Abstract
Arachidonic acid is one of the pivotal signaling molecules associated with inflammation, pain and homeostatic function. Drugs specifically targeting these signaling pathways represent more than 25% of annual pharmaceutical sales worldwide. However, chronic administration of nonsteroidal anti-inflammatory drugs (NSAIDs) and rofecoxib (Vioxx), a potent cyclooxygenase-2 inhibitor, have been associated with adverse cardiovascular events. Understanding the possible mechanisms underlying these adverse events is critical for evaluating the risks and benefits of this group of drugs and for development of safer drugs. Using a powerful metabolomics approach, 20-hydroxyeicosatetraenoic acid (20-HETE) was identified among many of arachidonic acid metabolic products as a likely culprit for adverse cardiovascular side effect associated with rofecoxib and NSAIDs. In addition, using a similar metabolomic approach, epoxyeicosatrienoic acids (EETs), which are lipid mediators derived from arachidonic acid through the cytochrome P450 epoxygenase pathway, have been shown to exhibit cardioprotective effects in a murine myocardial infarction (MI) model. Inhibitors of the soluble epoxide hydrolase increase titers of epoxy fatty acids and both block and reverse cardiac hypertrophy in rodent models. These highly potent, orally available compounds may be promising for treating heart failure and other cardiovascular disease. In this review, we will summarize some of the recent advances using metabolomic profiling to gain insights into the involvement of arachidonic acid pathways in cardiovascular disease.
Introduction
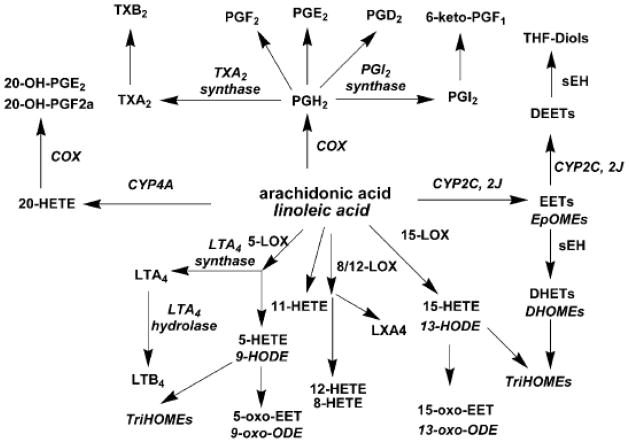
Arachidonic acid is a polyunsaturated omega-6 fatty acid which is released in response to tissue injury. Arachidonic acid represents one of the pivotal signaling molecules involved in the initiation and propagation of diverse signaling cascades regulating inflammation, pain and homeostatic function. Drugs developed to target these signaling pathways represent more than 25% of annual pharmaceutical sales worldwide. Arachidonic acid is metabolized through three enzymatic pathways. The cyclooxygenase (COX) pathway produces prostanoids. The lipoxygenase (LOX) pathway yields monohydroxy compounds and leukotrienes, while the cytochrome P450 (CYP450) epoxygenase pathway generates hydroxy and epoxyeicosanoids. This group of lipid mediators, which are derived from the 20-carbon atom arachidonic acid or similar fatty acids, is collectively referred to as eicosanoids (“eicosa” means 20 in Greek). A schematic metabolic pathway of arachidonic acid is shown in Figure 1. There is mounting evidence that some of these metabolic products play critical roles in cardiovascular disease.
Figure 1. Diagram illustrating the metabolic pathways for arachidonic acid and linoleic acid.
Arachidonic acid is metabolized through three enzymatic pathways. The cyclooxygenase (COX) pathway produces prostanoids. The lipoxygenase (LOX) pathway yields monohydroxy compounds and leukotrienes, while the cytochrome P450 (CYP) epoxygenase pathway generates hydroxy and epoxyeicosanoids.
Cardiovascular disease remains one of the leading causes of death in the Western societies [1]. Cardiac failure is the final consequence of a variety of etiologies including coronary heart disease, myocardial infarction (MI), hypertension, arrhythmia, viral myocarditis, and genetic cardiomyopathies. Once heart failure develops, the condition is for the most part irreversible. Although considerable progress has been made in the pharmacologic and device management of heart failure in recent decades, the mortality in heart failure patients remains significant. Moreover, the incidence and prevalence of cardiac failure are increasing as the population ages [2].
Recently, our laboratories have taken advantage of a new technique of metabolomic profiling using liquid chromatography-tandem mass spectrometry (LC-MS/MS) to elucidate the contribution of arachidonic acid metabolism in cardiovascular diseases. Metabolomics is a promising approach that has been widely used as a powerful tool in disease diagnosis [3], biomarker discovery [4], toxicity evaluation [5], gene function [6], and pharmacological research [7, 8]. In this review, we will provide examples of the use of metabolomic profiling in our two recent studies. Liu et al used a broad metabolomics approach to quantify the representative oxylipin mediators derived from arachidonic and linoleic acids mediated by COXs, LOXs, and CYP450s [9]. Oxylipins are oxygenated lipids and one of the most biologically important groups of oxylipins is the eicosanoid family. Specifically, Liu et al applied metabolomic profiling in a murine model and identified a link between the administration of rofecoxib (Vioxx) and adverse cardiovascular events. They found a significant increase in 20-hydroxyeicosatetraenoic acid (20-HETE), a potent vasoconstrictor and the culprit for increasing risk for MI and stroke. This mechanism may be shared among other non-aspirin non-steroidal anti-inflammatory drugs (NSAIDs). In the second instance, Li et al, using a similar approach, demonstrated the beneficial effects of increasing epoxyeicosatrienoic acid (EETs) levels and the EETs/dihydroxyeicosatrienoic acids (DHETs) ratio by application of soluble epoxide hydrolase (sEH) inhibitors in a murine MI model.
20-Hydroxyeicosatetraenoic acid (20-HETE)
Rofecoxib is a potent, orally active, and selective COX-2 inhibitor and was previously approved by the US Food and Drug Administration to treat a wide variety of pain ranging from arthritis, dysmenorrhea, and migraine. However, rofecoxib was voluntarily withdrawn from the worldwide market in 2004 because it was found to be associated with a higher risk for adverse cardiovascular events and stroke in arthritic patients compared with those on the control naproxen [10, 11]. This resulted in lawsuits involving an almost $5 billion settlement [12]. In addition, high doses of other coxibs such as valdecoxib and celecoxib are also associated with adverse cardiovascular events.
The current theory on the possible mechanisms responsible for the observed adverse effects of rofecoxib is that rofecoxib reduces the production of prostacyclin I2, an inhibitor of platelet aggregation (PGI2 see Figure 1). This results in an increase in platelet aggregation and may predispose patients for adverse cardiovascular events including MI or stroke. On the other hand, it has long been known that NSAIDs inhibit the production of the potent platelet activator thromboxane (TX) A2 [13, 14], so these agents may have thrombolytic activities. Hence, one might expect that conventional NSAIDs are “neutral” or even beneficial to the cardiovascular system. However, a significantly increased risk for cardiovascular diseases such as MI, hypertension, and heart failure has been observed to be associated with the administration of some of the non-aspirin NSAIDs, including but not limited to diclofenac, ibuprofen, naproxen, and indomethacin [15]. Thus, current hypothesis provides an incomplete explanation for the observed adverse cardiovascular events associated with the use of NSAIDs. We reason that this may result from the fact that the dominant theory is based on monitoring only a few arachidonate metabolites. To evaluate the risks and benefits of selective COX-2 inhibitors and to develop safe coxibs or adjuvants to improve the safety of existing coxibs, it is critical to understand the possible interactions among different arachidonic acid metabolic pathways.
We utilized a murine model which was administered with rofecoxib for a period of 3 months. In this model, there was a dramatic decrease in bleeding time which reflected an increase in platelet aggregability. Increased platelet aggregability has been associated with the pathogenesis of MI and stroke [16–19]. The quantitative levels of 27 oxylipin mediators of the plasma from treated animals were determined using metabolomic profiling. There was a greater than 120-fold increase in the plasma concentration of 20-HETE in the mice treated with rofecoxib. Moreover, a direct infusion of 20-HETE in mice also resulted in shortened bleeding time. Taken together, our data may provide a link between the use of rofecoxib and related compounds and the reported adverse cardiovascular events. This hypothesis suggests 20-HETE as a biomarker for cardiovascular risk from coxibs as well as possible strategies for attenuation of their adverse effects. For example, we predict that inhibition or down-regulation of CYP4A and or CYP4F may ablate the cardiovascular events of coxibs. In addition, this study exemplifies the use of metabolomic profiling as a promising tool to gain a more comprehensive understanding of biological processes.
Epoxyeicosatrienoic acids (EETs)
The CYP450 epoxygenase products, the epoxyeicosanoids, also known as EETs, are major anti-inflammatory arachidonic acid metabolites with a variety of biological effects [20]. There is growing evidence supporting the notion that EETs and other epoxy and diol fatty acids play a significant protective role in the cardiovascular system. EETs have been identified as potential endothelium-derived hyperpolarizing factors (EDHFs) [21, 22]. Major roles of EETs include modulation of both blood pressure and inflammatory signaling cascades. EETs are also associated with a number of other physiological functions including modulation of ion channel activity, angiogenesis, cell proliferation, vascular smooth muscle cell migration, leukocyte adhesion, platelet aggregation and thrombolysis, and neurohormone release [23, 24]. It has been proposed that diminished production or concentration of EETs contributes to cardiovascular disorders [25]. A polymorphism of the human CYP2J2 gene, which is highly expressed in heart and active in the biosynthesis of EETs, encodes variants with reduced catalytic activity and is independently associated with an increased risk of coronary artery disease [26]. Transgenic mice with cardiomyocyte-specific over-expression of human CYP2J2 demonstrate enhanced post-ischemic functional recovery [27] and significant protection against doxorubicin-induced cardiotoxicity [28]. As the protective role of EETs in cardiovascular biology has been increasingly recognized, considerable interest has arisen in developing methods to enhance the bioavailability of these compounds.
There are a variety of pathways involved in the degradation of EETs, but the major pathway is catalyzed by the enzyme soluble epoxide hydrolase (sEH). sEH converts EETs to their corresponding diols, dihydroxyeicosatrienoic acids (DHETs), thus modifying the function of these oxylipins [29]. Over the last few years, sEH has gained considerable attention as a therapeutic target for cardiovascular diseases [30–33]. Pharmacological inhibition of sEH has emerged as an intriguing approach to enhance the bioavailability of EETs and EET-mediated cardiovascular protective effects [29, 34–42]. The beneficial effects of several potent, orally available sEH inhibitors (sEHIs) in the prevention and reversal of cardiac remodeling due to maladaptive hypertrophy and myocardial ischemia/reperfusion have been demonstrated in several studies, including those from our laboratory [37, 40, 43, 44].
Specifically, we tested the effects of sEHIs on prevention and reversal of cardiac hypertrophy and post-ischemia remodeling, which are among the most common causes leading to heat failure. We demonstrated that sEHIs can prevent the development of pressure-induced cardiac hypertrophy using a murine model of thoracic aortic constriction (TAC) [43]. In addition, sEHIs reversed the pre-established cardiac hypertrophy caused by chronic pressure overload, in which a high level of expression of sEH in mouse atrial and ventricular myocytes was documented [43]. More recently, our laboratory has also demonstrated the beneficial effects of sEHIs on the progression of cardiac remodeling using a clinically relevant murine model of MI [44].
Using LC-MS/MS based techniques, we documented a significant decrease in the EETs/DHETs ratio in a MI model, indicating increased sEH activity, which may play a role in the progression of post-ischemia remodeling [44]. Treatment with sEHIs resulted in the normalization of the EETs/DHETs ratio and a reduction in post-ischemia LV remodeling [44]. Moreover, we have documented that the significant decrease in the EETs/DHETs ratio in the MI model showed a striking parallel with the changes in inflammatory cytokines at 3 weeks post MI, which indicated a heightened inflammatory state [44]. Additionally, the normalization of the EET/DHET ratios by sEHIs results in a reversal of the elevated cytokine levels in the MI model. Persistent inflammation, involving increased levels of inflammatory cytokines, plays a potential pathogenic role in the progression of LV dysfunction and remodeling in heart failure [45, 46]. The sEHIs appear to change the pattern of inflammatory mediators from a state which promotes the propagation of inflammation toward one promoting resolution.
sEH has been shown to be expressed in cardiomyocytes [37]. The expression of sEH is upregulated by angiotensin II in cardiac myocytes in vitro and in vivo, suggesting a potential regulatory role of sEH in angiotensin II-induced maladaptive hypertrophy [35]. Finally, recent human epidemiological studies have identified associations between variations in EET metabolic pathway genes and increased cardiovascular risk. A polymorphism leading to reduced gene activity of CYP2J2 is associated with an increased risk of coronary artery disease [26], and EPHX2 has also been identified as a susceptibility factor for heart failure [47]. Taken together, these findings suggest that increased sEH activity and reduced bioavailability of EETs may play a significant role in the pathogenesis of heart failure.
Interestingly, sEHIs have been shown to indirectly down regulate the expression of COX-2 and synergize with NSAIDs towards the reduction of inflammation [48, 49]. This suggests that these drug combinations (NSAIDs and sEHIs) may produce a beneficial anti-inflammatory effect while reducing the required dose of COX-2 inhibitors, thus avoiding the adverse cardiovascular side effects attributed to COX-2 inhibitors.
Future Directions
Both studies on 20-HETE and EETs demonstrate the use of metabolomic profiling as a promising tool to gain a more comprehensive understanding of the biological processes. An increased sEH activity has been demonstrated in an animal model of MI, supporting the notion that sEH may play an important role in the progression of post-ischemia remodeling. However, increased expression level of this enzyme has not been directly detected in the heart. Further studies to explore the mechanism by which sEH activity is dysregulated in MI and possible involvement of other organs such as liver and kidney may help to shed new light on the molecular defects in the pathogenesis of myocardial failure. Moreover, In order to definitively determine the best therapeutic utility for sEHIs, future studies to evaluate the potential interactions of sEHIs with other pharmaceuticals are warranted. It has been shown that regulation of sEH is intimately tied to the rennin-angiotensin-aldosterone system (RAAS) in animal models of hypertension and cardiac hypertrophy. sEHIs also synergize with COX-2 inhibitors and other modulators of the arachidonic acid cascade to exert anti-inflammatory effects. Thus, the combination of sEHIs and angiotensin converting enzyme inhibitors or COX inhibitors may provide powerful combination drug therapies with more favorable side effect profiles. Since heart failure is a complex clinical syndrome with diverse etiology and a wide array of pathophysiology, in order to translate the observed beneficial effects of sEHIs into clinical intervention in patient care, additional information is needed to identify whether the observed beneficial effects can be generalized to other causes of heart failure, such as idiopathic dilated cardiomyopathy, drug-induced heart failure,
Heart failure and its co-morbidities represent a major market and one of the paths to the clinic for sEHI that is best supported by mechanism, animal studies and human data. However, the length, high cost, and high risk of clinical trials for heart failure make this path unattractive to many pharmaceutical companies. Investigational new drug (IND) status for an sEHI could permit investigator initiated clinical trials to address this problem. Finally, other oxylipins apart from the ω-6 arachidonic acid metabolites may be relevant in cardiovascular disease. For example, the ω-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) accumulate in the heart [50] and the epoxides of EPA and DHA are analogs of the EETs. In fact, DHA and EPA epoxides share some of the vasoactive and anti-inflammatory properties of the EETs in vitro and in some cases have been shown to be more potent [51, 52]. DHA and EPA epoxides are, in general, better substrates for sEH than the EETs [Morisseau and Inceoglu, unpublished], so it is possible that some of the cardioprotective effects of sEH inhibition are due to reduction in DHA and EPA epoxide metabolism in the heart. Intervention studies with ω-3 lipids could test the hypothesis that these natural products have a protective effect on cardiac hypertrophy.
In summary, metabolomic profiling has been shown to not only identify a potential marker of risk or effect of rofecoxib and possibly other drugs, but also to demonstrate paths to mitigate the risk of these valuable pharmaceuticals. In addition, metabolomic profiling expanded our knowledge of the role of eicosanoids in the progression of cardiac hypertrophy. This knowledge from profiling pointed to inhibitors of the sEH as possible therapeutic agents for the prevention or even treatment of this and other cardiovascular diseases. Over the past few years, the use of sEHIs in animal models have demonstrated that sEHI have therapeutic potential in a broad range of cardiac diseases, many of which are co-morbidities with hypertrophy. Although possible side effects associated with the inhibition or genetic deletion of sEH have been reported [53, 54], the data obtained from several laboratories employing animal models of cardiac hypertrophy and ischemia/reperfusion support the notion that sEHIs and possibly EET mimics represent promising therapeutic targets for combating detrimental cardiac remodeling, heart failure and related diseases.
Acknowledgments
This work was supported by the Department of Veteran Affairs Merit Review Grant and the National Institutes of Health Grants (HL85844, HL85727) to N.C. Partial support was provided by NIEHS Grant R37 ES02710, the NIEHS Superfund Basic Research Program (P42 ES04699), the NIEHS Center for Children’s Environmental Health & Disease Prevention (P01 ES11269) and a Technology Translational Grant from UCDHS to B.D.H. H.Q. is supported by an American Heart Association Postdoctoral Fellowship. T.R.H. is supported by NIH T32 Training Grant in Basic and Translational Cardiovascular Science (T32 HL86350). BDH is a George and Judy Marcus Fellow of the American Asthma Society.
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, et al. Heart Disease and Stroke Statistics--2007 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006 doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 3.Brindle JT, Antti H, Holmes E, Tranter G, Nicholson JK, Bethell HW, Clarke S, Schofield PM, et al. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat Med. 2002;8:1439–44. doi: 10.1038/nm1202-802. [DOI] [PubMed] [Google Scholar]
- 4.Lewis GD, Wei R, Liu E, Yang E, Shi X, Martinovic M, Farrell L, Asnani A, et al. Metabolite profiling of blood from individuals undergoing planned myocardial infarction reveals early markers of myocardial injury. J Clin Invest. 2008;118:3503–12. doi: 10.1172/JCI35111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindon JC, Keun HC, Ebbels TM, Pearce JM, Holmes E, Nicholson JK. The Consortium for Metabonomic Toxicology (COMET): aims, activities and achievements. Pharmacogenomics. 2005;6:691–9. doi: 10.2217/14622416.6.7.691. [DOI] [PubMed] [Google Scholar]
- 6.Fiehn O. Metabolomics--the link between genotypes and phenotypes. Plant Mol Biol. 2002;48:155–71. [PubMed] [Google Scholar]
- 7.Wikoff WR, Pendyala G, Siuzdak G, Fox HS. Metabolomic analysis of the cerebrospinal fluid reveals changes in phospholipase expression in the CNS of SIV-infected macaques. J Clin Invest. 2008;118:2661–9. doi: 10.1172/JCI34138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu JY, Yang J, Inceoglu B, Qiu H, Ulu A, Hwang SH, Chiamvimonvat N, Hammock BD. Inhibition of soluble epoxide hydrolase enhances the anti-inflammatory effects of aspirin and 5-lipoxygenase activation protein inhibitor in a murine model. Biochem Pharmacol. 79:880–7. doi: 10.1016/j.bcp.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu JY, Li N, Yang J, Qiu H, Ai D, Chiamvimonvat N, Zhu Y, Hammock BD. Metabolic profiling of murine plasma reveals an unexpected biomarker in rofecoxib-mediated cardiovascular events. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1011278107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–9. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 11.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520–8. doi: 10.1056/NEJM200011233432103. 2 p following 1528. [DOI] [PubMed] [Google Scholar]
- 12.Wadman M. Merck settles Vioxx lawsuits for $4.85 billion. Nature. 2007;450:324–325. [Google Scholar]
- 13.Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, Lawson JA, FitzGerald GA. Role of prostacyclin in the cardiovascular response to thromboxane A(2) Science. 2002;296:539–541. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- 14.FitzGerald GA. Coxibs and cardiovascular disease. New England Journal of Medicine. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 15.Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. British Medical Journal. 2005;330:1366–1369. doi: 10.1136/bmj.330.7504.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tofler GH, Brezinski D, Schafer AI, Czeisler CA, Rutherford JD, Willich SN, Gleason RE, Williams GH, et al. Concurrent Morning Increase in Platelet Aggregability and the Risk of Myocardial-Infarction and Sudden Cardiac Death. New England Journal of Medicine. 1987;316:1514–1518. doi: 10.1056/NEJM198706113162405. [DOI] [PubMed] [Google Scholar]
- 17.Kalendov Z, Austin J, Steele P. Increased Platelet Aggregability in Young Patients with Stroke. Neurology. 1974;24:373–373. doi: 10.1001/archneur.1975.00490430035004. [DOI] [PubMed] [Google Scholar]
- 18.Milner PC, Martin JF. Shortened Bleeding-Time in Acute Myocardial-Infarction and Its Relation to Platelet Mass. British Medical Journal. 1985;290:1767–1770. doi: 10.1136/bmj.290.6484.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalby Kristensen S, Milner PC, Martin JF. Bleeding time and platelet volume in acute myocardial infarction--a 2 year follow-up study. Thromb Haemost. 1988;59:353–6. [PubMed] [Google Scholar]
- 20.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–9. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang X, Kaduce TL, Weintraub NL, Spector AA. Cytochrome P450 metabolites of arachidonic acid: rapid incorporation and hydration of 14,15-epoxyeicosatrienoic acid in arterial smooth muscle cells. Prostaglandins Leukot Essent Fatty Acids. 1997;57:367–71. doi: 10.1016/s0952-3278(97)90412-9. [DOI] [PubMed] [Google Scholar]
- 22.Eckman DM, Hopkins N, McBride C, Keef KD. Endothelium-dependent relaxation and hyperpolarization in guinea-pig coronary artery: role of epoxyeicosatrienoic acid. Br J Pharmacol. 1998;124:181–9. doi: 10.1038/sj.bjp.0701778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs) Prostaglandins Other Lipid Mediat. 2007;82:42–9. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiecker M, Liao JK. Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch Biochem Biophys. 2005;433:413–20. doi: 10.1016/j.abb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–85. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 26.Spiecker M, Darius H, Hankeln T, Soufi M, Sattler AM, Schaefer JR, Node K, Borgel J, et al. Risk of coronary artery disease associated with polymorphism of the cytochrome P450 epoxygenase CYP2J2. Circulation. 2004;110:2132–6. doi: 10.1161/01.CIR.0000143832.91812.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, Gooch R, Foley J, et al. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res. 2004;95:506–14. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, El-Sikhry H, Chaudhary KR, Batchu SN, Shayeganpour A, Jukar TO, Bradbury JA, Graves JP, et al. Overexpression of CYP2J2 provides protection against doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol. 2009;297:H37–46. doi: 10.1152/ajpheart.00983.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87:992–8. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 30.Marino JP., Jr Soluble epoxide hydrolase, a target with multiple opportunities for cardiovascular drug discovery. Curr Top Med Chem. 2009;9:452–63. doi: 10.2174/156802609788340805. [DOI] [PubMed] [Google Scholar]
- 31.Gross GJ, Nithipatikom K. Soluble epoxide hydrolase: a new target for cardioprotection. Curr Opin Investig Drugs. 2009;10:253–8. [PMC free article] [PubMed] [Google Scholar]
- 32.Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol. 2007;50:225–37. doi: 10.1097/FJC.0b013e3181506445. [DOI] [PubMed] [Google Scholar]
- 33.Imig JD. Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors. Cardiovasc Drug Rev. 2006;24:169–88. doi: 10.1111/j.1527-3466.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 34.Larsen BT, Gutterman DD, Hatoum OA. Emerging role of epoxyeicosatrienoic acids in coronary vascular function. Eur J Clin Invest. 2006;36:293–300. doi: 10.1111/j.1365-2362.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- 35.Ai D, Pang W, Li N, Xu M, Jones PD, Yang J, Zhang Y, Chiamvimonvat N, et al. Soluble epoxide hydrolase plays an essential role in angiotensin II-induced cardiac hypertrophy. Proc Natl Acad Sci U S A. 2009;106:564–9. doi: 10.1073/pnas.0811022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batchu SN, Law E, Brocks DR, Falck JR, Seubert JM. Epoxyeicosatrienoic acid prevents postischemic electrocardiogram abnormalities in an isolated heart model. J Mol Cell Cardiol. 2009;46:67–74. doi: 10.1016/j.yjmcc.2008.09.711. [DOI] [PubMed] [Google Scholar]
- 37.Motoki A, Merkel MJ, Packwood WH, Cao Z, Liu L, Iliff J, Alkayed NJ, Van Winkle DM. Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemia-reperfusion injury in vivo. Am J Physiol Heart Circ Physiol. 2008;295:H2128–34. doi: 10.1152/ajpheart.00428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulu A, Davis BB, Tsai HJ, Kim IH, Morisseau C, Inceoglu B, Fiehn O, Hammock BD, et al. Soluble epoxide hydrolase inhibitors reduce the development of atherosclerosis in apolipoprotein e-knockout mouse model. J Cardiovasc Pharmacol. 2008;52:314–23. doi: 10.1097/FJC.0b013e318185fa3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Carroll MA, Chander PN, Falck JR, Sangras B, Stier CT. Soluble epoxide hydrolase inhibitor, AUDA, prevents early salt-sensitive hypertension. Front Biosci. 2008;13:3480–7. doi: 10.2741/2942. [DOI] [PubMed] [Google Scholar]
- 40.Gross GJ, Gauthier KM, Moore J, Falck JR, Hammock BD, Campbell WB, Nithipatikom K. Effects of the selective EET antagonist, 14,15-EEZE, on cardioprotection produced by exogenous or endogenous EETs in the canine heart. Am J Physiol Heart Circ Physiol. 2008;294:H2838–44. doi: 10.1152/ajpheart.00186.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, Goralski K, Carey MA, et al. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res. 2006;99:442–50. doi: 10.1161/01.RES.0000237390.92932.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–4. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 43.Xu D, Li N, He Y, Timofeyev V, Lu L, Tsai HJ, Kim IH, Tuteja D, et al. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103:18733–8. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li N, Liu JY, Timofeyev V, Qiu H, Hwang SH, Tuteja D, Lu L, Yang J, et al. Beneficial effects of soluble epoxide hydrolase inhibitors in myocardial infarction model: Insight gained using metabolomic approaches. J Mol Cell Cardiol. 2009;47:835–845. doi: 10.1016/j.yjmcc.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satoh M, Minami Y, Takahashi Y, Nakamura M. Immune modulation: role of the inflammatory cytokine cascade in the failing human heart. Curr Heart Fail Rep. 2008;5:69–74. doi: 10.1007/s11897-008-0012-2. [DOI] [PubMed] [Google Scholar]
- 46.Sekiguchi K, Li X, Coker M, Flesch M, Barger PM, Sivasubramanian N, Mann DL. Cross-regulation between the renin-angiotensin system and inflammatory mediators in cardiac hypertrophy and failure. Cardiovasc Res. 2004;63:433–42. doi: 10.1016/j.cardiores.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Monti J, Fischer J, Paskas S, Heinig M, Schulz H, Gosele C, Heuser A, Fischer R, et al. Soluble epoxide hydrolase is a susceptibility factor for heart failure in a rat model of human disease. Nat Genet. 2008;40:529–37. doi: 10.1038/ng.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmelzer KR, Inceoglu B, Kubala L, Kim IH, Jinks SL, Eiserich JP, Hammock BD. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103:13646–51. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci U S A. 2005;102:9772–7. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Owen AJ, Peter-Przyborowska BA, Hoy AJ, McLennan PL. Dietary fish oil dose- and time-response effects on cardiac phospholipid fatty acid composition. Lipids. 2004;39:955–61. doi: 10.1007/s11745-004-1317-0. [DOI] [PubMed] [Google Scholar]
- 51.VanRollins M. Epoxygenase metabolites of docosahexaenoic and eicosapentaenoic acids inhibit platelet aggregation at concentrations below those affecting thromboxane synthesis. J Pharmacol Exp Ther. 1995;274:798–804. [PubMed] [Google Scholar]
- 52.Ye D, Zhang D, Oltman C, Dellsperger K, Lee HC, VanRollins M. Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J Pharmacol Exp Ther. 2002;303:768–76. doi: 10.1124/jpet.303.2.768. [DOI] [PubMed] [Google Scholar]
- 53.Pokreisz P, Fleming I, Kiss L, Barbosa-Sicard E, Fisslthaler B, Falck JR, Hammock BD, Kim IH, et al. Cytochrome P450 epoxygenase gene function in hypoxic pulmonary vasoconstriction and pulmonary vascular remodeling. Hypertension. 2006;47:762–70. doi: 10.1161/01.HYP.0000208299.62535.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hutchens MP, Nakano T, Dunlap J, Traystman RJ, Hurn PD, Alkayed NJ. Soluble epoxide hydrolase gene deletion reduces survival after cardiac arrest and cardiopulmonary resuscitation. Resuscitation. 2008;76:89–94. doi: 10.1016/j.resuscitation.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]