Abstract
Cannabinoid receptor 1 (CB1) inverse agonists (e.g., rimonabant) have been reported to produce adverse effects including nausea, emesis, and anhedonia that limit their clinical applications. Recent laboratory studies suggest that the effects of CB1 neutral antagonists differ from those of such inverse agonists, raising the possibility of improved clinical utility. However, little is known regarding the antagonist properties of neutral antagonists. In the present studies, the CB1 inverse agonist SR141716A (rimonabant) and the CB1 neutral antagonist AM4113 were compared for their ability to modify CB1 receptor–mediated discriminative stimulus effects in nonhuman primates trained to discriminate the novel CB1 full agonist AM4054. Results indicate that AM4054 serves as an effective CB1 discriminative stimulus, with an onset and time course of action comparable with that of the CB1 agonist Δ9-tetrahydrocannabinol, and that the inverse agonist rimonabant and the neutral antagonist AM4113 produce dose-related rightward shifts in the AM4054 dose-effect curve, indicating that both drugs surmountably antagonize the discriminative stimulus effects of AM4054. Schild analyses further show that rimonabant and AM4113 produce highly similar antagonist effects, as evident in comparable pA2 values (6.9). Taken together with previous studies, the present data suggest that the improved safety profile suggested for CB1 neutral antagonists over inverse agonists is not accompanied by a loss of antagonist action at CB1 receptors.
Introduction
The cannabinoid receptor 1 (CB1) inverse agonist SR141716A (rimonabant) has antagonist actions that have been valuable for pharmacologically assessing the role of CB1 receptors in the in vitro and in vivo effects of Δ9-tetrahydrocannabinol (Δ9-THC) as well as other cannabinergic ligands including synthetic cannabinoids (e.g., CP 55,940, WIN 55,212-2) and the endogenous ligands anandamide and 2-arachidonoylglycerol (Nakamura-Palacios et al., 1999; Pertwee, 2005). From a clinical perspective, rimonabant has also been shown to have beneficial effects in the management of obesity and smoking cessation, presumably as a result of its antagonist actions (Pacher et al., 2006; Padwal and Majumdar, 2007; Le Foll et al., 2008; Rigotti et al., 2009). Unfortunately, numerous reports describing gastrointestinal side effects such as nausea and emesis as well as mood-depressant actions followed the introduction of rimonabant, hampering its utility and eventually resulting in its removal from clinical practice (Després et al., 2005; Van Gaal et al., 2005; Traynor, 2007). The adverse effects reported, such as nausea and/or emesis and anhedonia or depression-related effects, are opposite to effects in humans commonly attributed to CB1 agonists and, moreover, are not unique to rimonabant. Evidence that other CB1 inverse agonists such as AM251 or taranabant have rimonabant-like profiles of action, including potential adverse effects, have similarly precluded their clinical application (Pertwee, 2005; Addy et al., 2008; Aronne et al., 2010; Proietto et al., 2010).
Although the cause of the above-mentioned adverse effects of rimonabant and other CB1 inverse agonists remains unknown, one possibility that has received some attention is that they result from inverse agonist actions at CB1 receptors, as reviewed by Ward and Raffa (2011), Kirilly et al. (2012), and McLaughlin (2012). According to this idea, similar adverse effects might not be observed with CB1 antagonists lacking inverse agonist properties. In this regard, recent data suggest that newly developed CB1 neutral antagonists, in contrast to CB1 inverse agonists, may not have rimonabant-like effects in laboratory studies. For example, CB1 inverse agonists like rimonabant reduce food intake and body weight but also produce nausea-related effects—gaping in rats or vomiting in ferrets—and prodepressant activity in a modified forced swim test. However, the peripherally restricted CB1 neutral antagonist AM6545 has been shown to reduce food intake and body weight without inducing nausea (or gaping) in rats (Cluny et al., 2010). Likewise, the centrally acting CB1 neutral antagonist AM4113 has been shown to reduce food intake in rats (Cluny et al., 2011) without causing nausea/gaping in rats (Salamone et al., 2007; Sink et al., 2008), vomiting in ferrets (Chambers et al., 2007; Salamone et al., 2007), or prodepressant effects in the rat forced swim test (Jutkiewicz et al., 2010). On the basis of such observations, neutral CB1 antagonists have been forwarded as a promising avenue of drug development that provide clinical benefits like those of rimonabant but, potentially, without its liability for adverse gastrointestinal and/or mood-altering effects (Meye et al., 2012).
The ability of the inverse agonist rimonabant to dose-dependently antagonize the behavioral effects, including discriminative stimulus effects, of CB1 agonists has been well documented in rodents and nonhuman primates (Wiley et al., 1995; Compton et al., 1996; Järbe et al., 2001; McMahon et al., 2005). However, comparable information is not available for CB1 neutral antagonists, and it is unknown whether the two types of CB1 ligands are similarly effective as antagonists. Consequently, the present studies were conducted to directly compare the antagonist properties of the CB1 inverse agonist rimonabant and the CB1 neutral antagonist AM4113 in drug discrimination studies in nonhuman primates. In these studies, subjects were trained to discriminate the novel CB1 agonist AM4054 (Desai et al., 2012; G.A. Thakur et al., submitted manuscript) from saline, and several doses of each antagonist were evaluated to permit Schild analysis of their antagonist properties. Schild analysis using data from behavioral studies previously has proven useful for revealing similarities in the receptor-mediated mechanisms of agonist and antagonist action (Dykstra et al., 1988; Woods et al., 1992; Paronis and Bergman, 1999; McMahon, 2006a). AM4054, like Δ9-THC, is a cannabinoid with <3-fold CB1/CB2 selectivity but with high affinity for the CB1 receptor (Ki = 4.9 ± 1.8 nM). Unlike Δ9-THC, which has partial agonist actions in stimulating guanosine 5′-3-O-(thio)triphosphate binding or inhibiting adenylyl cyclase activity, however, AM4054 is a cannabinoid that is characterized as a CB1 full agonist in functional assays measuring the decrease in forskolin-stimulated cAMP as well as the efficacy of translocation after exposure to CB1 agonists in U2OS cell lines—indicated by the ability to form membrane or cytosolic clusters of cannabinoid receptor complexes in CB1-E/β-arrestin-green fluorescent protein (Sim et al., 1996; Breivogel and Childers, 2000; G.A. Thakur et al., submitted manuscript). Inasmuch as this is the first report of the discriminative stimulus effects of AM4054, pharmacological studies were conducted to examine its potency by i.m. and i.v. routes of administration, its time course of action, and generalization to other CB1 and non-CB1 drugs.
Materials and Methods
Subjects
Nine adult male squirrel monkeys (Saimiri sciureus) were individually housed in a temperature- and humidity-controlled vivarium with a 12-hour light/dark cycle (7 AM–7 PM). Subjects had unlimited access to water in the home cage and were maintained at approximate free-feeding weights by postsession access to a nutritionally balanced diet of high-protein banana-flavored biscuits (Purina Monkey Chow, St. Louis, MO) supplemented daily with fresh fruit. Five subjects (31, 101, 103, 115, and 140) were drug-naïve prior to this study, whereas the remaining subjects (36, 83, 134, and 136) previously served in studies of behaviorally active drugs (e.g., dopamine agonists and antagonists, opioids) but had not received drug treatments for at least 2 months prior to the present studies. Experimental sessions were conducted 5 days a week (Monday–Friday). The experimental protocol for the present studies was approved by the Institutional Animal Care and Use Committee at McLean Hospital. Subjects were maintained in a facility licensed by the U.S. Department of Agriculture and in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003).
Apparatus
During experimental sessions, subjects were seated in a Plexiglas chair (Spealman et al., 1977) within a ventilated sound- and light-attenuating enclosure. The front panel of the chair was outfitted with two response levers that were positioned 6 cm left and right of center. Each lever-press with a force of at least 0.25 N closed a microswitch, produced an audible relay click, and was recorded as a response. Red stimulus lights were mounted behind the front panel of the chair, and were positioned 10 cm above each response lever. Before each session, a shaved portion of each subject’s tail was coated with electrode paste and placed under brass electrodes for the delivery of brief, low-intensity current (see below). Experimental events and data collection were controlled by Med Associates (St. Albans, VT) interfacing equipment and operating software.
Behavioral Procedure
CB1 Discrimination.
Subjects initially were trained to terminate visual stimuli associated with the delivery of a brief, low-intensity current (200 ms; 3 mA) across the electrodes and, subsequently, to identify injection of the cannabinoid CB1 agonist AM4054 (0.01 mg/kg i.m.) in a two-lever drug discrimination procedure. The two levers were designated as the drug (AM4054) and saline levers, with assignment counterbalanced across subjects but remaining the same for a subject throughout the study. AM4054 or saline was administered via intramuscular injection 50 minutes prior to all training sessions. Each training session began with a 10-minute timeout period during which all lights were extinguished and responding had no programmed consequences. After the timeout period, two red stimulus lights above each lever were illuminated and completion of 10 consecutive responses [fixed ratio (FR) 10] on the injection-associated (correct) lever extinguished all stimulus lights and initiated a 50-second timeout. Responses on the other (incorrect) lever reset the FR requirement. Current delivery was scheduled for delivery every 10 seconds until either the FR 10 was completed on the correct lever or 30 seconds elapsed, whichever came first. Sessions ended upon completion of 20 trials. A double-alternation injection schedule of drug-drug-saline-saline across training sessions was utilized throughout training, with a third drug or saline session programmed intermittently to avoid associations based on the regularity of the double-alternation schedule.
Drug Testing.
After initial training, each of five subjects (31, 101, 103, 115, and 140) was prepared with an intravenous catheter for i.v. drug delivery, using procedures initially described by Herd et al. (1969). Briefly, under isoflurane anesthesia and in aseptic conditions, one end of a hydrophilically coated polyurethane catheter (inside diameter, 0.635 mm; outside diameter, 1.2 mm) was inserted into a femoral vein and the other end was connected to a subdermal vascular access port (Access Technologies, Skokie, IL) placed in the subject’s mid-lumbar region. In test sessions involving i.v. drug administration in these subjects, the port was accessed by syringe from outside the experimental chamber via a catheter tubing/Huber needle assembly.
Tests for generalization to the training stimulus were conducted when a subject’s discrimination performance was at least 90% accurate for four of the last five sessions and on the immediately preceding session. Procedurally, test sessions differed from training sessions in three ways. First, 10 consecutive responses on either lever extinguished the stimulus lights and associated program of current delivery, and initiated the 50-second timeout. Second, cumulative dosing procedures were used to establish dose-response relationships for the discriminative stimulus effects of CB1 agonists administered either i.m. or i.v. through the vascular access port. Thus, a test session consisted of four components of 10 trials, each component beginning with a 10-minute timeout period. This procedure permitted the determination of the effects of up to four incremental i.v. doses of a drug delivered during the sequential timeout periods of a single test session (Spealman, 1985; Bergman and Spealman, 1988; Lamb et al., 2000). Third, no current deliveries were scheduled during test sessions so as to preclude possible stimulus-induced enhancement of responding. Other schedule contingencies were unchanged.
In initial experiments, a full range of cumulative doses of the training drug AM4054 (0.001–0.01 mg/kg) was administered i.m. or i.v. in catheterized subjects to compare potency via the two routes of administration. Additional studies to establish the slope and position of the dose-effect function for CB1 agonists including the cannabinoids Δ9-THC (0.01–0.3 mg/kg), AM2389 (0.0003–0.01 mg/kg; Järbe et al., 2012), and the stable endocannabinoid analog methanandamide (0.3–5.6 mg/kg) were conducted in catheterized subjects by administering up to four cumulative i.v. doses of each drug at the onset of sequential 10-minute timeout periods. The effects of five or more i.v. doses of a drug were determined by administering overlapping ranges of cumulative doses during separate test sessions. To assess behavioral onset and time course of action, effects of the training dose of AM4054 (0.01 mg/kg) and a comparable dose of Δ9-THC (0.3 mg/kg) were determined i.m. in subjects using single-component sessions that began 5, 15, 60, 120, 240, 480, and 960 minutes after injection on separate test days. Next, to assess the selectivity of discrimination performance, the indirect monoamine agonist cocaine (0.03–1.0 mg/kg), the N-methyl-d-aspartic acid noncompetitive antagonist ketamine (0.3–3.2 mg/kg), and the opioid agonist morphine (0.32–3.2 mg/kg) also were studied. Doses of each drug ranged from those with no effect on response rate to those that nearly or completely abolished lever pressing. Finally, modification of the discriminative stimulus effects of i.v. AM4054 by CB1 antagonists was studied by determining how pretreatment with the inverse agonist antagonist rimonabant (0.03–1.0 mg/kg i.m.) and neutral antagonist AM4113 (0.32-5.6 mg/kg i.m.) altered the position and/or slope of the AM4054 dose-effect function. Studies were conducted with several doses of rimonabant and AM4113 to permit Schild analysis of their antagonist effects (see below). Pretreatment times were based on the results of preliminary experiments and were 30 minutes for rimonabant and AM4113.
Data Analysis
The two primary dependent measures in the present experiments were response distribution across the two levers and overall response rate. Response distribution (percent AM4054 lever) was calculated by dividing the number of responses on the lever associated with the injection of AM4054 by the total number of responses (excluding any responses during timeout periods). Response rate was calculated by dividing the total number of responses on both levers by the total session time (excluding all timeout periods). Doses of drugs were considered to substitute fully when response distribution was >90% AM4054 lever responding and response rates were >0.2 responses/s
To quantify alteration in the effects of AM4054 by rimonabant and AM4113, ED50 values (i.e., the dose resulting in 50% responding on the drug lever) of AM4054 alone and after doses of each pretreatment drug were calculated by interpolation for each subject. Dose ratios were then determined by dividing the ED50 value when AM4054 was administered with rimonabant or AM4113 by its ED50 value when administered alone, and Schild plots were constructed by plotting the log (dose ratio −1) as a function of the negative log of molar dose of the pretreatment drug (Arunlakshana and Schild, 1959; Neubig et al., 2003). If slopes did not differ significantly from unity (i.e., 95% confidence limits included −1 and did not include 0 (Paronis and Bergman, 1999), and an apparent pA2 value (i.e., the dose of pretreatment drug yielding a dose ratio of 2) was then determined.
Drugs
AM4054, AM2389, AM4113, and methanandamide (AM356) were prepared by the present authors (A.M., G.A.T., S.P.N., V.K.V., K.V.S., V.G.S.) in the Center for Drug Discovery at Northeastern University (Boston, MA). Rimonabant, Δ9-THC, and cocaine, were provided by the National Institutes of Health National Institute on Drug Abuse Drug Supply Program (Rockville, MD); ketamine and morphine were purchased from Sigma-Aldrich (St. Louis, MO). All CB1 agonists and antagonists were prepared for administration in a 20:20:60 mixture of 95% ethanol, Emulphor (Alkamuls EL-620; Rhone-Poulenc, Cranbury, NJ), and saline. Cocaine, ketamine, and morphine were prepared for administration in saline solutions. All drug solutions were refrigerated and protected from light. Injections of drug or saline were prepared in volumes of 0.3 ml/kg body weight or less and were given in calf or thigh muscle when administered i.m.
Results
Control Performance.
All subjects learned to discriminate injections of 0.01 mg/kg AM4054 from saline, with time to criterion performance ranging from approximately 30 to 60 sessions among subjects. During control sessions after training, injections of the training dose of AM4054 produced on average >99% responding on the AM4054-associated lever, whereas injections of saline produced <1% on the AM4054-associated lever. Response rates after i.m. training doses of AM4054 were somewhat lower than those after saline administration in all subjects, with group averages of 3.1 ± 0.6 and 3.6 ± 0.7 responses/s, respectively (mean ± S.E.M.). This small (<20%) difference in response rate was evident at the outset and persisted over the course of the present experiments.
Discriminative-Stimulus Effects of AM4054.
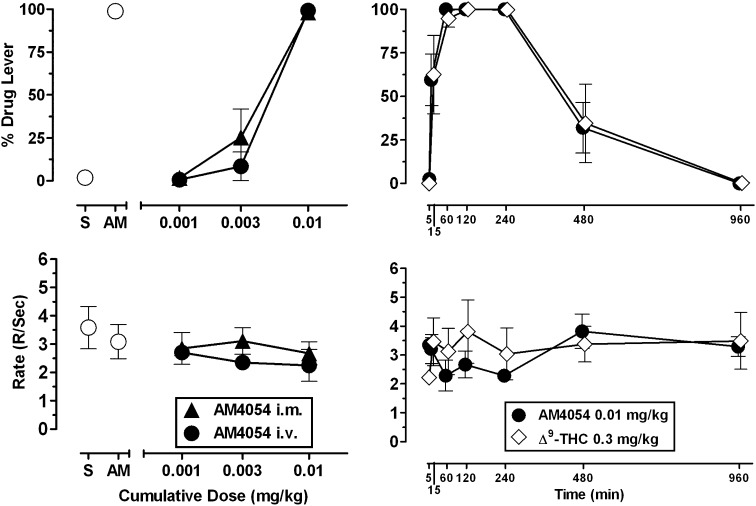
The left panels of Fig. 1 show the averaged cumulative dosing effects of AM4054 via i.v. and i.m. routes of administration. As shown, AM4054 displayed no significant difference in potency across the tested dose range when administered i.m. or i.v. (P > 0.51). Likewise, response rates averaged for the group of subjects overlapped after i.m. and i.v administration of AM4054, reflecting comparable rates of responding in individual subjects under the two test conditions. Overall, these data disclose no systematic effects of AM4054 on response distribution or rate measures as a function of route of administration.
Fig. 1.
(Left panels) Dose-effects functions of AM4054 delivered either i.m. or i.v. in subjects trained to discriminate 0.01 mg/kg AM4054 from saline. Abscissae, cumulative dose, log scale; ordinate, percent of responses on the AM4054-associated lever (top-left panel), response rate (bottom-left panel). Open symbols left of abscissae break indicate performance during saline (S) and AM4054 (AM) control sessions. Points represent averages (± S.E.M.) for the groups of subjects. (Right panels) Time course of 0.01 mg/kg AM4054 (filled symbols) and 0.3 mg/kg Δ9-THC (open symbols) in subjects trained to discriminate 0.01 mg/kg AM4054 from saline. Abscissae, time interval after injection in which discrimination session occurred; ordinate, percent of responses on the AM4054-associated lever (top-right panel), response rate (bottom-right panel). Points represent averages (± S.E.M.) for the groups of subjects.
The right panels of Fig. 1 present response distribution (upper panel) and response rate (lower panel) data for the training dose of 0.01 mg/kg AM4054 and the smallest dose of Δ9-THC that fully substituted [0.3 mg/kg (see Fig. 2)] at 5, 15, 60, 120, 240, 480, and 960 minutes after i.m. injection. As the figure indicates, a remarkably similar time course was observed for AM4054 and Δ9-THC. As shown in the top panel, the full time course of action for the CB1 discriminative stimulus effects of both AM4054 and Δ9-THC was captured within a 16-hour time frame. All subjects responded exclusively on the saline lever 5 minutes after injection. Three of four subjects responded on both levers at 15 minutes, and responding by all subjects occurred exclusively on the AM4054 lever at 1, 2, and 4 hours. Three of four subjects again responded on both levers after 8 hours, and all subjects responded exclusively on the saline-associated lever 16 hours after injection. As shown in the bottom panel, the averaged effects of Δ9-THC on response rates did not differ substantively from those of AM4054, reflecting similar results for the two drugs among individual subjects. Small decreases in response rates were observed 60 minutes after AM4054 injection, but all effects on response rate throughout the time course determinations for both drugs were slight and within the range of control values for all subjects.
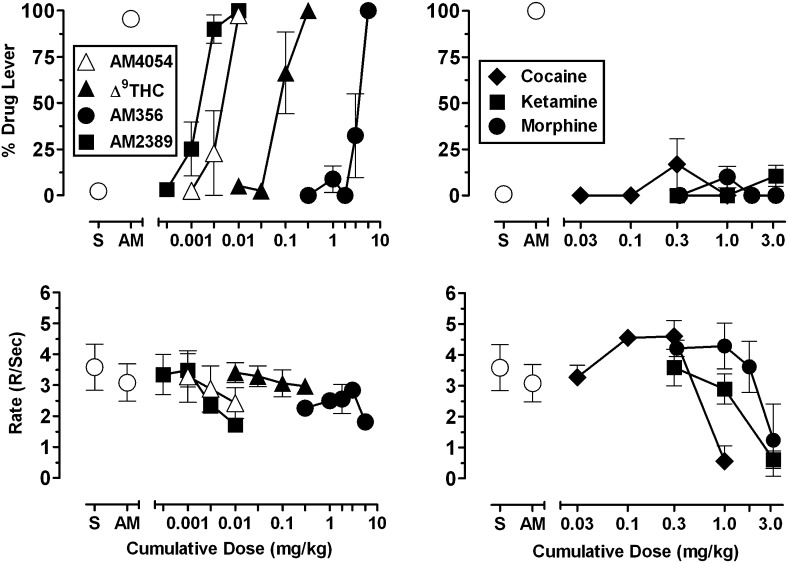
Fig. 2.
(Left panels) Dose-effect functions of CB1 agonist ligands in subjects trained to discriminate 0.01 mg/kg AM4054 from saline. Abscissae, cumulative dose, log scale; ordinate, percent of responses on the AM4054-associated lever (top-left panel), response rate (bottom-left panel). Open symbols left of abscissae break indicate performance during saline (S) and AM4054 (AM) control sessions. Points represent averages (± S.E.M.) for the groups of subjects. (Right panels) Dose-effect functions of non-CB1 agonist ligands in subjects trained to discriminate 0.01 mg/kg AM4054 from saline. Abscissae, cumulative dose, log scale; ordinate, percent of responses on the AM4054-associated lever (top-right panel), response rate (bottom-right panel). Open symbols left of abscissae break indicate performance during saline (S) and AM4054 (AM) control sessions. Points represent averages (± S.E.M.) for the groups of subjects.
Substitution with CB1 and Non-CB1 Agonist Ligands.
The left panels of Fig. 2 show the averaged effects of cumulative i.v. doses of four CB1 agonists. As displayed in the top-left panel, all four ligands produced dose-related increases in responding on the AM4054-associated lever, with full substitution after the cumulative doses of 0.01 mg/kg AM4054, 0.01 mg/kg AM2389, 0.3 mg/kg Δ9-THC, and 5.6 mg/kg AM356. The dose-effect functions relating dose and CB1-like stimulus effects were characterized by relatively steep slopes, as evident in the data indicating that 3-fold lower doses of each CB1 agonist except Δ9-THC produced <50% responding on the AM4054 lever. Grouped data reflect effects of CB1 agonists that were consistent among subjects for the highest doses, but varied somewhat among subjects for intermediate doses. For example, 0.003 mg/kg AM4054 and 3.0 mg/kg AM356 each evoked >90% responding on the AM4054 lever in one subject but >85% responding on the saline lever in the remaining three subjects. Similarly, one subject responded 100% on the saline-associated lever after 0.1 mg/kg Δ9-THC, whereas the remaining three subjects responded >85% on the AM4054-associated lever. As shown in the bottom-left panel of Fig. 2, cumulative doses of the CB1 agonists had small effects on rates of responding but none were significantly different than response rates during training sessions.
The right panels of Fig. 2 display the effects of cocaine, ketamine, and morphine on response distribution (upper panel) and response rate (lower panel). As the figure indicates, all three drugs failed to substitute for the training dose of 0.01 mg/kg AM4054 over a wide range of behaviorally active doses. Intermediate doses of cocaine (0.3 mg/kg) and morphine (1.0 mg/kg) increased response rates significantly above vehicle values (P < 0.001 and P < 0.05, respectively), whereas ketamine produced only dose-related decreases in response rate.
Pretreatment with CB1 Inverse Agonist and Antagonist Ligands.
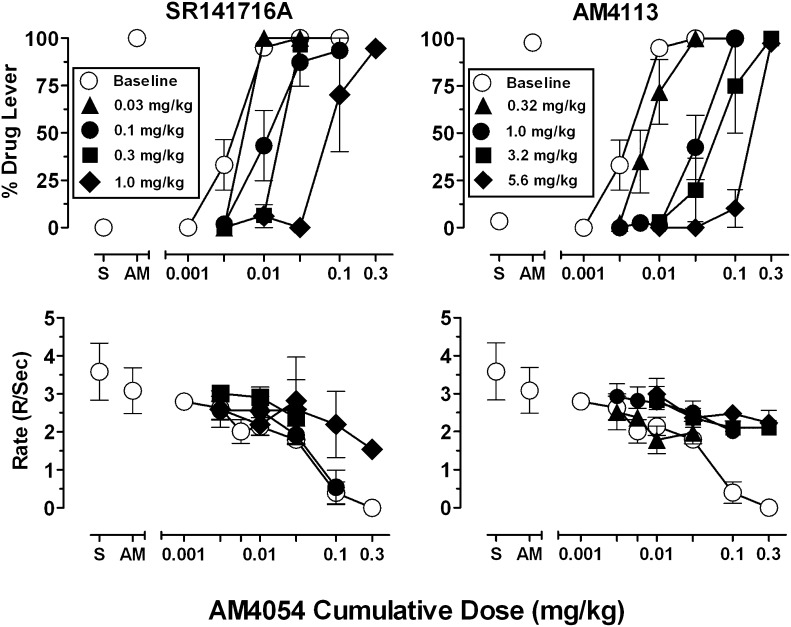
Figure 3 presents AM4054 cumulative dose-effect functions after pretreatment with the CB1 inverse agonist rimonabant and the CB1 neutral antagonist AM4113. Pretreatment with each ligand surmountably antagonized the discriminative stimulus effects of AM4054, shifting the dose-effect curve rightward in a dose-related manner and rimonabant was somewhat more potent than AM4113. For example, after a 1.0 mg/kg pretreatment dose for each antagonist, group average AM4054 ED50 values (see Table 1) reveal a 22-fold increase with rimonabant, and an 11-fold increase with AM4113. Response rates that were not altered by lower cumulative doses of AM4054 remained unchanged during antagonism studies. However, the rate-decreasing effects of higher cumulative doses of AM4054 (0.1 and 0.3 mg/kg) were attenuated after pretreatment with 1.0 mg/kg rimonabant and 1.0–5.6 mg/kg AM4113.
Fig. 3.
Dose-effect functions of AM4054 in subjects trained to discriminate 0.01 mg/kg AM4054 from saline either alone (open symbols) or after several pretreatment doses of SR141716A (left panels) and AM4113 (right panels). Abscissae, cumulative dose, log scale; ordinate, percent of responses on the AM4054-associated lever (top-left panel), response rate (bottom-left panel). Open symbols left of abscissae break indicate performance during saline (S) and AM4054 (AM) control sessions. Points represent averages (± S.E.M.) for the groups of subjects.
TABLE 1.
ED50 values for AM4054 administered alone (baseline) or after pretreatment with various doses of SR141716A and AM4113
Values are given in milligrams per kilogram.
| SR141716A | ED50 | AM4113 | ED50 |
|---|---|---|---|
| Baseline | 0.004 | Baseline | 0.004 |
| 0.03 | 0.006 | 0.32 | 0.019 |
| 0.1 | 0.014 | 1.0 | 0.036 |
| 0.3 | 0.017 | 3.2 | 0.168 |
| 1.0 | 0.095 | 5.6 | 0.164 |
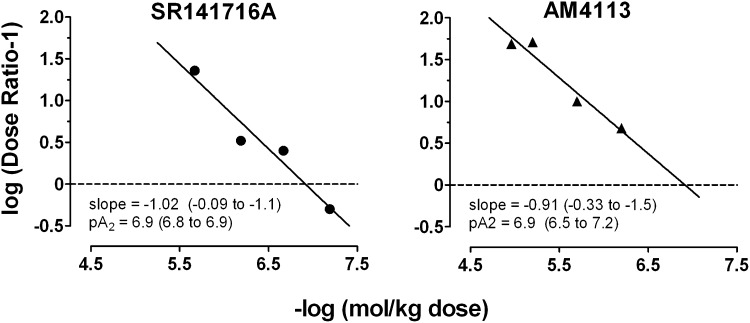
Figure 4 shows Schild plots for antagonism of AM4054′s discriminative stimulus effects by rimonabant (left panel) and AM4113 (right panel). The coefficients of determination (r2) for the regressions were 0.95 and 0.94, respectively, indicating that the quantification of antagonism was orderly across doses. The apparent pA2 value was 6.9 for both rimonabant (95% confidence limit, 6.8–6.9) and AM4113 (95% confidence limit, 6.5–7.2), with slopes that did not differ significantly from unity (i.e., –1). These analyses are consistent with the view that rimonabant and AM4113 comparably antagonized the CB1 receptor–mediated discriminative stimulus effects of AM4054.
Fig. 4.
Schild plots for SR141716A and AM4113 derived from data presented in Fig. 3. Abscissa, negative log of the molar dose of AM4054; ordinate, log (dose ratio –1). See Data Analysis in Materials and Methods for additional details.
Discussion
Results of the present studies indicate that the novel CB1 agonist AM4054 serves as a highly effective discriminative stimulus in nonhuman primates. Consistent with studies using the related cannabinoid Δ9-THC as a discriminative stimulus (Wiley et al., 1993; McMahon, 2006a, 2006b), subjects trained to discriminate 0.01 mg/kg AM4054 from saline in the present study reliably generalized drug lever responding across several common CB1 ligands (Δ9-THC, methanandamide, AM2389). However, drug lever responding was not generalized to non-CB1 drugs (cocaine, ketamine, morphine), and demonstrated good selectivity for cannabinoid receptor activity. In addition, AM4054 had comparable potency by both i.m. and i.v. routes of administration, and displayed a time course for CB1 discrimination that was remarkably similar to that of an equivalent dose of Δ9-THC.
Information regarding the extent to which AM4054 and Δ9-THC inhibit adenylate cyclase activity indicates that AM4054 has higher functional efficacy than Δ9-THC, consistent with the widespread characterization of Δ9-THC as a CB1 partial agonist (Sim et al., 1996; Burkey et al., 1997; Petitet et al., 1998; Shen and Thayer, 1999; G.A. Thakur et al., submitted manuscript). However, the in vitro distinction in CB1 efficacy between these drugs was not evident in the present data, showing that Δ9-THC substituted fully for AM4054. The full effectiveness of Δ9-THC is consistent with the results of previous drug discrimination studies comparing its effects with those of other ligands that are presumed to be CB1 full agonists. For example, Δ9-THC also produced full CB1-like effects in rats trained with either a low or high training dose of the CB1 full agonist AM5983—a methodological approach that has been used successfully to distinguish low- and high-efficacy agonists in other pharmacological classes (Järbe et al., 2012). Taken together, the present and previous drug discrimination studies of Δ9-THC and other CB1 agonists are consistent with the idea that, as reported for many behavioral effects of cannabinergic drugs, the receptor occupancy required for CB1-mediated discriminative stimulus effects is relatively low (see Gifford et al., 1999).
The CB1 inverse agonist rimonabant, in addition to serving as an antagonist in pharmacological studies of cannabinergic drugs, showed initial promise as a novel type of therapeutic for appetitive suppression and smoking cessation (Pacher et al., 2006; Padwal and Majumdar, 2007; Le Foll et al., 2008; Rigotti et al., 2009). However, reports of gastrointestinal side effects and mood-depressant actions (Després et al., 2005; Van Gaal et al., 2005; Traynor, 2007) cut short its use in clinical populations, leaving the future development of this class of drugs in doubt. Some investigators have suggested that the therapeutic effects of CB1 inverse agonists like rimonabant are related solely to their antagonist activity, whereas their undesirable effects stem from the direct consequences of their inverse agonist actions (Ward and Raffa, 2011; Kirilly et al., 2012). From this perspective, the development of neutral CB1 antagonists might yield safer, yet still effective therapeutics for appetite suppression and, possibly, smoking cessation. Indeed, recent laboratory data appear to support this suggestion, indicating that AM4113 and other newly developed CB1 neutral antagonists may not produce rimonabant-like effects of nausea, emesis, and anhedonia in laboratory animals (Chambers et al., 2007; Salamone et al., 2007; Sink et al., 2008; Cluny et al., 2010; Jutkiewicz et al., 2010; Cluny et al., 2011). Importantly, AM4113, like rimonabant, also has been shown to reliably reduce weight gain in laboratory animals, consistent with the idea that its potentially beneficial effects are linked to CB1 receptor blockade (Chambers et al., 2007; Sink et al., 2008; Cluny et al., 2011). The present results complement earlier comparisons of CB1 inverse agonists and neutral antagonists by showing that, notwithstanding differences in efficacy, rimonabant and AM4113 appear to have equally effective antagonist actions, that is, they surmountably antagonized CB1-mediated discriminative stimulus effects in nonhuman primates with comparable dose ratio relationships and pA2 values. In conjunction with available data discussed above, the reduced side-effect liability of therapeutically relevant doses of neutral antagonists, if confirmed in clinical studies, may be linked to the absence of inverse agonist activity at CB1 receptors.
Acknowledgments
The authors thank Ani Zakarian and Michael Z. Leonard for assistance with conducting these studies, and Carol A. Paronis and Rajeev I. Desai for comments on an earlier version of this manuscript.
Abbreviations
- Δ9-THC
Δ9-tetrahydrocannabinol
- AM251
1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-(1-piperidyl)pyrazole-3-carboxamide
- AM356
(R)-(+)-arachidonyl-1'-hydroxy-2'-propylamide
- AM2389
9β-Hydroxy-3-(1-hexyl-cyclobut-1-yl)-hexahydrocannabinol
- AM4054
9β-(hydroxymethyl)-3-(1-adamantyl)-hexahydrocannabinol
- AM4113
5-(4-alkylphenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide
- AM6545
5-(4-[4-cyanobut-1-ynyl]phenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(1,1-dioxo-thiomorpholino)-1H-pyrazole-3-carboxamide
- CB1
cannabinoid receptor 1
- CP 55,940
2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]-5-(2-methyloctan-2-yl)phenol
- FR
fixed ratio
- SR141716A
5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide
- Taranabant
N-[(2S,3S)-4-(4-chlorophenyl)-3-(3-cyanophenyl)-2-butanyl]-2-methyl-2-{[5-(trifluoromethyl)-2-pyridinyl]oxy}propanamide
- WIN 55,212-2
R-(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate
Authorship Contributions
Participated in research design: Kangas, Bergman.
Conducted experiments: Kangas, Delatte.
Contributed new reagents or analytic tools: Vemuri, Thakur, Nikas, Makriyannis, Subramanian, Shukla.
Performed data analysis: Kangas, Delatte.
Wrote or contributed to the writing of the manuscript: Kangas, Bergman.
Footnotes
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA023142 (to J.B.) and DA26795 (to A.M.)]; and the Ruth L. Kirschstein National Service Award (to B.D.K.).
References
- Addy C, Wright H, Van Laere K, Gantz I, Erondu N, Musser BJ, Lu K, Yuan J, Sanabria-Bohórquez SM, Stoch A, et al. (2008) The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab 7:68–78 [DOI] [PubMed] [Google Scholar]
- Aronne LJ, Tonstad S, Moreno M, Gantz I, Erondu N, Suryawanshi S, Molony C, Sieberts S, Nayee J, Meehan AG, et al. (2010) A clinical trial assessing the safety and efficacy of taranabant, a CB1R inverse agonist, in obese and overweight patients: a high-dose study. Int J Obes (Lond) 34:919–935 [DOI] [PubMed] [Google Scholar]
- Arunlakshana O, Schild HO. (1959) Some quantitative uses of drug antagonists. Br Pharmacol Chemother 14:48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Spealman RD. (1988) Behavioral effects of histamine H1 antagonists: comparison with other drugs and modification by haloperidol. J Pharmacol Exp Ther 245:471–478 [PubMed] [Google Scholar]
- Breivogel CS, Childers SR. (2000) Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J Pharmacol Exp Ther 295:328–336 [PubMed] [Google Scholar]
- Burkey TH, Quock RM, Consroe P, Roeske WR, Yamamura HI. (1997) delta 9-Tetrahydrocannabinol is a partial agonist of cannabinoid receptors in mouse brain. Eur J Pharmacol 323:R3–R4 [DOI] [PubMed] [Google Scholar]
- Chambers AP, Vemuri VK, Peng Y, Wood JT, Olszewska T, Pittman QJ, Makriyannis A, Sharkey KA. (2007) A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol 293:R2185–R2193 [DOI] [PubMed] [Google Scholar]
- Cluny NL, Chambers AP, Vemuri VK, Wood JT, Eller LK, Freni C, Reimer RA, Makriyannis A, Sharkey KA. (2011) The neutral cannabinoid CB₁ receptor antagonist AM4113 regulates body weight through changes in energy intake in the rat. Pharmacol Biochem Behav 97:537–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT, Lutz B, Zimmer A, Parker LA, Makriyannis A, et al. (2010) A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol 161:629–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton DR, Aceto MD, Lowe J, Martin BR. (1996) In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of delta 9-tetrahydrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther 277:586–594 [PubMed] [Google Scholar]
- Desai RI, Thakur GA, Vemuri VK, Bajaj S, Makriyannis A, Bergman J. (2012) Behavioral effects of cannabinoid and non-cannabinoid drugs during chronic CB1 agonist treatment. J Pharmacol Exp Ther DOI:10.1124/jpet.112.198374 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després JP, Golay A, Sjöström L, Rimonabant in Obesity-Lipids Study Group (2005) Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 353:2121–2134 [DOI] [PubMed] [Google Scholar]
- Dykstra LA, Bertalmio AJ, Woods JH. (1988) Discriminative and analgesic effects of mu and kappa opioids: In vivo pA2 analysis, in Transduction Mechanisms of Drug Stimuli (Colpaert FC, Balster RL. eds) pp 107–121, Springer-Verlag, Berlin: [DOI] [PubMed] [Google Scholar]
- Gifford AN, Bruneus M, Gatley SJ, Lan R, Makriyannis A, Volkow ND. (1999) Large receptor reserve for cannabinoid actions in the central nervous system. J Pharmacol Exp Ther 288:478–483 [PubMed] [Google Scholar]
- Herd JA, Morse WH, Kelleher RT, Jones LG. (1969) Arterial hypertension in the squirrel monkey during behavioral experiments. Am J Physiol 217:24–29 [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Tai S, LeMay BJ, Nikas SP, Shukla VG, Zvonok A, Makriyannis A. (2012) AM2389, a high-affinity, in vivo potent CB1-receptor-selective cannabinergic ligand as evidenced by drug discrimination in rats and hypothermia testing in mice. Psychopharmacology (Berl) 220:417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TUC, Lamb RJ, Lin S, Makriyannis A. (2001) (R)-methanandamide and Delta 9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology (Berl) 156:369–380 [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Makriyannis A, Vemuri K, and Bergman J (2010) Pro-depressant-like effects of CB1 receptor inverse agonists/antagonists in male Sprague-Dawley rats (Abstract). FASEB J 24 (Suppl):581.7.
- Kirilly E, Gonda X, Bagdy G. (2012) CB1 receptor antagonists: new discoveries leading to new perspectives. Acta Physiol (Oxf) 205:41–60 [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Järbe TUC, Makriyannis A, Lin S, Goutopoulos A. (2000) Effects of Δ 9-tetrahydrocannabinol, (R)-methanandamide, SR 141716,and d-amphetamine before and during daily Δ 9-tetrahydrocannabinol dosing. Eur J Pharmacol 398:251–258 [DOI] [PubMed] [Google Scholar]
- Le Foll B, Forget B, Aubin HJ, Goldberg SR. (2008) Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: insights from pre-clinical and clinical studies. Addict Biol 13:239–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin PJ. (2012) Reports of the death of CB1 antagonists have been greatly exaggerated: recent preclinical findings predict improved safety in the treatment of obesity. Behav Pharmacol 23:537–550 [DOI] [PubMed] [Google Scholar]
- McMahon LR. (2006a) Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther 319:1211–1218 [DOI] [PubMed] [Google Scholar]
- McMahon LR. (2006b) Discriminative stimulus effects of the cannabinoid CB1 antagonist SR 141716A in rhesus monkeys pretreated with Δ9-tetrahydrocannabinol. Psychopharmacology (Berl) 188:306–314 [DOI] [PubMed] [Google Scholar]
- McMahon LR, Amin MR, France CP. (2005) SR 141716A differentially attenuates the behavioral effects of delta9-THC in rhesus monkeys. Behav Pharmacol 16:363–372 [DOI] [PubMed] [Google Scholar]
- Meye FJ, Trezza V, Vanderschuren LJ, Ramakers GM, and Adan RA (2012) Neutral antagonism at the cannabinoid 1 receptor: a safer treatment for obesity. Mol Psychiatry DOI: 10.1038/mp.2012.145 [published ahead of print]. [DOI] [PubMed]
- Nakamura-Palacios EM, Moerschbaecher JM, Barker LA. (1999) The pharmacology of SR 141716A: A Review. CNS Drug Rev 5:43–58 [Google Scholar]
- National Research Council (2003) Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research, National Academies Press, Washington, DC: [PubMed] [Google Scholar]
- Neubig RR, Spedding M, Kenakin T, Christopoulos A, International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification (2003) International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol Rev 55:597–606 [DOI] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G. (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58:389–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padwal RS, Majumdar SR. (2007) Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet 369:71–77 [DOI] [PubMed] [Google Scholar]
- Paronis CA, Bergman J. (1999) Apparent pA2 values of benzodiazepine antagonists and partial agonists in monkeys. J Pharmacol Exp Ther 290:1222–1229 [PubMed] [Google Scholar]
- Pertwee RG. (2005) Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci 76:1307–1324 [DOI] [PubMed] [Google Scholar]
- Petitet F, Jeantaud B, Reibaud M, Imperato A, Dubroeucq MC. (1998) Complex pharmacology of natural cannabinoids: evidence for partial agonist activity of delta9-tetrahydrocannabinol and antagonist activity of cannabidiol on rat brain cannabinoid receptors. Life Sci 63:PL1–PL6. [DOI] [PubMed] [Google Scholar]
- Proietto J, Rissanen A, Harp JB, Erondu N, Yu Q, Suryawanshi S, Jones ME, Johnson-Levonas AO, Heymsfield SB, Kaufman KD, et al. (2010) A clinical trial assessing the safety and efficacy of the CB1R inverse agonist taranabant in obese and overweight patients: low-dose study. Int J Obes (Lond) 34:1243–1254 [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Gonzales D, Dale LC, Lawrence D, Chang Y, CIRRUS Study Group (2009) A randomized controlled trial of adding the nicotine patch to rimonabant for smoking cessation: efficacy, safety and weight gain. Addiction 104:266–276 [DOI] [PubMed] [Google Scholar]
- Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. (2007) Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav 91:383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Thayer SA. (1999) Delta9-tetrahydrocannabinol acts as a partial agonist to modulate glutamatergic synaptic transmission between rat hippocampal neurons in culture. Mol Pharmacol 55:8–13 [DOI] [PubMed] [Google Scholar]
- Sim LJ, Hampson RE, Deadwyler SA, Childers SR. (1996) Effects of chronic treatment with delta9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci 16:8057–8066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, Peng Y, Olszewska T, Thakur GA, Makriyannis A, et al. (2008) The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology 33:946–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spealman RD, Goldberg SR, Kelleher RT, Goldberg DM, Charlton JP. (1977) Some effects of cocaine and two cocaine analogs on schedule-controlled behavior of squirrel monkeys. J Pharmacol Exp Ther 202:500–509 [PubMed] [Google Scholar]
- Spealman RD. (1985) Discriminative-stimulus effects of midazolam in squirrel monkeys: comparison with other drugs and antagonism by Ro 15-1788. J Pharmacol Exp Ther 235:456–462 [PubMed] [Google Scholar]
- Traynor K. (2007) Panel advises against rimonabant approval. Am J Health Syst Pharm 64:1460–1461 [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S, RIO-Europe Study Group (2005) Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365:1389–1397 [DOI] [PubMed] [Google Scholar]
- Ward SJ, Raffa RB. (2011) Rimonabant redux and strategies to improve the future outlook of CB1 receptor neutral-antagonist/inverse-agonist therapies. Obesity (Silver Spring) 19:1325–1334 [DOI] [PubMed] [Google Scholar]
- Wiley JL, Barrett RL, Britt DT, Balster RL, Martin BR. (1993) Discriminative stimulus effects of delta 9-tetrahydrocannabinol and delta 9-11-tetrahydrocannabinol in rats and rhesus monkeys. Neuropharmacology 32:359–365 [DOI] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR. (1995) Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther 275:1–6 [PubMed] [Google Scholar]
- Woods JH, Winger G, France CP. (1992) Use of in vivo apparent pA2 analysis in assessment of opioid abuse liability. Trends Pharmacol Sci 13:282–286 [DOI] [PubMed] [Google Scholar]