Abstract
Osteoarthritis (OA) is a chronic joint disorder whose principal symptom is chronic pain. Current analgesics are inadequate and the mechanisms contributing to this pain are poorly understood but likely to include both local joint changes and central consequences. These studies used monoamine receptor agents combined with behavioral studies and single-unit dorsal horn recordings to examine whether descending noradrenergic and serotonergic inhibitions are altered in the monosodium iodoacetate model of OA pain, and whether increasing these inhibitions with the serotonin/noradrenaline reuptake inhibitor milnacipran can attenuate the attendant hypersensitivity. Early and late in the course of this model, milnacipran (s.c.) reduced behavioral hypersensitivity, and inhibited evoked responses from sensitized dorsal horn neurons. In naïve animals and the early, but not late, phase of the model, spinal administration of the α2-adrenoceptor antagonist atipamezole fully reversed this neuronal inhibition, whereas atipamezole administered alone revealed that endogenous noradrenergic inhibition was reduced in the late phase. Blocking spinal 5-hydroxytryptamine-7 receptors with (2R)-1-[(3-hydroxyphenyl)sulfonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl]pyrrolidine hydrochloride suggested that the effects of milnacipran in the late phase were partly mediated by these receptors, and that descending serotonergic inhibition was increased in this phase. An opioidergic mechanism behind the effects of milnacipran was indicated by a partial reversal of these effects with naloxone. These studies demonstrate antinociceptive effects for milnacipran in a model of OA pain, whose effects come via descending serotonergic and noradrenergic, as well as opioidergic, pathways. Variations in the activity of these pathways over the course of this model may contribute to the presence of behavioral hypersensitivity and determine through which endogenous systems milnacipran exerts its effects.
Introduction
Osteoarthritis (OA) is a chronic joint disorder affecting more adults globally than any other rheumatic condition. The principal symptom of this condition is movement-evoked or ongoing joint pain; as a result, OA can profoundly affect the quality of life of the sufferer (Hunter et al., 2008). Understanding the mechanisms driving this pain is important since the efficacy of current analgesics, which principally target the peripheral sensitization in the joint driving much of this pain, is limited (Zhang et al., 2010). One possible reason for this inadequacy is that OA pain can be driven by central as well as local joint mechanisms, the former supported by a number of clinical studies that report signs of central sensitization (Imamura et al., 2008; Gwilym et al., 2009; Lee et al., 2011) including abnormal wind-up in OA sufferers (Arendt-Nielsen et al., 2010).
Changes in spinal processing via central sensitization may lead to consequent changes in brainstem function, and a neuroimaging study has highlighted abnormal activation in the periaqueductal gray (PAG) in OA sufferers compared with controls (Gwilym et al., 2009). The PAG receives inputs from spinal nociceptive neurons (Hylden et al., 1986), and coordinates activity in descending noradrenergic (NA) and serotonergic (5-hydroxytryptamine [5-HT]) pathways that project back to the spinal cord to modulate nociceptive processing (Jensen and Yaksh, 1984; Aimone et al., 1987; Peng et al., 1996). As a result, changes in the PAG may result in a dysregulation of descending modulation, “opening the gate” in the spinal cord for increased nociceptive traffic to the brain and thus increased pain.
One preclinical study suggests that changes in the activity of descending pathways may be a feature of OA, contributing to neuronal hyperexcitability in the dorsal horn (Rahman et al., 2009). This showed that development of an OA-like state prompts an increased descending serotonergic facilitatory drive, occurring in parallel to sensitization of dorsal horn neurons. This latter phenomenon is also reported by Harvey and Dickenson (2009) and Sagar et al. (2010). Whether serotonergic or noradrenergic inhibitions are also altered in models of OA pain is not known, although such changes are a notable feature of a number of pain models (Bannister et al., 2009).
Targeting descending controls may also be an effective strategy to reduce OA pain, since a 5-HT/NA reuptake inhibitor (SNRI), duloxetine, has proved an effective analgesic in this condition (Chappell et al., 2009). Antinociceptive effects from SNRIs are thought to be mediated centrally, by an action on descending inhibitory controls, and therefore could be particularly efficacious in OA sufferers whose pain is centrally mediated.
Milnacipran is another SNRI that is effective in a number of models of pain (King et al., 2006; Berrocoso et al., 2011). Given its similar mechanism of action to duloxetine, it too may attenuate OA pain, and is currently the subject of a phase IV trial to examine this possibility (Harden, 2011; http://clinicaltrials.gov/ct2/show/NCT01510457). One of the benefits that milnacipran has is its balanced affinity for the noradrenergic and serotonergic reuptake transporters (Moret et al., 1985; Vaishnavi et al., 2004); since antidepressants that balance effects on noradrenergic and serotonergic systems are particularly effective in chronic pain conditions, this feature could confer superior efficacy on milnacipran compared with other SNRIs (Onghena and Van Houdenhove, 1992).
In the following studies, the antinociceptive effects of milnacipran were examined in the monosodium iodoacetate (MIA) model of OA pain. MIA is a chondrotoxin, causing the degradation of articular cartilage and subchondral bone similar to that seen in OA (Guzman et al., 2003). The efficacy of milnacipran in this model was assessed in an early phase and a late phase of the model, associated with hypersensitivity driven by inflammatory (Bove et al., 2003) and noninflammatory damage to the joint, respectively, a contrast potentially resulting in a differential efficacy of this drug. The effect of milnacipran in these studies was assessed on behavioral hypersensitivity, and on responses from neurons in the deep dorsal horn, to assess whether this drug modulated spinal nociceptive processing. In these electrophysiological studies, the involvement of descending monoaminergic controls in the effects of milnacipran was examined by blocking spinal α2-adrenoceptors and 5-HT7 receptors with selective antagonists. In addition, the involvement of endogenous opioids in the effects of milnacipran was also assessed, since previous studies have implicated these in the antinociceptive effects of SNRIs (e.g., Wattiez et al., 2011).
An additional aim was to examine the basal level of descending monoaminergic inhibition, mediated by α2-adrenoceptors and 5-HT7 receptors. It was posited that a reduction in monoaminergic inhibition would be present, which could drive the behavioral and neuronal hypersensitivity present in this model.
Materials and Methods
Animals.
Male Sprague-Dawley rats (Central Biologic Services, University College London, London, UK) were used for all experiments. They were housed at a maximum of five per cage on a 12-hour day/night cycle. Food and water were available ad libitum. All experimental procedures were approved by the UK Home Office and follow the guidelines of the International Association for the Study of Pain (Zimmermann, 1983).
The MIA Model.
All animals were assessed for their baseline sensitivity to mechanical stimuli 3 days prior to i.a. injection of MIA (Sigma-Aldrich, Poole, UK). Those with normal baseline responses were used for these studies. Animals were first anesthetized with isoflurane (4%) in a 2:1 mix of N2O and O2. Once withdrawal reflexes were absent, they were placed in a supine position onto a thermo-regulated heat mat and into a nose-cone for constant anesthetic delivery (isoflurane: 2.5–3%). The ventral surface of the right hind-limb was clipped of hair and the skin was wiped with an antibacterial chlorhexidine solution. Two milligrams MIA in 25 µl sterile saline (0.9%) was injected through the infrapatellar ligament of the right knee using a 27G needle. On removal of the needle, the limb was flexed to distribute this agent in the joint cavity. The health of the animals was assessed regularly by the experimenter and animal care staff thereafter. The day of MIA injection is day 0.
Drugs and Their Administration.
Milnacipran (Tocris, Bristol, UK) was dissolved in physiologic saline. In behavioral experiments, after baseline testing, milnacipran was injected i.p. at a dose of 10 mg/kg in a volume of 1 ml/kg. Effects were assessed at 20, 50, 90, 120, 150, and 180 minutes postinjection. In electrophysiological studies, after baseline tests were completed, milnacipran was injected s.c. at a dose of 5 mg/kg and its effects were assessed at 20, 50, and 90 minutes postinjection. A higher dose (10 mg/kg) was then injected and its effects assessed at these same time points. In other electrophysiological studies, a 10 mg/kg dose only was used.
In some electrophysiological experiments, atipamezole or SB-2699670 [(2R)-1-[(3-hydroxyphenyl)sulfonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl]pyrrolidine hydrochloride] was applied spinally alone; in other experiments, they were applied spinally after milnacipran had been administered to test whether they could antagonize the effects of the latter. Atipamezole is a selective α2-adrenoceptor antagonist, with a >8000-fold selectivity for this subtype over α1-adrenoceptors (Pertovaara et al., 2005). SB-269970 (Tocris) is a 5-HT7 receptor antagonist (Thomas et al., 2000) displaying over 100-fold selectivity versus other 5-HT receptors (Lovell et al., 2000). SB-269970 has proven to bind with high affinity to 5-HT7 receptors in the brain tissue of a variety of species including rodents and primates (Thomas et al., 2000). Doses of 10 µg of atipamezole and 1 µg of SB-269970 were used to antagonize the effects of milnacipran based on previous studies (e.g., Kalso et al., 1991) and pilot experiments, and were administered spinally in 50 µl saline. These were administered after three stable sets of baseline recordings from each neuron were made, and the effect of each drug was followed for 1 hour (tests at 15, 30, 45 minutes).
Behavioral Pharmacology.
Two groups of animals were used for these studies. One group comprised animals selected 2–4 days after MIA injection (early phase MIA), and another tested at days 14–18 (late phase MIA). After a 30-minute acclimatization period, sensitivity to mechanical stimuli was assessed through the use of von Frey (vF) filaments (Touch-Test; North Coast Medical Inc., San Jose, CA) applied to the plantar surface of the hind-paw using innocuous 1-, 6-, and 8-g filaments (9.8, 58.9, and 78.5 mN, respectively). Each vF hair was applied 10 times to the ipsilateral (IL) and contralateral (CL) paw, each application for a duration of 2 seconds from the bowing of the filament or until a withdrawal occurred. The following order of stimulus application was used:
1 g (CL) →1 g (IL) →6 g (CL) →6 g (IL) →8 g (CL) →8 g (IL)
A withdrawal response was noted if the animal actively lifted the whole paw upon the bending of the vF hair, bit or licked the paw, or shook the paw with high amplitude movements in response to the stimulus. For other types of response to stimulation, the test was deemed negative (e.g., the toe being lifted). Animals were included in the study if during baseline testing they displayed ≥5 limb withdrawals from 10 applications of an 8-g vF hair
After baseline testing, animals were removed from their Perspex container and given an i.p. injection of either saline or milnacipran before being replaced. The experimenter was blinded to the agent injected. Drug administration was counterbalanced such that in each phase of the model, drug A was administered to the first and then every other animal (e.g., animals 1, 3, and 5), and drug B was given to the second and then every other animal (e.g., animals 2, 4, and 6).
Single-Unit Dorsal Horn Recordings.
In vivo electrophysiological experiments followed the same protocol as described previously (Urch and Dickenson, 2003). Animals were anesthetized via a constant delivery of isoflurane (1.2–1.5%) (Baxter International Inc., Northampton, UK) in a 2:1 mix of N2O:O2 through a tracheal cannula. Body temperature was maintained via a rectal probe connected to a heating blanket placed under the animal (Harvard Homeothermic Blanket Control Unit, Edenbridge, UK). Recordings were made using a parylene-coated tungsten electrode (125 µm diameter, 2 MΩ; A-M Systems, Sequim, Washington,). Electrophysiological studies were carried out on naïve animals and early phase and late phase MIA animals. All MIA animals displayed mechanical hypersensitivity on the IL hind-paw in response to mechanical stimuli when tested, defined as in behavioral pharmacology studies (above). Naïve animals weighed 220–300 g at the time of experimentation, early phase MIA animals weighed 140–180 g, and late phase MIA animals weighed 220–300 g. For each recorded neuron, the depth of the recording site from the surface of the spinal cord was noted.
To test the response of neurons to mechanical stimuli, vF filaments at forces of 1-, 4-, 8-, 15-, 26-, and 60-g (9.8, 39.2, 78.4, 147.1, 254.9, and 588.2 mN, respectively) were applied to the center of the receptive field on the IL hind-paw, Thermal stimuli were applied by water jets at 35, 40, 45, and 48°C. Stimuli were applied for 10 seconds and the number of action potentials (APs) evoked during this period was recorded and quantified using Spike 2 software (Cambridge Electronic Design, Cambridge UK).
Responses to electrical stimulation of the receptive field were assessed by inserting transcutaneous stimulating electrodes into the receptive field and applying a current (2-ms wide rectangular pulses) at three times that required to evoke a C-fiber response. Neurons were stimulated with a train of 16 stimuli at this intensity (frequency: 0.5 Hz) and a poststimulus time histogram was constructed allowing the response of the neuron to be separated based on the poststimulus latency to fire; APs recorded at 0–20, 20–90, and 90–300 milliseconds were defined as responses evoked by Aβ-fibers, A∂-fibers, and C-fibers, respectively. Responses occurring at 300–800 milliseconds were labeled “postdischarge.” The wind-up of the cell was quantified as the cumulative total number of APs over the 16 stimuli minus [16 × the number of APs evoked by the first of the 16 stimuli]. The latter was referred to as the “input” to the neuron.
Stimuli were applied in the following order: electrical, mechanical, and thermal, with a 2-minute interval separating the electrical and the natural stimuli. For predrug responses, there was a 5-minute interval between testing rounds.
Data Analysis.
Statistical analyses were carried out using GraphPad Prism (version 4.0; GraphPad Software, Inc., La Jolla, CA) and SPSS 20.0 (IBM SPSS Statistics, Chicago, IL) software. Where appropriate, datasets were tested for normality (Kolmogorov–Smirnov test), sphericity (Mauchly’s test), and/or homogeneity of variance (Bartlett’s test). In the case of non-normality or a lack of homoscedasticity in between-subjects data, nonparametric tests were carried out. Violations of the assumption of sphericity in repeated-measures (RM) data were addressed by applying the Greenhouse–Geisser correction.
For behavioral studies, the raw number of withdrawals per paw was recorded and difference scores calculated by subtracting withdrawals on the CL side from those on the IL side at each time point. These were normalized to the predrug difference score to assess change in hypersensitivity over time; thus, values displayed represent the median normalized difference scores (and interquartile range). Drug effects were assessed in two ways: by comparing difference scores to the predrug baseline with Friedman tests and Dunn’s post hoc comparisons, and by assessing the difference between scores in the milnacipran and saline groups at individual time points postinjection using Bonferroni-corrected Mann–Whitney U tests. In addition, the area under the curve (AUC) was computed by the trapezoidal method, and for display purposes, values were inverted such that negative AUCs are displayed as positive.
For data collected in electrophysiological experiments, predrug values represented the mean number of APs (± S.E.M.) from three sets of stable control responses, where stable refers to a <10% variation in the C-fiber–evoked response and <20% variation in responses to noxious thermal and mechanical stimuli. Drug effects were displayed as the maximal change in the number of APs evoked after each drug dose relative to predose values, and are therefore graphed as the mean maximal change (± S.E.M.) from baseline. For electrically evoked responses, values recorded after each drug dose were normalized to the predrug mean (= 100%) and thus drug effects are shown as a mean percentage of predrug values (± S.E.M.). The effects of all drugs on neuronal responses to natural stimuli were assessed with two-way RM analysis of variance (ANOVA) with Bonferroni post hoc comparisons. The effects of milnacipran on electrically evoked responses were tested with one-way RM ANOVA with Bonferroni-corrected post hoc tests or Friedman tests with Dunn’s post hoc comparisons. The effects of atipamezole on electrically evoked responses were tested with paired t tests or Wilcoxon matched-pair signed-rank tests for naïve and early phase animals, and one-way RM ANOVA with Bonferroni-corrected post hoc tests or Friedman tests with Dunn’s post hoc comparisons. The effects of SB-269970 on electrically evoked responses were assessed with one-way RM ANOVA with Bonferroni-corrected post hoc tests or Friedman tests with Dunn’s post hoc comparisons.
Values were deemed significant at P < 0.05.
Results
Behavioral Studies
Mechanical hypersensitivity was present in both the early and late phases of the MIA model. In each phase and in both experimental (i.e., administered milnacipran) and control (i.e., administered saline) MIA groups (n = 8, per group per phase), prior to injection a greater frequency of limb withdrawal was seen on the IL compared with the CL paw when 6- and 8-g vF hairs were applied (Table 1). The level of hypersensitivity was comparable in animals subsequently treated with milnacipran or saline and the number of withdrawals on the IL side was not significantly different between the two phases of the model.
TABLE 1.
Baseline mechanical hypersensitivity prior to the injection of either milnacipran or saline (vehicle) in both phases of the MIA model
Data presented as the median number of hind-limb withdrawals from 10 applications to each paw of 1-, 6-, and 8-g vF filaments. n = 8 in each group, in both phases of the model.
| vF | +Milnacipran |
+Saline |
|||
|---|---|---|---|---|---|
| IL | CL | IL | CL | ||
| g | median (interquartile range) | ||||
| Early phase | 1 | 1.0 (0-1) | 0 (0-1) | 0.5 (0-1) | 0 (0-1) |
| 6 | 5.5 (3-6) | 0.0 (0-1) | 4.5 (4-7) | 0 (0-1) | |
| 8 | 8.0 (7-9) | 1.0 (0-1) | 8.0 (5-9) | 1 (0-2) | |
| Late phase | 1 | 0.0 (0-2) | 0.0 (0-1) | 1.0 (0-1) | 0.0 (0-1) |
| 6 | 3.5 (1-5) | 0.0 (0-0) | 4.0 (1-6) | 0.5 (0-1) | |
| 8 | 7.5 (4-8) | 0.0 (0-1) | 6.0 (5-9) | 1.0 (0-2) | |
CL, contralateral; MIA, monosodium iodoacetate; IL, ipsilateral; vF, von Frey.
Early Phase MIA.
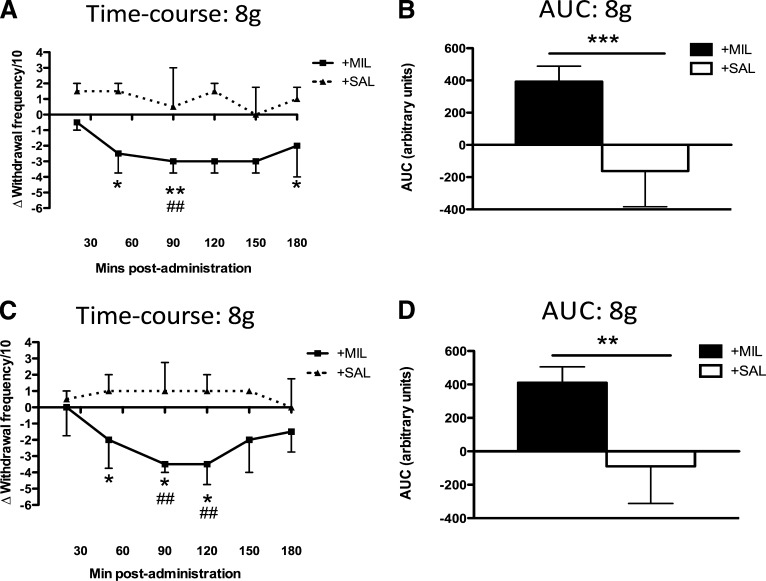
In the early phase (2–4 days) of the model, milnacipran reduced mechanical hypersensitivity at the hind-paw, whereas saline had no effect. Fewer withdrawal responses were evoked 50 minutes (P < 0.05), 90 minutes (P < 0.05), and 120 minutes (P < 0.05) after the injection of milnacipran when a 6-g vF was applied, compared with the same values after saline (Bonferroni-corrected Mann–Whitney U tests). Responses to an 8-g vF hair after milnacipran were reduced compared with baseline at 90 minutes postinjection (Friedman test; P < 0.05), with no effect of saline (Friedman test; P = 0.44). Comparing responses to 8-g vF in the two groups revealed that fewer were evoked after milnacipran compared with saline at 50, 90, and 180 minutes postinjection (Fig. 1). AUCs for responses to a 6-g vF hair (median 243 versus –108; P < 0.01; Mann–Whitney U test) and 8-g vF hair (Fig. 1) across the experimental period were significantly larger after milnacipran than saline.
Fig. 1.
Mechanical hypersensitivity of the ipsilateral hind-paw is attenuated by milnacipran in both the early (A and B) and late phases (C and D) of the MIA model. (A and C) Injection of milnacipran (10 mg/kg i.p.) caused a significant reduction in mechanical hypersensitivity compared with predrug values (#P < 0.05; ##P < 0.01; Friedman test and Dunn’s post hoc comparisons) and with the effects of saline (*P < 0.05; **P < 0.01; Mann–Whitney U tests with the Bonferroni correction) in the early (A) (n = 8) and late (C) (n = 8) phases of the model. Data are displayed as median difference scores, with postinjection scores normalized to the predrug baseline. (B and D) AUCs were significantly larger after milnacipran than after saline (**P < 0.01; ***P < 0.001; Mann–Whitney U test) in both the early (B) and late (D) phases of the MIA model. MIL, milnacipran; SAL, saline.
Late Phase MIA.
In the late phase of the model (14–18 days), neither milnacipran (P = 0.16; Friedman test) nor saline (P = 0.20; Friedman test) affected mechanical hypersensitivity to a 6-g vF, compared with that seen at preinjection baseline. However, responses to an 8-g vF hair were reduced from baseline after milnacipran was injected (P < 0.001; Friedman test), with Dunn’s post hoc tests demonstrating that responses were reduced at 90 and 120 minutes postinjection (Fig. 1). Saline caused no significant change in responses to an 8-g vF hair (P = 0.42; Friedman test). Comparing the two groups directly, responses were reduced after milnacipran compared with saline at 20, 50, and 90 minutes postinjection (Fig. 1). AUCs representing the effect of milnacipran on responses to 6-g vF hairs (median 118 versus −73; P < 0.05; Mann–Whitney U test) and 8-g vF hairs (Fig. 1) across the whole experimental period were larger after milnacipran than after saline.
Electrophysiological Studies
Across all studies, the mean depth of the recorded neurons in the spinal cord was consistent with an origin in the deep dorsal horn (mean 719 µm; range, 440–1020 µm; n = 63) and their general electrophysiological characteristics were similar in all groups with responses to innocuous and noxious mechanical and thermal stimuli, and a graded increase in response to stimuli that was related to stimulus intensity—features characteristic of wide dynamic range (WDR) neurons. However, there were between-group differences in responses to mechanical and thermal stimuli, with the number of spikes evoked by a 60-g vF hair (P < 0.001) applied to the plantar surface of the hind-paw greater in the late phase of the model than in naïve animals. Responses to 48°C were also greater in the late phase of the model, compared with in naïve animals (P < 0.01). Electrically evoked Aβ-fiber responses were greater in the late phase of the model compared with in naive animals (P < 0.05), whereas A∂-fiber–evoked responses (both P < 0.01) and C-fiber–evoked responses (both P < 0.001) were higher in both the early and late phases of the model compared with naive animals. Postdischarge was greater in the late phase of the model than in naive animal (P < 0.01), and wind-up was also greater in these animals, although this effect was not significant (P = 0.06).
When between-subjects analyses were made, no significant differences were found in mean neuronal depth or in C-fiber thresholds across groups (unpublished data).
Milnacipran Inhibits Spinal Neuronal Responses to Noxious Stimuli.
In naïve animals (n = 24), injection of milnacipran (5 and 10 mg/kg s.c.) caused a significant reduction of neuronal responses evoked by noxious mechanical (two-way RM ANOVA; main factor: dose; P < 0.001) and thermal stimuli (two-way RM ANOVA; main factor: dose; P < 0.001). Specifically, the number of spikes evoked by the application of a 60-g vF hair [predrug (APs): 823 ± 41] was significantly reduced after administration of both the low dose (549 ± 29; P < 0.001) and the high dose of milnacipran (423 ± 30; P < 0.001). These effects were dose dependent, with responses significantly reduced to a greater extent after the high dose than the low dose (P < 0.001). In contrast, responses to the innocuous 8-g vF hair were not affected by milnacipran. For thermal stimuli, the number of spikes evoked by 48°C applied to the neuronal receptive field (predrug: 863 ± 45) was reduced after injection of both low-dose (591 ± 27; P < 0.001) and high-dose milnacipran (48°C) (410 ± 30; P < 0.001), and to a significantly greater extent after the 10 mg/kg dose than after the 5 mg/kg dose (P < 0.001). Responses to the innocuous 35°C temperature were unchanged after milnacipran was administered.
Milnacipran also inhibited neuronal responses in the early phase of the MIA model (n = 14), with responses to noxious, but not innocuous, mechanical (two-way RM ANOVA; main factor: dose; P < 0.001) and thermal stimuli (two-way RM ANOVA; main factor: dose; P < 0.001) reduced after its administration. In the former case, the number of spikes evoked by a 60-g vF hair (predrug: 935 ± 114) was reduced by both low (532 ± 55; P < 0.001) and high doses of the drug (485 ± 74; P < 0.001) to a similar degree. The number of spikes evoked by the innocuous 8-g stimulus after each dose of milnacipran was similar to predrug values. With respect to thermal stimuli, responses to 48°C (predrug: 1036 ± 83) were reduced after administration of 5 mg/kg (558 ± 47; P < 0.001) and 10 mg/kg (503 ± 61; P < 0.001), and this effect was of a similar magnitude with the low dose as with the high dose. No inhibitory effects upon responses to the innocuous 35°C stimulus were seen after milnacipran was administered at either dose.
In the late phase of the MIA model (n = 22), milnacipran had a dose-dependent inhibitory effect on responses to high-intensity mechanical (two-way RM ANOVA; main factor: dose; P < 0.001) and thermal stimuli (two-way RM ANOVA; main factor: dose; P < 0.001). The number of spikes evoked by the 60-g vF hair (predrug: 60-g; 1030 ± 52) was significantly reduced after the low dose (679 ± 48; P < 0.001) and high dose of the drug (498 ± 44; P < 0.001). The effect of the 10 mg/kg dose compared with the 5 mg/kg dose was significantly greater on responses to the 60-g vF hair (all P < 0.001). The number of spikes evoked by the innocuous 8-g vF hair was not changed by the administration of milnacipran. With respect to thermal stimuli, the application of 48°C (predrug: 1075 ± 82) resulted in fewer APs being fired after both 5 mg/kg (736 ± 65; P < 0.001) and 10 mg/kg milnacipran (515 ± 40; P < 0.001), and this inhibitory effect was significantly greater after 10 mg/kg than after 5 mg/kg milnacipran (P < 0.001). Responses to 35°C in the late phase of the MIA model were not significantly different after milnacipran was administered in either dose.
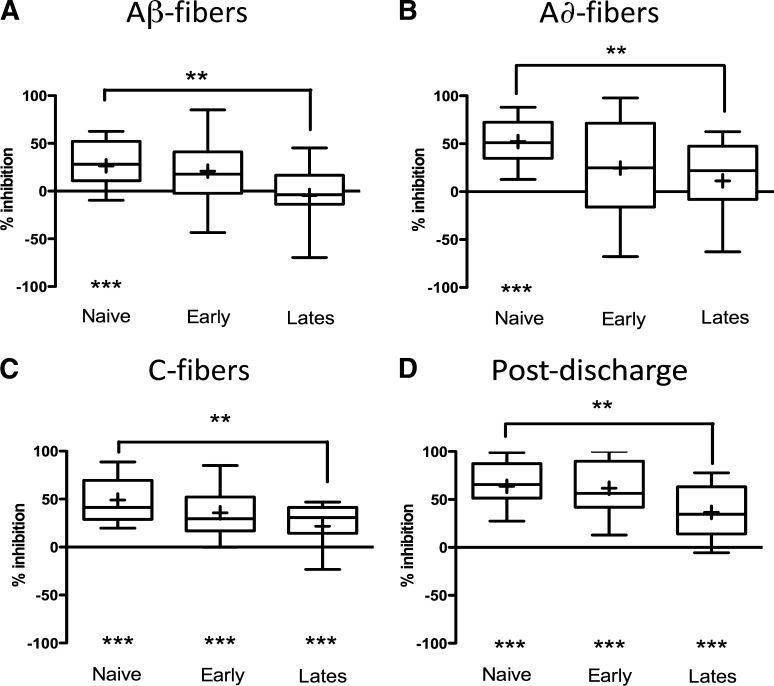
Milnacipran also inhibited electrically evoked responses of deep dorsal horn neurons. Injection of a 10 mg/kg dose caused a reduction of C-fiber–evoked responses and postdischarge in naïve and both early and late phase MIA groups (Fig. 2). Input was also reduced in all three groups (mean % inhibition: naïve, 57% ± 5%; early phase, 51% ± 10%; late phase, 27% ± 8%; all P < 0.001; paired t tests), with a significantly greater inhibitory effect in naïve compared with late phase MIA animals (P < 0.01; Kruskal–Wallis test). Similarly, wind-up was inhibited in all animal groups (naïve, 59% ± 5%; early phase, 49% ± 10%; late phase, 31% ± 7%; all P < 0.001; paired t tests), and the size of this effect was greater in naïve animals than in the late phase of the MIA model (one-way ANOVA; P < 0.01). In naïve animals only, A-fiber–evoked responses were significantly reduced. For all electrically evoked response parameters, direct comparisons revealed reduced inhibitory effects of milnacipran in the late phase of the model compared with naïve animals (Fig. 2).
Fig. 2.
Milnacipran inhibits electrically evoked responses from deep dorsal horn neurons in the early (n = 14) and late (n = 21) phases of the MIA model and in naïve animals (n = 24). In all three animal groups, milnacipran significantly reduced C-fiber–evoked responses (C) and postdischarge (D), although Aβ–evoked responses (A) and Aδ-fiber–evoked responses (B) were inhibited only in naïve animals (versus predrug baseline; ***P < 0.001; paired t test or Wilcoxon matched-pairs signed rank test; below graphs). Effects were consistently greater in naïve animals compared with the late phase of the model (naïve versus late phase MIA; **P < 0.01; Kruskal–Wallis tests with Dunn’s post hoc comparisons or one-way ANOVAs and Bonferroni post hoc tests; above graphs). Data are displayed as the mean % inhibition from predrug values in box and whisker plots apposing the level of inhibition caused by a dose of milnacipran (10 mg/kg s.c.) in the three animal groups. Plus symbols represent means, bars represent the median, and error bars mark the range of data.
Thus, milnacipran inhibited responses evoked from WDR neurons in the deep dorsal horn by mechanical, thermal, and electrical stimuli in both the early and late phases of the MIA model, but also in naïve animals. These inhibitory effects on electrically evoked responses were greater in naïve animals compared with those seen in animals studied 14–18 days into the model.
Blocking Spinal α2-Adrenoceptors Can Reverse the Effects of Milnacipran.
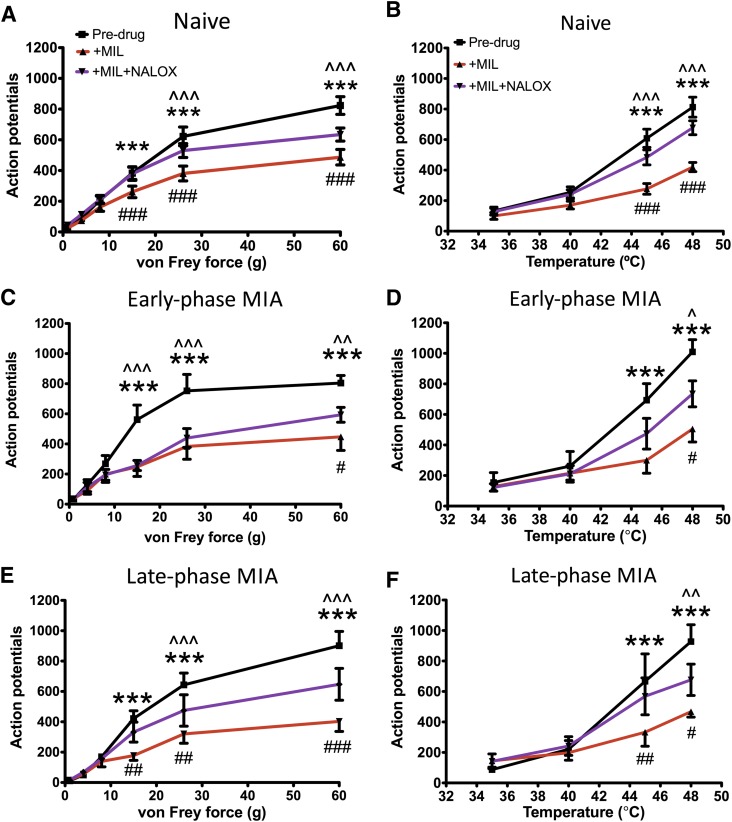
In a subset of naïve animals (n = 7), the α2-adrenoceptor antagonist, atipamezole (10 µg), was applied spinally in an attempt to reverse the inhibitory effects of milnacipran. This caused a full reversal of these effects on neuronal responses to mechanical stimuli (two-way RM ANOVA; main factor: dose; P < 0.001). Whereas 10 mg/kg milnacipran inhibited responses to 8-, 15-, 26-, and 60-g vF hairs, after atipamezole responses to these stimuli were significantly increased, and restored to predrug baseline values. A similar effect was seen for responses to thermal stimuli (two-way RM ANOVA; main factor: dose; P < 0.001); milnacipran reduced the number of spikes evoked by temperatures of 45°C and 48°C, and atipamezole fully reversed this effect (Fig. 3).
Fig. 3.
Blocking spinal α2-adrenoceptors reverses the inhibitory effects of milnacipran in naive animals (A and B) and in the early phase (C and D) but not in the late phase (E and F) of the MIA model (n = 7 per group). Milnacipran (s.c., 10 mg/kg) reduced responses from deep dorsal horn neurons to noxious mechanical and thermal stimuli in all three groups. In naïve animals (A and B) and in the early phase of the model (C and D), responses were reversed to predrug baseline values when the α2-adrenoceptor antagonist atipamezole (10 µg/50 µl) was administered (spinal). Atipamezole had no similar effect in the late phase of the model (E and F). Data are displayed as mean number of APs (± S.E.M.) evoked, plotted as a function of intensity of mechanical (A, C, and E) or thermal stimuli (B, D, and F) applied to the IL hind-paw. Data were analyzed with two-way RM ANOVAs and Bonferroni post hoc comparisons. Predrug versus +MIL: *P < 0.05; **P < 0.01; ***P < 0.001. +MIL versus +MIL+ATI: ^^^P < 0.001. Predrug versus +MIL+ATI: #P < 0.05; ###P < 0.001. MIL, milnacipran; ATI, atipamezole.
Similar effects were seen in animals (n = 7) studied in the early phase of the MIA model (two-way RM ANOVA; main factor: dose; P < 0.001). Responses evoked by 15-, 26-, and 60-g vF hairs and temperatures of 45°C and 48°C were each attenuated after the administration of milnacipran, whereas the subsequent spinal application of atipamezole increased responses back to predrug values (Fig. 3). The increase in response when atipamezole was administered after milnacipran was greater than the effect of atipamezole alone (60-g vF: mean, +149% versus +27%; 48°C: mean, +142% versus +10%), confirming that this reflected a true reversal of the effects of milnacipran.
In contrast to naïve and early phase MIA animals, atipamezole did not reverse the effects of milnacipran in the late phase of the MIA model (n = 7). Whereas milnacipran produced an inhibitory effect on responses to mechanical (two-way RM ANOVA; main factor: dose; P < 0.001) and thermal stimuli (two-way RM ANOVA; main factor: dose; P < 0.001), atipamezole had no effect on this inhibition (Fig. 3). The number of spikes evoked by the application of 26- and 60-g vF hairs and by temperatures of 45°C and 48°C remained significantly lower than predrug values, and not significantly different from the number recorded after milnacipran administration (Fig. 3).
Examination of electrically evoked responses from these neurons also demonstrated a time-related change in the ability of atipamezole to reverse the effects of milnacipran over the course of the model. In all groups of animals, milnacipran inhibited C-fiber–evoked responses (one-way RM ANOVA; Bonferroni post hoc tests; naïve: 46%, P < 0.001; early phase: 48%, P < 0.05; late phase: 74%, P < 0.01), although this measure was significantly increased after the administration of atipamezole in the naïve group and the early phase of the MIA model only (naïve: 103%, P < 0.001; early phase: 88%, P < 0.05; late phase: 65%, not significant). Similarly, milnacipran reduced postdischarge from neurons sampled from all three animal groups (one-way RM ANOVA; Bonferroni post hoc tests; naïve: 39%, P < 0.01; early phase: 18%, P < 0.001; late phase: 47%, P < 0.01). Whereas atipamezole significantly increased postdischarge in naïve animals (118%, P < 0.001), in the early phase of the model this was only partially reversed (57%, P < 0.05), remaining significantly lower than predrug values (P < 0.01). In the late phase of the model, atipamezole did not change the level of postdischarge when administered spinally in the presence of milnacipran (41%, not significant).
Hence, whereas the inhibitory effect of spinally released NA mediated by its action at α2-adrenoceptors, can account for the effects of milnacipran in naïve and early phase MIA animals, it does not appear to contribute to these effects in the later phase of the model.
Blocking Spinal 5-HT7 Receptors Reverses the Effect of Milnacipran in the Late Phase of the MIA Model on the Processing of Thermal Stimuli.
In the late phase of the MIA model (n = 9), spinal application of the 5-HT7 receptor antagonist SB-269970 (1 µg) reversed the inhibitory effect of milnacipran on the processing of thermal stimuli (two-way RM ANOVA; main factor: dose; P < 0.001). Whereas the number of spikes during the application of 45°C and 48°C was significantly reduced after the injection of 10 mg/kg milnacipran, a subsequent application of SB-269970 reversed the effects to predrug values. The inhibitory effect of milnacipran on neuronal responses to mechanical stimuli was, in contrast, only partially reversed by blocking 5-HT7 receptors (two-way RM ANOVA; main factor: dose; P < 0.001). Milnacipran attenuated the number of spikes during the application of 15- to 60-g vF hairs; although SB-269970 caused responses to 26- and 60-g to increase significantly, the number of spikes recorded remained significantly lower than predrug (Fig. 4).
Fig. 4.
Blocking spinal 5-HT7 receptors in the late phase of the MIA model (n = 9) reversed the inhibitory effect of milnacipran on responses from deep dorsal horn neurons to noxious thermal stimuli. Milnacipran (10 mg/kg s.c.) had attenuated responses to high-intensity mechanical (A) and thermal stimuli (B), but responses to the latter were fully reversed to baseline values after spinal administration of the 5-HT7 receptor antagonist SB-269970 (1 µg/50 µl). In contrast, SB-269970 had only a partial reversal effect on the inhibition of mechanical responses. Data are plotted as the mean number of APs (± S.E.M) as a function of stimulus intensity, two-way RM ANOVAs with Bonferroni post hoc tests. The inhibitory effects of milnacipran on C-fiber–evoked responses (C) and postdischarge (D) were also fully reversed after SB-269970 administration. Data are plotted as the mean % of predrug values and displayed as box and whisker plots, with plus symbols representing means, bars representing the median, and error bars marking the range of data (analyzed with one-way RM ANOVAs and Bonferroni post hoc tests). Predrug versus +MIL: **P < 0.01; ***P < 0.001. Predrug versus MIL +SB-269970: ^^^P < 0.001. +MIL +SB-269970 versus +MIL: #P < 0.05; ###P < 0.001. MIL, milnacipran.
Responses recorded during the application of 48°C after were increased by a significantly greater degree when SB-269970 was applied spinally after the administration of milnacipran (91%) compared with when it was administered alone (29%). This confirmed that blocking 5-HT7 receptors reversed the effects of milnacipran as opposed to merely having a direct excitatory effect on WDR neurons.
Examination of the effects of milnacipran and SB-269970 on electrically evoked responses suggested that the inhibitory effect of milnacipran on both C-fiber input and the development of spinal hyperexcitability is mediated by spinal 5-HT7 receptors; milnacipran significantly reduced both C-fiber–evoked responses (one-way RM ANOVA; P < 0.01) and postdischarge (one-way RM ANOVA; P < 0.05); however, the spinal application of SB-269970 caused each of these to increase significantly to a level no different from predrug values (Fig. 4). Friedman tests also demonstrated significant drug effects on wind-up (P < 0.05) and on input (P < 0.05). Input was reduced by milnacipran to 64% (±9%) of predrug values (Dunn’s post hoc test; P < 0.05) and spinal application of the 5-HT7 receptor antagonist SB-269970 increased this to 113% (±22%) of predrug baseline. Milnacipran inhibited wind-up (64% ± 6%; Dunn’s post hoc test; P < 0.05), but SB-269970 subsequently increased this to 94% (±16%) of predrug values.
Blocking Spinal Opioid Receptors Partially Reverses the Effect of Milnacipran.
In naïve animals (n = 9), the opioid receptor antagonist naloxone was applied spinally (50 µg) in an attempt to reverse the effects of milnacipran (10 mg/kg). Naloxone partially reversed the inhibitory effect of milnacipran on responses to noxious thermal (two-way RM ANOVA; main factor: dose; P < 0.001) and mechanical stimuli (two-way RM ANOVA; main factor: dose; P < 0.001). Milnacipran attenuated responses to 15- to 60-g vF hairs, and naloxone significantly increased the number of spikes evoked by these stimuli. However, responses to 26- and 60-g vF hairs remained significantly below predrug values. Similar effects were seen on responses to thermal stimuli, with milnacipran reducing the number of spikes evoked by 45°C and 48°C, and the subsequent spinal application of naloxone increasing responses, although to a level below predrug values (Fig. 5).
Fig. 5.
Spinal administration of the opioid receptor antagonist naloxone partially reverses the inhibitory effect of milnacipran in the early and late phases of the MIA model and in naïve animals (n = 9 per group). Milnacipran (10 mg/kg s.c.) reduced responses to noxious mechanical and thermal stimuli in all three animal groups and spinal application of the opioid receptor antagonist naloxone (50 µg/50 µl) significantly increased responses. In all groups, however, this reversal was only partial, with responses from deep dorsal horn neurons still significantly lower than predrug values. Data plotted as the mean number of APs (± S.E.M.) as a function of stimulus intensity. Analysis with two-way RM ANOVAs and Bonferroni post hoc tests. Predrug versus +MIL: ***P < 0.001. Predrug versus +MIL+NALOX: ^^P < 0.01; ^^^P < 0.001. +MIL versus +MIL+NALOX: #P < 0.05; ##P < 0.01; ###P < 0.001. MIL, milnacipran; NALOX, naloxone.
In the early phase of the MIA model (n = 9), similar effects were seen (two-way RM ANOVA; main factor: dose; P < 0.001) with milnacipran reducing responses to 15-, 26-, and 60-g vF hairs and naloxone significantly increasing responses to a 60-g vF. Much like in naïve animals, the number of spikes recorded to this stimulus remained lower than that seen predrug. For thermal stimuli, milnacipran attenuated responses evoked by 45 and 48°C. The subsequent spinal application of the opioid receptor antagonist naloxone significantly increased responses to 48°C to a level still below predrug values (Fig. 5).
Naloxone also partially reversed the effects of milnacipran on noxious mechanical (two-way RM ANOVA; main factor: dose; P < 0.001) and thermal stimuli (two-way RM ANOVA; main factor: dose; P < 0.001) in the late phase of the MIA model (n = 9). Responses to 15-, 26-, and 60-g vF hairs were reduced, whereas naloxone increased these significantly. However, in the case of 26- and 60-g, these remained lower than predrug values. Milnacipran also reduced responses to 45°C and 48°C, whereas naloxone significantly increased responses to both temperatures. Responses to 48°C remained significantly lower than predrug (Fig. 5).
Hence, the effects of milnacipran in normal animals and those studied in two phases of this model of OA pain are partly mediated by endogenous opioids in the spinal cord, and this effect appears similar in all three groups.
Blocking Spinal α2-Adrenoceptors Reveals a Tonic Noradrenergic Inhibition Present Only in the Early Phase of the MIA Model.
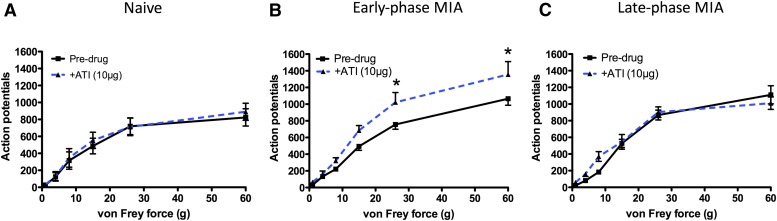
In a group of naïve animals (n = 7), the α2-adrenoceptor antagonist atipamezole (10 µg) was applied spinally to examine the presence of a tonic noradrenergic inhibition of spinal nociceptive processing mediated by these receptors. This caused no change in the response from deep dorsal horn neurons to mechanical (Fig. 6) and thermal stimuli, nor to electrical stimuli.
Fig. 6.
Blocking spinal α2-adrenoceptors reveals an engagement of descending noradrenergic inhibition in the early phase of the MIA model that relies on spinal α2-adrenoceptors (n = 7 per group). Spinal application of the α2-adrenoceptor antagonist atipamezole (10 µg) caused an increase in the response to noxious mechanical stimuli applied to the IL hind-paw in the early phase of the MIA model (B), but not naïve (A) or late phase MIA animals (C). Data are presented as mean number of APs (± S.E.M.) plotted as a function of stimulus intensity. Data were analyzed with two-way RM ANOVAs and Bonferroni-corrected post hoc comparisons. Predrug versus +ATI (10 µg): *P < 0.05. ATI, atipamezole.
By contrast, atipamezole increased responses to mechanical stimuli (two-way RM ANOVA; main factor: dose; P < 0.001) in the early phase of the MIA model (n = 7) indicative of reversal of an ongoing α2-mediated control. A greater number of spikes were evoked by the application of a 26- and a 60-g vF hair, compared with predrug values, after atipamezole was applied to the spinal cord. This drug also increased responses to thermal stimuli (two-way RM ANOVA; main factor: dose; P < 0.05) (Fig. 6).
Atipamezole also caused Aδ-evoked responses to increase significantly in the early phase of the model to a mean of 131% (±15) of predrug values (paired t test; P < 0.05) and C-fiber–evoked responses were also significantly increased to 129% (±13) of predrug values (P < 0.05; Wilcoxon paired-match signed rank test).
In the late phase of the model (n = 7), blocking α2-adrenoceptors with atipamezole at 10 µg or a higher dose of 100 µg had no effect on responses to mechanical (Fig. 6), thermal, or electrical stimuli, indicative of a loss of this noradrenergic inhibitory control later in the course of the model.
Blocking Spinal 5-HT7 Receptors Reveals a Descending Serotonergic Inhibition.
Spinal application of SB-269970 (1 and 5 µg) in naïve animals (n = 6) had a facilitatory effect on neuronal responses to mechanical stimuli (two-way RM ANOVA; main factor: dose; P < 0.001). Compared with predrug responses (318 ± 84), the number of spikes evoked by the innocuous 8-g vF hair was increased after the 5-µg dose (529 ± 123; P < 0.01) but no effect of the 1-µg dose was seen. Responses to the noxious 26-g vF stimulus were also increased after 5 µg of SB-269970 compared with predrug (1036 ± 90 versus 852 ± 99, respectively; P < 0.05). Responses to thermal stimuli were also increased (two-way RM ANOVA; main factor: dose; P < 0.001), with responses to the noxious 45°C stimulus increased only after the 5-µg dose, compared with predrug (1137 ± 145 versus 696 ± 118, respectively; P < 0.001). None of the electrically evoked response parameters were altered by the administration of SB-269970 at either dose in naïve animals.
In the late phase of the MIA model (n = 8), SB-269970 facilitated responses to mechanical stimuli (two-way RM ANOVA; main factor: dose; P < 0.001), with responses to the noxious 26-g vF hair increased compared with predrug values (896 ± 53), but only after the 5-µg dose (1018 ± 120; P < 0.05). A greater facilitatory effect was seen on responses to thermal stimuli (two-way RM ANOVA; main factor: dose; P < 0.001). Compared with predrug values (231 ± 51), an increased number of spikes was seen after 1 µg of SB-269970 during the application of the innocuous temperature 35°C (471 ± 64; P < 0.05), an increase also seen after the higher dose of the drug (612 ± 48; P < 0.001). Responses to the noxious temperatures 45°C and 48°C were also increased by SB-269970, with the former increased after both the low (1238 ± 131; P < 0.001) and the higher dose of this 5-HT7 antagonist (1520 ± 143; P < 0.001), compared with predrug values (888 ± 105). Responses to 48°C were also increased after both the low (1616 ± 77; P < 0.01) and high dose of this drug (1906 ± 122; P < 0.001), compared with predrug responses (1304 ± 129) Effects were greater after 5 g than 1 µg for response to 45°C and 48°C (both P < 0.01).
In late phase MIA animals, one-way ANOVAs revealed that SB-269970 increased C-fiber–evoked responses (P < 0.001), postdischarge (P < 0.05), and input (P < 0.01). Input increased to a mean of 136% (±7%) of predrug values after 1 µg (P < 0.01), whereas 5 µg resulted in values 134% (±12%) of baseline (P < 0.05). C-fiber–evoked responses increased to 124% (±7%) of predrug values after the lower dose of SB-269970 (P < 0.05). The higher dose significantly increased responses in the C-fiber range to 150% (±12%) of predrug (P < 0.01). Postdischarge was increased after the higher dose only, to 150% (±12%) of predrug values (P < 0.05).
Discussion
Milnacipran is an SNRI that has antinociceptive effects in the musculoskeletal pain condition, fibromyalgia (Arnold et al., 2010), and models of inflammatory and neuropathic pain (Iyengar et al., 2004; King et al., 2006; Mico et al., 2011). This is the first published study examining whether milnacipran also has antinociceptive effects in a model of OA, and demonstrates that this antidepressant reduces the mechanical hypersensitivity that presents at the hind-paw in both the early and late phases of the MIA model. Electrophysiological studies conducted at these same time points indicated that these effects were mediated by an action on descending monoaminergic controls, resulting in the inhibition of spinal nociceptive processing. Of those measures that were elevated in this model of pain, and which confirmed the presence of central sensitization, many were reduced after administration of this SNRI.
These findings also suggest that the relative importance of descending NA and 5-HT to the inhibitory effects of milnacipran changes over the course of the model; NA via its interaction with spinal α2-adrenoceptors mediated these effects in naïve animals and the early, inflammatory phase of the model, whereas at a later time point —when joint damage is advanced and not driven by inflammation—the inhibitory effects of milnacipran were mediated at least partly by spinal 5-HT7 receptors. In fact, the level of ongoing serotonergic and noradrenergic inhibition altered over the course of the model, with an increased noradrenergic inhibition engaged in the early but not late phase, and a 5-HT7 receptor–mediated inhibition of the processing of thermal stimuli engaged in the late phase. Thus, changes in the mechanisms behind the effect of milnacipran over the course of the model appeared connected to changes in the relative importance of endogenous noradrenergic and serotonergic inhibitory controls over the same period.
The efficacy of milnacipran in the early phase of the model adds to previous data demonstrating antinociceptive effects in postoperative (Obata et al., 2010), neuropathic (King et al., 2006), and polyarthritis pain models (Mico et al., 2011). The reduction of mechanical hypersensitivity in both phases of the MIA model is noteworthy because this has not been found to be the case for analgesics like paracetamol and diclofenac (Fernihough et al., 2004). Since duloxetine is also effective in the MIA model (Chandran et al., 2009) and OA sufferers (Chappell et al., 2009), descending monoaminergic pathways appear a relevant target for producing analgesia in this pain state.
Other behavioral studies also note that mechanical hypersensitivity is present at the hind-paw in the MIA model (e.g., Rahman et al., 2009; Vonsy et al., 2009) and that deep dorsal horn neurons display hyperexcitability in the late phase of the model when this hind-paw region is stimulated (Harvey and Dickenson, 2009; Sagar et al., 2010). Since the hind-paw itself is not affected by an i.a. injection of MIA, these results indicate that the development of an osteoarthritic state in the joint can prompt the development of central sensitization. Signs of central sensitization are present in some OA sufferers, with areas of referred pain present in regions away from the affected joint that are not explicable by the peripheral damage alone (Kosek and Ordeberg, 2000; Bajaj et al., 2001; Imamura et al., 2008; Gwilym et al., 2009; Lee et al., 2011). The increased wind-up of pain in other clinical studies—at the osteoarthritic joint and at a distal region—is also consistent with the presence of central sensitization in OA (Arendt-Nielsen et al., 2010), and plausibly leads to the central amplification of inputs from uninjured structures, resulting in the patterns of referred pain seen in studies that utilize quantitative sensory testing (e.g., Gwilym et al., 2009). In this light, the inhibitory effect of milnacipran on the wind-up of dorsal horn neurons in the late phase of the model may suggest a particular efficacy when joint damage is advanced and central sensitization is present; a plausible example of a population of OA sufferers who may benefit from centrally acting agents such as milnacipran being the significant minority whose pain is refractory to joint replacement (Beswick et al., 2012).
In all three groups of animals, milnacipran reduced input and C-fiber–evoked responses from dorsal horn neurons, suggesting that the inhibitory effects of this drug are mediated by receptors on the central terminals of unmyelinated afferents. α2-adrenoceptors are present on C-fiber terminals (Stone et al., 1998) and NA binds to these to reduce excitatory transmitter release (Kuraishi et al., 1985), providing a physiologic substrate for the effects of milnacipran in naïve animals and the early phase of the MIA model. Reducing excitatory transmitter release from C-fibers would likely reduce postsynaptic hyperexcitability in the dorsal horn, and, consistent with this idea, milnacipran reduced both postdischarge and wind-up in all animal groups.
There was agreement between the results from electrophysiological and behavioral studies in MIA-injected animals, with reductions in neuronal and behavioral hypersensitivity seen with milnacipran in both phases of the model. Systemic injection of milnacipran increases monoamine concentrations in the dorsal horn by 100–200% (Obata et al., 2010). Therefore, given their inhibitory effects in the spinal cord (Engberg and Ryall, 1966; Bannister et al., 2009) and the well described involvement of deep dorsal horn neurons in nociception (Mayer et al., 1975; Price and Dubner, 1977; D'Mello and Dickenson, 2008), this coherence is perhaps unsurprising.
Antagonizing spinal monoamine receptors can block the antinociceptive effects of milnacipran, and the noradrenergic system in particular appears important to these effects (Onal et al., 2007; Obata et al., 2010; Nakajima et al., 2012). In the present studies, whereas blocking spinal α2-adrenoceptors with atipamezole reversed the effects of milnacipran in naïve animals and the early phase of the model, this was not so in the late phase. The effect of atipamezole alone in the early phase suggested a descending noradrenergic inhibition engaged as a result of joint inflammation (Bove et al., 2003), although this was lost in the late phase of the model. These results and the failure of atipamezole to reverse the effects of milnacipran in the late phase could indicate reduced NA release in the dorsal horn as the condition becomes chronic, or a reduction in α2-adrenoceptor density (Stone et al., 1999). Whatever the mechanism, the reduced descending noradrenergic inhibition could foreshadow the emergence of central sensitization and hypersensitivity in the MIA model, and may also therefore explain the lack of contribution of NA to the effects of milnacipran in the late phase of the model.
These results also suggest that a descending serotonergic inhibition of the processing of thermal stimuli is engaged at an advanced stage of the model, mediated by 5-HT7 receptors. Spinal 5-HT7 receptors were also involved in the inhibitory effects of milnacipran at this same time point, and contribute to the effects of a variety of other analgesics, including opioids (Dogrul and Seyrek, 2006; Seyrek et al., 2010), and may thus represent a novel target for analgesia. The inhibitory effects of milnacipran were also partly opioid dependent, since spinal administration of naloxone partially reversed these effects. This finding contrasts with a study in a postoperative pain model that found that the effects of milnacipran were unchanged by intrathecal naloxone (Obata et al., 2010). At least in the case of OA pain then, milnacipran may produce some of its inhibitory effects by stimulating the release of endogenous opioids at the spinal level, perhaps from inhibitory interneurons that contain these peptides (Hokfelt et al., 1977). Hence, the mechanisms behind the inhibitory effects of milnacipran appear dynamic, and intimately related to the level of activity in descending monoaminergic pathways over the course of the model, although spinal release of opioids consequent to monoamine release also contributes to these effects and is unchanged after OA-like joint damage.
Although the effects of milnacipran seen here were after acute dosing, it is worth emphasizing that antidepressants may produce antinociceptive effects clinically after prolonged administration. There has been no published study of the antinociceptive effects of milnacipran in OA pain; thus, it is possible that the effects seen here suggest this SNRI has a rapid onset of action in this condition. The effectiveness of milnacipran merits further study in OA, and it would be insightful to examine whether its efficacy differs with chronic dosing. However, in light of the pervasive effects of duloxetine in OA pain (Chappell et al., 2009) and animal studies demonstrating that the antinociceptive effects of milnacipran increase or are similar when given chronically (King et al., 2006; Depoortère et al., 2011; Wattiez et al., 2011), there is reason to think that its efficacy would maintain over the long term.
To summarize, milnacipran has antinociceptive effects in a model of OA pain, produced by attenuating the spinal processing of noxious stimuli via an action on descending monoaminergic controls. NA acting at spinal α2-adrenoceptors is involved in these effects, although it does not contribute late in the model, when a 5-HT7 receptor–mediated effect predominates. The efficacy of milnacipran both early and late in this model could indicate an analgesic effect whether inflammation in the joint is a feature of the OA “phenotype.” Furthermore, given that milnacipran reduced the hypersensitivity referred to the hind-paw, this SNRI may be particularly effective when OA pain is underpinned by central sensitization.
Abbreviations
- 5-HT
5-hydroxytryptamine (serotonin)
- ANOVA
analysis of variance
- AP
action potential
- AUC
area under the curve
- CL
contralateral
- IL
ipsilateral
- MIA
monosodium iodoacetate
- NA
noradrenaline
- OA
osteoarthritis
- PAG
periaqueductal gray
- SNRI
serotonin-noradrenaline reuptake inhibitor
- RM
repeated measures
- SB-269970
(2R)-1-[(3-hydroxyphenyl)sulfonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl]pyrrolidine hydrochloride
- vF
von Frey
- WDR
wide dynamic range
Authorship Contributions
Participated in research design: Burnham, Dickenson.
Conducted experiments: Burnham.
Performed data analysis: Burnham.
Wrote or contributed to the writing of the manuscript: Burnham, Dickenson
Footnotes
This research was supported by Research Councils UK [Medical Research Council Doctoral Training Account Grant G0700020]; Medical Research Council quota award; and the London Pain Consortium.
References
- Aimone LD, Jones SL, Gebhart GF. (1987) Stimulation-produced descending inhibition from the periaqueductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain 31:123–136 [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. (2010) Sensitization in patients with painful knee osteoarthritis. Pain 149:573–581 [DOI] [PubMed] [Google Scholar]
- Arnold LM, Gendreau RM, Palmer RH, Gendreau JF, Wang Y. (2010) Efficacy and safety of milnacipran 100 mg/day in patients with fibromyalgia: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 62:2745–2756 [DOI] [PubMed] [Google Scholar]
- Bajaj P, Bajaj P, Graven-Nielsen T, Arendt-Nielsen L. (2001) Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain 93:107–114 [DOI] [PubMed] [Google Scholar]
- Bannister K, Bee LA, Dickenson AH. (2009) Preclinical and early clinical investigations related to monoaminergic pain modulation. Neurotherapeutics 6:703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocoso E, Mico JA, Vitton O, Ladure P, Newman-Tancredi A, Depoortère R, Bardin L. (2011) Evaluation of milnacipran, in comparison with amitriptyline, on cold and mechanical allodynia in a rat model of neuropathic pain. Eur J Pharmacol 655:46–51 [DOI] [PubMed] [Google Scholar]
- Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. (2012) What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2:e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS. (2003) Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage 11:821–830 [DOI] [PubMed] [Google Scholar]
- Chandran P, Pai M, Blomme EA, Hsieh GC, Decker MW, Honore P. (2009) Pharmacological modulation of movement-evoked pain in a rat model of osteoarthritis. Eur J Pharmacol 613:39–45 [DOI] [PubMed] [Google Scholar]
- Chappell AS, Ossanna MJ, Liu-Seifert H, Iyengar S, Skljarevski V, Li LC, Bennett RM, Collins H. (2009) Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trial. Pain 146:253–260 [DOI] [PubMed] [Google Scholar]
- D’Mello R, Dickenson AH. (2008) Spinal cord mechanisms of pain. Br J Anaesth 101:8–16 [DOI] [PubMed] [Google Scholar]
- Depoortère R, Meleine M, Bardin L, Aliaga M, Muller E, Ardid D, Newman-Tancredi A. (2011) Milnacipran is active in models of irritable bowel syndrome and abdominal visceral pain in rodents. Eur J Pharmacol 672:83–87 [DOI] [PubMed] [Google Scholar]
- Dogrul A, Seyrek M. (2006) Systemic morphine produce antinociception mediated by spinal 5-HT7, but not 5-HT1A and 5-HT2 receptors in the spinal cord. Br J Pharmacol 149:498–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I, Ryall RW. (1966) The inhibitory action of noradrenaline and other monoamines on spinal neurones. J Physiol 185:298–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, Kidd B, Bevan S, Winter J. (2004) Pain related behaviour in two models of osteoarthritis in the rat knee. Pain 112:83–93 [DOI] [PubMed] [Google Scholar]
- Guzman RE, Evans MG, Bove S, Morenko B, Kilgore K. (2003) Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol Pathol 31:619–624 [DOI] [PubMed] [Google Scholar]
- Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, Tracey I. (2009) Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum 61:1226–1234 [DOI] [PubMed] [Google Scholar]
- Harvey VL, Dickenson AH. (2009) Behavioural and electrophysiological characterisation of experimentally induced osteoarthritis and neuropathy in C57Bl/6 mice. Mol Pain 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T, Ljungdahl A, Terenius L, Elde R, Nilsson G. (1977) Immunohistochemical analysis of peptide pathways possibly related to pain and analgesia: enkephalin and substance P. Proc Natl Acad Sci USA 74:3081–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DJ, McDougall JJ, Keefe FJ. (2008) The symptoms of osteoarthritis and the genesis of pain. Rheum Dis Clin North Am 34:623–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden JLK, Hayashi H, Dubner R, Bennett GJ. (1986) Physiology and morphology of the lamina I spinomesencephalic projection. J Comp Neurol 247:505–515 [DOI] [PubMed] [Google Scholar]
- Imamura M, Imamura ST, Kaziyama HH, Targino RA, Hsing WT, de Souza LP, Cutait MM, Fregni F, Camanho GL. (2008) Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: a controlled analysis. Arthritis Rheum 59:1424–1431 [DOI] [PubMed] [Google Scholar]
- Iyengar S, Webster AA, Hemrick-Luecke SK, Xu JY, Simmons RM. (2004) Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther 311:576–584 [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. (1984) Spinal monoamine and opiate systems partly mediate the antinociceptive effects produced by glutamate at brainstem sites. Brain Res 321:287–297 [DOI] [PubMed] [Google Scholar]
- Kalso EA, Pöyhiä R, Rosenberg PH. (1991) Spinal antinociception by dexmedetomidine, a highly selective alpha 2-adrenergic agonist. Pharmacol Toxicol 68:140–143 [DOI] [PubMed] [Google Scholar]
- King T, Rao S, Vanderah T, Chen Q, Vardanyan A, Porreca F. (2006) Differential blockade of nerve injury-induced shift in weight bearing and thermal and tactile hypersensitivity by milnacipran. J Pain 7:513–520 [DOI] [PubMed] [Google Scholar]
- Kosek E, Ordeberg G. (2000) Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. Eur J Pain 4:229–238 [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Hirota N, Sato Y, Kaneko S, Satoh M, Takagi H. (1985) Noradrenergic inhibition of the release of substance P from the primary afferents in the rabbit spinal dorsal horn. Brain Res 359:177–182 [DOI] [PubMed] [Google Scholar]
- Lee YC, Lu B, Bathon JM, Haythornthwaite JA, Smith MT, Page GG, Edwards RR. (2011) Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res (Hoboken) 63:320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell PJ, Bromidge SM, Dabbs S, Duckworth DM, Forbes IT, Jennings AJ, King FD, Middlemiss DN, Rahman SK, Saunders DV, et al. (2000) A novel, potent, and selective 5-HT(7) antagonist: (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl) phen ol (SB-269970). J Med Chem 43:342–345 [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Price DD, Becker DP. (1975) Neurophysiological characterization of the anterolateral spinal cord neurons contributing to pain perception in man. Pain 1:51–58 [DOI] [PubMed] [Google Scholar]
- Mico JA, Berrocoso E, Vitton O, Ladure P, Newman-Tancredi A, Bardin L, Depoortère R. (2011) Effects of milnacipran, duloxetine and indomethacin, in polyarthritic rats using the Randall-Selitto model. Behav Pharmacol 22:599–606 [DOI] [PubMed] [Google Scholar]
- Moret C, Charveron M, Finberg JP, Couzinier JP, Briley M. (1985) Biochemical profile of midalcipran (F 2207), 1-phenyl-1-diethyl-aminocarbonyl-2-aminomethyl-cyclopropane (Z) hydrochloride, a potential fourth generation antidepressant drug. Neuropharmacology 24:1211–1219 [DOI] [PubMed] [Google Scholar]
- Nakajima K, Obata H, Iriuchijima N, Saito S. (2012) An increase in spinal cord noradrenaline is a major contributor to the antihyperalgesic effect of antidepressants after peripheral nerve injury in the rat. Pain 153:990–997 [DOI] [PubMed] [Google Scholar]
- Obata H, Kimura M, Nakajima K, Tobe M, Nishikawa K, Saito S. (2010) Monoamine-dependent, opioid-independent antihypersensitivity effects of intrathecally administered milnacipran, a serotonin noradrenaline reuptake inhibitor, in a postoperative pain model in rats. J Pharmacol Exp Ther 334:1059–1065 [DOI] [PubMed] [Google Scholar]
- Onal A, Parlar A, Ulker S. (2007) Milnacipran attenuates hyperalgesia and potentiates antihyperalgesic effect of tramadol in rats with mononeuropathic pain. Pharmacol Biochem Behav 88:171–178 [DOI] [PubMed] [Google Scholar]
- Onghena P, Van Houdenhove B. (1992) Antidepressant-induced analgesia in chronic non-malignant pain: a meta-analysis of 39 placebo-controlled studies. Pain 49:205–219 [DOI] [PubMed] [Google Scholar]
- Peng YB, Lin Q, Willis WD. (1996) Involvement of alpha-2 adrenoceptors in the periaqueductal gray-induced inhibition of dorsal horn cell activity in rats. J Pharmacol Exp Ther 278:125–135 [PubMed] [Google Scholar]
- Pertovaara A, Haapalinna A, Sirviö J, Virtanen R. (2005) Pharmacological properties, central nervous system effects, and potential therapeutic applications of atipamezole, a selective alpha2-adrenoceptor antagonist. CNS Drug Rev 11:273–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD, Dubner R. (1977) Neurons that subserve the sensory-discriminative aspects of pain. Pain 3:307–338 [DOI] [PubMed] [Google Scholar]
- Rahman W, Bauer CS, Bannister K, Vonsy JL, Dolphin AC, Dickenson AH. (2009) Descending serotonergic facilitation and the antinociceptive effects of pregabalin in a rat model of osteoarthritic pain. Mol Pain 5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar DR, Staniaszek LE, Okine BN, Woodhams S, Norris LM, Pearson RG, Garle MJ, Alexander SP, Bennett AJ, Barrett DA, et al. (2010) Tonic modulation of spinal hyperexcitability by the endocannabinoid receptor system in a rat model of osteoarthritis pain. Arthritis Rheum 62:3666–3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyrek M, Kahraman S, Deveci MS, Yesilyurt O, Dogrul A. (2010) Systemic cannabinoids produce CB₁-mediated antinociception by activation of descending serotonergic pathways that act upon spinal 5-HT(7) and 5-HT(2A) receptors. Eur J Pharmacol 649:183–194 [DOI] [PubMed] [Google Scholar]
- Stone LS, Broberger C, Vulchanova L, Wilcox GL, Hökfelt T, Riedl MS, Elde R. (1998) Differential distribution of alpha2A and alpha2C adrenergic receptor immunoreactivity in the rat spinal cord. J Neurosci 18:5928–5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, Vulchanova L, Riedl MS, Wang J, Williams FG, Wilcox GL, Elde R. (1999) Effects of peripheral nerve injury on alpha-2A and alpha-2C adrenergic receptor immunoreactivity in the rat spinal cord. Neuroscience 93:1399–1407 [DOI] [PubMed] [Google Scholar]
- Thomas DR, Atkinson PJ, Ho M, Bromidge SM, Lovell PJ, Villani AJ, Hagan JJ, Middlemiss DN, Price GW. (2000) [(3)H]-SB-269970—A selective antagonist radioligand for 5-HT(7) receptors. Br J Pharmacol 130:409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urch CE, Dickenson AH. (2003) In vivo single unit extracellular recordings from spinal cord neurones of rats. Brain Res Brain Res Protoc 12:26–34 [DOI] [PubMed] [Google Scholar]
- Vaishnavi SN, Nemeroff CB, Plott SJ, Rao SG, Kranzler J, Owens MJ. (2004) Milnacipran: a comparative analysis of human monoamine uptake and transporter binding affinity. Biol Psychiatry 55:320–322 [DOI] [PubMed] [Google Scholar]
- Vonsy JL, Ghandehari J, Dickenson AH. (2009) Differential analgesic effects of morphine and gabapentin on behavioural measures of pain and disability in a model of osteoarthritis pain in rats. Eur J Pain 13:786–793 [DOI] [PubMed] [Google Scholar]
- Wattiez AS, Libert F, Privat ante meridian, Loiodice S, Fialip J, Eschalier A, Courteix C. (2011) Evidence for a differential opioidergic involvement in the analgesic effect of antidepressants: prediction for efficacy in animal models of neuropathic pain? Br J Pharmacol 163:792–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, et al. (2010) OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 18:476–499 [DOI] [PubMed] [Google Scholar]
- Zimmermann M. (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110 [DOI] [PubMed] [Google Scholar]