Abstract
Recognition of the cytoprotective functions of autophagy that occur in tumor cells exposed to various forms of chemotherapy or radiation has generated intense interest in the possibility that pharmacological interference with autophagy could provide a clinical strategy for overcoming therapeutic resistance. Multiple clinical trials are currently in progress to evaluate the antimalarial agent chloroquine (generally in its clinical formulation as hydroxychloroquine) and its impact on various forms of cancer therapy. In this commentary/review, we focus on the relatively limited number of studies in the literature where chloroquine has been tested in combination with chemotherapy or radiation in experimental tumor-bearing animal models. We also present recent data from our own laboratories, in cell culture experiments as well as in vivo studies, which demonstrate that neither chloroquine nor silencing of an autophagy regulatory gene was effective in conferring radiation sensitivity in an experimental model of breast cancer. The capacity for sensitization by chloroquine appears to be quite wide-ranging, with dramatic effects for some drugs/tumor models and modest or minimal effects in others. One possible caveat is that, with only a few exceptions, experiments have generally been performed in xenograft models, thereby eliminating the involvement of the immune system, which might ultimately be proven to play a central role in determining the effectiveness of autophagy inhibition in chemosensitization or radiosensitization. Nevertheless, a careful review of the current literature suggests that caution is likely to be warranted in translating preclinical findings relating to autophagy inhibition as an adjunctive therapeutic strategy.
Introduction
In recent years, it has been recognized that one potential mechanism of resistance to chemotherapy as well as radiotherapy in cancer could be the promotion of protective autophagy; this recognition has generated interest in the possibility that interference with autophagy could enhance sensitivity to treatment (Paglin et al., 2001; Kanzawa et al., 2004; Boya et al., 2005; Kondo et al., 2005; Sotelo et al., 2006; Abedin et al., 2007; Amaravadi et al., 2007; Djavaheri-Mergny et al., 2007; Apel et al., 2008; Qadir et al., 2008; Wilson et al., 2011; Bristol et al., 2012). An extensive number of studies in cell culture (for example, among many others, Paglin et al., 2001; Kanzawa et al., 2004; Boya et al., 2005; Zhao et al., 2005; Amaravadi et al., 2007; Apel et al., 2008; Qadir et al., 2008; Livesey et al., 2009; Solomon and Lee, 2009; Ma et al., 2011; Wilson et al., 2011; Bristol et al., 2012), as well as a limited number of studies in animal models (Fu et al., 2009; Carew et al., 2010; Jiang et al., 2010; Wu et al., 2010; Ding et al., 2011; Lopez et al., 2011; Mirzoeva et al., 2011; Pan et al., 2011; Shi et al., 2011; Xu et al., 2011; Ghadimi et al., 2012; Godbole et al., 2012; Guo et al., 2012; Hu et al., 2012; Liang et al., 2012; Loehberg et al., 2012; Rao et al., 2012; Sasaki et al., 2012), have been performed combining chloroquine or hydroxychloroquine with chemotherapeutic drugs or radiation. Furthermore, a number of clinical trials have been initiated to test this premise in patients (Sotelo et al., 2006; Solomon and Lee, 2009).
In view of the fact that therapeutic efficacy of both antitumor drugs and radiation may be highly dependent on the immune system (Michaud et al., 2011; Golden et al., 2012; Martins et al., 2012), and since, with few exceptions, studies in the current literature have been performed using tumor xenografts, we assessed the influence of treatment with chloroquine on sensitivity to radiation in the 4T1 syngeneic murine breast tumor model. Complementary experiments were performed in cell culture evaluating the impact of chloroquine as well as genetic silencing of the autophagy regulatory gene, Atg12, on sensitivity to radiation in murine 4T1 breast tumor cells. Given that our studies, both in cell culture as well as in vivo, appeared to indicate that either pharmacologic or genetic interference with autophagy fails to confer radiation sensitization, it appeared to be relevant to review the literature relating to animal model systems combining chloroquine with chemotherapy and radiotherapy to evaluate the preclinical evidence that might support the ongoing clinical trials.
Materials and Methods
Cell Culture and Treatment.
4T1 Cells were obtained from American Type Culture Collection (Manassas, VA). 4T1-derived cell lines were grown from frozen stocks in basal RPMI 1640 supplemented with 5% fetal bovine serum, 5% bovine calf serum, 2 mmol/l l-glutamine, and penicillin/streptomycin (0.5 ml/100 ml medium). All cells were maintained at 37°C under a humidified, 5% CO2 atmosphere. Cells were routinely subcultured by trypsinization (0.25% trypsin, 0.03% EDTA; Gibco, Grand Island, NY) upon reaching confluence. All cell cultures were examined by microscope for bacterial and fungal contamination prior to experiments. Additionally, all cell lines were determined to be free of mycoplasma.
For in vitro radiation experiments, 4T1 cells were treated for 1 hour with chloroquine before radiation in an X-ray irradiator (RS2000; Rad Source Technologies, Suwannee, GA) at the indicated doses. After treatment, medium was changed and protein was collected 4 hours after treatment of Western blotting. For clonogenic assays, cells were plated in 12-well plates at a density of 300 cells per well, treated as described above, and allowed to recover for 7 days after treatment. Cells were then fixed and stained with crystal violet (Becton Dickinson, Franklin Lakes, NJ), the stain was solubilized with 30% acetic acid, and absorbance was measured at 540 nm. 4T1 Atg12 small-hairpin RNA (shRNA)-inducible cells were generated as described previously (Maycotte et al., 2012) . Briefly, 4T1 cells were transduced with lentiviruses containing a pTRIPZ nonsilencing shRNA or an Atg12 mouse shRNA cloned from a pGIPZ shRNAmir (V2LMM_72549) plasmid (Open Biosystems/Thermo Fisher Scientific Inc., Waltham, MA). Cells were grown in Dulbecco’s modified Eagle’s medium with 10% tetracycline-free fetal bovine serum (Hyclone/Thermo Scientific), selected with puromycin, and clones were isolated and validated for ATG12 knockdown. For shRNA induction, cells were treated with 1 μg/ml doxycycline (Clontech, Mountain View, CA) for 72 hours, replacing doxycycline every 24 hours. For radiation treatment, cells were plated in 12-well plates at a density of 200 cells per well, treated ± doxycycline for 72 hours, and irradiated at the indicated doses. After a 7-day recovery period, cells were fixed and clonogenic growth was evaluated as described above.
Western Blotting.
Cells were washed with phosphate-buffered saline and lysed with radioimmunoprecipitation assay buffer. Protein was quantitated using Bradford reagent (Bio-Rad, Hercules, CA). Twenty micrograms protein was loaded in a 10% SDS-PAGE, and polyvinylidene difluoride membranes (Millipore, Billerica, MA) were probed with anti-ATG12 (D88H11; Cell Signaling, Danvers, MA) or actin antibodies (A5441; Sigma-Aldrich, St. Louis, MO). For LC3 Western blots, 7.5 μg protein was used and probed with anti-LC3 antibody (NB100-2220; Novus Biologicals, Littleton, CO).
Animal Studies.
Female BALB/c mice, 8-weeks-old, were purchased from Jackson Laboratories (Bar Harbor, ME) and were maintained in groups of four per cage with access to food and water. The animals were acclimated at least 2 days before use and maintained throughout at standard conditions: 24 ± 2°C temperature; 50 ± 10% relative humidity. All studies involving mice were approved by the Animal Care and Use Committee at the University of Colorado School of Medicine.
Mice were challenged s.c. with 5 × 104 4T1 mouse breast tumor cells, transfected with luciferase, in the right flank on day 0. On day 3, animals were injected with 150 mg/kg luciferin and the presence of primary tumors was determined by bioluminescence signal as imaged using an IVIS 50 (Xenogen, Caliper Life Sciences, Hopkinton, MA). Of note, the bioluminescent signal could be detected in the animal before primary tumors were palpable and could be measured with a caliper. Tumor-bearing mice were randomly assigned into groups and were injected i.p. as indicated: (a) 25 mg/kg CQ (chloroquine), (b) 50 mg/kg CQ, (c) 100 mg/kg CQ, or (d) phosphate-buffered saline. The CQ doses chosen were based on the available literature, where studies have generally been performed using CQ at 50–60 mg/kg (Fu et al., 2009; Carew et al., 2010; Jiang et al., 2010; Wu et al., 2010; Ding et al., 2011; Lopez et al., 2011; Mirzoeva et al., 2011; Pan et al., 2011; Shi et al., 2011; Xu et al., 2011; Ghadimi et al., 2012; Godbole et al., 2012; Guo et al., 2012; Hu et al., 2012; Liang et al., 2012; Loehberg et al., 2012; Rao et al., 2012; Sasaki et al., 2012). Additional experiments were conducted where the mice were exposed to γ-irradiation using a 137Cs irradiator as indicated: (e) 5 Gy IR (ionizing radiation), (f) 10 Gy IR, (g) 15 Gy IR, (h) 10Gy IR + 14 days CQ i.p. Each group consisted of 6–8 mice. Irradiation was performed on day 3 and chloroquine (50 mg/kg) or saline treatment was initiated on day 3 and repeated every day for 14 days. Tumor growth was monitored multiple times per week by bioluminescence. Upon sacrifice, tumors were resected and weighed.
Statistical Analysis.
All of the data are represented as means ± S.E. Statistical differences were determined using StatView statistical software. Comparisons were made using a one-way analysis of variance followed by Tukey Kramer’s post-hoc test. For density analysis, mean comparison was done with a Student’s t test. P values of ≤0.05 were taken as indicating statistical significance.
Results
Studies in 4T1 Cells in Culture.
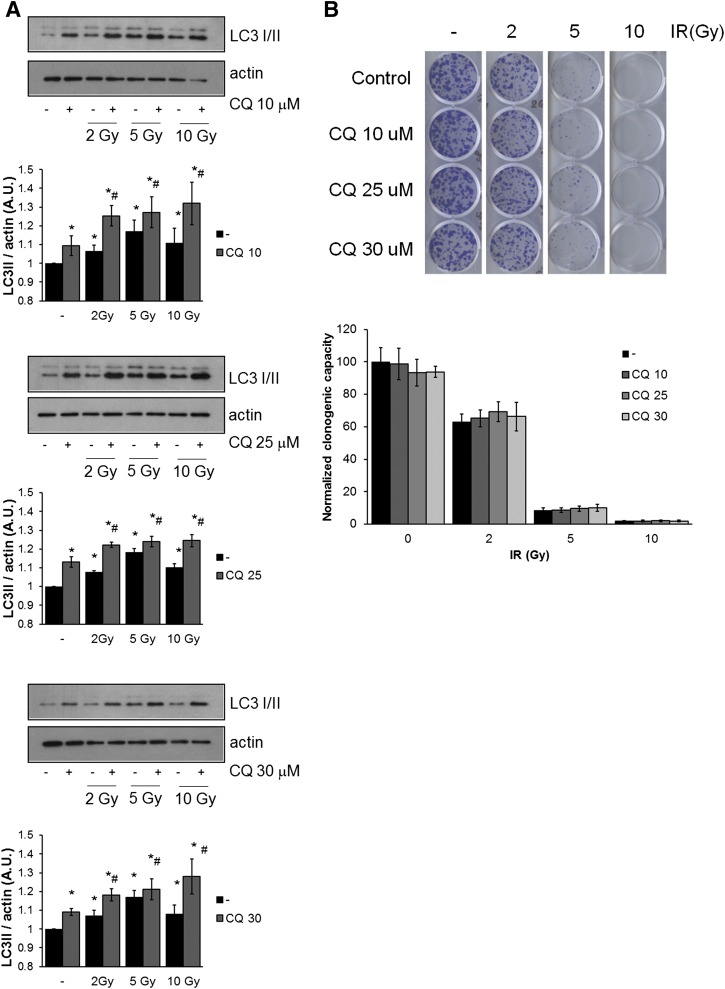
Extensive evidence in the literature supports a role for autophagy as a cytoprotective tumor survival pathway that is induced by exposure to chemotherapy or radiation (Paglin et al., 2001; Kanzawa et al., 2004; Boya et al., 2005; Kondo et al., 2005; Sotelo et al., 2006; Abedin et al., 2007; Amaravadi et al., 2007; Djavaheri-Mergny et al., 2007; Apel et al., 2008; Qadir et al., 2008; Wilson et al., 2011; Bristol et al., 2012). In the current work we treated 4T1 cells with radiation and different doses of chloroquine to block autophagy. Figure 1A shows induction of autophagy in 4T1 cells after radiation treatment (2, 5, and 10 Gy with and without exposure to chloroquine (10, 25, and 30 μM) when evaluated by Western blotting for LC3 (Microtubule-associated protein 1A/1B light chain 3), the mammalian homolog of ATG8. During autophagy, LC3 is cleaved and conjugated to phosphatidylethanolamine. This modified form, termed LC3II, is involved in the elongation of the autophagosome (Mizushima et al., 2010). However, LC3II is also degraded in the lysosome after autophagosome-lysosome fusion (Mizushima et al., 2010; Klionsky et al., 2012). Therefore, to measure autophagic flux, lysosomal inhibitors such as chloroquine are used to inhibit LC3II degradation, since in the absence of these inhibitors, an autophagy-inducing treatment can result in a modest increase or even a decrease in the amount of LC3II. The relevant parameter in this assay is the difference in the amount of LC3II in the presence and absence of lysosomal inhibitors (Klionsky et al., 2012). In Fig. 1A, chloroquine by itself increased LC3II due to inhibition of basal autophagy, and LC3II was increased when compared with untreated controls at the three different radiation doses. A greater increase in LC3II was evident in irradiated cells in the presence of chloroquine, indicative of autophagic flux induced by irradiation, which can be blocked by chloroquine treatment. Despite autophagy induction via irradiation, blocking autophagy with chloroquine did not result in a reduction in cell viability compared with radiation treatment alone when measured by clonogenic survival (Fig. 1B).
Fig. 1.
Chloroquine treatment does not affect clonogenic survival in 4T1 cells treated with radiation. 4T1 Cells were irradiated at 2, 5, or 10 Gy +/− CQ (10, 25, or 30 μM) and (A) evaluated for autophagy induction by Western blotting for LC3 or (B) allowed to recover for 7 days, when clonogenic growth was assessed. Graphs in (A) show density analysis of the mean ± S.E. of three independent experiments. *Different from untreated control; #different from control + CQ (P < 0.05). Graph in (B) shows quantification of clonogenic assays, where control was normalized to 100%. Graph shows mean ± S.E. of three independent experiments performed in triplicate. No statistical differences were found between untreated and CQ treated samples (P < 0.05).
Genetic inhibition of autophagy via silencing of Atg12 was performed to confirm the results of the experiments utilizing pharmacological inhibition. In a previous report, we developed 4T1 cells with an inducible Atg12 shRNA and complementary controls with nonsilencing shRNA (Maycotte et al., 2012). Figure 2 presents the results of studies assessing radiation sensitivity in these 4T1 cells by a clonogenic survival assay. Doxycycline treatment decreased ATG12 protein levels (Fig. 2A), with the consequent suppression of radiation-induced autophagy in 4T1 cells at all radiation doses used in this study (Fig. 2B). However, these experiments clearly demonstrate lack of sensitization by Atg12 silencing as the sensitivity to doses of 2, 5, and 10 Gy is essentially identical in the silenced and nonsilenced cells with and without doxycycline (Fig. 2C).
Fig. 2.
Atg12 knockdown does not affect clonogenic survival in 4T1 cells treated with radiation. (A) 4T1 cells expressing an Atg12 or nonsilencing shRNA were treated with doxycycline (doxy) at the indicated concentrations for 72 hours and tested for Atg12 knockdown. (B) Autophagic flux measured by LC3II Western blot was evaluated in Atg12 shRNA-expressing cells treated ± irradiation, 1 μM doxycycline, or 10 μM chloroquine. (C) Survival was assessed as clonogenic growth of cells treated ± doxycycline 1 μg/ml, exposed to radiation (Rad) and allowed to recover for 7 days. Graphs show mean ± S.E. of three independent experiments performed in triplicate. Graphs in (B) show density analysis of the mean ± S.E. of three independent experiments. *Different from untreated control; #different from same treatment without doxycycline (P < 0.05).
Studies of 4T1 Cells in an Animal Model.
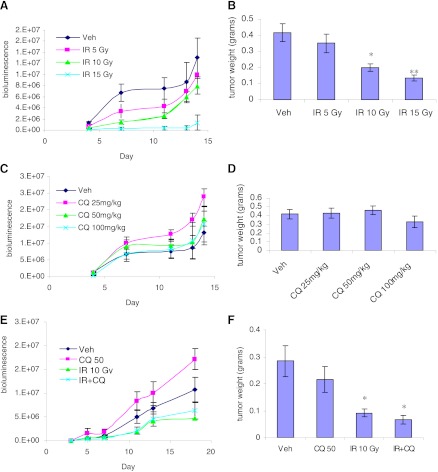
A quite extensive body of evidence has been generated in support of the presumption that chloroquine has the potential to sensitize tumor cells to radiation or chemotherapy, ostensibly via the inhibition of autophagy (Paglin et al., 2001; Kanzawa et al., 2004; Boya et al., 2005; Zhao et al., 2005; Amaravadi et al., 2007; Apel et al., 2008; Qadir et al., 2008; Livesey et al., 2009; Solomon and Lee, 2009; Ma et al., 2011; Wilson et al., 2011; Bristol et al., 2012). In conjunction with the genetic and pharmacological studies that were performed in cell culture to inhibit autophagy induced by radiation, experiments were designed to assess the impact of chloroquine on the response to radiation using the murine syngeneic 4T1 breast tumor cells in immunocompetent Balb/c mice. Varying doses of radiation alone (individual doses of 5, 10, and 15 Gy) as well as chloroquine alone (at concentrations of 25, 50, and 100 mg/kg) were monitored for effects on tumor volume by bioluminescence and final tumor weight. A radiation-dose–dependent decrease in tumor size was detected by bioluminescence measurement (Fig. 3A), which was supported by assessment of tumor weight at the termination of the study (Fig. 3B). Chloroquine alone had no impact on tumor weight (Fig. 3D); although the bioluminescence assay seemed to suggest acceleration of tumor growth, this was not significant for any dose of the drug (Fig. 3C). When 50 mg/kg chloroquine (the dose generally used in vivo) was combined with 10 Gy radiation, both the bioluminescence (Fig. 3E), and final tumor-weight determination (Fig. 3F) indicated that the impact of the combination treatment was no greater than for radiation alone. Of note, with all treatments, animal weights remained consistent throughout the study (not shown).
Fig. 3.
Influence of IR, CQ, or the combination on (A, C, and E) bioluminescence and (B, D, and F) final tumor weight. Animals were injected with luciferin, and bioluminescence was monitored at the indicated times (A-IR, C-CQ; E-CQ + IR). Animals were sacrificed and tumors were resected and weighed (B-IR; D-CQ; F-CQ + IR). *P < 0.05; **P < 0.001.
Discussion
Studies in the Literature Relating to Sensitization by Chloroquine.
Multiple studies have been published indicating that genetic or pharmacological interference with autophagy can enhance the response to radiation (for example, Gaudin and Yielding, 1969; Pazmino and Yuhas, 1974; Zhao et al., 2005; Apel et al., 2008; Livesey et al., 2009; Solomon and Lee, 2009; Jiang et al., 2010; Chaachouay et al., 2011; Bristol et al., 2012; Lim et al., 2012; Wilson et al., 2011). In contrast, our studies indicate that 4T1 cells could not be sensitized to radiation in vitro by pharmacological inhibition of autophagy with chloroquine treatment, by silencing of the autophagy-related gene Atg12, or by chloroquine in vivo. These findings stand in dramatic contrast to much of the previous literature cited above as well as findings from our own laboratories demonstrating that ionizing radiation promotes a cytoprotective form of autophagy in MCF-7 and ZR-75-1 breast tumor cells that can be inhibited by drugs such as chloroquine or by the silencing of autophagy-regulatory genes, with the consequent enhancement of radiation sensitivity (Wilson et al., 2011; Bristol et al., 2012).
These findings raise the compelling question as to whether the efforts to use autophagy inhibition as a clinical strategy for chemosensitization and radiosensitization might be somewhat premature in the absence of sufficient and rigorous supporting preclinical data. In reviewing the available literature, there are clearly studies in which the potential of chloroquine was realized in terms of the impact on tumor growth; however, in a number of studies, only very modest effects are evident in animal models assessing chemosensitization by chloroquine. Furthermore, few studies have demonstrated prolongation of survival in tumor-bearing animals. A review of the relevant literature is presented below.
In recent studies by Ghadimi et al. (2012), chloroquine clearly enhanced sensitivity to a dual PI3K/mTOR inhibitor in a xenograft model of malignant peripheral nerve sheath tumors. In contrast, Mirzoeva et al. (2011) demonstrated minimal sensitization to an agent with a similar mechanism of action in a xenograft model of pancreatic cancer. These differences could be related to the tumor models used, as Lopez et al. (2011) reported quite pronounced sensitization to a histone deacetylase inhibitor in studies that were also performed using malignant peripheral nerve sheath tumors; in this latter report, the combination with chloroquine also reduced lung tumor metastases.
With regard to the combination of chloroquine with some of the more conventional antitumor drugs, Pan et al. (2011) showed potentiation of the effectiveness of both doxorubicin and melphalan in a xenograft model of multiple myeloma. In contrast, quite modest and limited effects were evident in models of hepatocellular carcinoma utilizing oxaliplatin (Ding et al., 2011), sorafenib (Shi et al., 2011), or cisplatin, although the combination with 5-fluorouracil did appear to be quite effective (Guo et al., 2012). Again, one might ascribe these diverse findings to the generally high degree of resistance to therapy in hepatocellular carcinoma, which may not be susceptible to sensitization solely through the suppression of autophagy; that is, drug resistance could arise, in part, from drug degradation by hepatic enzymes.
With regard to a number of other agents of varying mechanisms of action, enhanced effectiveness of a Src family kinase inhibitor was observed when combined with chloroquine in a xenograft model of prostate cancer (Wu et al., 2010), to interleukin-2 in a model of murine colorectal carcinoma (Liang et al., 2012), and to bevacizumab in glioblastoma (Hu et al., 2012); the latter two findings are of particular interest and relevance and will be addressed in more detail below. Conversely, only quite modest improvement in response to chemotherapeutic agents was detected for chloroquine in combination with an mTOR inhibitor in MCF-7 xenografts (Loehberg et al., 2012), with voranistat in colon carcinoma (Carew et al., 2010), with perifosine in lung cancer xenografts (Fu et al., 2009), with a dual phosphatidylinositide 3-kinase/protein kinase B/mTOR inhibitor in non-small cell lung cancer xenografts (Xu et al., 2011), with a retinoic acid metabolism blocker in breast tumor cells (Godbole et al., 2012), and with panobinostat in breast cancer (Rao et al., 2012); in the latter studies, the lack of impact could be ascribed to the use of an unusually low dose of chloroquine of 10 mg/kg, which was apparently necessitated by the high degree of toxicity that was observed with chloroquine alone in this work.
A particularly relevant issue is that it is frequently unclear whether chloroquine has achieved levels in the tumor cells that are likely to be therapeutically effective in inhibiting autophagy. Although many investigators assess the promotion of apoptosis in the tumor cells, suppression of autophagy has not been uniformly tested in all of the cited studies, which may relate in large part to the lack of uniformly accepted protocols for monitoring autophagy suppression in vivo.
The studies by Liang et al. are noteworthy in being virtually the only ones that demonstrated a clear increase in long-term survival for the combination treatment with chloroquine (Liang et al., 2012). It is possible that this may be related to the experiments having been performed in a syngeneic, immune-competent system. However, the use of immune-competent animals was necessitated by the fact that these studies involved interleukin-2, a component of the immune system; therefore, at this juncture it cannot be convincingly argued that immune-competent animals would necessarily prove to be more predictive of the impact of autophagy inhibition on chemotherapeutic sensitivity than xenograft models. In fact, the studies by Hu et al. (2012), where genetic inhibition of autophagy demonstrated marked effects on tumor growth delay as well as survival of the tumor-bearing animals, were performed in xenograft models.
With respect to the studies of Hu et al. (2012) combining chloroquine with bevacizumab in glioblastoma, while an increase in survival of the tumor-bearing animals is evident, it is critical to note that this occurred only when the implanted tumor cells had been genetically engineered for silencing of ATG7; surprisingly, no survival studies were presented for the combination treatment with chloroquine, raising the possibility that chloroquine might have failed to achieve the desired outcome in this experimental system. As with virtually all of the data reported in the literature, the outcome of the combination treatment with chloroquine was essentially one of tumor growth delay without any substantive decline in tumor size over time. In contrast, when utilizing tumor xenografts with silencing of ATG7, Hu et al. (2012) provided clear evidence of tumor-cell killing (a reduction in tumor size over time), which may account for the observed increase in survival of the tumor-bearing animals.
In comparing the pharmacological experiments with the genetic silencing studies in the animal models, it can be argued that the studies by Hu et al. (2012) do in fact appear to provide proof of principle for autophagy inhibition as a potentially effective therapeutic strategy. However, it can also be concluded that chloroquine is unlikely to be uniformly effective in suppressing autophagy in tumors in vivo. Interestingly, although there is fairly extensive literature on the pharmacokinetics of chloroquine in humans, there is relatively limited information from animal studies. Pharmacokinetic studies of chloroquine in both animal models and in human subjects indicate that chloroquine has quite a long half-life (Mzayek et al., 2007; Lim et al., 2009; Karunajeewa et al., 2010; Moore et al., 2011), indicating that drug clearance is unlikely to be a limiting factor in the potential capacity of chloroquine to suppress of autophagy. However, the maximal concentrations of chloroquine achieved in the plasma fall only within the range of 1.5 and 3 μM, even with daily administration of the drug, concentrations which are unlikely to be sufficient to effectively interfere with autophagy (based on the concentrations required for autophagy inhibition in cell culture). It should however be noted that chloroquine accumulates in the lysosomes within cells and plasma levels may therefore not accurately reflect the levels in tumor tissues, especially when chloroquine treatment occurs over a long period of time. Overall, the variable effectiveness of chloroquine in enhancing sensitivity to chemotherapy and radiation in animal studies may relate to differences in the capacity of tumor models to accumulate chloroquine as well as differential sensitivity of tumors to autophagy inhibition by chloroquine. Consequently, a more potent autophagy inhibitor that might be developed for clinical use could actually prove to be quite effective as an adjunctive therapy.
Finally, although chloroquine is perhaps the most widely used drug to inhibit autophagy in vitro, and its effects on cell death have been directly attributed to its inhibition of autophagy, the mechanism by which chloroquine induces cell death could involve other functions independent of its ability to block autophagy that are less well characterized. Among these, chloroquine can decrease sequestration of anticancer drugs such as doxorubicin and mitoxantrone in endosomes by raising endosomal pH, thereby increasing their availability and cytotoxicity (Solomon and Lee, 2009). It can also promote cell cycle arrest (Jiang et al., 2010), intercalate into DNA (Solomon and Lee, 2009), activate ataxia telangiectasia mutated and p53 pathways and induce apoptosis (Bakkenist and Kastan, 2003). These actions are likely to confuse interpretation of studies in the literature where different outcomes are observed for the combination treatment of chloroquine with chemotherapeutic drugs or radiation.
Relevance of the Current Experimental Findings.
We observed that pharmacological inhibition of autophagy with chloroquine and genetic silencing of the autophagy gene Atg12 failed to influence radiation sensitivity in 4T1 breast tumor cells in vitro. In complementary experiments, the autophagy inhibitor, chloroquine, failed to influence sensitivity to radiation in 4T1 cells grown in a syngeneic animal model of breast cancer. The lack of radiation sensitization in vitro complements earlier studies where chloroquine failed to sensitize tumor cells to cisplatin (Maycotte et al., 2012), a chemotherapeutic agent that, like radiation, has DNA as its primary target. The lack of radiosensitization in vivo may be due to additional factors such as the fact that 4T1 cells proliferate rapidly and palpable tumors quickly become necrotic and ulcerated, preventing long-term observational studies. In one similar study in which the effect of chloroquine on radiation sensitivity was investigated in 4T1 cells (Jiang et al., 2010), and where the animals were injected with twice the number of cells as in the current work, no impact of chloroquine treatment on radiation-inhibited tumor growth was detected until 37 days post-challenge. This may relate to the development of hypoxia, as proposed by Rouschop et al. (2010) and that is further discussed below.
Studies of radiation sensitization by chloroquine that were performed in HCT-116 colon carcinoma and U373 glioblastoma cells (Rouschop et al., 2010) provide unequivocal evidence of tumor growth delay by the combination treatment, which is ascribed to a reduction of hypoxia-induced autophagy by chloroquine. Of note, the growth curves for radiation alone and for chloroquine plus radiation did not begin to diverge until after 14 days, which presumably reflects the time required for the tumors to become hypoxic. This may explain, in part, the lack of sensitization to radiation in our own animal studies, where the experiments were necessarily terminated after 15–18 days due to animal health and safety concerns.
It is further of interest that in the studies by Rouschop et al. (2010) sensitization to radiation did not occur in cell culture under either normoxic or hypoxic conditions, which is consistent with our findings in 4T1 cells. We have also observed that neither chloroquine nor 3-methyladenine conferred radiation sensitization in Hs578t breast tumor cells (unpublished observations). Taken together, these studies argue that radiation does not uniformly promote a cytoprotective form of autophagy that can be exploited for the purpose of tumor cell sensitization.
Conclusions
It is worth considering that studies in xenograft models alone may be insufficient for accurately determining the capacity of autophagy inhibition to sensitize tumors to chemotherapy or radiation, as a recent elegant study has shown that therapeutic success may be highly dependent on the immune system. In this work, anthracyclines and oxaliplatin did not alter the proliferation of tumors growing in athymic mice but only in immune-competent animals; furthermore, autophagy was determined to be essential for the release of immunogenic ATP from dying cells (Michaud et al., 2011; Martins et al., 2012); these findings indicate that syngeneic models of cancer and xenografts are likely to demonstrate uniquely different responses to therapeutic approaches involving autophagy induction or inhibition.
In additional preliminary studies in both non-small cell lung cancer cell lines and head and neck cancer cell lines, we have observed that autophagy inhibition by chloroquine does not consistently enhance radiation sensitivity (unpublished observations). Taken together with the studies in animal models discussed in this review, it can be predicted that autophagy inhibition is unlikely to be uniformly effective in increasing radiation or chemotherapeutic sensitivity across the tumor spectrum. However, this is not unusual or unexpected given that the sensitivities of malignancies of different origins to chemotherapeutic approaches can differ by orders of magnitude.
It is possible, but as yet untested, that the strength of autophagy induction in each system might determine its susceptibility to autophagy inhibition and sensitization. However, as autophagy is a dynamic process and as autophagy-related proteins are expressed and regulated differently among tissues (Klionsky et al., 2012), it is currently quite difficult to quantify and compare the degree of induction caused by different drugs, especially in vivo.
Accepting the premise that autophagy inhibition can potentially serve to promote chemosensitization and radiosensitization, it follows that it will be critical to identify biomarkers that will distinguish between malignancies that are likely to benefit from autophagy inhibition as a strategy for chemosensitization or radiation sensitization and those tumors which are unlikely to benefit from this therapeutic approach. Furthermore, it will be necessary to establish that the drug(s) used to modulate autophagy are capable of achieving and maintaining concentrations in the tumor cells that will suppress autophagy, possibly throughout the course of treatment. However, in considering the variability and uncertainty in the current literature relating to autophagy inhibition as a therapeutic strategy, caution appears to be warranted in extrapolating to the clinic from the limited preclinical data in animal models of cancer that have combined chloroquine with chemotherapy or radiotherapy.
Acknowledgments
The parental 4T1 cells were generously provided by Dr. Fred Miller at the Karmanos Cancer Institute at Wayne State University School of Medicine. The 4T1-luc cells were generously provided by Dr. Kazuaki Takabe at Virginia Commonwealth University.
Abbreviations
- CQ
chloroquine
- IR
ionizing radiation
- mTOR
mammalian target of rapamycin
- shRNA
small-hairpin RNA
Authorship Contributions
Participated in research design: Bristol, Emery, Gewirtz, Maycotte, Chakradeo.
Conducted experiments: Bristol, Emery, Maycotte, Chakradeo.
Wrote or contributed to the writing of the manuscript: Bristol, Emery, Maycotte, Thorburn, Gewirtz.
Footnotes
This work was supported in part by the National Institutes of Health [Grant 5R01CA135043]; by the Department of Defense [Grants W81XWH-09-1-0020 and W81XWH-11-1-016] (to M.L.B. and S.M.E., respectively); and by the National Institutes of Health [Grants R01CA150925 and R01CA111421] (to A.T.).
References
- Abedin MJ, Wang D, McDonnell MA, Lehmann U, Kelekar A. (2007) Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ 14:500–510 [DOI] [PubMed] [Google Scholar]
- Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB. (2007) Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest 117:326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. (2008) Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res 68:1485–1494 [DOI] [PubMed] [Google Scholar]
- Bakkenist CJ, Kastan MB. (2003) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421:499–506 [DOI] [PubMed] [Google Scholar]
- Boya P, González-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Métivier D, Meley D, Souquere S, Yoshimori T, et al. (2005) Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 25:1025–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol ML, Di X, Beckman MJ, Wilson EN, Henderson SC, Maiti A, Fan Z, Gewirtz DA. (2012) Dual functions of autophagy in the response of breast tumor cells to radiation: Cytoprotective autophagy with radiation alone and cytotoxic autophagy in radiosensitization by vitamin D 3. Autophagy 8:739–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew JS, Medina EC, Esquivel JA, 2nd, Mahalingam D, Swords R, Kelly K, Zhang H, Huang P, Mita AC, Mita MM, et al. (2010) Autophagy inhibition enhances vorinostat-induced apoptosis via ubiquitinated protein accumulation. J Cell Mol Med 14:2448–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaachouay H, Ohneseit P, Toulany M, Kehlbach R, Multhoff G, Rodemann HP. (2011) Autophagy contributes to resistance of tumor cells to ionizing radiation. Radiother Oncol 99:287–292 [DOI] [PubMed] [Google Scholar]
- Ding ZB, Hui B, Shi YH, Zhou J, Peng YF, Gu CY, Yang H, Shi GM, Ke AW, Wang XY, et al. (2011) Autophagy activation in hepatocellular carcinoma contributes to the tolerance of oxaliplatin via reactive oxygen species modulation. Clin Cancer Res 17:6229–6238 [DOI] [PubMed] [Google Scholar]
- Djavaheri-Mergny M, Botti J, Codogno P. (2007) Autophagy and autophagic cell death, in Apoptosis, Senescence, and Cancer (Gewirtz DA, Holt SE, Grant S eds), pp 93–107, Springer, New York. [Google Scholar]
- Fu L, Kim YA, Wang X, Wu X, Yue P, Lonial S, Khuri FR, Sun SY. (2009) Perifosine inhibits mammalian target of rapamycin signaling through facilitating degradation of major components in the mTOR axis and induces autophagy. Cancer Res 69:8967–8976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin D, Yielding KL. (1969) Response of a “resistant” plasmacytoma to alkylating agents and x-ray in combination with the “excision” repair inhibitors caffeine and chloroquine. Proc Soc Exp Biol Med 131:1413–1416 [DOI] [PubMed] [Google Scholar]
- Ghadimi MP, Lopez G, Torres KE, Belousov R, Young ED, Liu J, Brewer KJ, Hoffman A, Lusby K, Lazar AJ, et al. (2012) Targeting the PI3K/mTOR axis, alone and in combination with autophagy blockade, for the treatment of malignant peripheral nerve sheath tumors. Mol Cancer Ther 11:1758–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbole AM, Purushottamachar P, Martin MS, Daskalakis C, Njar VC. (2012) Autophagy inhibition synergistically enhances anticancer efficacy of RAMBA, VN/12-1 in SKBR-3 cells, and tumor xenografts. Mol Cancer Ther 11:898–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden EB, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC. (2012) The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol 2:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo XL, Li D, Hu F, Song JR, Zhang SS, Deng WJ, Sun K, Zhao QD, Xie XQ, Song YJ, et al. (2012) Targeting autophagy potentiates chemotherapy-induced apoptosis and proliferation inhibition in hepatocarcinoma cells. Cancer Lett 320:171–179 [DOI] [PubMed] [Google Scholar]
- Hu YL, DeLay M, Jahangiri A, Molinaro AM, Rose SD, Carbonell WS, Aghi MK. (2012) Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res 72:1773–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang PD, Zhao YL, Deng XQ, Mao YQ, Shi W, Tang QQ, Li ZG, Zheng YZ, Yang SY, Wei YQ. (2010) Antitumor and antimetastatic activities of chloroquine diphosphate in a murine model of breast cancer. Biomed Pharmacother 64:609–614 [DOI] [PubMed] [Google Scholar]
- Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. (2004) Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ 11:448–457 [DOI] [PubMed] [Google Scholar]
- Karunajeewa HA, Salman S, Mueller I, Baiwog F, Gomorrai S, Law I, Page-Sharp M, Rogerson S, Siba P, Ilett KF, et al. (2010) Pharmacokinetics of chloroquine and monodesethylchloroquine in pregnancy. Antimicrob Agents Chemother 54:1186–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, et al. (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8:445–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Kanzawa T, Sawaya R, Kondo S. (2005) The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 5:726–734 [DOI] [PubMed] [Google Scholar]
- Liang X, De Vera ME, Buchser WJ, Romo de Vivar Chavez A, Loughran P, Beer Stolz D, Basse P, Wang T, Van Houten B, Zeh HJ, 3rd, et al. (2012) Inhibiting systemic autophagy during interleukin 2 immunotherapy promotes long-term tumor regression. Cancer Res 72:2791–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HS, Im JS, Cho JY, Bae KS, Klein TA, Yeom JS, Kim TS, Choi JS, Jang IJ, Park JW. (2009) Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by Plasmodium vivax. Antimicrob Agents Chemother 53:1468–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y, Hedayati M, Merchant AA, Zhang Y, Yu HH, Kastan MB, Matsui W, Deweese TL. (2012) Chloroquine improves survival and hematopoietic recovery after lethal low-dose-rate radiation. Int J Radiat Oncol Biol Phys 84:800–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey KM, Tang D, Zeh HJ, Lotze MT. (2009) Autophagy inhibition in combination cancer treatment. Curr Opin Investig Drugs 10:1269–1279 [PubMed] [Google Scholar]
- Loehberg CR, Strissel PL, Dittrich R, Strick R, Dittmer J, Dittmer A, Fabry B, Kalender WA, Koch T, Wachter DL, et al. (2012) Akt and p53 are potential mediators of reduced mammary tumor growth by cloroquine and the mTOR inhibitor RAD001. Biochem Pharmacol 83:480–488 [DOI] [PubMed] [Google Scholar]
- Lopez G, Torres K, Lev D. (2011) Autophagy blockade enhances HDAC inhibitors’ pro-apoptotic effects: potential implications for the treatment of a therapeutic-resistant malignancy. Autophagy 7:440–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XH, Piao S, Wang D, McAfee QW, Nathanson KL, Lum JJ, Li LZ, Amaravadi RK. (2011) Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clin Cancer Res 17:3478–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins I, Michaud M, Sukkurwala AQ, Adjemian S, Ma Y, Shen S, Kepp O, Menger L, Vacchelli E, Galluzzi L, et al. (2012) Premortem autophagy determines the immunogenicity of chemotherapy-induced cancer cell death. Autophagy 8:413–415 [DOI] [PubMed] [Google Scholar]
- Maycotte P, Aryal S, Cummings CT, Thorburn J, Morgan MJ, Thorburn A. (2012) Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy 8:200–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al. (2011) Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334:1573–1577 [DOI] [PubMed] [Google Scholar]
- Mirzoeva OK, Hann B, Hom YK, Debnath J, Aftab D, Shokat K, Korn WM. (2011) Autophagy suppression promotes apoptotic cell death in response to inhibition of the PI3K-mTor pathway in pancreatic adenocarcinoma. J Mol Med (Berl) 89:877–889 [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. (2010) Methods in mammalian autophagy research. Cell 140:313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BR, Page-Sharp M, Stoney JR, Ilett KF, Jago JD, Batty KT. (2011) Pharmacokinetics, pharmacodynamics, and allometric scaling of chloroquine in a murine malaria model. Antimicrob Agents Chemother 55:3899–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mzayek F, Deng H, Mather FJ, Wasilevich EC, Liu H, Hadi CM, Chansolme DH, Murphy HA, Melek BH, Tenaglia AN, et al. (2007) Randomized dose-ranging controlled trial of AQ-13, a candidate antimalarial, and chloroquine in healthy volunteers. PLoS Clin Trials 2:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J. (2001) A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res 61:439–444 [PubMed] [Google Scholar]
- Pan Y, Gao Y, Chen L, Gao G, Dong H, Yang Y, Dong B, Chen X. (2011) Targeting autophagy augments in vitro and in vivo antimyeloma activity of DNA-damaging chemotherapy. Clin Cancer Res 17:3248–3258 [DOI] [PubMed] [Google Scholar]
- Pazmiño NH, Yuhas JM. (1974) Chloroquine: nonselective inhibition of recovery from radiation injury in tumors and normal tissues. Radiat Res 60:54–61 [PubMed] [Google Scholar]
- Qadir MA, Kwok B, Dragowska WH, To KH, Le D, Bally MB, Gorski SM. (2008) Macroautophagy inhibition sensitizes tamoxifen-resistant breast cancer cells and enhances mitochondrial depolarization. Breast Cancer Res Treat 112:389–403 [DOI] [PubMed] [Google Scholar]
- Rao R, Balusu R, Fiskus W, Mudunuru U, Venkannagari S, Chauhan L, Smith JE, Hembruff SL, Ha K, Atadja P, et al. (2012) Combination of pan-histone deacetylase inhibitor and autophagy inhibitor exerts superior efficacy against triple-negative human breast cancer cells. Mol Cancer Ther 11:973–983 [DOI] [PubMed] [Google Scholar]
- Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, et al. (2010) The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest 120:127–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Tsuno NH, Sunami E, Kawai K, Hongo K, Hiyoshi M, Kaneko M, Murono K, Tada N, Nirei T, et al. (2012) Resistance of colon cancer to 5-fluorouracil may be overcome by combination with chloroquine, an in vivo study. Anticancer Drugs 23:675–682 [DOI] [PubMed] [Google Scholar]
- Shi YH, Ding ZB, Zhou J, Hui B, Shi GM, Ke AW, Wang XY, Dai Z, Peng YF, Gu CY, et al. (2011) Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy 7:1159–1172 [DOI] [PubMed] [Google Scholar]
- Solomon VR, Lee H. (2009) Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol 625:220–233 [DOI] [PubMed] [Google Scholar]
- Sotelo J, Briceño E, López-González MA. (2006) Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 144:337–343 [DOI] [PubMed] [Google Scholar]
- Wilson EN, Bristol ML, Di X, Maltese WA, Koterba K, Beckman MJ, Gewirtz DA. (2011) A switch between cytoprotective and cytotoxic autophagy in the radiosensitization of breast tumor cells by chloroquine and vitamin D. Horm Cancer 2:272–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Chang PC, Yang JC, Chu CY, Wang LY, Chen NT, Ma AH, Desai SJ, Lo SH, Evans CP, et al. (2010) Autophagy blockade sensitizes prostate cancer cells towards Src family kinase inhibitors. Genes Cancer 1:40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CX, Zhao L, Yue P, Fang G, Tao H, Owonikoko TK, Ramalingam SS, Khuri FR, Sun SY. (2011) Augmentation of NVP-BEZ235’s anticancer activity against human lung cancer cells by blockage of autophagy. Cancer Biol Ther 12:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Cai Y, Santi S, Lafrenie R, Lee H. (2005) Chloroquine-mediated radiosensitization is due to the destabilization of the lysosomal membrane and subsequent induction of cell death by necrosis. Radiat Res 164:250–257 [DOI] [PubMed] [Google Scholar]