Abstract
A single metabolic path leading to synthesis of ether lipids is known in animal cells, the major products of which are plasmalogens. To learn whether this peroxisomal path is also responsible for the synthesis of base-resistant lipid components of glycosylphosphoinositol (GPI)-anchored membrane proteins, we have investigated the structure of anchor precursor mannolipids both in wild-type cells (CHO-K1 and a macrophage-like line, RAW 264.7) and in two corresponding mutant cells in which ether lipid biosynthesis is severely impaired. We observe that the precursor mannolipids of both the wild-type and mutant cells do not include alkylglycerol. Nevertheless, both wild-type and mutant cells express cell surface GPI-anchored placental alkaline phosphatase (AP) which includes alkali-resistant hydrophobic chains in its anchor moiety. Thus, (i) in normal AP GPI anchor synthesis, any ether-linked substituents must be added either immediately before, during, or after anchor addition to AP, and (ii) the classical peroxisomal path for ether lipid synthesis appears not to contribute to the synthesis of GPI anchors.
Full text
PDF




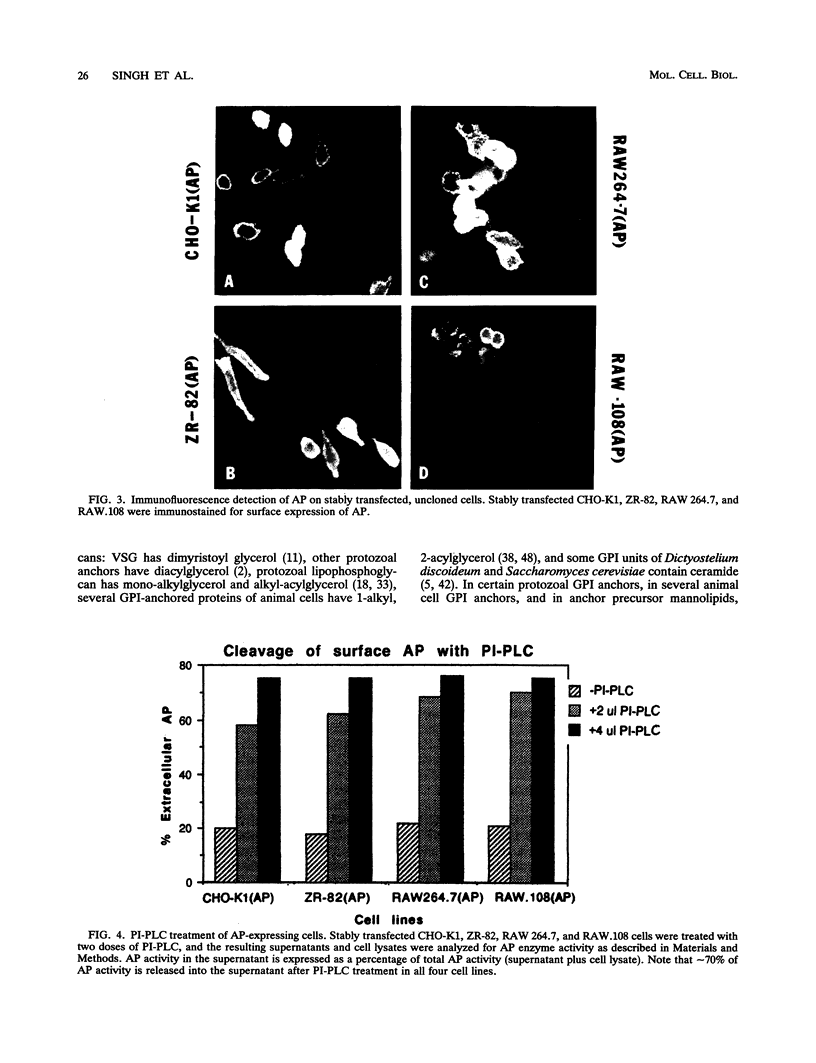
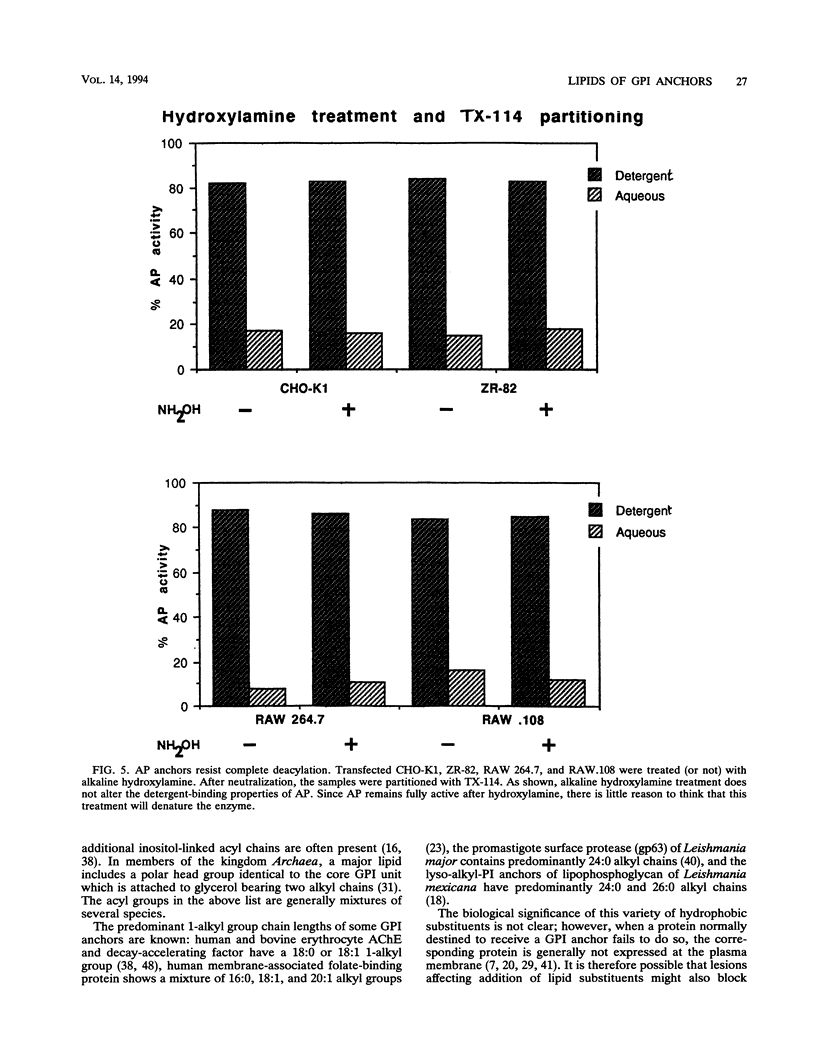



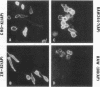
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bütikofer P., Kuypers F. A., Shackleton C., Brodbeck U., Stieger S. Molecular species analysis of the glycosylphosphatidylinositol anchor of Torpedo marmorata acetylcholinesterase. J Biol Chem. 1990 Nov 5;265(31):18983–18987. [PubMed] [Google Scholar]
- Chaney W. G., Howard D. R., Pollard J. W., Sallustio S., Stanley P. High-frequency transfection of CHO cells using polybrene. Somat Cell Mol Genet. 1986 May;12(3):237–244. doi: 10.1007/BF01570782. [DOI] [PubMed] [Google Scholar]
- Clarke N. G., Dawson R. M. Alkaline O leads to N-transacylation. A new method for the quantitative deacylation of phospholipids. Biochem J. 1981 Apr 1;195(1):301–306. doi: 10.1042/bj1950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann A., Puoti A., Lester R. L., Desponds C. Two different types of lipid moieties are present in glycophosphoinositol-anchored membrane proteins of Saccharomyces cerevisiae. EMBO J. 1992 Feb;11(2):457–466. doi: 10.1002/j.1460-2075.1992.tb05075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S., Aldrich T. H., Valenzuela D. M., Wong V. V., Furth M. E., Squinto S. P., Yancopoulos G. D. The receptor for ciliary neurotrophic factor. Science. 1991 Jul 5;253(5015):59–63. doi: 10.1126/science.1648265. [DOI] [PubMed] [Google Scholar]
- Delahunty M. D., Stafford F. J., Yuan L. C., Shaz D., Bonifacino J. S. Uncleaved signals for glycosylphosphatidylinositol anchoring cause retention of precursor proteins in the endoplasmic reticulum. J Biol Chem. 1993 Jun 5;268(16):12017–12027. [PubMed] [Google Scholar]
- Englund P. T. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu Rev Biochem. 1993;62:121–138. doi: 10.1146/annurev.bi.62.070193.001005. [DOI] [PubMed] [Google Scholar]
- Fatemi S. H., Haas R., Jentoft N., Rosenberry T. L., Tartakoff A. M. The glycophospholipid anchor of Thy-1. Biosynthetic labeling experiments with wild-type and class E Thy-1 negative lymphomas. J Biol Chem. 1987 Apr 5;262(10):4728–4732. [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Haldar K., Cross G. A. Trypanosoma brucei variant surface glycoprotein has a sn-1,2-dimyristyl glycerol membrane anchor at its COOH terminus. J Biol Chem. 1985 Apr 25;260(8):4963–4968. [PubMed] [Google Scholar]
- Gennarini G., Cibelli G., Rougon G., Mattei M. G., Goridis C. The mouse neuronal cell surface protein F3: a phosphatidylinositol-anchored member of the immunoglobulin superfamily related to chicken contactin. J Cell Biol. 1989 Aug;109(2):775–788. doi: 10.1083/jcb.109.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra A. K. Acyl dihydroxyacetone phosphate: precursor of alkyl ethers. Biochem Biophys Res Commun. 1970;39(6):1037–1044. doi: 10.1016/0006-291x(70)90663-7. [DOI] [PubMed] [Google Scholar]
- Hardeman D., van den Bosch H. Topography of ether phospholipid biosynthesis. Biochim Biophys Acta. 1989 Nov 6;1006(1):1–8. doi: 10.1016/0005-2760(89)90315-9. [DOI] [PubMed] [Google Scholar]
- Hirose S., Prince G. M., Sevlever D., Ravi L., Rosenberry T. L., Ueda E., Medof M. E. Characterization of putative glycoinositol phospholipid anchor precursors in mammalian cells. Localization of phosphoethanolamine. J Biol Chem. 1992 Aug 25;267(24):16968–16974. [PubMed] [Google Scholar]
- Ilg T., Etges R., Overath P., McConville M. J., Thomas-Oates J., Thomas J., Homans S. W., Ferguson M. A. Structure of Leishmania mexicana lipophosphoglycan. J Biol Chem. 1992 Apr 5;267(10):6834–6840. [PubMed] [Google Scholar]
- Jemmerson R., Low M. G. Phosphatidylinositol anchor of HeLa cell alkaline phosphatase. Biochemistry. 1987 Sep 8;26(18):5703–5709. doi: 10.1021/bi00392a019. [DOI] [PubMed] [Google Scholar]
- Jost C. R., Gaillard M. L., Fransen J. A., Daha M. R., Ginsel L. A. Intracellular localization of glycosyl-phosphatidylinositol-anchored CD67 and FcRIII (CD16) in affected neutrophil granulocytes of patients with paroxysmal nocturnal hemoglobinuria. Blood. 1991 Dec 1;78(11):3030–3036. [PubMed] [Google Scholar]
- Lemansky P., Gupta D. K., Meyale S., Tucker G., Tartakoff A. M. Atypical mannolipids characterize Thy-1-negative lymphoma mutants. Mol Cell Biol. 1991 Aug;11(8):3879–3885. doi: 10.1128/mcb.11.8.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhrs C. A., Slomiany B. L. A human membrane-associated folate binding protein is anchored by a glycosyl-phosphatidylinositol tail. J Biol Chem. 1989 Dec 25;264(36):21446–21449. [PubMed] [Google Scholar]
- Masterson W. J., Raper J., Doering T. L., Hart G. W., Englund P. T. Fatty acid remodeling: a novel reaction sequence in the biosynthesis of trypanosome glycosyl phosphatidylinositol membrane anchors. Cell. 1990 Jul 13;62(1):73–80. doi: 10.1016/0092-8674(90)90241-6. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Blackwell J. M. Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovani. Characterization of the promastigote and amastigote glycolipids. J Biol Chem. 1991 Aug 15;266(23):15170–15179. [PubMed] [Google Scholar]
- McConville M. J., Homans S. W., Thomas-Oates J. E., Dell A., Bacic A. Structures of the glycoinositolphospholipids from Leishmania major. A family of novel galactofuranose-containing glycolipids. J Biol Chem. 1990 May 5;265(13):7385–7394. [PubMed] [Google Scholar]
- Mentlein R., Schumann M., Heymann E. Comparative chemical and immunological characterization of five lipolytic enzymes (carboxylesterases) from rat liver microsomes. Arch Biochem Biophys. 1984 Nov 1;234(2):612–621. doi: 10.1016/0003-9861(84)90311-4. [DOI] [PubMed] [Google Scholar]
- Mikol D. D., Gulcher J. R., Stefansson K. The oligodendrocyte-myelin glycoprotein belongs to a distinct family of proteins and contains the HNK-1 carbohydrate. J Cell Biol. 1990 Feb;110(2):471–479. doi: 10.1083/jcb.110.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P., Caras I. W. Proteins containing an uncleaved signal for glycophosphatidylinositol membrane anchor attachment are retained in a post-ER compartment. J Cell Biol. 1992 Nov;119(4):763–772. doi: 10.1083/jcb.119.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand O. H., Zoeller R. A., Raetz C. R. Disappearance of plasmalogens from membranes of animal cells subjected to photosensitized oxidation. J Biol Chem. 1988 Aug 15;263(23):11597–11606. [PubMed] [Google Scholar]
- Nishihara M., Utagawa M., Akutsu H., Koga Y. Archaea contain a novel diether phosphoglycolipid with a polar head group identical to the conserved core of eucaryal glycosyl phosphatidylinositol. J Biol Chem. 1992 Jun 25;267(18):12432–12435. [PubMed] [Google Scholar]
- Ogata S., Hayashi Y., Takami N., Ikehara Y. Chemical characterization of the membrane-anchoring domain of human placental alkaline phosphatase. J Biol Chem. 1988 Jul 25;263(21):10489–10494. [PubMed] [Google Scholar]
- Orlandi P. A., Jr, Turco S. J. Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1987 Jul 25;262(21):10384–10391. [PubMed] [Google Scholar]
- Owens K. A two-dimensional thin-layer chromatographic procedure for the estimation of plasmalogens. Biochem J. 1966 Aug;100(2):354–361. doi: 10.1042/bj1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puoti A., Conzelmann A. Characterization of abnormal free glycophosphatidylinositols accumulating in mutant lymphoma cells of classes B, E, F, and H. J Biol Chem. 1993 Apr 5;268(10):7215–7224. [PubMed] [Google Scholar]
- Puoti A., Conzelmann A. Structural characterization of free glycolipids which are potential precursors for glycophosphatidylinositol anchors in mouse thymoma cell lines. J Biol Chem. 1992 Nov 5;267(31):22673–22680. [PubMed] [Google Scholar]
- Roberts W. L., Myher J. J., Kuksis A., Low M. G., Rosenberry T. L. Lipid analysis of the glycoinositol phospholipid membrane anchor of human erythrocyte acetylcholinesterase. Palmitoylation of inositol results in resistance to phosphatidylinositol-specific phospholipase C. J Biol Chem. 1988 Dec 15;263(35):18766–18775. [PubMed] [Google Scholar]
- Rosen C. L., Lisanti M. P., Salzer J. L. Expression of unique sets of GPI-linked proteins by different primary neurons in vitro. J Cell Biol. 1992 May;117(3):617–627. doi: 10.1083/jcb.117.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P., Ferguson M. A., McConville M. J., Mehlert A., Homans S. W., Bordier C. Structure of the glycosyl-phosphatidylinositol membrane anchor of the Leishmania major promastigote surface protease. J Biol Chem. 1990 Oct 5;265(28):16955–16964. [PubMed] [Google Scholar]
- Singh N., Singleton D., Tartakoff A. M. Anchoring and degradation of glycolipid-anchored membrane proteins by L929 versus by LM-TK- mouse fibroblasts: implications for anchor biosynthesis. Mol Cell Biol. 1991 May;11(5):2362–2374. doi: 10.1128/mcb.11.5.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Keenan T. W., Bauer G., Gerisch G. The contact site A glycoprotein of Dictyostelium discoideum carries a phospholipid anchor of a novel type. EMBO J. 1989 Feb;8(2):371–377. doi: 10.1002/j.1460-2075.1989.tb03387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens V. L., Raetz C. R. Class F Thy-1-negative murine lymphoma cells are deficient in ether lipid biosynthesis. J Biol Chem. 1990 Sep 15;265(26):15653–15658. [PubMed] [Google Scholar]
- Stevens V. L., Raetz C. R. Defective glycosyl phosphatidylinositol biosynthesis in extracts of three Thy-1 negative lymphoma cell mutants. J Biol Chem. 1991 Jun 5;266(16):10039–10042. [PubMed] [Google Scholar]
- Tartakoff A. M., Singh N. How to make a glycoinositol phospholipid anchor. Trends Biochem Sci. 1992 Nov;17(11):470–473. doi: 10.1016/0968-0004(92)90491-q. [DOI] [PubMed] [Google Scholar]
- Toutant J. P., Roberts W. L., Murray N. R., Rosenberry T. L. Conversion of human erythrocyte acetylcholinesterase from an amphiphilic to a hydrophilic form by phosphatidylinositol-specific phospholipase C and serum phospholipase D. Eur J Biochem. 1989 Apr 1;180(3):503–508. doi: 10.1111/j.1432-1033.1989.tb14674.x. [DOI] [PubMed] [Google Scholar]
- Walter E. I., Roberts W. L., Rosenberry T. L., Ratnoff W. D., Medof M. E. Structural basis for variations in the sensitivity of human decay accelerating factor to phosphatidylinositol-specific phospholipase C cleavage. J Immunol. 1990 Feb 1;144(3):1030–1036. [PubMed] [Google Scholar]
- Wanders R. J., Schumacher H., Heikoop J., Schutgens R. B., Tager J. M. Human dihydroxyacetonephosphate acyltransferase deficiency: a new peroxisomal disorder. J Inherit Metab Dis. 1992;15(3):389–391. doi: 10.1007/BF02435984. [DOI] [PubMed] [Google Scholar]
- Wong Y. W., Low M. G. Phospholipase resistance of the glycosyl-phosphatidylinositol membrane anchor on human alkaline phosphatase. Clin Chem. 1992 Dec;38(12):2517–2525. [PubMed] [Google Scholar]
- Wood R., Snyder F. Quantitative determination of alk-1-enyl- and alkyl-glyceryl ethers in neutral lipids and phospholipids. Lipids. 1968 Mar;3(2):129–135. doi: 10.1007/BF02531729. [DOI] [PubMed] [Google Scholar]
- Zoeller R. A., Morand O. H., Raetz C. R. A possible role for plasmalogens in protecting animal cells against photosensitized killing. J Biol Chem. 1988 Aug 15;263(23):11590–11596. [PubMed] [Google Scholar]
- Zoeller R. A., Raetz C. R. Isolation of animal cell mutants deficient in plasmalogen biosynthesis and peroxisome assembly. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5170–5174. doi: 10.1073/pnas.83.14.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller R. A., Rangaswamy S., Herscovitz H., Rizzo W. B., Hajra A. K., Das A. K., Moser H. W., Moser A., Lazarow P. B., Santos M. J. Mutants in a macrophage-like cell line are defective in plasmalogen biosynthesis, but contain functional peroxisomes. J Biol Chem. 1992 Apr 25;267(12):8299–8306. [PubMed] [Google Scholar]
- van den Bosch H., Schutgens R. B., Wanders R. J., Tager J. M. Biochemistry of peroxisomes. Annu Rev Biochem. 1992;61:157–197. doi: 10.1146/annurev.bi.61.070192.001105. [DOI] [PubMed] [Google Scholar]