Abstract
Until recently, the lens was thought to express only two Aquaporin (AQP) water channels, AQP1 and AQP0. In this study we confirm lenticular AQP5 protein expression by Western blotting and mass spectrometry in lenses from a variety of species. In addition, confocal microscopy was used to map cellular distributions of AQP5 in mouse, rat and human lenses. Tandem mass spectrometry of a human lens membrane preparation revealed extensive sequence coverage (56.2%) of AQP5. Western blotting performed on total fiber cell membranes from mouse, rat, bovine and human lenses confirmed AQP5 protein expression is conserved amongst species. Western blotting of dissected lens fractions suggests that AQP5 is processed in the lens core by C-terminal truncation. Immunohistochemistry showed that AQP5 signal was most abundant in the lens outer cortex and decreased in intensity in the lens core. Furthermore, AQP5 undergoes differentiation-dependent changes in subcellular location from an intracellular localization in differentiating fiber cells to the plasma membrane of mature fiber cells upon the loss of fiber cell nuclei. Our results show that AQP5 is a significant component of lens fiber cell membranes, representing the second most abundant water channel in these cells. Together, the changes to AQP5 distribution and structure are likely to modulate the functional role of AQP5 in different regions of the lens.
Keywords: Lens, aquaporins, immunohistochemistry, proteomics, water permeability
1. INTRODUCTION
It has been proposed that to compensate for the absence of a blood supply the lens operates an internal microcirculation system that convects nutrients into and waste products out of the lens faster than can be achieved by passive diffusion alone.(Mathias et al., 2007; Mathias et al., 1997) The fluid fluxes associated with this circulation system are thought to cross cell membranes in the lens via two different types of water channel from the aquaporin (AQP) family of proteins. The highly water permeable AQP1 is expressed exclusively in lens epithelial cells, which cover the anterior surface of the lens. At the equator of the lens these epithelial cells differentiate into the highly elongated fiber cells that comprise the majority of the lens. The fiber cells express AQP0 which, relative to AQP1, is a poor water channel but because it is abundantly expressed it has been shown to account for up to 80% of the water permeability of fiber cells.(Varadaraj et al., 2005) This process of epithelial to fiber cell differentiation occurs throughout life causing the older, more differentiated fiber cells to be internalized by the more peripheral younger cells. A key component of this differentiation process is the loss of cellular organelles and nuclei, which removes these light scattering elements from the path of light, but also removes protein turnover and synthesis machinery.(Bassnett and Beebe, 1992)
It is becoming apparent that lens fiber cells modify their existing membrane proteins to alter protein function as the cellular environment changes during the course of differentiation.(Donaldson et al., 2004; Donaldson and Lim, 2008) In this regard AQP0 is believed to act not only as a water channel,(Kushmerick et al., 1995; Mulders et al., 1995) but is also thought to act as an adhesion protein which forms junctional structures.(Kumari and Varadaraj, 2009; Michea et al., 1995; Sas et al., 1985; Varadaraj et al., 2010) AQP0 undergoes C-terminal truncation in mature fiber cells but the effect this truncation has on water permeability and the junction forming properties of AQP0 remains unclear.(Ball et al., 2003; Gonen et al., 2004; Harries et al., 2004; Palanivelu et al., 2006) However, post-translational phosphorylation appears to modulate AQP0 water permeability through interaction with calmodulin.(Kalman et al., 2008; Rose et al., 2008)
Until recently, AQP0 was thought to be the only aquaporin expressed in lens fiber cells. However, AQP5 mRNA has previously been detected in rat lens,(Patil et al., 1997) and AQP5 transcripts were found in expressed sequence tag analysis of adult human lenses.(Wistow et al., 2002) Recent studies of bovine and mouse lens membranes using a proteomics approach detected AQP5 at the protein level in the lens fiber cell plasma membrane.(Bassnett et al., 2009; Wang et al., 2008) and this result was confirmed by confocal microscopy (Kumari et al., 2012). In the present study we have employed a similar proteomics approach to identify AQP5 in human lenses. In addition, we have used confocal microscopy, previously used to map the distribution and post-translational truncation of AQP0 across large distances at high resolution in the rat lens,(Grey et al., 2009) to map the cellular localization of AQP5 throughout rodent and human lenses. Our results confirm that AQP5 is expressed in the lenses of a variety of species and show that AQP5 expression changes as a function of fiber cell differentiation.
2. MATERIALS AND METHODS
2.1 Animals and Reagents
All animals were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Human lens tissue was obtained from donor eyes courtesy of the New Zealand National Eye Bank within 24 hr of death or from the Lion’s Eye Bank of Oregon. Human lens work was conducted in compliance with the Declaration of Helsinki and was approved by the Northern X Regional ethics Committee (ref: NTX/07/08/079). An affinity purified anti-AQP5 antibody (Cat #: AB15858), directed against a synthetic rat sequence peptide from the C terminus of the protein, was obtained from Millipore (Billerica, MA). Affinity purified anti-AQP0, directed against a 17 amino acid peptide (human sequence) from the C terminus of the protein, was obtained from Alpha Diagnostic, Inc. (San Antonio, TX). For labelling of the cell membrane, wheatgerm agglutinin conjugated to a fluorophore (WGA-TRITC) was obtained from Invitrogen (Carlsbad, CA). Phosphate buffered saline (PBS) was prepared fresh from PBS tablets (Sigma Chemical Company, Australia). Unless otherwise stated all other chemicals were from Sigma.
2.2 Tissue Preparation for Mass Spectrometry
Tissue samples were prepared as published by Bassnett et al.(Bassnett et al., 2009) and membrane proteins isolated by the method of Russell.(Russell et al., 1981) Briefly, human lenses (1 day old, 4 days old, and 8 years old) were thawed, pooled and homogenized in 6 mL of 20 mM sodium phosphate buffer (pH 7.0) containing 1 mM EGTA. The insoluble plasma membrane was pelleted by centrifugation at 20,000× g for 30 min and then suspended by brief vortexing in 6.0 ml of homogenization buffer containing 50 mM dithiothreitol (DTT).
Followed centrifugation as above, the washed pellet was resuspended in 0.6 ml of 7 M urea with the aid of probe sonication for 10 s at a setting of 3 using a model 60 Sonic Dismembrator (Fisher Scientific, Pittsburgh, PA). Water (0.6 mL) was added and the pellet isolated by centrifugation as above. To further remove associated soluble proteins, the pellet was then suspended in 0.5 mL of ice cold 0.1 M NaOH containing 1 mM DTT, kept on ice for 15 min, and isolated by centrifugation as above. The resulting pellet was neutralized by washing with 0.5 mL of 0.5 M Tris (pH 6.8). For protein assays, a fraction of the resulting membrane proteins was resuspended in 0.25 ml of homogenization buffer with brief sonication, and the protein content was measured using a BCA assay with bovine serum albumin as the standard (Pierce Chemical, Rockford, IL).
2.3 Pepsin Digestion
A total of 357 μg of urea extracted and alkali washed lens membrane proteins were used for a limited pepsin digestion using a modification of the method of Han and Schey.(Han and Schey, 2004) In brief, the pellet was resuspended in 0.4 mL of 1.5 M Tris (pH 7.4)/ n-propanol (1:3 v/v) and reduction/alkylation of cysteine residues performed by addition of 10 μL of 0.9 M DTT, incubation at 37 °C for 15 min, followed by addition of 10 μL of 1.0 M iodoacetamide (IAA), and incubation for 15 min at room temperature. An additional 10 μL of 0.9 M DTT were then added to assure all excess IAA was quenched, the membrane pelleted, washed with 0.5 mL of water, and proteins delipidated by suspension in 0.5 mL of 95% ethanol at −20 °C overnight. The delipidated proteins were then pelleted and dissolved in 45 μL of 88% formic acid. The acid-solubilized membrane proteins were then diluted by ten stepwise additions of 35.5 μL of water to achieve a final formic acid concentration of 10%. Sixteen μL of freshly prepared 1 mg/mL porcine pepsin A (Worthington Biochemical, Lakewood, NJ) were then added (1:50 enzyme: substrate ratio), and the proteins incubated at 37 °C for 5 hr in a shaking water bath to partially proteolyze the membrane proteins with the acid attenuated pepsin. The digests were then diluted by addition of 0.4 mL of water, and the peptides desalted by solid phase extraction (SPE) using a Sep-Pak C-18 Light cartridge (Waters, Milford, MA).
2.4 Proteomics Analysis
Two-dimensional liquid chromatography-tandem mass spectrometry (2D LC-MS/MS) was used for proteomic analysis of the peptic peptides of lens membrane proteins. The SPE-desalted peptides were first fractionated into 39 fractions on a 2.1×100 mm polysulfoethyl A strong cation exchange LC column (PolyLC Inc., Columbia, MD) with a KCl elution gradient, as described previously.(Wilmarth et al., 2006) These fractions were then dried by vacuum centrifugation and reconstituted in 40–100 μL of 0.02% trifluoroacetic acid (TFA). Aliquots (20 μL) of each fraction were then loaded at 30 μL/min onto a 300 μm × 0.5 cm C-18 PeptideMap trap cartridge with 0.02% TFA as the delivery solvent on an LC Packings capillary high performance LC system (Dionex Inc., Amsterdam, Netherlands). Following sample loading, the trapped peptides were directed, at a flow rate of 200 nL/min, to a 75 μm × 150 mm capillary C-18 analytical column with a 300 μm particle size (CVC Technologies, Fontana, CA). The analytical column was interfaced via a nanoflow electrospray ion source with an LTQ linear ion trap mass spectrometer (ThermoFisher, San Jose, CA). Peptides were chromatographed with 0.1% formic acid as mobile phase A and 98% acetonitrile containing 0.1% formic acid as mobile phase B. The binary-solvent gradient elution started at 4% B for 3 min, then linearly increased from 4% to 60% over 90 min, 60% to 90% over 20 min and maintained at 95% for an additional period of 5 min. The column was reconditioned at 4% B for 12 min after every two injections. The eluted peptides were sprayed through a 10-μm PicoTip fused silica spray emitter (New Objective Inc., Woburn, MA) with a static electrospray potential of 1.8 kV. Data-dependent collection of MS/MS spectra was conducted for the three most abundant parent ions following each survey scan over m/z of 400–2000 and the dynamic exclusion feature of the instrument control software (repeat count equal to 1, exclusion list size of 50, exclusion duration of 30 s, and exclusion mass width of −1 to +4) was applied. The tune file was configured with no averaging of microscans, a maximum inject time of 200 msec, and AGC targets of 33104 in MS mode and 1×104 in MS/MS mode.
2.5 Data Analysis
The analysis of the lens membrane fraction resulted in 265,071 MS/MS spectra. These spectra were used to create DTA files using BioWorks 3.2 (ThermoFisher) with a molecular weight range of 550 to 4,000, an absolute intensity threshold of 500, group scan setting of 1, a minimum group count of 1, a minimum of 25 ions, and charge state analysis using the ZSA algorithm. A human species subset of the Sprot (10/31/2008 download) protein database (19,038 sequences) was prepared with concatenated reversed entries (and common contaminants) and searched with SEQUEST (ThermoFisher). Parent ion and fragment ion tolerances of 2.5 and 1.0 Da were used with calculated average and monoisotopic masses, respectively. Cysteine had a static modification mass of +57 Da, and no enzyme specificity was chosen. An in-house suite of programs(Wilmarth et al., 2009) was used to calculate discriminant function values from SEQUEST scores to identify “correct” peptides and discard “incorrect” peptides using sequence-reversed matches to estimate false discovery rates. Of the 265,071 membrane protein spectra, 13,360 passed thresholds, with 246 matches to reversed sequences, giving an estimated peptide false discovery rate of 1.6%. A protein summary with peptide subset removal (Parsimony principle) was performed where proteins were required to have two or more peptides with distinct sequences. Different charge states of the same peptide sequence were not considered as unique peptides. There were 216 non-redundant proteins identified in the membrane protein data set with nine protein matches to reversed sequences (4% FDR). All AQP5 peptide sequences were verified by manual inspection of the spectra.
2.6 Western blotting
Lenses from C57 Black mice (8 weeks old), Wistar rats (21 days old), cows (~2 years old), and human (63 years old) were decapsulated and either processed as total lens fiber preparations, or microdissected into outer cortex, inner cortex, and core regions (rat, bovine, human) or cortex and core regions (mouse) due to the small size of the mouse lenses. Fiber cell preparations were then homogenized in 5 mM Tris–HCl, 5 mM EDTA, 5 mM EGTA (pH 8.0) containing protease inhibitors (Complete™; Roche Applied Science, Indianapolis, IN). Lens homogenates were centrifuged at 14,000 g for 30 min using an Eppendorf 5415R centrifuge (Eppendorf AG, Hamburg, Germany) to pellet cellular membranes. The pellets were washed three times in storage buffer (5 mM Tris (pH 8.0), 2 mM EDTA, 2 mM EGTA) containing protease inhibitors. Resultant ‘crude’ lens fiber cell membranes then underwent a urea/alkali washing procedure to remove peripheral membrane proteins and bound crystallins. Membranes were washed with 4M urea, 7M urea, then three times with 20mM NaOH, before three final washes in storage solution. The concentration of protein in each sample was determined using the BCA Protein Assay Reagent kit (Pierce, Rockford, USA). Total fiber cell membrane protein loadings were: mouse (0.06 μg), rat (8 μg), bovine (0.04 μg), human (0.2 μg), and rat cornea (0.012 μg). For microdissected regions of fiber cell membrane protein, protein loadings were: mouse (0.02 μg /lane), rat (3 μg/lane), bovine (0.1 μg/lane), and human (0.2 μg/lane). Proteins were separated using a 4–15% TGX gradient gel (Bio-Rad Laboratories, Inc., Hercules, CA) and transferred onto a hydrophobic polyvinylidene difluoride (PVDF) membrane (Immun-Blot™, Bio-Rad Laboratories, Hercules, CA) by electrophoresis for 1 h at 170 mA. The membrane was blocked for 1 h at room temperature in 5% skim milk in 1× Tris-buffered saline (TBS) (2 mM Tris–HCl, 140 mM NaCl, pH 7.6) with 0.1% Tween20, and then incubated with anti-AQP5 primary antibody overnight at 4 °C (1:500). The protein blot was then incubated with biotinylated secondary antibody (1:1000 dilution; GE Healthcare UK Ltd, Buckinghamshire, UK) for 1 h and then with streptavidin-horse radish peroxidase (1:2000; GE Healthcare UK Ltd, Buckinghamshire, UK) for 30 min, at room temperature. The bands were visualized using chemiluminescence detection (ECL plus™; GE Healthcare UK Ltd, Buckinghamshire, UK).
2.7 Tissue preparation for microscopy
Lenses extracted from either 21-day old Wistar rats or 6week old C57BL/6 mice were immediately fixed with 0·75% paraformaldehyde (PFA) at room temperature (r.t.) for 24 hr using previously developed protocols.(Jacobs et al., 2003) Human lenses (68 years old) were prepared for sectioning using a two-step fixation process described previously.(Lim et al., 2009) Briefly, whole human lenses were fixed for 24 hr in 0.75% PFA in PBS, encased in 6% agarose, and then cut in half perpendicular to the optical axis with a sharp blade. The halved lenses were fixed for a further 24 hr in 0.75% PFA in PBS. Rat and human lenses were then washed 3 times for 10 min in PBS and cryo-protected by incubation for 1 hr in a 10% sucrose solution in PBS, followed by incubation in 20% sucrose solution in PBS for 1 hr. Both incubations were at room temperature. Finally, lenses were incubated overnight at 4°C in a 30% sucrose solution in PBS as a cryoprotectant. For sectioning, whole lenses were mounted with the equator parallel to the surface of pre-chilled chucks and encased in Tissue-Tek O.C.T. compound (Sakura Finetek, Zoeterwoude, The Netherlands). Lenses were cryosectioned at −18°C on a cryostat (CM3050, Leica, Germany) using disposable blades (S-35; Feather Safety Razor Co., Japan). 10 μm thick (rat) or 14 μm thick (human) equatorial sections were transferred onto poly-L-lysine coated microscope slides and washed three times in PBS. For corneal tissue, fresh whole rat eyes were encased in Tissue-Tek O.C.T. and plunge-frozen in liquid nitrogen. Corneal tissue was sectioned at 16 μm, thaw mounted onto microscope slides, and fixed with 0.75% PFA in PBS for 1 hr (r.t.). Corneal tissue was then washed three times in PBS and immunolabelled.
2.8 Immunolabelling
Sections were incubated in blocking solution (3% bovine serum albumin, 3% fetal calf serum in PBS) for 1 hr at room temperature and washed three times for 5 min in PBS. The tissue was incubated in a solution of primary antibody (anti-AQP5) at a dilution of 1:100 in blocking solution for 2 hr at room temperature. Slides were washed 3×5 min in PBS and incubated for 1·5 hr, in the dark at room temperature, with anti-rabbit immunoglobulins conjugated with Alexa 488 (Invitrogen, Carlsbad, CA). Cell nuclei were stained with 100 μM propidium iodide for 5 min, at room temperature. 10 μl of anti-fade reagent (Citifluor AF1, Leicester, UK) was added to the slides, and tissue sections were analysed by confocal microscopy.
2.9 Confocal Microscopy
Images of each fluorophore-staining pattern were recorded digitally using a laser scanning confocal microscope (TCS SP2, Leica Microsystems, Wetzlar, Germany). Adjacent regions were imaged at high resolution (0.59 – 0.19 μm/pixel). For high magnification images of AQP5 in each species, gain and offset were optimised for each channel to collect the entire dynamic range. Image tiles were stitched together, pseudo-colored and different color channels overlaid using Adobe Photoshop software (version CS3, Adobe Inc., San Jose, CA).
3. RESULTS
3.1 Verification of AQP5 protein expression in the lens of different species
Recent analysis of bovine and mouse lenses by mass spectrometry showed that AQP5 was present in fiber cell membranes.(Bassnett et al., 2009; Wang et al., 2008) To verify AQP5 expression in a variety of other species, mass spectrometry and Western blot analyses were performed. Proteomic analysis of the human lens membrane preparation revealed extensive sequence coverage (56.2%) of human AQP5. Fig. 1 shows those portions of the AQP5 sequence that were confidently identified by tandem mass spectrometry and Supplemental Table 1 indicates the 52 individual peptides observed. Note that these data were acquired from the whole lens membrane protein preparation, that is, in the presence of abundant AQP0, MP20, and connexin proteins. A total of 224 spectral counts were recorded for AQP5 and 4,473 for AQP0. Spectral counts, based on the number of times a peptide was detected and identified, provide a rough estimate of protein abundance and in this experiment indicate that the abundance of AQP5 is roughly 5% that of AQP0 in the human lens.
Figure 1. Human lens AQP5 identification by mass spectrometry.

Human AQP5 peptides were identified from proteomics analysis of a human lens membrane preparation. Tandem mass spectra were analyzed by Sequest and subsequently manually verified. The sequences corresponding to identified peptides in the proteomics analysis are underlined and correspond to 56.2% sequence coverage.
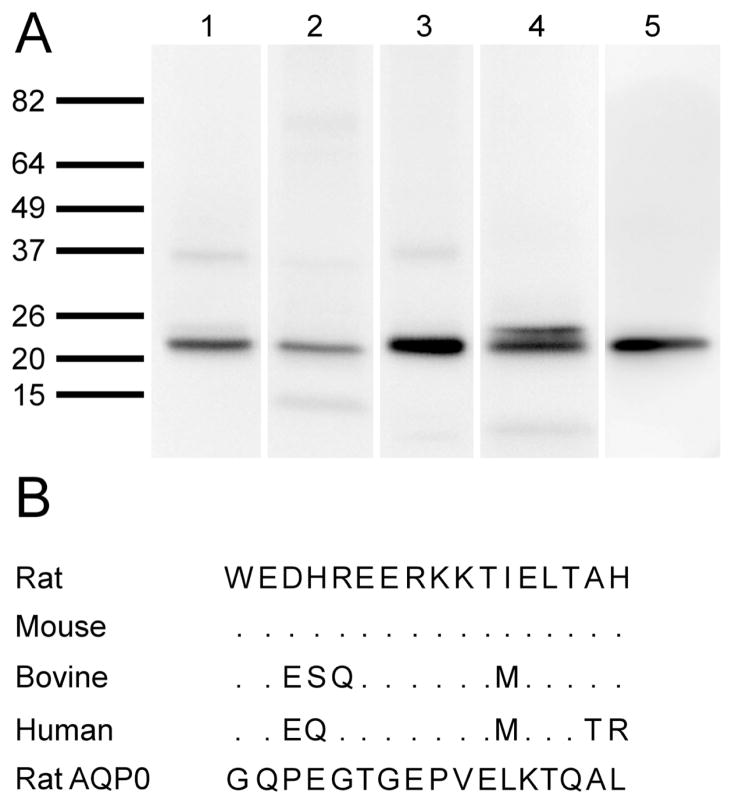
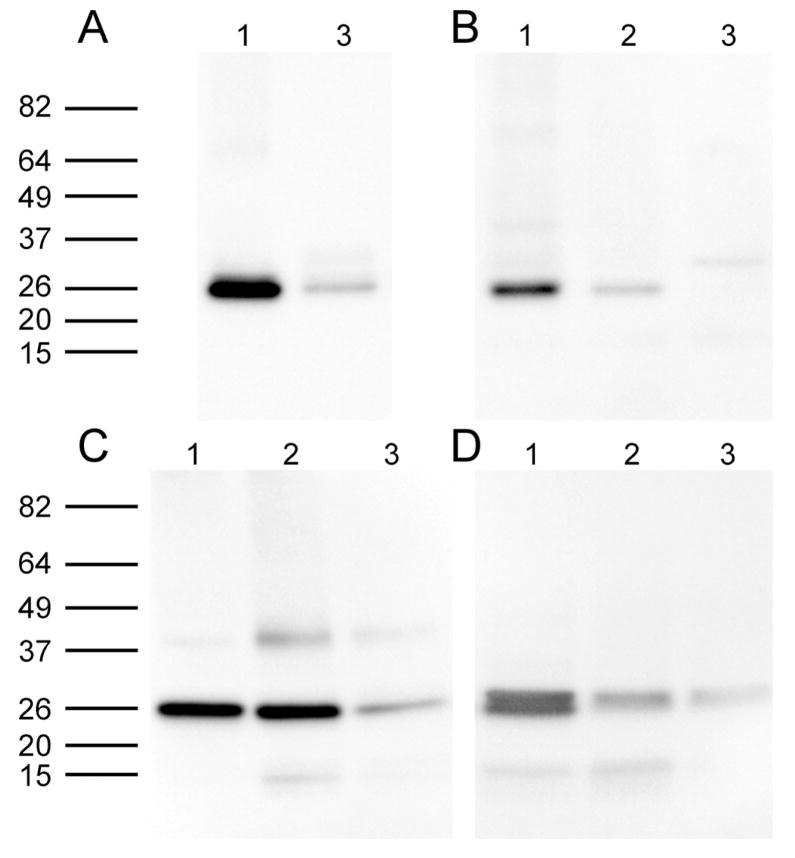
To show that expression of AQP5 is conserved amongst species, Western blotting was performed on total fiber cell membranes from mouse, rat, bovine and human lenses using an antibody directed against the C-terminus of rat AQP5 (Fig. 2). Protein loading was optimized for each species to give strong immunoreactivity. A band of the appropriate size for AQP5 was observed in all species of lens as well as the control tissue rat corneal epithelium that is known to express AQP5 (Fig. 2A). Western blotting of the same preparation using antibody pre-absorbed with control peptide resulted in elimination of all bands (SuppFig. 1). Sequence alignment of the antibody epitope region of the AQP5 C-terminus in the four analysed species indicates that the region is highly conserved (Fig. 2B). In addition, alignment with the C-terminus of AQP0 indicates no similarity between the two amino acid sequences, hence antibody cross-reactivity between AQP5 and AQP0 is unlikely to occur. These data, together with previous published results, indicate that AQP5 is expressed in lenses of rat, mouse, bovine and human species. However, this analysis does not tell us where in the lens AQP5 is expressed. Western blotting was used to investigate the expression of AQP5 in different regions of the lens in the four different species (Fig. 3). Lenses were dissected into two (mouse) or three (rat, bovine, human) regions to assess spatial differences in AQP5 expression. In the rodent lenses, strong AQP5 immunoreactivity was observed in the mouse cortex and rat outer cortex regions, which was greatly diminished in the mouse core and rat inner cortex (Fig. 3A and B). AQP5 immunoreactivity in the core of the rat lens was not detected by Western blotting (Fig. 3B) Interestingly, a second, higher molecular weight band was observed in the mouse cortex region, which was not present in the core region (Fig. 3A). In the bovine lens, AQP5 immunoreactivity was strong in the outer and inner cortex regions, and diminished in the core (Fig. 3C). Immunoreactivity for AQP5 in the human lens outer cortex was similar to mouse, where a doublet for AQP5 was observed (Fig. 3D). The occurrence of this doublet was found in an additional six human lenses ranging in age from 16 to 73 years of age (Supp. Fig. 2). However, in the inner cortex, immunoreactivity was diminished for the upper band and lost for the lower band. The upper band was further diminished in the lens core, which was in contrast to mouse immunoreactivity where the upper band was lost and the lower band remained. Taken together these observations suggest that full-length AQP5 protein is present throughout the lens, but its abundance is reduced in deeper regions of the lens. Furthermore, the appearance of a doublet in the mouse and human lens suggests either the expression of different forms of AQP5, or post translational modification to AQP5 occurs in these two species. Recent work by Kumari et al., (2012) suggests that this doublet is due to AQP5 phosphorylation.
Figure 2. AQP5 protein expression in lenses of several species.
(A) Western blot for AQP5 in total fiber cell membrane homogenates from mouse (Lane 1), rat (Lane 2), bovine (Lane 3) and human (Lane 4) lenses. Immunoreactivity for AQP5 from lens tissue matches that from the control tissue rat corneal epithelium (Lane 5). Interestingly, a doublet for AQP5 is observed in the human sample. (B) Comparison of the amino acid sequence used as the epitope for AQP5 antibody generation. AQP5 sequence is largely conserved amongst species, and is very different to the equivalent sequence in AQP0.
Figure 3. Differential immunoreactivity to the AQP5 C terminus in microdissected regions of the lens.
Western blots of AQP5 in the outer cortex (Lane 1), inner cortex (Lane 2) and core (Lane 3) show putative post-translational processing of the C terminus. In the mouse (A), immunoreactivity is present in the cortex (Lane 1) and reduced in the core (Lane 2). In the rat (B), immunoreactivity is greatly diminished in the inner cortex and lost in the core. In contrast, immunoreactivity for AQP5 is observed in all bovine (C) and human (D) lens regions, but is decreased in the lens core. Interestingly, an AQP5 doublet is apparent in the human lens outer cortex. The lower band, which migrates at the same molecular weight as in other species, is lost in inner cortical and core regions of the human lens.
3.2 Spatial mapping of AQP5 distribution in rodent and human lenses
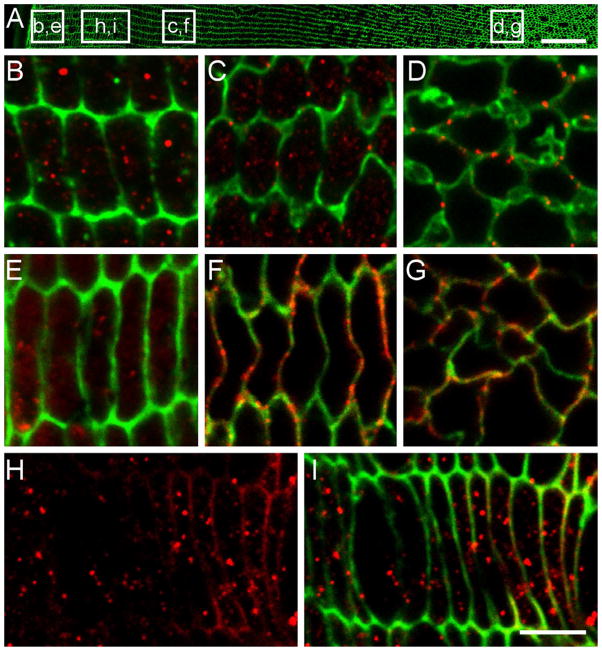
Previous immunomapping experiments have shown that the subcellular distribution of AQP0 changes as a function of fiber cell differentiation (Grey et al., 2009; Jacobs et al., 2003). Since we have optimized sectioning and labelling protocols to map the cellular distribution of specific membrane proteins at high resolution over large distances in both human and rat lenses (Jacobs et al., 2003; Lim et al., 2009), we applied these techniques to elucidate the cellular distribution of AQP5 in the different regions of rodent (Fig. 4) and human lenses (Fig. 5). Specificity of immunolabelling using the AQP5 antibody was established through our Western blot results (Figs 2 and 3), utilising rat corneal tissue as a positive control for in situ experiments since AQP5 is abundant in the corneal epithelium (Supp Fig. 3) and by the use of a control peptide preabsorption strategy in mouse equatorial lens sections (Fig. 4A). In addition, the specificity of antibodies made to the C-terminus of AQP5 was also demonstrated by Kumari et al., (2012) in AQP5 knockout mice where no labeling was detected in the knockout animals. The AQP5 antibody used in the current study was made to the same C-terminus region of the protein and we have aboserved the same AQP5 localization using both AQP5 C-terminus antibodies. Immunolabelling for AQP5 in rat lenses was intracellular in the most peripheral fiber cells located at r/a = 1.0 (where r is the distance of the cells from the lens center and a is the radius of the lens section) (Fig. 4B). A similar intracellular distribution of AQP5 was observed in mouse lens fibers at r/a = 1.0 (Fig. 4E). AQP5 was also predominantly intracellular in cortical rat fibers (r/a = 0.85) (Fig. 4C). However, in cortical fibers at an equivalent position in the mouse lens, AQP5 was associated with the cell membrane (Fig. 4F). In the lens core, at r/a = 0.4, AQP5 immunolabelling was membranous in rat (Fig. 4D) and mouse (Fig. 4G). The transition of intracellular to membranous labelling of AQP5 was captured in the mouse lens at r/a = 0.95 (Figs 4H, I). The transition of AQP5 labelling from intracellular to membranous in the rat occurred deeper into the lens (r/a = ~075 to ~0.65) but was difficult to precisely define due to the inherently lower AQP5 labelling observed in the rat lens. Clearly AQP5 subcellular distribution changes with fiber cell differentiation in rat and mouse lenses, but this change occurs at a different stage of fiber cell differentiation in each species.
Figure 4. Immunolocalization of AQP5 in rodent lenses.
(A) Overview of an equatorial section from a mouse lens double labelled with the membrane marker WGA (green) and the AQP5 antibody preabsorbed with its control peptide (red). The lack of AQP5 labelling indicates the specificity of antibody while the white boxes represent regions subsequently imaged at higher resolution in the rat (B–D) and mouse (E–I) lenses. To compensate for the differences in size of the two species of lens the distance from the centre (a) was expressed as a fraction of the lens radius (r), where r/a = 1 at the lens periphery and 0 at the lens centre. In the lens periphery (r/a = 1.0) AQP5 immunolabelling is predominantly intracellular in both the rat (B) and mouse (E). Deeper into the cortex (r/a = 0.85) this cytoplasmic labelling is still evident in the differentiating fiber cells in the rat (C), but shifts to a membrane labelling in the mouse (F) lens. In the core of the lens (r/a = 0.4) AQP5;labelling in the both the rat (D) and mouse lens (G) is associated with the membrane. Thus the transition of AQP5 from intracellular to membrane localization occurs at an earlier stage of in fiber cell differentiation in the mouse relative to the rat. In the mouse lens this transition was captured (H, I) and occurs abruptly at r/a = 0.95. Scale bars A, B–I = 50 μm, 5μm
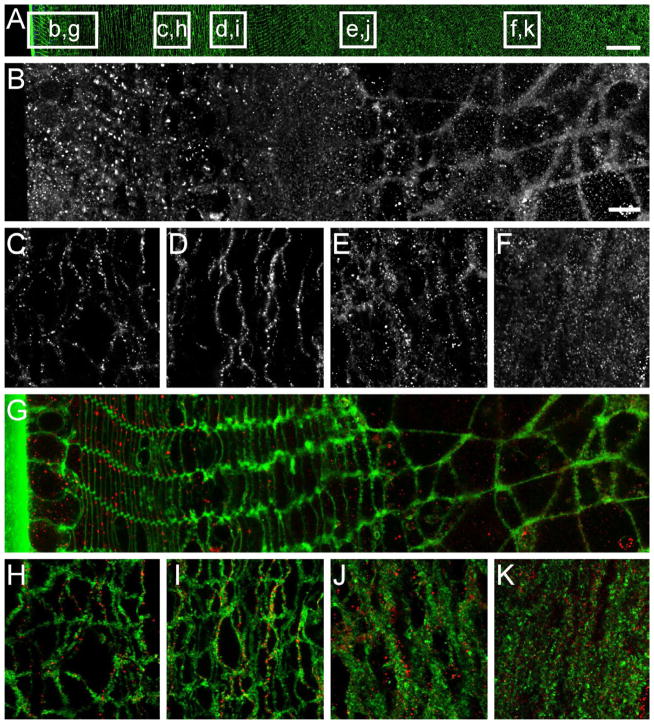
Figure 5. Immunolocalization of AQP5 in the human lens.
(A) Overview of equatorial human lens section from periphery to core showing cell membrane labelling (green) and cell nuclei (blue). White boxes represent regions subsequently imaged at higher resolution. (B–F) AQP5 labelling is generally punctate, and becomes more structured ~120 μm in from the lens capsule (B). The structured arrangement of AQP5 remains deeper in the lens (C–F). (G–K) Two channel images indicate that AQP5 (red) is located in the cell and associated with the fiber cell membrane (green) in puncta in the lens periphery (G), and becomes more uniformly distributed around fiber cell membranes with increasing depth in the lens (H–K). Scale bar A, B–K = 250 μm, 10 μm
An image montage from an equatorial section taken from a human lens has been labelled with the membrane marker WGA to indicate the position of higher magnification images (Fig. 5A). A single channel image of AQP5 immunolabelling shows how its distribution changes in the first 40–50 fiber cell layers (Fig. 5B). Initially diffuse punctate labelling is observed in peripheral fiber cells which, becomes more ordered approximately 120 μm in from the capsule. Outlines of deeper-lying fiber cells are evident in images of AQP5 immunolabelling (Fig. 5C–F), suggesting that AQP5 associates with the cell membranes in these regions. To confirm this, dual channel images containing AQP5 immunolabelling (red) and fiber cell membrane marker (green) are shown. The punctate AQP5 labelling in peripheral fiber cells is found inside the cell, but there is also some association with the cell membrane (Fig. 5G). At approximately 120 μm in from the capsule there is increased association of AQP5 labelling with cell membranes, although some intracellular labelling persists. Based on our previous studies of human lens morphology(Lim et al., 2009), this redistribution of AQP5 labelling to the membrane correlates with the loss of cell nuclei from differentiating fiber cells. In deeper fiber cells, AQP5 labelling is exclusively membranous (Fig. 5H and I) and in the core this membrane labelling is maintained although the membranes are more ruffled (Fig. 5J and K). Thus, consistent with our analysis of AQP5 expression in different regions of the lens by Western blotting (Fig. 3D), AQP5 labelling was detected in all regions of the lens. However, via immunohistochemistry it is evident that the subcellular distribution of AQP5 labelling changes from intracellular to membrane labelling at a discrete stage of fiber cell differentiation.
4. DISCUSSION
In this study we have confirmed recent proteomics studies that have reported AQP5 protein expression in mouse and bovine lenses(Bassnett et al., 2009; Wang et al., 2008) and have extended this finding to human and rat lenses. Furthermore, we have used high resolution fluorescence confocal microscopy to map the distribution of AQP5 in mouse, rat and human lenses, the latter for the first time, and shown that the majority of AQP5 in differentiating fiber cells of the outer cortex is located primarily intracellularly, before becoming associated with the membrane in mature fiber cells of the inner cortex and core. These results appear to be in contrast to a recent report in the mouse lens where only membrane localization was reported (Kumari et al., 2012). However, upon closer inspection it is evident that AQP5 labelling in peripheral fiber cells at the equator is predominately intracellular (see Figure 5G, Kumari et al., 2012) before transitioning into the plasma membrane in deeper fiber cells (see Figure 5H, Kumari et al., 2012). The AQP5 detected in mature fibers by both microscopy and Western blotting indicated that AQP5 antibody epitope levels were dramatically reduced in the core of all species of lenses. Thus our results show that in addition to AQP0, lens fiber cells express AQP5 and like AQP0 (Grey et al., 2009), AQP5 also exhibits differentiation-dependent changes in its subcellular localization.
Using an antibody-based detection system, it is difficult to quantify the relative amounts of each aquaporin in the lens due to the likelihood of each primary antibody having different affinities for their respective antigens. Previous detection of AQP5 in the rat lens by RT-PCR suggested that it was approximately 1000-fold lower in abundance than AQP1, and comparisons with AQP0 were not made.(Patil et al., 1997) Our proteomics results estimate the abundance of AQP5 at roughly 5% that of AQP0 in the human lens. Perhaps a better animal model for studies of AQP5 is the mouse, in which levels of AQP5 appear to be higher than in rat. Future experiments utilising the murine system will also allow access to gene knockout animals, in which the functional contribution of AQP5 to lens homeostasis can be more easily determined. To date, no cataract phenotype for AQP5 knockout animals has been reported, although it is possible that lens function is perturbed in less overt ways, which has been described for filensin and CP49 knockout animals in which cellular architecture of homozygous knockout animals was not initially affected but the optical properties were perturbed. (Alizadeh et al., 2003; Alizadeh et al., 2002)
In mouse, bovine and human lenses a decrease of AQP5 immunoreactivity was observed in the lens core by Western blot (Fig. 3). However, in the rat lens, AQP5 was found in cortical lens regions but not in the lens core (Fig. 3B), although AQP5 was detected in the core at low levels using immunohistochemistry (Fig. 5E,I). Despite the low AQP5 signal intensity in rat lenses the overall trend across species is clear, AQP5 is present in the core regions of lenses from 4 different species, but the levels, as detected by an antibody directed against the C-terminus of AQP5 are markedly reduced in the core relative to the outer cortex. This observed loss in AQP5 immunoreactivity in the deeper regions of the lens could be due to masking of the antibody epitope by an unknown protein that interacts with AQP5. However, since SDS-PAGE was run under denaturing and reducing conditions, masking of AQP5 the immunoreactivity is unlikely. It is more probable that the loss of AQP5 immunoreactivity in the lens core is due to a degradation of AQP5 and loss of the antibody epitope binding site on the C-terminal tail of AQP5, since similar age dependent post-translational truncation of the C terminus has been observed for AQP0(Grey et al., 2009) and lens connexins (Jacobs et al., 2004). Identifying the specific site(s) of this potential AQP5 truncation and determining its effects on AQP5 mediated water permeability are now required areas for future experimentation if we are to fully understand the contribution played by AQP5 to water transport in the different regions of the lens.
Western blots of total human and mouse fiber membrane protein also revealed a doublet for AQP5 (Fig. 2). This pattern was further explored using microdissection of lenses from each species into outer cortex, inner cortex, and core, a procedure that showed a doublet was present in the outer cortex of the human lens (Fig. 3D) and, to a lesser extent, in the mouse cortex (Fig. 3A). Interestingly, the lower band in the human matches the molecular weight of AQP5 in the other species analysed by Western blot, leaving the predominant form of AQP5 in the human lens a higher molecular weight. A report probing AQP5 presence in rabbit ocular tissue also reported two Western blot bands for AQP5 in the conjunctiva and cornea.(Oen et al., 2006), and suggested that phosphorylation maybe the cause for the shift in molecular weight of the AQP5 band. This result was confirmed in mouse lens where phosphatase treatment reduced the doublet to a single band (Kumari et al., 2012). The role of phosphorylation on AQP5 function in vivo remains to be elucidated; however, previous work suggests a role in AQP5 trafficking and internalization (Sidhaye et al., 2005; Kumari et al., 2012). The finding of persistent phosphorylation in human lens inner cortex and core regions, as evidenced by the upper band in Western blots (Fig. 3), is consistent with cell membrane localization reported by Sidhaye et al. (2005) and seen in Figure 5. We note however, that trafficking studies of phosphorylated AQP5 have yielded different results in different cell lines (Sidhaye et al., 2005; Kumari et al., 2012;
Our confocal images of AQP5 labelling in the outer cortex showed intracellular labelling for AQP5 in cortical lens fibers, suggesting AQP5 was present in intracellular vesicles or in organelle membranes (Bassnett, 1995) (Figs 4 and 5). In addition, intracellular labelling for AQP5 was observed in the human lens epithelium. This suggests that AQP5 is also expressed in the lens epithelium, consistent with the recent report by Kumari et al. (2012) in mouse lens epithelium but contradictory with previous Western blotting results (Varadaraj et al., 2007). Intracellular immunolabelling appeared stronger in the rat and mouse lens cortex than the human lens cortex, which may reflect species differences in the distribution of AQP5 in the lens and an increased ability of rodent lenses to store and traffic AQP5 to the fiber cell membrane.
Generally, aquaporins are constitutively expressed in the plasma membrane, although AQP2 in the renal collecting ducts is stored in intracellular vesicles that target to the apical membrane in response to anti-diuretic hormone stimulation to increase water absorption. AQP5, which is closely related to AQP2, has also been shown to traffic to the apical cell membrane of salivary gland cells in response to a number of stimuli to increase saliva production.(Ishikawa et al., 2006) Our initial observations in rodent (Fig. 4) and human (Fig. 5) lenses showed a shift in labelling for AQP5 from a putative vesicular pool to the membrane at discrete stages of fiber cell differentiation. This phenomenon has been observed previously for other rat lens membrane proteins including the second most abundant lens membrane protein MP20, the glycine transporter (GLYT1), and the glutamine/glutamate transporter (ASCT2).(Grey et al., 2003; Lim et al., 2006) It has been proposed that these proteins are first synthesized and stored in young differentiating fiber cells of the outer cortex that contain protein synthesis machinery, and are then inserted into the membranes of mature fiber cells that are incapable of de novo protein synthesis to change the membrane properties of cells in the lens core.(Donaldson and Lim, 2008) Since the single channel water permeability of AQP5 measured in Xenopus oocytes is high (5.0 × 10−14 cm3/s) (Yang and Verkman, 1997) relative to the more abundant fiber cell membrane protein AQP0 (2.8 × 10−16 cm3/s)(Chandy et al., 1997) it is reasonable to speculate that the observed insertion of AQP5 into the membranes of mature fiber cells would increase the water permeability of cells in the lens core. This increase would compensate for any reduction in water permeability caused by a switch in the function of AQP0 from a water channel to a junctional protein in this region of the lens. Since AQP5 is a mercury-sensitive water channel (Raina et al., 1995) and AQP0 is not,(Mulders et al., 1995) this hypothesis could be tested by comparing the ability of mercury compounds to inhibit water permeability in different regions of the lens. Unfortunately, to date, measurements of water permeability in membranes from the lens core have not been technically feasible.(Varadaraj et al., 2005) Confirmation of our hypothesis will require isolation of a membrane preparation from mature fiber cells that allows water permeability measurement to be performed.
5. Conclusions
In summary, we have confirmed that AQP5 protein expression occurs in mouse, rat, bovine and human lenses, indicating that lens fiber cells contain a second water channel. By mapping AQP5 labelling patterns in different regions of the lens we have shown that AQP5 is predominately located intracellularly, perhaps in vesicular storage pools, in differentiating fiber cells of the outer cortex, but in terminally differentiated mature fiber cells AQP5 is associated with the plasma membrane. Although observational in nature, our results lay the foundation for the advancement of the general hypothesis that AQP5 is a regulateable water channel whose differentiation-dependent recruitment to the plasma membranes of fiber cells can modulate water permeability in deeper regions of the lens. Having stated our working hypothesis, future work will be required to fully characterize specific AQP5 post translational modifications and to measure the relative contribution of AQP5 to water permeability in the different regions of the lens to test our initial hypothesis.
Supplementary Material
AQP5 is present in rat, mouse, cow and human lens fiber cells
AQP5 abundance decreases from cortical to core fiber cells in all species
Human AQP5 appears as a doublet by Western blot suggesting modification
AQP5 shifts from intracellular to membranous location with fiber cell age
Acknowledgments
This work was support by NIH grants EY-13462 (KLS), EY-07755 (LLD) and the MUSC Mass Spectrometry Facility, and the Health Research Council of New Zealand International Investment Opportunities Fund (PJD, KLS). The authors thank Michael Reviere for assistance in preparation of human lens membrane for proteomic analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alizadeh A, Clark JI, Seeberger T, Hess J, Blankenship T, FitzGerald PG. Targeted deletion of the lens fiber cell-specific intermediate filament protein filensin. Invest Ophthalmol Vis Sci. 2003;44:5252–5258. doi: 10.1167/iovs.03-0224. [DOI] [PubMed] [Google Scholar]
- Alizadeh A, Clark JI, Seeberger T, Hess J, Blankenship T, Spicer A, FitzGerald PG. Targeted genomic deletion of the lens-specific intermediate filament protein CP49. Invest Ophthalmol Vis Sci. 2002;43:3722–3727. [PubMed] [Google Scholar]
- Ball LE, Little M, Nowak MW, Garland DL, Crouch RK, Schey KL. Water permeability of C-terminally truncated aquaporin 0 (AQP0 1-243) observed in the aging human lens. Invest Ophthalmol Vis Sci. 2003;44:4820–4828. doi: 10.1167/iovs.02-1317. [DOI] [PubMed] [Google Scholar]
- Bassnett S. The fate of the Golgi apparatus and the endoplasmic reticulum during lens fiber cell differentiation. Invest Ophthalmol Vis Sci. 1995;36:1793–1803. [PubMed] [Google Scholar]
- Bassnett S, Beebe DC. Coincident loss of mitochondria and nuclei during lens fiber cell differentiation. Developmental Dynamics. 1992;194:85–93. doi: 10.1002/aja.1001940202. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Wilmarth PA, David LL. The membrane proteome of the mouse lens fiber cell. Molecular Vision. 2009;15:2448–2463. [PMC free article] [PubMed] [Google Scholar]
- Chandy G, Zampighi GA, Kreman M, Hall JE. Comparison of the water transporting properties of MIP and AQP1. Journal of Membrane Biology. 1997;159:29–39. doi: 10.1007/s002329900266. [DOI] [PubMed] [Google Scholar]
- Donaldson PJ, Grey AC, Merriman-Smith BR, Sisley AM, Soeller C, Cannell MB, Jacobs MD. Functional imaging: new views on lens structure and function. Clinical and Experimental Pharmacology and Physiology. 2004;31:890–895. doi: 10.1111/j.1440-1681.2004.04099.x. [DOI] [PubMed] [Google Scholar]
- Donaldson PJ, Lim J. Membrane transporters: new roles in lens cataract. In: Rizzo JF, Tombran-Tink J, Barnstable CJ, editors. Ocular Transporters in Ophthalmic Diseases and Drug Delivery. Humana Press Inc; Totawa: 2008. pp. 83–104. [Google Scholar]
- Gonen T, Cheng Y, Kistler J, Walz T. Aquaporin-0 membrane junctions form upon proteolytic cleavage. Journal of Molecular Biology. 2004;342:1337–1345. doi: 10.1016/j.jmb.2004.07.076. [DOI] [PubMed] [Google Scholar]
- Grey AC, Jacobs MD, Gonen T, Kistler J, Donaldson PJ. Insertion of MP20 into lens fibre cell plasma membranes correlates with the formation of an extracellular diffusion barrier. Experimental Eye Research. 2003;77:567–574. doi: 10.1016/s0014-4835(03)00192-1. [DOI] [PubMed] [Google Scholar]
- Grey AC, Li L, Jacobs MD, Schey KL, Donaldson PJ. Differentiation-dependent modification and subcellular distribution of aquaporin-0 suggests multiple functional roles in the rat lens. Differentiation. 2009;77:70–83. doi: 10.1016/j.diff.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Schey KL. Proteolysis and mass spectrometric analysis of an integral membrane: aquaporin 0. Journal of Proteome Research. 2004;3:807–812. doi: 10.1021/pr049945w. [DOI] [PubMed] [Google Scholar]
- Harries WE, Akhavan D, Miercke LJ, Khademi S, Stroud RM. The channel architecture of aquaporin 0 at a 2.2-A resolution. Proceedings of the National Academy of Sciences. 2004;101:14045–14050. doi: 10.1073/pnas.0405274101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Azlina A, Javkhlan P, Yao C, Akamatsu T, Hosoi K. Novel phosphorylation of aquaporin-5 at its threonine 259 through cAMP signaling in salivary gland cells. American Journal of Physiology Cell Physiology. 2011;301:C667–C678. doi: 10.1152/ajpcell.00058.2011. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Cho G, Yuan Z, Inoue N, Nakae Y. Aquaporin-5 water channel in lipid rafts of rat parotid glands. Biochimica et Biophysica Acta. 2006;1758:1053–1060. doi: 10.1016/j.bbamem.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Yuan Z, Inoue N, Skowronski MT, Nakae Y, Shono M, Cho G, Yasui MAP, Nielsen S. Identification of AQP5 in lipid rafts and its translocation to apical membranes by activation of M3 mAChRs in interlobular ducts of rat parotid gland. American Journal of Physiology - Cell Physiology. 2005;289:C1303–1311. doi: 10.1152/ajpcell.00211.2005. [DOI] [PubMed] [Google Scholar]
- Jacobs MD, Donaldson PJ, Cannell MB, Soeller C. Resolving morphology and antibody labeling over large distances in tissue sections. Microscopy Research and Techniques. 2003;62:83–91. doi: 10.1002/jemt.10360. [DOI] [PubMed] [Google Scholar]
- Jacobs MD, Soeller C, Sisley AM, Cannell MB, Donaldson PJ. Gap junction processing and redistribution revealed by quantitative optical measurements of connexin46 epitopes in the lens. Invest Ophthalmol Vis Sci. 2004;45:191–199. doi: 10.1167/iovs.03-0148. [DOI] [PubMed] [Google Scholar]
- Kalman K, Németh-Cahalan KL, Froger A, Hall JE. Phosphorylation determines the calmodulin-mediated Ca2+ response and water permeability of AQP0. Journal of Biological Chemistry. 2008;283:21278–21283. doi: 10.1074/jbc.M801740200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi-Tanaka C, Li X, Yao C, Akamatsu T, Kanamori N, Hosoi K. Protein kinase A-regulated membrane trafficking of a green fluorescent protein-aquaporin 5 chimera in MDCK cells. Biochimica et Biophysica Acta. 2006;1763:337–344. doi: 10.1016/j.bbamcr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj K. Intact AQP0 performs cell-to-cell adhesion. Biochemical and Biophysical Research Communications. 2009;390:1034–1039. doi: 10.1016/j.bbrc.2009.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj M, Yeramilli VS, Menon AG, Varadaraj K. Spatial expression of aquaporin 5 in mammalian cornea and lens, and regulation of its localization by phosphokinase A. Molecular Vision. 2012;18:957–967. [PMC free article] [PubMed] [Google Scholar]
- Kushmerick C, Rice SJ, Baldo GJ, Haspel HC, Mathias RT. Ion, water, neutral solute transport in Xenopus oocytes expressing frog lens MIP. Experimental Eye Research. 1995;61:351–362. doi: 10.1016/s0014-4835(05)80129-0. [DOI] [PubMed] [Google Scholar]
- Lim J, Lorentzen KA, Kistler J, Donaldson PJ. Molecular identification and characterisation of the glycine transporter (GLYT1) and the glutamine/glutamate transporter (ASCT2) in the rat lens. Experimental Eye Research. 2006;83:447–455. doi: 10.1016/j.exer.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Lim JC, Lam YC, Kistler J, Donaldson PJ. Molecular Characterization of the Cystine/Glutamate Exchanger and the Excitatory Amino Acid Transporters in the Rat Lens Invest. Ophthalmol Vis Sci. 2005;46:2869–2877. doi: 10.1167/iovs.05-0156. [DOI] [PubMed] [Google Scholar]
- Lim JC, Walker KL, Sherwin T, Schey KL, Donaldson PJ. Confocal microscopy reveals zones of membrane remodeling in the outer cortex of the human lens. Invest Ophthalmol Vis Sci. 2009;50:4304–4310. doi: 10.1167/iovs.09-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias RT, Kistler J, Donaldson P. The lens circulation. Journal of Membrane Biology. 2007;216:1–16. doi: 10.1007/s00232-007-9019-y. [DOI] [PubMed] [Google Scholar]
- Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiological Reviews. 1997;77:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- Michea LF, Andrinolo D, Ceppi H, Lagos N. Biochemical evidence for adhesion-promoting role of major intrinsic protein isolated from both normal and cataractous human lenses. Experimental Eye Research. 1995;61:293–301. doi: 10.1016/s0014-4835(05)80124-1. [DOI] [PubMed] [Google Scholar]
- Mulders SM, Preston GM, Deen PMT, Guggino WG, An OsCD, Agre P. Water channel properties of major intrinsic protein of lens. Journal of Biological Chemistry. 1995;270:9010–9016. doi: 10.1074/jbc.270.15.9010. [DOI] [PubMed] [Google Scholar]
- Oen H, Cheng P, Turner HC, Alvarez LJ, Candia OA. Identification and localization of aquaporin 5 in the mammalian conjunctival epithelium. Experimental Eye Research. 2006;83:995–998. doi: 10.1016/j.exer.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Palanivelu DV, Kozono DE, Engel A, Suda K, Lustig A, Agre P, Schirmer T. Co-axial association of recombinant eye lens aquaporin-0 observed in loosely packed 3D crystals. Journal of Molecular Biology. 2006;355:605–611. doi: 10.1016/j.jmb.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Patil RV, Saito I, Yang X, Wax MB. Expression of aquaporins in the rat ocular tissue. Experimental Eye Research. 1997;64:203–209. doi: 10.1006/exer.1996.0196. [DOI] [PubMed] [Google Scholar]
- Raina S, Preston GM, Guggino WB, Agre P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. Journal of Biological Chemistry. 1995;270:1908–1912. doi: 10.1074/jbc.270.4.1908. [DOI] [PubMed] [Google Scholar]
- Ren G, Cheng A, Reddy V, Melnyk P, Mitra A. Three-dimensional fold of the human AQP1 water channel determined at 4 Å resolution by electron crystallography of two-dimensional crystals embedded in ice. Journal of Molecular Biology. 2000;301:369–387. doi: 10.1006/jmbi.2000.3949. [DOI] [PubMed] [Google Scholar]
- Rose KM, Wang Z, Magrath GN, Hazard ES, Hildebrandt JD, Schey KL. Aquaporin 0-calmodulin interaction and the effect of aquaporin 0 phosphorylation. Biochemistry. 2008;47:339–347. doi: 10.1021/bi701980t. [DOI] [PubMed] [Google Scholar]
- Russell P, Robison WGJ, Kinoshita JH. A new method for rapid isolation of the intrinsic membrane proteins from lens. Experimental Eye Research. 1981;32:511–516. doi: 10.1016/s0014-4835(81)80030-9. [DOI] [PubMed] [Google Scholar]
- Sas DF, Sas J, Johnson KR, Menko AS, Johnson RG. Junctions between lens fiber cells are labeled with a monoclonal antibody shown to be specific for MP26. Journal of Cell Biology. 1985;100:216–225. doi: 10.1083/jcb.100.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhaye V, Hoffert JD, King LS. cAMP has distinct acute and chronic effects on aquaporin-5 in lung epithelial cells. Journal of BiologicalChemistry. 2005;280:3590–6. doi: 10.1074/jbc.M411038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadaraj K, Kumari S, Mathias RT. Functional expression of aquaporins in embryonic, postnatal, and adult mouse lenses. Developmental Dynamics. 2007;236:1319–1328. doi: 10.1002/dvdy.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadaraj K, Kumari S, Shiels A, Mathias RT. Regulation of aquaporin water permeability in the lens. Invest Ophthalmol Vis Sci. 2005;46:1393–1402. doi: 10.1167/iovs.04-1217. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Kumari SS, Mathias RT. Transgenic expression of AQP1 in the fiber cells of AQP0 knockout mouse: effects on lens transparency. Experimental Eye Research. 2010;91:393–404. doi: 10.1016/j.exer.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Han J, Schey KL. Spatial differences in an integral membrane proteome detected in laser capture microdissected samples. Journal of Proteome Research. 2008;7:2696–2702. doi: 10.1021/pr700737h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmarth PA, Riviere MA, David LL. Techniques for accurate protein identification in shotgun studies of human, mouse, bovine, and chicken lenses. Journal of Ocular Biology, Diseases, and Informatics. 2009;2:223–234. doi: 10.1007/s12177-009-9042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, Pevzner PA, David LL. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? Journal of Proteome Research. 2006;5:2554–2566. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G, Bernstein SL, Wyatt MK, Behal A, Touchman JW, Bouffard G, Smith D, Peterson K. Expressed sequence tag analysis of adult human lens for the NEIBank Project: Over 2000 non-redundant transcripts, novel genes and splice variants. Molecular Vision. 2002;8:171–184. [PubMed] [Google Scholar]
- Woo J, Chae YK, Jang SJ, Kim MS, Baek JH, Park JC, Trink B, Ratovitski E, Lee T, Park B, Park M, Kang JH, Soria JC, Lee J, Califano J, Sidransky D, Moon C. Membrane trafficking of AQP5 and cAMP dependent phosphorylation in bronchial epithelium. Biochemical and Biophysical Research Communications. 2008;366:321–327. doi: 10.1016/j.bbrc.2007.11.078. [DOI] [PubMed] [Google Scholar]
- Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. Journal of Biological Chemistry. 1997;272:16140–16146. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.