinard et al., 2010). GABAB receptors are heterodimers of GABAB1a or GABAB1b subunits where GABA and other GABAB receptor ligands bind, combined with GABAB2 subunits where allosteric modulators have been proposed to act (Calver et al., 2002; Bettler et al., 2004; Pin et al., 2004). Allosteric modulators alter effects of an endogenous transmitter or orthosteric agonist by binding to regions on the receptor that are different from the orthosteric site where the endogenous transmitter binds (Jensen and Spalding, 2004). By altering activated receptors only, allosteric modulators may have a broader therapeutic window than ligands that alter all receptors. Because GABAB receptors are thought to be involved in various psychiatric disorders (Kerr and Ong, 1995; Markou et al., 2004; Pilc and Nowak, 2005; Frankowska et al., 2007; Addolorato et al., 2009), allosteric modulation of these receptors could provide new treatments.
Several compounds have been shown to have positive GABAB modulatory activity in vitro [e.g., 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethylpropyl)phenol (CGP7930) (Urwyler et al., 2001; Adams and Lawrence, 2007), N,N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine (Urwyler et al., 2003), (R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one (rac-BHFF) (Malherbe et al., 2008), N-[(1R,2R,4S)-bicyclo[2.2.1]hept-2-yl]-2-methyl-5-[4-(trifluoromethyl)phenyl]-4-pyrimidinamine (BHF177) (Guery et al., 2007; Maccioni et al., 2009), methyl-2-(1-adamantanecarboxamido)-4-ethyl-5-methylthiophene-3-carboxylate and methyl-2-(cyclohexanecarboxamido)-4-ethyl-5-methyltiophene-3-carboxylate (Castelli et al., 2011), and 2-{1-[2-(4-chlorophenyl)-5-methylpyrazolo[1,5-a]pyrimidin-7-yl]-2-piperidinyl}ethanol (Perdona et al., 2011)]. Their positive GABAB modulatory activity in vitro was evidenced by enhancing GABA, and, for all compounds except BHF177, also by enhancing the GABAB receptor agonist baclofen. Several of these compounds have been shown to have positive modulatory properties not only in vitro but also in vivo. CGP7930 and rac-BHFF enhanced loss of righting in mice induced by baclofen (Carai et al., 2004; Malherbe et al., 2008; Koek et al., 2010), which probably involves cerebellar GABAB receptors that are especially sensitive to positive modulation (Hensler et al., 2012). GS39783 enhanced effects of baclofen on hamster circadian activity rhythms (Gannon and Millan, 2011). Recently, evidence was obtained in pigeons that CGP7930 and rac-BHFF also enhance the discriminative stimulus effects of baclofen (Koek et al., 2012). The generality of the positive modulatory effects of CGP7930 and rac-BHFF in vivo increases the likelihood that these effects are involved in their therapeutic-like activity.
Positive GABAB receptor modulators have anxiolytic- and antidepressant-like properties in elevated maze and forced swimming tests, respectively (Cryan et al., 2004; Frankowska et al., 2007; Jacobson and Cryan, 2008), and exhibit antipsychotic-like effects in (5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate (MK-801)- and amphetamine-induced hyperactivity tests (Wieronska et al., 2011). In addition, they reduce self-administration of alcohol (Orru et al., 2005, 2012; Liang et al., 2006; Maccioni et al., 2007, 2008, 2009, 2010a, b, 2012; Agabio et al., 2012), cocaine (Filip et al., 2007), and nicotine (Mombereau et al., 2007; Paterson et al., 2008; Vlachou et al., 2011). GABAB receptor-positive modulators are thought to have advantages as potential medications for anxiety, depression, and drug addiction, because they may have a better side-effect profile than GABAB receptor agonists, based on the notion that selective enhancement of activated receptors has effects that differ from indiscriminate activation of all receptors. Unlike baclofen, GABAB receptor-positive modulators do not seem to interfere with motor coordination (Cryan et al., 2004; Jacobson and Cryan, 2008), do not produce loss of righting (Carai et al., 2004; Malherbe et al., 2008; Koek et al., 2010) and, with the possible exception of CGP7930 (Koek et al., 2010), do not induce hypothermia. Also, CGP7930 does not have baclofen-like discriminative stimulus effects, and does not have discriminative stimulus effects similar to those of γ-hydroxybutyrate (GHB), a compound with GABAB receptor agonist properties (Koek et al., 2012). In contrast, rac-BHFF produced a level of drug-appropriate responding in baclofen-trained animals near the level observed with the training drug, and, like baclofen, substituted partially for GHB (Koek et al., 2012). Thus, the discriminative stimulus effects of rac-BHFF, unlike CGP7930, may be similar to those of baclofen. The present study was aimed at a more comprehensive characterization of the discriminative stimulus properties of rac-BHFF by attempting to use rac-BHFF as training drug. This study is the first to show that animals can discriminate effects of a GABAB receptor-positive modulator, and that these effects differ from those of GABAB receptor agonists.
Establishing rac-BHFF as training drug also made it possible to examine whether rac-BHFF shares discriminative stimulus effects with compounds other than GABAB receptor agonists. The present study examined the effects of the cholecystokinin (CCK) A receptor agonist CCK-8 (sulfated cholecystokinin-octapeptide), because in a broad radioligand binding screen rac-BHFF was found to be inactive at all non-GABAB targets tested except CCKA receptors (Malherbe et al., 2008). Also, because positive modulation of GABAB receptors has been reported to produce anxiolytic-like effects comparable with diazepam (Frankowska et al., 2007) and to decrease self-administration of alcohol, cocaine, and nicotine, the present study examined whether the discriminative stimulus effects of rac-BHFF resemble those of diazepam, ethanol, cocaine, or nicotine.
Materials and Methods
Animals.
Eight adult white Carneau pigeons (Columba livia; Palmetto, Sumter, SC) were individually housed under a 12/12-hour light/dark cycle. They had free access to water and were maintained between 80 and 90% of their free-feeding weight by food (Purina Pigeon Checkers, St. Louis, MO) received during experimental sessions and supplemental postsession feedings (Purina Pigeon Checkers or mixed grain). The animals were maintained and the experiments were conducted in accordance with the Institutional Animal Care and Use Committee (The University of Texas Health Science Center at San Antonio) and with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, 1996).
Apparatus.
Experiments were conducted in sound attenuating, ventilated chambers (BRS/LVE, Laurel, MD) equipped with two response keys that could be illuminated by a red light. After completion of each fixed ratio, the key light was extinguished for 4 seconds, during which time a white light illuminated the hopper where food (Purina Pigeon Checkers) was available. Chambers were connected by an interface (MED Associates Inc., St. Albans, VT) to a computer that used MED-PC IV software (MED Associates Inc.) to monitor and control inputs and outputs and to record the data.
Procedure.
The discrimination training and testing procedure was similar to that described in detail elsewhere (Koek et al., 2004). Briefly, before each daily session, subjects received either the training dose of rac-BHFF or vehicle using the same route (i.e., oral) and the same interval before being placed into the chambers (i.e., 45 minutes) that were used in the previous study of the effects of rac-BHFF in GHB- and in baclofen-discriminating pigeons (Koek et al., 2012). Drug and vehicle training sessions occurred with equal frequency. Sessions started with a period of 15 minutes, during which the lights were off and key pecks had no programmed consequence. Subsequently, the left and the right keys were illuminated red and 20 consecutive responses on the injection appropriate key resulted in 4-second access to food. These responses had to be consecutive because responses on the injection inappropriate key reset the fixed ratio requirement on the injection appropriate key. The response period ended after 30 food presentations or 15 minutes, whichever occurred first. Initially, pigeons had to satisfy the following criteria for at least seven of nine consecutive sessions: ≥90% of the total responses on the injection appropriate key and fewer than 20 responses on the injection inappropriate key before the first food presentation. Thereafter, tests were conducted when these criteria were satisfied during two consecutive (drug and vehicle) training sessions. Test sessions were the same as training sessions (i.e., a 15-minute period, followed by a response period that ended after 30 food presentations or 15 minutes, whichever occurred first), except that food was available after completion of 20 consecutive responses on either key. These responses had to be consecutive, because switching from responding on one of the keys to responding on the other key reset the fixed ratio requirement.
All compounds were injected i.m. [either immediately (agonists) or 45 minutes (antagonists) before the session], except GABAB receptor-positive modulators and ethanol, which were administered orally 45 minutes before the session. For each compound, administration routes and times were the same as used previously in GHB- and baclofen-discriminating pigeons (Koek et al., 2012).
The dose of rac-BHFF that was initially chosen for training (i.e., 178 mg/kg) occasioned the greatest amount of drug key-responding in pigeons discriminating baclofen from saline (Koek et al., 2012).
Data Analysis.
The mean percentage of responses on the training drug-appropriate key ± 1 S.E.M. was plotted as a function of dose. When an animal responded at a rate less than 20% of the vehicle control rate, discrimination data from that test were not included in the average. Mean percentages of responses on the training drug-appropriate key were calculated only when they were based on at least one-half of the animals tested.
Dose-response curves that attained at least 80% training drug-appropriate responding were analyzed by nonlinear regression of individual values by means of GraphPad Prism version 5.04 for Windows (GraphPad Software, San Diego, CA) using the sigmoid equation: response = bottom + (top – bottom)/(1+10^((logED50 – log(dose))*slope), with bottom = 0 and top = 100. F ratio tests in Prism were used to compare dose-response curves with respect to their slopes. Parallel shifts of dose-response curves were examined by simultaneously fitting sigmoid models to the control and the shifted curves and expressing the logED50 of the shifted curve as the sum of the logED50 of the control curve and the log of the potency ratio, which yielded an estimate of the potency ratio and its 95% confidence limits (see “EC50 shift” equation in GraphPad Prism). Shifts of dose-response curves were considered statistically significant if the 95% confidence interval of the potency ratio did not include 1. Dose-response curves that attained a maximum between 50 and 80% training drug-appropriate responding were analyzed in the same manner, except that instead of fitting a sigmoid curve to all dose-response data, a straight line was fitted only to the data at doses with effects immediately below and above 50%, to estimate the ED50 and slope. Possible deviations from the regression models were examined by the replicates test implemented in GraphPad Prism. None of the dose-response data obtained in the present study deviated significantly from the regression models used.
Drug effects on response rate were examined by calculating for each dose the 95% confidence interval around the mean rate of responding (expressed as percentage of vehicle control). If this interval did not contain 100, the response rate was considered significantly different from control.
Drugs.
Baclofen, diazepam, and (-)nicotine hydrogen tartrate were purchased from Sigma-Aldrich (St. Louis, MO), and sulfated CCK-8 from Tocris Bioscience (Bristol, UK). GHB and cocaine hydrochloride were provided by the National Institute on Drug Abuse (Bethesda, MD). CGP7930 and rac-BHFF were synthesized by K. Cheng at the National Institute on Drug Abuse, and CGP35348 (3-aminopropyl(diethoxymethyl) phosphinic acid) was synthesized by J. Agyin at the University of Texas Health Science Center (San Antonio, TX). All compounds were dissolved in sterile water or saline, except diazepam, which was dissolved in sterile water with 70% castor oil ethoxylate(30) [Rhodia (Novecare), Charleston, SC] and 10% ethanol (by volume); CGP7930, which was suspended in sterile water with 0.6% methylcellulose; and rac-BHFF, which was suspended in a 4:1:15 mixture containing Cremophor EL (Kolliphor EL; Sigma-Aldrich), 1,2-propanediol and sterile water (Malherbe et al., 2008). All compounds were injected intramuscularly in a volume of 0.1–1 ml, except CGP7930, rac-BHFF, and ethanol, which were administered orally by a feeding needle, in a volume of 0.5–5 ml. The dose of ethanol was manipulated by varying the volume of a 20% (v/v) solution of ethanol in sterile water. Doses are expressed as the form of the compound listed above.
Results
Because 178 mg/kg rac-BHFF had marked rate-decreasing effects in drug-naïve pigeons, for which little tolerance occurred within 20 sessions (unpublished data), the training dose was decreased to 100 mg/kg. At this dose, seven of the eight animals acquired the discrimination (median sessions to criterion 31, range 3–61, excluding sessions that were used to calculate criterion performance).
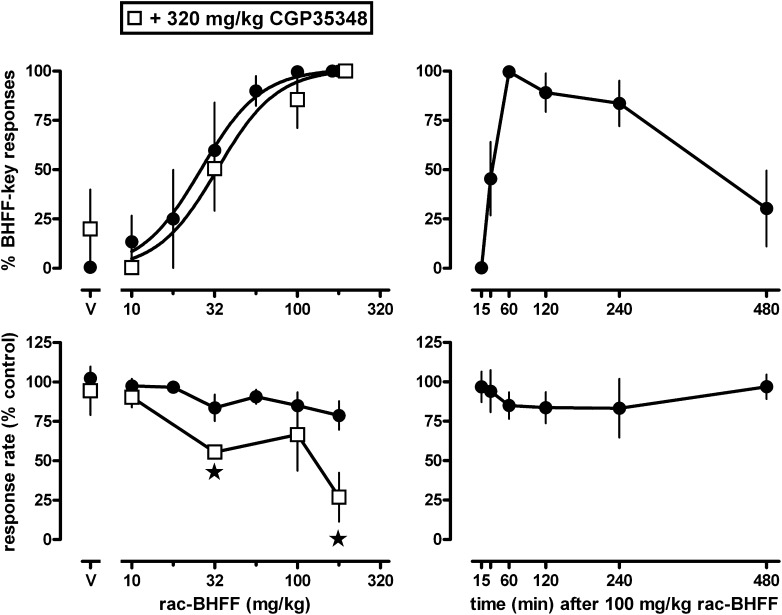
Rac-BHFF produced discriminative stimulus effects in a dose- and time-dependent manner, without affecting response rate (Fig. 1). Under test conditions, rac-BHFF dose-dependently increased responding on the rac-BHFF-appropriate key from 1% after vehicle to a maximum of 100% at the training dose of 100 mg/kg (upper left panel, solid circles), without significantly affecting the rate of responding (lower left panel, solid circles). A sigmoid curve fitted to the dose-response data yielded an ED50 value of 26 [95% confidence limits: 15–45] mg/kg rac-BHFF. The GABAB antagonist CGP35348, at a dose (320 mg/kg) that attenuated the effects of baclofen and GHB (see below), did not significantly shift the dose-response curve of rac-BHFF to produce training drug-appropriate responding (dose ratio: 1.3; 95% confidence limits: 0.7–2.2). However, CGP35348 together with rac-BHFF significantly decreased the rate of responding. When the training dose of rac-BHFF (i.e., 100 mg/kg) was given at various times before the session, its discriminative stimulus effects were not apparent after 15 minutes, were maximal after 1 hour, and decreased to 30% after 8 hours (upper right panel), without significantly affecting response rate at any of the intervals (lower right panel).
Fig. 1.
Effects of the GABAB receptor positive modulator rac-BHFF in pigeons trained to discriminate between 100 mg/kg rac-BHFF and vehicle using a two-key food-reinforced procedure. The mean (± S.E.M.; if not shown, S.E.M. values are contained by the symbol) percentage of responses on the rac-BHFF-appropriate key (top panels) and the mean (± S.E.M.) rate of responding (expressed as percentage control: bottom panels) are plotted as a function of dose (n = 4–6 per dose) in the left panels and as a function of time after the administration of 100 mg/kg rac-BHFF in the right panels. rac-BHFF was tested alone (solid circles) and together with the GABAB receptor antagonist CGP35348 (320 mg/kg; open squares). For dose-response data that attained the 80% level, nonlinear regression was used to obtain the best-fitting sigmoid curve; for other dose-response data, and for time-response data, the individual points were connected. Stars indicate mean rates of responding that were significantly (P < 0.05) different from control.
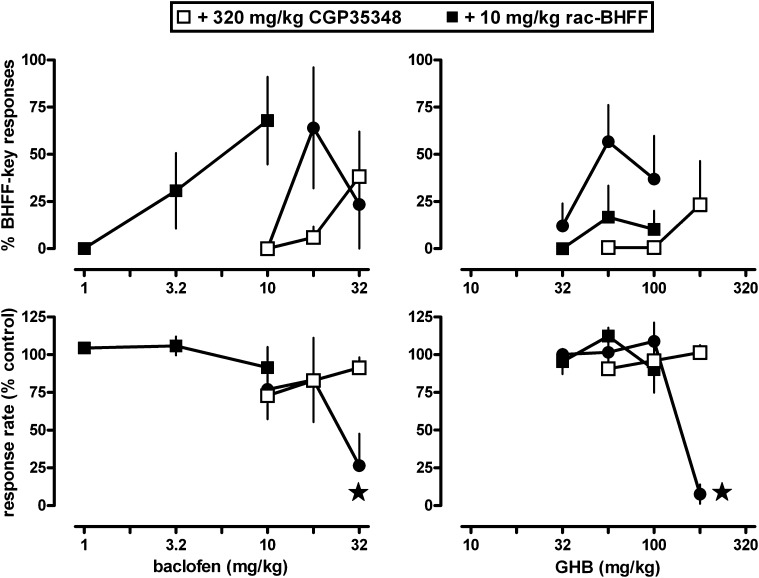
Baclofen and GHB did not produce full rac-BHFF-like discriminative stimulus effects (Fig. 2). They increased rac-BHFF-appropriate responding to at most 64% (baclofen) and 57% (GHB) (upper panels, solid circles) when tested at doses up to and including those that significantly decreased response rate (lower panels, solid circles). CGP35348 antagonized the discriminative stimulus- and response rate-decreasing effects of baclofen and GHB (Fig. 2, compare open squares with solid circles). Rac-BHFF, at a dose that when given alone produced little training drug-appropriate responding (i.e., 10 mg/kg; Fig. 1, upper left panel), enhanced the discriminative stimulus effects of baclofen (Fig. 2, upper left panel, compare solid squares with solid circles) and did not enhance, but appeared to attenuate, the discriminative stimulus effects of GHB (Fig. 2, upper right panel, compare solid squares with solid circles). A dose ratio for the enhancement of baclofen by rac-BHFF was not calculated, because the difference between the slopes of the ascending part of the dose-response curves approached statistical significance (P = 0.06); the enhancement was evidenced by the ED50 values of the dose-response curves being significantly different (P = 0.03).
Fig. 2.
Effects of the GABAB receptor agonist baclofen (left) and GHB (right) in pigeons trained to discriminate between 100 mg/kg rac-BHFF and vehicle using a two-key food-reinforced procedure. Baclofen and GHB were tested alone (solid circles), together with the GABAB receptor antagonist CGP35348 (320 mg/kg; open squares), and together with the GABAB receptor positive modulator rac-BHFF (10 mg/kg; solid squares). Stars indicate mean rates of responding that were significantly (P < 0.05) different from control. See Fig. 1 for other details.
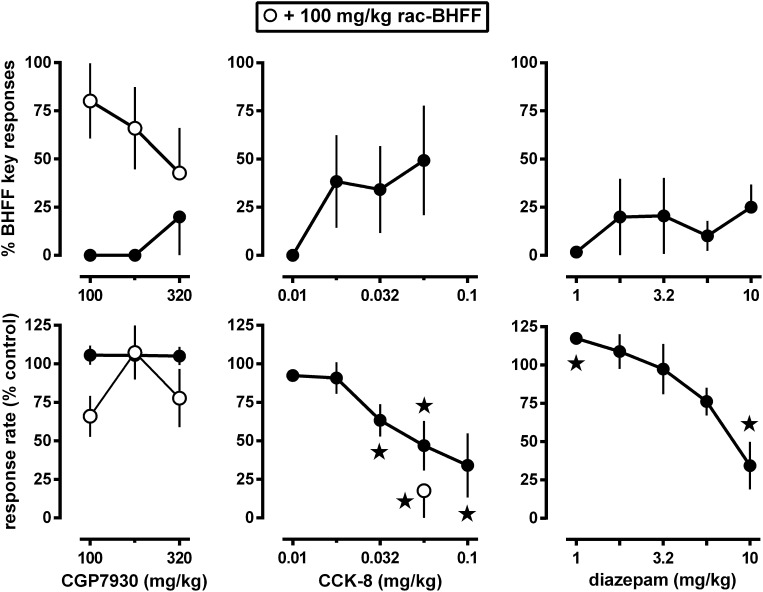
The discriminative stimulus effects of rac-BHFF were not mimicked by the GABAB receptor-positive modulator CGP7930 (Fig. 3, upper left panel). CGP7930 produced at most 20% rac-BHFF-appropriate responding at a dose of 320 mg/kg; because of limited solubility, higher doses could not be tested. When given together with the training dose, CGP7930 attenuated the discriminative stimulus effects of rac-BHFF.
Fig. 3.
Effects of the GABAB receptor positive modulator CGP7930 (left panels), the CCKA receptor agonist CCK-8 (middle panels), and the GABAA receptor positive modulator diazepam (right panels) in pigeons trained to discriminate between 100 mg/kg rac-BHFF and vehicle using a two-key food-reinforced procedure. All compounds were tested alone (solid circles), and CGP7930 and CCK-8 were also tested together with the training dose of rac-BHFF (open circles). Stars indicate mean rates of responding that were significantly (P < 0.05) different from control. See Fig. 1 for other details.
The CCKA receptor agonist CCK-8 did not produce full rac-BHFF-like responding (Fig. 3, upper middle panel). CCK-8 increased rac-BHFF-appropriate responding to at most 49% at doses that decreased response rate. To examine possible CCKA antagonist properties of rac-BHFF, it was administered together with a response rate-reducing dose of CCK-8. The training dose of rac-BHFF did not attenuate the effects of CCK-8 on response rate (Fig. 3, lower middle panel). Effects on rac-BHFF-appropriate responding were not calculated, because when rac-BHFF and CCK-8 were administered together, more than half of the animals responded at a rate less than 20% of the vehicle control rate.
The discriminative stimulus effects of rac-BHFF were not mimicked by the GABAA receptor-positive modulator diazepam (Fig. 3, upper right panel). At the lowest dose tested (i.e., 1 mg/kg), diazepam significantly increased the rate of responding (Fig. 3, lower right panel). When tested at doses up to and including those that significantly decreased response rate, diazepam increased rac-BHFF-appropriate responding to at most 25%.
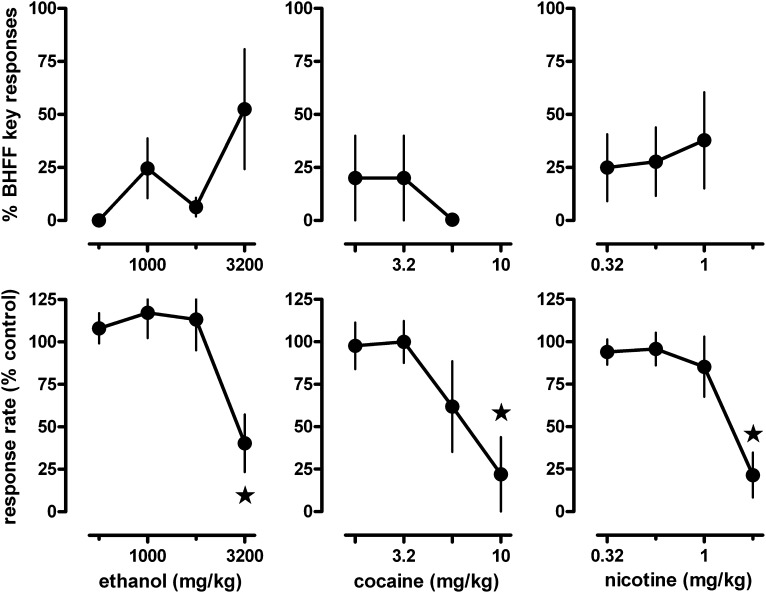
Ethanol, cocaine, and nicotine substituted at most partially for rac-BHFF (Fig. 4, upper panels). Rac-BHFF-appropriate responding was maximally increased to 52% by ethanol, to 20% by cocaine, and to 38% by nicotine when tested at doses up to and including those that significantly decreased response rate (Fig. 4, lower panels).
Fig. 4.
Effects of ethanol (left), cocaine (center), and nicotine (right) in pigeons trained to discriminate between 100 mg/kg rac-BHFF and vehicle using a two-key food-reinforced procedure. Stars indicate mean rates of responding that were significantly (P < 0.05) different from control. See Fig. 1 for other details.
Discussion
This study is the first to show that animals can be trained to discriminate a positive GABAB receptor modulator from vehicle. Seven of the eight pigeons met the training criterion after a median number of sessions (i.e., 31; range 3–61), similar to that observed previously with 100 mg/kg GHB (median 32, range 19–75; Koek et al., 2004), suggesting that rac-BHFF is as discriminable as GHB. Conceivably, interactions with CCKA receptors could mediate in the discriminative stimulus effects of rac-BHFF, because in a broad ligand binding screen, rac-BHFF was found to be inactive at all non-GABAB targets tested except CCKA receptors (Malherbe et al., 2008). However, the CCKA receptor agonist CCK-8 substituted only partially for rac-BHFF, suggesting that rac-BHFF does not have marked CCKA receptor agonist properties. Also, the response rate-decreasing effects of CCK-8 were not antagonized by rac-BHFF, suggesting that rac-BHFF does not have CCKA receptor antagonist properties. Thus, it appears unlikely that interactions of rac-BHFF with CCKA receptors play a major role in its discriminative stimulus effects.
To examine the role of GABAB receptors in the discriminative stimulus effects of rac-BHFF, antagonism tests were conducted with CGP35348, which acts at the GABA site of the GABAB receptor. CGP35348 did not antagonize the effects of rac-BHFF examined in the present study. Previously, CGP35348 did not antagonize the effects of rac-BHFF in baclofen-discriminating pigeons (Koek et al., 2012). In the present study, lack of antagonism of rac-BHFF by CGP35348 was observed at dose of CGP35348 (i.e., 320 mg/kg) that blocked the response rate-decreasing effects of the GABAB receptor agonists baclofen and GHB. Although CGP35348 generally acts as a silent antagonist, in vitro conditions have been reported in which it acts as a partial agonist (Urwyler et al., 2005; Hensler et al., 2012). Consistent with this, combining CGP35348 with rac-BHFF enhanced their effects on response rate. However, combining CGP35348 with rac-BHFF did not enhance the rac-BHFF-like discriminative stimulus effects (present study) or the baclofen-like discriminative stimulus effects (Koek et al., 2012) of CGP35348. Thus, the partial agonist properties of CGP35348 evidenced in vitro are not always apparent in vivo. Nevertheless, under conditions in which CGP35348 appears to have partial agonist properties, such as in the study by Urwyler et al. (2005), which showed that the maximum stimulation produced by CGP35348 in the presence of GABAB receptor positive modulators was at most 40% of the stimulation observed with GABA, CGP35348 should still behave as an antagonist of GABA. Taken together, the lack of antagonism of the discriminative stimulus effects of rac-BHFF by CGP35348 suggests that these effects do not result from enhanced activity of endogenous GABA at GABAB receptors. Instead, the discriminative stimulus effects of rac-BHFF could involve receptor activation through other sites of the GABAB receptor, consistent with in vitro evidence that rac-BHFF can activate GABAB receptors in the absence of GABA (Malherbe et al., 2008; Hensler et al., 2012).
The discriminative stimulus effects of rac-BHFF were not mimicked by the GABAB receptor agonist baclofen and GHB, consistent with the finding that rac-BHFF did not fully substitute for the training drug in pigeons trained to discriminate baclofen or GHB from vehicle (Koek et al., 2012). Together, these findings are further evidence that the effects of GABAB receptor positive modulators are not identical to those of GABAB receptor agonists. Interestingly, the GABAB receptor positive modulator CGP7930 produced little rac-BHFF-appropriate responding when tested at doses that take into account that CGP7930 is about 3-fold less potent than rac-BHFF (Koek et al., 2012). Instead, CGP7930 attenuated the discriminative stimulus effects of rac-BHFF. This suggests the possibility that both compounds have agonist activity at the receptor sites mediating the discriminative stimulus effects of rac-BHFF, but that CGP7930 has less intrinsic efficacy at these sites than rac-BHFF. In addition to enhancing endogenous GABA at GABAB1 subunits, CGP7930 also directly activates GABAB2 subunits (Binet et al., 2004). Like CGP7930, rac-BHFF is able to activate GABAB receptors in the absence of GABA, and is more efficacious than CGP7930 in stimulating GTP(γ)35S binding in membranes of transfected Chinese hamster ovary cells expressing GABAB receptors (Malherbe et al., 2008) and in brain using quantitative autoradiography (Hensler et al., 2012). Thus, although a role for non-GABAB receptors in the discriminative stimulus effects of rac-BHFF cannot be ruled out, the present findings suggest the possibility that at the training dose examined here (i.e., 100 mg/kg) rac-BHFF produces its discriminative stimulus effects not by enhancing endogenous GABA at GABAB1 subunits, but by directly activating GABAB2 subunits.
Rac-BHFF, at a dose 10-fold lower than the training dose, enhanced the discriminative stimulus effects of baclofen. This observation confirms and extends findings that rac-BHFF enhanced the discriminative stimulus effects of baclofen in pigeons trained to discriminate baclofen and in pigeons trained to discriminate GHB (Koek et al., 2012), and agrees with other evidence of the in vivo effectiveness of rac-BHFF as GABAB receptor-positive modulator (Malherbe et al., 2008; Koek et al., 2010). Here, rac-BHFF enhanced the potency of baclofen to substitute for rac-BHFF about 3-fold. However, rac-BHFF did not similarly enhance the potency of baclofen to decrease responding, because in the presence of rac-BHFF 10 mg/kg baclofen did not decrease responding, whereas 32 mg/kg baclofen given alone did. The observation that rac-BHFF did not similarly enhance different GABAB receptor-mediated effects is consistent with previous findings in mice that rac-BHFF enhanced baclofen-induced loss of righting but not baclofen-induced hypothermia, whereas both effects of baclofen were antagonized by CGP35348 (Koek et al., 2010). Using quantitative autoradiography, GABAB receptor-positive modulators have been found to act in a brain region-dependent manner (Hensler et al., 2012). Together, these observations suggest that rac-BHFF may preferentially modulate particular GABAB receptor populations.
Rac-BHFF enhanced the discriminative-stimulus effects of baclofen, but not of GHB. This differential enhancement, which suggests that the baclofen-enhancing effects of rac-BHFF do not result from the drug-appropriate responding it produces when given alone, is consistent with previous findings in baclofen- and GHB-discriminating pigeons (Koek et al., 2012). Effects of allosteric modulators can be agonist-dependent (e.g., Kenakin, 2009). Thus, the present and the previous results could be explained by assuming that the same GABAB receptors mediate effects of baclofen and GHB, and that rac-BHFF enhances effects at these receptors in an agonist-dependent manner. However, there is accumulating evidence that the GABAB receptor mechanisms underlying the effects of baclofen and GHB are not identical. Behavioral effects of baclofen and GHB are differentially enhanced by N-methyl-d-aspartate antagonists (Koek et al., 2007a; Koek and France, 2008) and differentially antagonized by CGP35348 (Koek et al., 2004, 2007b, 2009, 2012; Carter et al., 2006). In these studies, CGP35348 completely antagonized baclofen and GHB, but antagonized baclofen more potently than GHB. Because the antagonism of GHB, like that of baclofen, was complete, it seems unlikely that receptors other than GABAB receptors are involved in the effects of GHB examined in the aforementioned studies. Thus, the different potencies with which CGP35348 antagonized baclofen and GHB suggests that different GABAB receptor populations mediate these effects of baclofen and GHB. Therefore, the differential enhancement of effects of baclofen and GHB by rac-BHFF may not involve agonist-dependent enhancement of a single population of GABAB receptors, but may result from preferential modulation of different GABAB receptor populations. Consistent with this latter possibility, in vitro studies show that CGP7930 enhances activity at GABAB autoreceptors, but not at GABAB heteroreceptors (Chen et al., 2006; Parker et al., 2008), and show that CGP7930 and rac-BHFF enhance GTP(γ)35S binding stimulated by GABAB receptor agonists in a brain region-dependent manner (Hensler et al., 2012). Such selective enhancement is further evidence of pharmacologically distinct GABAB receptor populations.
GABAB receptor positive modulators are thought to have advantages as potential medications for anxiety, depression, and drug addiction (Cryan et al., 2004; Frankowska et al., 2007; Jacobson and Cryan, 2008; Vlachou and Markou, 2010). They may have a better side-effect profile than GABAB receptor agonists, based on the notion that selective enhancement of activated receptors has effects that differ from indiscriminate activation of all receptors. Unlike baclofen, GABAB receptor positive modulators do not appear to interfere with motor coordination (Cryan et al., 2004; Jacobson and Cryan, 2008), do not produce loss of righting (Carai et al., 2004; Malherbe et al., 2008; Koek et al., 2010) and, with the possible exception of CGP7930 (Koek et al., 2010), do not induce hypothermia (Jacobson and Cryan, 2008; Malherbe et al., 2008; Koek et al., 2010). rac-BHFF and CGP7930 did not mimic the discriminative-stimulus effects of baclofen and GHB (Koek et al., 2012) and in the present study, baclofen and GHB did not mimic the discriminative-stimulus effects of rac-BHFF. Also, the discriminative-stimulus effects of rac-BHFF were not mimicked by diazepam, suggesting that rac-BHFF can induce anxiolytic-like effects (Malherbe et al., 2008) without producing benzodiazepine-like discriminative-stimulus effects. The discriminative-stimulus effects of rac-BHFF were also not mimicked by alcohol, cocaine, and nicotine, whose self-administration has been reported to be attenuated by GABAB receptor-positive modulators. This suggests that rac-BHFF suppresses alcohol self-administration (Maccioni et al., 2010b) without producing alcohol-like discriminative stimulus effects. It suggests also, together with the finding that CGP7930 did not produce cocaine-like discriminative stimulus effects (Filip et al., 2007), that positive modulation of GABAB receptors does not produce discriminative stimulus effects similar to those of alcohol, cocaine, or nicotine.
In summary, the discriminative stimulus effects of the positive GABAB receptor modulator rac-BHFF differ from those of GABAB receptor agonists, of a benzodiazepine, and of drugs of abuse whose self-administration is reportedly attenuated by GABAB receptor-positive modulators. The discriminative stimulus effects of rac-BHFF were not antagonized by CGP35348 but were attenuated by CGP7930, suggesting the possibility that at the training dose examined here (i.e., 100 mg/kg) rac-BHFF produces its discriminative stimulus effects not by enhancing endogenous GABA at GABAB1 subunits, but by directly activating GABAB2 subunits. At a dose 10-fold lower than the training dose, rac-BHFF enhanced the discriminative stimulus effects of baclofen. Thus, rac-BHFF acts in vivo as GABAB receptor-positive modulator and, at higher doses, conceivably also as allosteric GABAB receptor agonist. Together with previous evidence that the GABAB receptor populations involved in the in vivo effects of baclofen and GHB are not identical, the findings that rac-BHFF enhanced effects of baclofen, but not of GHB, suggest these populations differ in their susceptibility to positive modulatory effects. Such differential susceptibility could allow for more selective therapeutic targeting of GABAB receptors.
Acknowledgments
The authors thank Jason Persyn and Christopher Limas for technical assistance.
Abbreviations
- GHB
γ-hydroxybutyrate
- CGP7930
2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethylpropyl)phenol
- rac-BHFF
(R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one
- BHF177
N-[(1R,2R,4S)-bicyclo[2.2.1]hept-2-yl]-2-methyl-5-[4-(trifluoromethyl)phenyl]-4-pyrimidinamine
- CGP35348
3-aminopropyl(diethoxymethyl)phosphinic acid
- CCK
cholecystokinin
- CCK-8
sulfated cholecystokinin-octapeptide
- GS39783
N,N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine
- GTP(γ)35S
guanosine 5′-O-(3-[35S]thiotriphosphate
- MK-801
(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate
Authorship Contributions
Participated in research design: Koek.
Contributed new reagents or analytic tools: Cheng, Rice.
Performed data analysis: Koek.
Wrote or contributed to the writing of the manuscript: Koek.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA15692]; and also, in part, by the Intramural Research Programs of the National Institutes of Health National Institute on Drug Abuse and the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism.
References
- Adams CL, Lawrence AJ. (2007) CGP7930: a positive allosteric modulator of the GABAB receptor. CNS Drug Rev 13:308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Cardone S, Ferrulli A, Gasbarrini G. (2009) Role of the GABA(B) receptor system in alcoholism and stress: focus on clinical studies and treatment perspectives. Alcohol 43:559–563 [DOI] [PubMed] [Google Scholar]
- Agabio R, Maccioni P, Carai MA, Gessa GL, Froestl W, Colombo G. (2012) The development of medications for alcohol-use disorders targeting the GABAB receptor system. Recent Patents CNS Drug Discov 7:113–128 [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. (2004) Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev 84:835–867 [DOI] [PubMed] [Google Scholar]
- Binet V, Brajon C, Le Corre L, Acher F, Pin JP, Prézeau L. (2004) The heptahelical domain of GABA(B2) is activated directly by CGP7930, a positive allosteric modulator of the GABA(B) receptor. J Biol Chem 279:29085–29091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. (2002) International Union of Pharmacology. XXXIII. Mammalian γ-aminobutyric acid(B) receptors: structure and function. Pharmacol Rev 54:247–264 [DOI] [PubMed] [Google Scholar]
- Bowery NG. (2006) GABAB receptor: a site of therapeutic benefit. Curr Opin Pharmacol 6:37–43 [DOI] [PubMed] [Google Scholar]
- Calver AR, Davies CH, Pangalos M. (2002) GABA(B) receptors: from monogamy to promiscuity. Neurosignals 11:299–314 [DOI] [PubMed] [Google Scholar]
- Carai MA, Colombo G, Froestl W, Gessa GL. (2004) In vivo effectiveness of CGP7930, a positive allosteric modulator of the GABAB receptor. Eur J Pharmacol 504:213–216 [DOI] [PubMed] [Google Scholar]
- Carter LP, Chen W, Coop A, Koek W, France CP. (2006) Discriminative stimulus effects of GHB and GABA(B) agonists are differentially attenuated by CGP35348. Eur J Pharmacol 538:85–93 [DOI] [PubMed] [Google Scholar]
- Castelli MP, Casu A, Casti P, Lobina C, Carai MAM, Colombo G, Solinas M, Giunta D, Mugnaini C, Pasquini S, et al. (2011) Characterization of COR627 and COR628, two novel positive allosteric modulators of the GABAB receptor. J Pharmacol Exp Ther 340:529–538 [DOI] [PubMed] [Google Scholar]
- Chen Y, Menendez-Roche N, Sher E. (2006) Differential modulation by the GABAB receptor allosteric potentiator 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethylpropyl)-phenol (CGP7930) of synaptic transmission in the rat hippocampal CA1 area. J Pharmacol Exp Ther 317:1170–1177 [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, Froestl W, Bettler B, Kaupmann K, Spooren WP. (2004) Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N’-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther 310:952–963 [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kaupmann K. (2005) Don’t worry ‘B’ happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci 26:36–43 [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Przegaliński E. (2007) Effects of GABA(B) receptor antagonist, agonists and allosteric positive modulator on the cocaine-induced self-administration and drug discrimination. Eur J Pharmacol 574:148–157 [DOI] [PubMed] [Google Scholar]
- Frankowska M, Filip M, Przegaliński E. (2007) Effects of GABAB receptor ligands in animal tests of depression and anxiety. Pharmacol Rep 59:645–655 [PubMed] [Google Scholar]
- Gannon RL, Millan MJ. (2011) Positive allosteric modulators at GABAB receptors exert intrinsic actions and enhance the influence of baclofen on light-induced phase shifts of hamster circadian activity rhythms. Pharmacol Biochem Behav 99:712–717 [DOI] [PubMed] [Google Scholar]
- Guery S, Floersheim P, Kaupmann K, Froestl W. (2007) Syntheses and optimization of new GS39783 analogues as positive allosteric modulators of GABAB receptors. Bioorg Med Chem Lett 17:6206–6211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler JG, Advani T, Burke TF, Cheng K, Rice KC, Koek W. (2012) GABAB receptor-positive modulators: brain region-dependent effects. J Pharmacol Exp Ther 340:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. (2008) Evaluation of the anxiolytic-like profile of the GABAB receptor positive modulator CGP7930 in rodents. Neuropharmacology 54:854–862 [DOI] [PubMed] [Google Scholar]
- Jensen AA, Spalding TA. (2004) Allosteric modulation of G-protein coupled receptors. Eur J Pharm Sci 21:407–420 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2009) A Pharmacology Primer: Theory, Application and Methods, 3rd ed, Elsevier Academic Press, Burlington, MA. [Google Scholar]
- Kerr DI, Ong J. (1995) GABAB receptors. Pharmacol Ther 67:187–246 [DOI] [PubMed] [Google Scholar]
- Koek W, France CP. (2008) Cataleptic effects of gamma-hydroxybutyrate (GHB) and baclofen in mice: mediation by GABA(B) receptors, but differential enhancement by N-methyl-d-aspartate (NMDA) receptor antagonists. Psychopharmacology (Berl) 199:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Flores LR, Carter LP, Lamb RJ, Chen W, Wu H, Coop A, France CP. (2004) Discriminative stimulus effects of gamma-hydroxybutyrate in pigeons: role of diazepam-sensitive and -insensitive GABA(A) and GABA(B) receptors. J Pharmacol Exp Ther 308:904–911 [DOI] [PubMed] [Google Scholar]
- Koek W, Khanal M, France CP. (2007a) Synergistic interactions between ‘club drugs’: gamma-hydroxybutyrate and phencyclidine enhance each other’s discriminative stimulus effects. Behav Pharmacol 18:807–810 [DOI] [PubMed] [Google Scholar]
- Koek W, Mercer SL, Coop A. (2007b) Cataleptic effects of gamma-hydroxybutyrate (GHB), its precursor gamma-butyrolactone (GBL), and GABAB receptor agonists in mice: differential antagonism by the GABAB receptor antagonist CGP35348. Psychopharmacology (Berl) 192:407–414 [DOI] [PubMed] [Google Scholar]
- Koek W, Mercer SL, Coop A, France CP. (2009) Behavioral effects of gamma-hydroxybutyrate, its precursor gamma-butyrolactone, and GABA(B) receptor agonists: time course and differential antagonism by the GABA(B) receptor antagonist 3-aminopropyl(diethoxymethyl)phosphinic acid (CGP35348). J Pharmacol Exp Ther 330:876–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, France CP, Cheng K, Rice KC. (2010) GABAB receptor-positive modulators: enhancement of GABAB receptor agonist effects in vivo. J Pharmacol Exp Ther 335:163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, France CP, Cheng K, Rice KC. (2012) Effects of the GABAB receptor-positive modulators CGP7930 and rac-BHFF in baclofen- and γ-hydroxybutyrate-discriminating pigeons. J Pharmacol Exp Ther 341:369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JH, Chen F, Krstew E, Cowen MS, Carroll FY, Crawford D, Beart PM, Lawrence AJ. (2006) The GABA(B) receptor allosteric modulator CGP7930, like baclofen, reduces operant self-administration of ethanol in alcohol-preferring rats. Neuropharmacology 50:632–639 [DOI] [PubMed] [Google Scholar]
- Maccioni P, Pes D, Orrù A, Froestl W, Gessa GL, Carai MA, Colombo G. (2007) Reducing effect of the positive allosteric modulator of the GABA(B) receptor, GS39,783, on alcohol self-administration in alcohol-preferring rats. Psychopharmacology (Berl) 193:171–178 [DOI] [PubMed] [Google Scholar]
- Maccioni P, Fantini N, Froestl W, Carai MA, Gessa GL, Colombo G. (2008) Specific reduction of alcohol’s motivational properties by the positive allosteric modulator of the GABAB receptor, GS39783—comparison with the effect of the GABAB receptor direct agonist, baclofen. Alcohol Clin Exp Res 32:1558–1564 [DOI] [PubMed] [Google Scholar]
- Maccioni P, Carai MA, Kaupmann K, Guery S, Froestl W, Leite-Morris KA, Gessa GL, Colombo G. (2009) Reduction of alcohol’s reinforcing and motivational properties by the positive allosteric modulator of the GABA(B) receptor, BHF177, in alcohol-preferring rats. Alcohol Clin Exp Res 33:1749–1756 [DOI] [PubMed] [Google Scholar]
- Maccioni P, Flore P, Carai MA, Mugnaini C, Pasquini S, Corelli F, Gessa GL, Colombo G. (2010a) Reduction by the positive allosteric modulator of the GABA(B) receptor, GS39783, of alcohol self-administration in Sardinian alcohol-preferring rats exposed to the “sipper” procedure. Front Psychiatry 1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni P, Thomas AW, Carai MA, Gessa GL, Malherbe P, Colombo G. (2010b) The positive allosteric modulator of the GABA(B) receptor, rac-BHFF, suppresses alcohol self-administration. Drug Alcohol Depend 109:96–103 [DOI] [PubMed] [Google Scholar]
- Maccioni P, Zaru A, Loi B, Lobina C, Carai MA, Gessa GL, Capra A, Mugnaini C, Pasquini S, Corelli F, et al. (2012) Comparison of the effect of the GABA(B) receptor agonist, baclofen, and the positive allosteric modulator of the GABA(B) receptor, GS39783, on alcohol self-administration in 3 different lines of alcohol-preferring rats. Alcohol Clin Exp Res 36:1748–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe P, Masciadri R, Norcross RD, Knoflach F, Kratzeisen C, Zenner MT, Kolb Y, Marcuz A, Huwyler J, Nakagawa T, et al. (2008) Characterization of (R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one as a positive allosteric modulator of GABAB receptors. Br J Pharmacol 154:797–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Paterson NE, Semenova S. (2004) Role of gamma-aminobutyric acid (GABA) and metabotropic glutamate receptors in nicotine reinforcement: potential pharmacotherapies for smoking cessation. Ann N Y Acad Sci 1025:491–503 [DOI] [PubMed] [Google Scholar]
- Mombereau C, Lhuillier L, Kaupmann K, Cryan JF. (2007) GABAB receptor-positive modulation-induced blockade of the rewarding properties of nicotine is associated with a reduction in nucleus accumbens DeltaFosB accumulation. J Pharmacol Exp Ther 321:172–177 [DOI] [PubMed] [Google Scholar]
- Orrù A, Fujani D, Cassina C, Conti M, Di Clemente A, Cervo L. (2012) Operant, oral alcoholic beer self-administration by C57BL/6J mice: effect of BHF177, a positive allosteric modulator of GABA(B) receptors. Psychopharmacology (Berl) 222:685–700 [DOI] [PubMed] [Google Scholar]
- Orrù A, Lai P, Lobina C, Maccioni P, Piras P, Scanu L, Froestl W, Gessa GL, Carai MA, Colombo G. (2005) Reducing effect of the positive allosteric modulators of the GABA(B) receptor, CGP7930 and GS39783, on alcohol intake in alcohol-preferring rats. Eur J Pharmacol 525:105–111 [DOI] [PubMed] [Google Scholar]
- Parker DA, Marino V, Ong J, Puspawati NM, Prager RH. (2008) The CGP7930 analogue 2,6-di-tert-butyl-4-(3-hydroxy-2-spiropentylpropyl)-phenol (BSPP) potentiates baclofen action at GABA(B) autoreceptors. Clin Exp Pharmacol Physiol 35:1113–1115 [DOI] [PubMed] [Google Scholar]
- Paterson NE, Vlachou S, Guery S, Kaupmann K, Froestl W, Markou A. (2008) Positive modulation of GABA(B) receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats. J Pharmacol Exp Ther 326:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdona’ E, Costantini VJ, Tessari M, Martinelli P, Carignani C, Valerio E, Mok MH, Zonzini L, Visentini F, Gianotti M, et al. (2011) In vitro and in vivo characterization of the novel GABAB receptor positive allosteric modulator, 2-1-[2-(4-chlorophenyl)-5-methylpyrazolo[1,5-a]pyrimidin-7-yl]-2-piperidinylethanol (CMPPE). Neuropharmacology 61:957–966 [DOI] [PubMed] [Google Scholar]
- Pilc A, Nowak G. (2005) GABAergic hypotheses of anxiety and depression: focus on GABA-B receptors. Drugs Today (Barc) 41:755–766 [DOI] [PubMed] [Google Scholar]
- Pin J-P, Kniazeff J, Binet V, Liu J, Maurel D, Galvez T, Duthey B, Havlickova M, Blahos J, Prézeau L, et al. (2004) Activation mechanism of the heterodimeric GABA(B) receptor. Biochem Pharmacol 68:1565–1572 [DOI] [PubMed] [Google Scholar]
- Pinard A, Seddik R, Bettler B. (2010) GABAB receptors: physiological functions and mechanisms of diversity. Adv Pharmacol 58:231–255 [DOI] [PubMed] [Google Scholar]
- Urwyler S, Mosbacher J, Lingenhoehl K, Heid J, Hofstetter K, Froestl W, Bettler B, Kaupmann K. (2001) Positive allosteric modulation of native and recombinant gamma-aminobutyric acid(B) receptors by 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Mol Pharmacol 60:963–971 [PubMed] [Google Scholar]
- Urwyler S, Pozza MF, Lingenhoehl K, Mosbacher J, Lampert C, Froestl W, Koller M, Kaupmann K. (2003) N,N’-Dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine (GS39783) and structurally related compounds: novel allosteric enhancers of gamma-aminobutyric acidB receptor function. J Pharmacol Exp Ther 307:322–330 [DOI] [PubMed] [Google Scholar]
- Urwyler S, Gjoni T, Koljatić J, Dupuis DS. (2005) Mechanisms of allosteric modulation at GABAB receptors by CGP7930 and GS39783: effects on affinities and efficacies of orthosteric ligands with distinct intrinsic properties. Neuropharmacology 48:343–353 [DOI] [PubMed] [Google Scholar]
- Vlachou S, Guery S, Froestl W, Banerjee D, Benedict J, Finn MG, Markou A. (2011) Repeated administration of the GABAB receptor positive modulator BHF177 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine seeking in rats. Psychopharmacology (Berl) 215:117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, Markou A. (2010) GABAB receptors in reward processes. Adv Pharmacol 58:315–371 [DOI] [PubMed] [Google Scholar]
- Wierońska JM, Kusek M, Tokarski K, Wabno J, Froestl W, Pilc A. (2011) The GABA B receptor agonist CGP44532 and the positive modulator GS39783 reverse some behavioural changes related to positive syndromes of psychosis in mice. Br J Pharmacol 163:1034–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]